Submitted:
18 April 2024
Posted:
18 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
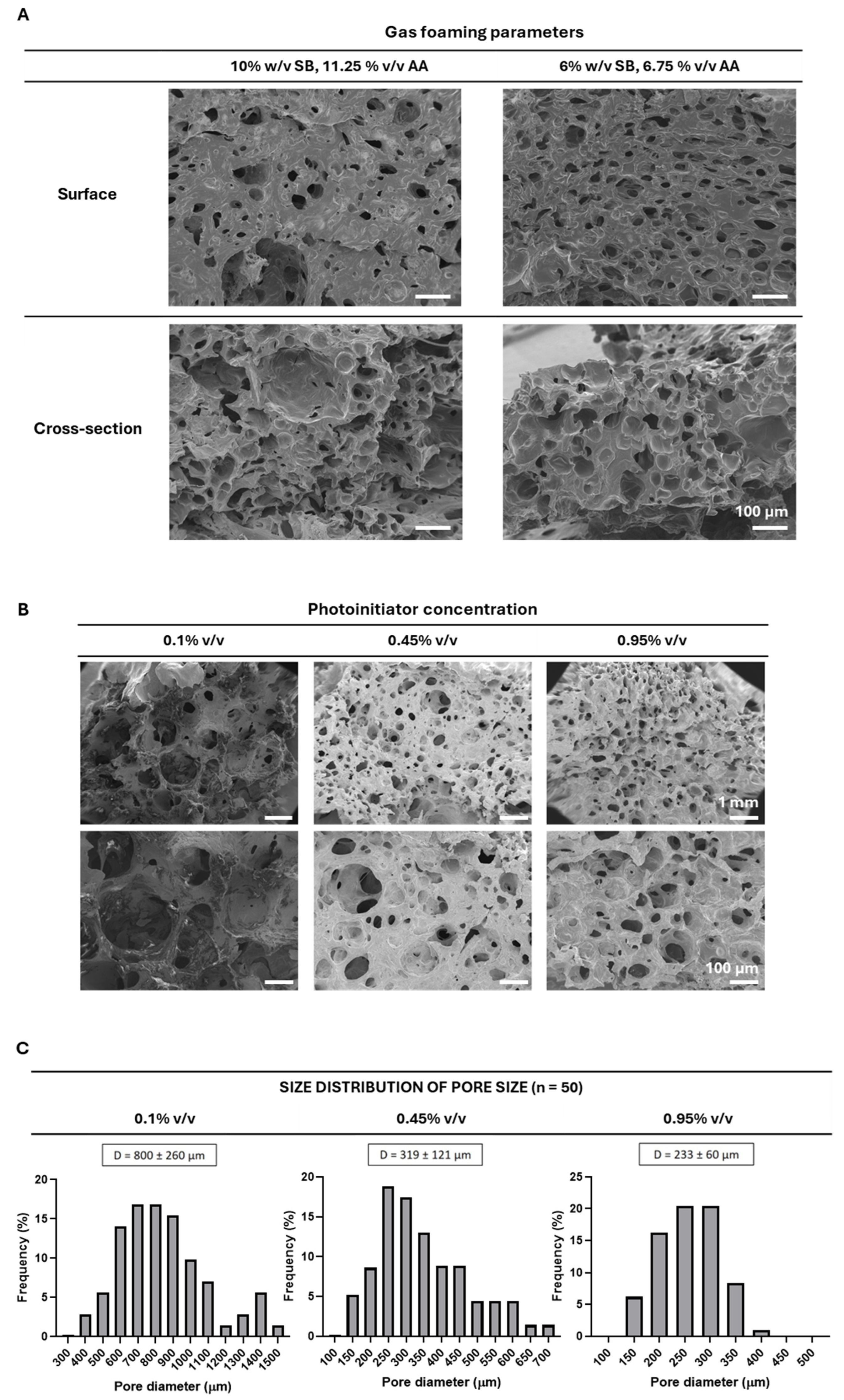
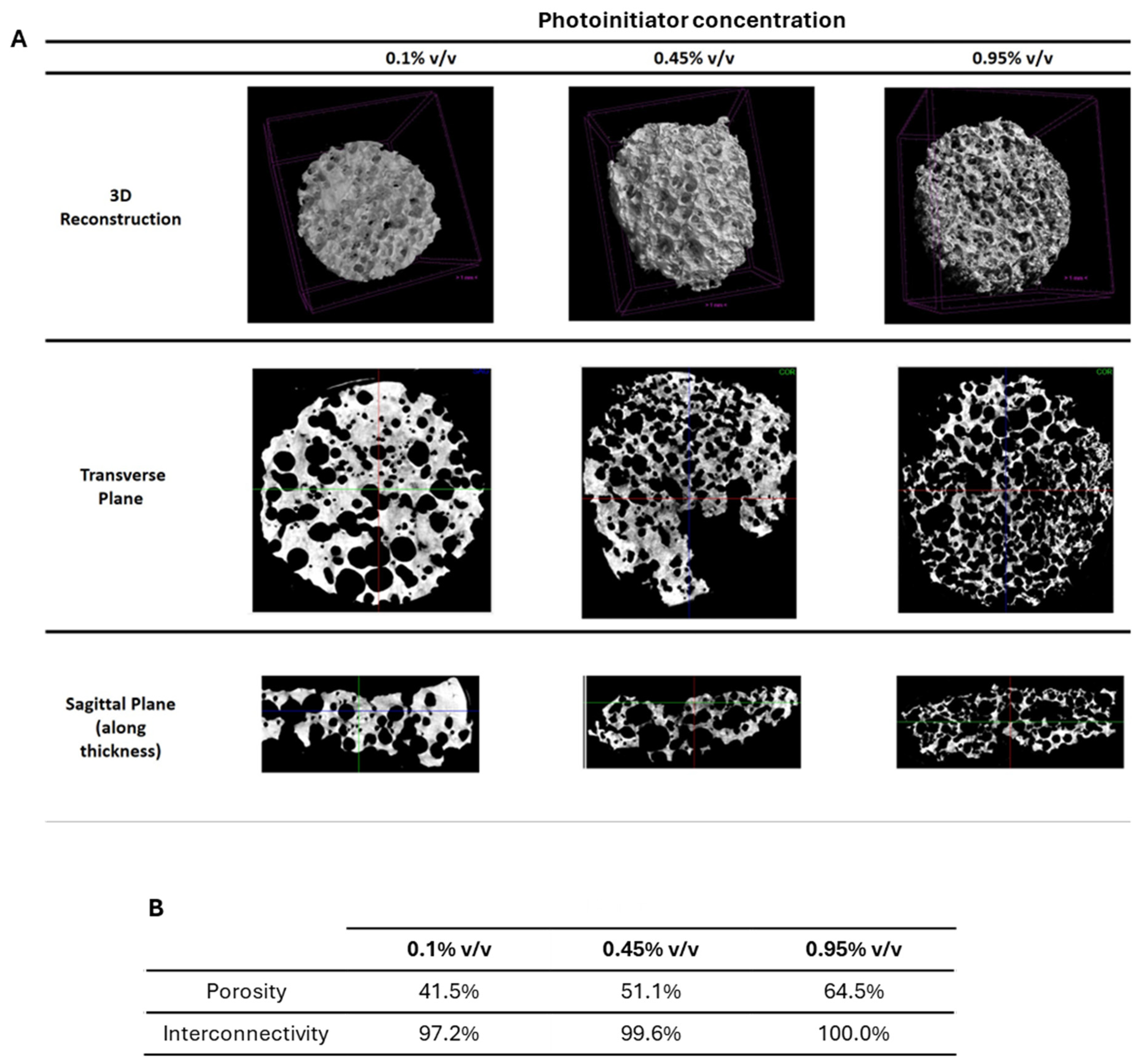
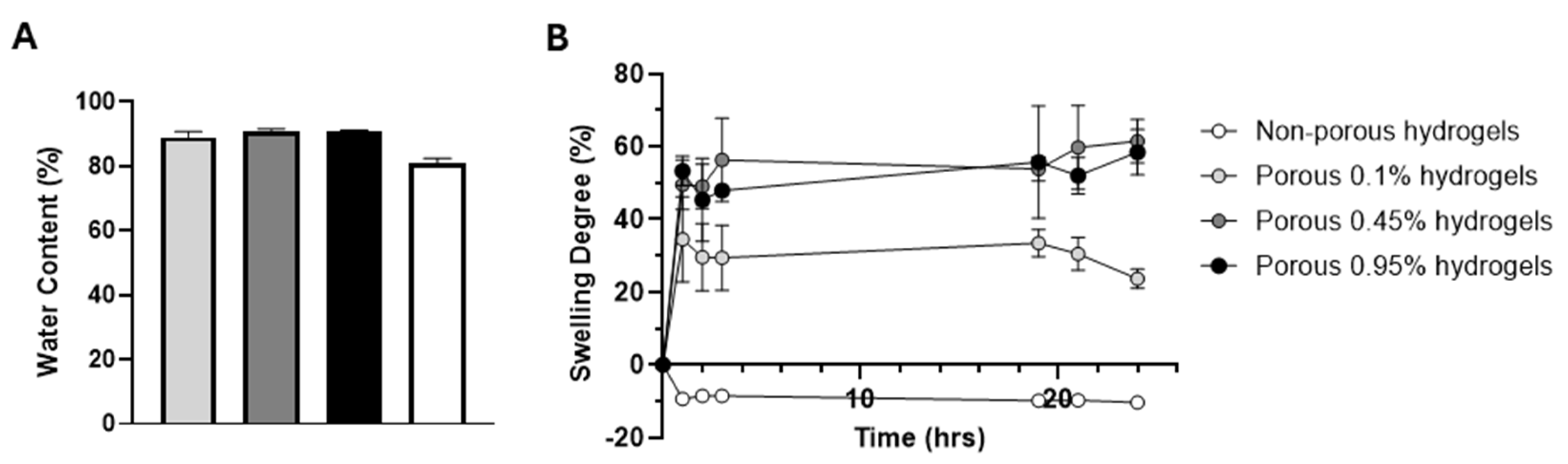
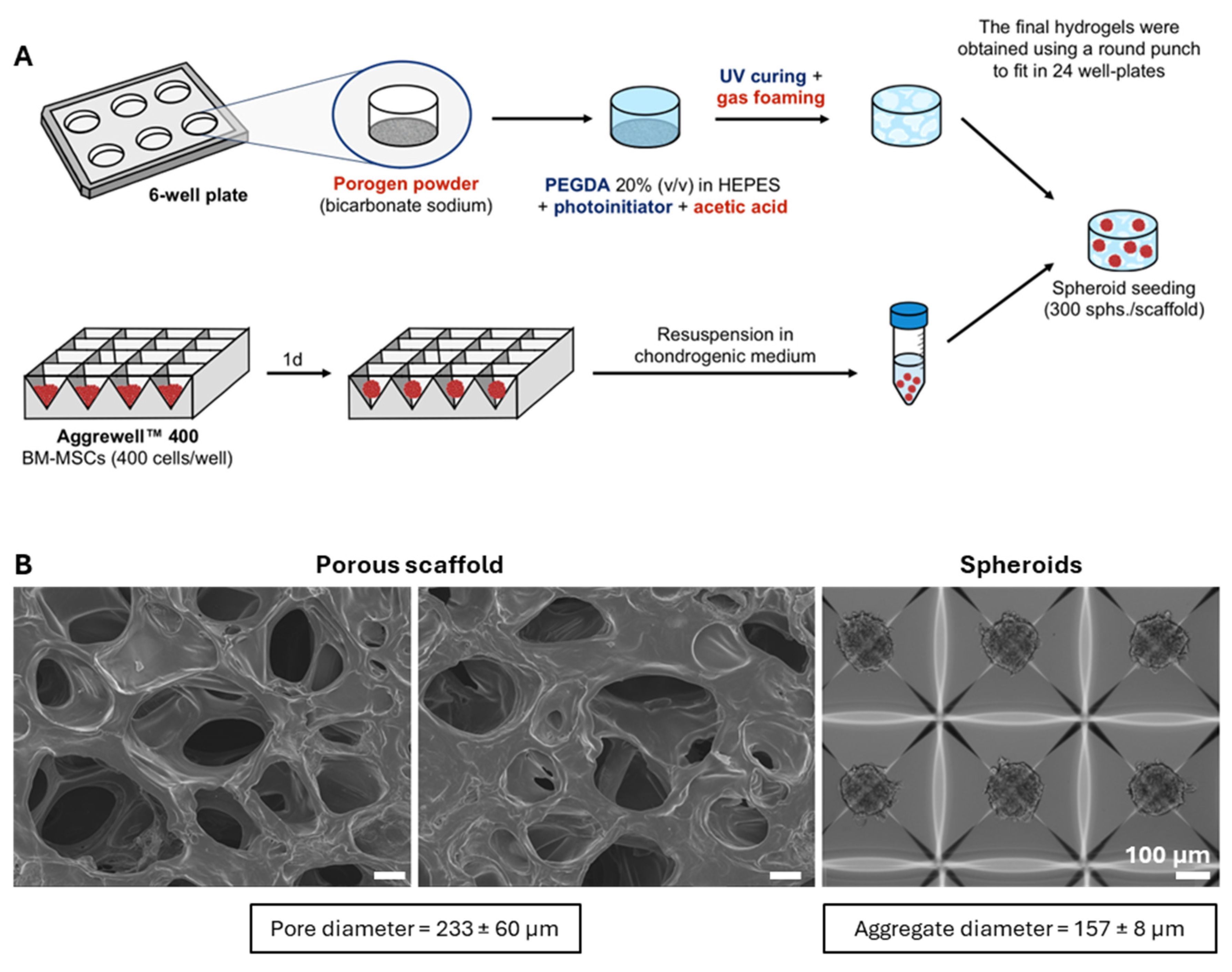
2.1. Production and Structural Characterization of 3D Porous PEGDA Hydrogels
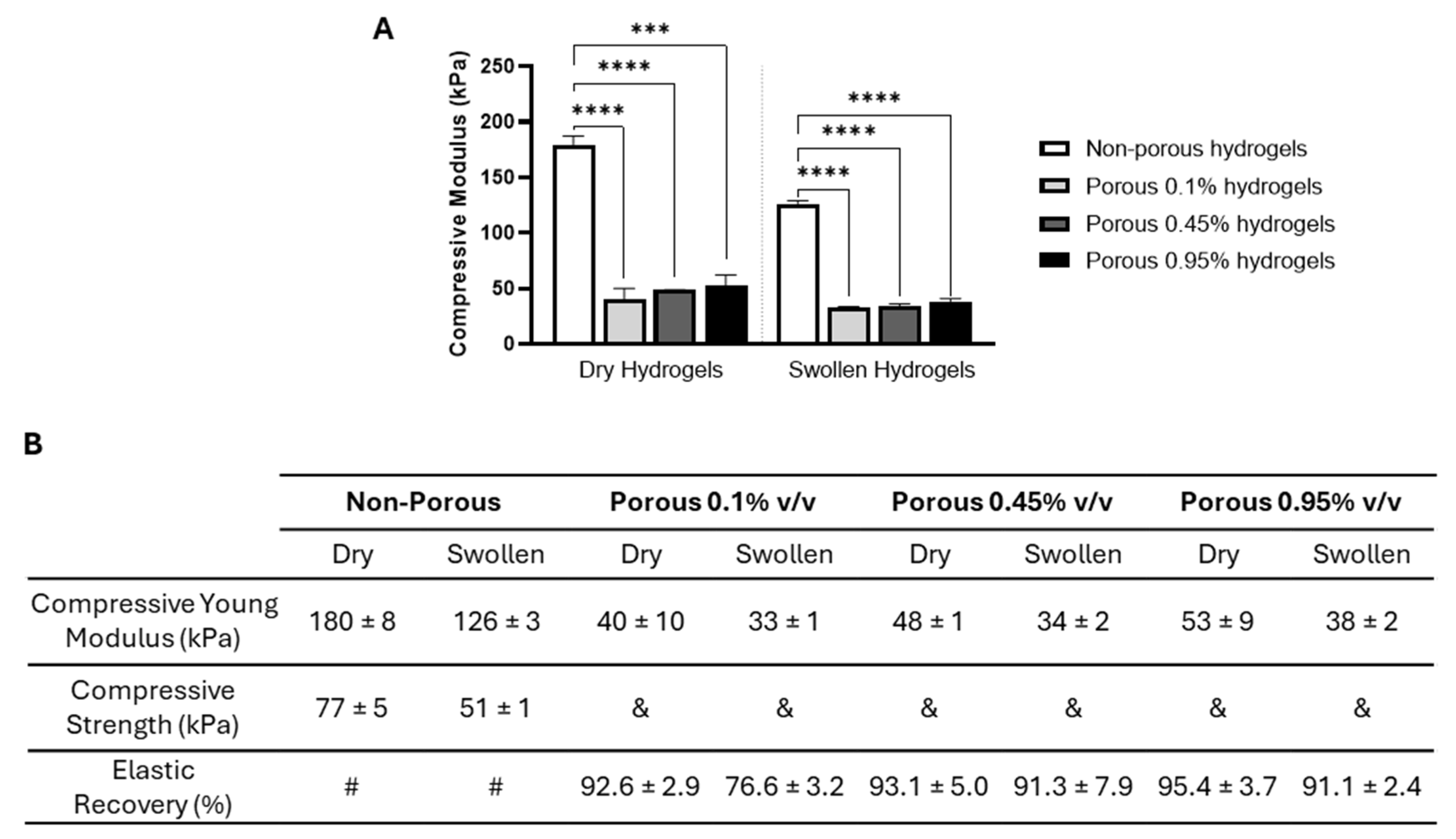
2.2. In Vitro Biocompatibility of 3D Porous PEGDA Hydrogels with Relevant Cell Populations
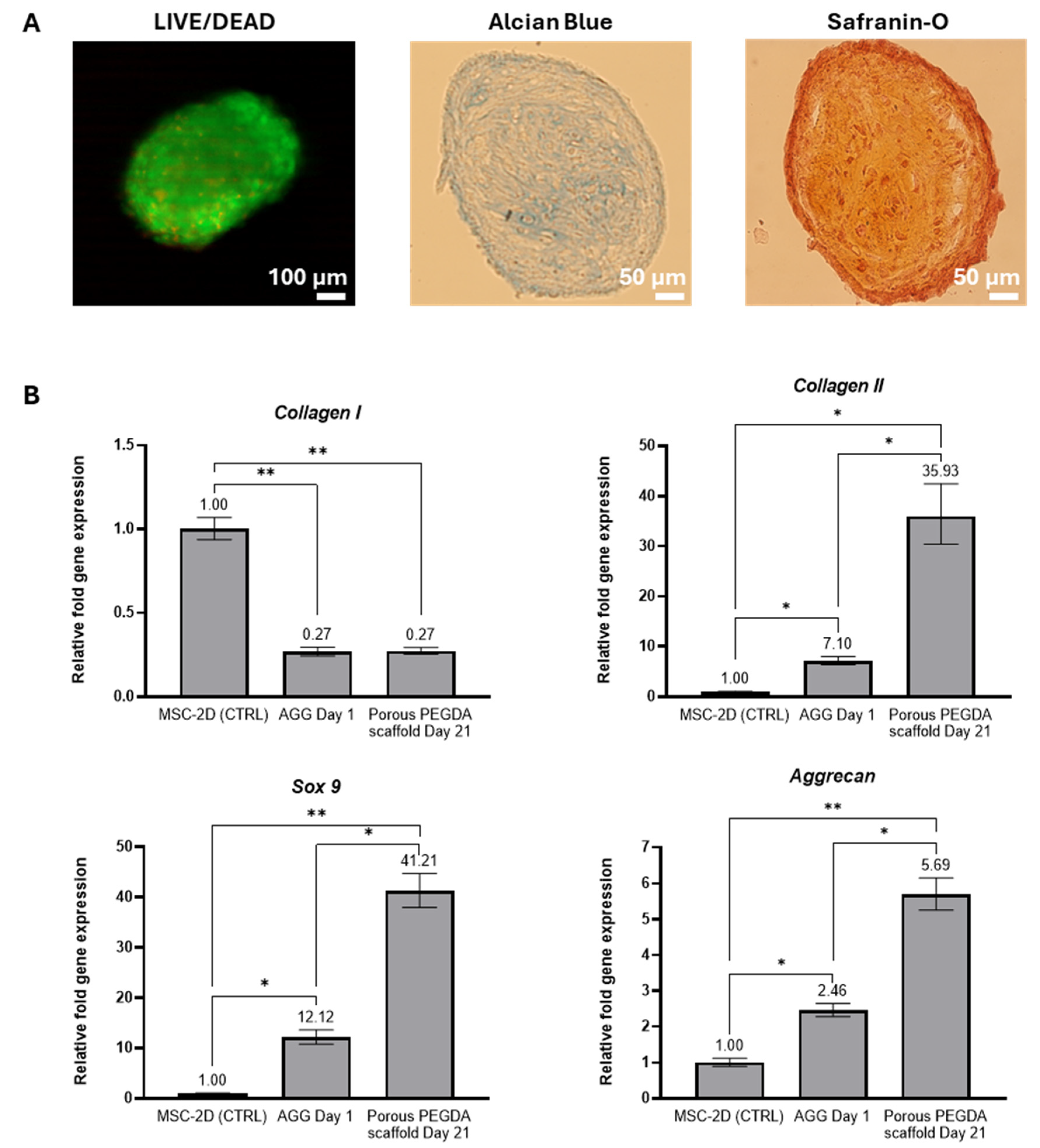
2.3. Chondrogenic Differentiation of MSC-Spheroids on 3D Porous PEGDA Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of 3D Porous PEGDA Hydrogels
4.3. Characterization of 3D Porous PEGDA Hydrogels
4.3.1. Scanning Electron Microscopy (SEM) and Pore Size Measurements
4.3.2. Micro-Computed Tomography (μ-CT) Analysis
4.3.3. Swelling Behaviour and Water Content
4.3.4. Mechanical Properties under Compressive Testing
4.4. In Vitro Cytotoxicity Tests
4.5. MSC Spheroids Culture and Differentiation on 3D Porous PEGDA Hydrogels
4.6. Assessment of Spheroid Viability and Chondrogenic Differentiation after Culture on 3D Porous PEGDA Hydrogels
4.6.1. Live/Dead Staining
4.6.2. Alcian Blue and Safranin-O Stainings
4.6.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, A.J. S.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar]
- Kempson, G.E. The mechanical properties of articular cartilage. In The Joints and Synovial Fluid, 1st ed.; Sokoloff, L., Ed.; Academic Press: New York, NY, USA, 1980; Volume 2, pp. 177–238. [Google Scholar]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular cartilage regeneration in osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr Course Lect 2005, 54, 465–480. [Google Scholar] [PubMed]
- Armiento, A.R.; Stoddart, M.J. ; Alini, M; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology, Acta Biomater 2018, 65, 1–20. [Google Scholar] [PubMed]
- Kwon, H.; Paschos, N.K.; Hu, J.C.; Athanasiou, K. Articular cartilage tissue engineering: the role of signaling molecules. Cell Mol Life Sci 2016, 73, 1173–1194. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Darling, E.M.; Hu, J.C. In vitro tissue engineering of hyaline articular cartilage. In Articular Cartilage Tissue Engineering, 1st ed.; Seidel, A., Ed.; Springer: Cham, Switzerland, 2010; Volume 1, pp. 31–53. [Google Scholar]
- Flik, K.R.; Lewis, P.; Kang, R.W.; Cole, B.J. Articular cartilage. In Clinical Sports Medicine, pp. 505–514, Elsevier, 2006.
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the global burden of disease study 2021. Lancet Rheumatol 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-T.; Chen, J.; Meng, Z.-L.; Ge, W.-Y.; Bian, Y.-Y.; Cheng, S.-W.; Xing, C.-K.; Yao, J.-L.; Fu, J.; Peng, L. Research progress on osteoarthritis treatment mechanisms. Biomed. Pharmacother 2017, 93, 1246–1252. [Google Scholar] [CrossRef]
- World Health Organization. Osteoarthritis. Available online: https://www.who.int/news-room/fact-sheets/detail/osteoarthritis (accessed on 21 February 2024).
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthritis Cartilage 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Plotnikoff, R.; Karunamuni, N.; Lytvyak, E.; Penfold, C.; Schopflocher, D.; Imayama, I.; Johnson, S.T.; Raine, K. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health 2015, 15, 1195. [Google Scholar] [CrossRef]
- Borrelli, J., Jr.; Olson, S.A.; Godbout, C.; Schemitsch, E.H.; Stannard, J.P.; Giannoudis, P.V. Understanding articular cartilage injury and potential treatments. J Orthop Trauma 2019, 33, S6–S12. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioactive Materials 2021, 6, 4830–4855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mu, Y.; Zhou, H.; Yao, H.; Wang, D.-A. Cartilage tissue engineering in practice: Preclinical trials, clinical applications, and prospects. Tissue Engineering. Part B, Reviews 2023, 29, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Udangawa, R.N.; Chen, J.; Mancinelli, C.D.; Garrudo, F.F. F.; Mikael, P.E.; Cabral, J.M. S.; Ferreira, F.C.; Linhardt, R.J. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Materials Science & Engineering. C, Materials for Biological Applications 2023, 107, 110291. [Google Scholar]
- Huang, Y.-Z.; Xie, H.-Q.; Silini, A.; Parolini, O.; Zhang, Y.; Deng, L.; Huang, Y.-C. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: Current status and future perspectives. Stem Cell Reviews 2017, 13, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R.; Hung, C.T. Concise review: Mesenchymal stem cells for functional cartilage tissue engineering: Taking cues from chondrocyte-based constructs. Stem Cells Translational Medicine 2017, 6, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Han, X.; Silva, T.P.; Xia, K.; Mikael, P.E.; Cabral, J.M. S.; Ferreira, F.C.; Linhardt, R.J. Glycosaminoglycan remodeling during chondrogenic differentiation of human bone marrow-/synovial-derived mesenchymal stem/stromal cells under normoxia and hypoxia. Glycoconjugate Journal 2020, 37, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Gäbler, S.; Stampfl, J.; Koch, T.; Seidler, S.; Schüller, G.; Redl, H.; Juras, V.; Trattnig, S.; Weidisch, R. Determination of the viscoelastic properties of hydrogels based on polyethylene glycol diacrylate (PEG-DA) and human articular cartilage. Int J Mater Eng Innov 2009, 1, 3–20. [Google Scholar] [CrossRef]
- Williams, C.G.; Kim, T.K.; Taboas, A.; Malik, A.; Manson, P.; Elisseeff, J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Engineering 2003, 9, 679–688. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous hydrogels: Present challenges and future opportunities. Langmuir: The ACS Journal of Surfaces and Colloids 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J Biomech 2008, 41, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Annabi, N. Engineering porous scaffolds using gas-based techniques. Curr Opin Biotechnol 2011, 22, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.M.; Park, K.; Son, J.S.; Kim, J.-J.; Rhie, J.-W.; Han, D.K. Beneficial effect of hydrophilized porous polymer scaffolds in tissue-engineered cartilage formation. Journal of Biomedical Materials Research. Part B, Applied Biomaterials 2008, 85, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Keskar, V.; Marion, N.W.; Mao, J.J.; Gemeinhart, R.A. In vitro evaluation of macroporous hydrogels to facilitate stem cell infiltration, growth, and mineralization. Tissue Engineering. Part A 2009, 15, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Gumiero, A.; Pecci, R.; Bedini, R.; Dentini, M. Gas-in-liquid foam templating as a method for the production of highly porous scaffolds. Biomacromolecules 2009, 10, 3188–3192. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Netti, P.A.; Madaghiele, M.; Coccoli, V.; Luciani, A.; Maffezzoli, A.; Nicolais, L. Synthesis and characterization of macroporous poly(ethylene glycol)-based hydrogels for tissue engineering application. Journal of Biomedical Materials Research. Part A 2006, 79, 229–236. [Google Scholar] [CrossRef]
- Oliviero, O.; Ventre, M.; Netti, P.A. Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomaterialia 2012, 8, 3294–3301. [Google Scholar] [CrossRef]
- Beaman, H.T.; Monroe, M.B. B. Highly porous gas-blown hydrogels for direct cell encapsulation with high cell viability. Tissue Engineering. Part A 2023, 29, 308–321. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Hatami, J.; Lehner, A.N.; da Silva, C.L.; Ferreira, F.C.; Antunes, A.P. M. Gelatin porous scaffolds fabricated using a modified gas foaming technique: characterisation and cytotoxicity assessment. Materials Science Engineering. C, Materials for Biological Applications.
- Lim, Y.-M.; Gwon, H.-J.; Shin, J.; Jeun, J.P.; Nho, Y.C. Preparation of porous poly(ɛ-caprolactone) scaffolds by gas foaming process and in vitro/in vivo degradation behavior using γ-ray irradiation. Journal of Industrial and Engineering Chemistry 2008, 14, 436–441. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. Journal of Biomedical Materials Research 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Amani, P.; Miller, R.; Javadi, A.; Firouzi, M. Pickering foams and parameters influencing their characteristics. Advances in Colloid and Interface Science 2022, 301, 102606. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, H.; Xu, T.; Li, J.; Guo, T.; Lu, X. , Ren, J.; Ren, X.; Weng, J. Effect of the interconnecting window diameter of hydroxyapatite scaffolds on vascularization and osteoinduction. Ceramics International 2022, 48, 25070–25078. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 33–6690. [Google Scholar] [CrossRef]
- Ricka, J.; Tanaka, T. Swelling of ionic gels: quantitative performance of the Donnan theory. Macromolecules 1984, 17, 2916–2921. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Engineering. Part B, Reviews 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomaterialia 2014, 10, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Draghi, L.; Giordano, C.; Pietrabissa, R. The effect of scaffold pore size in cartilage tissue engineering. Journal of Applied Biomaterials Functional Materials 2016, 14(3), e223–e229. [Google Scholar] [CrossRef] [PubMed]
- Stenhamre, H.; Nannmark, U.; Lindahl, A.; Gatenholm, P.; Brittberg, M. Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly(urethane urea) scaffolds. Journal of Tissue Engineering and Regenerative Medicine 2011, 5, 578–588. [Google Scholar] [CrossRef]
- Yamane, S.; Iwasaki, N.; Kasahara, Y.; Harada, K.; Majima, T.; Monde, K.; Nishimura, S.-I.; Minami, A. Effect of pore size on in vitro cartilage formation using chitosan-based hyaluronic acid hybrid polymer fibers. Journal of Biomedical Materials Research. Part A 2007, 81, 586–593. [Google Scholar] [CrossRef]
- Lim, S.M.; Jang, S.H.; Oh, S.H.; Yuk, S.H.; Im, G.I.; Lee, J.H. Dual-growth-factor-releasing PCL scaffolds for chondrogenesis of adipose-tissue-derived mesenchymal stem cells. Advanced Engineering Materials 2010, 12, B62–B69. [Google Scholar] [CrossRef]
- Ye, C.; Hu, P.; Ma, M.-X.; Xiang, Y.; Liu, R.-G.; Shang, X.-W. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials 2009, 30, 4401–4406. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Koo, Y.; Kim, G. ASC/chondrocyte-laden alginate hydrogel/PCL hybrid scaffold fabricated using 3D printing for auricle regeneration. Carbohydrate Polymers 2020, 248, 116776. [Google Scholar] [CrossRef] [PubMed]
- Im, G.-I.; Ko, J.-Y.; Lee, J.H. Chondrogenesis of adipose stem cells in a porous polymer scaffold: influence of the pore size. Cell Transplantation 2012, 21, 2397–2405. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Im, G.I.; Lee, J.H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; Hattori, K.; Ishimoto, Y.; Yamauchi, J.; Habata, T.; Takakura, Y.; Ohgushi, H.; Fukuchi, T.; Sato, M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 2005, 26, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Engineering. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Xiao, Y.; He, L.; Che, J. An effective approach for the fabrication of reinforced composite hydrogel engineered with SWNTs, polypyrrole and PEGDA hydrogel. Journal of Materials Chemistry 2012, 22, 8076. [Google Scholar] [CrossRef]
- Moura, C.; Trindade, D.; Vieira, M.; Francisco, L.; Ângelo, D.F.; Alves, N. Multi-material implants for temporomandibular joint disc repair: Tailored additive manufacturing production. Frontiers in Bioengineering and Biotechnology 2020, 8, 342. [Google Scholar] [CrossRef]
- Della Sala, F.; Biondi, M.; Guarnieri, D.; Borzacchiello, A.; Ambrosio, L.; Mayol, L. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. Journal of the Mechanical Behavior of Biomedical Materials 2020, 110, 103885. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.K.; von Halling Laier, C.; Kiziltay, A.; Wilson, S.; Larsen, N.B. 3D printed hydrogel multiassay platforms for robust generation of engineered contractile tissues. Biomacromolecules 2020, 21, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Larsen, N.B. Stereolithographic hydrogel printing of 3D culture chips with biofunctionalized complex 3D perfusion networks. Lab on a Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab on a Chip 2010, 10, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Hefferan, T.E.; Szatkowski, J.P.; Mishra, P.K.; Macura, S.I.; Lu, L.; Yaszemski, M.J. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials 2008, 29, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Larsen, L.B.; Trifol, J.; Szabo, P.; Burri, H.V. R.; Canali, C.; Dufva, M.; Emnéus, J.; Wolff, A. Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Materials Science & Engineering. C, Materials for Biological Applications 2015, 55, 569–578. [Google Scholar]
- Hwang, C.M.; Sant, S.; Masaeli, M.; Kachouie, N.N.; Zamanian, B.; Lee, S.-H.; Khademhosseini, A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010, 2, 035003. [Google Scholar] [CrossRef] [PubMed]
- Muniz, E.C.; Geuskens, G. Compressive elastic modulus of polyacrylamide hydrogels and semi-IPNs with poly(N-isopropylacrylamide). Macromolecules 2001, 34, 4480–4484. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Ng, T.Y.; Sharma, P. The effect of water content on the elastic modulus and fracture energy of hydrogel. Extreme Mechanics Letters 2020, 35, 100617. [Google Scholar] [CrossRef]
- Schinagl, R.M.; Gurskis, D.; Chen, A.C.; Sah, R.L. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society 1997, 15, 499–506. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.J.; Wagner, D.R.; Kelly, D.J. The pericellular environment regulates cytoskeletal development and the differentiation of mesenchymal stem cells and determines their response to hydrostatic pressure. European Cells & Materials 2013, 25, 167–178. [Google Scholar]
- Otero, M.; Favero, M.; Dragomir, C.; Hachem, K.E.; Hashimoto, K.; Plumb, D.A.; Goldring, M.B. Human chondrocyte cultures as models of cartilage-specific gene regulation. In Methods in Molecular Biology, 1st ed.; Picot, J., Ed.; Eds. Humana Press: Clifton, N.J.; U.S.A.; 2012; Volume 107, pp. 301–336. [Google Scholar]
- Surmeneva, M.A.; Kovtun, A.; Peetsch, A.; Goroja, S.N.; Sharonova, A.A.; Pichugin, V.F.; Grubova, I.Y.; Ivanova, A.A.; Teresov, A.D.; Koval, N.N.; et al. Preparation of a silicate-containing hydroxyapatite-based coating by magnetron sputtering: structure and osteoblast-like MG63 cells in vitro study. RSC Advances 2013, 3, 11240. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Strehin, I.; Elisseeff, J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histology and Histopathology 2011, 26, 1265–1278. [Google Scholar]
- Musumeci, G.; Carnazza, M.L.; Loreto, C.; Leonardi, R.; Loreto, C. β-Defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochemica 2012, 114, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Markway, B.D.; Tan, G.-K.; Brooke, G.; Hudson, J.E.; Cooper-White, J.J.; Doran, M.R. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplantation 2010, 19, 29–42. [Google Scholar] [CrossRef]
- Suzuki, S.; Muneta, T.; Tsuji, K.; Ichinose, S.; Makino, H.; Umezawa, A.; Sekiya, I. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Research & Therapy 2012, 14, R136. [Google Scholar]
- International Organization for Standardization. ISO 10993-5, Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity, 2009.

| PEGDA concentration | 20% v/v PEGDA in 10 mM HEPES solution |
| Photoinitiator concentration | 0.1% v/v 0.45% v/v 0.95% v/v |
| UV curing time | 45 seconds |
| Gas foaming parameters | 6% w/v Sodium bicarbonate + 6.75% v/v Acetic acid 10% w/v Sodium bicarbonate + 11.25% v/v Acetic acid |
| Gene | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| GADPH | 5’-GGTCACCAGGGCTGCTTTTA-3’ | 5’-CCTGGAAGATGGTGATGGGA -3’ |
| COL1A1 | 5’- CATCTCCCCTTCGTTTTTGA -3’ | 5’- CCAAATCCGATGTTTCTGCT-3’ |
| COL2A1 | 5’- GGAATTCCTGGAGCCAAAGG-3’ | 5’- AGGACCAGTTCTTGAG-3’ |
| SOX9 | 5’- TACGACTACACCGACCACCA-3’ | 5’- TTAGGATCATCTCGCCCATC-3’ |
| ACAN | 5’- CACTGGCGAGCACTGTAACAT-3’ | 5’- TCCACTGGTAGTCTTGGGCAT-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





