1. Introduction
One of the promising areas in the contemporary cardiac surgery is the development of new methods to treat pathologies in the aortic valve and thoracoabdominal aorta [
1,
2,
3].
In preclinical studies on prosthetic heart valves and thoracic aorta, the most commonly used model is pigs [
4,
5,
6]. The stiffness of the aortic tissue in a young pig is comparable to the stiffness of the aorta in a healthy person under 60 years, especially in the ascending aorta [
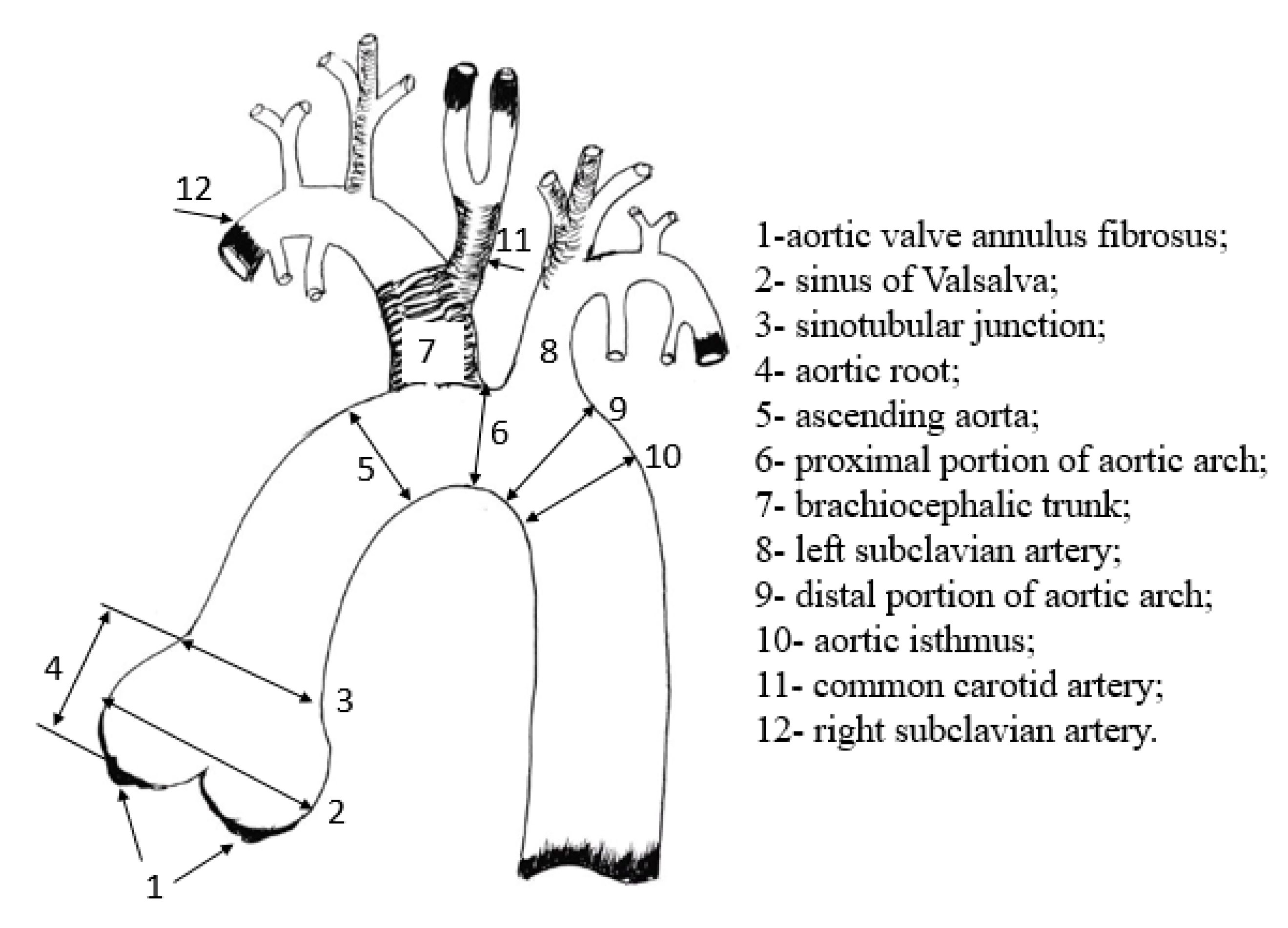
7]. The anatomical structure of the aorta in pigs is close to that in humans, but it has some specific differences (
Figure 1).
At the branching point the brachiocephalic trunk and the left subclavian artery branch off the aortic arch. In pigs the common carotid artery with its branches arises from the brachiocephalic trunk. In a human the most common anatomical variant is when the aortic arch gives off the brachiocephalic trunk, the left common carotid and left subclavian arteries. In humans, such an anatomical variant of the arch similar to that in pigs occurs only in 8-30% of cases [
8]
.
When planning preclinical trials of implantable cardiovascular devices, it is very important to understand the dimensions and geometric features of the aorta in animals. A properly selected animal model will make it possible to install the implant being tested with great success, avoid fatal complications and death of experimental animals due to the discrepancy in the size of the structures in their hearts, blood vessels and medical devices. The most advantageous method to accurately measure the size of the heart and its structures is undoubtedly multispiral computed tomography [
9,
10]. However, in daily experimental practice, preference should be given to the simplest and fastest method to evaluate the anatomy of the target zone. In this case, echocardiography (TEE or transthoracic) or angiography can be of use. Echocardiography is by far the simplest and most non-invasive approach, but it is unclear if the data obtained using this method are comparable with the X-ray examination data, which are considered to be more accurate.
The other important issue under discussion, is whether the mass-growth indicators of an adult animal correlate with the size of cardiac structures. These correlations have been established for young growing animals and humans [
11,
12,
13], but not for the pig model. Every author studying the relationship between these indicators has conducted experiments on pigs aged from 0 to 3-4 years. This makes correlations between the size of internal organs and height-weight indicators obvious due to intensive growth at an early age [
11,
14,
15]. However, the growth of internal organs stops when the animal reaches its sexual maturity. So the question is if a larger size of cardiovascular structures corresponds to a larger body weight.
In different countries, researchers use laboratory pigs of different breeds (about 30 breeding groups) [
15]. It might be possible that for different breeds, different variations in the ratio between height-weight indicators and the size of cardiac structures can be observed. However, to ensure the integrity of the experiments and to increase their efficiency, these ratios are sure to be verified before the start of a serial experiment.
Our institute carries out research mainly on mini-pigs bred in the nursery in the Institute of Cytology and Genetics SB RAS. Therefore, this study was also done on this breed of animals. In 1992 under the supervision of I. G. Gorelov his team started to form the breeding group of these mini-pigs. The group was based on Svetlogorsk mini-pigs (54%), large white breed (18%), Landrace (8%) and Vietnamese breed (20%). Breeding these laboratory animals is possible in a pig farm, unlike Svetlogorsk mini-pigs, which can only be kept in a vivarium. This breed has a strong constitution and a harmonious exterior, good reproductive function, and it is also capable of rapid adaptation. The live weight of these mini pigs reaches 110-120 kg in boars and 70 kg in sows, but the main herd is maintained within the weight range 50-80 kg. The growth period of these animals is 6.6 years in wild boars and 3 years in sows [
16], while mini-pigs reach their main size by the age of 2. The age dynamics of growth and weight in mini pigs of this breeding are described in detail in the available literature, including the growth dynamics of some internal organs of piglets in the postnatal period [
15,
16]. However, main morphometric parameters of the heart and main vessels of these pigs have not been described yet.
The purpose of our study is to characterize the anatomy of the aorta in mini-pigs, to evaluate the relationship between the size and age of animals and the size of the main structures in the aorta and to determine the role of TEE and angiography while preparing the animal for the experiment.
2. Materials and Methods
The study was performed on 28 laboratory mini-pigs at the Institute of Cytology and Genetics SB RAS. According to the weight at the time of the study, three groups were identified: 40-70 kg, 71-90 kg and more than 91 kg.
The animals were taken from the nursery in the Federal Research Center Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia.
The work with animals was carried out in accordance with the requirements of the European Convention for the Protection of Vertebrates Used for Experiments and Other Scientific Purposes (Strasbourg, 1986).
Pre-experimental manipulations. 12 hours before the study, the animals were not fed, but they had free access to water.
For the premedication, the drug Zoletil-100 (Virbac Sante Animale, France) was administered at a dose of 6 mg /kg.
An 18G or 20G peripheral catheter was inserted into the marginal vein of the ear. Animals were measured (weight, chest volume, body length). Afterwards, transesophageal echocardiography was performed. To maintain anesthesia, animals were bolus-injected with "Zoletil-100" at a dose of 2 mg / kg, propofol 10 mg / ml (the dose was adjusted according to body weight and depth of introductory anesthesia, on average 2-2.5 mg / kg). For subsequent angiography, the animals were transferred to the operating room. Orotracheal intubation was performed in the animal's "lying on its back" position. Anesthesia was maintained with sevoflurane (3-4 vols.%) with additional administration of zoletil if necessary.
Animal body weight measuring. The age of the animals was determined in accordance with their accompanying documents. Twenty-eight pigs were measured according to the study plan. Weighing the animals was performed on scales designed for weighing animals VSP4-150 ZHSO VSP4-150 (Neva Scales, Russia manufacturer Nevsky Scales, Russia, accuracy class according to GOST OML R76-1-2011: III (medium).
The volume of the chest behind the shoulder blades was measured with a centimeter tape. The length of the trunk was fixed with a measuring stick (cattle height hip measuring stick) (manufactured by JSC Vetzootechnika Vetzooequipment, Russia) from the occipital protuberance to the tail base.
Ultrasound study. Echocardiography was performed according to the methods described for animals [
17,
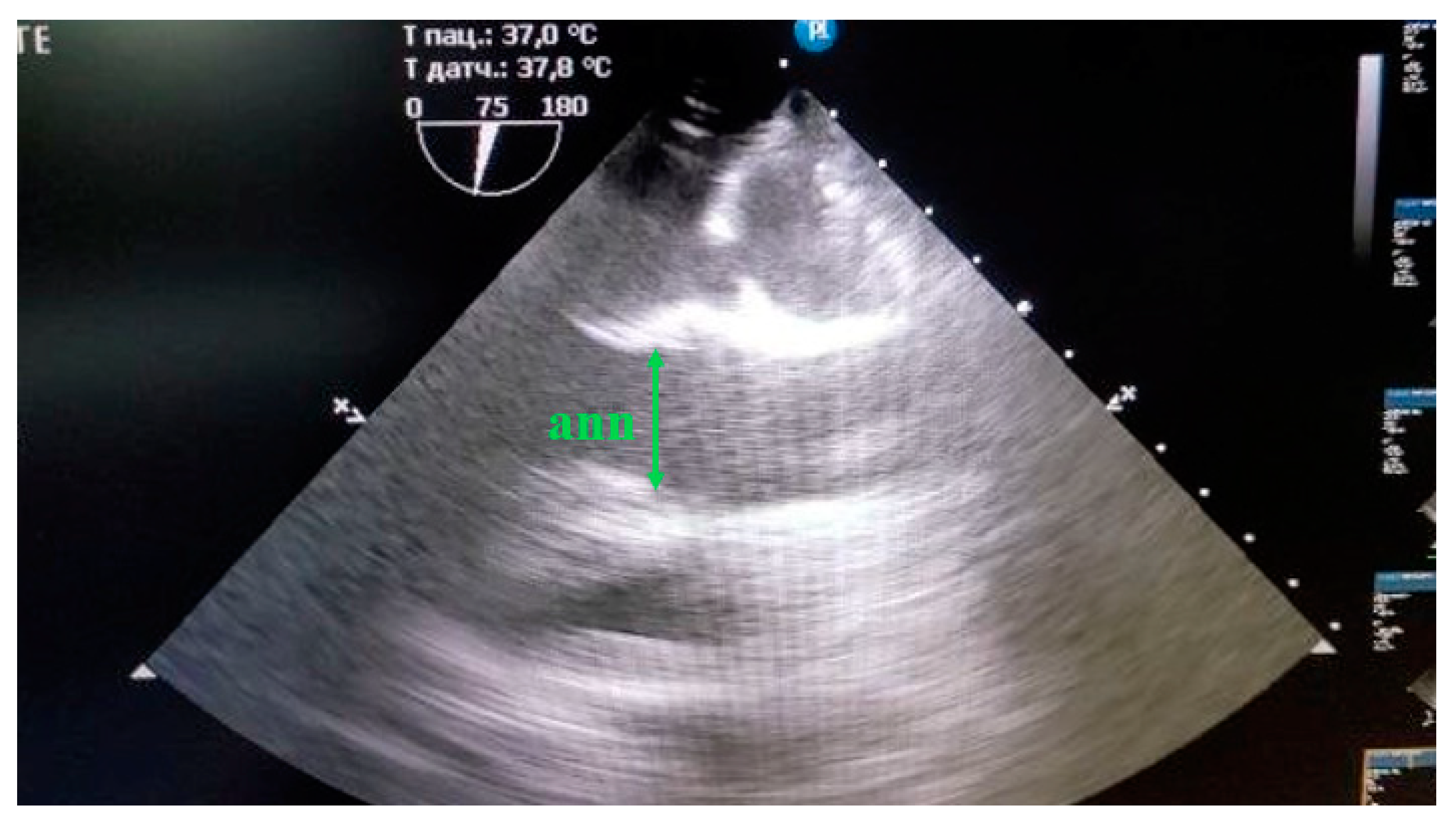
18] on a Philips CX-50 ultrasound machine (Revision 3.1.2) with an X7-2t transesophageal sensor. The structures of the aorta were evaluated from the fibrous ring of the aortic valve to the ascending part of the aortic arch. The structures of the aorta located below either were not visualized at all or were poorly distinguished by echocardiography. So, the size of the aorta below the distal arch was estimated by angiography. The fibrous ring of the aorta was measured at the maximum ring size during systole, with the cursor placed from the inner edge to the inner edge at the point of attachment of the flaps (
Figure 2).
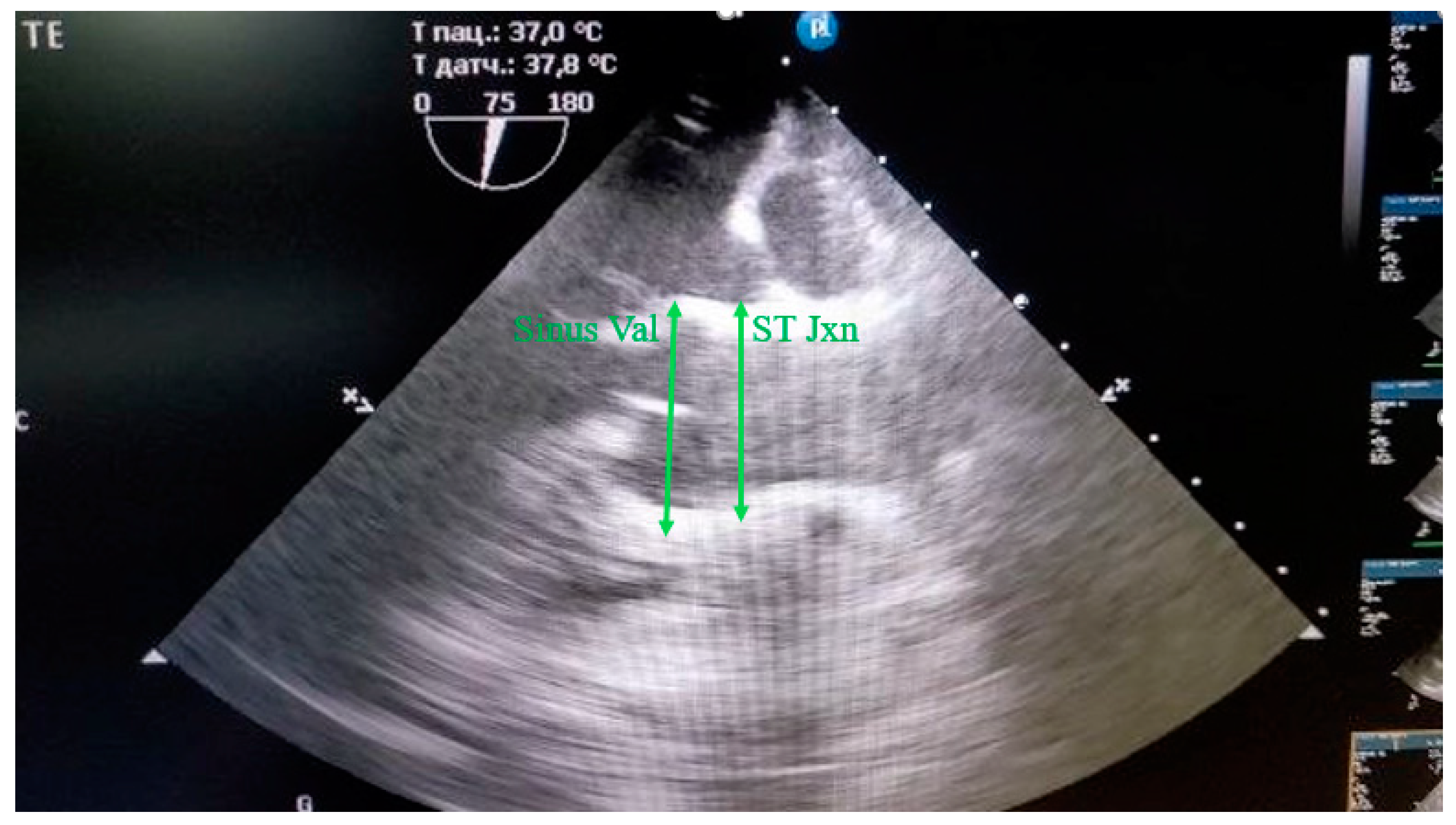
The sinus of Valsalva, the sinotubular junction was measured during diastole from the outer contour to the outer contour (
Figure 3).
The height of the aortic root was measured from the fibrous ring to the line of the sinotubular junction.
Angiography examination. Although angiography is not the gold standard so that to assess the morphometric parameters of the aorta, some researchers evaluate the results obtained with angiography as more accurate than the results obtained using computed tomography or magnetic resonance angiography [
19,
20] By means of angiography one can well visualize the ascending aorta, the proximal part of the aortic arch and the main vessels arising from it, as well as the distal part of the aortic arch, isthmus, thoracic and abdominal arteries. The diameter of these vessels and their length can be measured. After echocardiography had been performed, the animals were taken to the operating room to perform angiography. To carry out angiography of the aorta, a 7Fr section introducer was inserted into the left femoral artery. Supportive infusion therapy was administered into the marginal vein of the ear or into the jugular vein through an introducer. Before endovascular instruments were introduced, the animal was injected with heparin at a dose of 100 units / kg of weight. A diagnostic Cordis 6F diagnostic catheter, USA, was inserted into the area of interest using a diagnostic hydrophilic conductor (0.035” diameter, 260cm long, with a J-3mm tip). The examination/ test began from the aortic root, then, the diagnostic catheter was gradually moved, with a series of straight and oblique images being taken. The diameter of the ascending and descending aortic arch, the main vessels arising from it - the brachiocephalic trunk and the left subclavian artery, the thoracic aorta at the diaphragm level, in the abdominal department at the bifurcation level were estimated. For angiography, the C-Arc OEC 9900 Elit (General Electric, USA) was used, a series of images were taken using the contrast agent Omnipak LLC Scientific and Technological Pharmaceutical Company POLISAN, Russia). At the end of the examination, the femoral artery was ligated with the absorbable suture material Safil 0 (3,5) (Safil, B Braun, Germany), the wound was stitched layer by layer with the same suture material. The obtained images were processed using the program RadiAnt DICOM Viewer 4.6.9 (64-bit) Medixant, Poland.
The type of aortic root was determined according to the procedure which was proposed in 2022 in order to determine the type of aortic root in humans [
21]. The aortic root was thought to be a part of the ascending aorta from the fibrous ring of the aortic artery to the plane of the sinotubular junction. Taking into account the ratio of the height of the aortic root and the diameter of the fibrous ring, the authors identified three variants of the structure of the aortic root: A, B and C. Type A is characterized by a large value of the height of the of the Valsalva sinuses in relation to the diameter of the fibrous ring (K>1.05). In the aortic root of Type B, the height of the sinuses of the Valsalva is close in magnitude to the diameter of the fibrous ring (0.95≤K≤1.05). In the root of Type C aorta, the height of the sinuses of the Valsalva is less than the diameter of the fibrous ring (K≤0.95). The coefficient is calculated by the formula: K= h/D, where h is the distance from the fibrous ring to the sinotubular joint (mm); D is the average diameter of the fibrous ring of this animal (mm). The K coefficient was calculated for each mini-pig.
Statistical analysis. Quantitative data were processed using Dell Statistica 13.0 (Dell Software Inc., USA). As most groups’ distributions were not typical, non-parametric statistics were used. Quantitative data are reported as medians and interquartile ranges (25% - 75%) (IQRs). The Kruskal-Wallis (K-W) test was used to compare three or more groups. Correlation Spearman was used to identify the relationships between the values. The level of significance was set to p < 0.05.
3. Results
As can be seen from
Table 1, the average weight of mini pigs reaches 65 kg by the age of one year, and about 80 kg by the age of two. Their growth does not end then. At the age of three years, laboratory mini-pigs bred by ICiG SB RAS can weigh about 100 kg. At the same time, the ratio of their chest volume behind the shoulder blades to their body length (the index of animal fatness) was within the range of 0.87-1.07 (transitional or eirisomal constitutional type) in all three groups, regardless of age (
Table 1).
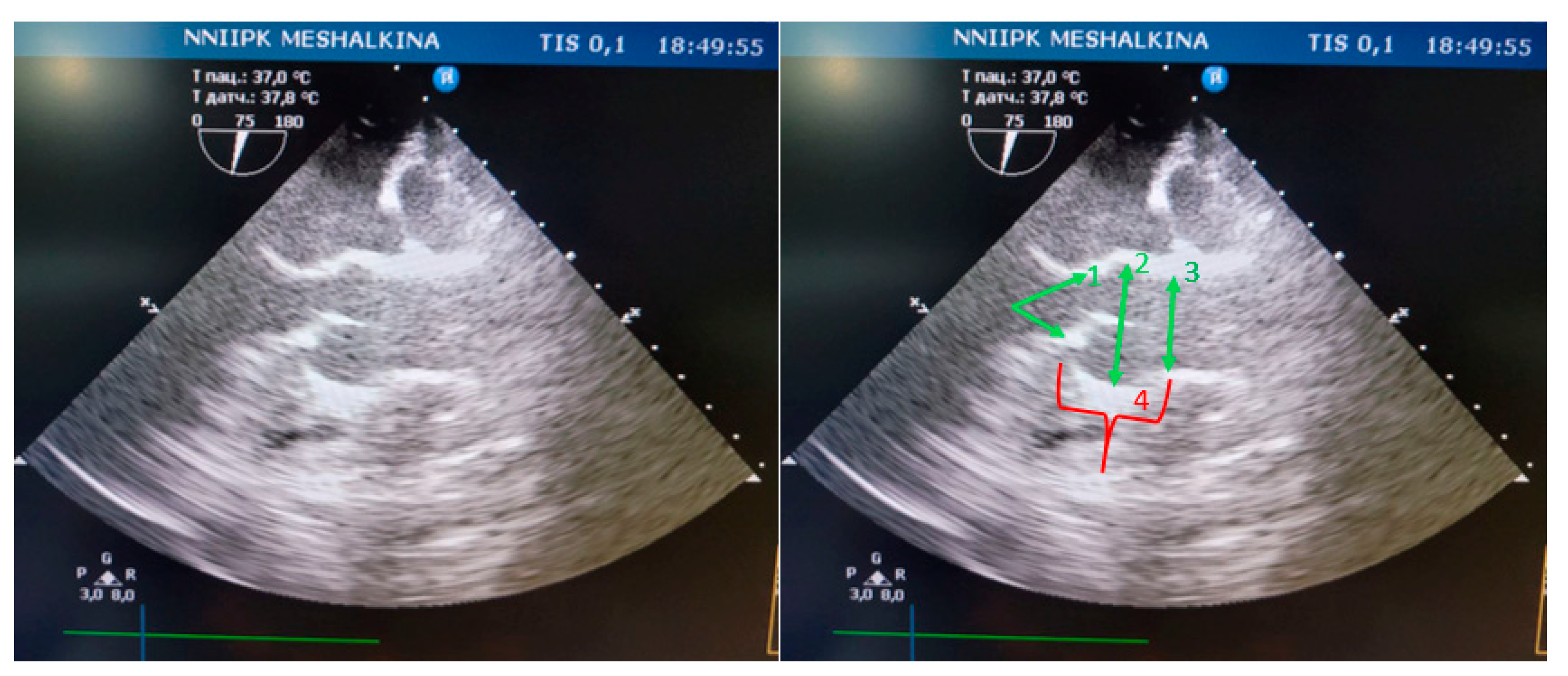
In all animals structures such as the fibrous ring of the aortic valve, the sinus of the Valsalva, and the sinotubular junction were clearly identified by transesophageal echocardiography (
Figure 4).
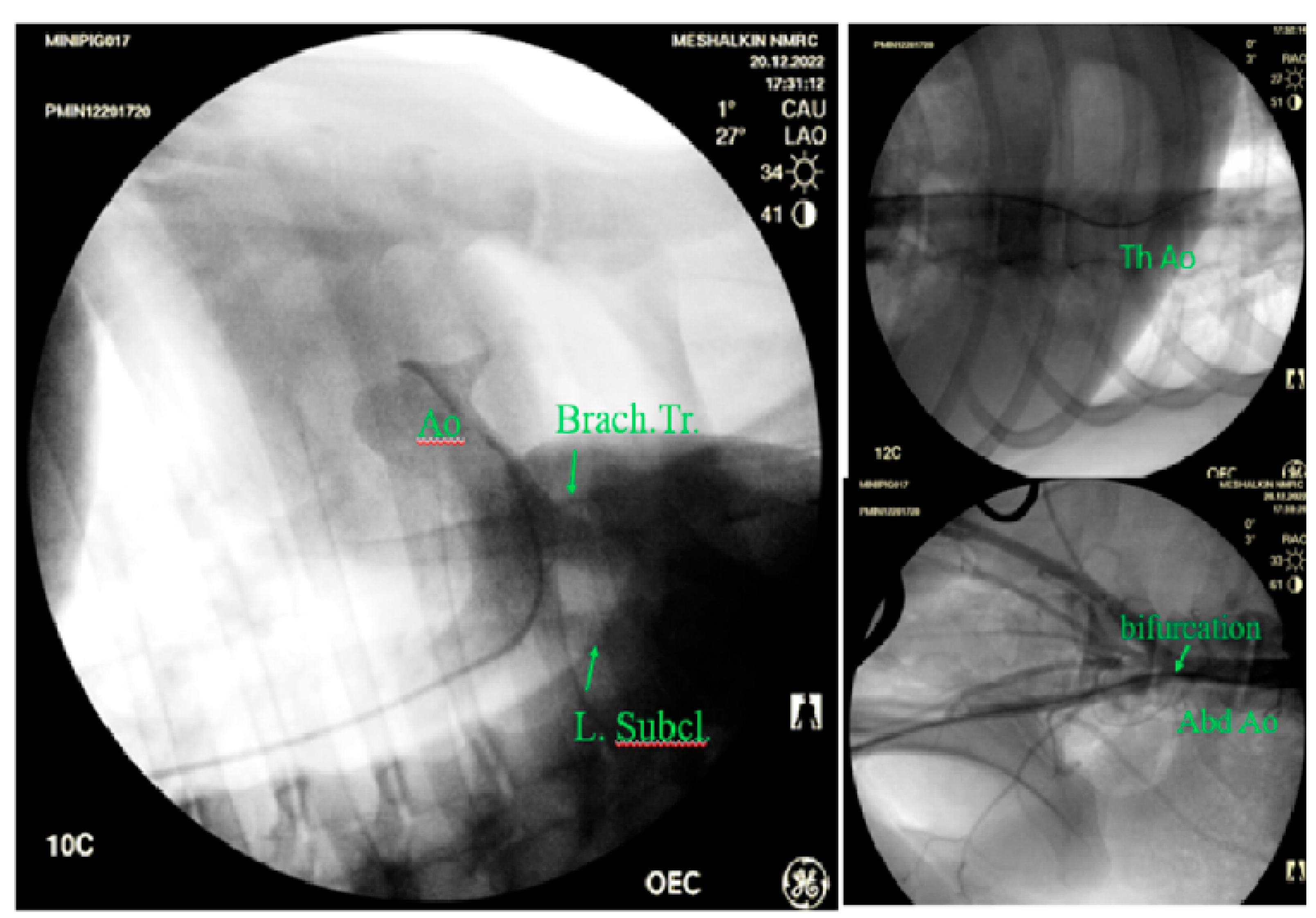
Visualization of the ascending aortic arch, the proximal part of the arch, the subclavian artery and the brachiocephalic trunk was difficult or impossible. Meanwhile angiography made it possible to visualize both the aortic root and its thoracic and abdominal sections at any level before bifurcation (
Figure 5).
When comparing the echocardiogram data with the results obtained by angiography (
Table 1), it is obvious that the differences between them are insignificant and, in general, do not exceed 1 mm. The Spearman coefficient between the data obtained by these methods was p=0.84-0.98 at p<0.05,
Figure 6).
Thus, when the parameters of the aortic root are measured, - echocardiography proves to be the most convenient, quickly feasible and much less invasive method.
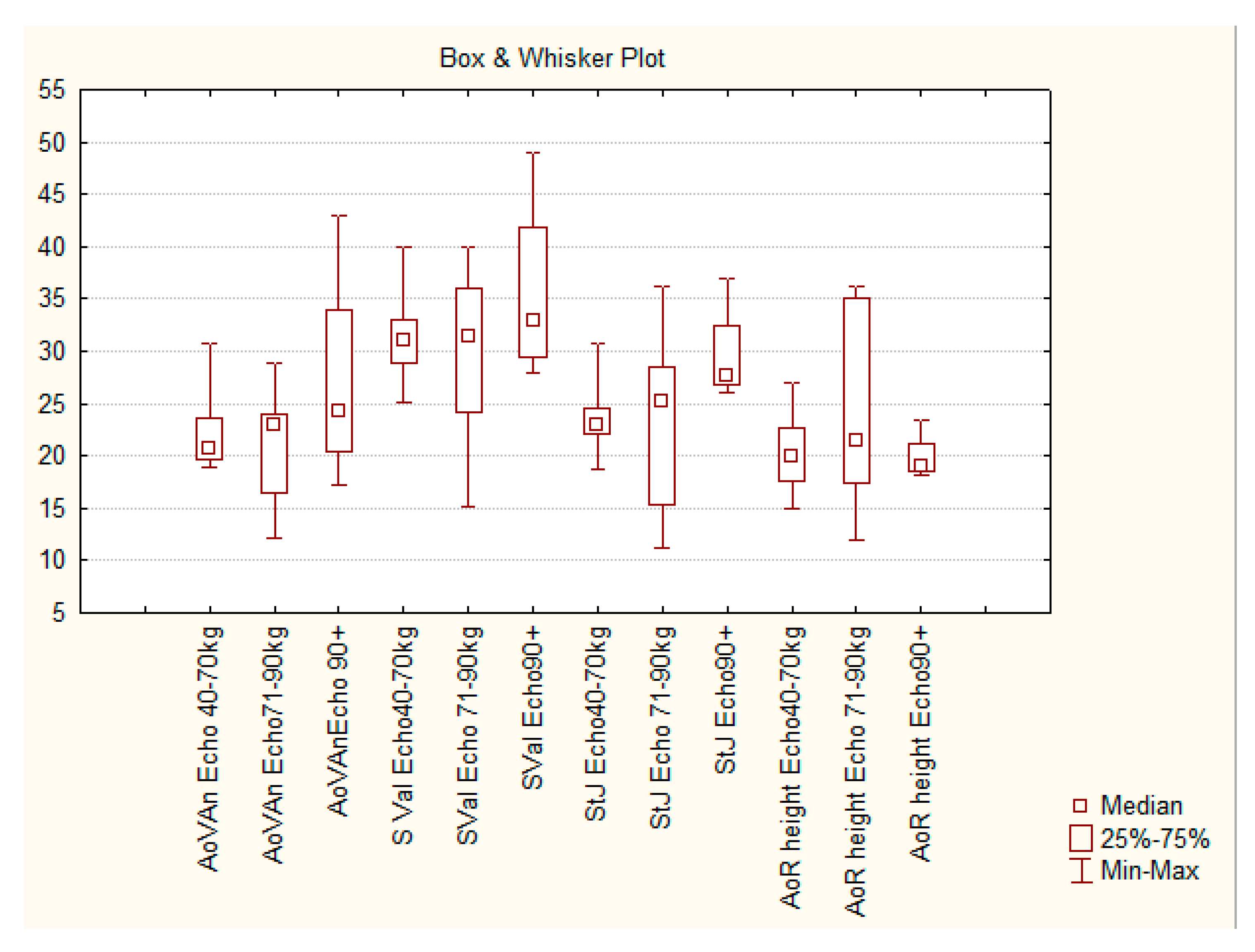
Given the data obtained by these methods the diameter of the fibrous ring of the aortic valve in Group I pigs, averaged 20.7 (19.6/23.7) mm, in Group II pigs - 22.8 (16.4/24.0) mm and in Group III pigs– 25.0 (23.5/28.8) mm. At the level of the sinuses of Valsalva the diameter of the aorta was 31.0 (28.9/33.0) mm in group I, 29.8 (24.1/36.0) mm in Group II and 34.8 (30.9/40.0) mm in group III. At the level of the sino-tubular junction the median values of the aortic diameter in Group I were 23 mm, in Group II – 24.7 mm, in Group III - 27.9 mm (
Table 1). The table? shows that with the increase of weight and age of animals the median values of the diameters of aortic structures enhance as well. However, the differences between them are invalid. (the P value according to the Kraskel-Wallis criterion was more than 0.999, while the statistically significant P value should be less than 0.017 when comparing these three groups).
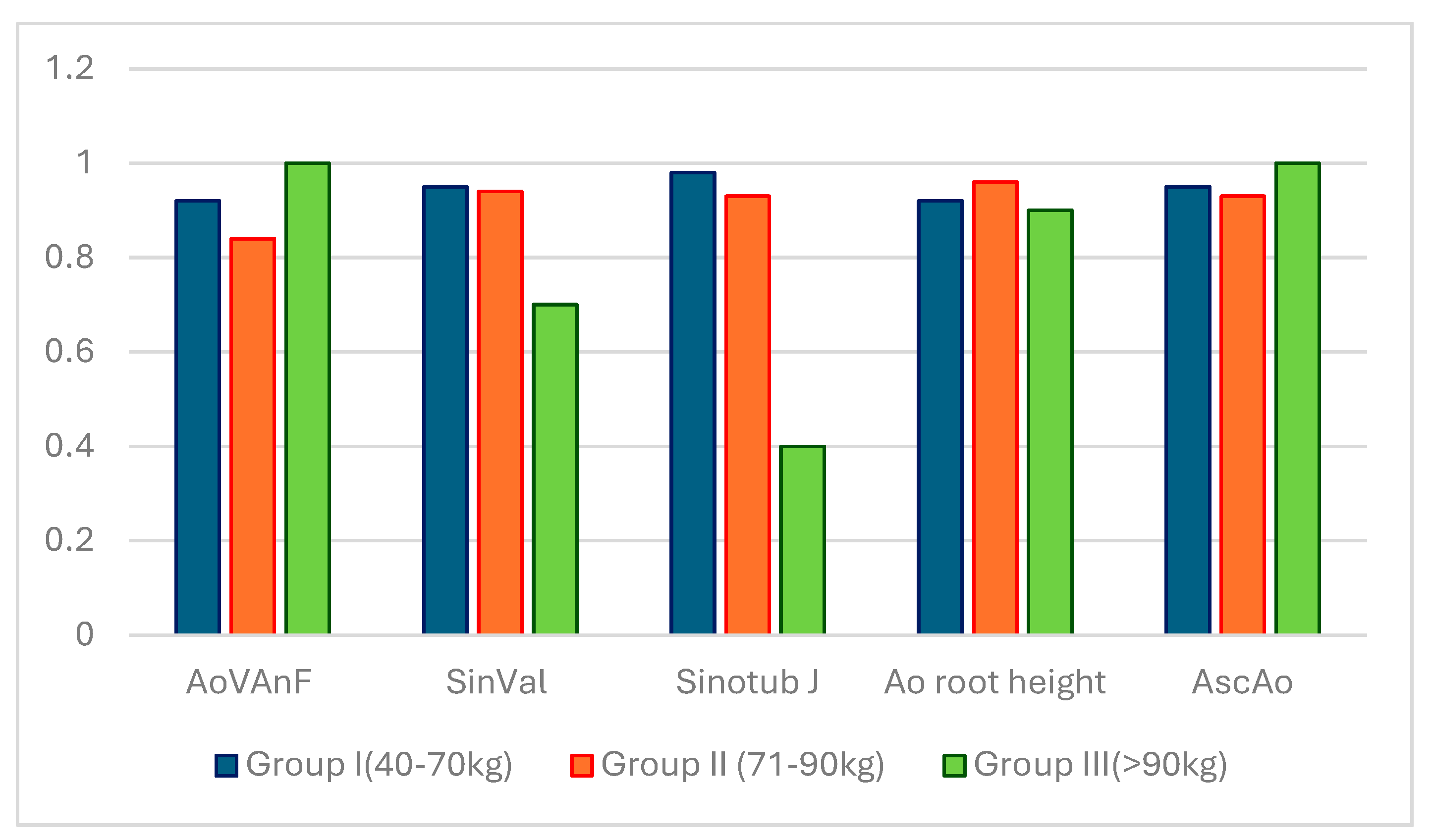
The significant individual variability of indicators in each group of animals is well demonstrated in
Figure 7.
The aorta was not always larger in larger animals. A strong direct positive correlation between somatometric parameters is intuitively comprehensible and it is confirmed by Spearman's nonparametric method (body length and breast volume (p=0.82, at p<0.05), body length and weight (p=0.78, at p<0.05) and between weight and breast volume (p=0.87, at p<0.05)). It was also found that there was a strong direct positive relationship between some morphological structures of the aortic root Namely, between the diameter of the fibrous ring of the aorta and the sinuses of the Valsalva the Spearman correlation was p=0.73 (at p<0.05); between the diameter of the fibrous ring of the aorta and the sinotubular junction the correlation was p=0.80 (at p<0.05); the correlation between the sizes of the sinuses of the Valsalva and the ascending aorta was p=0.80 (at p<0.05), between the sizes of the sinotubular junction and the ascending aorta it was p=0.72 (at p<0.05).
We observed a gradual decrease in its diameter from the distal part of the aortic arch to the bifurcation in the abdominal region. Moreover, the most severe reduction in diameter was observed in Group I animals. Notably, in the isthmus zone, the diameter of the aorta decreased relative to the distal part of the arch in Group I animals by 18%, in Group II by 7%, in Group III by 0.6%. In the diaphragm area, the diameter of the aorta decreased in relation to the distal part of its arch in each group (Group I, Group II, Group III) by 45%, 23.5% and 13.6%, respectively. In the bifurcation area, the reduction in the diameter of the aorta relative to the distal part of its arch in Groups I, II, and III was 51%, 52%, and 36%, respectively. (
Figure 8).
It was found that there was a moderate direct positive correlation between the external parameters of the animals and the diameter of the abdominal aorta. Namely, there was a correlation between the volume of the breast and the diameter of the aorta at the diaphragm level (p=0.54, at p<0.05), between the weight of pigs and the diameter of the aorta at the diaphragm point (p=0.46, at p<0.05). Spearman's rank correlation was p=0.5, at p<0.05 and it involved body length and aortic diameter at the bifurcation level, breast volume and aortic diameter at the bifurcation level, weight, and aortic diameter at the bifurcation level.
4. Discussion
It is believed that in order to visualize the ascending section and the aortic root in animals with a small body weight, it is possible to use a parasternal position along the long axis of the left ventricle [
22,
23]. However, in pigs weighing more than 60 kg, transthoracic echocardiography is vague due to poor visualization, even when using a specialized echocardiographic table for animals. Therefore, in our study we used a transesophageal sensor. It allowed us to assess the geometry of the aortic root from the middle parts of the esophagus. Angiography can be performed if it is necessary to accurately measure these parameters in pigs, as well as the diameters of the thoracic and abdominal aorta at any level before bifurcation.
According to the published data, in humans, the diameter of the sinotubular junction remains larger relative to the fibrous ring of the aorta by 10-20% [
24]. The diameter of the Valsalva sinuses in humans is 19% larger than that of the ascending aorta, and the diameter of the descending aorta is 24% smaller on average [
25]. We have not observed similar patterns for pigs. Our results showed that the diameter of the sinotubular junction was 8-11% larger than that of the fibrous ring of the aorta, the diameter of the sinuses of the Valsalva exceeded the diameter of the ascending aorta in pigs weighing up to 70 kg by 29%, and in pigs weighing more than 70 kg by 48%. The diameter of the ascending aorta was 11-26% larger than that of the descending one.
However, unlike other researchers, we have not found a relationship between the length of the body, chest volume, weight of adult mini pigs and the size of the thoracic aorta of animals, the structures of its root. For example, in large Swiss white pigs, the length of the ascending aorta correlates with body weight [
14] or the body length of miniature inbred pigs from Allan’s study [
11] correlates with the aortic ring and the diameter of the aortic root.
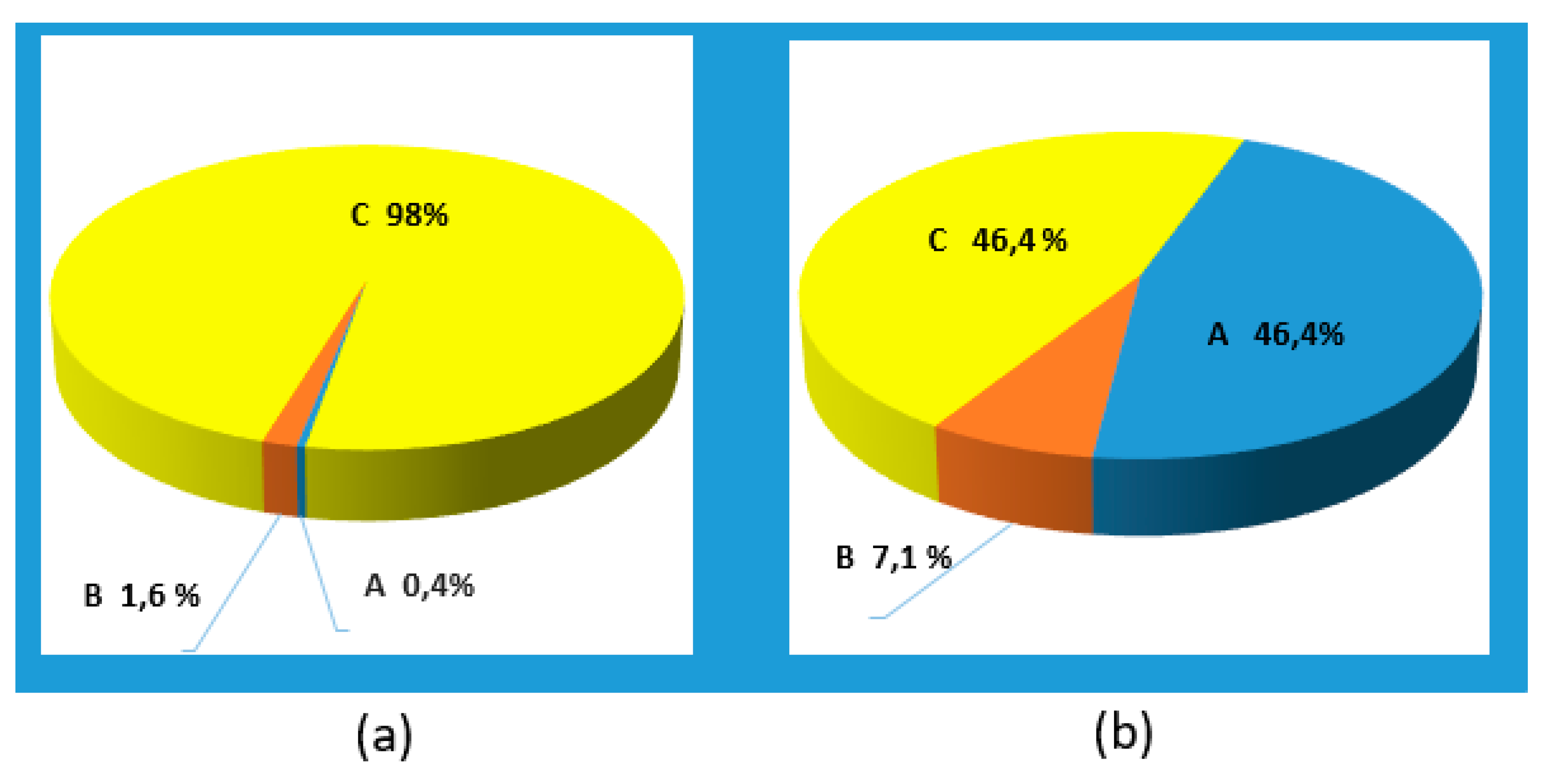
As for the structural variant of the aortic root, in about half of the studied animals (46.43%), the height of the aortic root was less than the fibrous ring, the so-called root type "C" (K≤0.95). The second half (46.43%) showed type "A" root (the height of the sinuses is greater than the fibrous ring). In 7.14% (in two animals out of 28) it turned out to be type "B" root (the height of the sinuses is approximately equal to the diameter of the fibrous ring in the aorta). Moreover, type "A" was found mainly in pigs weighing 71-90 kg, and type "C" in animals weighing 40-60 and more than 90 kg. In a human the aortic type C root can be found in 98% of cases, type B - in 1.6% of cases, type A almost never occurs (0.4%) (Kobelev E. et al., [
21]),
Figure 9.
Special attention could be paid to one or another type of root in an experimental animal when developing new models of transcatheter aortic valves for humans. The purpose is to be able to bring the preclinical model as close as possible to an upcoming end product.
Our results are consistent with the data obtained at the Quebec Heart and Lung Institute, QC, Canada [
26]. Georges G et al noted that the reduction in the diameter of the aorta in pigs in the abdominal part compared to the thoracic was from 19% to 48%. In our mini pigs, the diameters of the thoracic and abdominal aorta were slightly smaller than those described by Georges G. It was stated there that in animals weighing 40-59 kg, the diameter of the thoracic aorta was on average 20.0±1.7mm, abdominal 10.5±3.8mm; in pigs weighing 60-79 kg, the same diameters were 18.6± 2.0 mm and 15.1±2.2 mm, respectively; pigs weighing more than 80 kg had a thoracic aorta with a diameter of 21.4 ± 2.2 mm, an abdominal aorta of 11.0 mm. However, their study did not show at which points the measurements of the thoracic and abdominal aorta were made.
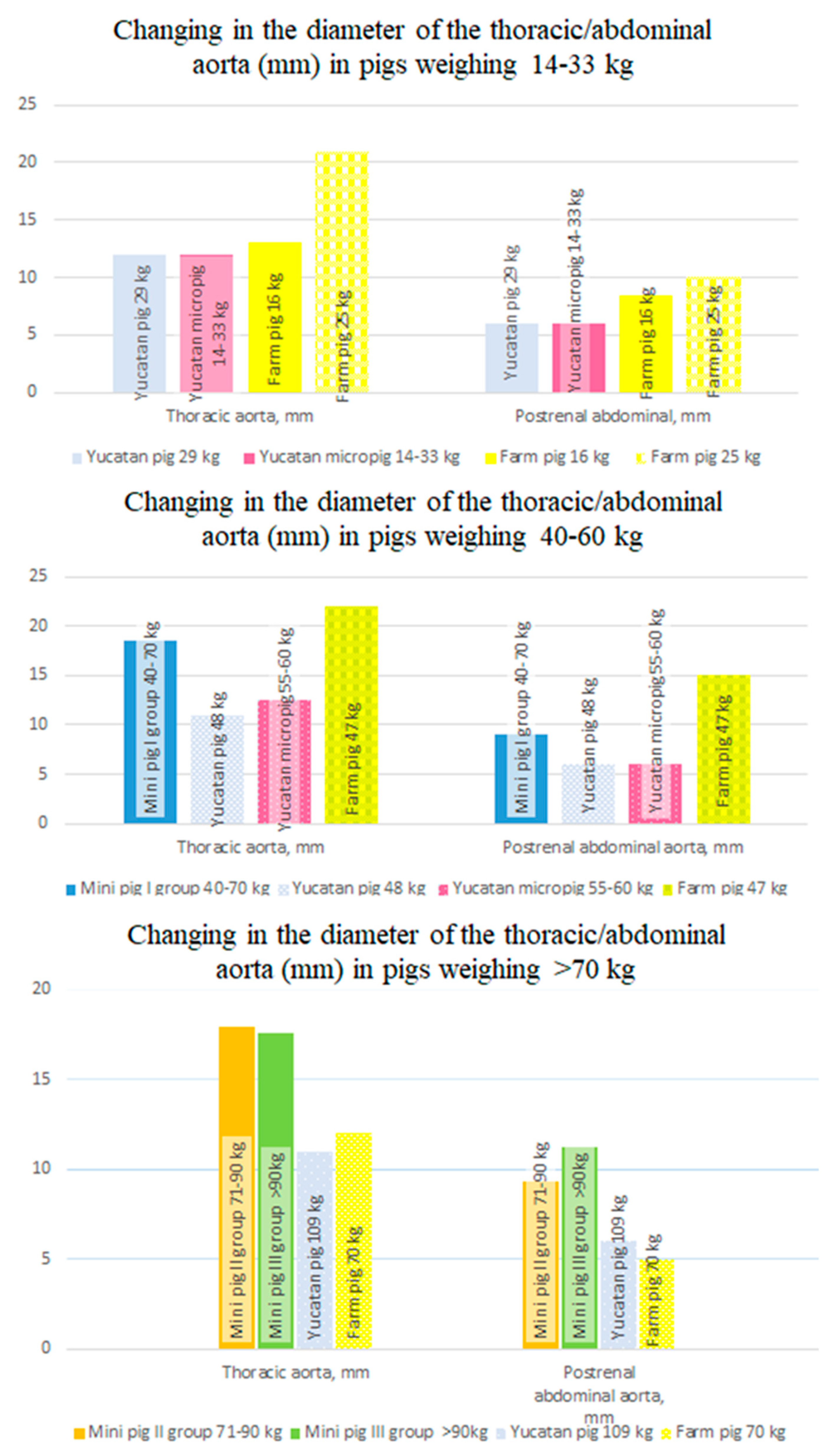
The thing that there is no correlation between the weight of pigs and the size of the thoracic/abdominal aorta is supported by the data provided in the manual on anesthesia, surgery, and experimental work with laboratory pigs [
27]. It was suggested there that Yucatan pigs weighing 29 kg had a diameter of 12 mm in their thoracic aorta, 6mm in their abdominal in the postrenal section/segment, and Yucatan pigs weighing 109 kg had a diameter of 11mm in their thoracic and 6 mm in their abdominal region. The manual for other pig breeds also noted that there is no relationship between the weight of animals and the diameter of their aorta (
Figure 10).
Thus, given the individual variability of the structure in the aortic root in pigs, it is difficult to overestimate the role of preoperative echocardiography in experimental cardiac surgery [
28,
29,
30]
We performed a morphometric analysis of the aorta in laboratory mini pigs. The results obtained have demonstrated an individual variability in the geometry of the aortic root, irrelevant to the somatometric characteristics of animals. This indicates the need for individual selection of laboratory animals as models, and implementation of high-tech innovative methods for preoperative planning, such as computed tomography, into experimental research practice. If the latter is not possible, echocardiography and angiography will make it possible to preliminarily assess the anatomical structures in potential models. Unfortunately, there are currently no pig breeds with standardized aortic sizes and geometries. The anatomy of each animal is individual and does not depend on its weight. Hence, the number of animals under study should be enhanced in groups substantially to properly identify a correlation between the somatometric characteristics of animals and the diameter of their main vessels and the valvular apparatus of the heart.
Author Contributions
Conceptualization, Y.L.R. и I.Yu.Z.; Resources Y.L.R., D.S.G; Formal analysis, Y.L.R.; Investigation, Y.R.L., D.S.G., K.S.P.; Writing - original draft, Y.L.R.; Writing - review & editing, Y.L.R., I.Y.Z.; Visualization, Y.L.R.; Project administration, Y.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of E. Meshalkin National Medical Research Center (protocol No. 3 of ethical committee meeting, approved 25 August 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
Conflicts of Interest: The authors declare that they have no conflict of interest.
References
- Jahanyar J., Mastrobuoni S., Munoz D.E. Aortic annulus elevation for aortic valve and aortic root replacement. J Card Surg. 2022, 37(4), 1101-1103. [CrossRef]
- Odinokova S.N. Nikolenko V.N., Komarov R N. et all. The correlations of morphometric parameters of structures of the aortic root having practical significance in the surgical correction of the aortic valve. Morphological Newsletter. 2020, 28(1):30-36. [CrossRef]
- Ramot Y. Rousselle S.D., Yellin N. et all. Biocompatibility and systemic safety of a novel implantable annuloplasty ring for the treatment of mitral regurgitation in a minipig model. Toxicologic Pathology. 2016, 44(5), 655-662. [CrossRef]
- Miller J.R., Henn M.C., Lancaster T.S. et all. Pulmonary Valve Replacement With Small Intestine Submucosa-Extracellular Matrix in a Porcine Model. World J Pediatr Congenit Heart Surg. 2016, 7(4), 475-483. [CrossRef]
- Salameh A., Greimann W., Vondrys D., Kostelka M. Calcification or Not. This Is the Question. A 1-Year Study of Bovine Pericardial Vascular Patches (CardioCel) in Minipigs. Semin Thorac Cardiovasc Surg. 2018, 30 (1), 54-59. [CrossRef]
- de Beaufort H. W., Ferrara A., Conti M., Moll F.L. et all. Comparative Analysis of Porcine and Human Thoracic Aortic Stiffness. Eur J Vasc Endovasc Surg. 2018, 55(4), 560-566. [CrossRef]
- Brenner G.B., Giricz Z., Garamvölgyi R. et all. Post- Myocardial Infarction Heart Failure in Closed-chest Coronary Occlusion. Reperfusion Model in Göttingen Minipigs and Landrace Pigs. J Vis Exp. 2021, 17 (170). [CrossRef]
- Uchino A., Saito N., Okada Y., Kozawa E., Nishi N., Mizukoshi W., Nakajima R., Takahashi M., Watanabe Y. Variation of the origin of the left common carotid artery diagnosed by CT angiography. Surg Radiol Anat. 2013, 35(4), 339-342. PMID: 23129264. [CrossRef]
- Radike M., Sutelman P., Ben-Aicha S., Gutiérrez M., Mendieta G. et all. A comprehensive and longitudinal cardiac magnetic resonance imagin study of the impact of coronary ischemia duration on myocardial damage in a highly translatable animal model. Europian Journal of Clinical Investigation. 2023, 53,-I.1. [CrossRef]
- Meissner F., Galbas M.C., Szvetics S., von zur Mühlen C. et all. Cardioaortic dimensions in German landrace pigs derived from cardiac magnetic resonance imaging. Sci Rep. 2024, 14, 1869. [CrossRef]
- Allan J.S., Rose G.A., Choo J.K., Arn J.S. et all. Morphometric analysis of miniature swine hearts as potential human xenografts. Xenotransplantation. 2002, 8, I.2, 90-93. [CrossRef]
- Mirea O., Maffessanti F.,Gripani P., Tamborini G., Muratori M. et all. Effects of aging and body size on proximal and ascending aorta and aortic arch: inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J Am Soc Echocardiogr. 2013, 26(4), 419-427. [CrossRef]
- Sündermann S. H, Cesarovic N, Falk V, Bettex D. Two- and three-dimensional transoesophageal echocardiography in large swine used as model for transcatheter heart valve therapies: standard planes and values. Interact Cardiovasc Thorac Surg. 2016, 2(5), 580-586. [CrossRef]
- Lipiski M. Eberhard C. M., Fleischmann T. Computed Tomography-based evaluation of porcine cardiac dimensions to assist in pre-study planning and optimized model selection for pre-clinical research. Scientific reports. 2020, 7;10(1), 6020. [CrossRef]
- Shatokchin K. Problems of mini-pig breeding. Vavilov Journal of Genetics and Breeding. 2021, 25(3), 284-291. [CrossRef]
- Nikitin S.V., Knyazev S.P., Shatokhin K.S. Miniature Pigs of ICG as a Model Object for Morphogenetic Research. Russian Journal of Genetics: Applied Research. 2014, 4 (6), 511-522. [CrossRef]
- Huenges K., Pokorny S., Berndt R. et all. Transesophageal echocardiography in swine: establishment of a baseline. Ultrasound in Medicine & Biology. 2017, 43, 974-980. [CrossRef]
- Billing S., Zayat R., Ebeling A. et all. Transesophageal echocardiography in swine: evaluation of left and right ventricular structure, function and myocardial work. Int J Cardiovasc Imaging. 2021, 37(3), 835-846. [CrossRef]
- Edwards J., Abdou H., Patel N., Madurska M.J., Poe K. et all. The functional vascular anatomy of the swine for research. Vascular. 2022, 30(2), 392-402. [CrossRef]
- Heo Y-C., Lee H-K., Yang H-J., Cho J-H. Analysis of enlarged images using time-of-flight magnetic resonance angiography, computed tomography, and conventional angiography. J Med Syst. 2014, 38(12), 146. [CrossRef]
- Kobelev E., Bergen T.A., Tarkova A.R. et all. A new look at structural changes in the aortic root in aortic valve stenosis. Modern Technologes in Medicine. 2022, 14. (2), P.51-56. [CrossRef]
- Grau-Mercier L., Coisy F., Markarian T., Muller L. et all. Can blood loss be assessed by echocardiography? An experimental study on a controlled hemorrhagic shock model in piglets. J Trauma Acute Care Surg. 2022, 1;92(5), 924-930. [CrossRef]
- Schwarz S., Kalbitz M., Hummler H. D., Mendler M. R. Transthoracic Echocardiography of the Neonatal Laboratory Piglet. Front Pediatr. 2019, 31(7), 318. [CrossRef]
- Contino M, Mangini A, Lemma M. G, Romagnoni C. et all. A geometric approach to aortic root surgical anatomy. Eur J Cardiothorac Surg. 2016, 49(1), 93-100. [CrossRef]
- Ladouceur M., Kachenoura N., Lefort M., Redheuil A. et all. Structure and function of the ascending aorta in palliated transposition of the great arteries. International Journal of Cardiology. 2013, 165, I. 3, 458-462. [CrossRef]
- Georges G, Couture T, Voisine P. Assessment of Large Animal Vascular Dimensions for Intra-Aortic Device Research and Development: A Systematic Review. Innovations (Phila). 2023,18(2), 144-151. [CrossRef]
- Swindle M.M., Smith A.S. Swine in the laboratory surgery, anesthesia, imaging and experimental techniques, 3rd ed.; CRC Press Taylor&Francis Group Boca Ration London New York, USA, 2016; p.213.
- Galbas M.C., Meissner F., Asmussen A., Straky H. C., Schimmel M., et all. A systematic methodology for epicardial and epiaortic echocardiography in swine research models. Health Sci Rep. 2024, 5;7(1), e1777. [CrossRef]
- Zacchigna S., Paldino A., Falcão-Pires I., Daskalopoulos E. P., et all. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: a position paper of the ESC Working Group on Myocardial Function. Cardiovasc Res. 2021, 1;117(1), 43- 59. [CrossRef]
- Hogen T., Li J., Balmaceda P., Ha T. et all. Echocardiography Recording in Awake Miniature Pigs. J Vis Exp. 2023, 26, 195. [CrossRef]
Figure 1.
The scheme of branching of the main arteries in pigs.
Figure 1.
The scheme of branching of the main arteries in pigs.
Figure 2.
Measurement of the fibrous aortic ring (ann) in mini pigs from the "inner edge to the inner edge" at the site of flap attachment, in the position with the maximum ring size.
Figure 2.
Measurement of the fibrous aortic ring (ann) in mini pigs from the "inner edge to the inner edge" at the site of flap attachment, in the position with the maximum ring size.
Figure 3.
Measurement of the Sinus Valsalva (Sinus Val) and sinotubular junction (ST Jxn) in mini pigs from the outer contour to the outer contour during diastole.
Figure 3.
Measurement of the Sinus Valsalva (Sinus Val) and sinotubular junction (ST Jxn) in mini pigs from the outer contour to the outer contour during diastole.
Figure 4.
Visualization of the aortic root in mini pigs on TЕE : 1- fibrous ring of the aortic valve; 2- sinus Valsalva; 3- sinotubular junction; 4- height of the aortic root.
Figure 4.
Visualization of the aortic root in mini pigs on TЕE : 1- fibrous ring of the aortic valve; 2- sinus Valsalva; 3- sinotubular junction; 4- height of the aortic root.
Figure 5.
Visualization of the aorta in its various sections by angiography: Ao -aorta, Brach. Tr.- brachiocephalic trunk, L. Subcl.-left subclavian artery, bifurcation- bifurcation, Abd.Ao - abdominal aorta, Tr Ao- thoracic aorta.
Figure 5.
Visualization of the aorta in its various sections by angiography: Ao -aorta, Brach. Tr.- brachiocephalic trunk, L. Subcl.-left subclavian artery, bifurcation- bifurcation, Abd.Ao - abdominal aorta, Tr Ao- thoracic aorta.
Figure 6.
Correlations of Spearman's rank order between the results obtained by echocardiography and angiography; the noted correlations are significant at p<0.05.
Figure 6.
Correlations of Spearman's rank order between the results obtained by echocardiography and angiography; the noted correlations are significant at p<0.05.
Figure 7.
The main parameters of the aortic root in mini pigs in groups of 40-70 kg, 71-90 kg and more than 90 kg (90+). AoVAn Echo - diameter of the fibrous ring of the aorta according to TEE, SVal Echo - sinus Valsalva according to TEE, StJ Echo - sinotubular junction according to TEE, AoR height Echo -height of the aortic root according to TEE.
Figure 7.
The main parameters of the aortic root in mini pigs in groups of 40-70 kg, 71-90 kg and more than 90 kg (90+). AoVAn Echo - diameter of the fibrous ring of the aorta according to TEE, SVal Echo - sinus Valsalva according to TEE, StJ Echo - sinotubular junction according to TEE, AoR height Echo -height of the aortic root according to TEE.
Figure 8.
The change in the diameter of the aorta (mm) from the distal part of the aortic arch to the bifurcation in the abdominal region.
Figure 8.
The change in the diameter of the aorta (mm) from the distal part of the aortic arch to the bifurcation in the abdominal region.
Figure 9.
The occurrence of different types of aorta in humans and in mini-pigs: (a) Percentage of occurrence in humans; (b) Percentage of occurrence in pigs.
Figure 9.
The occurrence of different types of aorta in humans and in mini-pigs: (a) Percentage of occurrence in humans; (b) Percentage of occurrence in pigs.
Figure 10.
The change in the diameter of the thoracic and abdominal aorta in mini pigs of different weights compared to pigs of other breeds.
Figure 10.
The change in the diameter of the thoracic and abdominal aorta in mini pigs of different weights compared to pigs of other breeds.
Table 1.
Measurements of height and weight parameters and aortic structures in miniature pigs by using transesophageal echocardiography and angiography*.
Table 1.
Measurements of height and weight parameters and aortic structures in miniature pigs by using transesophageal echocardiography and angiography*.
| Parameter |
I group
(40-70 kg)
Median (Q25%/Q75%) |
II group
(71-90 kg)
Median (Q25%/Q75%) |
III group
(>90 kg)
Median (Q25%/Q75%) |
| Male / Female, n, (%) |
4/5
(44,4/55,6%) |
3/11
(21,4/78,6%) |
0/5
(0/100%) |
| Age, month |
12,0 (8/18) |
24,0 (24/36) |
36,0 (32/36) |
| Weight, kg |
65,0 (53/68) |
76,50 (76/86) |
98,0 (93/101) |
| Body length, cm |
94,0 (90/95) |
99,0 (97/105) |
111,0 (110/118) |
| Breast volum, cm |
90,0 (86/95) |
101,5 (98/109) |
114,0 (114/120) |
| Aortic valve annulus fibrosus, Echo, mm |
20,70 (19,6/23,7) |
22,8 (16,4/24,0) |
25,0 (23,5/28,8) |
| Aortic valve annulus fibrosus, A, mm |
21,1 (20,0/23,5) |
22,5 (17,0/23,3) |
24,6 (21,2/28,2) |
| Diaphragmatic part of the aorta, A, mm |
10,2 (9,0/12,0) |
13,7 (11,0/16,6) |
15,2 (13,0/15,5) |
| Sinus of Valsalva, Echo, mm |
31,0 (28,9/33,0) |
29,8 (24,1/36,0) |
34,8 (30,9/40,0) |
| Sinus of Valsalva, A, mm |
31,5 (28,9/32,6) |
28,2 (24,3/35,8) |
29,6 (27,1/40,3) |
| Aortic root height, Echo, mm |
19,9 (17,7/22,6) |
21,6 (17,5/35,0) |
19,0 (18,9/21,2) |
| Aortic root height, A, mm |
18,5 (16,0/20,9) |
21,9 (18,0/35,2) |
20,8 (20,2/22,9) |
| Ascending aorta, Echo, mm |
24,0 (22,2/25,4) |
20,1(16,0/25,3) |
23,7 (19,3/28,0) |
| Ascending aorta,A, mm |
23,8 (22,0/32,4) |
19,6 (17,5/25,0) |
23,4 (19,3/25,0) |
| Proximal portion of aortic arch, A, mm |
22,4 (19,6/24,0) |
20,2 (16,0/25,5) |
24,0 (20,8/25,0) |
| Distal portion of aortic arch, A, mm |
18,5 (17,9/22,5) |
17,9 (16,8/22,0) |
17,6 (17,0/18,2) |
| Aortic Isthmus A,mm |
15,2 (15,0/22,4) |
16,7 (15,0/19,6) |
17,5 (16,5/18,3) |
| Aortic bifurcation, A, mm |
9,0 (7,7/9,0) |
9,3 (9,0/10,0) |
11,2 (10,0/12,2) |
| Brachiocephalic trunk, A, mm |
10,0 (9,0/10,0) |
10,9 (10,0/11,1) |
14,7 (10,2/15,1) |
| Left subclavian artery, A, mm |
9,0 (8,8/9,0) |
9,6 (8,0/10/0) |
11,8 (11,2/12,0) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).