1. Introduction
Neonatal screening for congenital hypothyroidism (CH) is a milestone in the prevention of severe neurological sequelae, enabling the timely identification and treatment of affected newborns [
1,
2,
3]. Immediately after screening was implemented, the incidence of the disease increased from 1:6,700 to approximately 1:3,500 live births. In recent decades, however, higher incidences have been reported, between 1:1,400 and 1:3,000, depending on the region, ethnicity, and screening methods [
1,
2,
4,
5,
6,
7].
The reason for this increase is still debated. Longer survival of premature and low-birthweight newborns, advanced maternal age, and ethnic changes in the population have been suggested as contributing factors[
7,
8,
9,
10]. Nonetheless, a decreased thyroid-stimulating hormone (TSH) cut-off at blood-spot screening (b-TSH) is considered the main factor for the increased incidence[
2,
11,
12,
13]. This rise occurs primarily, but not exclusively, because of the detection of mild cases with eutopic glands[
4].
Treatment with levothyroxine for moderate to severe CH has unquestionable benefits. However, the natural course of the disease and the need for treatment in mild cases are not fully understood[
2,
14,
15,
16,
17]. This is one of the reasons why it is necessary to discuss the need and cost-benefit of decreasing the b-TSH cut-off level in neonatal screening programs for CH.
With this in mind, we evaluated the impact of b-TSH cut-off reduction on a government funded neonatal screening program for CH.
2. Materials and Methods
This universal prospective cohort study was conducted between November 2021 and August 2022 in the Neonatal Screening Program funded by the Brazilian State of Minas Gerais (PTN-MG, acronym in Portuguese).
Minas Gerais is the 4th largest and 2nd most populated Brazilian state with 853 municipalities. It is located in the south-eastern region of Brazil. Approximately 18,000–20,000 newborns are screened per month by the program.
The study was approved by the Research Ethics Committee of Universidade Federal de Minas Gerais (UFMG). The parents or legal guardians received relevant information and signed an informed consent form.
Before the study, the PTN-MG used the protocol established by the Brazilian Ministry of Health to measure filter-paper bloodspot TSH (b-TSH) with a cut-off of 10 mIU/L. During the study, the protocol for newborn screening was modified, and the reference value for b-TSH was reduced from 10 mIU/L to 6 mIU/L.
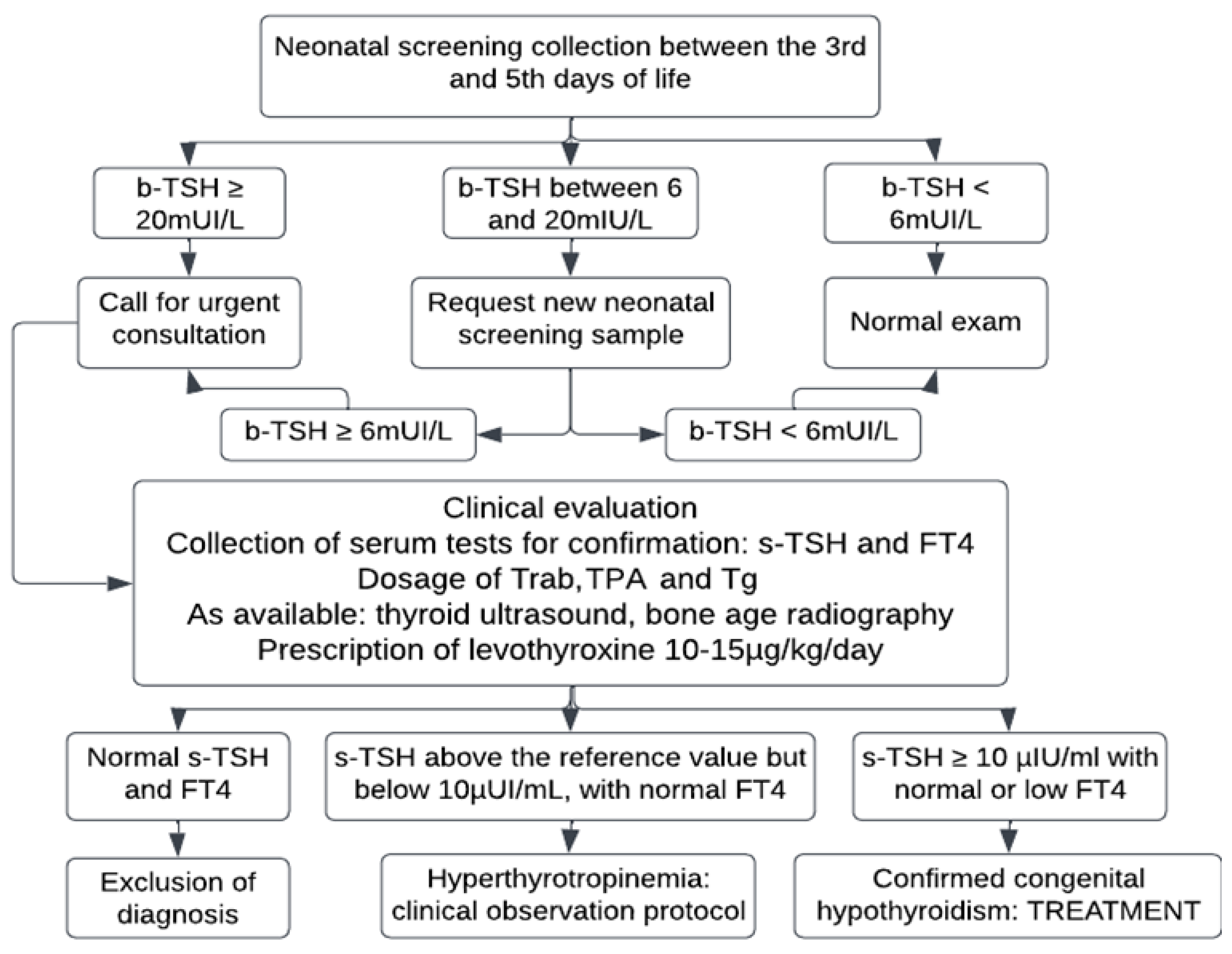
The technical operation of the PTN-MG is carried out by the Núcleo de Ações e Pesquisa em Apoio Diagnóstico (NUPAD) [Center for Diagnostic Support Action and Research] of the Medical School of the Universidade Federal de Minas Gerais (FM-UFMG). General management and coordination are performed by the Health Secretariat of the State of Minas Gerais. Screening is performed on samples collected between the third and fifth day of life, at primary healthcare units (UBS, acronym in Portuguese) or maternity wards, from newborns who were not discharged by the fifth day of life for some relevant reason associated with their clinical picture.
b-TSH is evaluated using the fluoroimmunoassay method at the NUPAD laboratory. According to the protocol, children with b-TSH ≥ 20 mIU/L were contacted for an emergency appointment at the reference center to confirm the diagnosis. Those who presented borderline results (between 6 and 20 mIU/L) had a second sample collected on a filter paper and were asked to come to an appointment if the result remained ≥ 6 mIU/L (
Figure 1).
Extreme preterm babies, newborns with hemodynamic instability, and those who received a transfusion before sample collection for screening, who were at risk for a false-negative result, underwent serial collections even if the first sample was normal (special protocols).
Diagnosis is confirmed based on serum TSH levels (s-TSH – reference values (RV): 0.69 to 8.55 μIU/mL) and free thyroxine (FT4 – RV: 0.89 to 1.76 ng/dl) by means of chemiluminescence immunometric assay (ICMA). Newborns with b-TSH between 6 and <10 mIU/L underwent thyroid ultrasound by one single trained radiologist, with a reference value for thyroid volume of 0.45 to 1.34 cm³ [
18].
The criteria used to confirm cases and initiate treatment were s-TSH ≥10 µIU/mL and normal or low FT4 or borderline s-TSH (above the reference value, but lower than 10 µIU/mL) associated with low FT4. Patients diagnosed based on this last criterion were assessed for central CH.
Newborns presenting with s-TSH ≥10 µIU/mL and FT4 below the reference value were classified as decompensated CH.
According to the sample calculation, with 95% confidence and 97% accuracy, a minimum sample size was established for 60 detected cases of CH and at least 150,000 negative tests.
The analyses were performed using software R 4.1.2 and IBM SPSS Statistics 26. A significance level of 5% was considered for all the tests. Variables were verified for normality using the Kolmogorov-Smirnov or Shapiro-Wilk tests. Continuous variables were characterized by medians, minimum and maximum values, and percentiles. Comparisons between continuous variables were performed using the Kruskal-Wallis test with the Bonferroni post-hoc test. Categorical variables were represented by their absolute values and percentages and were compared using Pearson's chi-square test. The incidence of CH was calculated, as well as the recall rates for new sample collections and calls for appointments in the period. Using the ROC curve, the sensitivity and specificity of b-TSH cut-off values of 6, 7, 8, 9, and 10 mIU/L were calculated. Moreover, the respective positive and negative predictive values (PPV and NPV) were calculated to compare the performance. Patients who were screened using special protocols were excluded.
For clinical and laboratory comparisons, patients referred for medical appointments during the study period were divided into two groups, stratified according to b-TSH values at first sampling: 1) newborns with b-TSH between 6 and <10 mIU/L and 2) newborns with b-TSH ≥10 mIU/L.
3. Results
During the study period, 162,730 patients were screened using the PTN-MG, and 3,070 newborns were screened using special protocols. A total of 123 cases of CH were diagnosed, and 50 were identified due to a change in the b-TSH cut-off. The incidence for the cut-off of 6 mIU/L was 1 case for every 1,323 newborns, whereas for the cut-off of 10 mIU/L, it was 1:2,229 in the study period.
The recall rate was calculated for a b-TSH cutoff of 6 mUI/L at the first sampling. The recall rate for the second sample of neonatal screenings was 0.57%, and for medical appointments was 0.08%.
The area under the ROC curve for the first sample was 0.999, with a 95% confidence interval (95%CI) between 0.999 and 0.999; for the second sample, it was 0.991 (CI 0.986–0.996). The projected performances for the different cut-offs are summarized in
Table 1. All the cut-offs assessed had adequate specificity and NPV. Lower cut-offs were related to better sensitivity, but with a decrease in PPV and increase in recall rates.
Group 1 consisted of 50 newborns, with 27.6% preterm children and Group 2 had 58 children, 20% premature. The prematurity rate did not differ significantly between the groups (p= 0.378). Newborns called for a medical appointment showed a median of b-TSH in the first neonatal screening sample of 7.44 mIU/L (Q1, 6.02; Q3, 9.91) in Group 1, and of 39.88 mIU/L (Q1, 10.90; Q3, 313) in Group 2 (p<0.01). In the second sample, Group 1 had a b-TSH median of 9.53 mIU/L (Q1, 4.69; Q3, 24.34), at a median age of 14 days, and Group 2, at 13 days, had a median of 21.40 mIU/L (Q1, 10.68; Q3, 77.46) (p<0.01). It is noteworthy that among Group 2 patients, only those whose first b-TSH sample was between 10 and 20 mIU/L had a second sample evaluated. Patients with a b-TSH ≥20 mIU/L in the first sample were referred for emergency appointments.
Group 2 patients presented significantly more clinical signs, such as prolonged jaundice, dry skin, poor weight gain, and large fontanelles and cranial sutures, than Group 1 (p=0.02), even though they were assessed earlier (median age of 16- vs. 25-day-old).
Among the patients with confirmed CH, Group 1 presented significantly lower levels of s-TSH and significantly higher levels of FT4 than those in Group 2 (p<0.001 for both analyses), as summarized in
Table 2. Of the patients in Group 1, 21% presented with FT4 values below the reference value used in the program.
A total of 18 patients from Group 1 underwent thyroid ultrasound upon diagnosis: three (16.7%) with tests suggestive of dysgenesis – two with hypoplasia, and one who showed hemiagenesis – and 15 (83.3%) were inconclusive – two with goiter, and 13 with normal thyroid.
4. Discussion
The decrease of the b-TSH cut-off from 10 to 6 mIU/L represented a significant increase in the test sensitivity, allowing detection of more newborns with CH, but, also, a high rate of false positives cases. The unequivocal benefits of treating children precociously, and reports of undiagnosed CH with current cut-offs of b-TSH have been led many newborn screening programs to reduce the thresholds resulting in higher incidence of the disease [
2,
11,
12,
13].
The incidence of CH in Brazil ranges from 1:2,500 to 1:4,800 of live births with a b-TSH cut-off of 10 mIU/L[
19]. Similar to what was found by the present study, the incidence of CH drop sharply to 1:950 - 1:1,560 [
20,
21,
22] in some states that reduced the cut-offs to 4.5 to 6 mIU/L.
The same have been reported in several parts of the world, paralleled by a change in newborns profile, with identification of mild CH cases and reduction in percentage of dysgenetic glands [
4,
11].
In accordance to these reports, we observed a greater proportion of patients with less severe conditions, with few or no symptoms and compatible laboratory findings. Most patients assessed did not present anatomical abnormalities on thyroid ultrasound, a frequent finding related to CH diagnoses with thyroid gland in situ after a reduction in the cut-off points [
4,
11,
16,
23,
24]. In a survey conducted at the PTN-MG between 2018 and 2019, with 44 children under the unchanged protocol, the proportion of newborns with thyroid with no anatomical alterations on ultrasound in the first appointment was 58% (unpublished data), which is lower than the current findings.
However, more severe cases of CH have been reported with lower cut-off points, and approximately 40% of patients with permanent and decompensated CH upon diagnosis were found with b-TSH between 8 and 10 mIU/L [
25].
We observed although most newborns in Group 1 presented mild laboratory alterations, 21% of the sample presented decompensated CH, which is an unequivocal indication for treatment in all consensuses on the disease [
1,
3,
26].
We show that if the cut-off of 10 mIU/L had been kept, 40% of newborns would not have been diagnosed with the disease, which seems an unacceptable rate. A significant number of children with transient or permanent CH being left undiagnosed using the cut-off of 10 mIU/L (18%) led the authors suggesting a value of 6 mIU/L to be more appropriate for the screening program in the United Kingdom [
13].
On the other hand, a problem related to the reduction of the b-TSH cutoff is a great number of children called for medical assessment.
Keeping higher cut-offs, as seen in some high income countries, can reduce the number of false-positive results [
27]. However, active vigilance and quality infant care must be guaranteed to diagnose possible cases missed during screening. A Swedish study showed that using a b-TSH cut-off of 15 mIU/L, 50% of detected CH cases had come back negative during neonatal screening and were identified during pediatric care [
28]. Taking care of these children at the right time is certainly not possible in many regions.
There is no consensus on the ideal recall rate. Just as the cut-off value, this rate depends on several factors, such as the geographical coverage of the program, available infrastructure, financial resources, among others. The worldwide rate of calls for medical appointments varies between 0.01% and 13.3%, depending on the adopted b-TSH cut-off [
29].
We observed the reduction in the b-TSH cut-off was associated with a 5.2-fold increase in the rate of recall for a second sample, and as the result was normal for most children, the rate of calls for appointments increased by 30%. A similar reduction, from 10 to 6 mIU/L in the United Kingdom, led this rate to increase from 0.08% to 0.23% [
12]. High recall rates represent higher costs for screening programs, in addition to a higher number of healthy children undergoing needless exams and appointments, with a relevant emotional impact for the patients and their families. The recall rate increased in all reported programs that implemented a reduction in the cut-off screening [
30,
31], up to 10 times [
24,
32].
In the United States, costs incurred from repeated tests can reach up to US
$ 2 million annually [
29], the burden can be expected to be bigger for disadvantaged populations. It is clear, however, that the lower the recall rate – without leaving sick children undiagnosed – the better.
Besides the costs, a definition of the appropriate cut-off point for a neonatal screening program must consider the methods used; the median age of the newborns during sample collection; ethnicity of the population and degree of iodine sufficiency; capacity to process the tests; availability of specialized centers; and socioeconomic and cultural factors. This definition should aim for a sensitivity close to 100%, but without overburdening the program with too many false positive results.
Herein, the different cut-off points presented good specificity and a high NPV, which were expected based on the low prevalence of CH in the population [
29]. Therefore, sensitivity and PPV assessments are more important for suitability analysis. Despite a higher sensitivity of the 6 mIU/L cut-off in comparison to the 10 mIU/L, the low PPV in the first sample – which generated over 88% of false-positive results – is not attractive to a government funded program. In Australia, reducing the cut-off from 15 to 8 mIU/L caused the PPV to drop from 74.3% to 12.8% [
32]. The same was reported in Brazilian regions – Rio de Janeiro and Campinas – which showed false positive results close to 90% [
20,
33].
Analyzing the performances of the different cut-offs, we found an intermediate value of 8 mIU/L was related to good sensitivity and a PPV over 2 times higher than the cut-off of 6 mIU/L, which potentially makes it more adequate for the first sample.
Although a higher prevalence of preterm infants could have been expected in Group 1, the similar number of preterm newborns observed in both groups precludes any effect of prematurity on the performance analysis of different b-TSH cutoff points, potentially increasing false negative results.
Multiple strategies have been attempted to enhance the effectiveness of screening programs. An advantage arises from implementing second screening for extremely preterm and very low birth weight infants due to the risk of delayed TSH rise [
1], as is currently practiced in the Brazilian program protocol.
Re-screening children with borderline results reduced the recall rate of false-positives without delaying the assessment of urgent cases [
34]. One of the strategies useful to improve the performance of the programs, with fewer expenses to families and a better cost-benefit, is the use of cut-offs adjusted for age, which can reduce by up to 50% the number of false results [
29].
Another one is the use of lower cut-off points for the second sample, when recommended [
34,
35]. Decreasing the cut-off only in the second sample doubled the incidence of newborns diagnosed with dyshormonogenesis and dysgenesis [
16], and allowed the diagnosis of 43.7% of CH cases in another report [
36]. The use of these lower cut-off points takes into consideration the physiological changes of thyroid function, increase in TSH in the first 48 hours after birth, and progressive decrease during the first weeks of life. As we observed, a reduced cut-off in the second sample can increase test sensitivity without compromising the PPV, that reached 80% using the 6 mIU/L cut-off.
The disadvantages of using different cut-off points in the subsequent samples include the additional complexity of the screening process, which requires different criteria to interpret results according to the sample. This can increase the possibility of interpretation errors and demands more training of the healthcare professionals involved in the program.
The main objective of CH screening is to prevent intellectual deficit associated with the disease. Therefore, the main argument of authors who are against reducing cut-off points is that the benefit of treatment for very mild and subclinical cases – which comprise the majority of cases that have been identified – is still under investigation. The results obtained in the treatment of moderate to severe CH since screenings were implemented should not be simply extrapolated [
37,
38]. Natural evolution studies have been conducted to show the developmental impacts suffered by children with untreated mild CH, but results are still conflicting. Some reports suggest differences in the neurodevelopment of children with borderline b-TSH were not clinically relevant to recommend changes in the cut-off [
39,
40,
41], while others show that these children had an increased risk of poor school performance, developmental changes, reduced memory span, and a higher chance to develop special needs [
42,
43]. This assessment is complex since cognitive deficits can be associated to multiple factors, including socioeconomic and environmental factors [
44,
45].
It is important, also, to consider that excessive treatment in CH patients seems to be associated to unfavorable cognitive outcomes. There are reports of an increase in cases of attention deficit hyperactivity disorder and even a decrease in intelligence quotient related to excessive treatment [
46,
47,
48]. There is yet no consensus on this subject, more research is needed.
The main limitation of this study is a potential underestimation of false-negative cases since all patients with a neonatal b-TSH <6 mIU/L were considered normal. Furthermore, few patients underwent thyroid ultrasound. However, the sample size and rigorous protocol are strengths supporting the results found are highly consistent.
5. Conclusions
There was an increase in the diagnosis of CH after reducing the b-TSH cut-off to 6 mIU/L, mainly attributed to the detection of children with mild cases. Maintaining a cut-off of 10 mIU/L would have resulted in 40% of newborns with CH going undiagnosed, which appears to be an unacceptable rate. Since the b-TSH cut-off of 6 mIU/L for the first sample exhibited a very low PPV, an intermediate value of 8 mIU/L is proposed, along with a threshold of 6 mIU/L for subsequent samples, to achieve an appropriate cost-benefit balance. Continuous longitudinal monitoring of newborn screening programs utilizing lower cut-off points is essential to evaluate their efficacy over time.
Author Contributions
Conceptualization, methodology N.T.P.B, V.M.A.D and I.N.S; investigation, N.T.P.B and J.M.V.A; formal analysis, N.T.P.B and E.A.C; original draft preparation, N.T.P.B; writing—review and editing, I.N.S; V.M.A.D and J.N.J; supervision, I.N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (UFMG), (number 4,983,456, in 09/17/2021).
Informed Consent Statement
Informed consent was obtained from all parents/legal guardians of the patients involved in the study. Written informed consent has been obtained from parents/legal guardians of the patients to publish this paper.
Data Availability Statement
Data is not available due to ethical reasons. Further enquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Núcleo de Ações e Pesquisa em Apoio Diagnóstico da Faculdade de Medicina da Universidade Federal de Minas Gerais – Nupad for the great contribution on this project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Trotsenburg, P.V.; Stoupa, A.; Léger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; et al. An ENDO-EUROPEAN REFERENCE NETWORK ( ERN ) initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [PubMed]
- Wassner, A.J.; Brown, R.S. Congenital hypothyroidism: Recent advances. Curr Opin Endocrinol Diabetes Obes. 2015, 22, 407–12. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K.; Minamitani, K.; Anzo, M.; Adachi, M.; Ishii, T.; Onigata, K.; et al. Guidelines for Mass Screening of Congenital Hypothyroidism (2014 revision) Mass Screening Committee, Japanese Society for Pediatric Endocrinology, and Japanese Society for Mass Screening. Clin Pediatr Endocrinol. 2015, 24, 107–133. [Google Scholar]
- Chiesa, A.; Prieto, L.; Mendez, V.; Papendieck, P.; Calcagno, M.D.L.; Gruñeiro-Papendieck, L. Prevalence and etiology of congenital hypothyroidism detected through an argentine neonatal screening program (1997-2010). Horm Res Paediatr. 2013, 80, 185–92. [Google Scholar] [CrossRef] [PubMed]
- Barry, Y.; Bonaldi, C.; Goulet, V.; Coutant, R.; Léger, J.; Paty, A.C.; et al. Increased incidence of congenital hypothyroidism in France from 1982 to 2012: A nationwide multicenter analysis. Ann Epidemiol [Internet], 2016; 26, 100–105.e4. [Google Scholar]
- Peters, C.; Brooke, I.; Heales, S.; Ifederu, A.; Langham, S.; Hindmarsh, P.; et al. Defining the newborn blood spot screening reference interval for TSH: Impact of ethnicity. J Clin Endocrinol Metab. 2016, 101, 3445–9. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Kato, T.; Harada, S.; Inomata, H.; Aoki, K. Time Trend and Geographic Distribution of Treated Patients with Congenital Hypothyroidism Relative to the Number of Available Endocrinologists in Japan. J Pediatr. 2010, 157, 153–7. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.L.; Derraik, J.G.B.; Webster, D.; Hofman, P.L. The impact of demographic factors on newborn TSH levels and congenital hypothyroidism screening. Clin Endocrinol (Oxf) 2019, 91, 456–63. [Google Scholar] [CrossRef] [PubMed]
- Albert, B.B.; Cutfield, W.S.; Webster, D.; Carll, J.; Derraik, J.G.B.; Jefferies, C.; et al. Etiology of increasing incidence of congenital hypothyroidism in New Zealand from 1993-2010. J Clin Endocrinol Metab. 2012, 97, 3155–60. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.B.; Pass, K.A. Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab. 2007, 91, 268–77. [Google Scholar] [CrossRef]
- Olivieri, A.; Fazzini, C.; Medda, E. Multiple factors influencing the incidence of congenital hypothyroidism detected by neonatal screening. Horm Res Paediatr. 2015, 83, 86–93. [Google Scholar] [CrossRef]
- Korada, S.M.; Pearce, M.; Ward, M.P.; Avis, E.; Turner, S.; Wastell, H. et al. Difficulties in selecting an appropriate neonatal thyroid stimulating hormone (TSH) screening threshold. Arch Dis Child, 2010; 95, 169–73. [Google Scholar]
- Langham, S.; Hindmarsh, P.; Krywawych, S.; Peters, C. Screening for Congenital Hypothyroidism: Comparison of Borderline Screening Cut-Off Points and the Effect on the Number of Children Treated with Levothyroxine. Eur Thyroid J. 2013, 180–6. [Google Scholar] [CrossRef]
- Cherella, C.E.; Wassner, A.J. Congenital hypothyroidism: insights into pathogenesis and treatment. Int J Pediatr Endocrinol. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Deladoëy, J.; Van Vliet, G. The changing epidemiology of congenital hypothyroidism: Fact or artifact? Expert Rev Endocrinol Metab. 2014, 9, 387–95. [Google Scholar] [CrossRef] [PubMed]
- Deladoëy, J.; Ruel, J.; Giguère, Y.; Van Vliet, G. Is the incidence of congenital hypothyroidism really increasing? A 20-year retrospective population-based study in Québec. J Clin Endocrinol Metab. 2011, 96, 2422–9. [Google Scholar] [CrossRef]
- Connelly, K.J.; Lafranchi, S.H. Detection of neonates with mild congenital hypothyroidism (primary) or isolated hyperthyrotropinemia: An increasingly common management dilemma. Expert Rev Endocrinol Metab. 2014, 9, 263–71. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.; Monte, O.; Tomimori, E.K.; Catarino, R.M.; Sterza, T.; Rocha, T.; et al. Sonographic evaluation of the thyroid size in neonates. J Clin Ultrasound 2015, 43, 224–9. [Google Scholar] [CrossRef]
- Brasil. Protocolo clínico e diretrizes terapêuticas para hipotireoidismo congênito. Portaria SAS/MS no 1161, de 18 de novembro de 2015. Ministério da Saúde. Available online (accessed on 02/28/2024) <http://portalarquivos.saude.gov.br/images/pdf/2015/novembro/26/Hipotiroidismo-congenito---PCDT-Formatado--.pdf>. 2015. p. 1–8.
- Barone, B.; Lopes, C.L. da S.; Tyszler, L.S.; do Amaral, V.B.; Zarur, R.H.C.; Paiva, V.N.; et al. Avaliação do valor de corte de TSH em amostras de filtro na triagem neonatal para diagnóstico de hipotireoidismo congênito no programa “Primeiros Passos” - IEDE/RJ. Arq Bras Endocrinol Metabol. 2013, 57, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.L.; Nascimento, A.L.; Dornbusch, P.; Ohira, M.; Simoni, G.; Cechinel, E.; et al. Impact of the reduction in TSH cutoff level to 6 mUI/L in neonatal screening for congenital hypothyroidism in Santa Catarina: final results. Arch Endocrinol Metab. 2020, 64, 816–23. [Google Scholar]
- Matos, D.M.; Ramalho, R.J.R.; Carvalho, B.M.; Almeida, M.A.C.T. ; Passos LFD, Vasconcelos TTS, et al. Evolution to permanent or transient conditions in children with positive neonatal TSH screening tests in Sergipe, Brazil. Arch Endocrinol Metab 2016, 60, 450-6. [Google Scholar] [CrossRef]
- Corbetta, C.; Weber, G.; Cortinovis, F.; Calebiro, D.; Passoni, A.; Vigone, M.C.; et al. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol (Oxf). 2009, 71, 739–45. [Google Scholar] [CrossRef]
- Mengreli, C.; Kanaka-Gantenbein, C.; Girginoudis, P.; Magiakou, M.A.; Christakopoulou, I.; Giannoulia-Karantana, A.; et al. Screening for congenital hypothyroidism: The significance of threshold limit in false-negative results. J Clin Endocrinol Metab. 2010, 95, 4283–90. [Google Scholar] [CrossRef] [PubMed]
- McGrath, N.; Hawkes, C.P.; Mayne, P.; Murphy, N.P. Permanent Decompensated Congenital Hypothyroidism in Newborns with Whole-Blood Thyroid-Stimulating Hormone Concentrations between 8 and 10 mU/L: The Case for Lowering the Threshold. Horm Res Paediatr. 2018, 89, 265–70. [Google Scholar] [CrossRef]
- Rose, S.R.; Wassner, A.J. Wintergerst KA, Yayah-Jones NH, Hopkin RJ, Chuang J, et al. American Academy of Pediatrics Section on Endocrinology, AAP Council on Genetics, Pediatric Endocrine Society, American Thyroid Association. Technical report. Congenital hypothyroidism: screening and management. Pediatrics [Internet] 2023, 151. [Google Scholar]
- Danner, E.; Niuro, L.; Huopio, H.; Niinikoski, H.; Viikari, L.; Kero, J.; et al. Incidence of primary congenital hypothyroidism over 24 years in Finland. Pediatr Res. 2022, 93, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Gunnerbeck, A.; Lundholm, C.; von Döbeln, U.; Zetterström, R.H.; Almqvist, C.; Nordenström, A. Neonatal screening for congenital hypothyroidism in Sweden 1980-2013: effects of lowering the thyroid-stimulating hormone threshold. Eur J Endocrinol. 2023, 188, 536–46. [Google Scholar] [CrossRef]
- Mehran, L.; Khalili, D.; Yarahmadi, S.; Amouzegar, A.; Mojarrad, M.; Ajang, N. et al. Worldwide recall rate in newborn screening programs for congenital hypothyroidism. Int J Endocrinol Metab. 2017, 15, e55451. [Google Scholar] [CrossRef]
- Anne, R.P.; Rahiman, E.A. Congenital hypothyroidism in India: A systematic review and meta-analysis of prevalence, screen positivity rates, and etiology. Lancet Reg Health Southeast Asia [Internet]. 2022, 5, 100040. [Google Scholar]
- Simonetti, S.; D’Amato, G.; Esposito, B.; Chiarito, M.; Dentico, D.; Lorè, T.; et al. Congenital hypothyroidism after newborn screening program reorganization in the Apulia region. Ital J Pediatr. 2022, 48, 131. [Google Scholar] [CrossRef]
- Yu, A.; Alder, N.; Lain, S.J.; Wiley, V.; Nassar, N.; Jack, M. Outcomes of lowered newborn screening thresholds for congenital hypothyroidism. J Paediatr Child Health. 2023, 59, 955–61. [Google Scholar] [CrossRef]
- Christensen-Adad, F.C. ; Mendes-dos-Santos; C.T.; Goto, M.M.F.; Sewaybricker, L.E.; D’Souza-Li, L.F.R.; Guerra-Junior, G.; et al. Neonatal screening: 9% of children with filter paper thyroid-stimulating hormone levels between 5 and 10 μIU/mL have congenital hypothyroidism. J Pediatr (Rio J), 2017; 93, 649–54. [Google Scholar]
- Odenwald, B.; Brockow, I.; Hanauer, M.; Lüders, A.; Nennstiel, U. Is Our Newborn Screening Working Well? A Literature Review of Quality Requirements for Newborn Blood Spot Screening (NBS) Infrastructure and Procedures. Int J Neonatal Screen. 2023, 9, 35. [Google Scholar]
- Dorreh, F.; Chaijan, P.Y.; Javaheri, J.; Zeinalzadeh, A.H. Epidemiology of congenital hypothyroidism in Markazi Province, Iran. J Clin Res Pediatr Endocrinol. 2014, 6, 105–10. [Google Scholar] [CrossRef]
- Caiulo, S.; Corbetta, C.; Di Frenna, M.; Medda, E.; De Angelis, S.; Rotondi, D.; et al. Newborn screening for congenital hypothyroidism: the benefit of using differential TSH cutoffs in a two-screen program. J Clin Endocrinol Metab. 2021, 106, e338–e349. [Google Scholar] [CrossRef]
- Krude, H.; Blankenstein, O. Treating patients not numbers: The benefit and burden of lowering TSH newborn screening cut-offs. Arch Dis Child. 2011, 96, 121–2. [Google Scholar] [CrossRef] [PubMed]
- Lain, S.; Trumpff, C.; Grosse, S.D.; Olivieri, A.; Van Vliet, G. Are lower TSH cutoffs in neonatal screening for congenital hypothyroidism warranted? Eur J Endocrinol. 2017, 177, D1–12. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; se Schepper, J.; Vanderfaeillie, J.; Vercruysse, N.; Tafforeau, J.; Van Oyen, H.; et al. No association between elevated thyroid-stimulating hormone at birth and parent-reported problem behavior at preschool age. Front Endocrinol (Lausanne). 2016, 7(DEC), 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; De Schepper, J.; Vanderfaeillie, J.; Vercruysse, N.; Van Oyen, H.; Moreno-Reyes, R.; et al. Neonatal thyroid-stimulating hormone concentration and psychomotor development at preschool age. Arch Dis Child. 2016, 101, 1100–6. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Hong, J.; Derraik, J.G.B.; Webster, D.; Heather, N.L.; Hofman, P.L. Newborn Screening TSH Values Less Than 15 mIU/L Are Not Associated With Long-term Hypothyroidism or Cognitive Impairment. J Clin Endocrinol Metab [Internet]. 2020, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Ramos, R.; Amaya, E.; Fernández, M.F.; Santiago-Fernández, P.; Lopez-Espinosa, M.J.; et al. Newborn TSH concentration and its association with cognitive development in healthy boys. Eur J Endocrinol. 2010, 163, 901–9. [Google Scholar] [CrossRef]
- Lain, S.J.; Wiley, V.; Jack, M.; Martin, A.J.; Wilcken, B. , Nassar, N. Association of elevated neonatal thyroid-stimulating hormone levels with school performance and stimulant prescription for attention deficit hyperactivity disorder in childhood. Eur J Pediatr, 2021; 180, 1073–80. [Google Scholar]
- de Andrade, J.E.; Dias, V.M.A.; Jardim de Paula, J.; Silva, I.N. Socioeconomic aspects are crucial to better intellectual outcome in early-treated adolescents with congenital hypothyroidism. Child Neuropsychol. 2021, 27, 587–600. [Google Scholar] [CrossRef]
- Pelaez, J.M.; Rojas-Ramos, J.C.R.; Domingos, M.T.; de Lima, M.R.; Kraemer, G.C.; Cardoso-Demartini, A.A.; et al. Cognitive outcome of 458 children over 25 years of neonatal screening for congenital hypothyroidism. J Pediatr (Rio J). 2023, 99, 478–484. [Google Scholar] [CrossRef]
- Bongers-Schokking, J.J.; Resing, W.C.M.; Oostdijk, W.; de Rijke, Y.B.; Keizer-Schramaa, S.M.P. F de M. Relation between Early Over- and Undertreatment and Behavioural Problems in Preadolescent Children with Congenital Hypothyroidism. Horm Res Paediatr 2019, 90, 247–56. [Google Scholar] [CrossRef] [PubMed]
- Villanger, G.D.; Ystrom, E.; Engel, S.M.; Longnecker, M.P.; Pettersen, R.; Rowe, A.D.; Reichborn-Kjennerud, T.; Aase, H. Neonatal thyroid-stimulating hormone and association with attention-deficit/hyperactivity disorder. Paediatr Perinat Epidemiol. 2020, 34, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Bongers-Schokking, J.J.; Resing, W.C.M.; Oostdijk, W.; De Rijke, Y.B.; De Muinck Keizer-Schrama, S.M.P.F. Individualized treatment to optimize eventual cognitive outcome in congenital hypothyroidism. Pediatr Res. 2016, 80, 816–23. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).