1. Introduction
Sophora flavescens exhibits a variety of pharmacological properties, including anti-inflammatory, antioxidant, and anticancer activities [
1,
2]. The root contains a range of secondary metabolites, including isoprenoid flavonoids such as kushenol, kurarinone, and maackiain, as well as alkaloids and other flavonoids [
3]. Some of these compounds have been shown to have specific pharmacological effects, such as anti-inflammatory and PPAR transactivational properties, estrogenic and anticarcinogenic properties, and antimalarial properties [
4,
5]. In addition,
S. flavescens has been traditionally used in Chinese medicine for the treatment of multiple conditions, including diarrhea, inflammation, and cancer [
6,
7].
S. flavescens root metabolites have been extensively studied, and ongoing research is exploring their potential therapeutic applications.
Eleutherococcus sessiliflorus, also known as
Acanthopanax sessiliflorus, is a shrub or small tree native to Eastern Asia [
8]. It has been used in traditional medicine for its various properties [
9]. Some of the properties associated with
E. sessiliflorus include immunostimulating, anti-inflammatory, and anticancer activities [
10]. It has been found to inhibit receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclast differentiation and has been evaluated for its potential to prevent osteoporosis [
11,
12]. The chemical constituents of
E. sessiliflorus include triterpenoids, phenolic compounds, alkaloids, flavonoids, and other bioactive compounds. These constituents have been found in various parts of the plant, including the leaves, roots, and fruits [
13]. Some specific compounds that have been identified include chiisanoside, chiisanogenin, eleutherosides, 3,4-seco-lupanetype triterpenoids, elesesterpenes AK, and lignans [
14].
Bacillus fermentation is a process where bacteria from the
Bacillus genus are used to produce a variety of products through the breakdown of organic materials.
Bacillus bacteria are aerobic, gram-positive soil bacteria that have been used for decades in the production of foods such as fermented soybean products, cheese, and yogurt [
15]. During fermentation, the bacteria utilize the nutrients in the organic material to produce various substances, including enzymes, organic acids, and other metabolites. These substances can have beneficial effects on health and are used in various applications, such as food, agriculture, and industry [
16]. Examples of substances produced through Bacillus fermentation include nattokinase, phytase, amylase, protease, and biosurfactants [
17,
18]. The process is generally carried out in liquid or solid-state fermentation systems under controlled conditions to maximize product yield and quality.
This study aimed to verify the antioxidant and antifungal activities of S. flavescens and E. sessiliflorus through fermentation by Bacillus species. A single microbial strain was isolated from the humus soil to ferment S. flavescens and E. sessiliflorus. The fermented extract confirmed the efficacy compared with unfermented plant extracts by TPC, TFC, and DPPH radical scavenging effects. Furthermore, it was measured antifungal activities against Phytophthora cactorum, Botrytis cinerea, Colletotrichum gloeosporioides, and Colletotrichum fructicola Sau-3. The plant extracts and their fermented extracts were analyzed using LC-Q-TOF/MS to identify the metabolite changes.
2. Materials and Methods
2.1. Isolation of Microbial Strains
The fermentation strain used in this study was isolated from the humus at filed soil in Gyeongsang National University (35°10’50.3”N 128°05’38.1”E, Jinju-si, Gyeongsang-nam-do, Republic of Korea). The sterilized water (9 mL) was added to the collected humus soil (1 g) using a sonicator. Then, 1 mL of the solution was diluted with 9 ml of sterilized water. The diluted humus solution (200 µL) was spread on starch plate medium, which was incubated for 18 hr at 37ºC. The colonies observed on the plate were segregated based on their type and then cultured. After the culturing, the amylase, CMCase, and protease activities were measured based on the size of the clear zone of the separated colonies, which were appeared to spray 0.05% KI-I solution. The single strains with high enzyme effects were isolated to use for fermentation of the plant sample.
2.2. 16S rRNA Sequence Analysis
Genomic DNA of the isolated microorganisms was extracted using the genomic DNA extraction kit (Bioneer, Daejeon, South Korea). The reaction mixture was consisted of genomic DNA (1 µL), universal primers (27F/1492R, 5 µM), DNA polymerase, dNTPs, PreMix (20 µL, AccuPower PCR PreMix, Bioneer) was performed PCR. The PCR condition was as follows; initial denaturation (95°C, 2 min), denaturation (95°C, 30 min), annealing (55°C, 30 sec), and extension (72°C, 30 sec) for a total of 30 cycle. After the cycles, the extension was carried out at 72°C for 5 min. Accuprep TM PCR purification Kit (Bioneer) was used to remove and purified the remaining primers, nucleotides, polymerase, and salts. Then, 30 µL of the elution buffer (10 mM Tris-HCl, pH 8.5) was added. The base sequences of purified PCR products were analyzed by Macrogen Co., Ltd. (Seoul, South Korea).
2.3. Fermentation and Cultivation of Plant Extracts
The dried roots of Sophora flavescens and Eleutherococcus sessiliflorus were purchased from a local market in Haman-gun, Gyeongsangnam-do, Republic of Korea. Each 200 g of the dried roots of S. flavescens and E. sessiliflorus were extracted with 1 L of distilled water using a sonicator for 7 h at 80°C to make S. flavescens and E. sessiliflorus extract samples. The LB broth (0.5 g) was added to 20 mL of the plant extracts (S. flavescens and E. sessiliflorus). The pre-cultured strain was inoculated with 1 mL based on the optical density (O.D.) at 0.7. The mixture was incubated for 48 hr at 37ºC. Then, it was obtained filtration through filter paper (No. 2, Whatman, UK).
2.4. Antifungal Activity
The plant extract, fermented plant extract, and pre-cultured strain broth were measured for antifungal activity against Phytophthora cactorum, Botrytis cinerea, Colletotrichum gloeosporioides, and Colletotrichum fructicola Sau-3 by monitoring the inhibition zone. Phytophthora cactorum (Lebert & Cohn) Schroeter (KACC 40166), Botrytis cinerea Pers. (KACC 40573), and Colletotrichum gloeosporioides (Penzig) Penzig & Saccardo (KACC 40003) were distributed from Korean Agricultural Culture Collection (KACC, Wanju-gun, Jeollabuk-do, Republic of Korea). Colletotrichum fructicola Sau-3 was provided by Gyeongsangnam-do Agricultural Research & Extension Services at Jinju-si, Gyeongsangnam-do, Republic of Korea. Cork borer (5 mm) holes were drilled in the center and edges of the high-pressure sterilized PDA (potato dextrose agar) plate. 180 ul of fungal strain was dispensed into the center hole. The plant extracts, fermented plant extracts, and pre-cultured strain medium were centrifuged at 12,000 rpm for 3 min to obtain the supernatant, which was filtered through a 0.45 um membrane filter. Then, the filtered samples (160 µL) were loaded into three edge holes on the fungal inoculated plate. The plate was incubated for 48 h at 25ºC to observe the inhibition zone as antifungal effects of the samples.
2.5. LC-Q-TOF/MS Analysis
Identification of the metabolites from the plant extract was performed by LC-Q-TOF/MS equipped with high performance liquid chromatography (HPLC, Shimadzu, Kyoto, Japan) and quadrupole-time of flight mass spectrometry (Q-TOF/MS, X500R, AB Sciex, Framingham, MA, USA). The mobile phase A and B were water containing 0.1% acetic acid and acetonitrile containing 0.1% acetic acid, respectively. Infinity Lab Poroshell 120 C18 column (2.1 × 100 mm, 2.7 um, Agilent Technology, Santa Clara, CA, USA) was used as the analytical column. The mass ionization was electrospray ionization (ESI) in a positive mode with source parameters as follows: ionspray voltage, 5500 V; ion source temperature, 450ºC; curtain gas pressure, 30 psi; nebulizer gas pressure, 50 psi; heating gas pressure, 50 psi; declustering potential, 50 V. The mass detection range was configured from m/z 50 to 1000. The resulting MS data were acquired utilizing SCIEX OS software.
3. Results and Discussion
3.1. Identification of the Isolated Microbial Strains
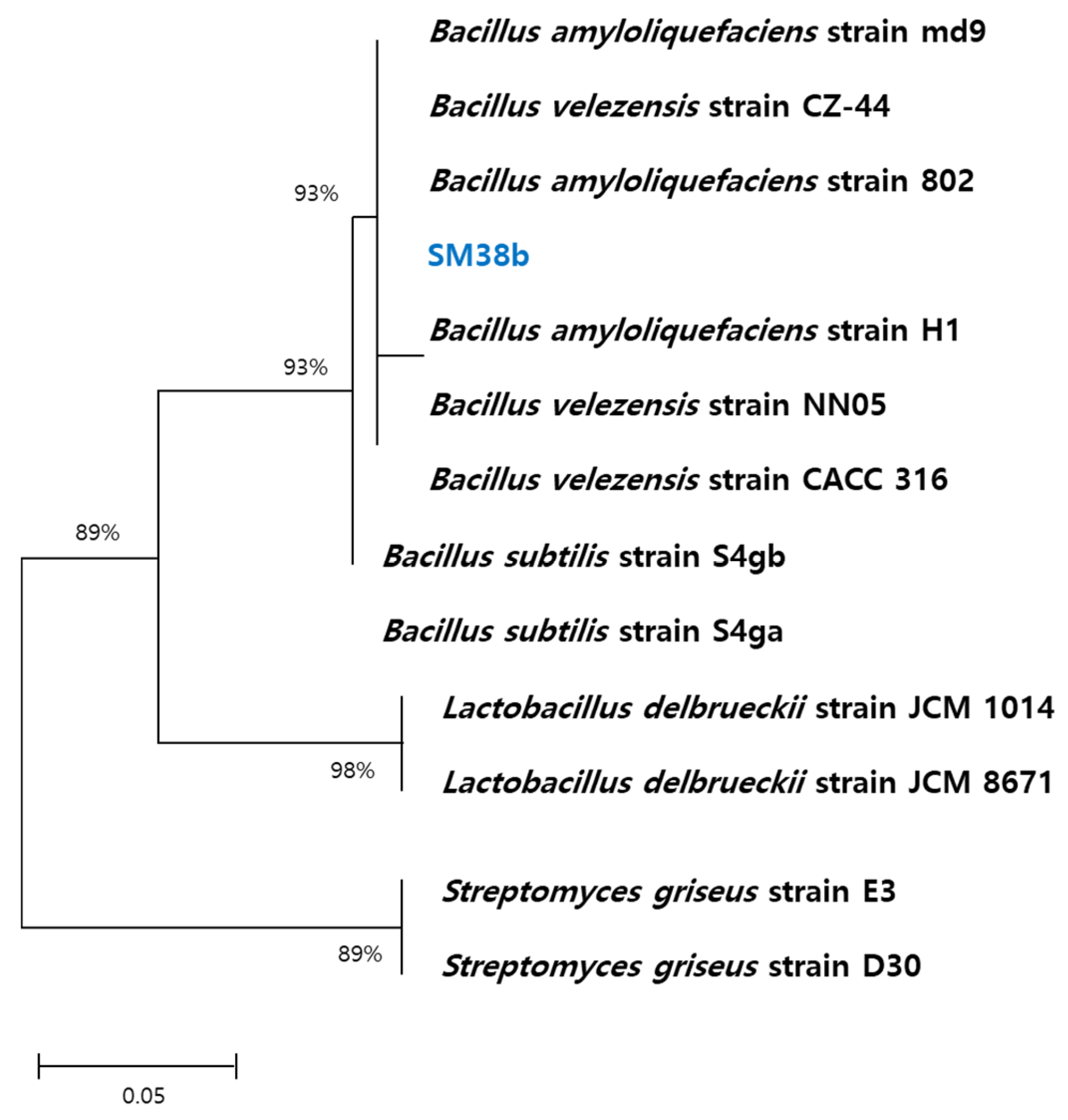
The amplified products by 16S ribosomal RNA PCR were determined by DNA sequencing data identification of GenBank database (
http://www.ncbi.nlm.nih.gov). As shown in
Figure 1, a phylogenetic tree was observed with
Bacillus,
Lactobacillus, and
Streptomyces genus. The dominance of
Bacillus,
Lactobacillus, and
Streptomyces was 93%, 98%, and 89%, respectively. Furthermore, the isolated bacteria (SM38b) was expressed to be a similar strain to
Bacillus amyloliquefaciens and
Bacillus velezensis.
Bacillus genus is a diverse group of bacteria that play important roles in various environments.
Bacillus subtilis and
Bacillus cereus have been reported that found in grass, soil, gastrointestinal tracts of ruminants, humans, and sponges, respectively. In this study,
Bacillus amyloliquefaciens and
Bacillus velezensis were isolated from humus, a nutrient-rich layer of soil. These bacteria are frequently found in soil environments and have been found to have beneficial effects on plant growth.
Bacillus amyloliquefaciens as a Gram-positive bacterium is known as a biocontrol abilities. It has been shown to effectively combat plant root pathogens in various agricultural settings. Similarly,
Bacillus velezensis has the ability to inhibit the growth of plant pathogens by competing with other bacteria. These bacteria can also produce bacterial metabolic active substances that help strengthen the plant’s immune system. Thus,
Bacillus velezensis can contribute to promoting plant growth, suppressing pathogens, and maintaining the balance of the soil ecosystem.
3.2. Antifungal Effects of the Fermented and Unfermented Plant Extracts
S. flavescens and E. sessiliflorus extracts were inoculated with the isolated microbial strain to prepare the fermented plant extracts. The fermented and unfermented S. flavescens and E. sessiliflorus extracts were tested to antifungal effects against four plant pathogens including Phytophthora cactorum, Botrytis cinerea, Colletotrichum fructicola Sau-3, and Colletotrichum gloeosporioides according to incubation times of 24, 48, 72, 96, and 120 hr, respectively.
P. cactorum is a soilborne pathogen that can infect many plant species. It causes various types of damage, including leaf spots, shoot blight, root rots, and crown rots [
19,
20].
B. cinerea is a destructive fungal pathogen that affects numerous plant hosts. It causes fuzzy gray-brown mold and soft decay of plant tissues [
21,
22].
C. fructicola is known to cause anthracnose, bitter rot, and leaf spotting diseases on over 90 cultivated and non-cultivated woody or herbaceous plant species. The pathogen has been reported from all five continents and affects a wide range of crops, including apple, pear, strawberry, mango, avocado, and coffee [
23,
24].
C. gloeosporioides is a fungal pathogen that causes anthracnose and fruit rotting diseases in a wide range of host plants [
25].
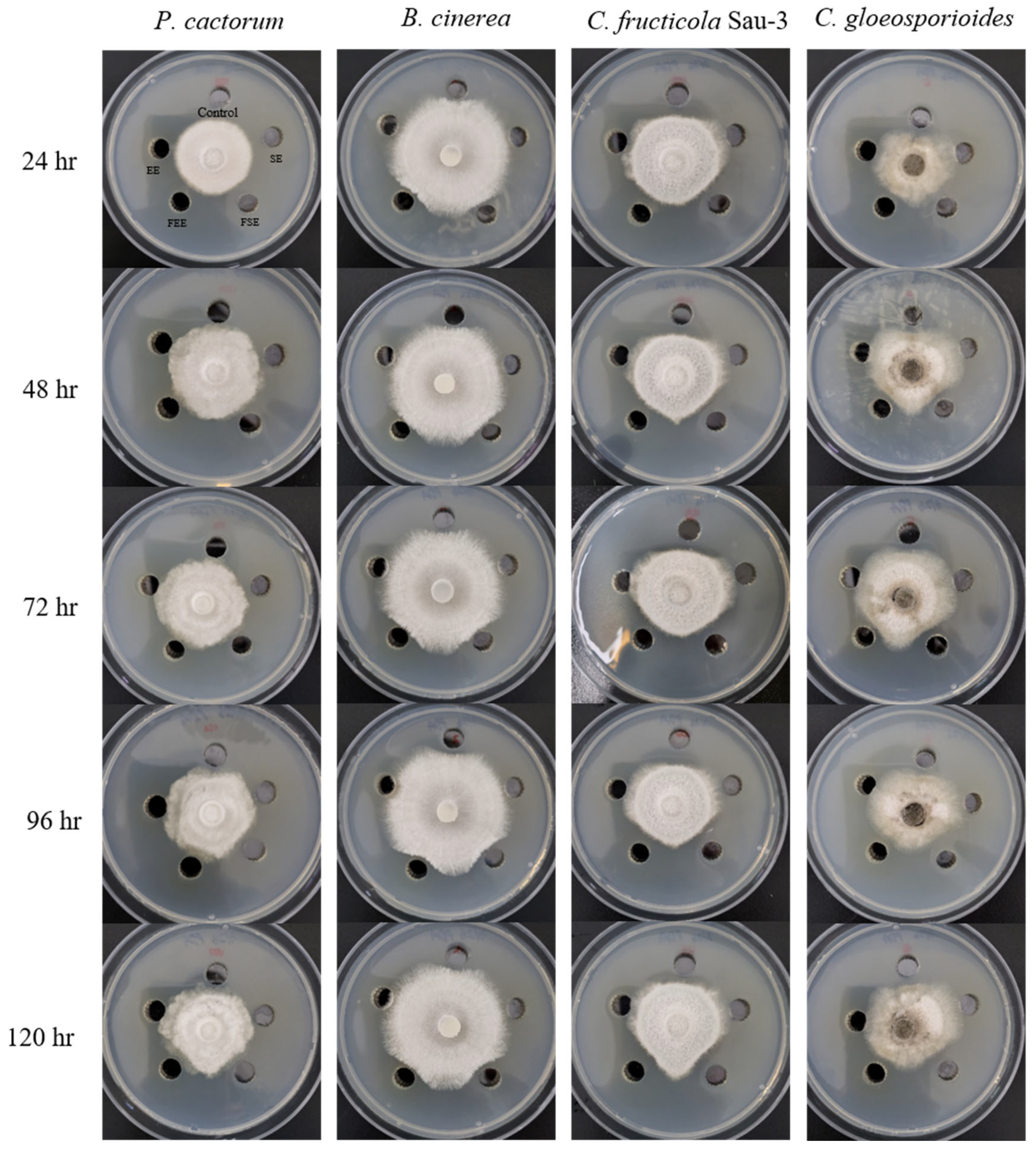
As shown in
Figure 2 and
Table 1, the fermented
S. flavescens and
E. sessiliflorus extracts showed more active antifungal activities against four fungi than the unfermented extracts. The growth inhibitory potential of
P. cactorum was slightly exhibited by the effects of fermented
S. flavescens extract (FSE) and fermented
E. sessiliflorus extract (FEE). FSE and FEE exhibited the same inhibition patterns against
P. cactorum; no inhibition at 24 hr, mycelial growth inhibition with less than 2 mm at 48 hr, and more than 2 mm from 96 hr. Similarly,
B. cinerea growth was inhibited by less than 2 mm up to 72 hr and more than 2 mm thereafter by FSE and FEE treatments. The most inhibition of FSE and FEE was observed against
Colletotrichum genus (
C. fructicola Sau-3 and
C. gloeosporioides). For the inhibition of
C. fructicola Sau-3, the results were observed that mycelial growth was hindered at a level of more than 2 mm by 96 hr, moreover it was inhibited by more than 3 mm when cultured for up to 120 hr. The growth of
C. gloeosporioides was inhibited by approximately 2 mm for up to 72 hr, but FSE inhibited its growth by over 3 mm. The effects of FEE confirmed that
C. gloeosporioides was suppressed at the 2 mm level throughout the entire culture period. These findings suggested that the fermented extracts of
S. flavescens and
E. sessiliflorus have potential as antifungal agents against these plant pathogenic fungi.
3.3. LC-Q-TOF/MS Analysis
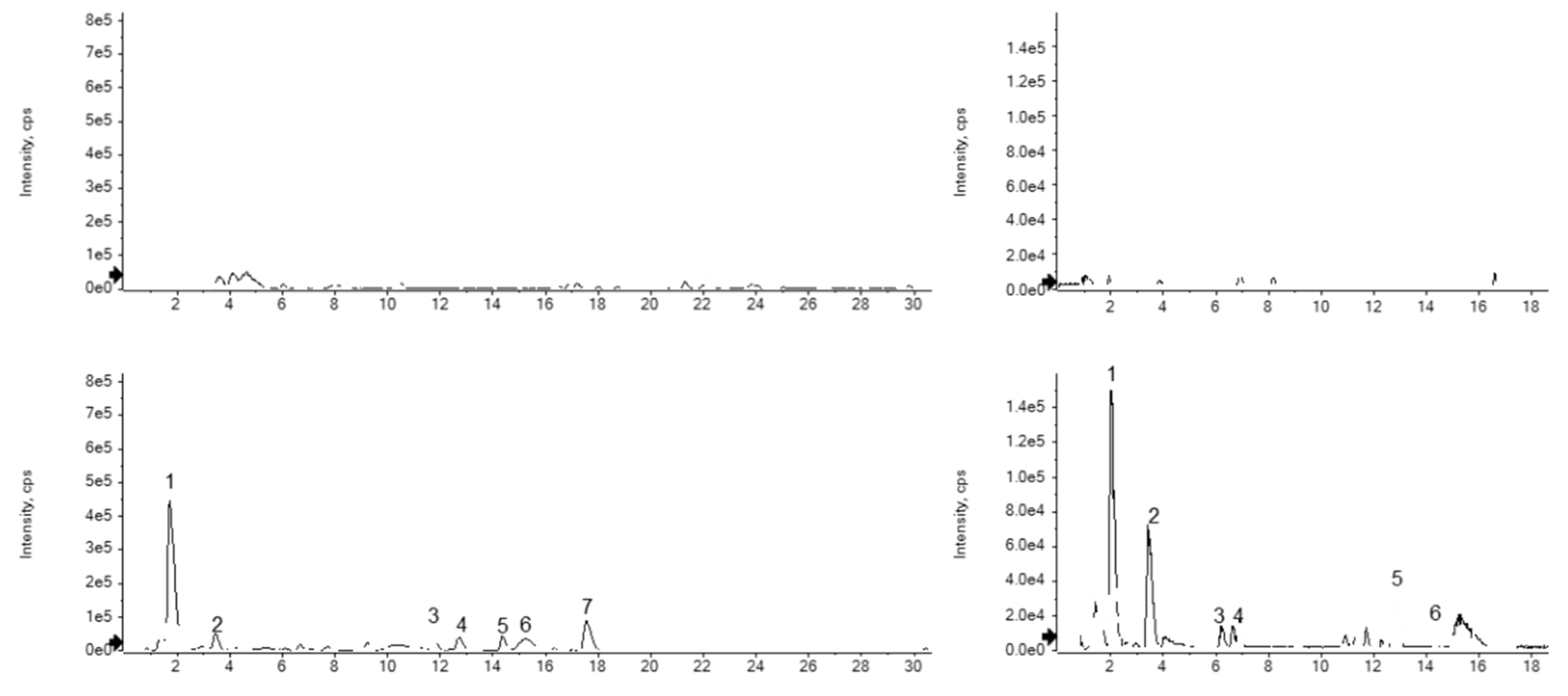
Figure 3 showed that the peaks from the BPI gram were well separated in the unfermented and fermented
S. flavescens and
E. sessiliflorus extracts by a positive mode of LC-Q-TOF/MS. The different patterns of the peaks represented the metabolite changes of the fermented extracts compared to the control.
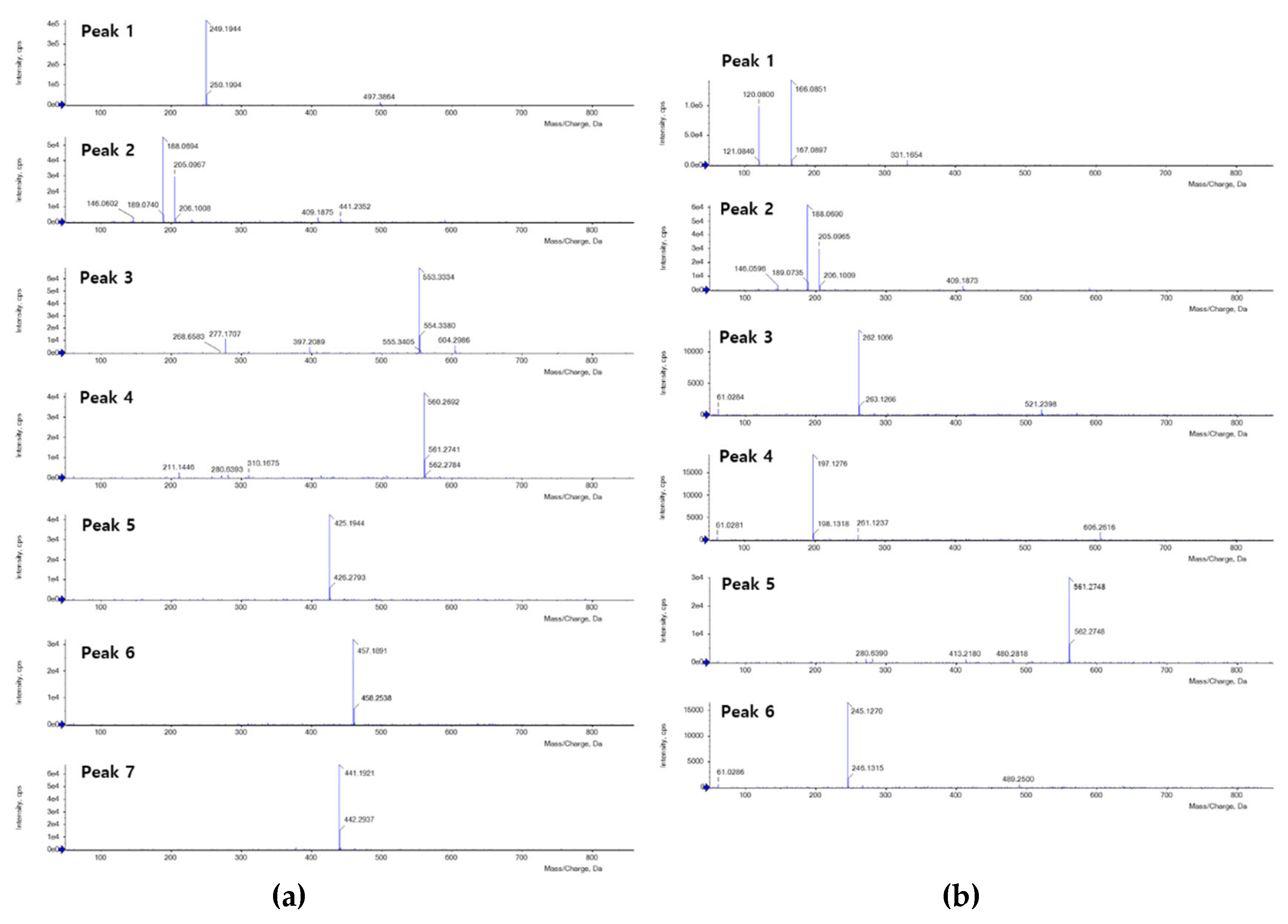
The BPI gram of FSE displayed (
Figure 3c) predominant seven peaks (peaks 1-7), but only five peaks (peaks 1, 2, 5-7) were identified by comparing observed and theoretical mass in the individual mass gram (
Table 2 and
Figure 4a). Peak 1 of FSE (
tR = 1.79 min) had an observed ion mass at
m/
z 249.1944 compared with the theoretical mass (
m/
z 249.1967) to annotate matrine. Peak 2 of FSE (
tR = 3.46 min) possessed a reasonable error value of -4.87 ppm to be presented as tryptophan. Peaks 5-7 of FSE were confirmed as flavonoids named as kushenol F, G, and L, respectively. Peak 5 (
tR = 14.39 min) had an error value of -4.7 ppm between the observed
m/
z 425.1944 and the theoretical mass at
m/
z 425.1964, indicated as kushenol F. Kushenol G with the chemical formula C
25H
28O
8 conformed the molecular ion peaks at
m/
z 457.1891 [M+H]
+ in the mass gram of peak 6 (
tR = 15.21 min). Peak 7 (
tR = 17.58 min) showed the observed mass at
m/
z 441.1921 with +1.81 ppm of error value compared with the theoretical mass to annotate as kushenol L. Based on the identification of five peaks, FSE was found to contain one alkaloid, one amino acid, and three flavonoids.
FEE had six peaks (peaks 1-6) detected in the BPI gram within 30 min (
Figure 3d). Among the peaks, five peaks were annotated by molecular ion peaks with individual mass grams (
Table 2 and
Figure 4b). Peak 1 (
tR = 2.06 min) and peak 2 (
tR = 3.48 min) were determined as phenylalanine and tryptophan, respectively, to confirm the theoretical mass by examining the molecular ion peaks in mass gram. Based on the compatibility between their observed and theoretical masses, peaks 3, 4, and 6 were expected as peptide derivatives. Peak 3 (
tR = 6.24 min) displayed molecular ion peaks at
m/
z 262.1066 [M+H]
+, indicating the presence of Asn-Glu with a theoretical mass of
m/
z 262.1039. Peak 4 (
tR = 6.87 min) was expected as cyclo(-Pro-Val) by having an error value of -7.10 ppm, which was determined by comparing the theoretical mass from the chemical formula (C
10H
16N
2O
2) with the observed one. Similarly, cyclo(-Phe-Pro) was also detected molecular ion peak at
m/
z 245.1270 [M+H]
+, which was consistent with its theoretical mass of
m/
z 245.1290. Thus, it was confirmed that peptide derivatives were enhanced in FEE than unfermented one.
4. Conclusions
The isolated microorganism from humus soil was identified by resulting to belong to the Bacillus genus such as Bacillus amyloliquefaciens and Bacillus velezensis with a high dominance based on phylogenetic tree through 16S rRNA sequence analysis. The isolated Bacillus was inoculated to S. flavescens and E. sessiliflorus extracts for fermentation. The fermented plant extracts showed the antifungal effects against P. carotorum, B. cinerea, C. fructicola Sau-3, and C. gloeosporioides as plant pathogens depending on the incubation time (24, 48, 72, 96, and 120 hr). The fermented plant extracts showed more active anti-fungal effects than unfermented ones, which was especially effective on Colletotrichum genus growth inhibition. Furthermore, the metabolites in the fermented plant extracts were identified by LC-Q-TOF/MS to determine the causality associated with improved biological effects. It was confirmed that the fermented S. flavescens and E. sessiliflorus extracts improved the flavonoids and peptide derivatives, respectively, based on BPI and individual mass gram. Overall, this study suggested that the potential of fermentation enhanced anti-fungal effects of S. flavescens and E. sessiliflorus extracts by resulting in the production of specific metabolites.
Author Contributions
Ju Yeon Kim and Min Joo Chae: conceptualization, formal analysis, investigation; Yun Gon Son, Su Min Jo, Na Rae Kang, Seong Doo Kang, and Yun Gon Son: formal analysis, validation; Kwang Dong Kim: writing-Review & Editing, Funding acquisition; Sang Won Lee: resources, Writing - Original Draft; Jeong Yoon Kim: Writing - Original Draft, Supervision.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) 2021R1A5A8029490 and 2022R1F1A1063786.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hong, M.H.; Lee, J.Y.; Jung, H.; Jin, D.H.; Go, H.Y.; Kim, J.H.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Sophora Flavescens Aiton Inhibits the Production of Pro-Inflammatory Cytokines through Inhibition of the NF ΚB/IκB Signal Pathway in Human Mast Cell Line (HMC-1). Toxicol. Vitr. 2009, 23, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Sa, K.; Li, H.; Chen, L. Alkaloids from the Roots of Sophora Flavescens and Their Anti-Tumor Activity. Fitoterapia 2023, 171, 105685. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, X.J.; Li, J.J.; He, L.; Yang, Y.R.; Zhong, F.; He, M.H.; Shen, Y.T.; Tu, B.; Zhang, X.; et al. A Novel Type Lavandulyl Flavonoid from Sophora Flavescens as Potential Anti-Hepatic Injury Agent That Inhibit TLR2/NF-ΚB Signaling Pathway. J. Ethnopharmacol. 2023, 307, 116163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, K.Z.; Yang, Y.N.; Feng, Z.M.; Yuan, X.; Jiang, J.S.; Zhang, P.C. Six Undescribed Lavandulylated Flavonoids with PTP1B Inhibition from the Roots of Sophora Flavescens. Phytochemistry 2023, 216, 113889. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora Flavescens Ait.: Traditional Usage, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef]
- Alfajaro, M.M.; Rho, M.C.; Kim, H.J.; Park, J.G.; Kim, D.S.; Hosmillo, M.; Son, K.Y.; Lee, J.H.; Park, S.I.; Kang, M. Il; et al. Anti-Rotavirus Effects by Combination Therapy of Stevioside and Sophora Flavescens Extract. Res. Vet. Sci. 2014, 96, 567–575. [Google Scholar] [CrossRef]
- Li, Z.; Lin, M.; Li, Y.; Shao, J.; Huang, R.; Qiu, Y.; Liu, Y.; Chen, L. Total Flavonoids of Sophora Flavescens and Kurarinone Ameliorated Ulcerative Colitis by Regulating Th17/Treg Cell Homeostasis. J. Ethnopharmacol. 2022, 297, 115500. [Google Scholar] [CrossRef]
- Shohael, A.M.; Chakrabarty, D.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Application of Bioreactor System for Large-Scale Production of Eleutherococcus Sessiliflorus Somatic Embryos in an Air-Lift Bioreactor and Production of Eleutherosides. J. Biotechnol. 2005, 120, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Olech, M.; Verpoorte, R.; Khan, I.; Kuźniewski, R.; Nowak, R. Phytoconstituents and Nutritional Properties of the Fruits of Eleutherococcus Divaricatus and Eleutherococcus Sessiliflorus: A Study of Non-European Species Cultivated in Poland. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Załuski, D.; Smolarz, H.D.; Gawlik-Dziki, U. Bioactive Compounds and Antioxidative, Antileukemic and Anti-MMPs Activity of Eleutherococcus Species Cultivated in Poland. https://doi.org/10.1177/1934578X1200701118. [CrossRef]
- Rimondi, E.; Zweyer, M.; Ricci, E.; Fadda, R.; Secchiero, P. Receptor Activator of Nuclear Factor Kappa B Ligand (RANKL) Modulates the Expression of Genes Involved in Apoptosis and Cell Cycle in Human Osteoclasts. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 838–845. [Google Scholar] [CrossRef]
- Muratovic, D.; Atkins, G.J.; Findlay, D.M. Is RANKL a Potential Molecular Target in Osteoarthritis? Osteoarthr. Cartil. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Li, W.; Yan, X.T.; Yang, S.Y.; Kim, Y.H.; Sun, Y.N.; Li, W.; Yan, X.T.; Yang, S.Y.; Kim, Y.H. Chemical Constituents from the Stems of Acanthopanax Divaricatus Var. Albeofructus. BioSE 2014, 57, 164–168. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, W.J.; Lee, S.U.; Kim, M.O.; Park, M.H.; Song, S.; Kim, D.Y.; Lee, S.M.; Yuk, H.J.; Lee, D.Y.; et al. Optimization of Chiisanoside and Chiisanogenin Isolation from Eleutherococcus Sessiliflorus (Rupr. & Maxim.) Leaves for Industrial Application: A Pilot Study. Ind. Crops Prod. 2022, 185, 115099. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus Species in Food Fermentations: An Underappreciated Group of Organisms for Safe Use in Food Fermentations. Curr. Opin. Food Sci. 2023, 50, 101007. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Ning, C.; Liu, Z.; Liang, Q.; Fu, X.; Tian, M.; Zhu, C.; Mou, H. High-Efficiency Heterologous Expression of Nattokinase Based on a Combinatorial Strategy. Process Biochem. 2023, 133, 65–74. [Google Scholar] [CrossRef]
- Sardar, R.; Asad, M.J.; Ahmad, M.S.; Ahmad, T.; Mehr, P.; Shah, A.; Alberti, A. Optimization of Phytase Production by Bacillus Sp. (HCYL03) under Solid-State Fermentation by Using Box-Behnken Design. Brazilian Arch. Biol. Technol. 2022, 65, e22210307. [Google Scholar] [CrossRef]
- Toljamo, A.; Koistinen, V.; Hanhineva, K.; Kärenlampi, S.; Kokko, H. Terpenoid and Lipid Profiles Vary in Different Phytophthora Cactorum – Strawberry Interactions. Phytochemistry 2021, 189, 112820. [Google Scholar] [CrossRef]
- Grenville-Briggs, L.J.; Kushwaha, S.K.; Cleary, M.R.; Witzell, J.; Savenkov, E.I.; Whisson, S.C.; Chawade, A.; Vetukuri, R.R. Draft Genome of the Oomycete Pathogen Phytophthora Cactorum Strain LV007 Isolated from European Beech (Fagus Sylvatica). Genomics Data 2017, 12, 155–156. [Google Scholar] [CrossRef]
- Jin, W.; Wu, F. Characterization of MiRNAs Associated with Botrytis Cinerea Infection of Tomato Leaves. BMC Plant Biol. 2015, 15, 1–14. [Google Scholar] [CrossRef]
- Shi, S.; Wang, J.; Liu, C.; Zheng, L. Alleviative Effects of Quercetin of Botrytis Cinerea-Induced Toxicity in Zebrafish (Danio Rerio) Larvae. Fish Shellfish Immunol. 2023, 142. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, Q.; Yuan, K.; Xing, C.; Qiao, Q.; Huang, X.; Zhang, S. PbrATL18, an E3 Ubiquitin Ligase Identified by Genome-Wide Identification, Is a Positive Factor in Pear Resistance to Drought and Colletotrichum Fructicola Infection. Hortic. Plant J. 2023. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.Y.; Liu, J.A.; Xu, J.X. Population Genetic Analyses of the Fungal Pathogen Colletotrichum Fructicola on Tea-Oil Trees in China. PLoS One 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, Q.; Luo, H.; He, C.; An, B. A Baeyer-Villiger Monooxygenase CgBVMO1 Is Involved in Superoxide Anion Metabolism, Cell Wall Synthesis, and Pathogenicity of Colletotrichum Gloeosporioides. Postharvest Biol. Technol. 2024, 210, 112786. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).