1. Introduction
Pulmonary fibrosis is a chronic, progressive lung disorder comprising a large number of chronic respiratory pathologies accompanied by connective tissue growth in various lung compartments and characterized by lung architecture disruption and respiratory failure [
1]. Pre-existing lung inflammation caused by a wide variety of etiological factors, including viral and bacterial infections, is considered to be a key factor in pulmonary fibrosis initiation and development [
2].
In response to lung injury or infection, macrophages, the first sentinel cells contacting external pathogens, undergo a transition into the pro-inflammatory M1 phenotype and secrete pro-inflammatory cytokines (TNF-α, IL-6, and IL-1) and chemokines (IL-8, CCL7, and CCL2), attracting monocytes and neutrophils to alveolar spaces [
3]. In turn, neutrophils release numerous inflammatory mediators, reactive oxygen species, and proteinases, which destroy surfactant, basal membranes, and the epithelia-endothelial barrier [
4]. The most common outcome of acute inflammation is its successful resolution and the restoration of altered tissues. However, acute inflammation transforms into chronic inflammation if the etiological factor cannot be eliminated.
One of the most dangerous consequences of chronic inflammation is the development of fibrosis, which, according to modern concepts, is associated with the deregulation of wound healing [
5]. After tissue damage, myofibroblasts migrate to the site of injury and synthesize extracellular matrix (ECM) components in response to cytokines and chemokines secreted by inflammatory and resident cells [
6]. After resolution of inflammation and restoration of lung tissue, myofibroblasts undergo elimination through apoptosis [
7]. However, under chronic inflammation, myofibroblasts evade apoptosis, inducing aberrant wound healing, hyperproduction of ECM, and, as a result, pulmonary fibrosis [
8].
Immunostimulatory nucleic acids (INAs), including immunostimulatory RNA (isRNA), can modulate the fibrotic process by stimulating the innate immune system, which participates in defense against pathogens and tissue damage. The innate immune system recognizes INAs as pathogen or danger signals and activates pathways leading to the expression and secretion of interferons and cytokines. Interferons have antiviral, antiproliferative, and immunomodulatory effects [
9]. Cytokines regulate inflammation, cell growth, differentiation, and survival [
10]. Some of the interferons and cytokines induced by INAs, including interferon gamma (IFN-γ), interleukin 10 (IL-10), and tumor necrosis factor alpha (TNF-α), have anti-fibrotic properties [
11].
Previously, we described a 19-base pair double-stranded RNA with 3’-trinucleotide overhangs, acting as isRNA. This molecule demonstrated notable antiproliferative effects on cancer cells, inhibited tumor growth, and elicited immunostimulatory and antiviral responses by inducing cytokine and interferon production [
12].
The impact of isRNA on cytokine levels, encompassing both pro-inflammatory and anti-inflammatory mediators, has been substantiated [
13]. Unraveling the mechanisms underlying the augmentation of cytokines assumes paramount significance. Given the propensity for heightened cytokine levels during inflammation, augmentation may precipitate a cytokine storm, culminating in an allergic shock and posing a life-threatening risk. On the other side, augmentation of IFN-α- levels by isRNA treatment unveils promising avenues for its application in combating infections and malignancies [
13]. These pathologies are closely related to inflammation and fibrosis, so the application of isRNA that affects the synthesis and secretion of inflammatory mediators, as well as anti-inflammatory and anti-proliferative cytokines and chemokines, can have both positive and negative effects on the development of fibrotic changes in tissues.
isRNA can interact with fibrosis by activating the innate immune system and inducing the expression of anti-fibrotic interferons and cytokines, which can inhibit the fibrotic process by targeting the fibroblasts and the ECM. However, there are also some challenges and limitations in the use of INAs for fibrosis prevention and treatment, such as increased inflammation or toxic effects. Therefore, a more detailed study of the safety of INAs and their preventive and therapeutic significance is required.
Currently, a number of pulmonary fibrosis mouse models have been successfully established. Bleomycin (BLM), an antitumor antibiotic synthesized by the bacteria
Streptomyces verticillus, and lipopolysaccharide (LPS), the main component of the outer membrane of Gram-negative bacteria, are the most commonly used inductors of this pathology [
14]. BLM damages the cells through single- or double-strand DNA breaks, leading to cell cycle arrest and direct cell damage followed by necrosis or apoptosis of epithelial and endothelial cells, lung inflammation, and, as a result, pulmonary fibrosis development [
15]. LPS acts as a powerful activator of innate immunity through the TLR4-dependent pathway, causes local and systemic inflammatory response, making this model appropriate for the study of lung inflammation and associated fibrosis, similar to the one during bacterial infections [
16].
Within this study, we compared the intensity of lung fibrosis induced in mice by BLM or LPS using different schemes of induction. Then we evaluated the effect of isRNA application in a preventive or therapeutic regimens on the development of fibrosis in selected BLM- and LPS-induced mouse models.
3. Discussion
Immunostimulatory nucleic acids (INAs) are low in toxicity and stimulate the body’s innate immunity by operating on natural mechanisms. INAs represents a promising class of prospective medications for the treatment of viral and tumor diseases [
30]. The repertoire of nucleic acids that directly interact with immune system components is growing with advancements in the processes of nucleic acid synthetic production and the elaboration of their derivatives with improved properties.
The effect of INAs depends on many factors, including their nature, structure, sequence, mode of delivery and intracellular localization. A particular 19-bp isRNA duplex with 3’-3-nt overhangs was previously described by our group [
12,
31,
32,
33]. This isRNA lacks significant similarity with human or mouse mRNAs and is one nucleotide longer than canonical siRNAs, since that it cannot alter the pattern of gene expression through RNA interference. The immunostimulatory, interferon-inducing, antiproliferative, anticancer, and antiviral properties of this isRNA were confirmed in vitro and in vivo. Despite the pronounced therapeutically beneficial effects of isRNA, increased expression of a number of cytokines may be of concern due to their potentially undesirable effects, including inflammation enhancement and fibrosis induction.
Previously, we analyzed the cytokines released in mouse blood after i.v. administration of isRNA pre-complexed with cationic liposomes [
13]. The main cytokines whose levels significantly increased in response to isRNA were found to be monocyte chemoattractant protein 1 (MCP-1) and interferons α and γ; the cytokines IL-6 and IL-10 were moderately activated, and a slight activation of IL-1α and TNF-α was also noted. Thus, agents with pro-fibrotic (MCP-1, IL-6, IL-1α), anti-fibrotic (interferons α and γ, IL-10) or dual (TNF-α) effects can be found among the cytokines whose expression is stimulated by isRNA.
Significant activation of MCP-1 expression, which may contribute to fibrosis development, is a cause for concern. It was demonstrated, that MCP-1 mediates and promotes renal [
34], liver [
35] and pulmonary [
36] fibrosis by recruiting monocyte, promoting the activation and transdifferentiation of macrophages. The infarcted myocardium exhibits a notable induction of MCP-1, which is crucial for both infarct healing and post-infarction remodeling [
37]. Important steps in the development of renal fibrosis may be influenced by IL-6 trans-signaling. The loss or inhibition of IL-6 was found to decrease renal fibrosis [
38] and to alleviate BLM-induced pulmonary fibrosis in mice [
39]. In several tissues, including the lungs, the IL-1 cytokine family is known to play an important role in mediating inflammation and fibrosis [
40]. It has been demonstrated that mice treated with BLM exhibit an increase in IL-1α; conversely, IL-1α knockout mice do not develop BLM-induced lung fibrosis [
41].
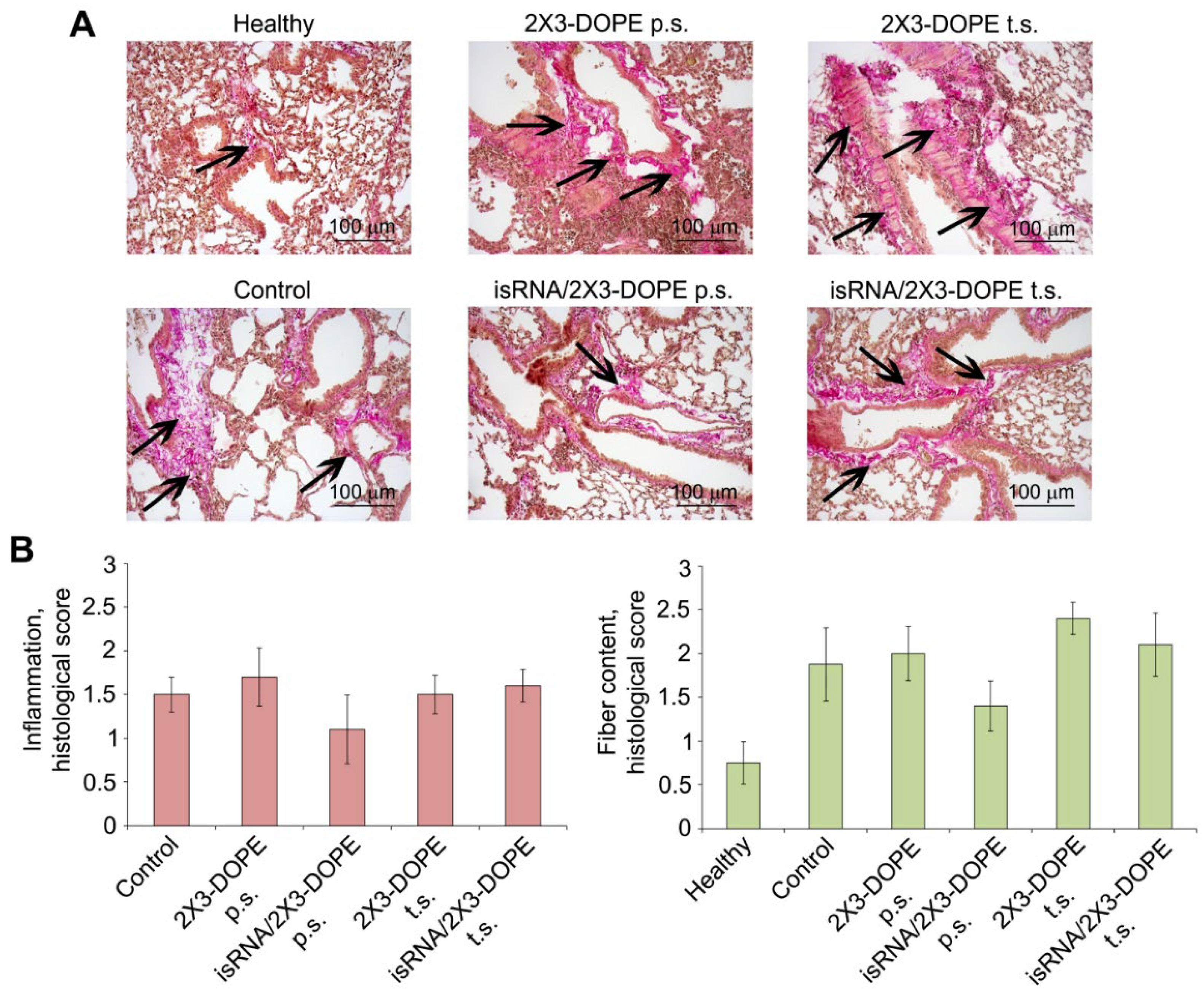
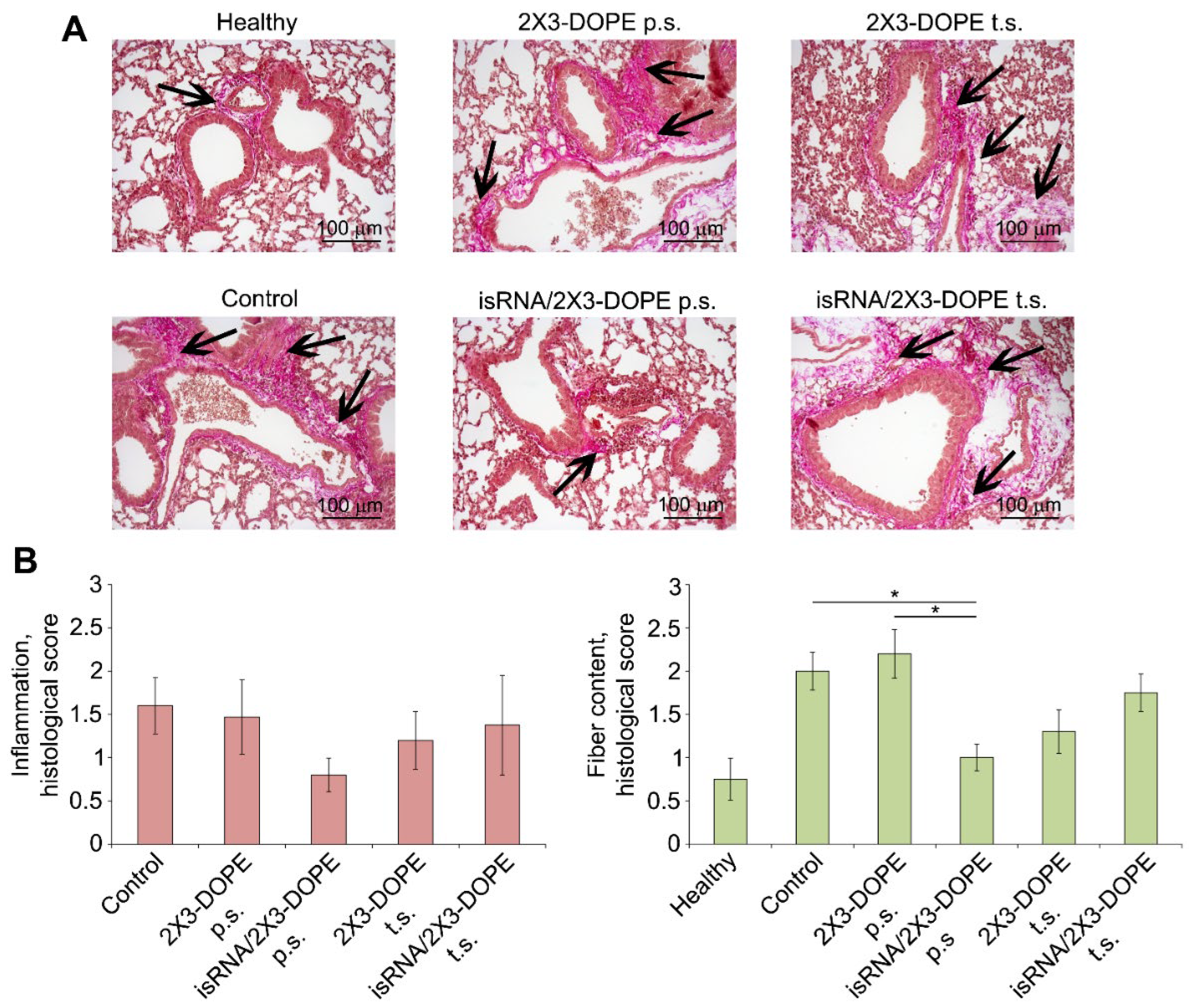
Our data showed that an increase in the levels of pro-fibrotic cytokines (MCP-1, IL-6, Il-1α) caused by exposure to isRNA does not increase inflammation or the development of fibrosis in the lungs, either with a single prophylactic administration or with a two-time therapeutic regimen (
Figure 4 and
Figure 5). This indicates the safety of isRNA application for the treatment of tumors and infectious diseases. Chemotherapy components used for the treatment of tumor diseases can damage non-malignant-sensitive cells and cause the subsequent development of fibrosis as side effects [
42] (as modeled in BLM-induced fibrosis). In infectious diseases, both viral and bacterial, the production of interferons and cytokines is induced by the pathogen itself; however, many pathogens are equipped with mechanisms to evade the immune response [
43], and in this case, INAs can activate the immune system without undesirable consequences (as modeled in LPS-induced fibrosis).
The anti-fibrotic molecules (interferons α and γ, IL-10) can exert their effects by directly targeting the fibroblasts or indirectly affecting the microenvironment [
11]. Thus, IFN-γ inhibits the differentiation of fibroblasts into myofibroblasts and reduces the expression of ECM proteins and profibrotic factors, including transforming growth factor beta (TGF-β) and connective tissue growth factor (CTGF) [
44]. It was documented, that idiopathic pulmonary fibrosis may benefit from inhaled interferon-γ aerosol [
45]. Adeno-associated virus expressing human interferon-gamma demonstrated potential effects, which could inhibit the progression of hepatic fibrosis in experimental hepatic fibrosis in vitro and in vivo [
46]. IL-10 suppresses the production of pro-inflammatory cytokines such as interleukin 1 beta (IL-1β) and interleukin 6 (IL-6), which can stimulate fibroblast activation and ECM synthesis [
47]. The hydrogel containing IL-10 suppressed TGF-β driven collagen production by lung fibroblasts and myofibroblasts and reduced collagen deposition in mice model of bleomycin-induced lung fibrosis [
48]. TNF-α induces apoptosis of fibroblasts and myofibroblasts and promotes the degradation of the ECM by activating matrix metalloproteinases (MMPs) [
49], however, according to recent studies, treating fibrotic disorders either directly or through targeting TNF or its receptors may be a promising strategy [
50].
Our data showed that significant decrease in the deposition of fibrotic fibers occurs only with the prophylactic regimen of isRNA application (
Figure 4 and
Figure 5). The advantage of the prophylactic regimen can be explained by the formation of a state of refractoriness to repeated immunostimulation after administration of isRNA, which alters the cytokine response to the stimuli. Previously, we observed the refractoriness to the induction of interferon α in mice by the subsequent isRNA injection within 72–96 hours after its first administration [
12]. These data are in good agreement with the observations of Jeljeli et al. [
51] on a mouse model of systemic sclerosis. They demonstrated, that low-dose LPS training reduces inflammation and fibrosis. Additionally, co-culturing fibroblasts from mice and patients with systemic sclerosis with low-dose LPS-trained macrophages lowers their fibro-inflammatory profile, suggesting that immunity training could be a way to treat autoimmune disorders and inflammatory fibrotic diseases.
Our data demonstrated, that the prophylactic isRNA treatment had a more notable favorable effect in the case of LPS-induced fibrosis, than in BLM-induced fibrosis. The observed variation could potentially result from distinct dynamics in the development of the pathological process induced by different stimuli, although, at the final point of the experiments, the parameters of fibrotic and inflammatory alterations in mice challenged with BLM or LPS were almost identical. The action of BLM primarily causes cell damage, after which they undergo apoptosis, in the later stages of which damage-associated patterns (DAMPs) are released. At the next stage, the signaling pathways leading to the synthesis of cytokines, chemokines and interferons are activated and inflammation develops. A different situation is observed when mouse lungs are exposed to LPS, which is a component of the bacterial wall belonging to the pathogen-associated pattern (PAMPs). PAMPs bind to pattern recognition receptors (PRRs) and immediately trigger the synthesis of signaling molecules and the development of inflammation. Presumably, in the case of BLM-induction, the major peak of cytokines occurs later, lasts longer, or is less pronounced, and so extends beyond the duration of the isRNA-induced refractory state. This assumption is supported by the data of Kimura et al. [
52], who studied the effect of BLM and LPS and their combined effect on the induction of fibrosis and showed that the levels of cytokines such as MCP-1, KC (keratinocyte-derived cytokine), and IL-6 in the bronchoalveolar fluid of mice were higher after exposure to LPS than to BLM. It can be assumed that the positive effect of isRNA in the case of BLM-induced fibrosis could be increased by varying the time intervals in the prophylactic regimen.
Thus, a comparison of the effects of immunostimulatory RNA used in a preventive or therapeutic regimen on the development of fibrosis in selected BLM- and LPS-induced mouse models showed that isRNA can be used in pathological conditions accompanied by the development of inflammation and the risk of developing fibrosis, without burden conditions. Prophylactic regimen of isRNA application can be beneficial for prevention of the development of pulmonary fibrosis. However, further research is needed to determine dosing regimens and select optimal lipid formulations to potentiate in full isRNA activity against specific pathology-inducing agents.
4. Materials and Methods
Mice
Female 6–8-week-old C57Bl6 mice with an average weight of 16-18 g were obtained from the Vivarium of Institute of Chemical Biology and Fundamental Medicine SB RAS (Novosibirsk, Russia). The mice were housed in plastic cages under standard 12/12 h light/dark conditions. Water and food were provided ad libitum. Experiments were carried out in accordance with the European Communities Council Directive (ECC Directive 2010/63/EU). The experimental protocols were approved by the Committee on the Ethics of Animal Experiments at the Institute of Cytology and Genetics SB RAS (Novosibirsk, Russia) (protocol No. 51 from May 23, 2019).
BLM- and LPS-Induced Lung Fibrosis
Mice (n = 5 in each group) were challenged with BLM (B5507, Sigma-Aldrich, USA) or LPS (055:B5, Sigma-Aldrich, USA). To select the optimal regimen for fibrosis induction, both agents were administered to mice according to three schemes. BLM was administered once at a dose of 1.5, 5, and 10 U/kg intranasally (i.n.). LPS was administered five times at a dose of 5 mg/kg intraperitoneally (i.p.) and once i.n. at the same dose. Combined route of administration consisted of consequent instillations of LPS at a dose of 1.5 mg/kg i.n., 5 mg/kg i.p. and 1.5 mg/kg i.n. each other day. All i.n. instillations were carried out under isoflurane anesthesia. On day 14 and 28 after induction mice were euthanized and lung tissue was collected for histological analysis.
Synthesis of isRNA
The synthesis of oligoribonucleotides (Strand 1: 5’-GUGUCAGGCUUUCAGAUUUUUU-3’, Strand2:5’-AAAUCUGAAAGCCUGACACUUA-3’) was performed as previously established described in [
34]. Subsequently, siRNAs (50 µM) were annealed in 30 mM HEPES-KOH (pH 7.4), 100 mM sodium acetate, and 2 mM magnesium acetate. The annealing process involved heating at 90 °C for 5 minutes, followed by gradual cooling to room temperature. The resulting siRNA preparations were stored at -20 °C until used.
isRNA/Liposome Complexes Preparation
The isRNA/liposome complexes were formed at N/P ratios of 6/1 before use. The equal volumes of isRNA solution, with a final concentration of 3.5 µM in serum-free OptiMEM medium (Invitrogen, Waltham, MA, USA) and the liposomes solution, with a final concentration of 150 µM in the same medium, were mixed and incubated for 20 minutes at room temperature.
Histology
For the histological study, lung specimens were fixed in 10% neutral-buffered formalin (BioVitrum, Moscow, Russia), dehydrated in ascending ethanols and xylols and embedded in HISTOMIX paraffin (BioVitrum, Russia). Paraffin sections (up to 5 µm) were sliced on a Microm HM 355 S microtome (Thermo Fisher Scientific, Waltham, MA, USA). The extracellular matrix deposition and fiber expansion was determined using van Gieson’s staining. All the images were examined and scanned using an Axiostar Plus microscope equipped with an Axiocam MRc5 digital camera (Zeiss, Oberkochen, Germany) at magnifications of × 200.
The intensity of inflammatory infiltration and fibrotic changes in the lung tissue was assessed by a semi-quantitative scoring system where 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis. The quantification was performed at a magnification of × 200 in 5 test fields for each lung sample; the number of samples studied was five for each experimental group.
Statistical Analysis
The variables were presented as the mean ± standard deviation (SD). In the case of in vivo experiments, statistical analysis was performed using the Mann–Whitney unpaired test. GraphPad Prism version 9.5.1 (528) (GraphPad Software, Inc., San Diego, CA, USA) was used for the statistical analyses.
Figure 1.
Morphological changes in the lung tissue of mice during the development of lung fibrosis induced by bleomycin (BLM) and lipopolysaccharide (LPS). (A) Experimental setup. BLM was administered intranasally (i.n.) one time at a dose of 1.5, 5, or 10 U/kg. LPS was administered five days at a dose of 5 mg/kg daily intraperitoneally (i.p.) or one time i.n. at the same dose. Combined route of administration consisted of three consequent instillations of LPS at a dose of 1.5 mg/kg i.n., 5 mg/kg i.p. and 1.5 mg/kg i.n. every other day. On day 14 and 28 after induction mice were euthanized and lung tissue was collected for histological analysis. (B, C) Inflammatory and fibrotic changes in the lung tissue of mice with BLM- and LPS-induced fibrosis with different schemes of induction. To assess the intensity of inflammatory and fibrotic changes in the lungs, the following semi-quantitative histological scoring system was used: 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis.
Figure 1.
Morphological changes in the lung tissue of mice during the development of lung fibrosis induced by bleomycin (BLM) and lipopolysaccharide (LPS). (A) Experimental setup. BLM was administered intranasally (i.n.) one time at a dose of 1.5, 5, or 10 U/kg. LPS was administered five days at a dose of 5 mg/kg daily intraperitoneally (i.p.) or one time i.n. at the same dose. Combined route of administration consisted of three consequent instillations of LPS at a dose of 1.5 mg/kg i.n., 5 mg/kg i.p. and 1.5 mg/kg i.n. every other day. On day 14 and 28 after induction mice were euthanized and lung tissue was collected for histological analysis. (B, C) Inflammatory and fibrotic changes in the lung tissue of mice with BLM- and LPS-induced fibrosis with different schemes of induction. To assess the intensity of inflammatory and fibrotic changes in the lungs, the following semi-quantitative histological scoring system was used: 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis.
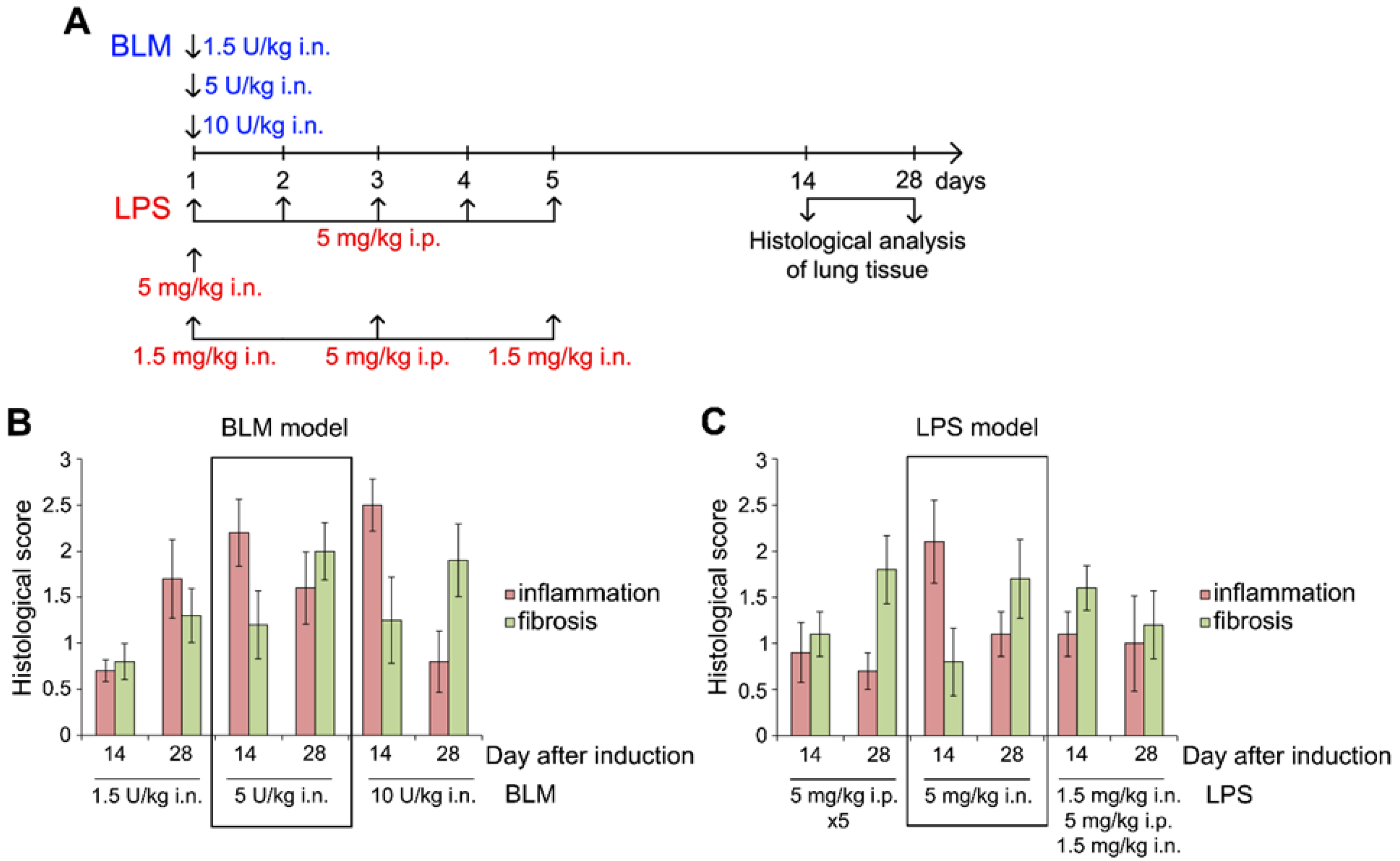
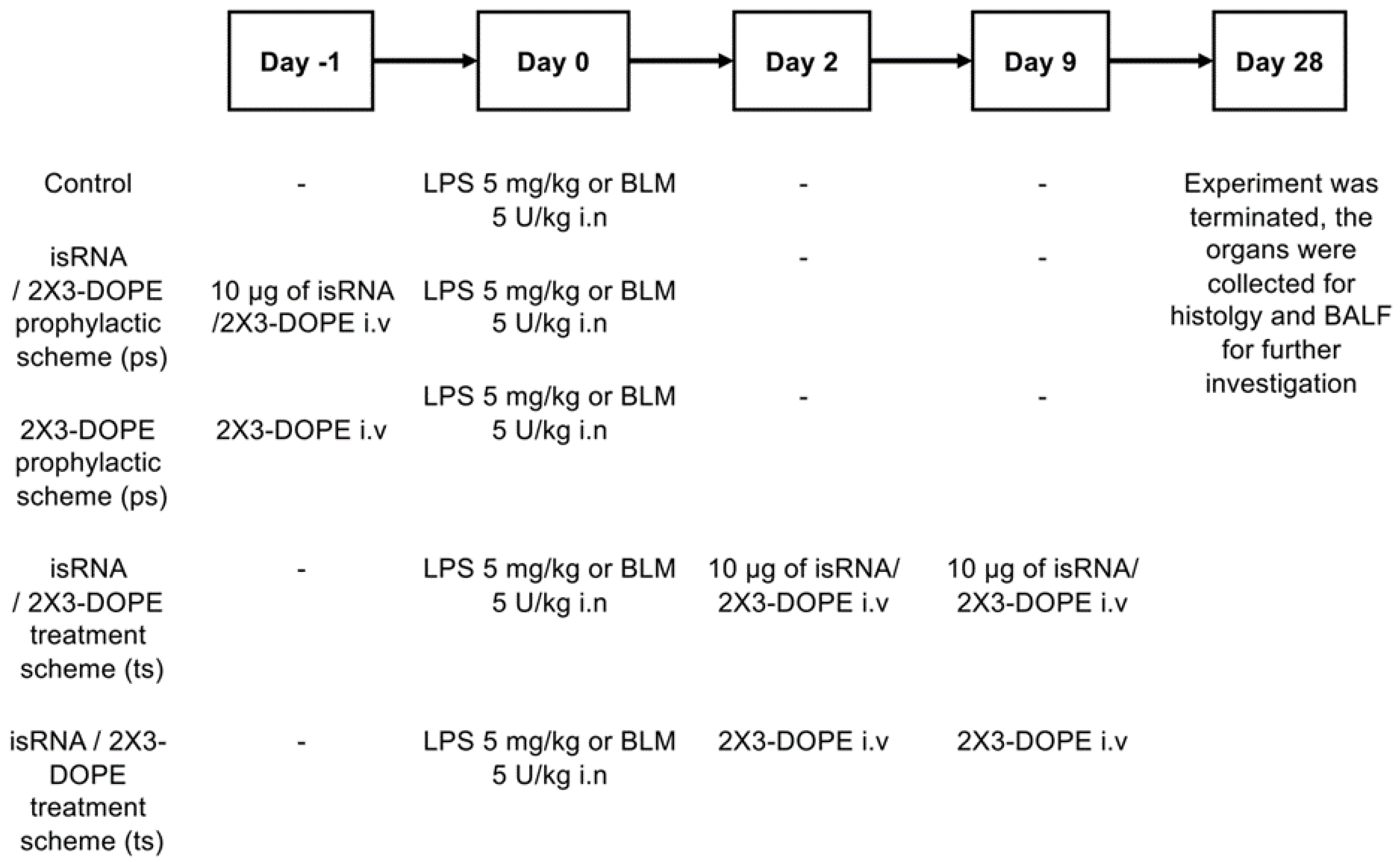
Figure 2.
Experimental scheme. One day before the induction of fibrosis (day -1), two distinct groups of mice received 10 μg of isRNA/2X3-DOPE or 2X3-DOPE i.v. (prophylactic scheme, p.s.). Subsequently, on day 0, all experimental groups were subjected to the introduction of fibrosis by either lipopolysaccharide (LPS) at a dose of 5 mg/kg i.n. or bleomycin (BLM) at a dose of 5 U/kg i.n. In the treatment scheme (t.s.), on days 2 and 9, two other groups of mice received 10 μg of isRNA/2X3-DOPE or 2X3-DOPE i.v. Termination of the experiment on the day 28th to ascertain the progression of fibrosis. Histological analysis of the lungs and the analysis of bronchoalveolar lavage fluid (BALF) were conducted post-termination. Body weight measurements were recorded bi-daily for all experimental groups over the duration of the experiment.
Figure 2.
Experimental scheme. One day before the induction of fibrosis (day -1), two distinct groups of mice received 10 μg of isRNA/2X3-DOPE or 2X3-DOPE i.v. (prophylactic scheme, p.s.). Subsequently, on day 0, all experimental groups were subjected to the introduction of fibrosis by either lipopolysaccharide (LPS) at a dose of 5 mg/kg i.n. or bleomycin (BLM) at a dose of 5 U/kg i.n. In the treatment scheme (t.s.), on days 2 and 9, two other groups of mice received 10 μg of isRNA/2X3-DOPE or 2X3-DOPE i.v. Termination of the experiment on the day 28th to ascertain the progression of fibrosis. Histological analysis of the lungs and the analysis of bronchoalveolar lavage fluid (BALF) were conducted post-termination. Body weight measurements were recorded bi-daily for all experimental groups over the duration of the experiment.
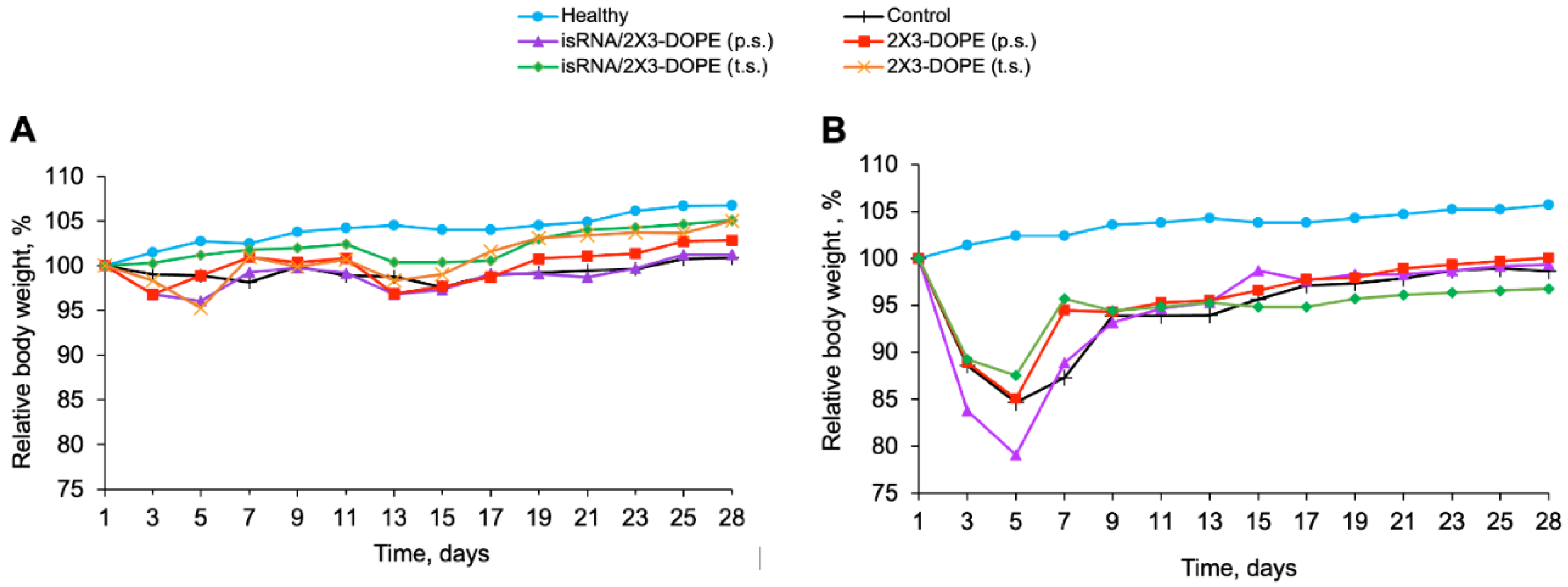
Figure 3.
The dynamic alterations of the body weight over a 28-day experimental period after administration of BLM (A) and LPS (B). Body weight measurements were performed each other day. The y-axis represents the percentage change in the body weight, calculated as [(Final Weight - Initial Weight)/Initial Weight] × 100.
Figure 3.
The dynamic alterations of the body weight over a 28-day experimental period after administration of BLM (A) and LPS (B). Body weight measurements were performed each other day. The y-axis represents the percentage change in the body weight, calculated as [(Final Weight - Initial Weight)/Initial Weight] × 100.
Figure 4.
The effect of isRNA on the inflammatory and fibrotic changes in the lung tissue of mice with BLM-induced lung fibrosis. (A) Representative histological images of lung sections of healthy and BLM-challenged mice without treatment and after isRNA/2X3-DOPE administration using prophylactic scheme (p.s.) and treatment scheme (t.s.) 4 weeks after induction. Van Gieson staining, original magnification ×200. The black arrows indicate the fiber expansion in the lung tissue. (B) The intensity of inflammatory and fibrotic changes in the lungs of mice of control and experimental groups, assessed by semi-quantitative method, where 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis. The number of samples studied was five for each experimental group.
Figure 4.
The effect of isRNA on the inflammatory and fibrotic changes in the lung tissue of mice with BLM-induced lung fibrosis. (A) Representative histological images of lung sections of healthy and BLM-challenged mice without treatment and after isRNA/2X3-DOPE administration using prophylactic scheme (p.s.) and treatment scheme (t.s.) 4 weeks after induction. Van Gieson staining, original magnification ×200. The black arrows indicate the fiber expansion in the lung tissue. (B) The intensity of inflammatory and fibrotic changes in the lungs of mice of control and experimental groups, assessed by semi-quantitative method, where 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis. The number of samples studied was five for each experimental group.
Figure 5.
The effect of isRNA on the inflammatory and fibrotic changes in the lung tissue of mice with LPS-induced lung fibrosis. (A) Representative histological images of lung sections of healthy and LPS-challenged mice without treatment and after isRNA/2X3-DOPE administration using prophylactic scheme (p.s.) and treatment scheme (t.s.) 4 weeks after induction. Van Gieson staining, original magnification ×200. The black arrows indicate the fiber expansion in the lung tissue. (B) The intensity of inflammatory and fibrotic changes in the lungs of mice of control and experimental groups, assessed by semi-quantitative method, where 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis. The number of samples studied was five for each experimental group. The statistical analysis was performed using the two-tailed unpaired t-test; * p ≤ 0.05.
Figure 5.
The effect of isRNA on the inflammatory and fibrotic changes in the lung tissue of mice with LPS-induced lung fibrosis. (A) Representative histological images of lung sections of healthy and LPS-challenged mice without treatment and after isRNA/2X3-DOPE administration using prophylactic scheme (p.s.) and treatment scheme (t.s.) 4 weeks after induction. Van Gieson staining, original magnification ×200. The black arrows indicate the fiber expansion in the lung tissue. (B) The intensity of inflammatory and fibrotic changes in the lungs of mice of control and experimental groups, assessed by semi-quantitative method, where 0 – no pathological changes, 1 – mild inflammation and fibrosis, 2 – moderate inflammation and fibrosis, 3 – severe inflammation and fibrosis. The number of samples studied was five for each experimental group. The statistical analysis was performed using the two-tailed unpaired t-test; * p ≤ 0.05.