1. Introduction:
Global food security is threatened by climate change, both directly through responses of crop physiology and productivity, and indirectly through responses of plant-associated microbiota, including plant pathogens. Climate change further increases outbreak risks by altering pathogen evolution and host–pathogen interactions and facilitating the emergence of new pathogenic strains. Pathogen range can shift, increasing the spread of plant diseases in new areas according to Raza and Bebber [
1].
The root-knot nematodes (
Meloidogyne spp.) attack the roots of various trees, shrubs and herbaceous plants. Infested roots become distorted and develop rounded or irregular galls. The nematodes also increase the deleterious effects of pathogenic bacteria and fungi. There are millions of
$ dollars (
$118 billion) are loss annually because of the root-knot nematodes as reported by Atkinson et al., [
2]. Therefore, management of Plant parasitic nematodes (PPN) is a vital process which must contains multi-measures to keep the soil population below the economical threshold level according to Khalil et al., [
3].
Root-knot nematodes enter roots as larvae, causing lumps or nodules to form on the plant's root system, blocking the pathways of water and nutrients transport through the plant [
4]. Also, Gamalero and Glick [
5] reported that once nematodes are present in soil, they are almost impossible to eliminate, but their damage to plants can be reduced. Moreover, nematode strains that have developed a high level of resistance to chemical nematicides have emerged.
Due to the harmful effect of most chemical nematicides on humans and the environment, there is a global trend to use bio-pesticides as the perfect and new approach to avoid agro-chemicals problem in crop protection. It has been challenge for the pest control strategy in recent years. One source of potential new pesticides is natural products produced by plants such as natural oils as described by Osman et al., [
6]. Many plants, including neem (
Azadirachta indica) have the ability to generate secondary phytochemical metabolites useful for controlling nematodes and other parasites. In this context, Kumar [
7] reported that neem plant is one of the most important natural additives used to control the spread of nematodes in the soil as well as and pathogenic fungi in the soil. Moreover, Javed et al. [
8] mentioned that curative application of neem formulations (i.e. oil) significantly reduced the nematodes (
M. incognita) infection., neem oil is the most active component in Azadirachtin and alongin a with others like nimbin, meliantriol, nimbidin, nimbinin, salannin, nimbolides, and fatty acids (palmitic, stearic, and oleic), is responsible for its biochemical activity. Additionally, a research revealed that terpinenen-4-ol, allyl isopropyl sulphide, 1,2,4-trithiolane, 3,5-diethyl, and cycloisolongifolene are some of the main active volatile components in neem essential oil as shown by Mossa et al., [
9]. Nanomaterials typically display exceptional properties owing to their distinctive characteristics, including small dimensions and an extraordinarily high surface area-to-volume ratio according Darwesh et al., [
10]. The transport of bioactive compounds from plants using nano emulsions has received a lot of interest recently. The micro emulsions technique works best with bioactive essential oils that are transient and susceptible to deterioration as was shown by Ali et al., [
11].
With taking environmental dimension consideration, the current work aimed to investigate the direct effect of neem oil nano-emulsions on the controlling of nematode (M. incognita) infection for fig (Ficus carica cv. Black mission) seedlings and the influences of such applications on soil microbial activity. The effect of applying crude neem oil and nano-emulsion form on some important parameters of the plant were also studied, in compare Fosthiazate (Krenkel 75%EC).
2. Materials and Methods
2.1. Plant material
Current work was carried out during 2019/2020 at greenhouse of Pomology Dept., National Research Centre, and Zoology Department, Faculty of Science, Tanta University, Egypt. About eighty uniform fig seedlings (Ficus carica cv. Black mission) (one year old) grown in pots (25cm) felt in with sandy soil that infected with root-knot nematodes Meloidogyne incognita) were divided into 5 groups. Each group, that contains 16 seedlings. Pre-treatment soil samples were taken (250 gm) to count the larvae (Js) of M. incognita (Initial population). Fig groups were subjected to different treatment as the following:
Group 1: was served as control and received water only
Group 2: Fosthiazate (Krenkel 75%EC) applied for one time at the recommended dose of (4ml /L)
Group 3: crude neem oil applied at 10 ml/L for 30, 60 and 90 days
Group 4: neem oil nano-emulsion applied at 7 ml/L for 30, 60 and 90 days.
Group 5: neem oil nano-emulsion applied at 5 ml/L for 30, 60 and 90 days.
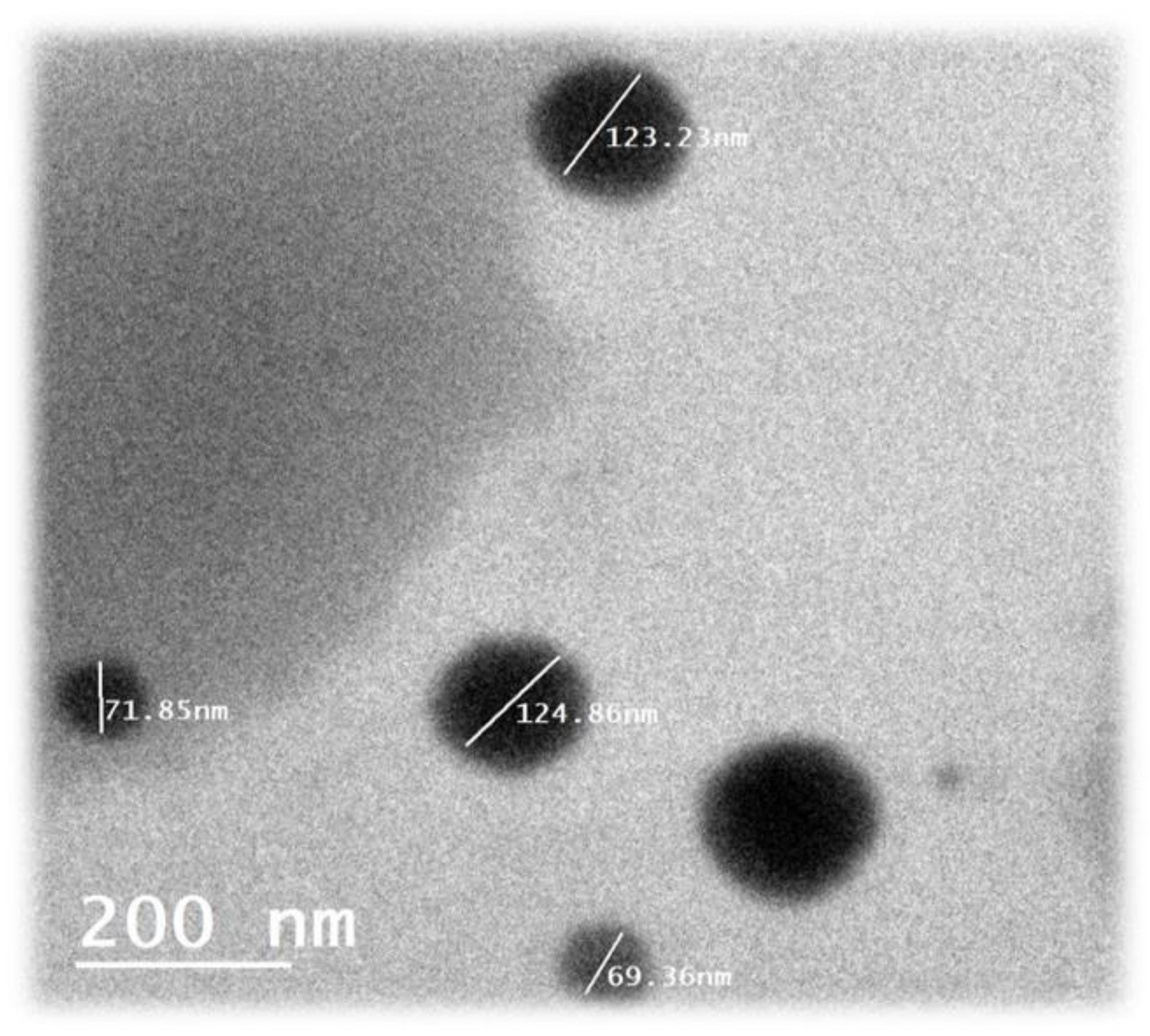
2.2. Nano-emulsion of neem oil source, preparation and SEM
The oil used in this study was neem oil that extracted from neem seeds by press cooling methods. The nano-emulsion of neem oil was prepared according to the method described by Ghotbi et.al [
12]. The SEM of nano-emulsion of neem oil was carried out in the electron microscopy unit of the National research centre.The nano-emulsion of neem oil was prepared by dissolving 0.225 g nano-emulsion of neem oil in 150 ml distilled water blended with 0.1% glacial acetic acid Khan et al., [
13] (
Figure 1).
2.3. Plant health measurements
To assess impact of studied treatments on the vegetative growth, leaf fresh and dry weight (g) were measured. Also, leaf water content was calculated. Also, leaf chlorophyll content was recorded in fresh leaves by using Minolta chlorophyll meter (SPAD – 501). In addition, leaf mineral content was determined at the end of the experiment in both seasons. Leaf samples were prepared to assessing nutrient content according to Chapman and Pratt [
14].
| Variable |
Methods used for sample preparation and analysis |
| Nitrogen |
(N) |
(%) |
Micro – Kjelahl method, using boric acid modification, and
distillation was done using Gerhardt apparatus |
| Phosphorus |
(P) |
(%) |
(NaHCO3-Extractable) and measured using Spectrophotometer (Perkin-Elmer Lambda-2) according Jakson [15] |
| Potassium |
(K) |
(%) |
(NH4OAC-Extractable) and measured using (Eppendorof Dr. Lang) Flame-photometer according to Chapman and Bratt [14] |
| Calcium |
(Ca) |
| Magnesium |
(Mg) |
(%) |
(DTPA-Extractable) and measured using Atomic absorption (Perkin-Elmer 1100 B) according to Lindsay and Norvell, [16] |
| Iron |
(Fe) |
|
| Manganese |
(Mn) |
(ppm) |
| Zinc |
(Zn) |
|
| Copper |
(Cu) |
|
2.4. Field Soil and Root Sampling Techniques
Soil and root samples were collected from the Rhizosphere of plants, composed of almost 250 gm soil in weight and were taken generally from 10-30 cm depth in soil into polyethylene – plastic bags. Bags were then sealed, carried to the laboratory and stored in a refrigerator on 5-7oC until processing.
2.5. Nematode inoculum
The egg inoculum of root-knot nematode (
Meloidogyne incognita) was isolated from eggplant roots (cv. Balady). The roots were cut into small segments (1–2 cm long), and shaken for 3 min in sodium hypochlorite (5%). The suspension was passed through 200 and 400 mesh sieves to obtain free eggs as reported by Hussey & Barker [
17]. The eggs were washed several times with water and then counted under a stereo-microscope. The second stage juveniles (J2) were obtained from the hatched eggs by Baermann plate technique as shown by Ayoub [
18]. The species of root-knot nematode (M. incognita) was identified by using the perineal patterns method according to Taylor and Nelscher [
19].
2.6. Nematode reduction
Whereas, PTA is the population no in the treated pots after application, PTB is the population no in the treated pots before application, PCB is the population no in the check pots before application, and PCA is the no. in the check pots after application. Percentages of nematode reduction were determined according to Henderson and Tilton formula as reported by Puntener [
20]. As follows:
2.7. Soil microbial activity
Soil samples were analyzed using the standard procedures in the laboratory at Microbial Genetics, National Research Centre (NRC).
The total microbial enzyme activities of soils were estimated based on the rate of fluorescein diacetate (FDA) hydrolytic activity according to Patle et al., (2018) [
21] with some modifications. Two gram of rhizosphere soil samples were placed (triplicates) into 50-ml capped centrifuge tubes. A volume of 15 ml potassium phosphate buffer (60 mm, pH 7.6) and 0.2 mL of 0.1% FDA (in acetone) were added to initiate the reaction. Tubes were incubated horizontally at 30°C for 20 min in a rotary shaker. After incubation and color development, the reaction stopped by adding 15 mL of chloroform/methanol (2:1) and vortexing for one min. Tubes were subjected to centrifuge (5000 rpm for 10 min) to spindown soil and turbidity and separate chloroform layer. The developed colored fluorescein in the chloroform layer was spectrophotometrically measured at 490 nm against fluorescein standers. Total soil microbial activity was expressed as FDA hydrolysis values (µg of released fluorescein g-1 soil).
2.8. Statistical analysis:
Data were analyzed as one way analysis of variance (ANOVA) and means were represented as combined analysis of both seasons. Data were statistically analyzed using SAS (Statistical Analysis System) version 9.1 (Gomez and Gomez, 1984). The least significant difference (L.S.D) at 0.05 was used to compare among the means of the different treatments according to Snedecor and Cochran, [
22].
3. Results
3.1. Impact of neem oil & nano-emulsion on Vegetative growth of infected fig seedlings
The data presented in
Table 1 reveals that most measured vegetative parameters exhibited increases in treatments compared to the control treatment. However, these increments varied depending on the type of applied treatment. Notably, neem oil treatments, including nano-emulsion, demonstrated superior results compared to other treatments. Crude neem oil recorded the highest values for both fresh weight and dry weight of leaves (10.56 and 4.1 g, respectively), while nano-emulsion at a concentration of 7 ml/l ranked second for both parameters (9.88 and 3.33 g, respectively). This supports the hypothesis that foliage and root growth, specifically in terms of length, are key parameters for evaluating the impact of nematode infection stress according to Wareing [
23].
Moreover, water content (%) reached its highest level when nano-emulsion of neem oil was applied at 7 ml/l (66.32%) compared to other treatments. This suggests that the efficiency of nutrient uptake through the infected root system treated with nano-emulsion of neem oil at 7 ml/l was significantly enhanced compared to other treatments.
In terms of chlorophyll content,
Table 1 indicates that all treatments led to an enhancement in chlorophyll content in the leaves of infected fig seedlings compared to control fig seedlings. Notably, there was no significant variation among treatments, particularly with nematicide and nano-emulsion.
3.2. Impact of neem oil & oil nano-emulsion on leaf nutrient content of infected fig seedlings
Analysis of
Table 2 data reveals that control plants (infected) receiving only water exhibited the lowest content of essential minerals (N, P, K, Ca, Mg, Fe, Mn, Zn, and Cu). Conversely, the leaf mineral content improved with various treatments, ranging from nematicide to neem oil treatment and nano-emulsion of neem oil at both 5 and 7 ml/l.
Upon closer examination of
Table 2, it becomes evident that neem oil outperformed nematicide in enhancing leaf mineral content across all levels. Particularly noteworthy is the fact that nano-emulsion of neem oil at 7 ml/l recorded the highest values for macro-nutrients (nitrogen, phosphorus, potassium, calcium, and magnesium) at 3.4, 1.01, 1.05, 2.4, and 0.315%, respectively. Additionally, nano-emulsion of neem oil at 5 ml/l secured the second position for nitrogen, phosphorus, potassium, calcium, and magnesium content, registering values of 3, 0.95, 0.77, 2, and 0.27%, respectively. Following closely was the crude neem oil treatment, which produced nutrient content values of 2.8, 0.9, 0.72, 2, and 0.27% for the same macro-nutrients.
Fosthiazate (Krenkel 75%EC) treatment claimed the fourth position, while control plants receiving water exhibited the lowest values for these macro-nutrients.
The trend observed with macro-nutrients extended to micro-elements, where nano-emulsion of neem oil that applied as soil treatment at 7 ml/l, recorded the highest values for Fe, Mn, Zn, and Cu at 118.8, 45, 25.2, and 7.5 ppm, respectively. Nano-emulsion of neem oil at 5 ml/l secured the second rank with values of 110.7, 38, 21.6, and 6 ppm for Fe, Mn, Zn, and Cu, respectively. Crude neem oil, when applied, ranked third after nano-emulsion of neem oil treatments, producing micro-nutrient values of 91.8, 33, 18, and 4.5 ppm for Fe, Mn, Zn, and Cu, respectively. Fosthiazate (Krenkel 75%EC treatment claimed the fourth position for these nutrients, and the control treatment ranked last, as illustrated in the table.
Table 2.
effect of Neem oil & oil nano-emulsion versus nematicid on nutrient content in leaves of nematode infected fig seedlings.
Table 2.
effect of Neem oil & oil nano-emulsion versus nematicid on nutrient content in leaves of nematode infected fig seedlings.
| |
Nutrients |
N |
P |
K |
Ca |
Mg |
Fe |
Mn |
Zn |
Cu |
| Treatments |
|
(%) |
(ppm) |
| Control (water) |
2.4 b
|
0.25 e
|
0.57 e
|
1.7 b
|
0.22 b
|
83.7 d
|
25 e
|
15.7 d
|
4.5 c
|
| Nematicide Fosthiazate |
2.5 b
|
0.48 d
|
0.67d
|
1.75b
|
0.26ba
|
86.4 d
|
31 d
|
17.5 c
|
4.5 c
|
| Crude neem oil (10ml/l) |
2.8 ba
|
0.9 c
|
0.72 c
|
2 ba
|
0.27ba
|
91.8 c
|
33 d
|
18 c
|
4.5 c
|
| Neem oil nano-emulsion (5ml/l) |
3 ba
|
0.95 b
|
0.77b
|
2 ba
|
0.27ba
|
110.7b
|
38 b
|
21.6 b
|
6 b
|
| Neem oil nano-emulsion (7ml/l) |
3.4 a
|
1.01 a
|
1.05a
|
2.4 a
|
0.31a
|
118.8a
|
45 a
|
25.2 a
|
7.5 a
|
3.3. Impact of neem oil &oil nano-emulsion on nematode Parameters
Obtained data in
Table 3 indicated that all treatments resulted in decreasing in
Meloidogyne incognita populations in treated soil comparing with control. Moreover, these reduction percentages increased with increasing number times of repeats for treatments. After, first month of treatments application, the highest reduction was recorded with crude neem oil at 10ml/l (89.4%) followed with nematicide (88.7%). Meanwhile, neem oil nano-emulsion treatments (5& 7 ml/l) recorded medium reduction percentage (65.2 & 74.5%).
Also, it was observed that reduction percentage was increased by increasing number times of treatments repeating whereas, reduction percentage for nematicide Fosthiazate (Krenkel 75%EC increased from 88.7& to 96.1% at the 3rd time of application (3rd month) and crude neem oil at 10ml/l increased from (89.4 to 90.9). For neem oil nano-emulsion (5 & 7ml/l) the reduction percentages increased from (65.2 to 68.8%) and (74.4 to 80.35%) respectively.
Table 4 shows that after first month of application, the only treatment which caused reduction in egg mass of
M. incognita was neem oil nano-emulsion at 7ml/l which emphasized the effectiveness on neem oil nano-emulsion at 7ml/l on decreasing reproduction rate of nematode. With increasing number of times repeats of applications, data showed that there were considerable reduction percentages in egg mass in all treatments comparing to control.
The highest reduction percentage in egg mass was recorded for neem oil nano-emulsion at 7ml/l.
In regard to number of gall that occurred on infected roots, data in
Table 5 revealed that after first month of applying studied treatments, only crude neem oil (10ml/l) treatment was caused reduction. After three month of repeating of applications the reduction percentages for nematicide
Fosthiazate (Krenkel 75%EC (66.5%) , crude neem oil (10ml/l)(64.6%), neem oil nano-emulsion (5ml/l) (64.2%) and neem oil nano-emulsion (7ml/l)(64.3%) were very closed, which indicates that there is no preference among tested treatments in reduction of galls on infected roots.
3.4. Soil microbial activity
In agricultural soils, rhizosphere bacteria play a key role in nutrient facilitation, the creation of plant growth stimulants, the bioremediation of toxic chemicals, and disease management. Total bacterial counts and enzymes activity are important parameters of soil quality. Both reflect the activity of the microbial population, which gave an indirect indication of soil nutrition and fertility according to Patle
et al., [
21].
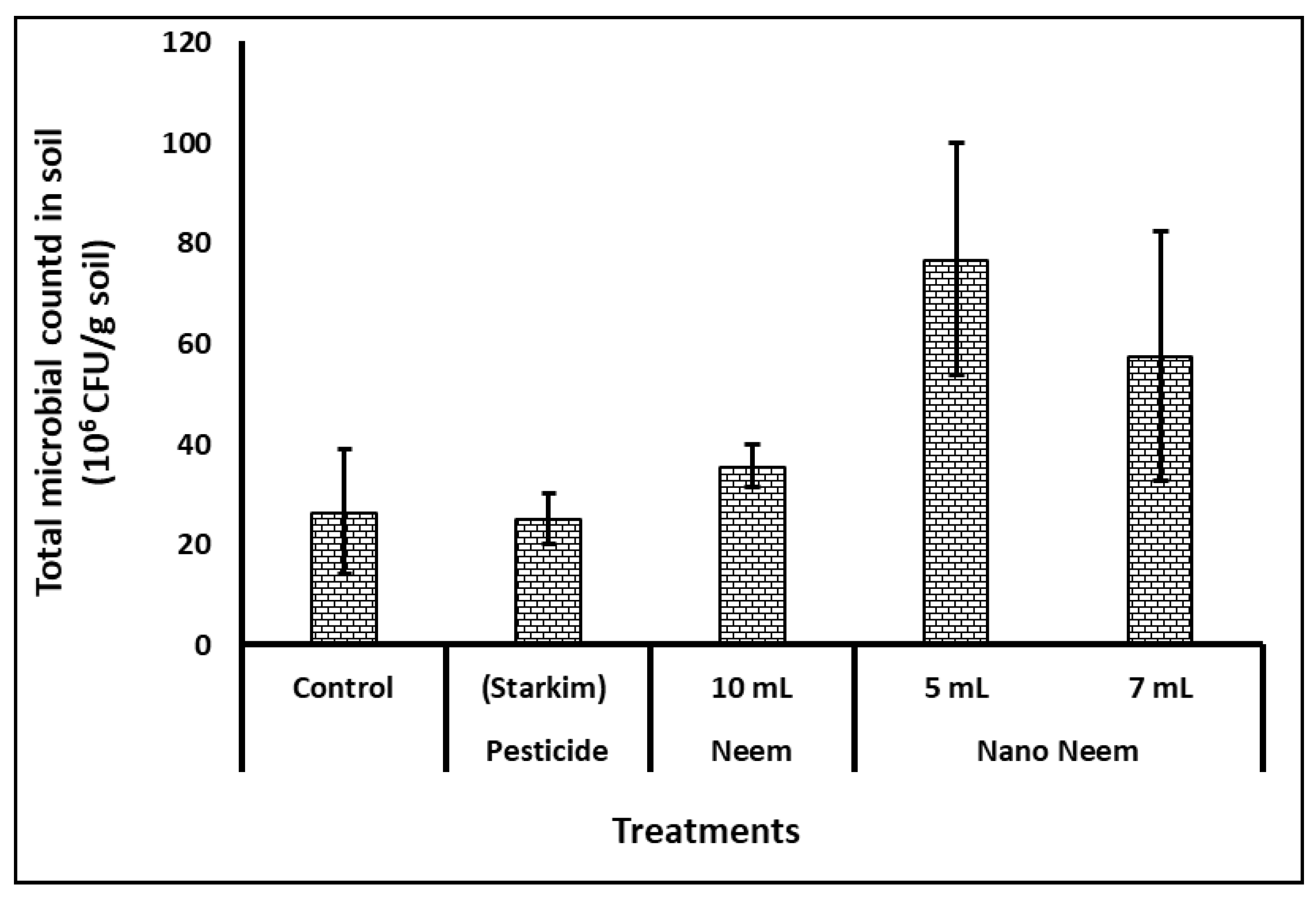
Regarding microbial counts, as presented in
Figure 1, the highest microbial populations were recognized in the rhizosphere of plants treated with five- and seven-mL of nano-emulsion of neem oil. While the microflora in Fig seedling’s (
Ficus carica Cv Black mission) soil that treated with the commercial pesticide
Fosthiazate (Krenkel 75%EC as well as that treated with 10 mL of neem oil were significantly lower than those of nano-emulsion of neem oil treated plants. However, the microbial populations of both 10-mL neem and commercial pesticide were close to the control treatment.
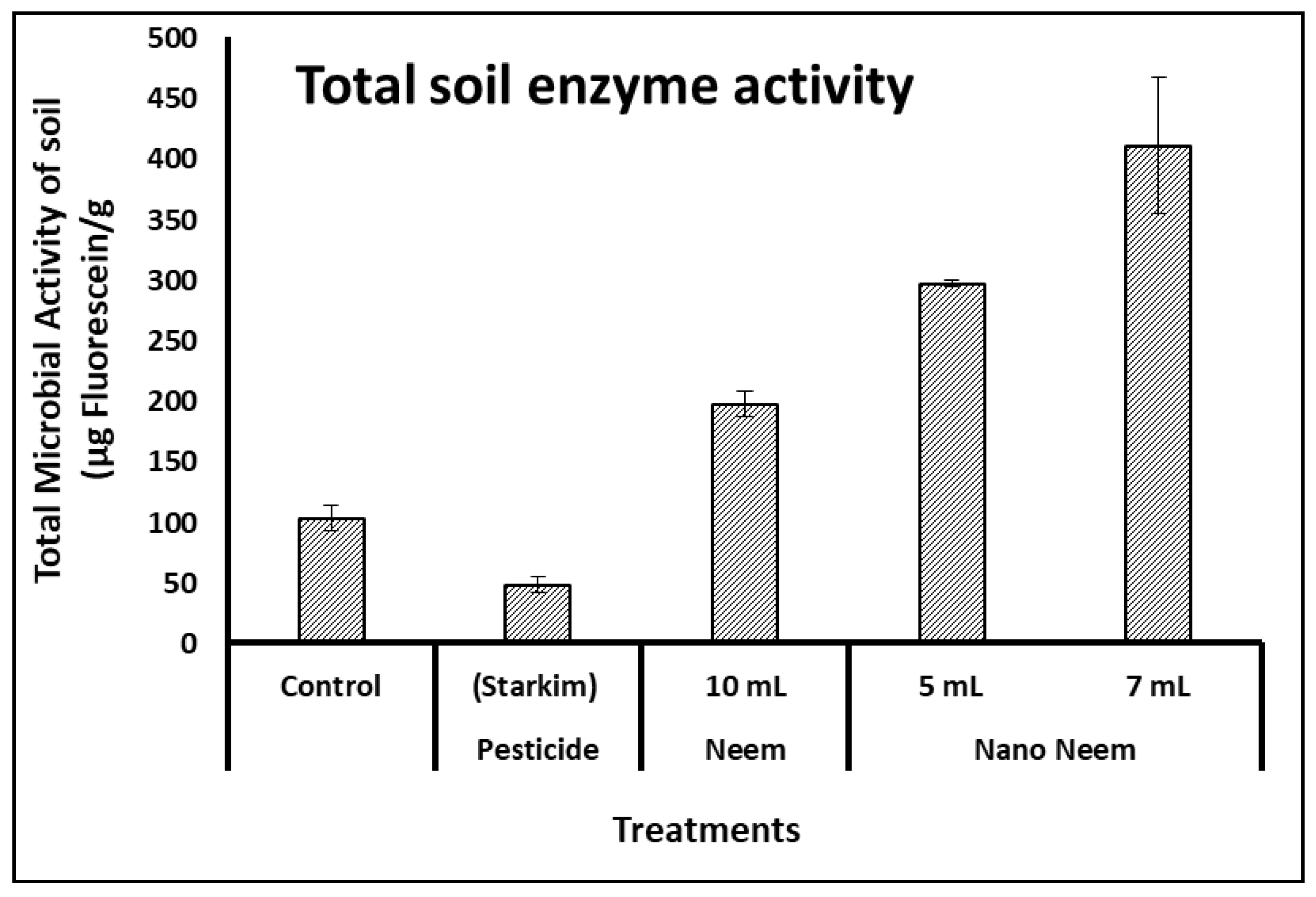
On the other hands, the enzymes produced by microbial populations in soil (such as proteases, lipases, and esterases) are capable of cleavage the colorless fluorescein diacetate into fluorescein (with a measurable fluorescent color). According to data illustrated in
Figure 2, the total microbial enzyme activity of soil measured as FDA activity revealed that the 7-ml and 5-ml neem oil nano-emulsion treatments were significantly higher than that of 10-mL neem and commercial pesticide as well as control.
4. Discussion
4.1. Impact of neem oil & nano-emulsion on Vegetative growth of infected fig seedlings
Table 1 data highlights the positive impact of various treatments on vegetative parameters in infected fig seedlings. Neem oil treatments, particularly nano-emulsion, show the most promising results, with significant increases in fresh and dry leaf weights. Nano-emulsion at 7 ml/l also enhances water content, indicating improved nutrient uptake. Additionally, all treatments boost chlorophyll content in leaves, with no notable differences among treatments, particularly with nematicide and nano-emulsion.
These results were supported with obtained results by Javed
et al., [
8], who reported that application of neem formulations significantly controlled nematode infestation which positively reflected on growth prformance. In addition Norhidayah
et al., [
24] showed that application of neem extract at (25%) resulted in better performance for pre-harvest parameters of chili plants compared to the chemical pesticide.
From other Angle, Usharani
et al., [
25] mentioned that neem extract considered as unique natural products for development of biological agrochemical (fertilizers, soil conditioner and pesticides against various diseases) that helps in improving the quality of soil, bacterial activity that is responsible for de-nitrification, hence thereby enhancing the growth of plants and fruits [
26,
27].
4.2. Impact of neem oil & oil nano-emulsion on leaf nutrient content of infected fig seedlings
Table 2 analysis indicates that control plants (infected) receiving only water show the lowest essential mineral content, while various treatments, including nematicide, neem oil, and nano-emulsion of neem oil at different concentrations, enhance leaf mineral content. Neem oil outperforms nematicide in boosting mineral content, with nano-emulsion of neem oil at 7 ml/l yielding the highest values for macro-nutrients (N, P, K, Ca, Mg) and micro-elements (Fe, Mn, Zn, Cu). Nano-emulsion at 5 ml/l and crude neem oil also demonstrate significant improvements, followed by the nematicide treatment and control plants.
Current results were supported with several studies whereas findings of Javed et al., [
28] emphasized on potential of the use of neem as biocide to manage the nematode populations and improving growth performance of infected tomato plants. Also, results of Saroj et al., [
29] who revealed that all plant growth parameters of tomato improved while the nematode reproduction factors were suppressed significantly in case of neem leaves and chemical checks as compare to untreated inoculated check.
4.3. Impact of neem oil &oil nano-emulsion on nematode Parameters
Data from
Table 3 demonstrates that all treatments led to a decrease in
Meloidogyne incognita populations compared to the control, with reduction percentages increasing as the number of treatment repeats increased. After the first month, crude neem oil at 10 ml/l showed the highest reduction (89.4%), followed by nematicide (88.7%). Neem oil nano-emulsion treatments at 5 and 7 ml/l exhibited moderate reduction percentages (65.2% and 74.5%, respectively). The reduction percentages increased with subsequent treatments; for instance, nematicide Fosthiazate (Krenkel 75%EC) increased from 88.7% to 96.1% after the third application, while crude neem oil at 10 ml/l increased from 89.4% to 90.9%. Similarly, neem oil nano-emulsion at 5 and 7 ml/l saw increases from 65.2% to 68.8% and from 74.4% to 80.35%, respectively.
The previous results strongly corresponds to the results of Joymatti et al. [
30], who reported that eggs exposed to some extracts for a longer period of time decreased in their rate. Although, nematicide (Fosthiazate (Krenkel 75%EC was recorded the highest reduction percentage in nematode population in the soil by increasing number of applications (repeat) however these results should not be the only essential factor to adopt this approach in controlling nematode population in soil. The decision maker should put in his mind effect of the treatment (nematicide) on reduction percentages in both of egg mass (the means of nematode reproduction) and number of galls on infected roots. Besides, the environmental dimension should be taken in the consideration which mean adopting any mechanism to control nematode infection should associated with impact of this treatment on soil microbial count and activity. Neem oil effect according to Gommers et al. [
31] may be due to the action of the extract releasing substances into the soil which inhibits the entry of root knot nematodes into the roots of plants.
In addition
Table 4 indicates that after the first month of application, the sole treatment resulting in a reduction in the egg mass of M. incognita was neem oil nano-emulsion at 7 ml/l, highlighting its effectiveness in decreasing the nematode's reproduction rate. With an increase in the number of treatment repetitions, significant reduction percentages in egg mass were observed across all treatments compared to the control. The highest reduction percentage in egg mass was consistently recorded for neem oil nano-emulsion at 7 ml/l.
Moreover,
Table 5 data indicates that after the first month of applying the treatments, only crude neem oil (10 ml/l) caused a reduction in the number of galls on infected roots. However, after three months of repeated applications, the reduction percentages for nematicide Fosthiazate (Krenkel 75%EC), crude neem oil (10 ml/l), neem oil nano-emulsion (5 ml/l), and neem oil nano-emulsion (7 ml/l) were very close (around 64%), suggesting no significant preference among the tested treatments in reducing galls on infected roots.
Obtained results in
Table 3,
Table 4 and
Table 5 are supported with results of several studies, Gommers et al. [
31] mentioned that neem oil may be suppressed nematodes penetration for roots by improving physical properties of root barriers and improving active post penetration bio-chemical defense which work on decreasing galls formation on infected roots which explain the considerable reduction in galls resulted by neem application that close to Fosthiazate (Krenkel 75%EC) reduction value. Also, Javed et al., [
8] showed that nematicidal metabolites of neem were absorbed by the root and they were able to disrupt the development and fecundity of nematodes that had already invaded the roots. Also, Yasmin et al., [
32] reported that neem treatments was caused significant reduction in population of adult females and number of egg-mass in soil of infected sweet gourd and that may be to the affectivity of toxicity of neem extract on nematodes or to affectivity of neem extract in suppressing the eggs to development. Study of Sankaram et al. [
33] indicated that neem synthesize more metabolic substances like azadirachtin and other closely related metabolites-vepaol, isovepaol and nimibidin which have been stated to be antifeedant and growth inhibitor of insects . Such synthesized metabolites in mature seeds of neem accumulate in more concentrate from and are likely to be more lethal to the plant pathogen including nematodes allowing better plant growth.
4.4. Soil microbial activity
Rhizosphere bacteria in agricultural soils play crucial roles in facilitating nutrient uptake, producing plant growth stimulants, bioremediating toxic chemicals, and managing diseases. Total bacterial counts and enzyme activity serve as important indicators of soil quality, reflecting microbial population activity and providing indirect insights into soil nutrition and fertility. Microbial counts, depicted in
Figure 1, show the highest populations in the rhizosphere of plants treated with five- and seven-milliliter nano-emulsion of neem oil. Conversely, the microflora in soil treated with the commercial pesticide Fosthiazate (Krenkel 75%EC) and 10 milliliters of neem oil were significantly lower than those treated with nano-emulsion of neem oil. However, the microbial populations of both 10-milliliter neem oil and commercial pesticide treatments were similar to the control. Meanwhile, microbial enzyme activity measured as FDA activity (
Figure 2) indicated that the 7- and 5-milliliter neem oil nano-emulsion treatments exhibited significantly higher enzyme activity compared to the 10-milliliter neem oil, commercial pesticide, and control treatments.
The effect of soil applications on microbial counts and activity can be attributed to the direct use of nutrients that reach the soil as reported by Luziatelli et al., [
34]. In addition, plant nutrition and health affects the microbial activity of the soil indirectly by stimulating root exudates that contain microbial growth stimulants. These results demonstrate the positive effect of neem oil nano-emulsion application to reduce the effect of nematode on fig plants without affecting the soil population or activity. These findings and recommendations are important in the applications of sustainable agriculture and clean environment practices that depend on the application of natural products for pest control.
5. Conclusions
Therefore, farmers could adopt neem oil nano-emulsion extract as alternative to synthetic nematicidal chemicals and eco-friendly strategy in controlling root-knot nematode on farmlands.
Author Contributions
Dr. Ashwaq M. Alnemari; Participating in Setting the plan of study, revising data and revise the manuscript, Prof. Nabil S.A. Mustafa; Setting the plan of work, monitoring conducting the expt., tabulating the data and writing the article, Prof. Mohamad F. El-Dahshouri & M. A. Hassoub; Carried out all chemical analysis and participation in writing the discussion section., Prof. Noweer E. M. A.& U. S. Elkelany; Participating in carrying out all nematode analysis and participation in writing the article., Dr. Ibrahim A. Matter; Participating in carrying out all Microbial analysis and participation in writing the article., Dr. H. H. Shaarawy; producing the nano-emulsion of neem oil , Monitoring the expt.,Dr. Rasha E. Selim; Participating in carrying out all nematode analysis and participation in writing the article., Dr. Zuhair Raghda M.;Participating in monitoring analysis and participation in writing the article.
Funding
This research received no external funding.
Data Availability Statement
We suggested Data is Available.
Acknowledgments
All Authors in current work would like to express their grateful to National research Centre, Egypt for supporting this work..
Conflicts of Interest
The authors declare there is no conflicts of interest.
References
- Raza MM,and Bebber DP. Climate change and plant pathogens. Curr Opin Microbiol. 2022 Dec;70:102233. Epub 2022 Nov 1. [CrossRef]
- Atkinson HJ, Lilley CJ, Urwin PE (2012) Strategies for transgenic nematode control in developed and developing world crops. Current Opinion in Biotechnology, 2012, 23( 2) 251-256. [CrossRef]
- Khalil, M. S.; M. H. Abd El-Aziz and A. M. El-khouly (2022). Optimization the Impact of Fluopyram and Abamectin against the Root-Knot Nematode (Meloidogyne incognita) on Tomato Plants by Using Trichoderma album. Egypt. J. Agronematol., 202, 21(2): 79 -90. [CrossRef]
- Mai, W. and Abawi, G. Interactions among root-knot nematodes and Fusarium wilt fungi on host plants. Annual Review of Phytopathology, 1987, 25, 317-338. In :plant parasitic nematodes. Vol.II. BM Zuckerman, W .F Mai and Rhode R. A. (eds.) Academic Press. New York. 347 pp. [CrossRef]
- Gamalero E. and B. R. Glick. The use of plant growth-promoting bacteria to prevent nematode damage to plants. Biology (Basel). 2020 Nov; 9(11): 381. [CrossRef]
- Osman HA, Ameen HH, Moawad M, Mohamedy R, Elkelany US. Field control of Meloidogyne incognita and root rot disease infecting eggplant using nematicide, fertilizers, and microbial agents. Egypt Biol Pest Control, 2018, 28:40. [CrossRef]
- Kumar C. V. Use of Neem Extracts (Azadirachta indica) in Control of Plant Diseases: A Review. Int. J. Curr. Microbiol .App. Sci. (2020) Special Issue-11: 3481-3487. (http://www.ijcmas.com).
- Javed N., S.R. Gowen; M.Inam-ul-Haq and S.A.Anwar. (2007). Protective and curative effect of neem (Azadirachta indica) formulations on the development of root-knot nematode Meloidogyne javanica in roots of tomato plants. Crop Protection,2007, Vol. 26(4) Pages 530-534. [CrossRef]
- Mossa, A.T.H., Mohamed, R.I. and Mohafrash, S.M.. Development of a ‘green’nanoformulation of neem oil-based nano-emulsion for controlling mosquitoes in the sustainable ecosystem. Biocatalysis and Agricultural Biotechnology, 2022, 46, p.102541.
- Darwesh, O.M., Ali, S.S., Matter, I.A. and Elsamahy, T., (2021). Nanotextiles waste management: Controlling of release and remediation of wastes. In Nanosensors and Nanodevices for Smart Multifunctional Textiles, Micro and Nano Technologies. 2021, pp. 267-286. [CrossRef]
- Ali, E.O.M., Shakil, N.A., Rana, V.S., Sarkar, D.J., Majumder, S., Kaushik, P., Singh, B.B. and Kumar, J. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii. Industrial crops and products, 2017,108, pp.379-387. [CrossRef]
- Ghotbi,S.R., Khatibzadeh,M. and Kordbacheh,S. Preparation of Neem Seed Oil Nanoemulsion. Proceedings of the 5 th International Conference on Nanotechnology: Fundamentals and Applications Prague, Czech Republic, August, 2014, 11-13, 2014 Paper No. 150.
- Khan, A., Tariq, M., Ahmad, F., Mennan, S., Khan, F., Asif, M.,Nadeem, H., Ansari, T., Shariq, M., & Siddiqui, M. A. (2021).Assessment of nematicidal efficacy of chitosan in combinationwith botanicals against Meloidogyne incognita on carrot. ActaAgriculturae Scandinavica, Section B — Soil and Plant Sci., 2021,71, 225–236. [CrossRef]
- Chapman, H.D. and P.F. Pratt. Methods of analysis for soils, plants and waters. Univ. of California, Dept. of Agric. Sci., Priced publication, 1978, pp: 4034.
- Jackson, M.L. (1973). Soil Chemical Analysis. Prentice-Hall, Inc. India.
- Lindsay, W.L., Norvell, W.A. Development of a DTPA micronutrient soil tests for Zinc, iron, manganese and copper. Soil Sci. Amer. 1978, J., 42: 421-428.
- Hussey, R. S. and Barker, K. R. A comparison of methods of collecting inocula on Meloidogyne spp., including a new technique. Plant Dis. Rept.1973, 57(12): 1025-1028.
- Ayoub, S. M. Plant nematology, an agricultural training aid. Secramanto, California, USA, Nema aid Publications, 1980, P. 195.
- Taylor, D. P. and Nelscher, C. An improved technique for preparing perineal patterns of Meloidogyne spp.. Nematol.,1974, 20: 268-269. [CrossRef]
- Puntener, W. Manual for field trials in plant protection. Agricultural Division, Ciba Geigy Limited, Basle, Switzerland, 1981; 205 pp.
- Patle, P.N., Navnage, N.P. and Barange, P.K., (2018). Fluorescein diacetate (FDA): measure of total microbial activity and as indicator of soil quality. Int. J. Curr. Microbiol. Appl. Sci, 7, pp.2103-2107. [CrossRef]
- Snedecor, G.W. and W.G. Cochran (1989). Statistical methods. (8th edn) Ames: Iowa State University Press, pp: 503.
- Wareing, P.. Growth and its co-ordination in trees, in: Luckwill, L.C. and Cutting, C.V. (Eds.), Physiology of Tree Crops, Long Ashton Research Station, University of Bristol, Academic Press, N.Y.,1970, pp. 1-21.
- Norhidayah C.S., N. S. Md Yusoff, S. Lob, N. f. Ibrahim, N. Che Soh and Johar Mohamed. Effect of Neem Extract on Growth Performance and Post-harvest Quality of Chili. Asian Journal of Plant Sci., 2021, 20(1):80-85. [CrossRef]
- Usharani KV, Dhananjay Naik and Manjunatha RL.Neem as an organic plant protectant in agriculture. Journal of Pharmacognosy and Phytochemistry 2019; 8(3): 4176-4184.
- Smith MAK, Tolorun TP, Adeniji OS. (2001). Effect of combined mulch and fertilizer on weed growth and okra (Abelmoshus esculentus L. Moench) yield in Tropical environment. In; Proceedings 35th Annual conference of the Agricultural Society of Nigeria held at University of Agriculture, Abeokuta, Nigeria Sept. 16-20, 2001, 103- 112.
- Mohanty S, Patra A, Chhonkar P.(2008). Neem (Azadirachta indica) seed kernel powder retards urease and nitrification activities in different soils at contrasting moisture and temperature regimes. Bioresearch Technology. 99:894-899. [CrossRef]
- Javed N., S.A. Anwar, S. Fayyaz, M. Khan. Effects of Neem formulations applied as soil drenching on the development of Meloidogyne javanica root-knot nematode on roots of tomato. Pak. J. Bot., 2008, (4092):905-910.
- Saroj Yadav, Jaydeep Patil, and Anil Kumar, (2018). Bio-nematicidal effect of Azadirachta indica, against Meloidogyne incognita in tomato. Int., J. Chem. Studies,2018, 6(3): 2757-2761.
- Joymatti, L., Dhanachand, C. and Devi, L.S. Effect of plant extracts on M. incognita. Indian J.Nematology 1998, 28:225-230.
- Gommers, F., Barker, J. and Wynberg, H., (1982). Dithiophenes as singlet oxygen sensitizers. Photoche. and photobio., 1982, 35, 615-619. [CrossRef]
- Yasmin L., M.H. Rashid, M. Nazim Uddin, M.S. Hossain, M.E. Hossain and M.U. Ahmed. Use of Neem Extract in Controlling Root-knot Nematode (Meloidogyne javanica) of Sweet-gourd. Plant Pathology J.,2003, vol 2(3)161-168.
- Sankaram, A.B., Reddy, V.V.N., Marthandamurthi, M. 13C NMR spectra of some naturally occurring binaphthoquinones and related compounds Phytochemistry 1986, 25(12) Pages 2867-2871.
- Luziatelli, F., Ficca, A.G., Colla, G., Baldassarre Švecová, E. and Ruzzi, M. Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Frontiers in plant sci.,2019, 10, p.60. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).