Submitted:
16 April 2024
Posted:
19 April 2024
You are already at the latest version
Abstract
Keywords:
1.1. Introduction
2.1. Results
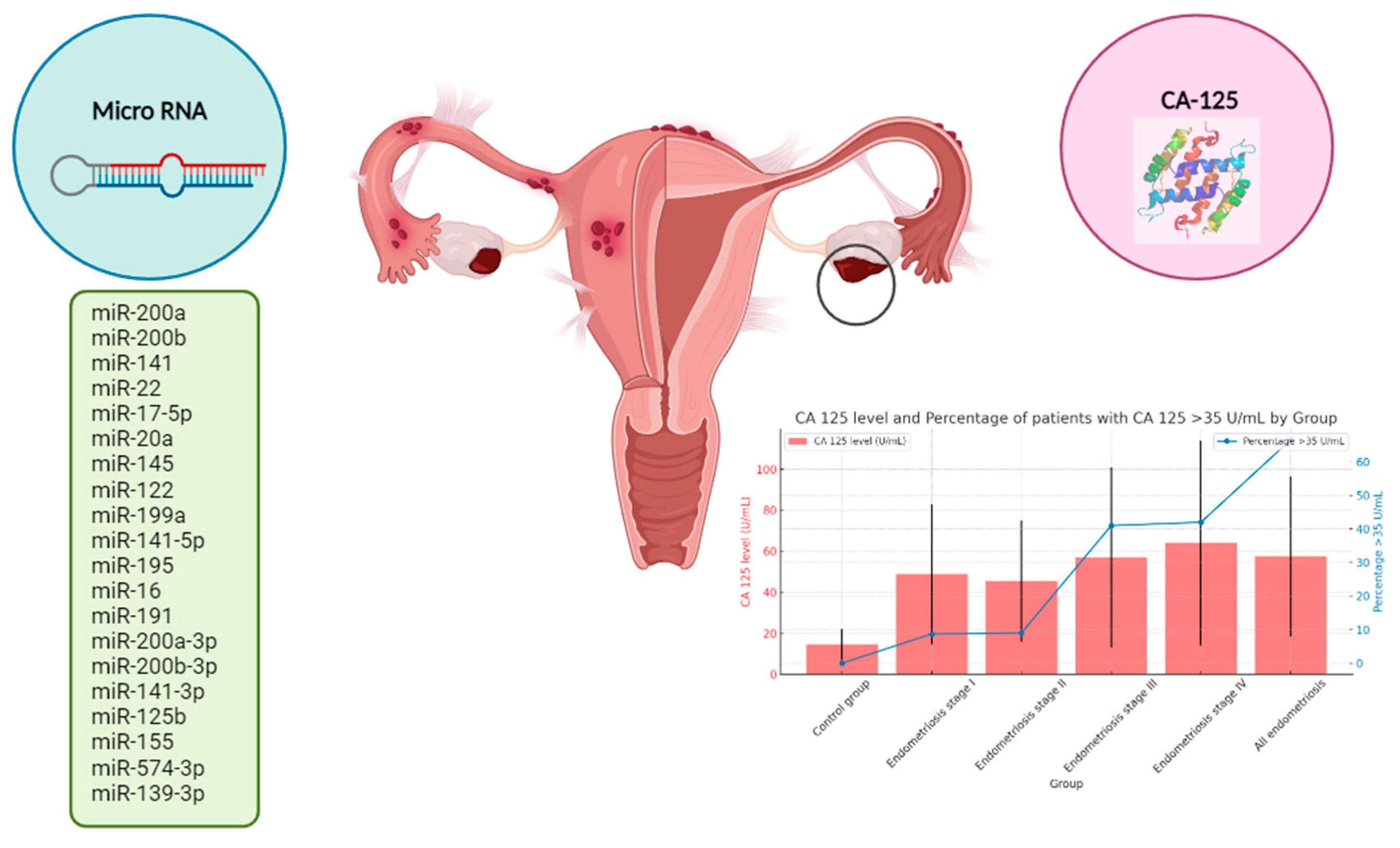
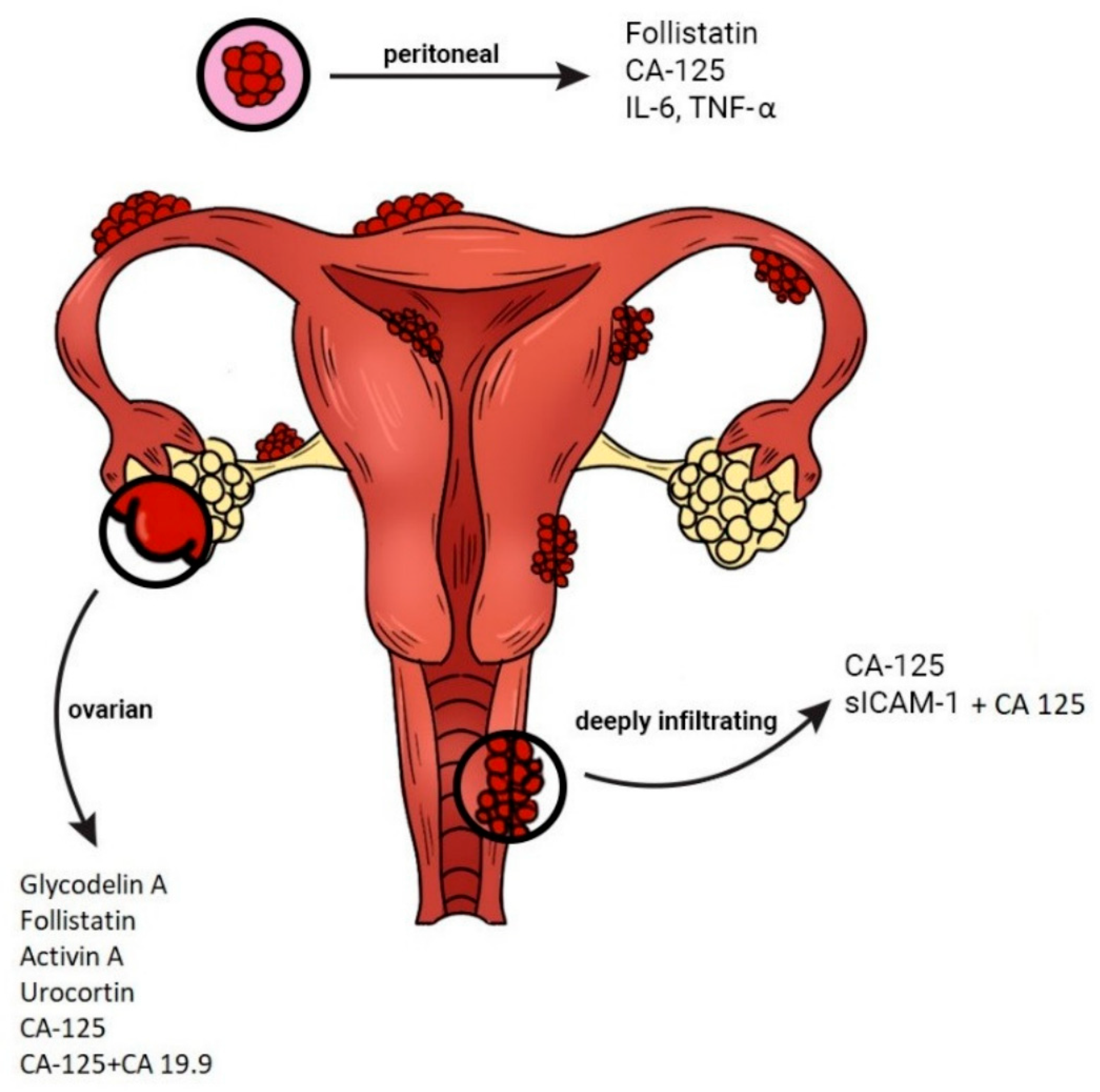
2.2. CA-125 in Endometriosis
2.2.1. Pathogenesis and Diagnosis
| Year | Main objective | Type of study Size of sample Diagnosis method |
Biomarkers | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 1996 | To evaluate the clinical utility of CA-125 in the diagnosis of endometriosis and to compare the sensitivity of the serum and the peritoneal test as an indicator of disease. | Case control 26 Laparoscopy |
Serum and peritoneal CA-125 | CA-125 levels in peritoneal fluid were higher than those found in serum and were significantly elevated (P < 0.05) | Levels of CA-125 in peritoneal fluid seem to be a more sensitive indicator of disease than serum levels (0.86 vs. 0.36), especially in early stage endometriosis (0.80 vs. 0.20) which tends to be overlooked by the CA-125 serum test. | [24] |
| 2004 | To elucidate whether endometriosis can be diagnosed clinically by assessing the differences between serum CA-125 levels during menstruation and during the rest of the menstrual cycle. | Case control 28 Laparoscopy |
CA-125 | The mean CA-125 concentrations of healthy women during menstruation and during the rest of the menstrual cycle were 12.2 and 10 U ml. | Assessment of changes in serum CA-125 levels during the menstrual cycle may be useful in the diagnosis of endometriosis. | [25] |
| 2008 | Investigated the possible use of CCR1 miRNA measurement in peripheral blood leukocytes with monocyte chemotactic protein-1 (MCP-1) and CA-125 protein in serum as a diagnostic test for endometriosis. | Case control (Retrospective) 151 Laparoscopy |
CCR1, HRPT, MCP1, CA-125 | The ratio of CCR1/HPRT miRNA in peripheral blood of patients with endometriosis and adenomyosis was significantly elevated compared with women without endometriosis. Additionally, serum levels of MCP-1 and CA-125 were significantly higher in patients with endometriosis. | Found increased levels of CA-125 in peripheral blood of patients with endometriosis compared with healthy controls. Nevertheless, CA-125 alone was above the threshold in only 28 out of 102 endometriosis patients (sensitivity 27.5%). Considering only CA-125, none of the controls showed false positive results (specificity 100%). | [26] |
| 2010 | To evaluate the combined performance of six potential plasma biomarkers in the diagnosis of endometriosis. | Case control 294 Laparoscopy |
CA-125, IL-6, IL-8, TNF-α, CA-19-9, hsCRP | Increased plasma levels: IL-6, IL-8, CA-125, Decreased plasma level: TNF-α | Results show that multivariate methods such as logistic regression and LSSVMs in general perform better than single protein models, suggesting that more than one protein is necessary to predict the presence of endometriosis. | [27] |
| 2011 | Analyzed selected well-known and less well-known serum markers that have been proposed for diagnosis and severity assessment of endometriosis. | Case control 48 Laparoscopy |
CA-125, IL6 | CA-125 levels were over the cut-off of 35 IU/l in 54% of patients (versus 8% of controls), averaging 67.5 (CI95: ±17.5). The sensitivity and specificity were 54% and 91%, respectively, with a p value of <0.001 (statistically significant). | CA-125 correlated with endometriosis screening and severity, indicating its superiority as a marker for further, larger studies. | [28] |
| 2012 | To develop and validate a non-invasive diagnostic test with a high sensitivity (80% or more). | Case control 353 Laparoscopy |
CA-125, CA 19–9, IL-1beta, IL-6, IL-8, IL-17, IL-21, RANTES, TNF-alpha, IFN-gamma, MCP-1, MIF, CRP, OPN, IL-4, IL-10, annexin V, sICAM-1, VCAM-1, VEGF, NGF, FGF-2, Leptin, IGFBP-3, glycodelin (PP-14), M-CSF, HGF | Increased plasma levels: IGFBP-3, CA-125, CA 19-9 and glycodelin, Decreased plasma level: IL-1beta, IFN-γ, TNF-alpha, Leptin and sICAM-1 | In plasma samples obtained during menstruation, multivariate analysis of four biomarkers (annexin V, VEGF, CA-125 and sICAM-1/or glycodelin) enabled the diagnosis of endometriosis undetectable by US with a sensitivity of 81–90% and a specificity of 63–81% in independent training- and test data set. | [29] |
| 2016 | To define the utility of serum carcinogenic antigen CA-125 and CA 19-9 combining pain score in the prediction of pelvic endometriosis in infertile women. | Case control 294 Laparoscopy |
CA-125, CA 19-9 | Preoperative serum CA-125 and CA 19-9 levels were significantly different between the two groups. | Preoperative CA-125 and CA 19-9 levels combining pain score can be useful for the prediction of pelvic endometriosis and may be included in the evaluation of unexplained infertile women. | [30] |
| 2017 | To evaluate the performance of CA-125 measurement in the menstrual and mid-cycle phases of the cycle, as well as the difference in its levels between the two phases, for the early diagnosis of DIE. | Case control (perspective) 54 Laparoscopy |
CA-125 | Area Under the Curve (AUC) of CA-125 in menstrual phase and of the difference between menstrual and midcycle phases had the best performance (both with AUC = 0.96), followed by CA-125 in the midcycle (AUC = 0.89). | CA-125 may be useful for the diagnosis of deep endometriosis, especially when both are collected during menstruation and in mid cycle. | [22] |
| 2019 | To identify potential biomarkers through proteomics profiling of eutopic and ectopic endometrial tissue specimens. | Case control (Retrospective) 46 Laparoscopy |
CA-125, sICAM1, FST, VEGF, MCP1, MIF, IL1R2, LUM, CPM, TNC, TPM2, PAEP | The best single marker for discriminating endometriosis from controls remained CA-125 (AUC = 0.63) | Data indicate that the markers tested, whilst not useful alone, have improved diagnostic accuracy when used in combination and demonstrate menstrual cycle specificity. | [31] |
| 2021 | To develop a noninvasive diagnostic method for endometriosis. | Case control 293 Laparoscopy |
CA-125, CA199, HE4, CYFRA 21-1, TNF-α, IL6, sflt-1, MCP-1 | In tumor markers, alpha fetoprotein (AFP), carcinoembryonic antigen (CA) 125, CA199 and human epididymis protein 4 (HE4) helped to diagnose endometriosis; CA-125, HE4, and cytokeratin 19 fragment (CYFRA 21-1) could differentiate stages. | Serological indicators in ovarian endometriosis patients were different from healthy women, which were of certain differential values in diagnosis and disease staging. | [32] |
| 2021 | To evaluate the diagnostic value of a quadruple panel of serum markers CA-125, endocan, YKL-40 and copeptin, for the prediction of endometriosis. | Case control 140 Laparoscopy |
CA-125, endocan, YKL-40, copeptin | Serum CA-125, endocan, copeptin and YKL-40 levels were significantly increased. | YKL-40, endocan and copeptin levels were significantly increased in the moderate-severe endometriosis group compared to the mild-moderate endometriosis group and CA-125 levels remained nonsignificant. A quadruple panel score (CA-125, endocan, YKL-4 and copeptin) had an AUC of 0.954, a sensitivity of 96.5% and specificity of 84.6% for prediction of moderate to severe endometriosis. |
[33] |
| 2021 | To analyze the differences in the peripheral blood cells and tumor biomarkers between the patients with endometriosis and healthy people. | Case control (Retrospective) 274 Laparoscopy |
CA-125, CA199, HE4, HGB | The ROC curve showed that the combined diagnostic model reached a sensitivity of 85.4%, a specificity of 78.83%, and an area under the curve of 0.900, which was significantly higher than that of the individual index in endometriosis diagnosis. | The combined diagnostic model of HGB, CA199, CA-125, and HE4 may provide a new approach for the early non-invasive diagnosis of endometriosis. | [34] |
| 2023 | To find an algorithm based on symptoms and laboratory tests that could diagnose endometriosis in a non-invasive way. | Retrospective analysis 101 Laparoscopy |
CA-125, VEGF | The strongest impact on endometriosis prediction had information about painful periods, CA-125 over 15 u/mL, and the lowest BMI, with a sensitivity of 0.8800 and a specificity of 0.8000, respectively. | An algorithm based on three easy features, including painful menses, BMI level, and CA-125 concentration could have an important place in the non-invasive diagnosis of endometriosis. If confirmed in a prospective study, implementing such an algorithm in populations with a high risk of endometriosis will allow us to cover patients suspected of endometriosis with proper treatment. | [35] |
| 2023 | To evaluate the prognostic value as diagnosis makers of cancer antigen (CA)125, human epididymis 4 (HE4), and CA72-4 serum levels in ovarian endometriosis. | Case control 55 Laparoscopy |
CA-125, CA72-4, HE4 | (i) For CA-125, a statistically significant difference in-between the mean serum levels of the two groups: 9.02 U/mL in the OvEndo group versus 7.1 U/mL in the CTR group (p=0.0158). (ii) For CA72-4 levels in the OvEndo group, where the mean serum level was 6.1 U/mL compared to 3.5 U/mL in the CTR group, (p=0.0185). (iii) The mean serum level of HE4 in the OvEndo group was 7.6 ng/mL versus 7.8 ng/mL in the CTR group, found highly significant (p=0.0001). HE4 levels were highly correlated with CA72-4 levels (p<0.0001), while CA-125 levels were not correlated with HE4 and CA72-4. |
Measurements of CA-125 can be used in the diagnosis of OvEndo mainly in association with HE4 serum levels, which are lower in endometriosis patients. | [36] |
| 2023 | To investigate the efficacy of new endometriosis biomarkers in diagnosis and treatment. | Case control (Cohort Retrospective) 79 Laparoscopy |
Annexin A5 (ANXA5), soluble intercellular adhesion molecule-1 (sICAM-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), soluble vascular cell adhesion molecule-1 (sVCAM-1), vascular endothelial growth factors (VEGF) and CA-125 | Only the AUC of the Ca-125 biomarker values were found to be significant with 73% sensitivity and 98% specificity (p < 0.001). However, when Ca-125 and ANXA5 were evaluated together, it was concluded that the diagnosis of endometriosis could be made with 73% sensitivity and 100% specificity. | When Ca-125 and ANXA5 are evaluated together, it seems to be more valuable than Ca-125 alone in diagnosing endometriosis. | [37] |
2.2.2. Cut-off Value for Diagnosis
| Year | Main objective | Type of study Size of sample Diagnosis method |
Biomarkers | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 1995 | To compare the serum CA-125 concentrations determined by both assays in women with and without endometriosis. | Cohort 123 Laparoscopy |
CA-125 | The CA-125 concentrations determined by the new assay were highly correlated with concentrations determined by the older assay in patients with and without endometriosis (r = 0.96). | This study indicates that while new CA-125 assay concentrations are closely correlated to those of the older CA-125 assay, the improvement in the ability to differentiate between patients with and without endometriosis is small. | [38] |
| 2005 | To evaluate the diagnostic significance of CA-125 for endometriosis without ovarian endometriomas. | Cohort 775 Laparoscopy |
CA-125 | Receiver operating characteristic curve analysis revealed that the area under the curve for endometriosis without endometriomas was 0.788, significantly smaller than that for endometriosis with endometriomas (0.935, P < 0.05). In diagnosis of endometriosis without endometriomas, both the maximal accuracy of 78.8% and the maximal diagnostic value of 61.2% were obtained at the cutoff value of 20 U/mL. Negative predictive value was 78.0% at the cutoff value of 20 U/mL, whereas positive predictive value was 92.9% at the cutoff value of 30 U/mL. This range is clearly superior to the empirical single cutoff of 35 U/mL. | In the diagnosis of endometriosis without endometriomas, combined use of two cutoff values for CA-125, 20 and 30 U/mL, provides improved diagnostic performance. However, the accuracy of using only CA-125 testing for diagnosis is still limited. Serum CA-125 testing can be done during initial screenings of women with possible endometriosis. | [23] |
| 2007 | To define the serum CA-125 values that best indicate the presence and stage of endometriosis. | Retrospective, cross-sectional 201 Laparoscopy |
CA-125 | Using a CA-125 serum concentration higher than 10 IU/mL as the cut-off value for the diagnosis of endometriosis yielded a sensitivity of 64.2% and a specificity of 81.1% (with a positive predictive value of 91.3% and a negative predictive value of 45.3%). | It is not advisable to use serum levels of CA-125 as a diagnostic tool. It should be borne in mind that the cut-off values proposed herein present low sensitivity, underscoring the role of laparoscopy in the definitive diagnosis. Combining serum CA-125 measurement with noninvasive methods such as transvaginal ultrasonography and magnetic resonance imaging might increase the sensitivity of this marker. | [39] |
| 2012 | To evaluate CA-125 in serum and peritoneal fluid (PF) as an indicator of endometriosis. | Case control 56 Laparoscopy |
CA-125 | The mean value of CA-125 concentration in the endometriosis group was 33.98 U/ml, vs. 9.3 U/ml in the control group. | Cancer antigen 125 is a well-known biomarker for endometriosis and helpful in daily clinical practice when endometriosis is suspected. The cut-off value in serum suggesting endometriosis with 68% sensitivity is 11 U/ml. This value is the normal range for Ca-125 concentration. | [40] |
| 2016 | To evaluate the association between preoperative serum CA-125 levels and clinic pathological characteristic in women with endometriosis and find out the best serum CA-125 levels cut-off in pre- and post-menopause women. | Cross-sectional analysis 87 Laparoscopy |
CA-125 | The mean serum CA-125 level was 49.93±4.30 U/mL. There was a significant correlation between the endometriosis stage, lesion size, adhesion score and preoperative CA-125 plasma concentration. | Preoperative serum CA-125 is an important predictor for patients with endometriosis and should be considered when surgical management is suspected, especially if stage of disease, lesion size and adhesion score are undertaken. | [41] |
2.2.3. Staging (Severity)
| Year | Main objective | Type of study Size of sample Diagnosis method |
Biomarkers | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 1989 | To test whether there was a relationship between CA-125 levels and the severity of endometriosis that would allow CA-125 to be used to monitor disease progression. | Cohort 60 Laparoscopy |
CA-125 | There was a positive correlation (r = 0.63; P less than .0001) between disease severity and CA-125 levels. CA-125 was also elevated, compared with women with a normal pelvis, in patients with mild and moderate disease. There was no relationship between CA-125 levels and the day of the menstrual cycle. | These results suggest that after malignancy has been excluded, CA-125 levels may offer a useful method of monitoring disease progress. | [42] |
| 1993 | To examine variations in CA-125 levels during the three phases of the menstrual cycle in women with and without endometriosis. | Case control 100 Laparoscopy |
CA-125 | In the endometriosis groups, there was a significant difference in the mean CA-125 levels drawn at menses and those drawn in the follicular phase. In patients with severe endometriosis, there was also a difference in the mean CA-125 levels drawn at menses and in the luteal phase. | CA-125 levels during menses are elevated compared with those during the follicular phase in patients with endometriosis. Screening tests based on the relationship of multiple CA-125 levels taken throughout the menstrual cycle were more sensitive for detection of endometriosis than tests based on a single CA-125 level. | [43] |
| 2006 | To correlate CA-125 levels in serum and peritoneal fluid from women with and without pelvic endometriosis. | Prospective, cross-sectional, controlled study 52 Laparoscopy |
CA-125 | CA-125 levels in serum and peritoneal fluid were higher in patients with advanced pelvic endometriosis (means of 39.1 ± 45.8 U/ml versus 10.5 ± 5.9 U/ml in serum, p < 0.005; 1,469.4 ± 1,350.4 U/ml versus 888.7 ± 784.3 U/ml in peritoneal fluid, p < 0.05), and showed a positive correlation between each other (correlation coefficient (r) = 0.4880). | There is a positive correlation between serum and peritoneal fluid values of CA-125 in women with and without endometriosis, and their levels are higher in peritoneal fluid. Advanced endometriosis is related to higher levels in both serum and peritoneal fluid. | [44] |
| 2007 | To investigate the clinical value of the serum CA-125 level for diagnosing and determining the severity of endometriosis and pelvic pain associated with endometriosis. | Case control 86 Laparoscopy |
CA-125 | The mean serum CA-125 levels of women with endometriosis were higher than those of the control group (p<0.050). | CA-125 serum levels were related to endometriosis and R-AFS score in the evaluated patient series. No correlation was found between serum levels of CA-125 and pelvic pain in patients with endometriosis. | [45] |
| 2014 | To investigate the diagnostic potentials of the serum levels of nine different biomarkers in endometriosis. | Case control 80 Laparoscopy |
α-enolase, macrophage migration inhibitory factor, leptin, interleukin-8, anti-endometrial antibody, phosphoinositide dependent protein kinase 1, CA-125, syntaxin-5, and laminin-1 | The serum levels of α-enolase, macrophage migration inhibitory factor, leptin, interleukin-8 and anti-endometrial antibodies showed a statistically significant difference neither between control and endometriosis groups nor among control group and endometriosis subgroups. The serum levels of CA-125, syntaxin-5 and laminin-1 showed a statistically significant difference both between the control and endometriosis groups (p<0.01) and among control group and endometriosis subgroups | The concurrent measurement of the three biomarkers including CA-125, STX-5 and LN-1 might be a useful non-invasive test in strengthening the diagnosis of endometriosis and in predicting its severity. | [46] |
| 2014 | To determine whether cancer antigen-125 (CA-125) levels are increased in women with endometriosis, especially in those with endometriomas (OMAs), deep infiltrating lesions (DIE), and superficial endometriosis (SUP). | Cross-sectional 679 Laparoscopy |
CA-125 | Women with endometriosis displayed higher mean serum CA-125 levels compared with disease-free controls (50.1 ± 62.4 U/mL vs 22.5 ± 25.2 U/mL; p ≤ .001). | Serum CA-125 levels were significantly increased in women with severe forms of endometriosis, OMA, and DIE lesions. In addition, elevated serum Ca-125 levels were associated with more severe and extended DIE lesions. In women with superficial peritoneal lesions, CA-125 levels were not different from women without endometriosis. | [47] |
| 2018 | To evaluate preoperative levels of CA-125 and HE4 in patients with endometriosis-like symptoms. | Case control (Prospective) 221 Laparoscopy |
CA-125, HE4 | CA-125 serum levels were significantly elevated in the endometriosis patients compared with the control patients (p = 1.3 × 10-10), while the difference in the HE4 serum levels did not reach significance (p > 0.05). | CA-125 levels were significantly elevated in endometriosis patients compared with control patients (p<0.001). | [48] |
| 2020 | To examine whether CA-125 correlates with different types and severity of pain among adolescents and young women with and without endometriosis and assess its performance as an endometriosis biomarker among those presenting with dysmenorrhea in this young population. | Cross-sectional analysis 575 Laparoscopy |
CA-125 | Average CA-125 values were 12.5 U/mL in controls and 12.1 U/mL in cases adjusted for age. CA-125 did not differ by pain type, its severity, or frequency in endometriosis cases or controls. | CA-125 did not efficiently discriminate endometriosis cases from controls, even when accounting for pain symptomatology. Average CA-125 values were low in adolescents and young women in both endometriosis cases and controls, suggesting cautious interpretation may be needed when measuring CA-125 in this population. | [49] |
| 2021 | To investigate the relationships of cancer antigen (CA) 125, CA 19-9, prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), fibrinogen (FIB), and D-dimer values with ovarian endometriosis (OEM). | Case control (Retrospective) 571 Laparoscopy |
CA-125, CA 19-9, aPTT, TT, PT, D-dimer, and FIB | The serum CA-125, aPTT, FIB and D-dimer levels were statistically different between OEM patients in the stages I to (and) II group and those in the stages III and IV group (P<0.05). However, a statistical difference in CA 19-9 levels and TT was only found between patients with stages III and IV OEM. | The combined index of CA-125, aPTT, and D-dimer is a valid noninvasive preoperative method for the evaluation of moderate and severe OEM, and may help to decrease the interval between the first complaint and a definitive diagnosis. | [50] |
| 2023 | To develop a diagnostic test based on the combination of serum biomarkers and clinical variables. | Case control 204 Laparoscopy |
CA-125, BDNF | CA-125 and BDNF, can distinguish endometriosis cases from controls with statistical significance. | Although no individual cut-off values were set, CA-125 and BDNF levels were demonstrated to be elevated in patients with endometriosis, with CA-125 mostly able to identify high-stage endometriosis and BDNF performing well for both low- and high-stage disease. | [51] |
2.2.4. Management (Treatment and Prognosis)
| Year | Objective | Type of study Size of sample Diagnosis method |
Biomarkers | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 1986 | To determine CA-125 potential usefulness in the diagnosis and management of endometriosis. |
Cohort 392 Laparoscopy |
CA-125 | In women with minimal, mild, moderate, and severe endometriosis, the mean CA-125 levels (+/- standard deviation) were 13.6 +/- 6.8, 22.8 +/- 15.5, 27 +/- 17, and 50 +/- 28 U/ml, respectively, and were significantly higher than mean levels (7.8 +/- 4.1) in 46 women with a normal laparoscopic examination. | Changes in the CA-125 levels correlated with the clinical course of endometriosis in 37 of 44 (84%) women (P less than 0.001). The determination of CA-125 levels may assist in the evaluation and treatment of women with endometriosis. | [52] |
| 1996 | To evaluate, a clinical examination during menstruation and plasma CA-125 concentrations to diagnose deep endometriosis. | Prospective, Retrospective, Clinical validation 217 Laparoscopy |
CA-125 | CA-125 concentrations were higher during menstruation and correlated with deep endometriosis and with deep and cystic ovarian endometriosis. Nodularities at clinical examination or follicular phase CA-125 concentrations > 35 U/mL are useful to decide that a bowel preparation should be given, achieving a sensitivity of 87% and a specificity of 83%. | Clinical examination during menstruation can diagnose reliably deep endometriosis, cystic ovarian endometriosis, or cul-de-sac adhesions. This test, preferentially combined with a follicular phase CA-125 assay, should be used to decide whether a preparation for bowel surgery should be given. | [53] |
| 1998 | To estimate the value of CA-125 for the diagnosis of endometriosis in women with dysmenorrhea, as well as its significance in monitoring therapy and follow-up. | Prospective 157 Laparoscopy |
CA-125 | The sensitivity and specificity of serum CA-125 for the diagnosis of endometriosis were 61.1% and 87.5% respectively. Elevated CA-125 (±35 U/ml) was noted in 65/75 cases (86.7%) with advanced endometriosis, but in only 15/56 patients (26.8%) with minimal and mild endometriosis. | For endometriosis, CA-125 is a valuable adjuvant in the follow-up of recurrence in patients with advanced endometriosis and initially elevated CA-125 levels. It is not an effective screening tool for patients with dysmenorrhea, or for monitoring therapy. | [54] |
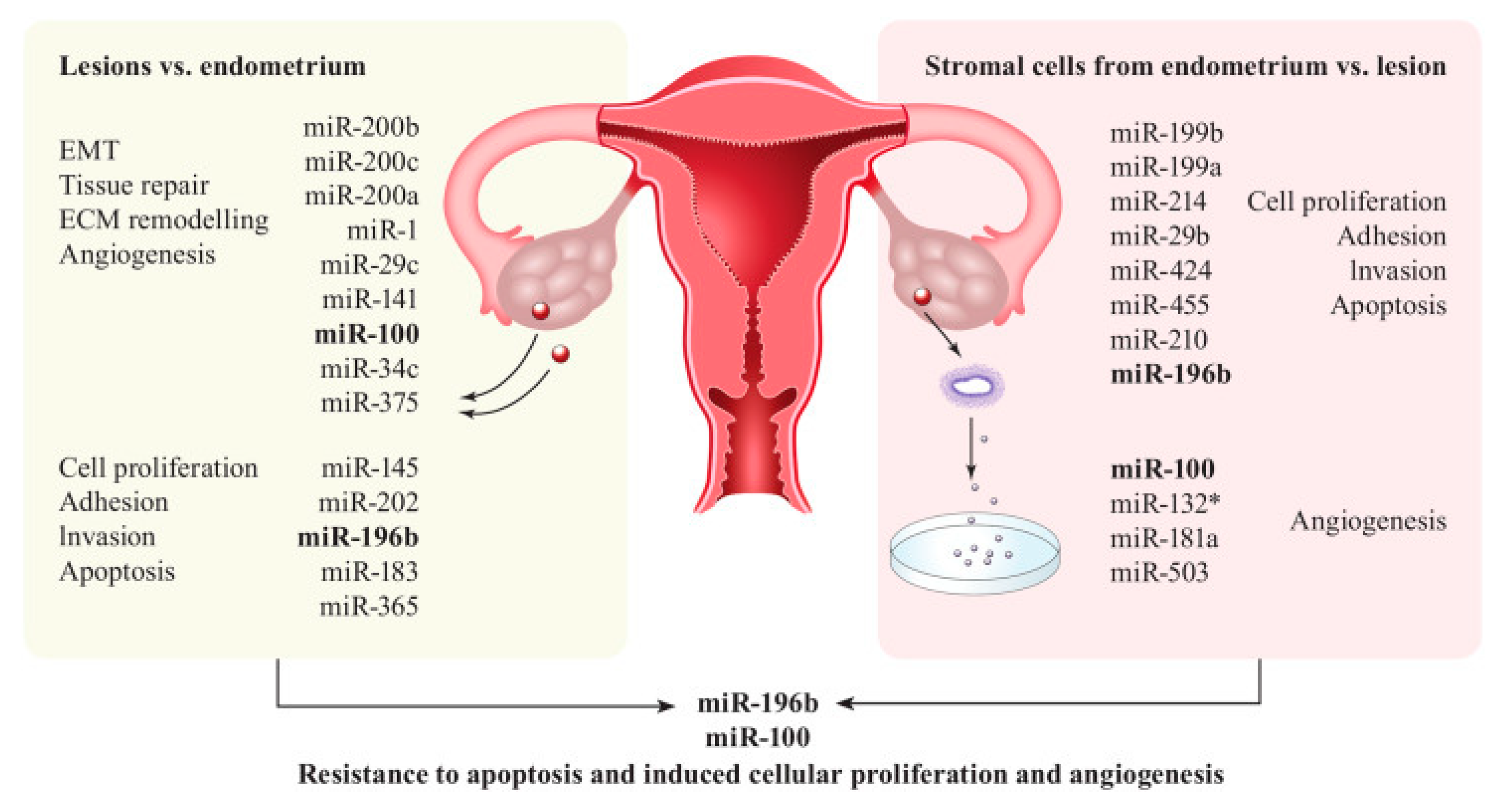
2.3. MiRNAs in Endometriosis
2.3.1. Pathogenesis and Diagnosis
| Year | Objective | Type of study Size of sample Diagnosis method |
miRNA type | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 2012 | To perform a combined miRNA microarray and proteomics analysis. | Case control 49 Laparoscopy |
Not mentioned | miRNA analysis of eutopic endometrium did not show any differentially expressed genes in women with endometriosis when compared with controls, regardless of endometriosis stage or cycle phase. | miRNA expression of eutopic endometrium was comparable in women with and without endometriosis, but different in menstrual endometrium when compared with luteal endometrium in women with endometriosis. | [56] |
| 2013 | Can plasma microRNAs be used as a non-invasive diagnostic test for the detection of endometriosis? | Prospective study 46 Laparoscopy |
miR-15b-5p, miR-17-5p, miR-20a, miR-21, miR-22 and miR-26a | miR-17-5p, miR-20a and miR-22 were significantly down-regulated. | Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis compared with those without endometriosis. | [57] |
| 2016 | To investigate serum microRNAs (miRNAs) in women with endometriosis. | Case control 48 Laparoscopy |
miR-3613-5p, miR-125b-5p, miR-150-5p, miR-342-3p, miR-143-3p, miR-145-5p, miR-500a-3p, miR-451a, miR-18a-5 piR-6755-3p, | miR-3613-5p, miR-6755-3p were down-regulated and miR-125b-5p, miR-150-5p, miR-342-3p, miR-143-3p, miR-145-5p, miR-500a-3p, miR-451a, miR-18a-5p were up-regulated more than 10-fold in the microarray. | Identified several miRNAs in serum that distinguished subjects with endometriosis from those without. miR-125b-5p had the greatest potential as a single diagnostic biomarker. A combination of that miRNA with miR-451a and miR-3613-5p further improved diagnostic performance. | [58] |
| 2016 | To further analyze the serum miRNAs profile in endometriosis. | Case control 50 Laparoscopy |
miR-30c-5p, miR-127-3p, miR-99b-5p, miRNA-15b-5p, miRNA-20a-5p, miR-424-3p, miR-185-5p | Only 21 of 98 significantly downregulated miRNAs, and none of significantly upregulated miRNAs were reported in published literature, which may be due to the differences in samples and analytical methods. | Circulating miRNAs may be useful as detection biomarkers for the early diagnosis of minimal-mild endometriosis. | [59] |
| 2018 | To profile the circular RNAs (circRNAs) expressed in eutopic endometrium from patients with ovarian endometriosis and explore potential clues to the pathogenesis of endometriosis, providing evidence for clinical diagnosis and treatment. | Case control 63 Laparoscopy |
Two upregulated circRNAs: 1.circ_0004712 2.circ_000219 | Among 88 differentially expressed circRNAs, 11 were upregulated and 77 were downregulated in the eutopic endometrium of patients with endometriosis. | Provides evidence that circRNAs are differentially expressed between eutopic and normal endometrium, which suggests that circRNAs are candidate factors in the activation of endometriosis. circ_0002198 and circ_0004712 may be potential novel biomarkers for the diagnosis of ovarian endometriosis. | [60] |
| 2018 | To determine differences in miRNA expression between eutopic endometrium. | Case control 47 Laparoscopy |
miR-21, miR-424, miR-10b | miRNA expression between ectopic implants Of the six miRNAs quantified, expression of miR-21, miR-424, and miR-10b was differentially regulated between endometriotic lesions from different anatomical sites. miR-21 expression was significantly lower in PE compared to OMA | miRNA expression differs between the eutopic endometrium of women with endometriosis compared to a symptomatic control population without endometriosis. | [61] |
| 2018 | This study tested whether they could serve as putative non-invasive biomarkers for endometriosis, and their expression differences between endometriosis patients and controls. | Case control (Prospective) 92 Laparoscopy |
hsa-miR-154-5p,hsa-miR-196b-5p, hsa-miR-378a-3p, hsa-miR-33a-5p | Data showed that a specific plasma miRNA signature is associated with endometriosis and that hsa-miR-154-5p, which alone or in combination with hsa-miR-196b-5p, hsa-miR-378a-3p, and hsa-miR-33a-5p and the clinical parameters of body mass index and age, are potentially applicable for non-invasive diagnosis of the disease. | miRNA seem to be promising candidates for the non-invasive diagnosis of endometriosis. | [62] |
| 2019 | To identify endometriosis-specific plasma miRNAs and determine their diagnostic test accuracy. | Case control and cohort studies 249 Laparoscopy |
miR-155, miR574-3p and miR139-3p | Forty-nine miRNAs were differentially expressed in women with endometriosis. Nine maintained dysregulation in the selection cohort, but only three (miR-155, miR574-3p and miR139-3p) did so in the validation cohort. | Plasma miRNAs demonstrated modest sensitivity and specificity as diagnostic tests or triage tools for endometriosis. | [63] |
| 2019 | Retrieving data. Wait a few seconds and try to cut or copy again. | Case control and Cohort 210 Laparoscopy |
hsa-miR-125b-5p, hsa-miR-28-5p and hsa-miR-29a-3p | hsa-miR-125b-5p, hsa-miR-28-5p and hsa-miR-29a-3p) had diagnostic power above chance performance in the independent validation (AUC = 60%) with an acceptable sensitivity (78%) but poor specificity (37%). | A possible biological link between certain miRNAs and endometriosis, but the potential of these miRNAs as clinically useful biomarkers is questionable in women with infertility. | [64] |
| 2020 | Exploring aberrant exosomal miRNA profiles by using miRNA microarray and at providing more accurate molecular biomarkers of endometriosis. | Case control 50 Laparoscopy |
miR-22-3p, miR-320a | miR-22-3p and miR-320a were significantly upregulated in serum exosomes from patients with endometriosis compared with negative individuals. ROC curve revealed that the serum exosomal miR-22-3p and miR-320a yielded the area under the curve values of 0.855 and 0.827. | Results demonstrated that exosomal miR-22-3p and miR-320a were significantly increased in the sera of patients with endometriosis. The two miRNAs may be useful potential biomarkers for endometriosis diagnosis. | [65] |
| 2020 | Evaluated the differential expression of circulating miRNA-185-5p (miR-185-5p), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) target genes between endometriosis and healthy women. | Case control 50 Laparoscopy |
miRNA-185-5p, VEGF, PDGF | miR-185-5p was significantly down-regulated in the case group compared with the controls. | The low expression of miR-185-5p in the plasma of women with endometriosis could be employed as an important non-invasive biomarker for early detection and screening of endometriosis by blood samples. | [66] |
| 2020 | To identify novel candidate diagnostic microRNA (miRNA) markers of endometriosis. | Retrospective cohort 20 Laparoscopy |
24 candidate miRNAs and 3 reference miRNA: hsa-miR-150-5p, hsa-miR-199a-3p, hsa-miR-143-3p, hsa-miR-199a-5p, hsa-miR-335-3p, hsa-miR-381-3p, hsa-miR-224-5p, hsa-miR-340-5p, hsa-let-7d-3p, hsa-miR-92a-3p, hsa-miR-221-3p, hsa-miR-486-5p, hsa-let-7b-5p, hsa-miR-122-5p, hsa-miR-21-5p, hsa-miR-133a-3p, hsa-miR-148a-5p, hsa-let-7a-3p, put-miR-5, put-miR-27, hsa-miR-125b-5p, hsa-miR-17-5p, hsa-miR-20a-5p, hsa-miR-3613-5p, hsa-miR-103a-3pa, hsa-miR-30e-5p, hsa-miR-148b-3p | Combination of five miRNAs (miR-17-5p, miR-20a-5p, miR-199a- 3p, miR-143-3p, and let-7b-5p) produced sensitivity and specificity of 0.96 and 0.79 with PPV and NPV of 0.80 and 0.96, respectively. | A panel of candidate miRNAs was comparable to laparoscopy in distinguishing between women with endometriosis and control women. | [19] |
| 2020 | To explore the diagnostic potency of this approach[67]. | Case control 66 Laparoscopy |
Up-regulated: miR-181a, miR-191, miR-195 and miR-200b Down-regulated: miR-10b, miR-200c, miR-10a, miR-221 and miR-31 |
mir-10b, miR-200c and miR-191 were significantly dysregulated in the eutopic endometrium of AM patients. The expression ratio of reciprocally dysregulated microRNAs allowed us to diagnose AM with a range of sensitivity from 65% to 74%, and of specificity from 72% to 86%. | The analysis of microRNAs from the eutopic endometrium might present a promising low-invasive method of AM diagnostics. | [67] |
| 2021 | To evaluate the level of serum miR-34a-5p and miR-200c from women with and without endometriosis[68]. | Case control 136 Laparoscopy |
CA-125, miR-34a-5p, miR-200c | miR-34a-5p expression levels were decreased and miR-200c expression levels were increased in the endometriosis patients compared to the control group. | Serum miRNAs may provide a promising opportunity for diagnosis of endometriosis. Understanding the role of circulating miRNAs will serve a better comprehension of the systemic effects of endometriosis and offer options for new treatments. | [68] |
| 2021 | To investigate whether the combination of miR-224-5p, miR-199-3p, and let-7d-3p is a suitable diagnostic panel for endometriosis[69]. | Case control 50 Laparoscopy |
miRNAs 199b-3p, 224-5p,Let-7d-3p | Upregulation of miRNAs 199b-3p (P value < 0.001) and down-regulation of 224-5p (P value < 0.001) and miRNA let-7d-3p (P value < 0.05) in women with endometriosis compared to non-endometriosis women. | The levels of miRNAs 199b-3p, 224- 5p, and Let-7d-3p in plasma are potential diagnostic biomarkers for endometriosis patients. | [69] |
| 2022 | To analyze the current human miRNAome to differentiate between patients with and without endometriosis, and to develop a blood-based miRNA diagnostic signature for endometriosis with internal cross-validation[70]. | Prospective trial 200 Laparoscopy |
miR-3622a-,miR-504-3p,miR-526a-3p,miR-124-3p,miR-3923, miR-5004-3p,miR-520h, miR-5700, miR-6502-5p, miR-6799-3p,miR-6826-5p,miR-6837-5p,miR-7108-3p,miR-1180-5p,miR-3064-3p,miR-3168, miR-3185, miR-4674, miR-4764-5p, miR-516a-3p, miR-542-5p, miR-889-5p, miR-1253, miR-1292-5p, miR-138-1-3p,miR-1910-5p,miR-216b-3p,miR-26a-2-3p, miR-29b-1-5p, miR-30e-3p, miR-3117-5p, miR-3122, miR-3137, miR-4696, miR-4703-5p, miR-4715-5p, miR-4740-5p, miR-4749-5p, miR-4797-3p, miR-4804-5p, miR-4999-5p, miR-5681a, miR-6075, miR-6509-5p, miR-6824-3p, miR-6875-3p, miR-1278, miR-1343-5p, miR-1973, miR-203a-5p, miR-208a-3p, miR-208a-5p, miR-3124-5p,miR-3176, miR-3683, miR-3691-5p, miR-375-5p, miR-3939, miR-3975, miR-4260, miR-4295, miR-4296, miR-433-3p, miR-4445-3p, miR-4455, miR-4511, miR-4536-3p, miR-4655-5p, miR-4725-5p, miR-4738-5p, miR-4750-3p, miR-514b-5p, miR-548aw, miR-548w, miR-5572, miR-5702, miR-573, miR-6788-3p, miR-6811-3p, miR-6813-5p, miR-6830-5p, miR-6872-3p, miR-6888-5p,miR-7109-5p, miR-7150, miR-7152-5p |
The most accurate signature provides a sensitivity, specificity, and Area Under the Curve (AUC) of 96.8%, 100%, and 98.4%, respectively, and is sufficiently robust and reproducible to replace the gold standard of diagnostic surgery. | The present study supports the use of a blood-based miRNA signature of endometriosis. | [18] |
| 2022 | To evaluate the diagnostic potential of differentially expressed miRNAs in serum samples of women with endometriosis, ECO and EC to establish them as diagnostic biomarkers[71]. | Cohort 40 Laparoscopy |
miR-16, miR-20a, miR-99b, miR-125a, miR-143, miR-145 | miR-16 was downregulated (P<0.05) whereas miR-99b, miR-125a, miR-143 and miR-145 were upregulated (P<0.05). | Certain circulating miRNAs (miB99b, miR-16, miR-125a, miR-145) might act as indicators and discriminators of endometriosis and endometrioid subtypes of EC and ovarian cancer and might serve as potential biomarkers for early diagnosis and management of these debilitating diseases. | [70] |
| 2022 | To identify circulating miRNAs associated with ovarian endometriosis (OMA)[72[. | Case control 188 Laparoscopy |
miR-484, miR-192-5p, miR-16-5p, miR-215-5p, let-7b-5p, miR-92a-3p, miR-93-5p, miR-30a-5p, U6 | let-7b and miR-92a-3p, and miR-93-5p | Results suggested that KIAA1324 might be involved in endometriosis through the downregulating action of two circulating miRNAs. As these miRNAs were found to be overexpressed, their quantification in plasma could provide a tool for an early diagnosis of endometriosis. | [71] |
| 2023 | Investigated the diagnostic value of serum miR-17-5p, miR-424-5p, and their combined expressions for EMT[73]. | Case control 160 Laparoscopy |
miR-424-5p, miR-17-5p | miR-17-5p and miR-424-5p were down regulated in EMT patients. For diagnosing EMT, the AUC of miR-17-5p was 0.865 and cutoff value was 0.890 (91.3% sensitivity and 85% specificity). | miR-424-5p combined with miR-17-5p has high diagnostic efficacy for EMT. | [72] |
2.3.2. Staging (Severity)
| Year | Objective | Type of study Size of sample Diagnosis method |
miRNA type | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 2018 | To examine the association of miR-31 and miR-145 expression in plasma with the presence of endometriosis[74]. | Case control 88 Laparoscopy |
miR-145 and miR-31 | miR-31 expression levels in stage 3 or 4 and stage 1 or 2 were significantly down-regulated (less than 0.01-fold, P<0.05), while the expression level of miR-145 was significantly up-regulated in women with endometriosis in stage 1 or 2. | miR- 31 was under-expressed in patients with endometriosis, while miR-145 was over-expressed in stage 1 or 2, indicating that they were relatively down-regulated in the more severe forms. | [73] |
| 2020 | To validate the use of a microRNA panel as a noninvasive diagnostic method for detecting endometriosis[10]. | Prospective study 100 Laparoscopy |
miR-125b-5p, miR-150-5p, miR-342-3p, and miR-451a, miR-3613-5p and let-7b | Upregulated: miR-125b-5p, miR-150-5p, miR-342-3p, and miR-451a. Down regulated: miR-3613-5p and let-7b. |
This is the first report showing that microRNA biomarkers can reliably differentiate between endometriosis and other gynecological pathologies, with an area under the curve >0.9 across 2 independent studies. | [10] |
| 2022 | Analyzed miRNA expression in saliva of women with and without endometriosis using a FireFly custom multiplex circulating miRNA assay[75]. | Case control 34 Laparoscopy |
hsa-mir-135a | A significant upregulation of plasma hsa-mir-135a was only observed in the secretory phase, although the proliferative phase showed a similar trend. | hsa-mir-135a was expressed significantly higher in the saliva of women with endometriosis, independent of disease stage and menstrual cycle phase. Also confirmed that hsa-mir-135a showed significantly elevated expression in the plasma of endometriosis patients. | [74] |
2.3.3. Management (Treatment and Prognosis)
| Year | Objective | Type of study Size of sample Diagnosis method |
miRNA type | Main result | Main outcome | Ref |
|---|---|---|---|---|---|---|
| 2014 | Could an aberrant microRNA (miRNA) expression profile be responsible for the changes in the angiogenic and fibrino- lytic states observed in endometriotic lesions? | Case control 83 Laparoscopy |
miR-202-3p, miR-424-5p, miR-449b-3p and miR-556-3p | Patient endometrial tissue showed significantly lower levels of miR-202-3p, miR-424-5p, miR-449b-3p and miR-556-3p, and higher levels of VEGF-A and uPA than healthy (control) endometrium. | Differences in miRNA levels could modulate the expression of VEGF-A and TSP-1, which may play an important role in the pathogenesis of endometriosis. The higher angiogenic and proteolytic activities observed in eutopic endometrium from patients might facilitate the implantation of endometrial cells at ectopic sites. | [21] |
| 2020 | To investigate exosomal RNAs (long noncoding RNAs (lncRNAs), microRNAs (miRNAs) and messenger RNAs (miRNAs)) profiling and their related networks in endometriosis (EMs). | Case control 30 Laparoscopy |
KRAS, RAB5B, BIRC2, TRAK1, PSMC5, MIB2, ATP6V1A, ADCY3, HMGCR, SMO, ATF2. RBM19 HERC3 MIB2 DMXL1 TRAK1 QKI PSMC5 DDX55 AGFG1 UBR4 PUS7L BIRC2 PSMD8 SKP1 WDR75 RAB38 DDX47 KRAS PSMD14 RAB12 PAPSS1 RAB5B OXR1 ATP6V1A SMO ADCY3 ATF2 PRKACB MZT2A HMGCR MDC1 NAPB | Overlapped differentially expressed 938 lncRNAs, 39 miRNAs and 1449 miRNAs were identified. 13 co-expression modules and 61 ceRNA networks were constructed. | Revealed exosomal lncRNA, miRNA and miRNA expression profiles in EMs, and identified EMs-associated exosomal co-expression networks and ceRNA networks. It is a novel and comprehensive research of EMs related RNAs. | [75] |
| 2020 | Identified aberrant high expression of circ_0007331 in ectopic endometrial cells by comparing the endometrial samples from patients with and without endometriosis. | Case control 50 Laparoscopy |
circ_0007331, MiR-200c-3p, HIF-1α | 1. The expression of circ_0007331 is abnormally elevated in endometriosis. 2. Circ_0007331 knock-down results in decreased cell proliferation and invasion of the EE cells in endometriosis. 3. Circ_0007331 knock-down suppresses the proliferation of EE cells by down-regulating the expression of HIF-1α. 4. MiR-200c-3p is predicted to mediate the regulation of HIF-1α by circ_0007331. 5. Circ_0007331 affects the proliferation and invasion of EE cells by sponging miR-200c-3p to regulate HIF-1α. 6. Circ_0007331 knock-down suppresses the progression of endometriosis via miR-200c-3p/HIF-1α axis in vivo |
Results show that circ_0007331 is abnormally highly expressed in patients with endometriosis. Both in vitro or in vivo, circ_0007331 knock-down suppresses the progression of endometriosis by inhibiting the proliferation and invasion of ectopic endometrial cells. Further mechanism studies confirmed that the molecular basis of this function is the circ_0007331/miR-200c-3p/HIF-1α axis. | [76] |
| 2020 | Analyzed the miRNA expressions in patients with ovarian endometriosis and healthy controls with microarray analysis to identify differentially expressed miRNAs. | Case control 60 Laparoscopy |
hsa-let-7i-5p, hsa-let-7a-5p, hsa-let- 7b-5p, hsa-let-7d-5p, hsa-let-7f-5p, hsa-let-7g-5p, hsa-miR-199a-3p, hsa-miR- 320a, hsa-miR-320b, hsa-miR-320c, hsa-miR-320d, hsa-miR-328-3p, hsa-miR- 331-3p, hsa-miR-320e | hsa-let-7i-5p showed the highest area under the ROC curve (AUC) with a value of 0.900. | The identified 14 differentially expressed miRNAs could be potential biomarkers and therapeutic targets for the diagnosis and treatment of endometriosis. | [77] |
| 2021 | To evaluate the possibility of using microrna let-7 and mir-9 as non-invasive biomarkers for the diagnosis and treatment of external genital endometriosis. | Case control 86 Laparoscopy |
mir-9, let-7 microRNA | Showed that the difference in mir-9 miRNA between the groups with and without endometriosis, as well as between the groups with more clinically and histologically severe and mild endometriosis, was statistically insignificant. In addition, a significant difference was noted regarding let-7 microRNA between the groups with and without endometriosis, as well as between the groups with more clinically and histologically severe and mild endometriosis. Comparison with cancer antigen-125 (CA-125) showed that let-7 microRNA was a more specific test than CA-125. | Believed that the measurement of microRNA let-7 is promising for routine use in patients with endometriosis. | [78] |
| 2022 | To analyze the human miRNAome to define a saliva-based diagnostic miRNA signature for endometriosis. | Case control 200 Laparoscopy |
Not mentioned | The respective sensitivity, specificity, and AUC for the diagnostic miRNA signature were 96.7%, 100%, and 98.3%. | Data support the use of a saliva-based diagnostic miRNA signature for endometriosis in the diagnosis care pathways after an external validation to confirm these results. | [79] |
| 2022 | Focused on examining the relationship between serum exosomal miRNA expression and the severity of endometriosis. | Case control 66 Laparoscopy |
miR-26b-5p, miR-215-5p, and miR-6795-3p | qRT-PCR analysis verified the differential expression of three miRNAs, miR-26b-5p, miR-215-5p, and miR-6795-3p. | Further analysis indicated that these differentially expressed miRNAs in serum exosomes may be involved in the pathogenesis of endometriosis and are related to the severity and certain symptoms of endometriosis. | [80] |
| 2022 | To investigate functions and pathways associated with the various miRNAs differentially expressed in patients with endometriosis. | Case control 200 Laparoscopy |
miR-124-3p, miR-6502-5p; miR-515-5p; miR-548j-5p; miR-29b-1-5p; miR-4748, miR-3137 and miR-3168. miRNA–548 family | Up-regulated: miR-6502-5p; miR-515-5p; miR-548j-5p; miR-29b-1-5p; miR-4748 Down regulated: miR-3137 and miR-3168. |
Results provide evidence of the relation between the miRNA profiles of patients with endometriosis and various signaling pathways implicated in its pathophysiology. | [81] |
| 2022 | To identify early follicular phase micro ribonucleic acids (miRNAs) that are altered in serum of women with endometriosis. | Case control 45 Laparoscopy |
hsa-miR-34c-3p | hsa-miR-34c-3p was significantly down-regulated in the follicular phase of patients with endometriosis. | These results support hsa-miR-34-3p as a potential therapeutic target in endometriosis. | [82] |
3.1. Discussion
3.2. Historical Context and the Role of CA-125
3.3. MiRNAs: A Molecular Revolution
3.4. Interplay between CA-125 and miRNAs
3.5. A Multidimensional Perspective on Endometriosis
4.1. Conclusion
4.2. miRNAs: The Molecular Narrators of Endometriosis
4.3. CA-125: The Enduring Biomarker
4.4. Synergistic Perspectives
4.5. Further Investigations
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N Engl J Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; A Flores, V. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.; Soliman, A.M.; Trenz, H.; Blauer-Peterson, C.; Sluis, A. Impact of Endometriosis Diagnostic Delays on Healthcare Resource Utilization and Costs. Adv. Ther. 2020, 37, 1087–1099. [Google Scholar] [CrossRef]
- Ghai, V.; Jan, H.; Shakir, F.; Haines, P.; Kent, A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J. Obstet. Gynaecol. 2019, 40, 83–89. [Google Scholar] [CrossRef]
- Moss, K.M.; Doust, J.; Homer, H.; Rowlands, I.J.; Hockey, R.; Mishra, G.D. Delayed diagnosis of endometriosis disadvantages women in ART: a retrospective population linked data study. Hum. Reprod. 2021, 36, 3074–3082. [Google Scholar] [CrossRef]
- Warzecha, D.; Szymusik, I.; Wielgos, M.; Pietrzak, B. The Impact of Endometriosis on the Quality of Life and the Incidence of Depression—A Cohort Study. Int. J. Environ. Res. Public Heal. 2020, 17, 3641. [Google Scholar] [CrossRef]
- Kamath, M.S.; Subramanian, V.; Antonisamy, B.; Sunkara, S.K. Endometriosis and oocyte quality: an analysis of 13 614 donor oocyte recipient and autologous IVF cycles. Hum. Reprod. Open 2022, 2022, hoac025. [Google Scholar] [CrossRef]
- Al Shukri, M.; Al Riyami, A.S.; Al Ghafri, W.; Gowri, V. Are There Predictors of Early Diagnosis of Endometriosis Based on Clinical Profile? A Retrospective Study. Oman Med J. 2023, 38, e458. [Google Scholar] [CrossRef]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, 557.e1–557.e11. [Google Scholar] [CrossRef]
- Kimber-Trojnar; Pilszyk, A.; Niebrzydowska, M.; Pilszyk, Z.; Ruszała, M.; Leszczyńska-Gorzelak, B. The Potential of Non-Invasive Biomarkers for Early Diagnosis of Asymptomatic Patients with Endometriosis. J. Clin. Med. 2021, 10, 2762. [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Emergencias 2016, 2016, CD012179. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chang, X.-H.; Zhao, Y.; Zhu, H.-L. Current biomarkers for the detection of endometriosis. Chin. Med J. 2020, 133, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Anastasiu, C.V.; Moga, M.A.; Neculau, A.E.; Bălan, A.; Scârneciu, I.; Dragomir, R.M.; Dull, A.-M.; Chicea, L.-M. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pan, M.; Zuo, Y.; Yang, B.; Wang, S. Research progress of CA125 in endometriosis: Teaching an old dog new tricks. Gynecol. Obstet. Clin. Med. 2022, 2, 191–198. [Google Scholar] [CrossRef]
- Azam, I.N.A.; Wahab, N.A.; Mokhtar, M.H.; Shafiee, M.N.; Mokhtar, N.M. Roles of microRNAs in Regulating Apoptosis in the Pathogenesis of Endometriosis. Life 2022, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in endometriosis: biological function and emerging biomarker candidates†. Biol Reprod. 2019, 100, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Dabi, Y.; Suisse, S.; Jornea, L.; Bouteiller, D.; Touboul, C.; Puchar, A.; Daraï, E. MicroRNome analysis generates a blood-based signature for endometriosis. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- Papari, E.; Noruzinia, M.; Kashani, L.; Foster, W.G. Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil. Steril. 2020, 113, 1232–1241. [Google Scholar] [CrossRef]
- Bendifallah, S.; Dabi, Y.; Suisse, S.; Delbos, L.; Spiers, A.; Poilblanc, M.; Golfier, F.; Jornea, L.; Bouteiller, D.; Fernandez, H.; et al. Validation of a Salivary miRNA Signature of Endometriosis — Interim Data. NEJM Évid. 2023, 2. [Google Scholar] [CrossRef]
- Braza-Boïls, A.; Marí-Alexandre, J.; Gilabert, J.; Sánchez-Izquierdo, D.; España, F.; Estellés, A.; Gilabert-Estellés, J. MicroRNA expression profile in endometriosis: its relation to angiogenesis and fibrinolytic factors. Hum. Reprod. 2014, 29, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.P.; Raymundo, T.S.; Soares, L.C.; Pereira, T.R.D.; Demôro, A.V.E. How to Use CA-125 More Effectively in the Diagnosis of Deep Endometriosis. BioMed Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, J.; Ishihara, H.; Koshiba, H.; Kiyomizu, M.; Teramoto, M.; Kitaoka, Y.; Honjo, H. Usefulness and limits of CA-125 in diagnosis of endometriosis without associated ovarian endometriomas. Hum. Reprod. 2005, 20, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Colacurci, N.; Fortunato, N.; De Franciscis, P.; Fratta, M.; Cioffi, M.; Zarcone, R.; Cardone, A. Serum and peritoneal CA-125 levels as diagnostic test for endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 66, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Kafali, H.; Artuc, H.; Demir, N. Use of CA125 fluctuation during the menstrual cycle as a tool in the clinical diagnosis of endometriosis; a preliminary report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Agic, A.; Djalali, S.; Wolfler, M.M.; Halis, G.; Diedrich, K.; Hornung, D. Combination of CCR1 mRNA, MCP1, and CA125 Measurements in Peripheral Blood as a Diagnostic Test for Endometriosis. Reprod. Sci. 2008, 15, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Mihalyi, A.; Gevaert, O.; Kyama, C.M.; Simsa, P.; Pochet, N.; De Smet, F.; De Moor, B.; Meuleman, C.; Billen, J.; Blanckaert, N.; et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum. Reprod. 2009, 25, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Socolov, R.; Butureanu, S.; Angioni, S.; Sindilar, A.; Boiculese, L.; Cozma, L.; Socolov, D. The value of serological markers in the diagnosis and prognosis of endometriosis: a prospective case–control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 154, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Vodolazkaia, A.; El-Aalamat, Y.; Popovic, D.; Mihalyi, A.; Bossuyt, X.; Kyama, C.M.; Fassbender, A.; Bokor, A.; Schols, D.; Huskens, D.; et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum. Reprod. 2012, 27, 2698–2711. [Google Scholar] [CrossRef]
- Zhu, H.; Lei, H.; Wang, Q.; Fu, J.; Song, Y.; Shen, L.; Huang, W. Serum carcinogenic antigen (CA)-125 and CA 19-9 combining pain score in the diagnosis of pelvic endometriosis in infertile women. Clin. Exp. Obstet. Gynecol. 2016, 43, 826–829. [Google Scholar] [CrossRef]
- Irungu, S.; Mavrelos, D.; Worthington, J.; Blyuss, O.; Saridogan, E.; Timms, J.F. Discovery of non-invasive biomarkers for the diagnosis of endometriosis. Clin. Proteom. 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Tang, T.; Lai, H.; Huang, X.; Gu, L.; Shi, H. Application of serum markers in diagnosis and staging of ovarian endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 1441–1450. [Google Scholar] [CrossRef]
- Guralp, O.; Kaya, B.; Tüten, N.; Kucur, M.; Malik, E.; Tüten, A. Non-invasive diagnosis of endometriosis and moderate-severe endometriosis with serum CA125, endocan, YKL-40, and copeptin quadruple panel. J. Obstet. Gynaecol. 2020, 41, 927–932. [Google Scholar] [CrossRef]
- Chen, T.; Wei, J.; Leng, T.; Gao, F.; Hou, S. The diagnostic value of the combination of hemoglobin, CA199, CA125, and HE4 in endometriosis. J. Clin. Lab. Anal. 2021, 35, e23947. [Google Scholar] [CrossRef]
- Szubert, M.; Rycerz, A.; Wilczyński, J.R. How to Improve Non-Invasive Diagnosis of Endometriosis with Advanced Statistical Methods. Medicina 2023, 59, 499. [Google Scholar] [CrossRef] [PubMed]
- Micu, R.; Gaia-Oltean, A.M.I.; Budişan, L.; Braicu, C.; Irimie, A.; Berindan-Neagoe, I. The added value of CA125, HE4, and CA72-4 as markers for ovarian endometriosis diagnosis. Romanian J. Morphol. Embryol. 2023, 64, 159–164. [Google Scholar] [CrossRef]
- Kovalak, E.E.; Karacan, T.; Zengi, O.; Akgül, K.; Özyürek, .E.; Güraslan, H. Evaluation of new biomarkers in stage III and IV endometriosis. Gynecol. Endocrinol. 2023, 39, 2217290. [Google Scholar] [CrossRef] [PubMed]
- Hornstein, M.D.; Harlow, B.; Thomas, P.; Check, J. Endometriosis: Use of a new CA 125 assay in the diagnosis of endometriosis*. Hum. Reprod. 1995, 10, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.S.A.C.; Rosa, E.S.J.C.; Ferriani, R.A. Serum CA-125 in the diagnosis of endometriosis. Int. J. Gynaecol. Obstet. 2007, 96, 206–207. [Google Scholar] [CrossRef]
- Szubert, M.; Suzin, J.; Wierzbowski, T.; Kowalczyk-Amico, K. CA-125 concentration in serum and peritoneal fluid in patients with endometriosis – preliminary results. Arch. Med Sci. 2012, 8, 504–508. [Google Scholar] [CrossRef]
- Karimi-Zarchi, M.; Dehshiri-Zadeh, N.; Sekhavat, L.; Nosouhi, F. Correlation of CA-125 serum level and clinico-pathological characteristic of patients with endometriosis. Int J Reprod Biomed. 2016, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.D.; Thornton, J.G.; Cooper, E.H. Serum CA 125 antigen levels and disease severity in patients with endometriosis. Obstet Gynecol. 1989, 73, 767–769. [Google Scholar] [PubMed]
- O'Shaughnessy, A.; Check, J.H.; Nowroozi, K.; Lurie, D. CA 125 levels measured in different phases of the menstrual cycle in screening for endometriosis. Obstet Gynecol. 1993, 81, 99–103. [Google Scholar] [PubMed]
- Amaral, V.F.D.; Ferriani, R.A.; de Sá, M.F.S.; Nogueira, A.A.; e Silva, J.C.R.; Silva, A.C.J.d.S.R.e.; de Moura, M.D. Positive correlation between serum and peritoneal fluid CA-125 levels in women with pelvic endometriosis. Sao Paulo Med J. 2006, 124, 223–227. [Google Scholar] [CrossRef]
- Maiorana, A.; Cicerone, C.; Niceta, M.; Alio, L. Evaluation of serum CA 125 levels in patients with pelvic pain related to endometriosis. Int. J. Biol. Markers. 2007, 22, 200-2. [Google Scholar] [CrossRef] [PubMed]
- Ozhan, E.; Kokcu, A.; Yanik, K.; Gunaydin, M. Investigation of diagnostic potentials of nine different biomarkers in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Streuli, I.; Melonio, I.; Marcellin, L.; M'Baye, M.; Bititi, A.; Borghese, B.; Pillet, M.-C.L.; Chapron, C. Increased Serum Cancer Antigen-125 Is a Marker for Severity of Deep Endometriosis. J. Minim. Invasive Gynecol. 2015, 22, 275–284. [Google Scholar] [CrossRef]
- Knific, T.; Vouk, K.; Vogler, A.; Osredkar, J.; Gstöttner, M.; Wenzl, R.; Rižner, T.L. Models including serum CA-125, BMI, cyst pathology, dysmenorrhea or dyspareunia for diagnosis of endometriosis. Biomarkers Med. 2018, 12, 737–747. [Google Scholar] [CrossRef]
- Sasamoto, N.; DePari, M.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A.; Shafrir, A.L.; Terry, K.L. Evaluation of CA125 in relation to pain symptoms among adolescents and young adult women with and without surgically-confirmed endometriosis. PLOS ONE 2020, 15, e0238043. [Google Scholar] [CrossRef]
- Zhao, K.; Qu, P. Noninvasive evaluation of ovarian endometriosis: a single-center experience. Ann. Palliat. Med. 2021, 10, 4728–4735. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Daoud, E.; Viganò, P.; García-Velasco, J.A.; Colli, E. Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables. Biomolecules 2023, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Pittaway, D.E.; Fayez, J.A. The use of CA-125 in the diagnosis and management of endometriosis. Fertil. Steril. 1986, 46, 790–795. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Meuleman, C.; Oosterlynck, D.; Cornillie, F.J. Diagnosis of deep endometriosis by clinical examination during menstruation and plasma CA-125 concentration. Fertil. Steril. 1996, 65, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Soong, Y.; Lee, N.; Lo, S.K. The use of serum CA-125 as a marker for endometriosis in patients with dysmenorrhea for monitoring therapy and for recurrence of endometriosis. Acta Obstet. et Gynecol. Scand. 1998, 77, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Kimber-Trojnar, Ż.; Pilszyk, A.; Niebrzydowska, M.; Pilszyk, Z.; Ruszała, M.; Leszczyńska-Gorzelak, B. The Potential of Non-Invasive Biomarkers for Early Diagnosis of Asymptomatic Patients with Endometriosis. J. Clin. Med. 2021, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, A.; Verbeeck, N.; Börnigen, D.; Kyama, C.; Bokor, A.; Vodolazkaia, A.; Peeraer, K.; Tomassetti, C.; Meuleman, C.; Gevaert, O.; et al. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Hum. Reprod. 2012, 27, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-Z.; Yang, Y.; Lang, J.; Sun, P.; Leng, J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum. Reprod. 2012, 28, 322–330. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef]
- Wang, L.; Huang, W.; Ren, C.; Zhao, M.; Jiang, X.; Fang, X.; Xia, X. Analysis of Serum microRNA Profile by Solexa Sequencing in Women With Endometriosis. Reprod. Sci. 2016, 23, 1359–1370. [Google Scholar] [CrossRef]
- Xu, X.X.; Jia, S.Z.; Dai, Y.; Zhang, J.J.; Li, X.Y.; Shi, J.H.; Leng, J.H.; Lang, J.H. Identification of Circular RNAs as a Novel Biomarker for Ovarian Endometriosis. Chin. Med. J. 2018, 131, 559–566. [Google Scholar] [CrossRef]
- E Haikalis, M.; Wessels, J.M.; A Leyland, N.; Agarwal, S.K.; Foster, W.G. MicroRNA expression pattern differs depending on endometriosis lesion type†. Biol. Reprod. 2018, 98, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Pateisky, P.; Pils, D.; Szabo, L.; Kuessel, L.; Husslein, H.; Schmitz, A. et al. hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod Biomed Online 2018, 37, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Sharkey, D.J.; Wang, Z.; Evans, S.F.; Healey, M.; Teague, E.M.C.O.; Print, C.G.; A Robertson, S.; Hull, M.L. Plasma miRNAs Display Limited Potential as Diagnostic Tools for Endometriosis. J. Clin. Endocrinol. Metab. 2019, 104, 1999–2022. [Google Scholar] [CrossRef] [PubMed]
- Vanhie, A.; O, D.; Peterse, D.; Beckers, A.; Cuéllar, A.; Fassbender, A.; Meuleman, C.; Mestdagh, P.; D’hooghe, T. Plasma miRNAs as biomarkers for endometriosis. Hum. Reprod. 2019, 34, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Hossein Razi M, Eftekhar M, Ghasemi N, Hasan Sheikhha M, Dehghani Firoozabadi A. Expression levels of circulatory mir-185-5p, vascular endothelial growth factor, and platelet-derived growth factor target genes in endometriosis. Int J Reprod Biomed. 2020;18(5):347-58.
- Borisov, E.; Knyazeva, M.; Novak, V.; Zabegina, L.; Prisyazhnaya, T.; Karizkiy, A.; Berlev, I.; Malek, A. Analysis of Reciprocally Dysregulated miRNAs in Eutopic Endometrium Is a Promising Approach for Low Invasive Diagnostics of Adenomyosis. Diagnostics 2020, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Misir, S.; Hepokur, C.; Oksasoglu, B.; Yildiz, C.; Yanik, A.; Aliyazicioglu, Y. Circulating serum miR-200c and miR-34a-5p as diagnostic biomarkers for endometriosis. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102092. [Google Scholar] [CrossRef] [PubMed]
- Zafari, N.; Tarafdari, A.M.; Izadi, P.; Noruzinia, M.; Yekaninejad, M.S.; Bahramy, A.; Mohebalian, A. A Panel of Plasma miRNAs 199b-3p, 224–225p and Let-7d-3p as Non-Invasive Diagnostic Biomarkers for Endometriosis. Reprod. Sci. 2021, 28, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Kumari, P.; Saha, S.; Srinivasan, R.; Bhardwaj, P. Role of serum microRNAs as biomarkers for endometriosis, endometrioid carcinoma of ovary & endometrioid endometrial cancer. Indian J. Med Res. 2022, 156, 516–523. [Google Scholar] [CrossRef]
- Abo C, Biquard L, Girardet L, Chouzenoux S, Just PA, Chapron C, et al. Unbiased In Silico Analysis of Gene Expression Pinpoints Circulating miRNAs Targeting KIAA1324, a New Gene Drastically Downregulated in Ovarian Endometriosis. Biomedicines. 2022;10(9).
- Lin, C.; Zeng, S.; Li, M. miR-424-5p combined with miR-17-5p has high diagnostic efficacy for endometriosis. Arch. Gynecol. Obstet. 2023, 307, 169–177. [Google Scholar] [CrossRef]
- Bashti, O.; Noruzinia, M.; Garshasbi, M.; Abtahi, M. miR-31 and miR-145 as Potential Non-Invasive Regulatory Biomarkers in Patients with Endometriosis. Cell J. 2018, 20, 84–89. [Google Scholar] [PubMed]
- Perricos, A.; Proestling, K.; Husslein, H.; Kuessel, L.; Hudson, Q.J.; Wenzl, R.; Yotova, I. Hsa-mir-135a Shows Potential as A Putative Diagnostic Biomarker in Saliva and Plasma for Endometriosis. Biomolecules 2022, 12, 1144. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Huang, W.; Wang, L.; Xia, X.; Fang, X. Analysis of exosomal lncRNA, miRNA and mRNA expression profiles and ceRNA network construction in endometriosis. Epigenomics 2020, 12, 1193–1213. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, L.; Liu, H.; Xie, M.; Gao, J.; Zhou, X. , et al. Circ_0007331 knock-down suppresses the progression of endometriosis via miR-200c-3p/HiF-1α axis. J Cell Mol Med. 2020, 24, 12656–12666. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.-L.; Zhang, Z.; Fan, W.-S.; Li, L.-A.; Ye, M.-X.; Zhang, Q.; Zhang, N.-N.; Li, Z.; Meng, Y.-G. Identification of MicroRNAs as Potential Biomarkers in Ovarian Endometriosis. Reprod. Sci. 2020, 27, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Pokrovenko, D.A.; Vozniuk, V.; Medvediev, M.V. MicroRNA let-7: A promising non-invasive biomarker for diagnosing and treating external genital endometriosis. J. Turk. Soc. Obstet. Gynecol. 2021, 18, 291–297. [Google Scholar] [CrossRef]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, W.; Ding, H.; Wu, X. Serum exosomal miRNA from endometriosis patients correlates with disease severity. Arch. Gynecol. Obstet. 2022, 305, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Dabi, Y.; Suisse, S.; Jornea, L.; Bouteiller, D.; Touboul, C.; Puchar, A., et al. Clues for Improving the Pathophysiology Knowledge for Endometriosis Using Serum Micro-RNA Expression. Diagnostics 2022, 12.
- Neuhausser, W.M.; Faure-Kumar, E.; Mahurkar-Joshi, S.; Iliopoulos, D.; Sakkas, D. Identification of miR-34-3p as a Candidate Follicular Phase Serum Marker for Endometriosis: a pilot study. F&S Sci. 2022, 3, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Saare, M.; Rekker, K.; Laisk-Podar, T.; Rahmioglu, N.; Zondervan, K.; Salumets, A.; Götte, M.; Peters, M. Challenges in endometriosis miRNA studies — From tissue heterogeneity to disease specific miRNAs. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2017, 1863, 2282–2292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





