Submitted:
18 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Advances of p-Toluenesulfonic Acid Promoted Reactions in Organic and Polymer Chemistry
2.1. Application of p-Toluenesulfonic Acid as a Catalyst
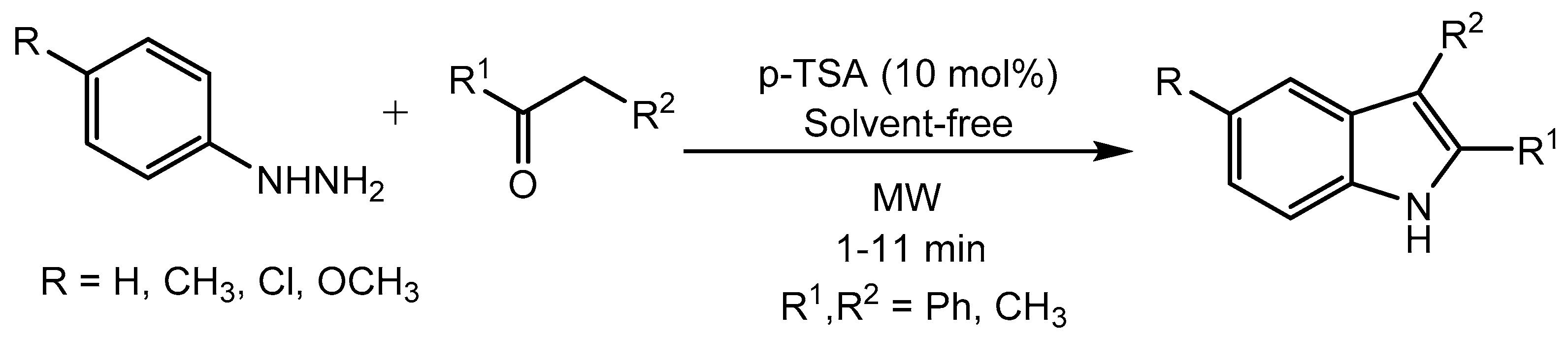
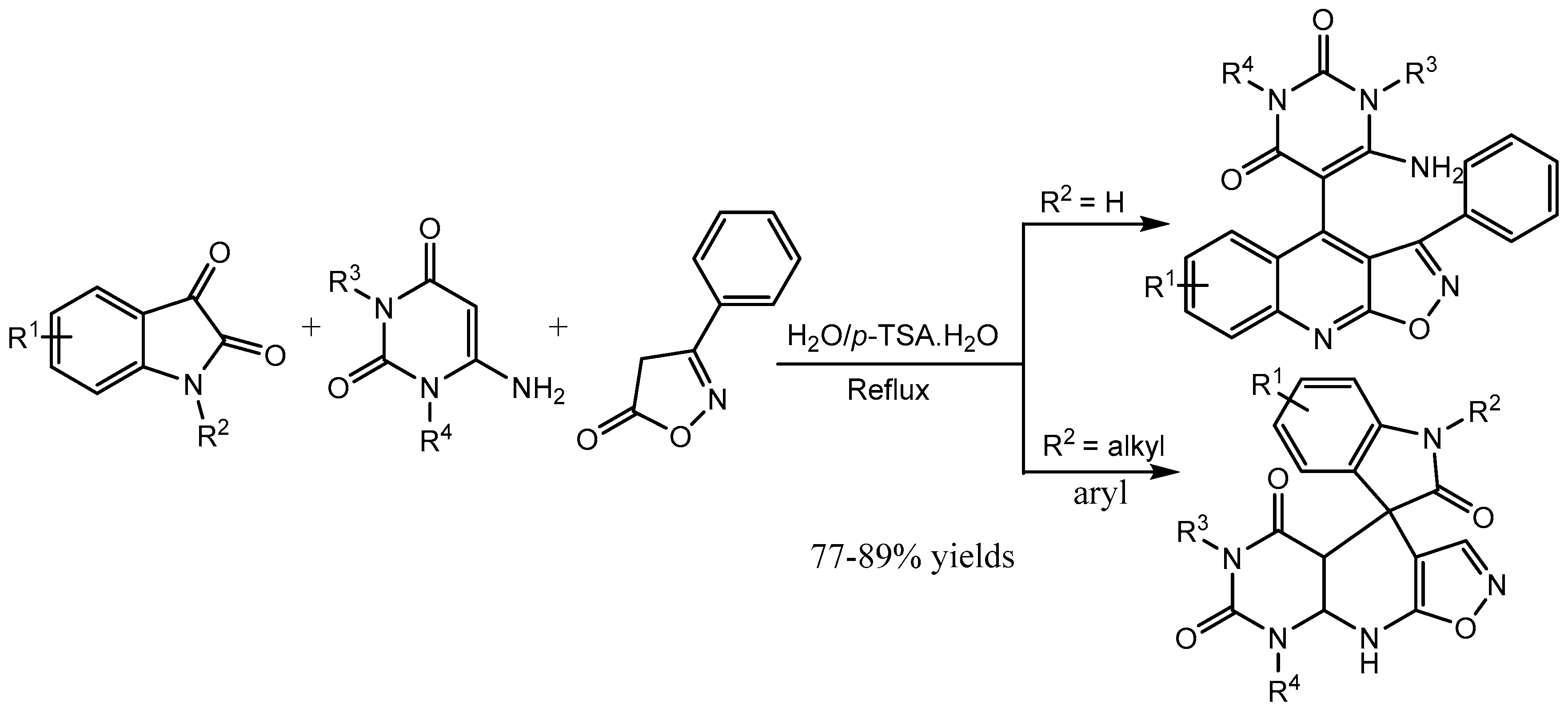
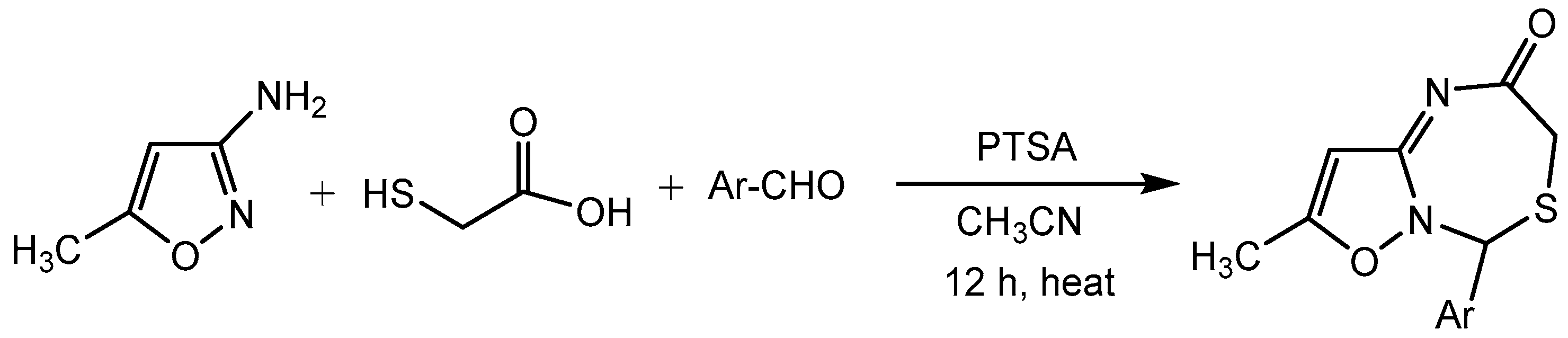
2.2. Application of p-Toluenesulfonic Acid as a Reagent
2.3. Application of p-Toluenesulfonic Acid in Combination with Metal Salts and Other Reagents
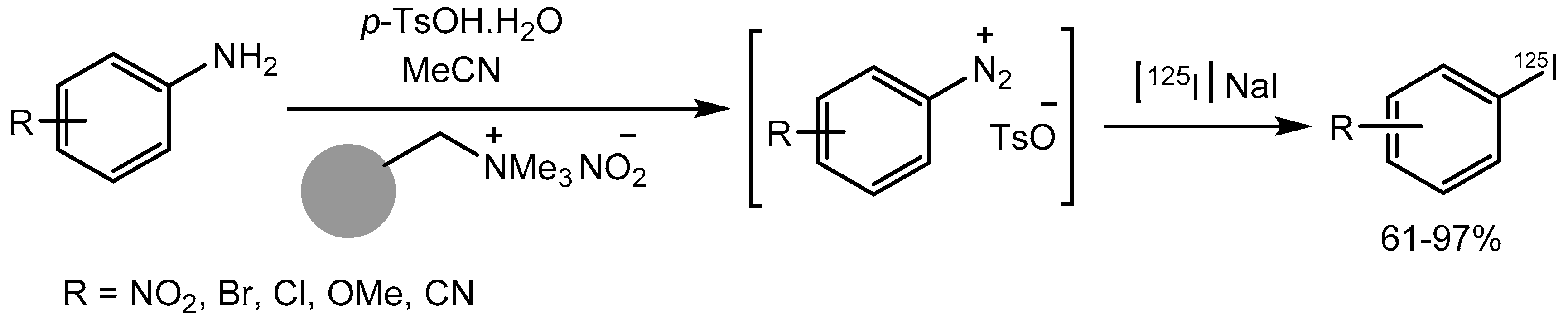
2.4. Application of p-Toluenesulfonic Acid in Ionic Liquids
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baghernejad, B. Application of p-toluenesulfonic Acid (PTSA) in Organic Synthesis. Current Organic Chemistry 2011, 15, 3091–3097. [Google Scholar] [CrossRef]
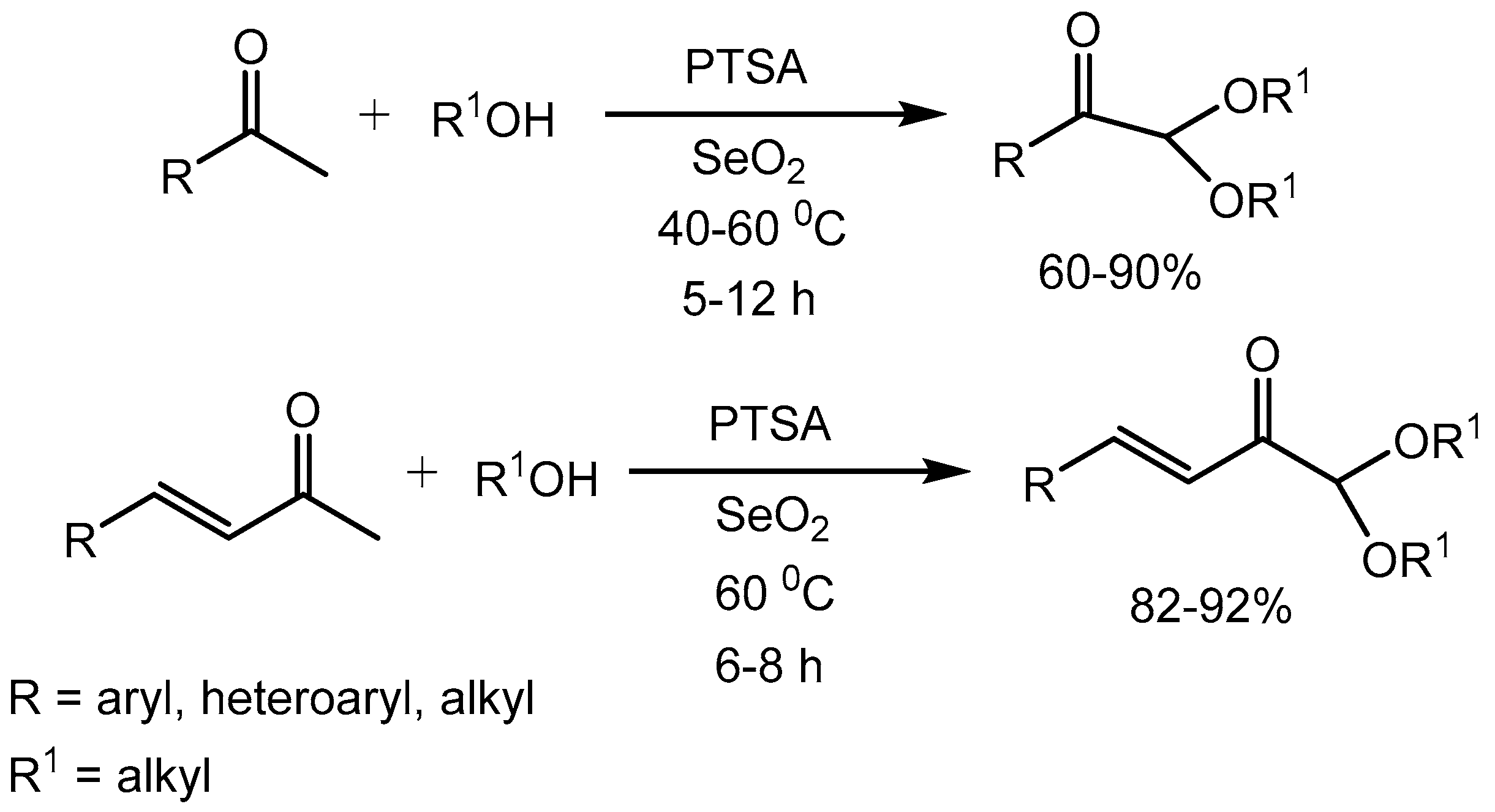
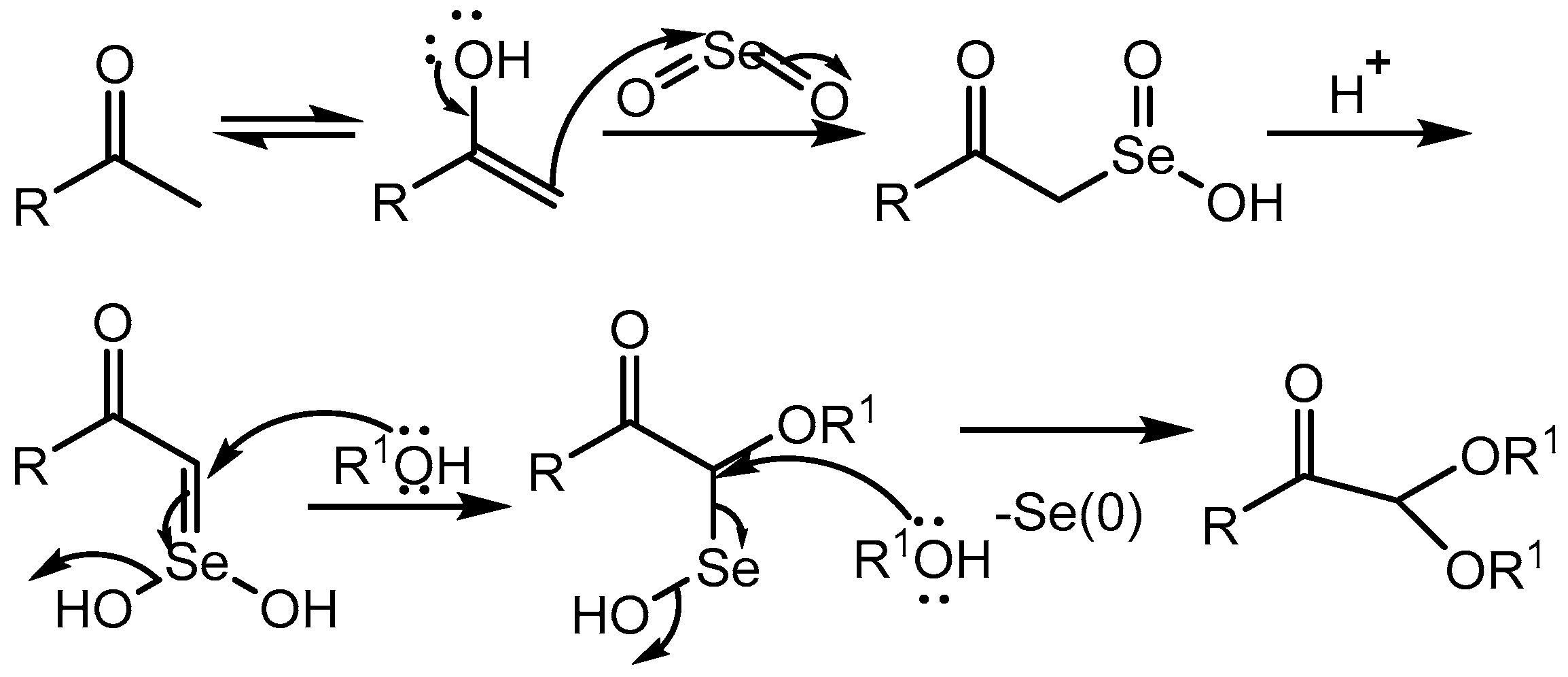
- Shangpliang, O.R.; Wanniang, K.; Kshiar, B.; Marpna, I.D.; Lipon, T.M.; Mizar, P.; Myrboh, B. PTSA-catalyzed reaction of alkyl/aryl methyl ketones with aliphatic alcohols in the presence of selenium dioxide: A protocol for the generation of an α-ketoacetals library. ACS omega 2019, 4, 6035–6043. [Google Scholar] [CrossRef] [PubMed]
- Kharkongor, I.; Myrboh, B. One-pot synthesis of α-ketoacetals from aryl methyl ketones in the presence of selenous acid catalyzed by boron trifluoride etherate. Tetrahedron letters 2015, 56, 4359–4362. [Google Scholar] [CrossRef]
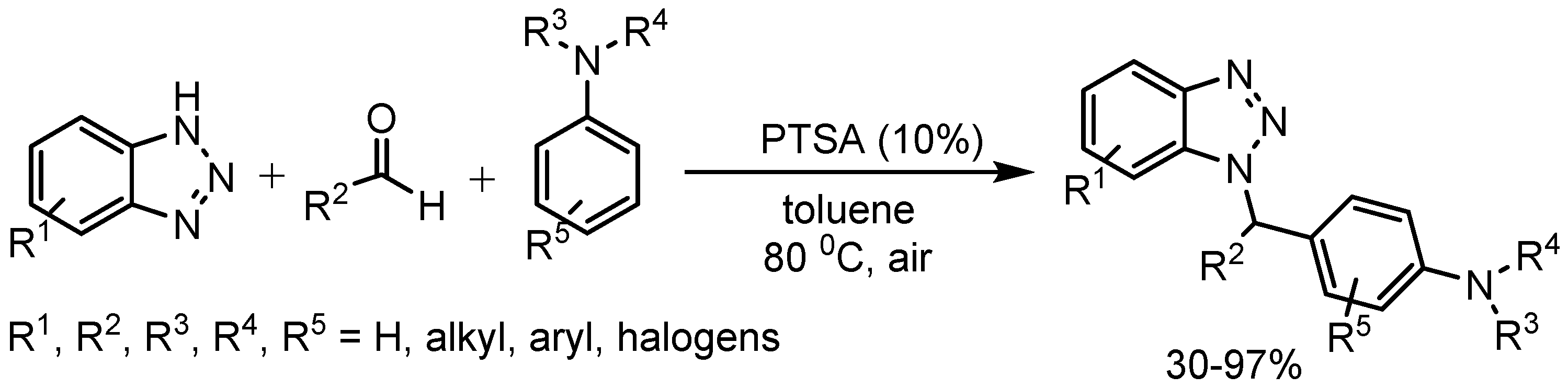
- Wang, X.; Jia, C.; Feng, Y.; Wang, L.; Cui, X. One-Pot Synthesis of N-Alkyl Benzotriazoles via a Brønsted Acid-Catalyzed Three-Component Reaction. Adv. Synth. Catal. 2018, 360, 374–378. [Google Scholar] [CrossRef]
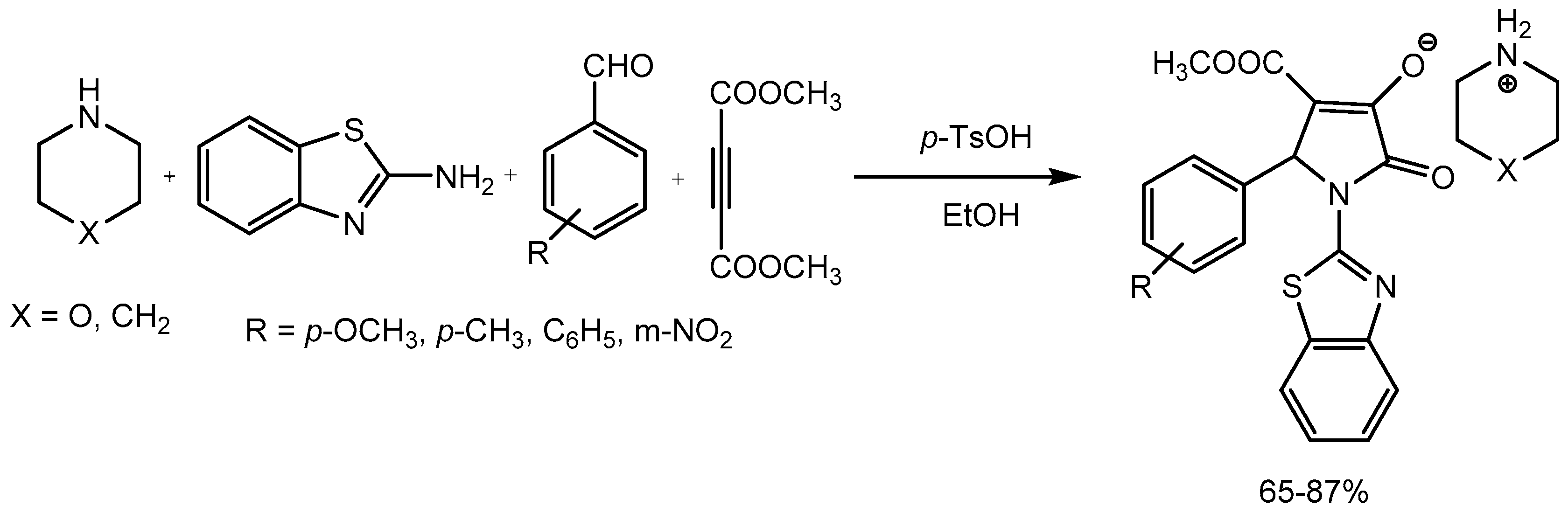
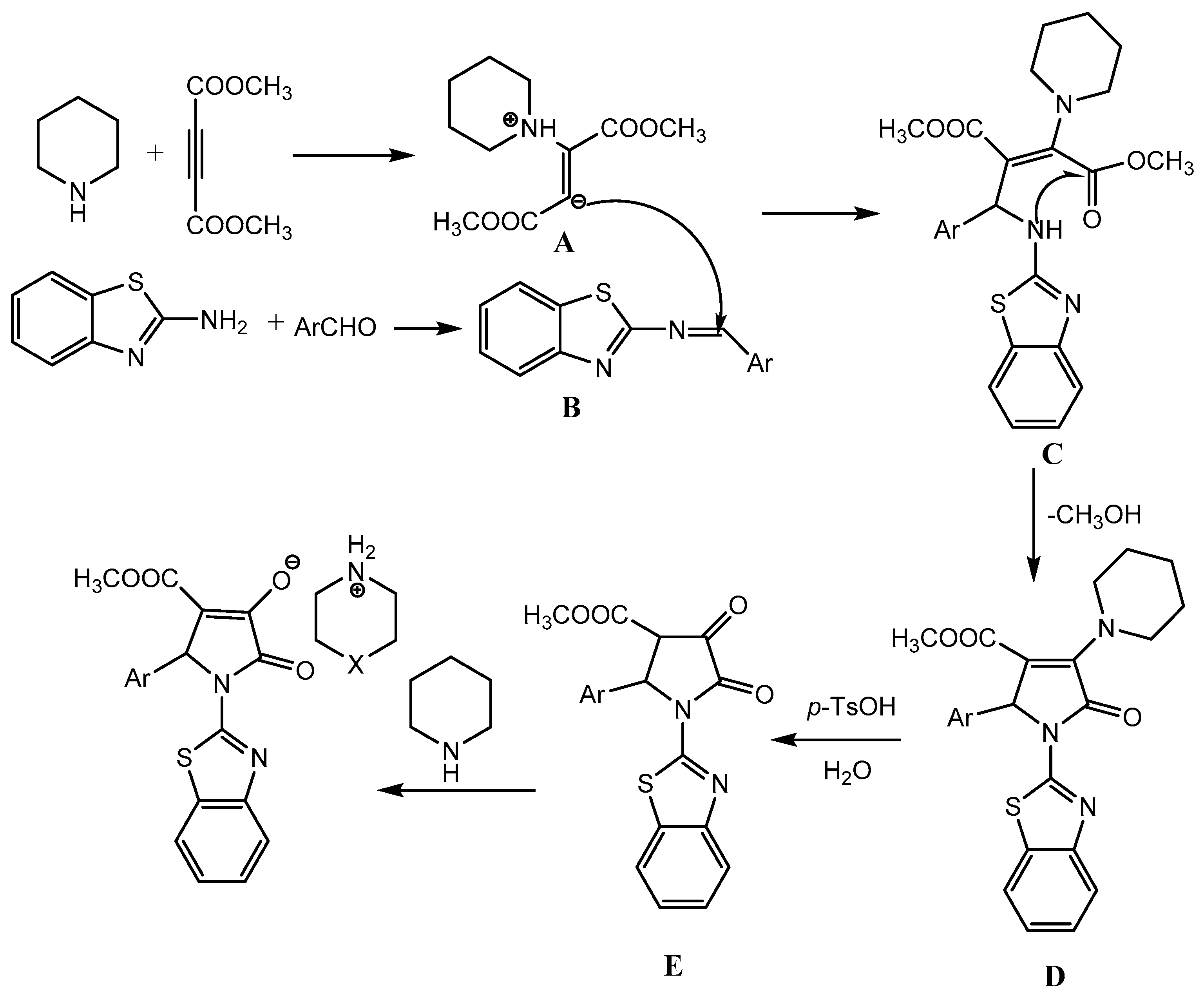
- Gao, H.; Sun, J.; Yan, C.-G. Four-component reaction of cyclic amines, 2-aminobenzothiazole, aromatic aldehydes and acetylenedicarboxylate. Beilstein J. Org. Chem. 2013, 9, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
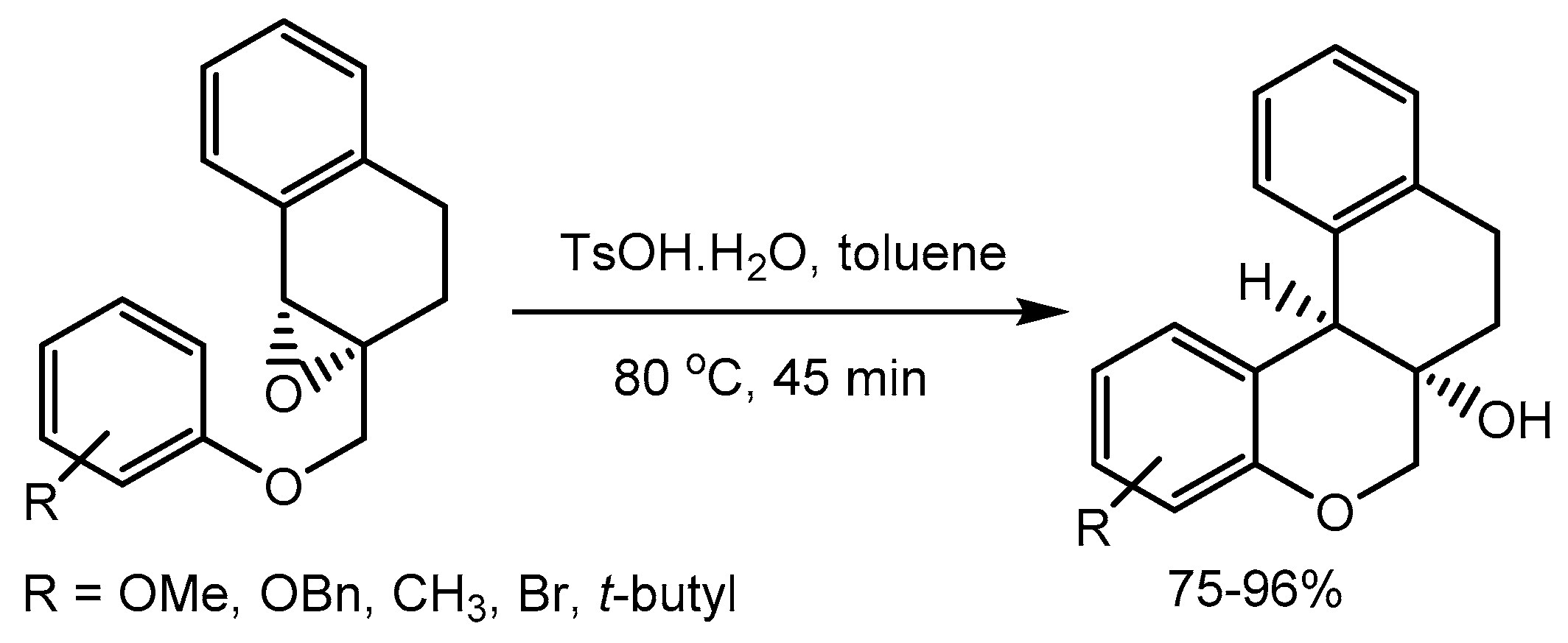
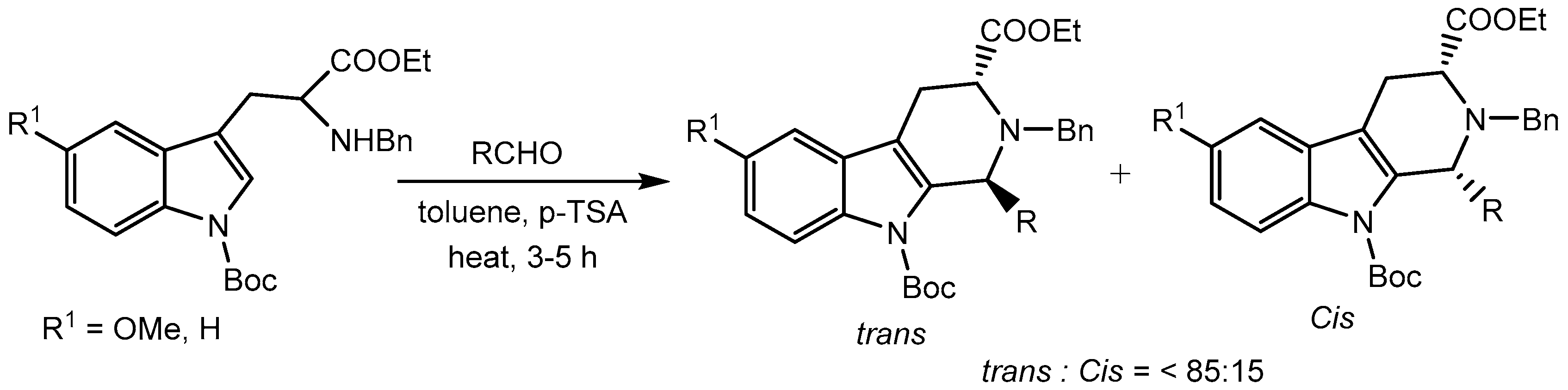
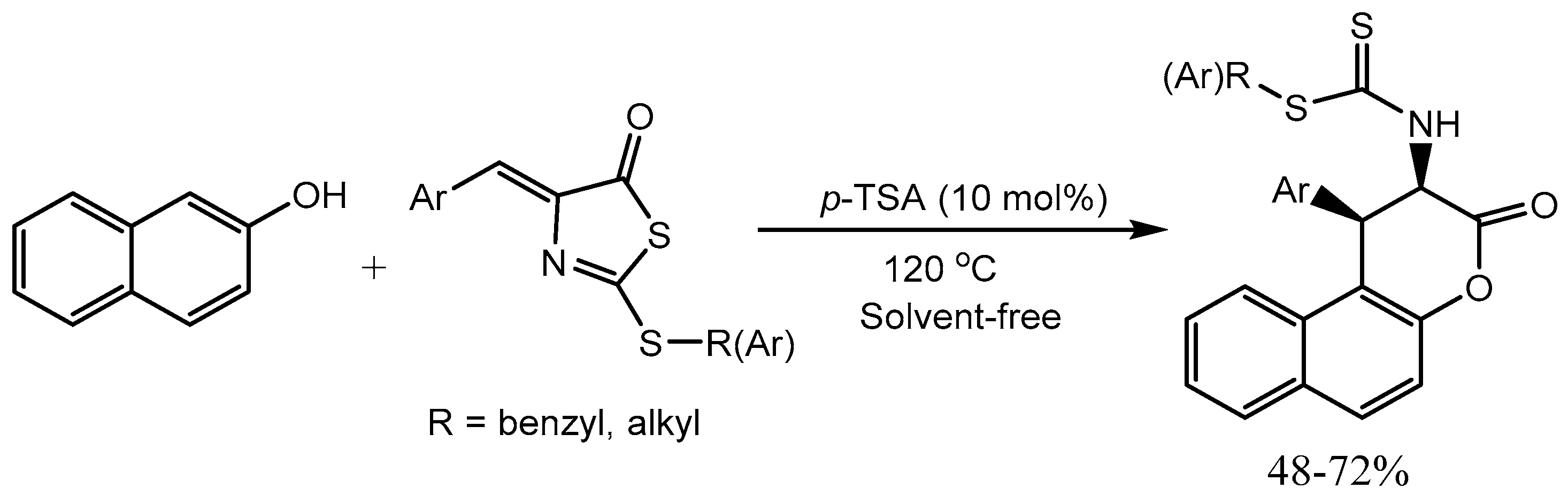
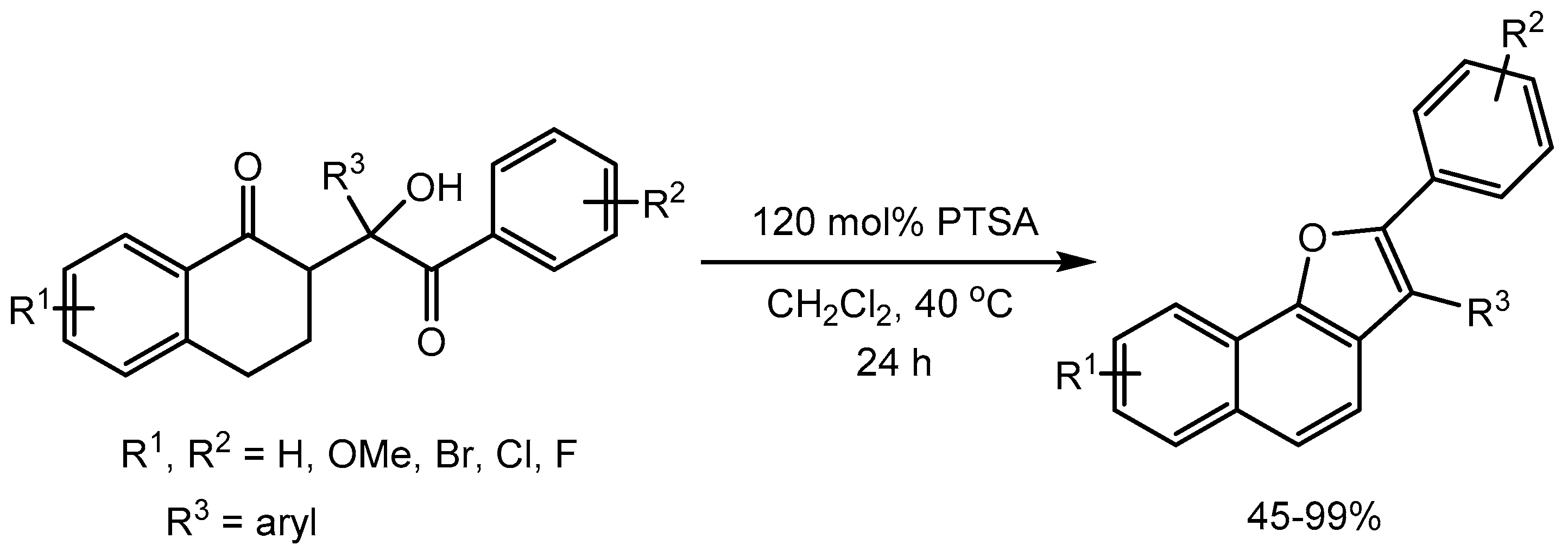
- Gogoi, D.; Devi, R.; Pahari, P.; Sarma, B.; Das, S.K. cis-Diastereoselective synthesis of chroman-fused tetralins as B-ring-modified analogues of brazilin. Beilstein J. Org. Chem. 2016, 12, 2816–2822. [Google Scholar] [CrossRef] [PubMed]
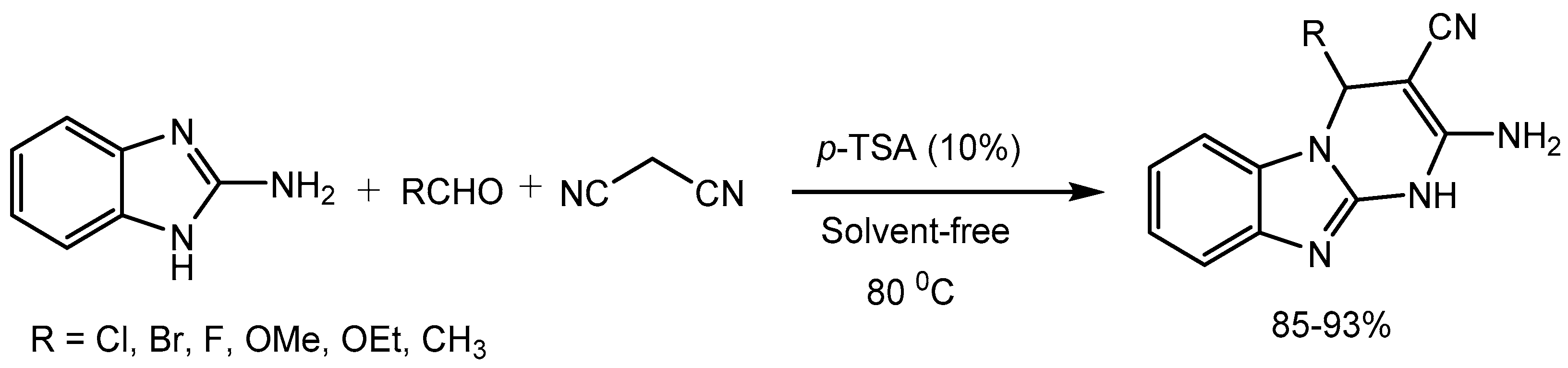
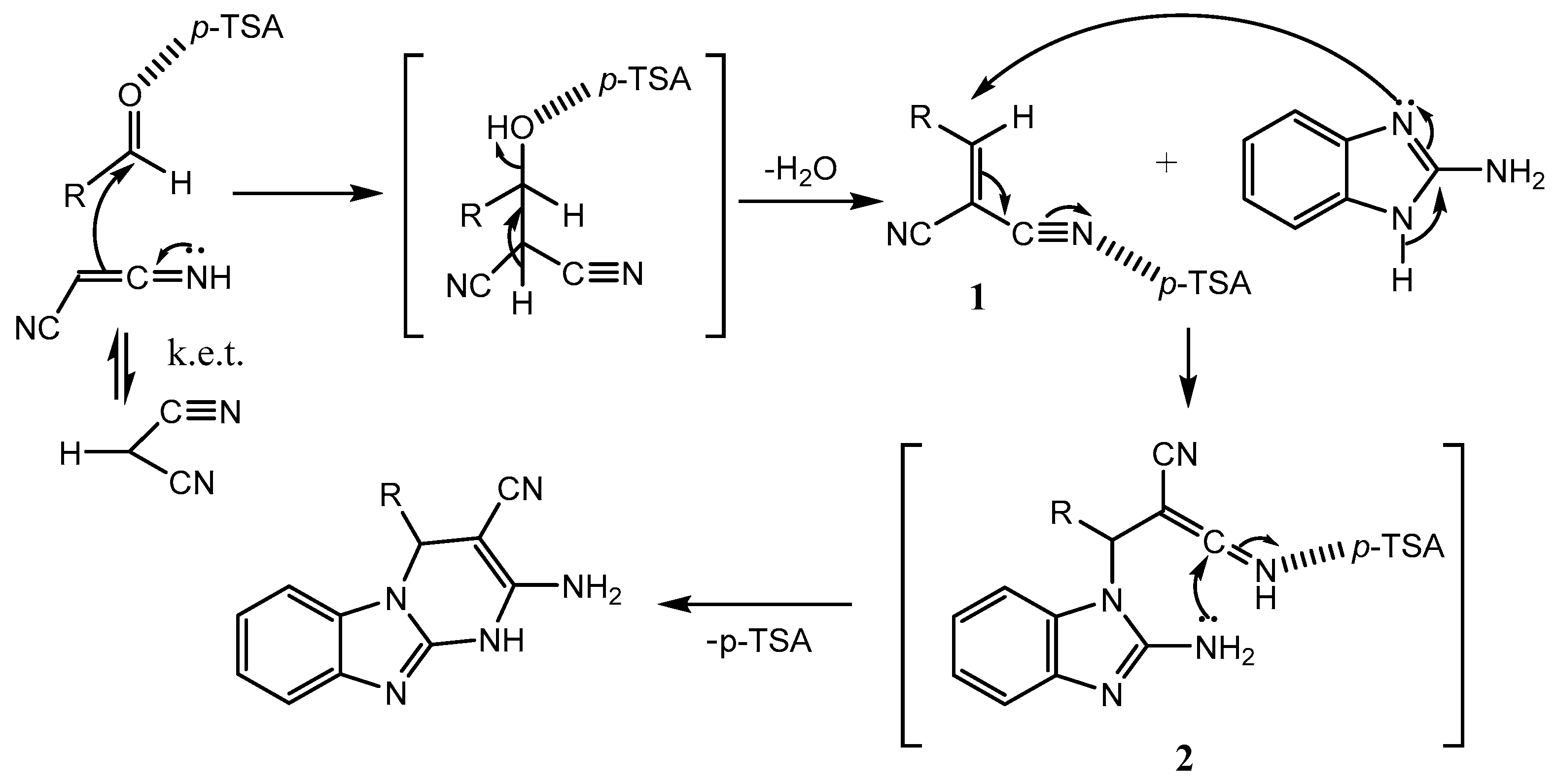
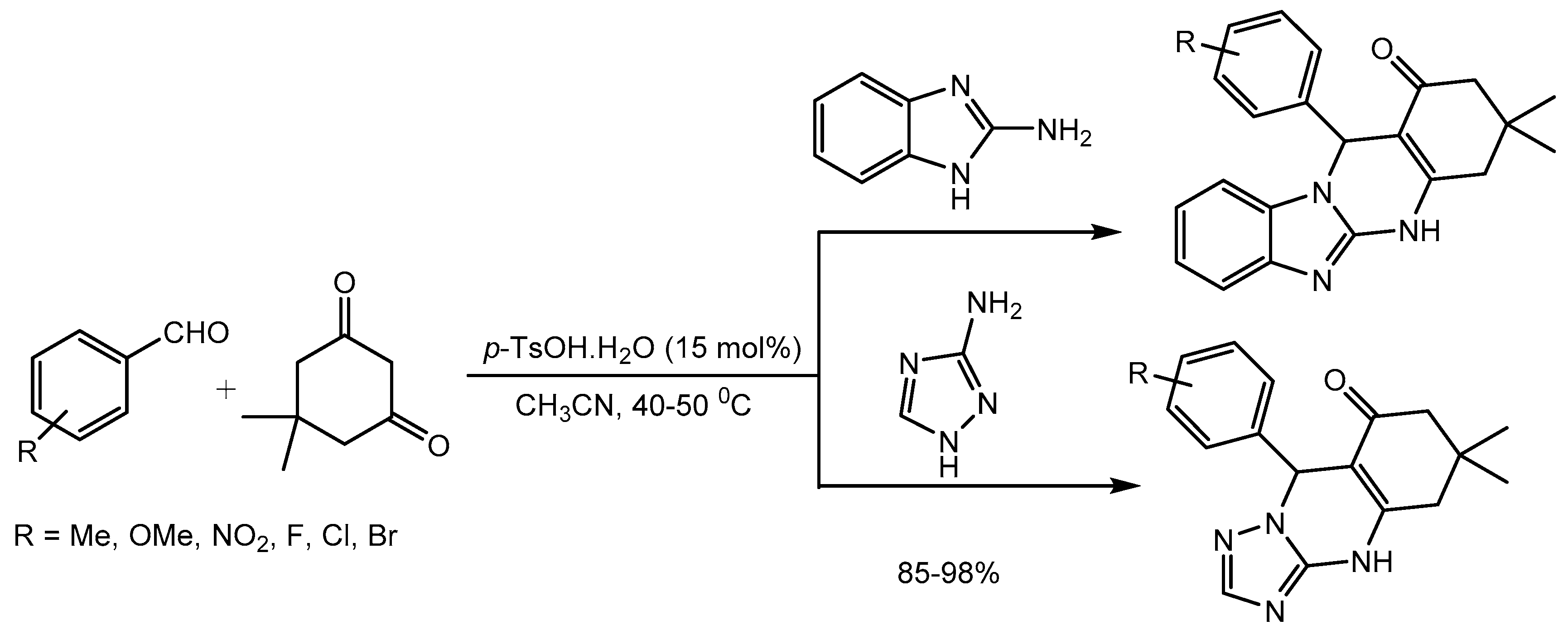
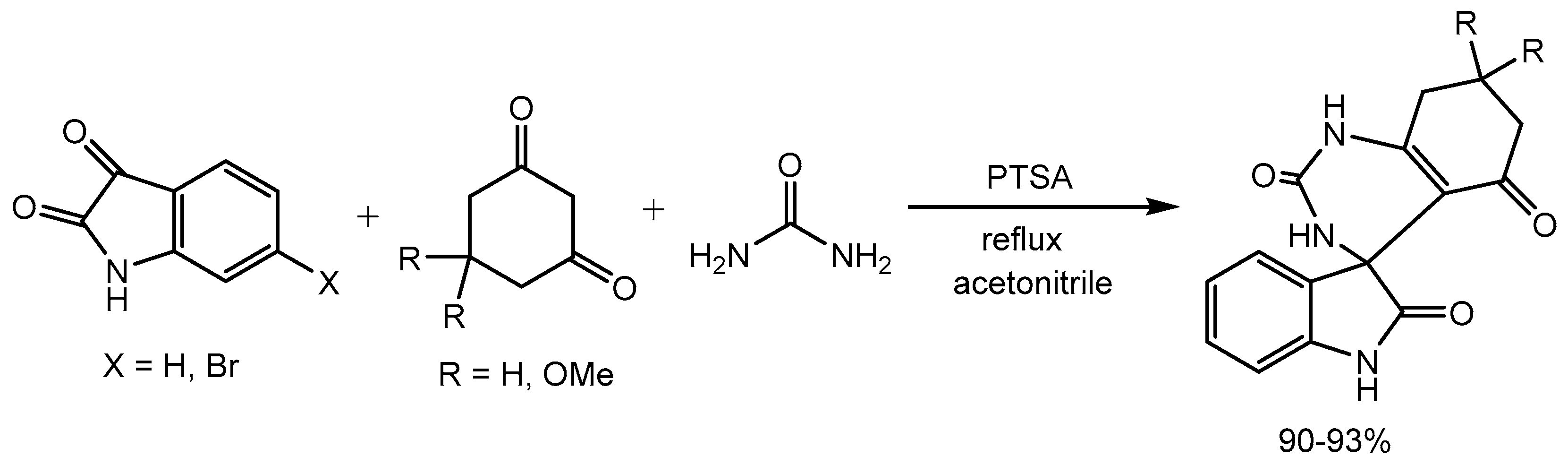
- Reddy, M.V.; Oh, J.; Jeong, Y.T. p-Toluenesulfonic acid-catalyzed one-pot synthesis of 2-amino-4-substituted-1,4-dihydrobenzo [4, 5] imidazolo [1, 2-a] pyrimidine-3-carbonitriles under neat conditions. C. R. Chimie 2014, 17, 484–489. [Google Scholar] [CrossRef]
- Nofal, Z.; Fahmy, H.; Mohamed, H. Synthesis, antimicrobial and molluscicidal activities of new benzimidazole derivatives. Arch. Pharm. Res. 2002, 25, 28–38. [Google Scholar] [CrossRef]
- Creencia, E. C.; Tsukamoto, M.; Horaguchi, T. , One-pot-one-step, microwave-assisted Fischer indole synthesis. Journal of Heterocyclic Chemistry 2011, 48, 1095–1102. [Google Scholar] [CrossRef]
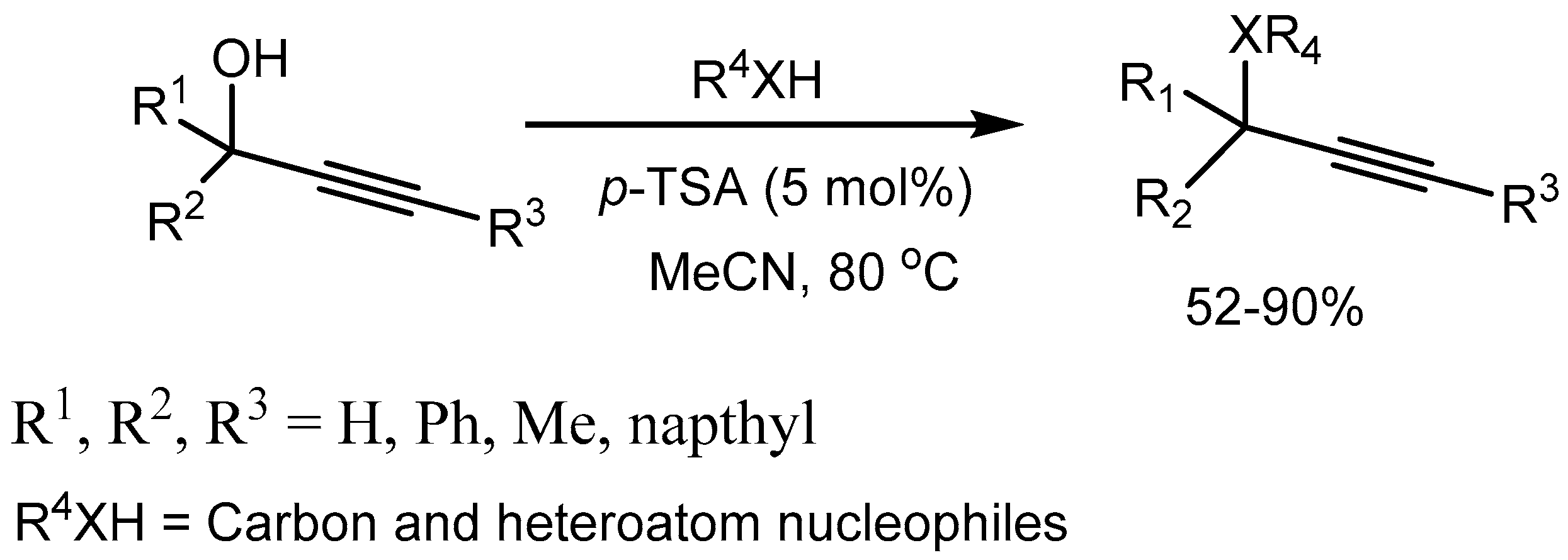
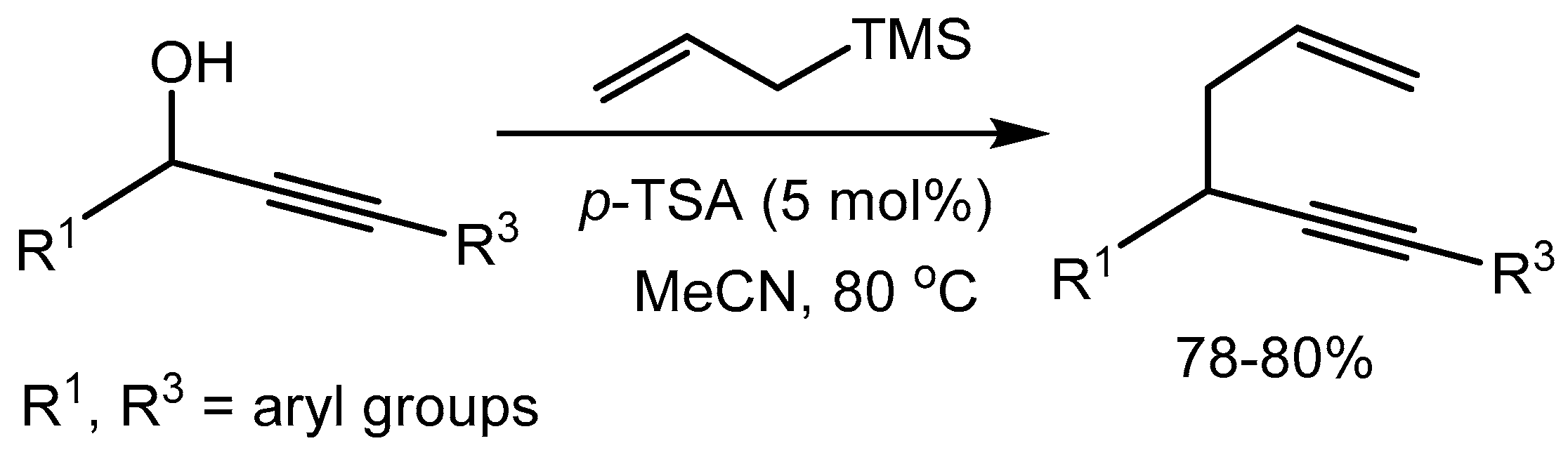
- Sanz, R.; Martínez, A.; Álvarez-Gutiérrez, J.M.; Rodríguez, F. Metal-Free Catalytic Nucleophilic Substitution of Propargylic Alcohols. Eur. J. Org. Chem. 2006, 1383–1386. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Li, C.; Yin, K.; Ye, D.; Jia, X. PTSA-catalyzed green synthesis of 1, 3, 5-triarylbenzene under solvent-free conditions. Green Chem. 2010, 12, 1370–1372. [Google Scholar] [CrossRef]
- Poomathi, N.; Mayakrishnan, S.; Muralidharan, D.; Srinivasan, R.; Perumal, P.T. Reaction of isatins with 6-amino uracils and isoxazoles: isatin ring-opening vs. annulations and regioselective synthesis of isoxazole fused quinoline scaffolds in water. Green Chem. 2015, 17, 3362–3372. [Google Scholar] [CrossRef]
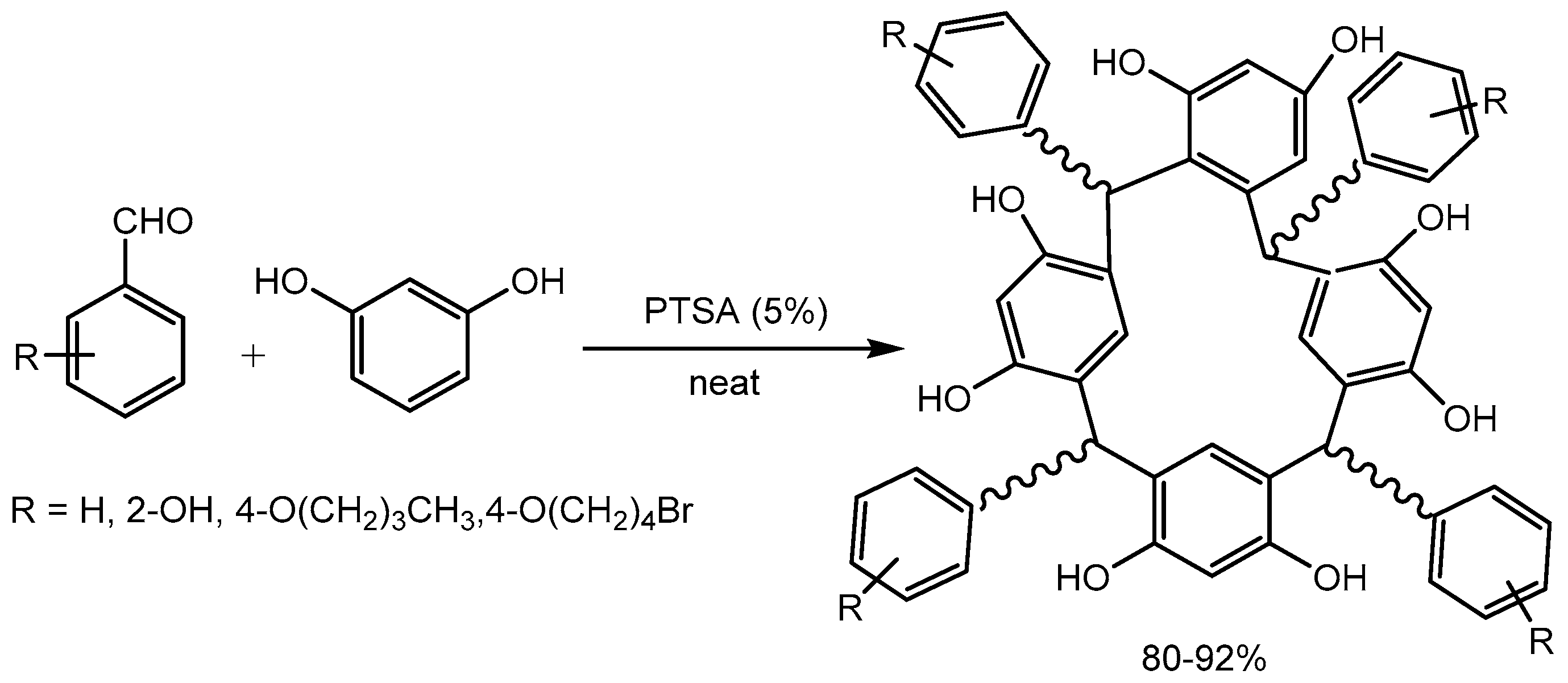
- Roberts, B.A.; Cave, G.W.; Raston, C.L.; Scott, J. L. Solvent-free synthesis of calix [4] resorcinarenes. Green Chem. 2001, 3, 280–284. [Google Scholar] [CrossRef]
- Antesberger, J.; Cave, G. W. V.; Ferrarelli, M. C.; Heaven, M. W.; Raston, C. L.; Atwood, J. L. Solvent-free, direct synthesis of supramolecular nano-capsules. Chem. Commun. 2005, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Rajanarendar, E.; Venkateshwarlu, P.; Krishna, S.R.; Reddy, K.G.; Thirupathaiah, K. One-Pot Three Component Domino Reaction for the Synthesis of Novel Isoxazolo [2, 3-c][1, 3, 5] Thiadiazepin-2-Ones Catalyzed by PTSA—A Green Chemistry Approach. Green and Sustainable Chemistry 2015, 5, 107–114. [Google Scholar] [CrossRef]
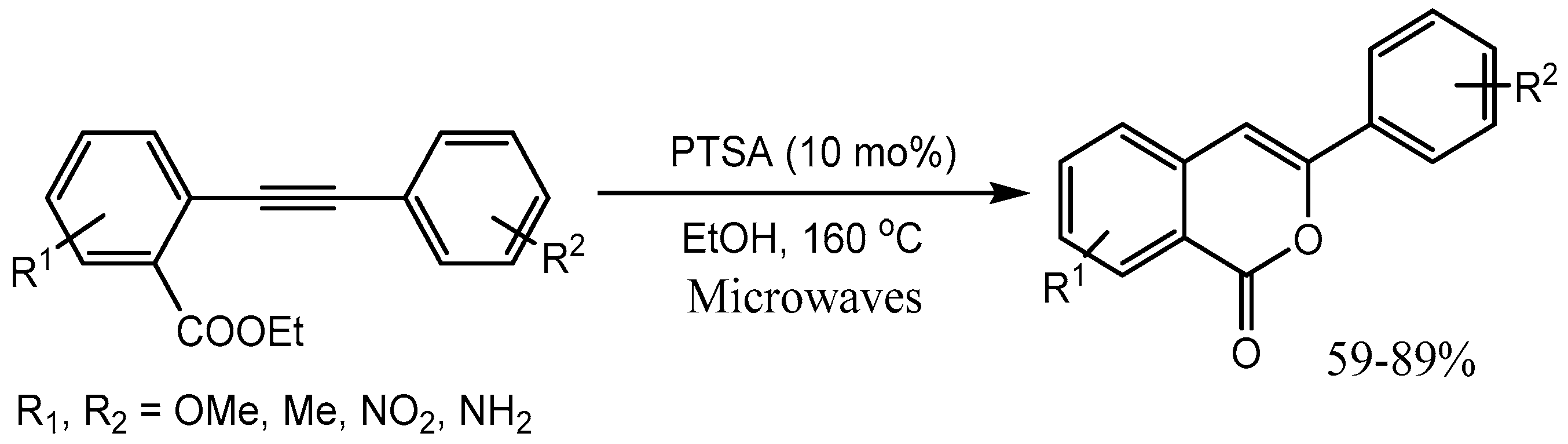
- Le Bras, G.; Hamze, A.; Messaoudi, S.; Provot, O.; Le Calvez, P.-B.; Brion, J.-D.; Alami, M. Synthesis of isocoumarin via PTSA-catalyzed annulation of diarylalkynes. Synthesis 2008, 1607–1611. [Google Scholar]
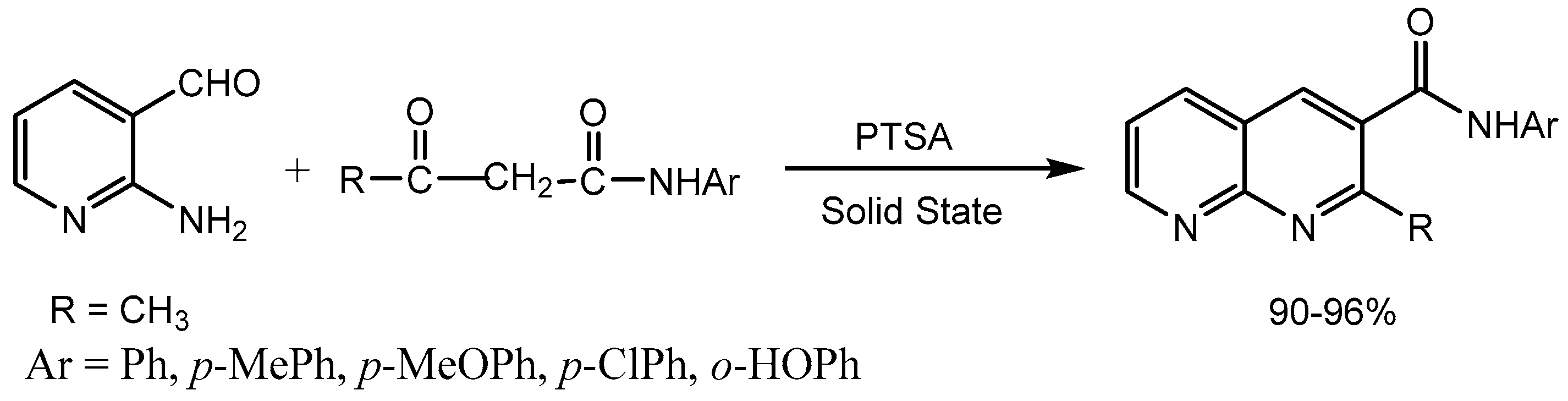
- Mogilaiah, K.; Sudhakar, G. R. PTSA-catalyzed Friedlander condensation in the solid state. Ind. J. Chem. 2003, 42B, 1170–1171. [Google Scholar]
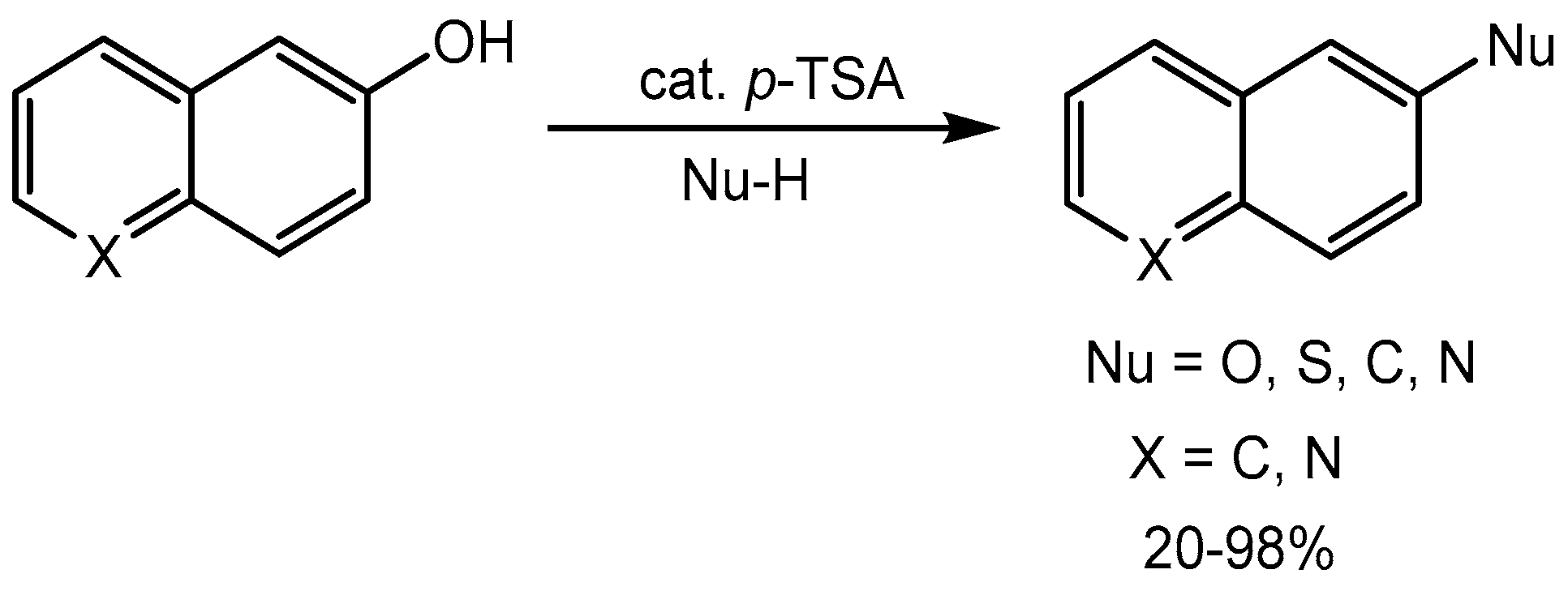
- Mishra, A.K.; Biswas, S. Brønsted acid catalyzed functionalization of aromatic alcohols through nucleophilic substitution of hydroxyl group. J. Org. Chem. 2016, 81, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
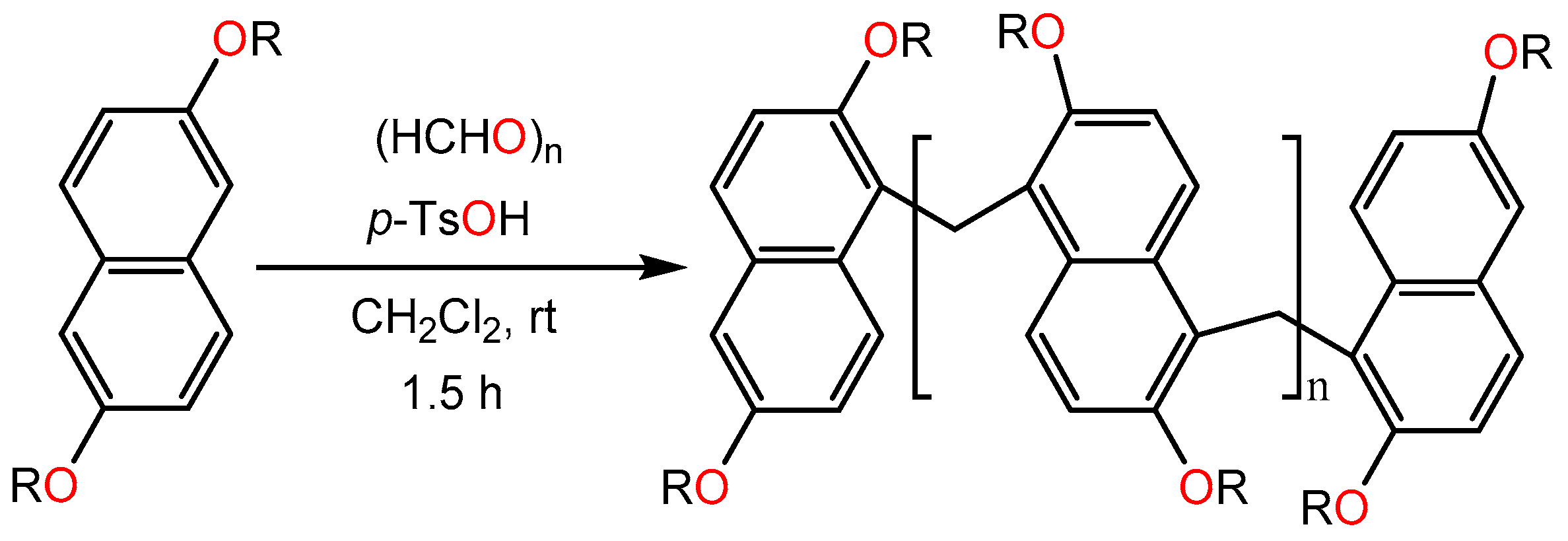
- Pan, S.-J.; Ye, G.; Jia, F.; He, Z.; Ke, H.; Yao, H.; Fan, Z.; Jiang, W. Regioselective synthesis of methylene-bridged naphthalene oligomers and their host–guest chemistry. J. Org. Chem. 2017, 82, 9570–9575. [Google Scholar] [CrossRef]
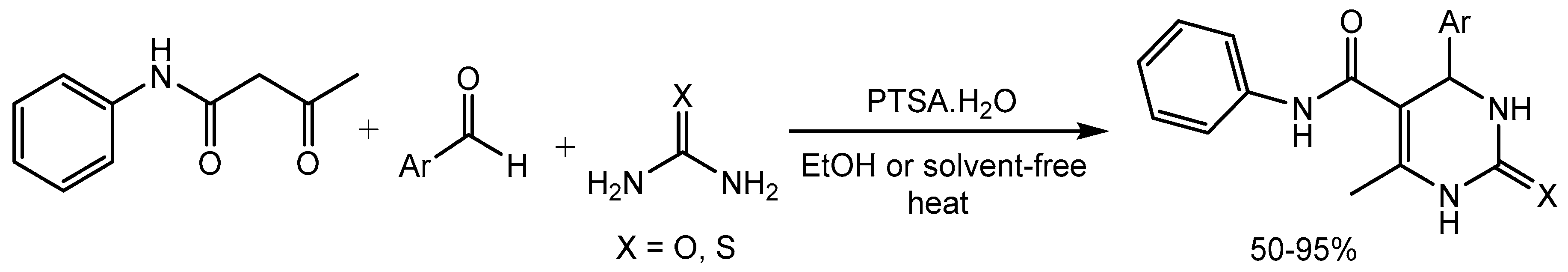
- Gülten, Ş.; Gezer, U.; Gündoğan, E.A. Fast and Efficient One-Pot Three-Component Synthesis of Some 1, 2, 3, 4-Tetrahydro-6-methyl-N-phenyl-5-pyrimidinecarboxamide Derivatives via Biginelli Condensation Reaction. Letters in Organic Chemistry 2020, 17, 366–371. [Google Scholar] [CrossRef]
- Mousavi, M.R.; Maghsoodlou, M.T. Catalytic systems containing p-toluenesulfonic acid monohydrate catalyzed the synthesis of triazoloquinazolinone and benzimidazoquinazolinone derivatives. Monatsh Chem 2014, 145, 1967–1973. [Google Scholar] [CrossRef]
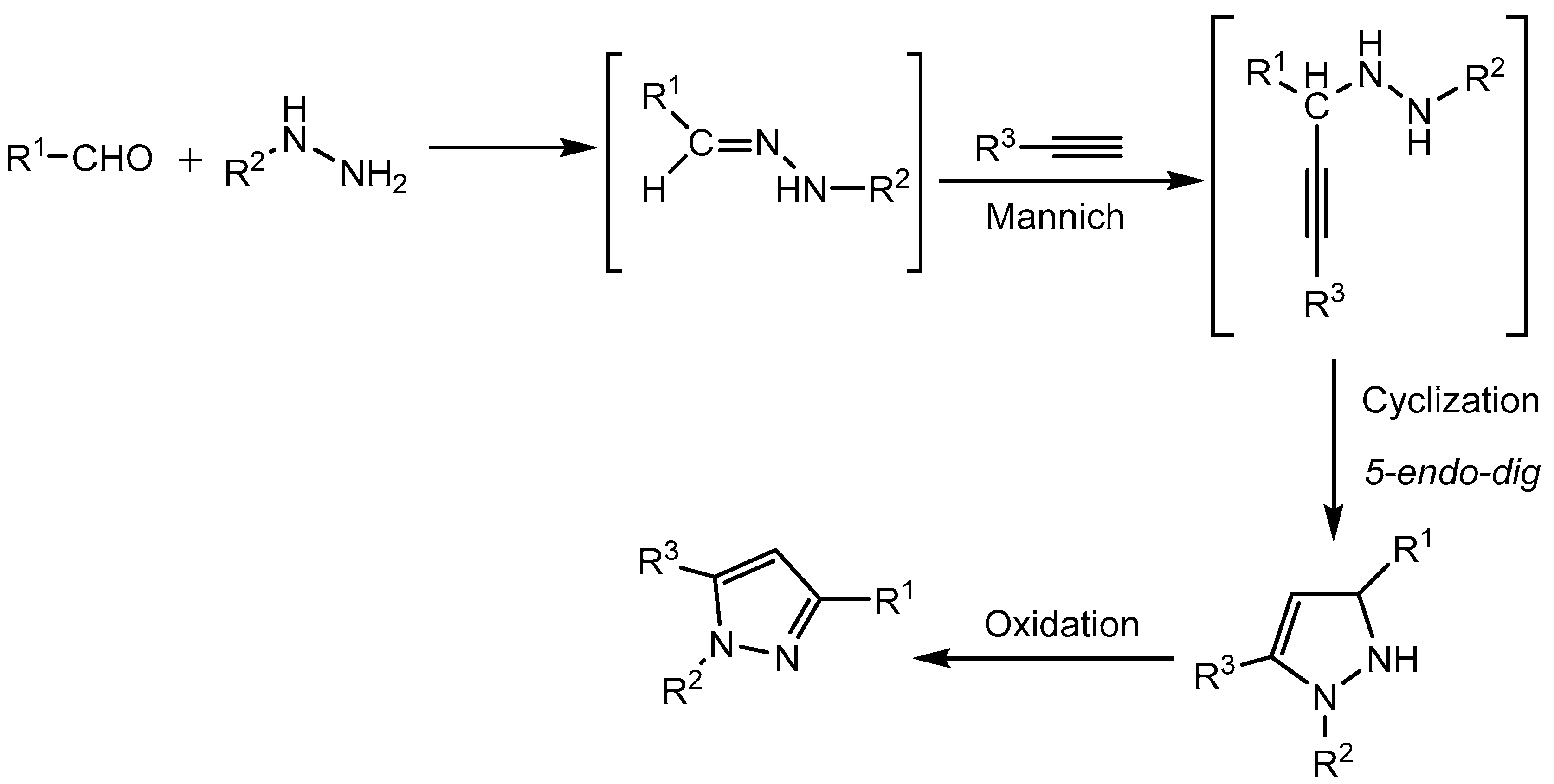
- Liu, P.; Pan, Y.-M.; Xu, Y.-L.; Wang, H.-S. PTSA-catalyzed Mannich-type–cyclization–oxidation tandem reactions: one-pot synthesis of 1, 3, 5-substituted pyrazoles from aldehydes, hydrazines and alkynes. Org. Biomol. Chem. 2012, 10, 4696–4698. [Google Scholar] [CrossRef] [PubMed]
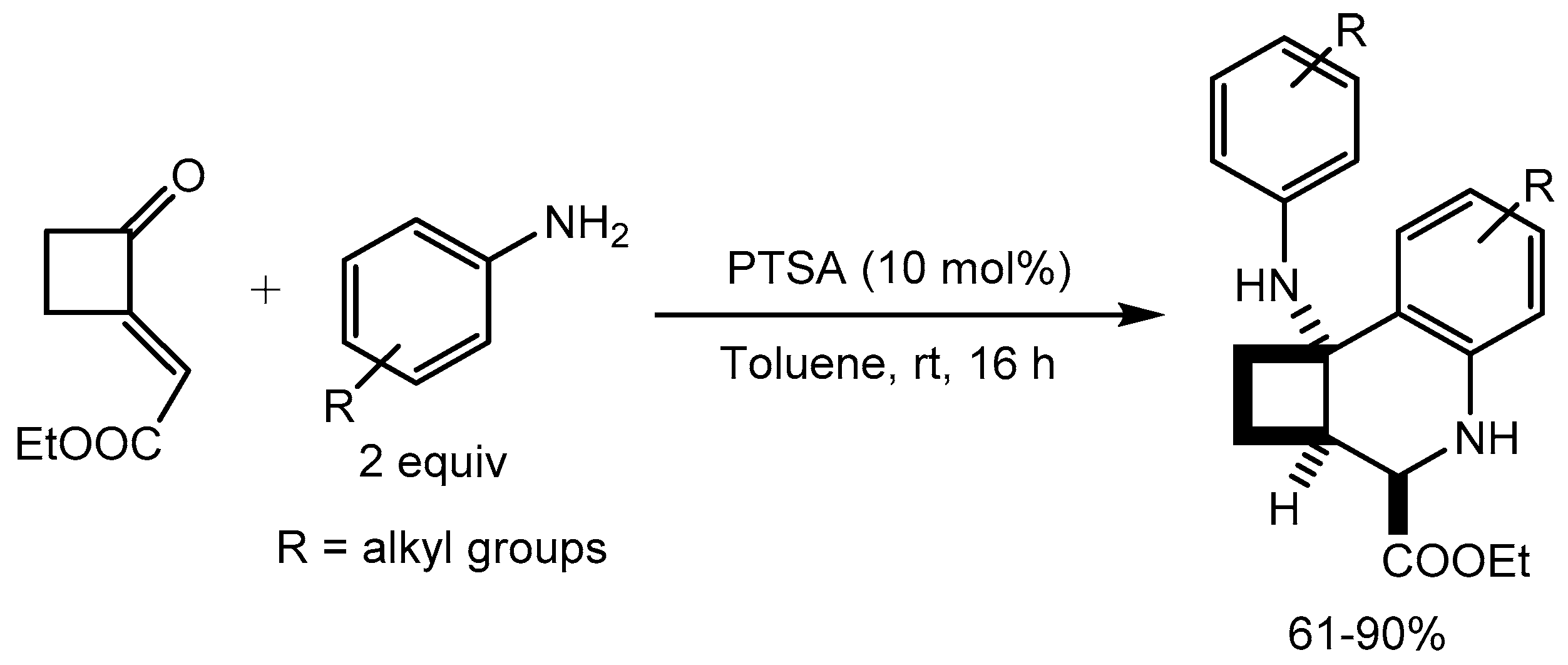
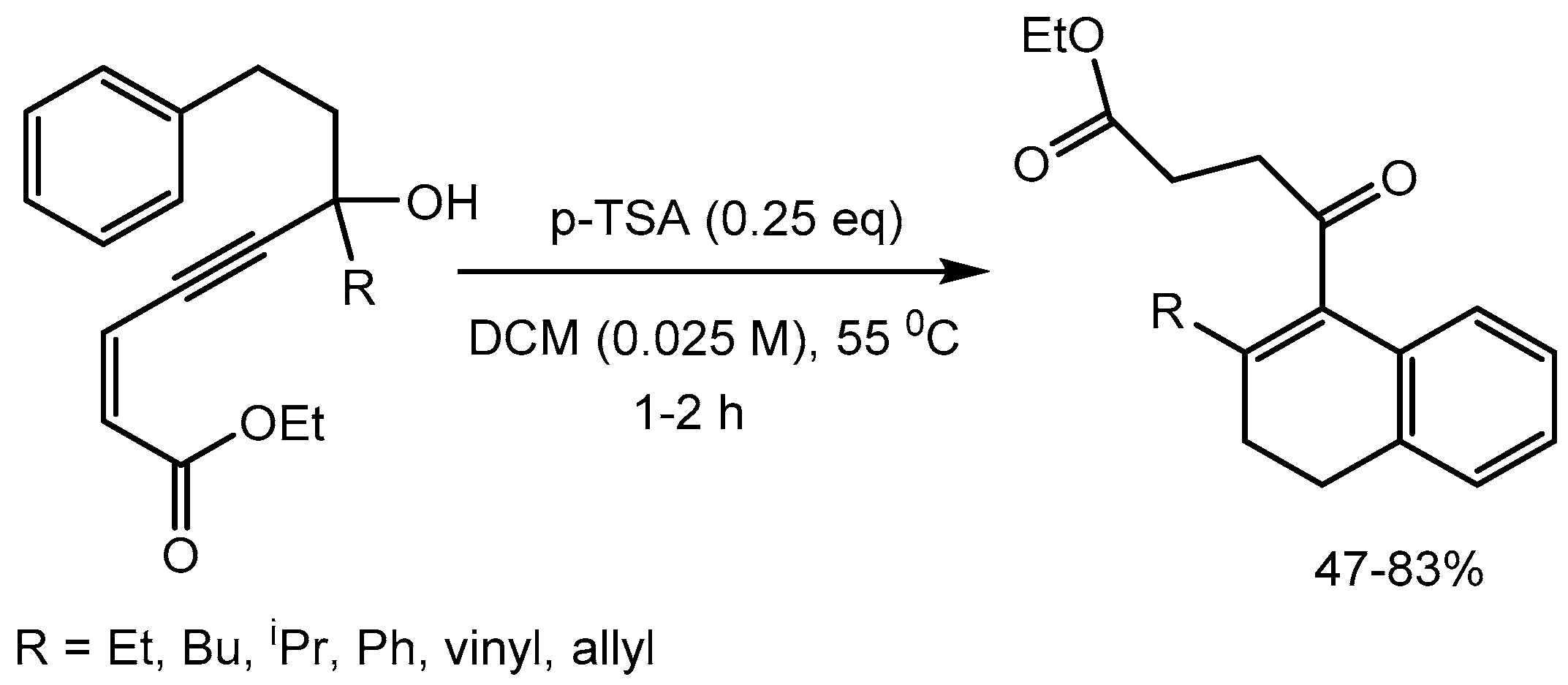
- Marras, V.; Caboni, P.; Secci, F.; Guillot, R.; Aitken, D.J.; Frongia, A. A Brønsted acid catalyzed tandem reaction for the diastereoselective synthesis of cyclobuta-fused tetrahydroquinoline carboxylic esters. Org. Biomol. Chem. 2021, 19, 8912–8916. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Tanaka, H. Syntheses of 1, 2, 3, 4-tetrahydroisoquinolines from N-sulfonyl-phenethylamines and aldehydes. Chem. Pharm. Bull. 1977, 25, 1732–1739. [Google Scholar] [CrossRef]
- Salama, S.K.; Darweesh, A.F.; Abdelhamid, I.A.; Elwahy, A.H. p-TSA Catalyzed One-Pot Synthesis of Some Novel Bis (Hexahydroacridine-1, 8-Diones) and Bis (Tetrahydrodipyrazolo [3, 4-b: 4′, 3′-e] Pyridines) Derivatives. Polycyclic Aromatic Compounds 2021, 41, 1392–1405. [Google Scholar] [CrossRef]
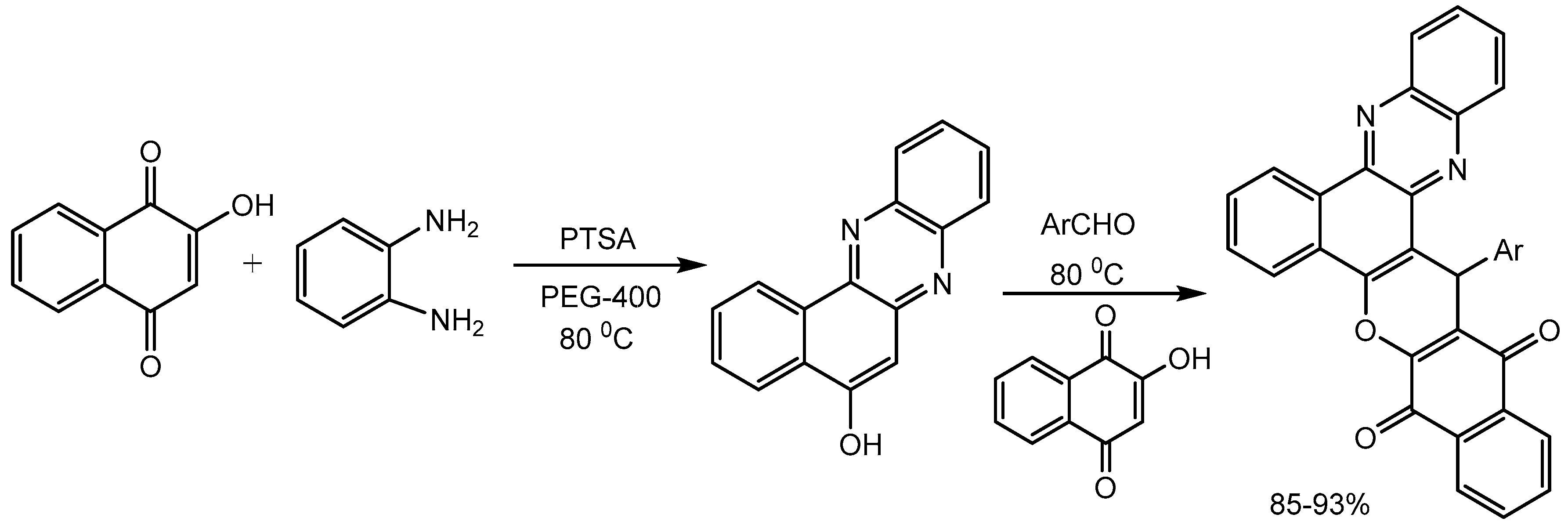
- Mohebat, R.; Yazdani Elah Abadi, A.; Maghsoodlou, M.-T.; Mohammadi, M. PTSA-catalyzed four-component domino reactions for the one-pot synthesis of functionalized 11H-benzo [a] benzo [6, 7] chromeno [2, 3-c] phenazine-11, 16 (17H)-diones in PEG. Res Chem Intermed 2016, 42, 5915–5926. [Google Scholar] [CrossRef]
- Khosropour, A.R.; Khodaei, M.M.; Moghannian, H. A facile, simple and convenient method for the synthesis of 14-alkyl or aryl-14-H-dibenzo [a, j] xanthenes catalyzed by pTSA in solution and solvent-free conditions. Synlett 2005, 0955–0958. [Google Scholar] [CrossRef]
- Khorshidi, M.; Heravi, M.M.; Beheshtia, Y.S.; Baghernejad, B. Novel One-Pot Synthesis of New Oxindole Derivatives Catalyzed by PTSA. Synth. Commun. 2011, 41, 2899–2904. [Google Scholar] [CrossRef]
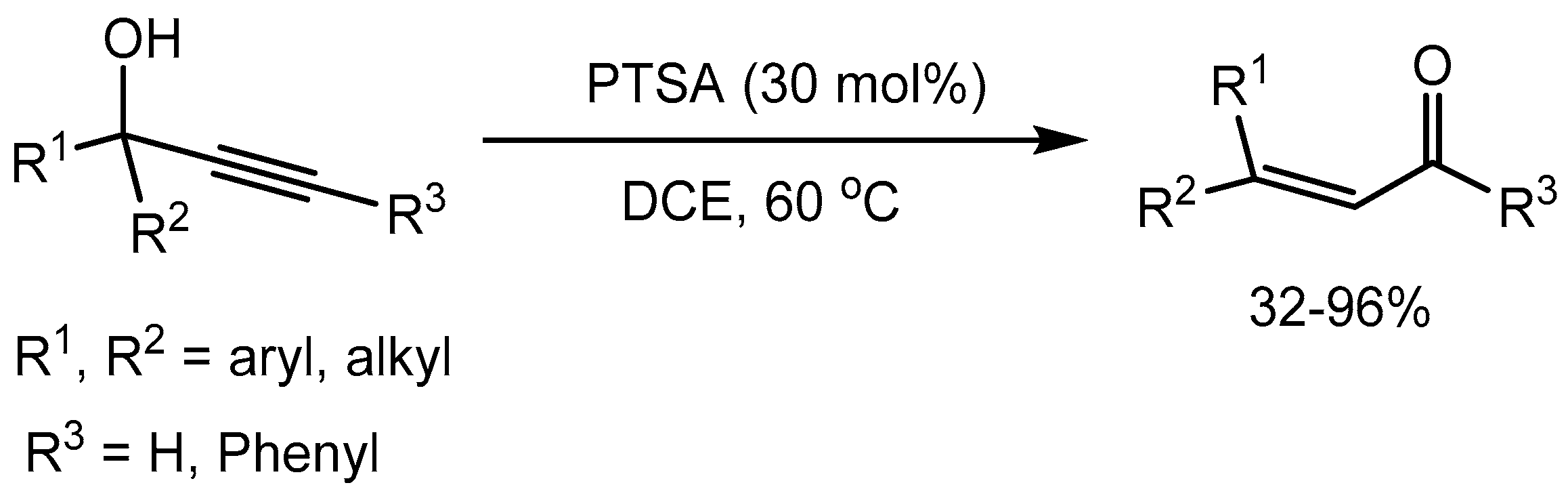
- Park, J.; Yun, J.; Kim, J.; Jang, D.-J.; Park, C.H.; Lee, K. Brønsted Acid–Catalyzed Meyer–Schuster rearrangement for the synthesis of α, β-unsaturated carbonyl compounds. Synth. Commun. 2014, 44, 1924–1929. [Google Scholar] [CrossRef]
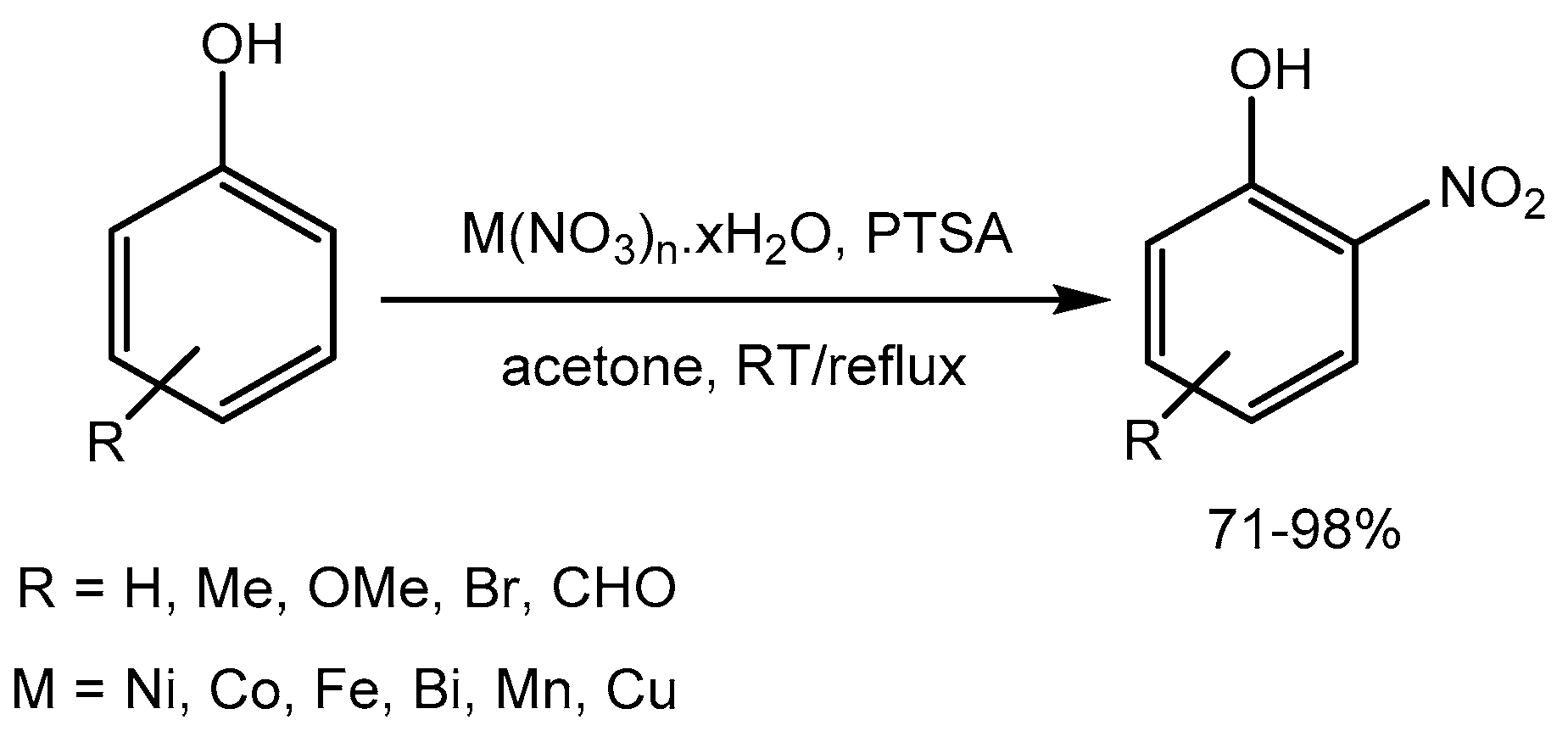
- Anuradha, V.; Srinivas, P.; Aparna, P.; Rao, J.M. p-Toluenesulfonic acid catalyzed regiospecific nitration of phenols with metal nitrates. Tetrahedron letters 2006, 47, 4933–4935. [Google Scholar] [CrossRef]
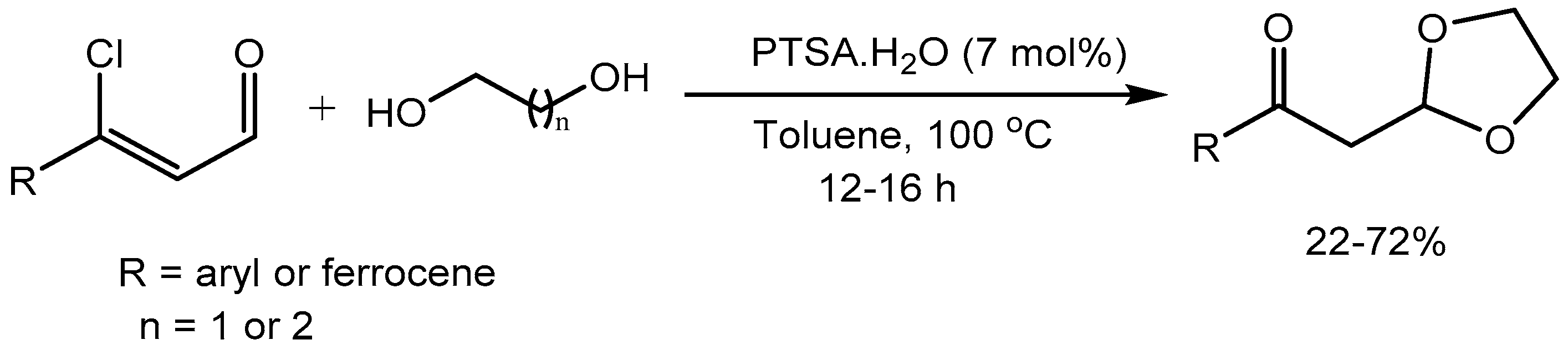
- Srivastava, A.K.; Ali, M.; Sharma, K.N.; Joshi, R.K. Metal-free, PTSA catalyzed facile synthesis of β-ketoacetal from β-chlorocinnamaldehyde. Tetrahedron Letters 2018, 59, 3188–3193. [Google Scholar] [CrossRef]
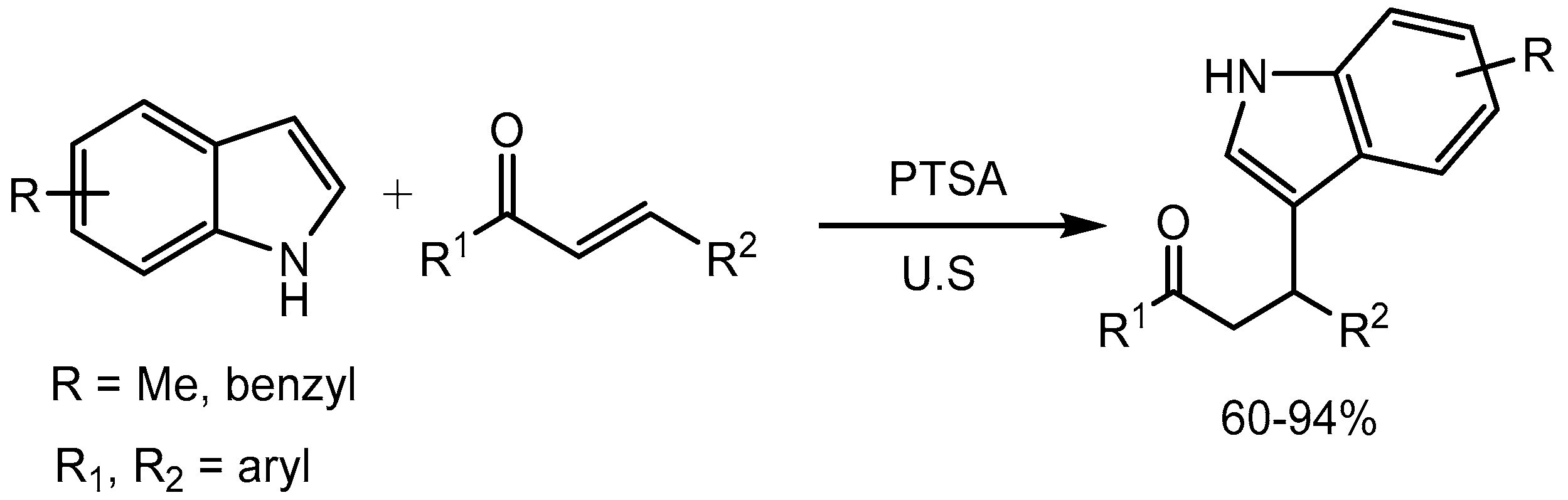
- Ji, S.-J.; Wang, S.-Y. An expeditious synthesis of β-indolylketones catalyzed by p-toluenesulfonic acid (PTSA) using ultrasonic irradiation. Ultrasonics sonochemistry 2005, 12, 339–343. [Google Scholar] [CrossRef] [PubMed]
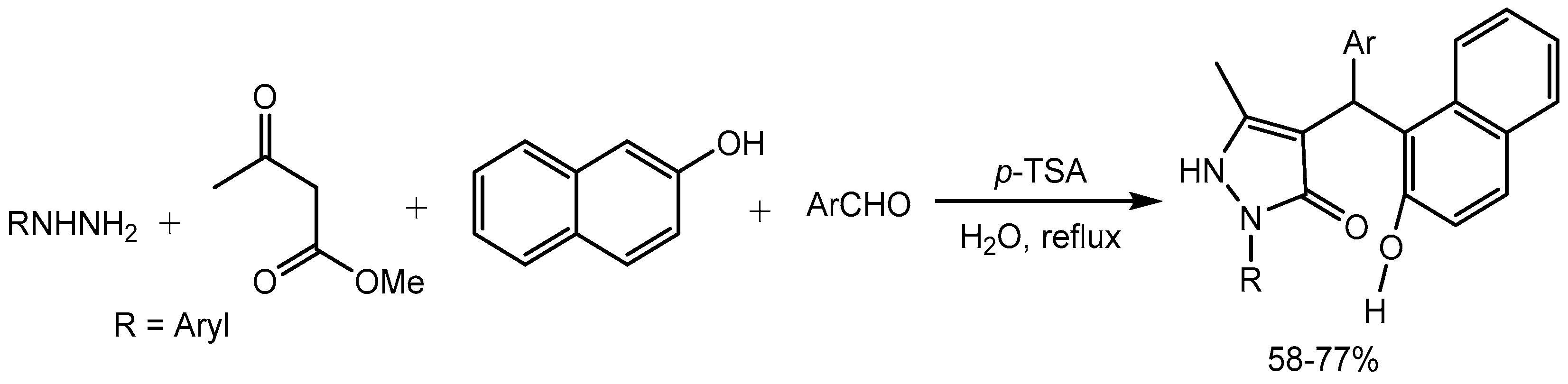
- Gunasekaran, P.; Perumal, S.; Yogeeswari, P.; Sriram, D. A facile four-component sequential protocol in the expedient synthesis of novel 2-aryl-5-methyl-2, 3-dihydro-1H-3-pyrazolones in water and their antitubercular evaluation. Eur. J. Med. Chem. 2011, 46, 4530–4536. [Google Scholar] [CrossRef] [PubMed]
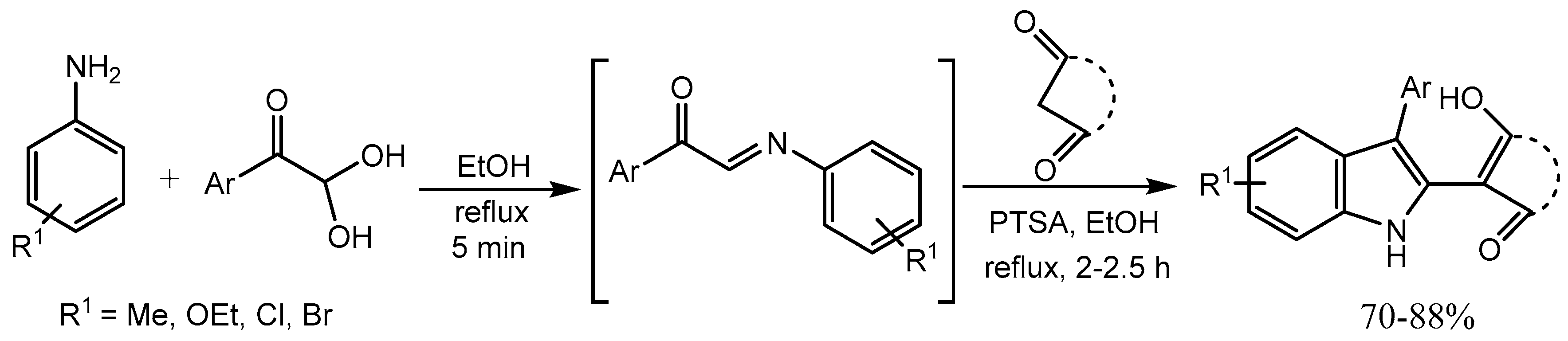
- Naidu, P.S.; Kolita, S.; Sharma, M.; Bhuyan, P.J. Reductive alkylation of α-keto imines catalyzed by PTSA/FeCl3: Synthesis of indoles and 2, 3′-biindoles. J. Org. Chem. 2015, 80, 6381–6390. [Google Scholar] [CrossRef] [PubMed]
- Ziyaei Halimehjani, A.; Khoshdoun, M. Tandem esterification/1, 4-addition-type Friedel–Crafts alkylation reactions of phenols/naphthols with olefinic thioazlactones: access to functionalized 1, 2-dihydrobenzo [f] chromen-3-ones and 3, 4-dihydrochromen-2-ones. J. Org. Chem. 2016, 81, 5699–5704. [Google Scholar] [CrossRef]
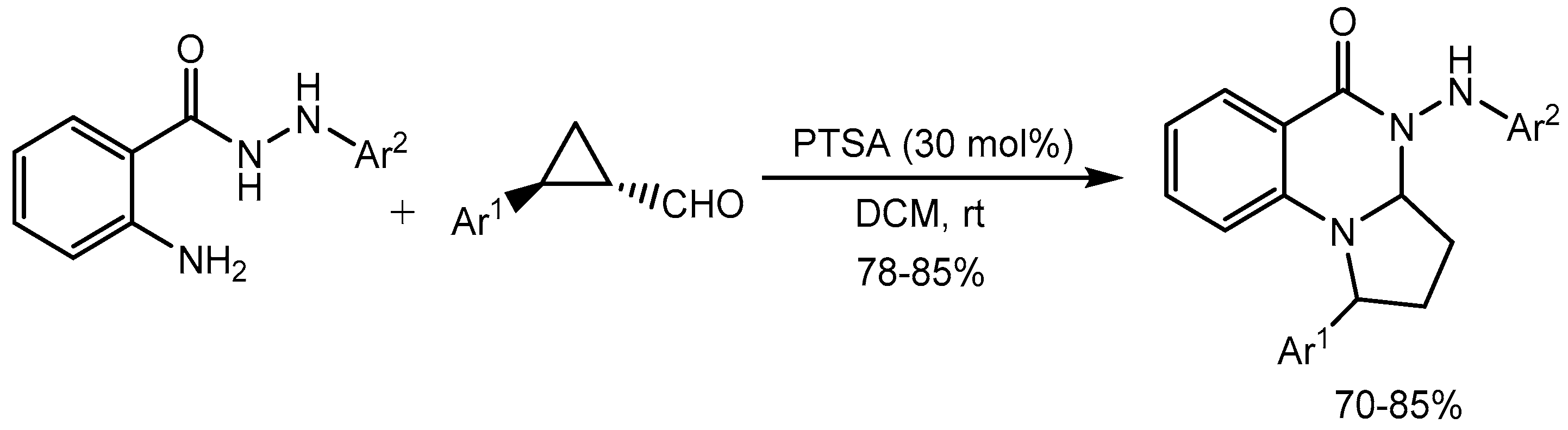
- Singh, P.; Kaur, N.; Banerjee, P. Regioselective Brønsted Acid-Catalyzed Annulation of Cyclopropane Aldehydes with N′-Aryl Anthranil Hydrazides: Domino Construction of Tetrahydropyrrolo [1, 2-a] quinazolin-5 (1 H) ones. J. Org. Chem. 2020, 85, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
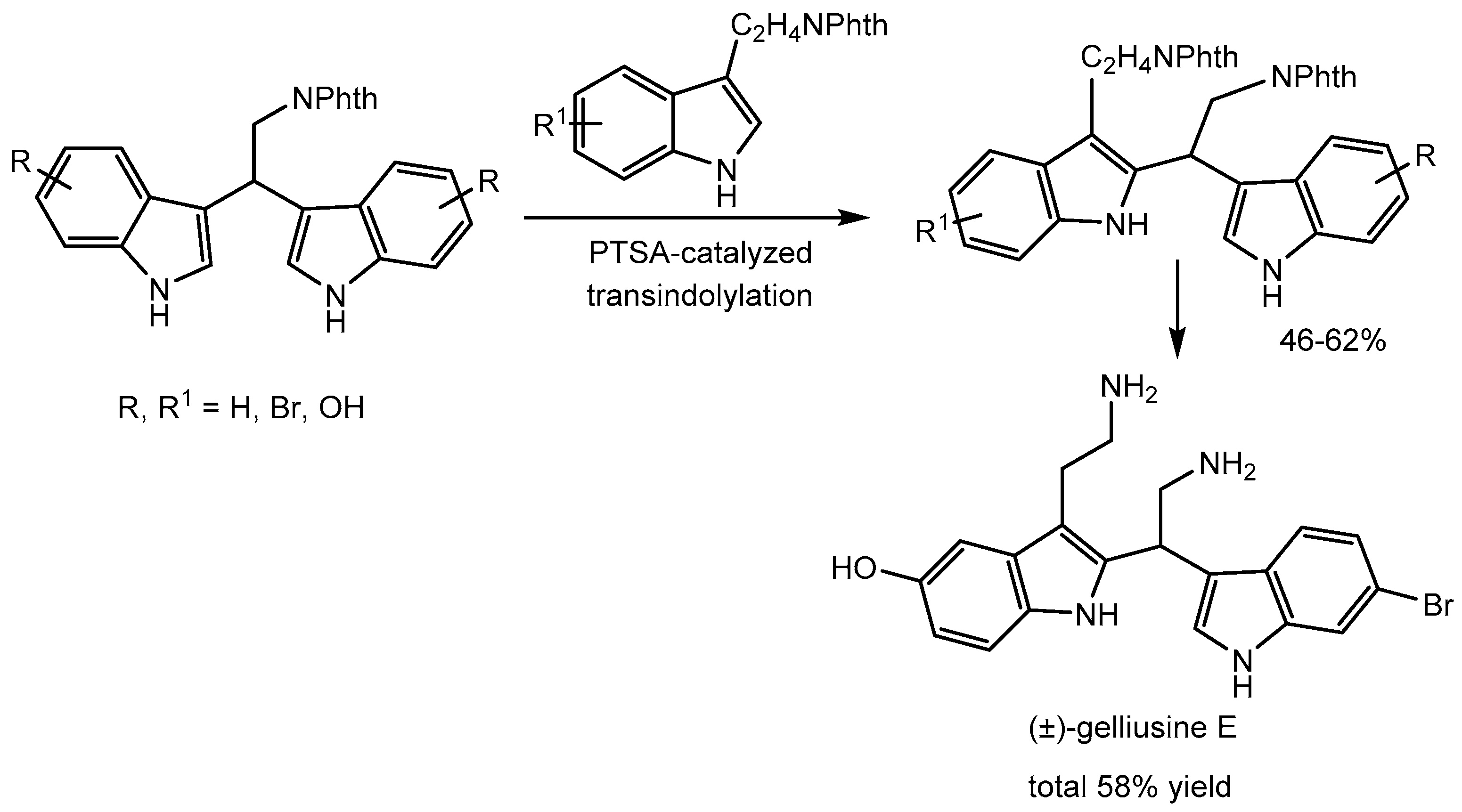
- Chantana, C.; Sirion, U.; Iawsipo, P.; Jaratjaroonphong, J. Short total synthesis of (±)-gelliusine E and 2, 3′-bis (indolyl) ethylamines via PTSA-catalyzed transindolylation. J. Org. Chem. 2021, 86, 13360–13370. [Google Scholar] [CrossRef] [PubMed]
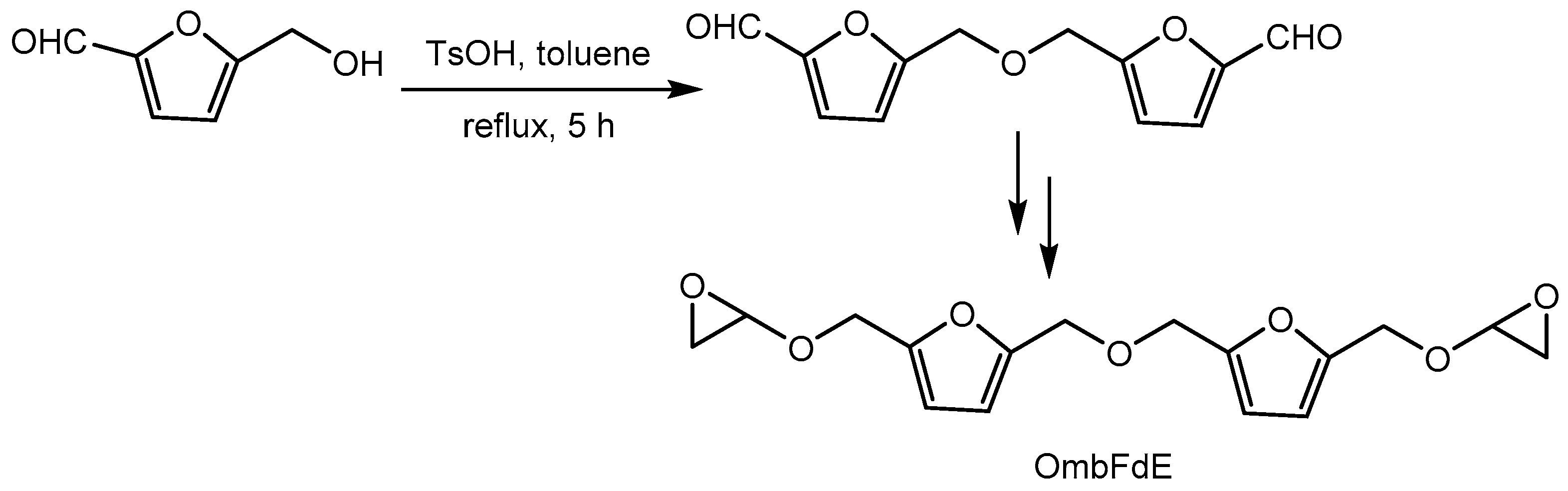
- Meng, J.; Zeng, Y.; Zhu, G.; Zhang, J.; Chen, P.; Cheng, Y.; Fang, Z.; Guo, K. Sustainable bio-based furan epoxy resin with flame retardancy. Polymer Chemistry 2019, 10, 2370–2375. [Google Scholar] [CrossRef]
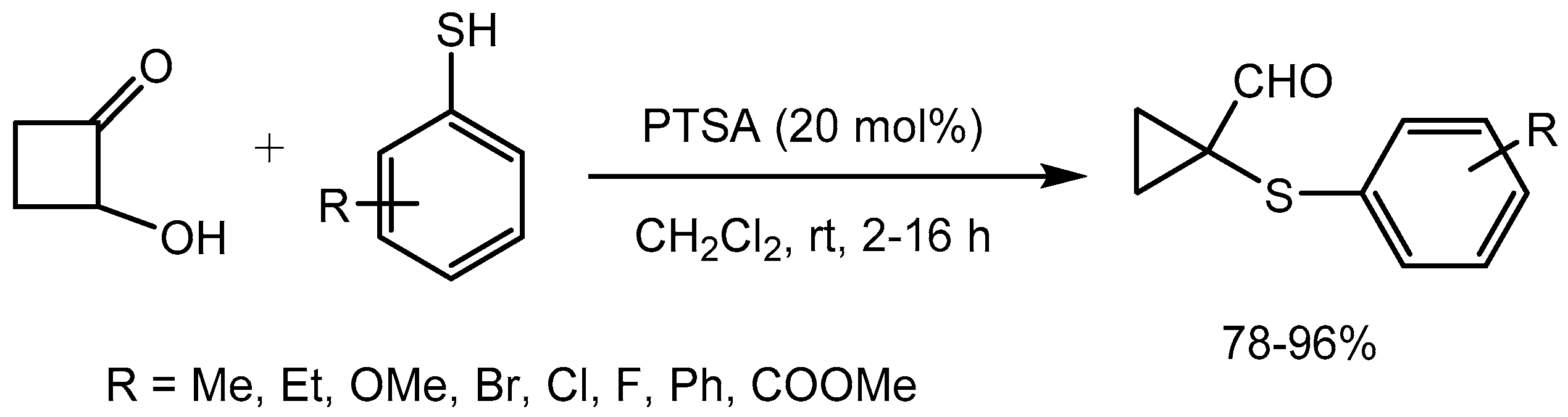
- Porcu, S.; Luridiana, A.; Martis, A.; Frongia, A.; Sarais, G.; Aitken, D.J.; Boddaert, T.; Guillot, R.; Secci, F. Acid-catalyzed synthesis of functionalized arylthio cyclopropane carbaldehydes and ketones. Chem. Commun. 2018, 54, 13547–13550. [Google Scholar] [CrossRef]
- Tharra, P.; Baire, B. The Z-enoate assisted, Meyer–Schuster rearrangement cascade: unconventional synthesis of α-arylenone esters. Chem. Commun. 2016, 52, 12147–12150. [Google Scholar] [CrossRef]
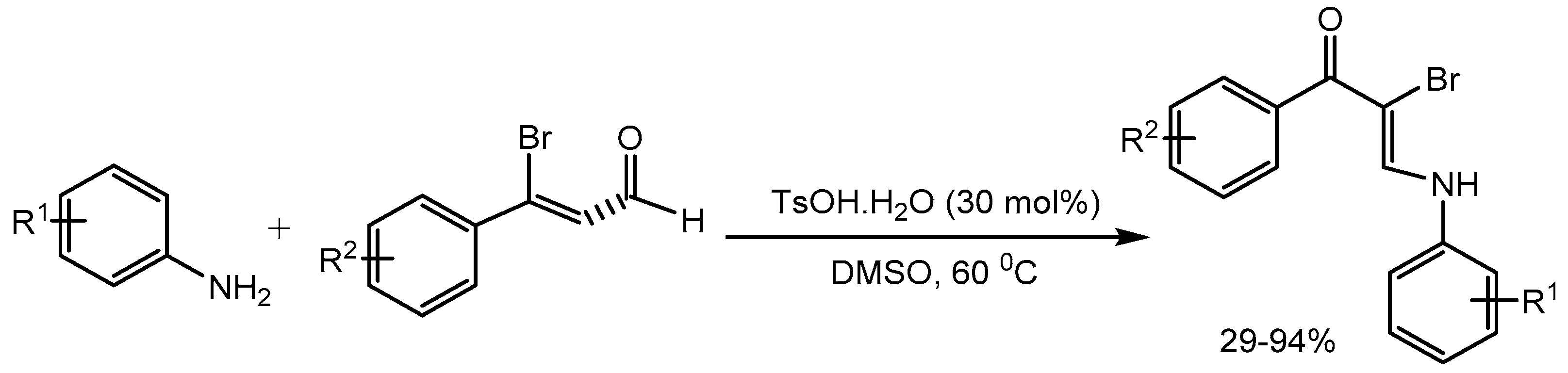
- Suresh, S.; Bhimrao Patil, P.; Yu, P.H.; Fang, C.C.; Weng, Y.Z. ; Kavala,V.; Yao, C.F. A Study of the Reactions of 3-Bromopropenals with Anilines for the Synthesis of α-Bromo Enaminones. Adv. Synth. Catal. 2021; 363, 4915–4925. [Google Scholar]
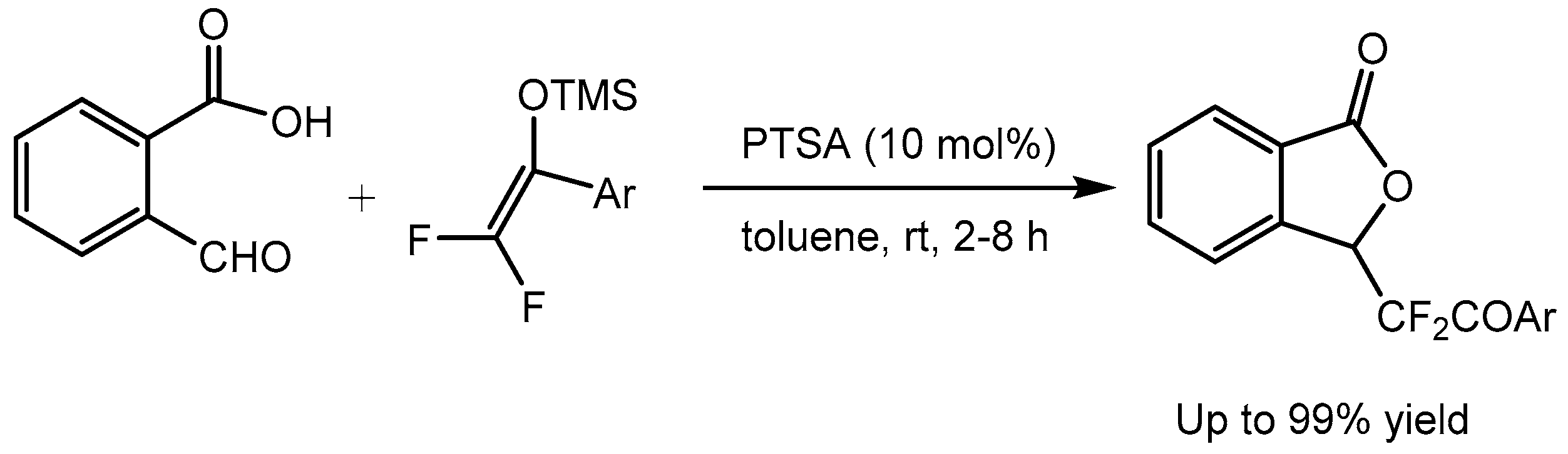
- Liu, S.; Li, Y.; Wang, F.; Ma, C.; Yang, G.; Yang, J.; Ren, J. p-Toluenesulfonic acid-catalyzed reaction of phthalaldehydic acids with difluoroenoxysilanes: Access to 3-difluoroalkyl phthalides. Synthesis 2022, 54, 161–170. [Google Scholar]
- Tang, L.; Jiang, S.; Huang, X.; Song, Z.; Wang, J.-b.; Ma, M.; Chen, B.; Ma, Y. Cascade of C(sp2)–H Addition to Carbonyl and C(sp2)–CN/C(sp2)–H Coupling Enabled by Brønsted Acid: Construction of Benzo [a] carbazole Frameworks. Org. Lett. 2022, 24, 3232–3237. [Google Scholar] [CrossRef] [PubMed]
- Cankařová, N.; Nemec, I.; Krchňák, V. p-TSA-Mediated Four-Component Reaction: One-Step Access to Mesoionic 1H-Imidazol-3-ium-4-olates, Direct NHC Precursors. Adv. Synth. Catal. 2022, 364, 2996–3003. [Google Scholar] [CrossRef]
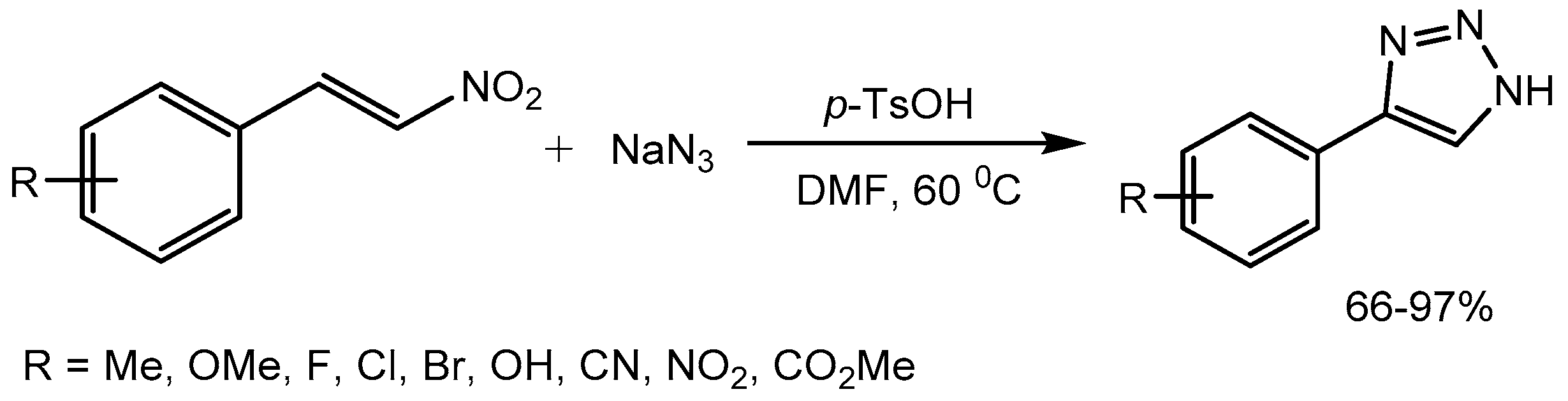
- Quan, X.-J.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. p-Toluenesulfonic acid mediated 1, 3-dipolar cycloaddition of nitroolefins with NaN3 for synthesis of 4-aryl-NH-1, 2, 3-triazoles. Org. Lett. 2014, 16, 5728–5731. [Google Scholar] [CrossRef]
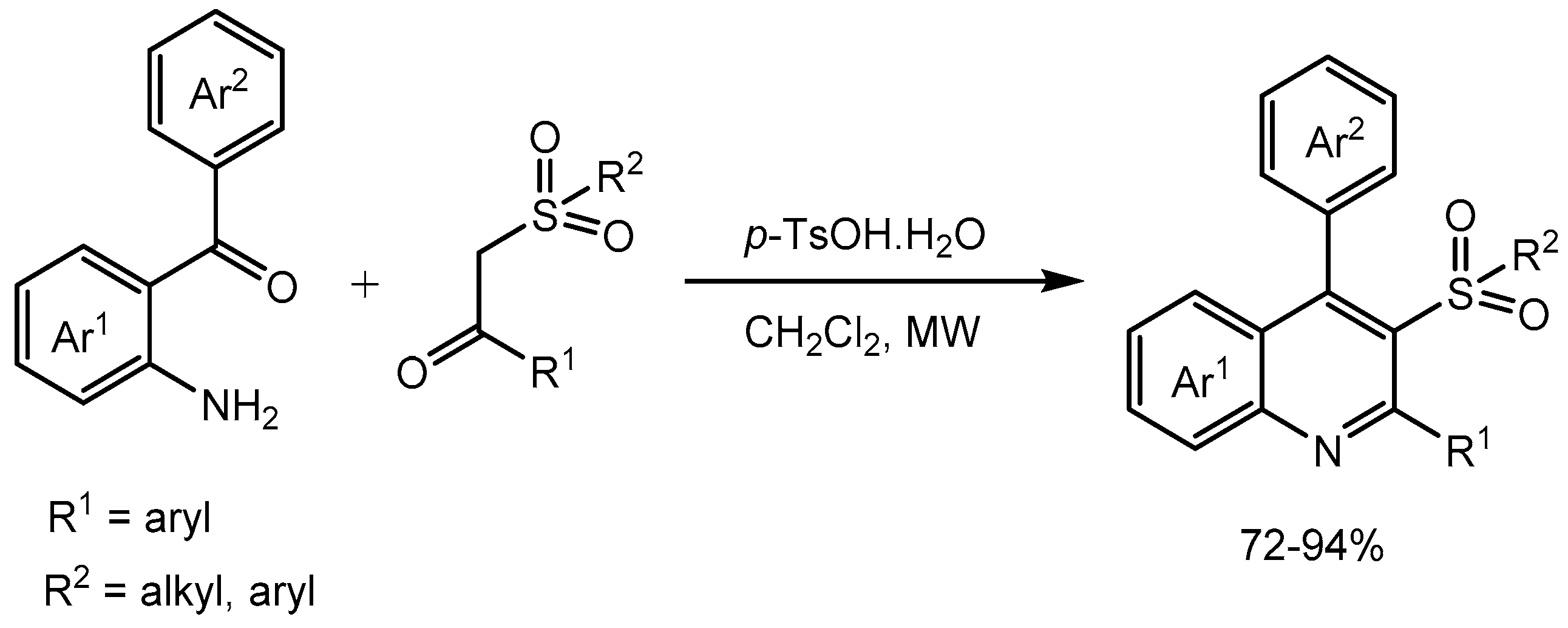
- Chan, C.-K.; Lai, C.-Y.; Lo, W.-C.; Cheng, Y.-T.; Chang, M.-Y.; Wang, C.-C. p-TsOH-mediated synthesis of substituted 2, 4-diaryl-3-sulfonylquinolines from functionalized 2-aminobenzophenones and aromatic β-ketosulfones under microwave irradiation. Org. Biomol. Chem. 2020, 18, 305–315. [Google Scholar] [CrossRef] [PubMed]
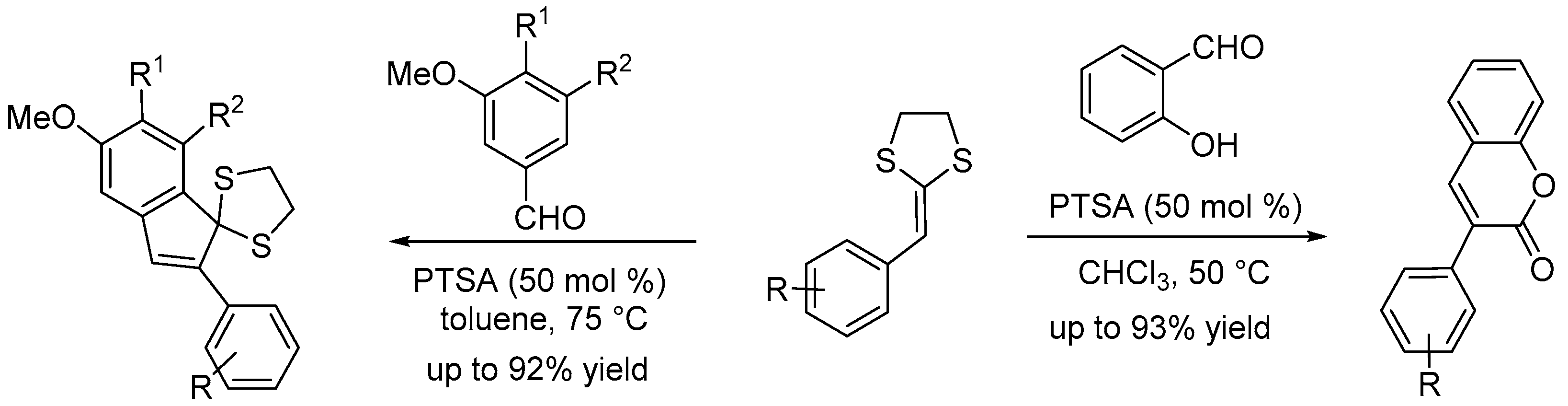
- Arora, S.; Singh, S. P.; Sharma, P.; Singh, A. , Brønsted acid catalyzed annulations of ketene dithioacetals: synthesis of 3-aryl coumarins and indenes. Org. Biomol. Chem. 2022, 20, 8907–8911. [Google Scholar] [CrossRef] [PubMed]
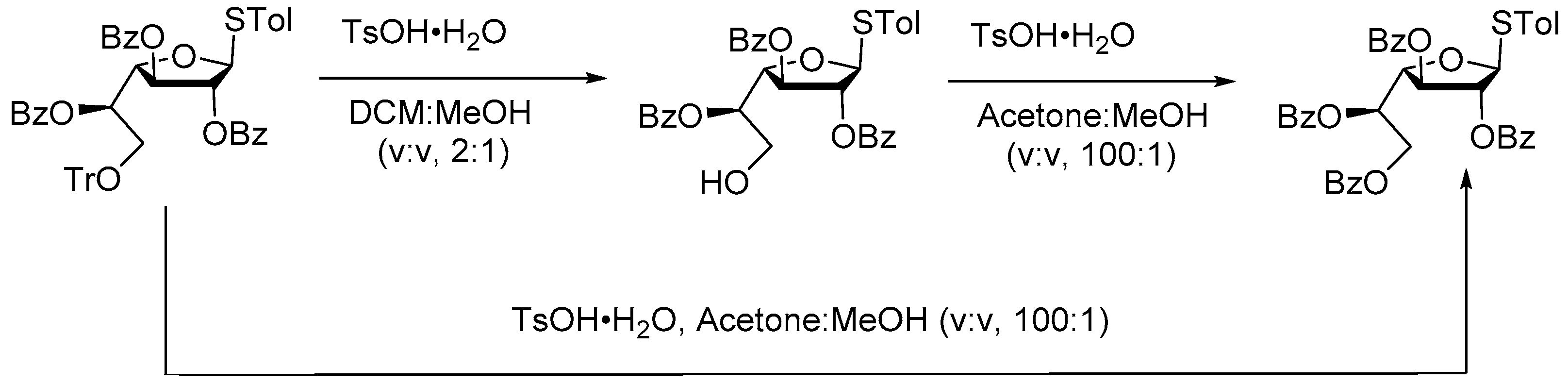
- Liang, X.-Y.; Liu, A.-L.; Fan, H.-J. S.; Wang, L.; Xu, Z.-N.; Ding, X.-G.; Huang, B.-S. , TsOH-catalyzed acyl migration reaction of the Bz-group: innovative assembly of various building blocks for the synthesis of saccharides. Org. Biomol. Chem. 2023, 21, 1537–1548. [Google Scholar] [CrossRef]
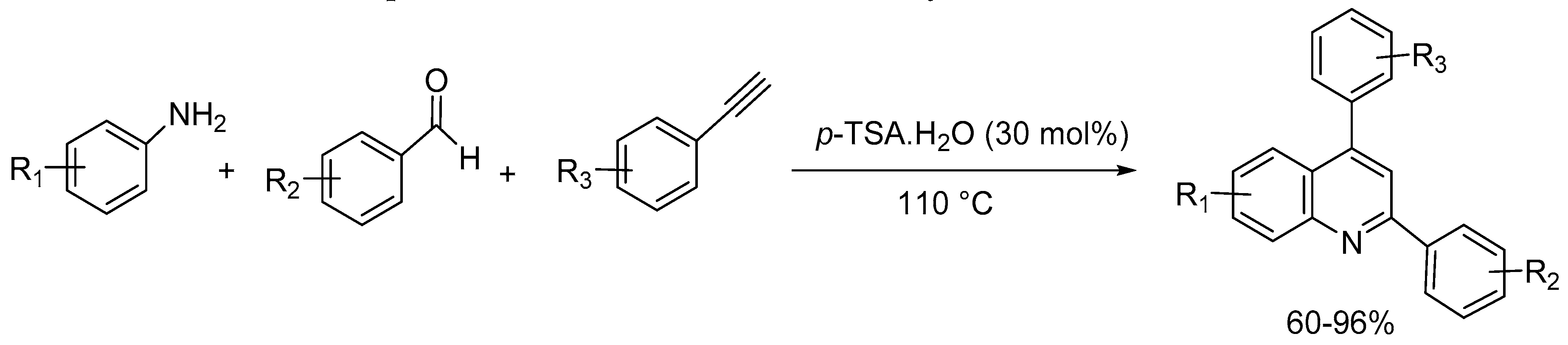
- Faraz, S.; Yashmin, S.; Marathe, M. D.; Khan, A. T. , Environmentally benign synthesis of 2, 4-diarylquinolines under metal-& solvent-free conditions. Tetrahedron Letters 2023, 120, 154433. [Google Scholar]
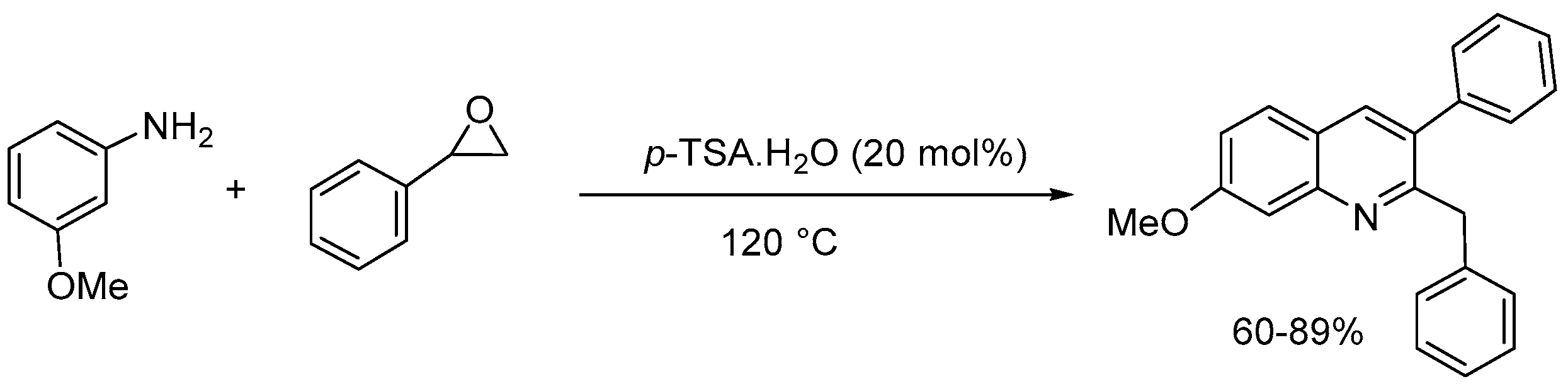
- Faraz, S.; Kumar, M.; Khan, A. T.; Ponneganti, S.; Radhakrishnanand, P. , Metal-and solvent-free synthesis of 2-benzyl-3-arylquinoline using a pseudo-three-component reaction. Tetrahedron Letters 2023, 115, 154283. [Google Scholar] [CrossRef]
- Yin, H.; Wu, Y.; Jiang, Y.; Wang, M.; Wang, S. , Synthesis of Cyclohepta [b] indoles and Furo [3, 4-b] carbazoles from Indoles, Tertiary Propargylic Alcohols, and Activated Alkynes. Org. Lett. 2023, 25, 3078–3082. [Google Scholar] [CrossRef]
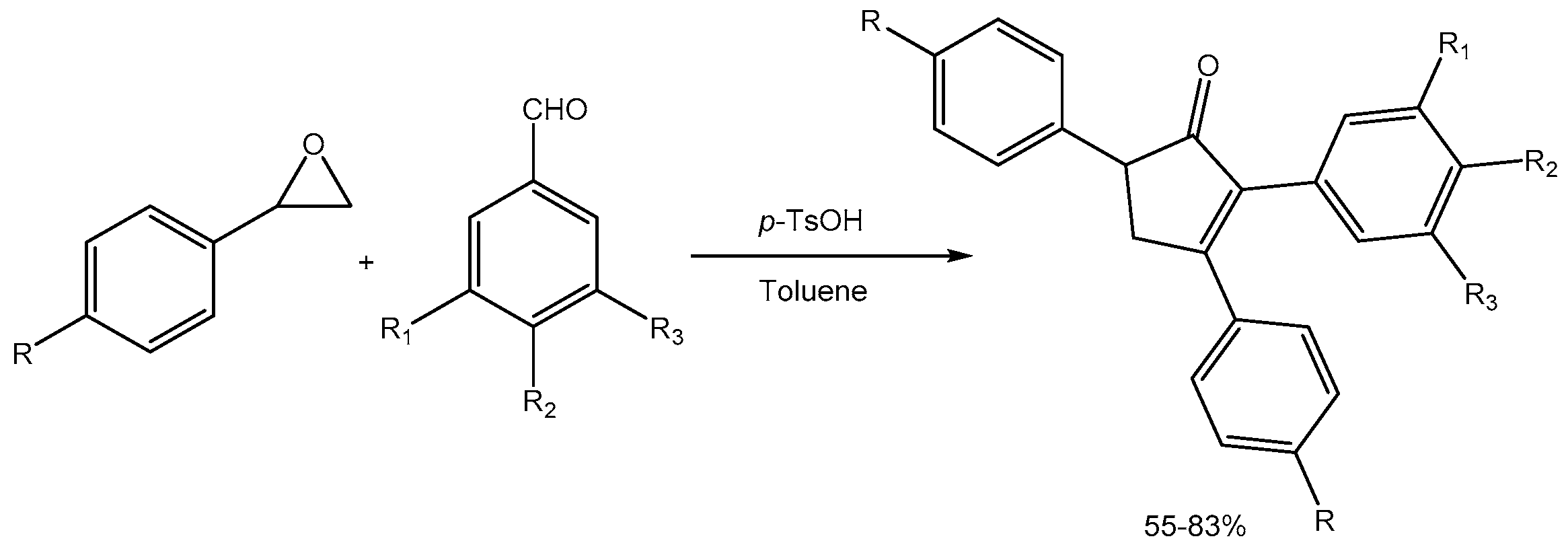
- Reddy, N. R.; Gouse, S.; Selvaraju, S.; Baskaran, S. , Domino Semipinacol/Iterative Aldol/Iso-Nazarov Cyclization to Triaryl-cyclopentenone: Enantioselective Synthesis of Combretastatin A-4 Analogues. Org. Lett. 2022, 24, 4240–4245. [Google Scholar] [CrossRef]
- Babcock, E. G.; Rahman, M. S.; Taylor, J. E. , Brønsted acid-catalysed desilylative heterocyclisation to form substituted furans. Org. Biomol. Chem. 2023, 21, 163–168. [Google Scholar] [CrossRef]
- Lv, X.; Gao, P.; Zhao, X.; Jiang, Z. , Metal-Free Construction of Multisubstituted Indolizines via Intramolecular Amination of Allylic Alcohols. J. Org. Chem. 2023, 88, 9459–9468. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Sueki, S.; Anada, M. , Brønsted Acid-Catalyzed Intramolecular 7-endo Hydroarylation Reaction of 1, 5-Diaryl-1-pentynes. Adv. Synth. Catal. 2023, 365, 1471–1476. [Google Scholar] [CrossRef]
- Das, A.; Ajarul, S.; Debnath, S.; Hota, P.; Maiti, D. K. , Bro̷nsted Acid-Catalyzed [5+ 1] and [4+ 1] Annulation of Cyclic Anhydrides with o-Alkynylanilines to Construct Fused-N-Heterocycles. J. Org. Chem. 2023, 88, 15073–15084. [Google Scholar] [CrossRef] [PubMed]
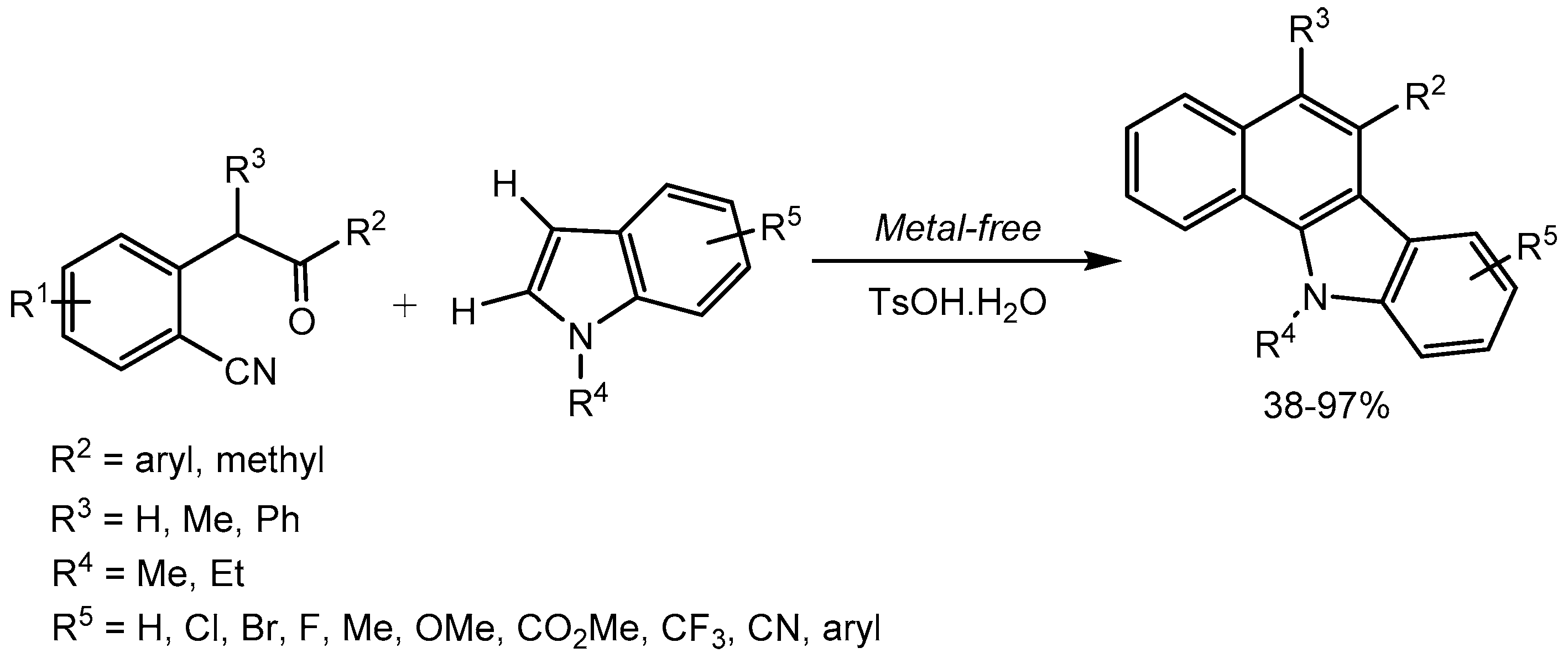
- Chaudhari, T. Y.; Bisht, S.; Chorol, S.; Bhujbal, S. M.; Bharatam, P. V.; Tandon, V. , Bronsted Acid-Catalyzed Regioselective Carboxamidation of 2-Indolylmethanols with Isonitriles. J. Org. Chem. 2023, 88, 10412–10425. [Google Scholar] [CrossRef] [PubMed]
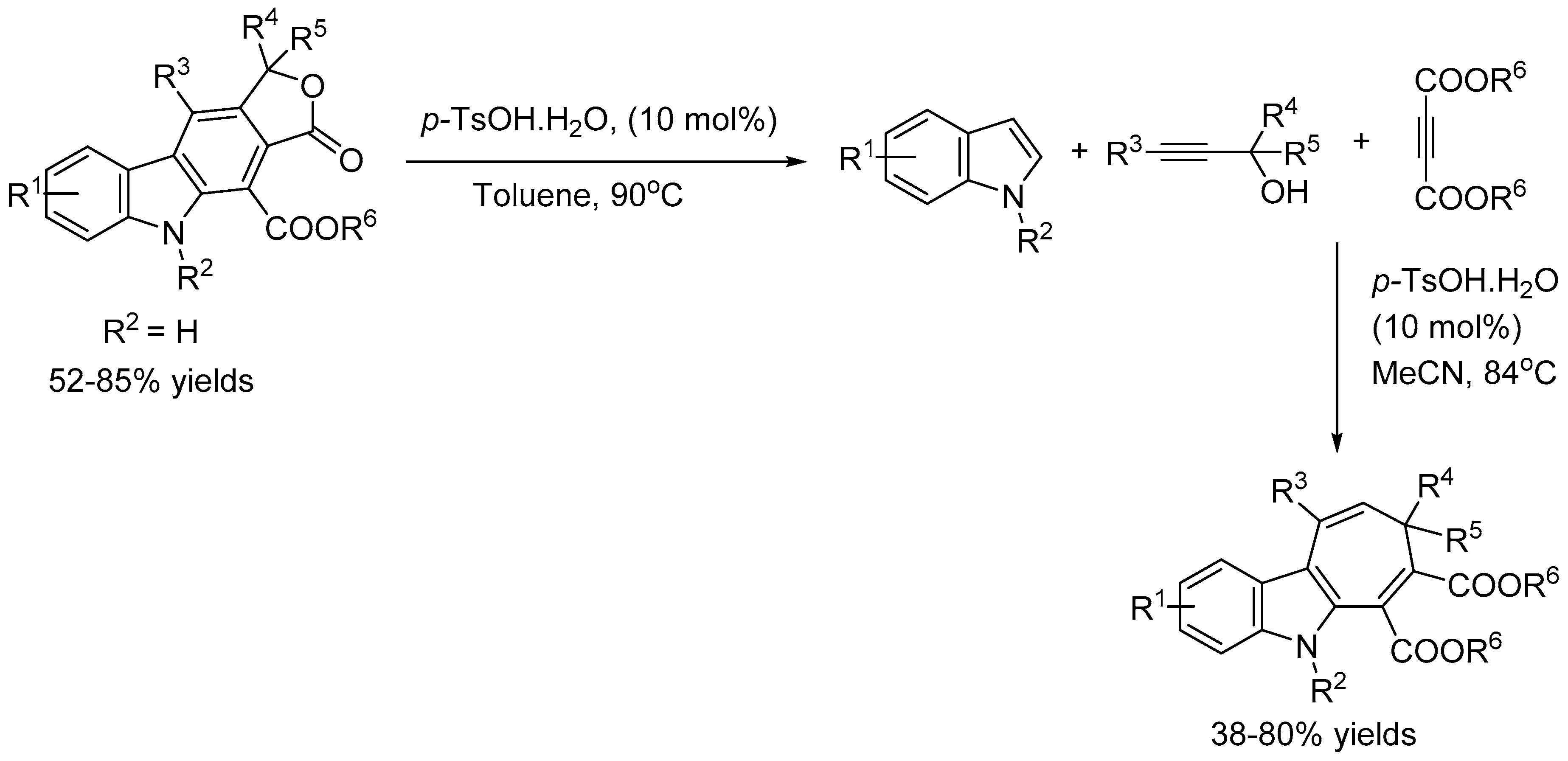
- Zhan, S.-C.; Sun, J.; Sun, Q.; Han, Y.; Yan, C.-G. , Acid-Modulated Construction of Cyclopenta [b] indole and Cyclohepta [b] indole via Unprecedented C3/C2 Carbocation Rearrangement. J. Org. Chem. 2023, 88, 5440–5456. [Google Scholar] [CrossRef] [PubMed]
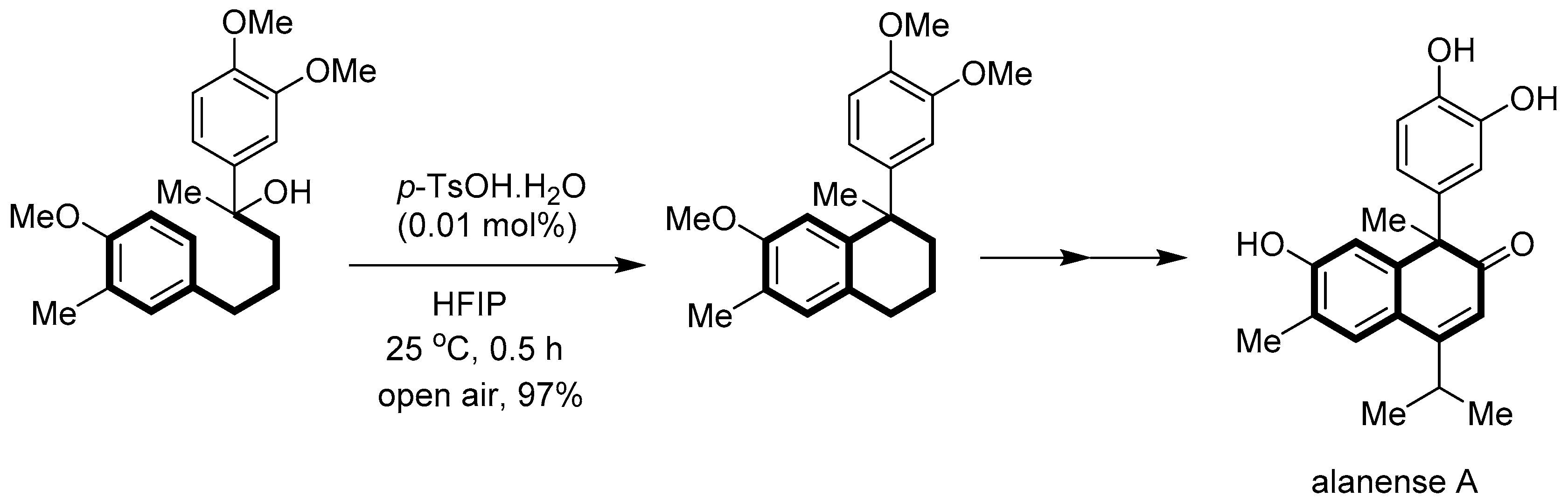
- Makino, K.; Fukuda, R.; Sueki, S.; Anada, M. , Total Synthesis of Alanense A through an Intramolecular Friedel–Crafts Alkylation. J. Org. Chem. 2024, 89, 2050–2054. [Google Scholar] [CrossRef]
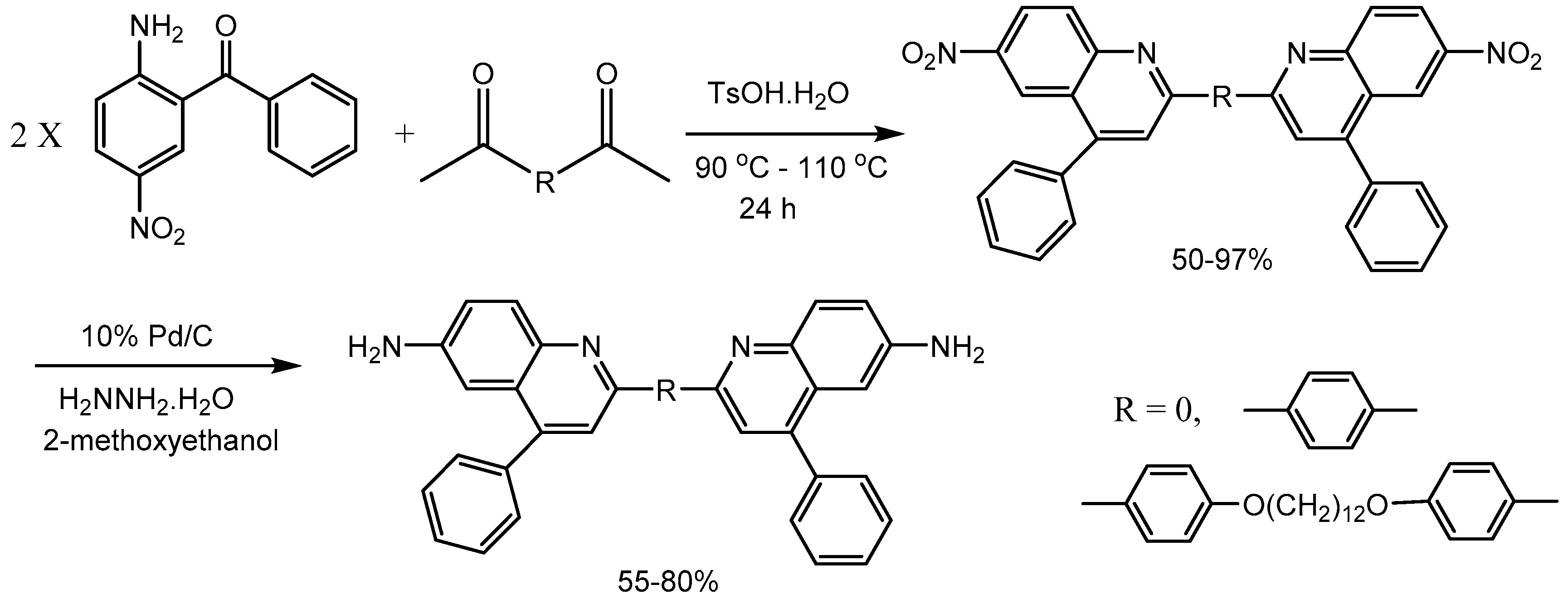
- Bhowmik, P.K.; Nedeltchev, A.K.; Han, H.; Jo, T.S.; Koh, J.J.; Senthilkumar, L. Umadevi, P. Photoactive amorphous molecular Materials based on bisquinoline diamines and their synthesis by Friedländer condensation reaction. J. Photochem. Photobiol. A: Chem. 2014; 283, 45–55. [Google Scholar]
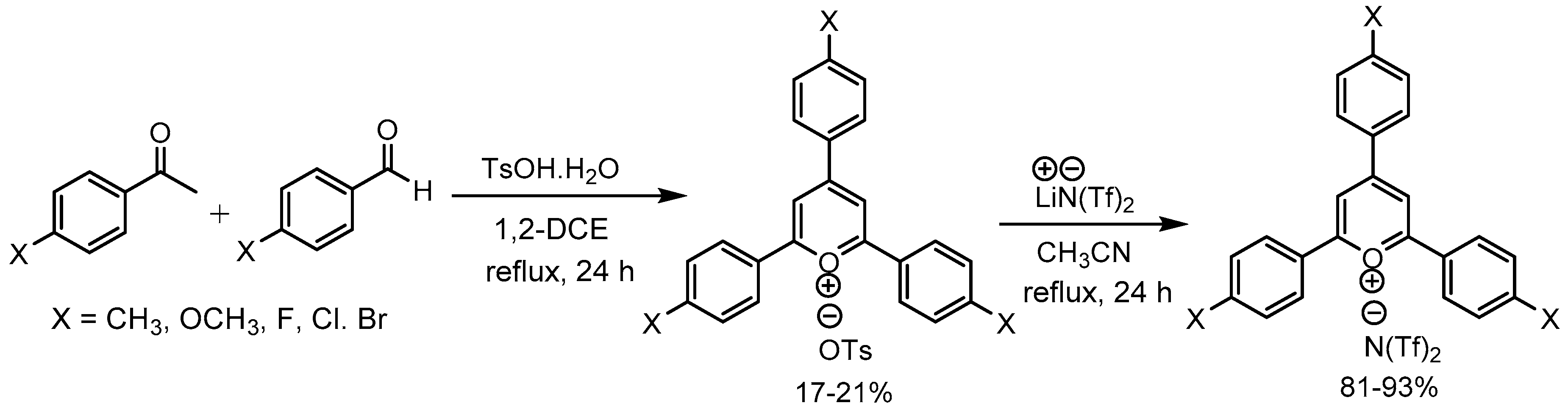
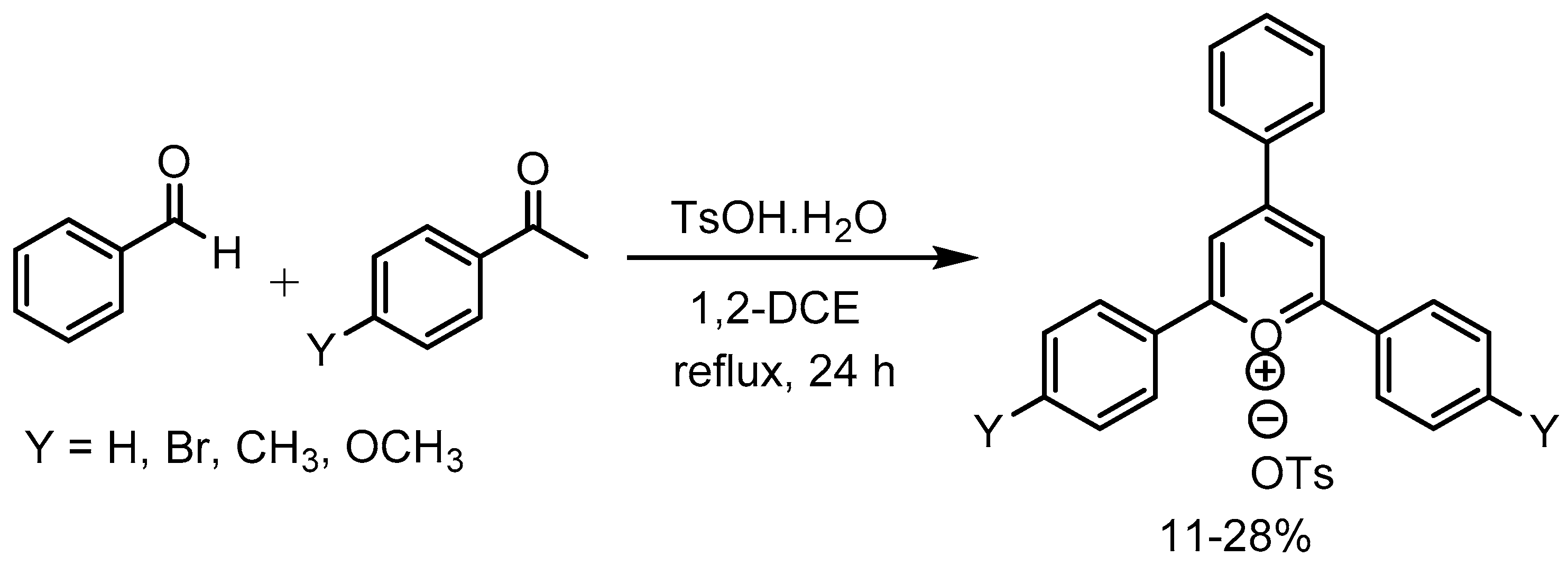
- Bhowmik, P.K.; Lee, C.I.; Koh, J.J.; Han, H.; Jubair, A.; Kartazaev, V.; Gayen, S.K. Synthesis, optical, and thermal properties of 2,4,6-tris(4-substituted phenyl)pyrylium tosylates and triflimides. J. Mol. Struc. 2020, 1202, 127325. [Google Scholar] [CrossRef]
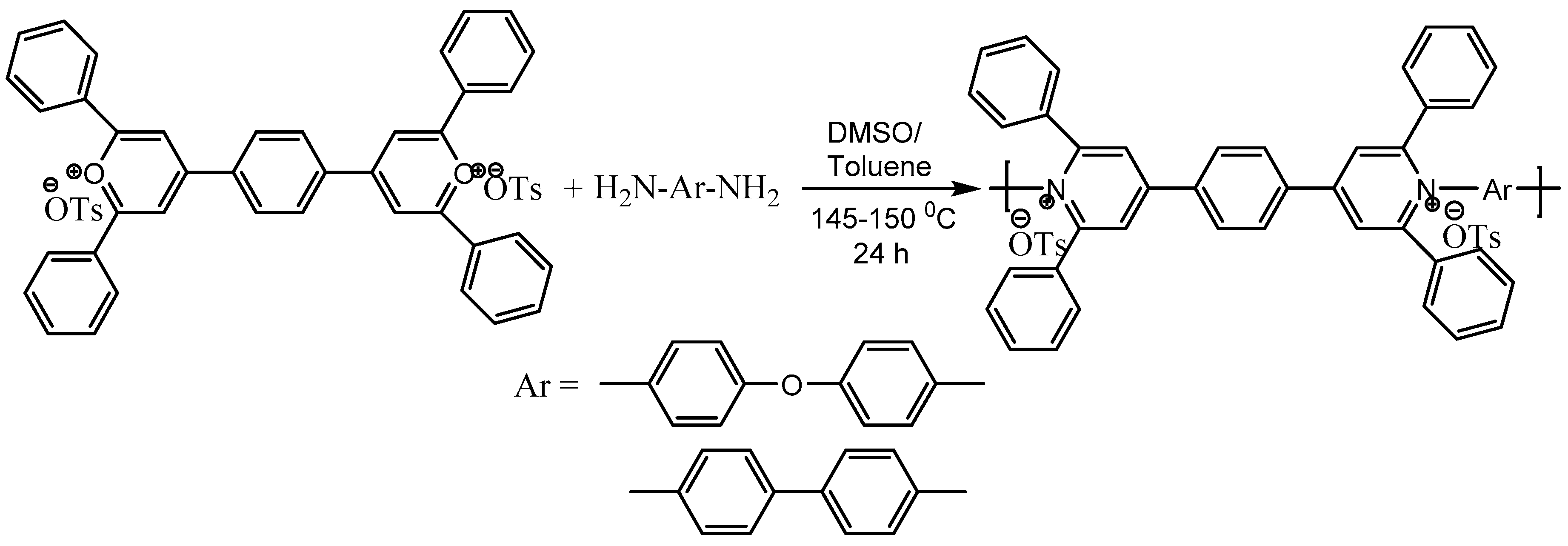
- Bhowmik, P.K.; Burchett, R.A.; Han, H.; Cebe, J.J. Synthesis and characterization of poly(pyridinium salt)s with organic counterion exhibiting both lyotropic liquid-crystalline and light-emitting properties. Macromolecules 2001, 34, 7579–7581. [Google Scholar] [CrossRef]
- Koh, J.J.; Lee, C.I.; Ciulei, M.A.; Han, H.; Bhowmik, P. K.; Kartazaev, V.; Gayen, S.K. Synthesis, optical spectroscopy and laser potential of pyrylium tosylates. J. Mol. Struc. 2018, 1171, 458–465. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Liu, L.; Wang, H.; Zhao, J.; Zhu, Q. p-Toluenesulfonic Acid Promoted Annulation of 2-Alkynylanilines with Activated Ketones: Efficient Synthesis of 4-Alkyl-2, 3-Disubstituted Quinolines. Eur. J. Org. Chem. 2010, 818–822. [Google Scholar] [CrossRef]
- Ao, J.; Liu, Y.; Jia, S.; Xue, L.; Li, D.; Tan, Y.; Qin, W.; Yan, H. Acid-promoted furan annulation and aromatization: An access to benzo [b] furan derivatives. Tetrahedron 2018, 74, 433–440. [Google Scholar] [CrossRef]
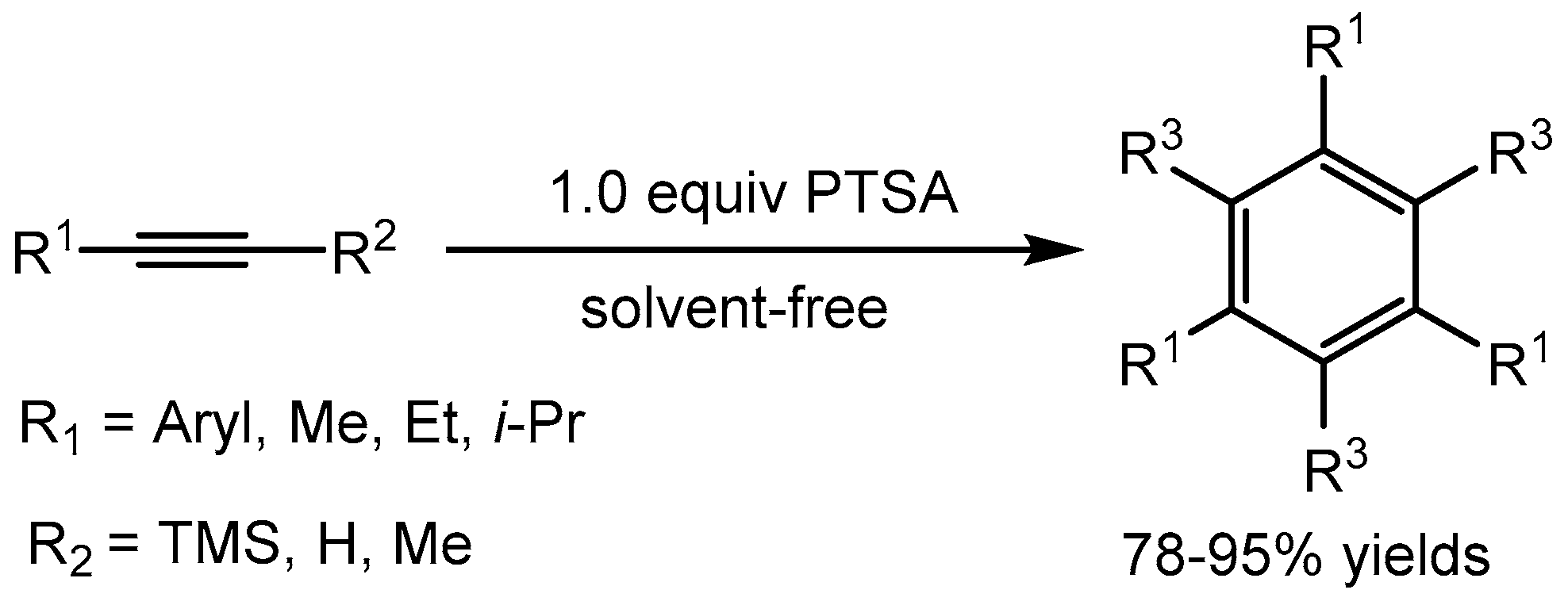
- Gao, Q.; Bao, F.-p.; Feng, X.-j.; Pan, Y.-m.; Wang, H.-s.; Li, D.-p. An efficient approach to the cyclotrimerisation of alkynes: solvent-free synthesis of 1, 3, 5-trisubstituted benzenes using p-toluenesulfonic acid monohydrate. Arkivoc 2013, 3, 49–60. [Google Scholar] [CrossRef]
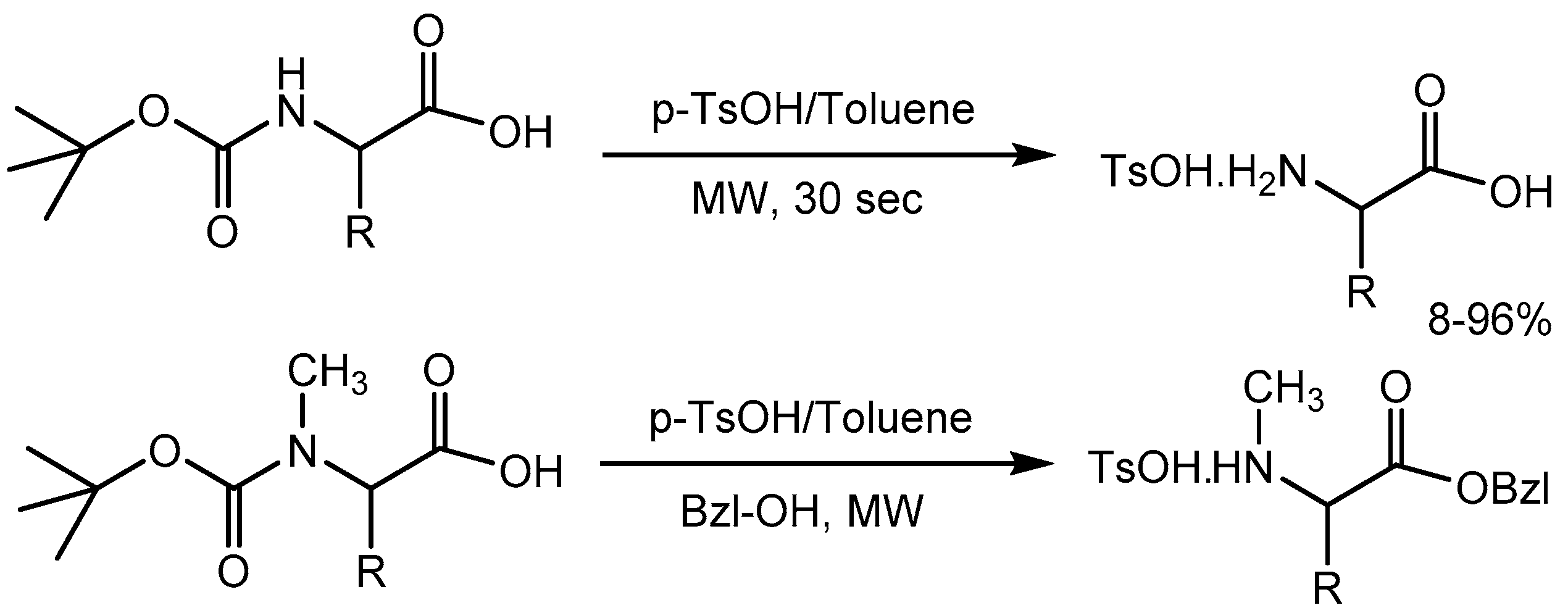
- Babu, V.V.S.; Patil, B.S.; Vasanthakumar, G.R. MW-Enhanced High-Speed Deprotection of Boc Group Using p-TsOH and Concommitant Formation of N-Me-Amino Acid Benzyl Ester p-TsOH Salts. Synth. Commun. 2005, 35, 1795–1802. [Google Scholar] [CrossRef]
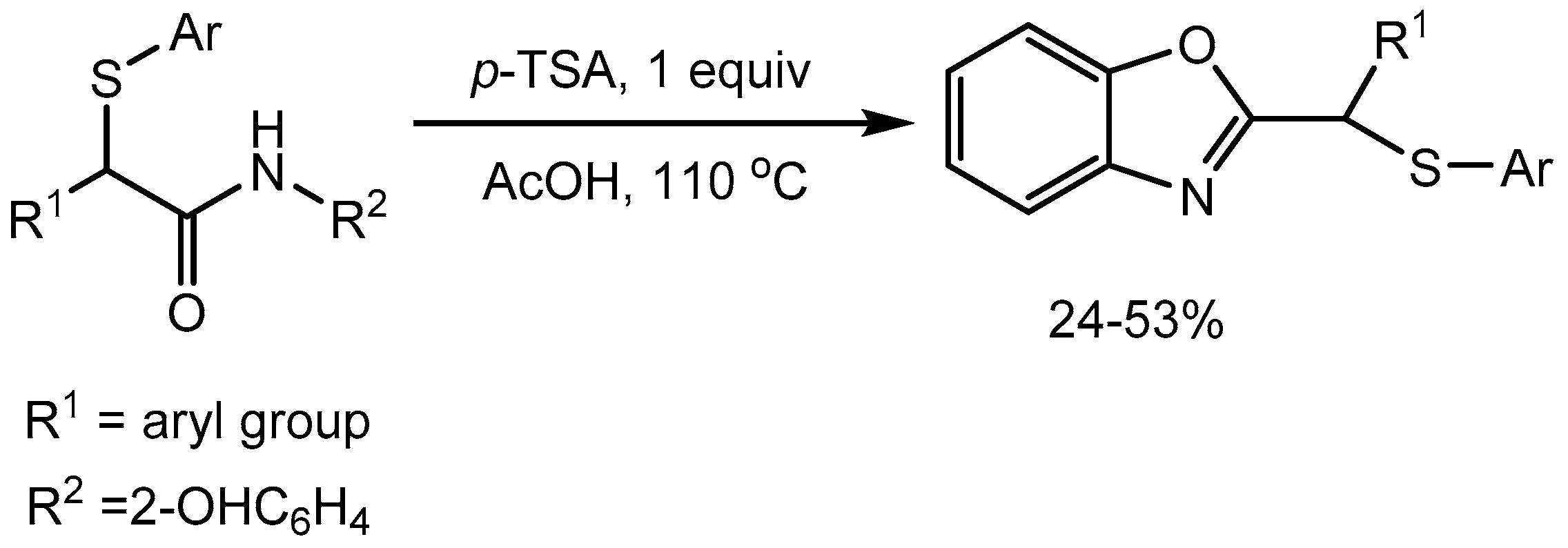
- Tian, S.; Wang, C.; Xia, J.; Wan, J.P.; Liu, Y. Transition Metal-Free, Free-Radical Sulfenylation of the α-C (sp3)− H Bond in Arylacetamides and Its Application Toward 2-Thiomethyl Benzoxazoles Synthesis. Adv. Synth. Catal. 2021, 363, 4627–4631. [Google Scholar] [CrossRef]
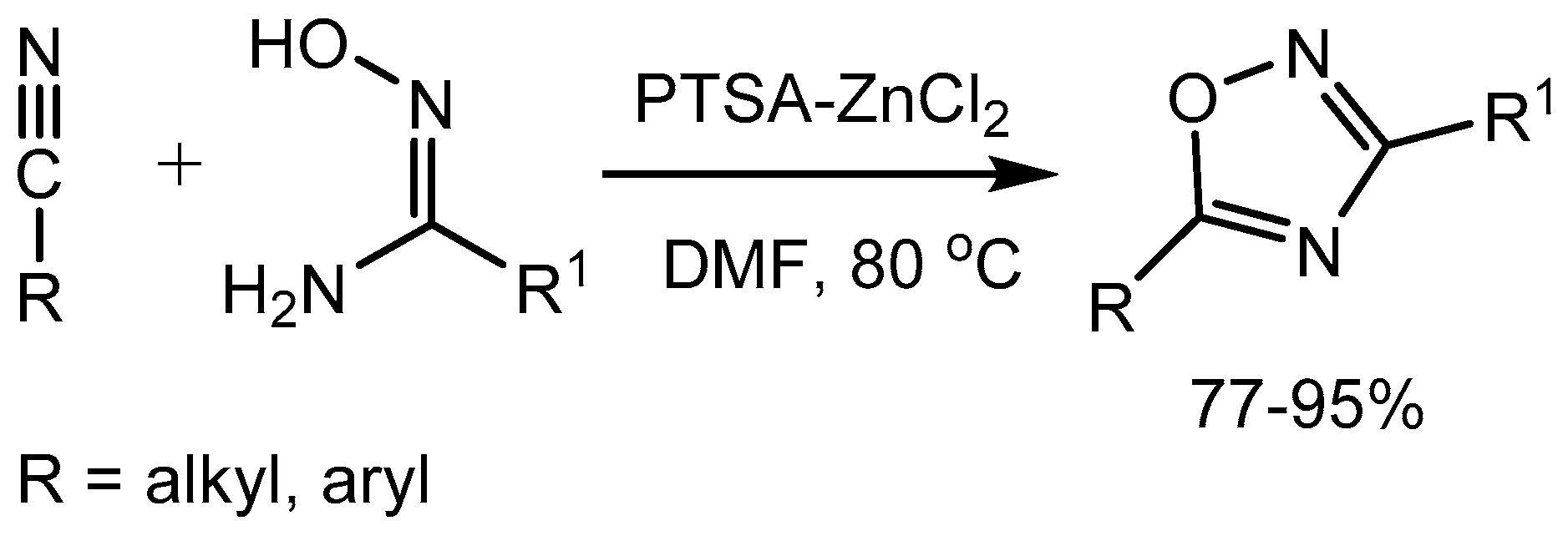
- Augustine, J.K.; Akabote, V.; Hegde, S.G.; Alagarsamy, P. PTSA− ZnCl2: An efficient catalyst for the synthesis of 1, 2, 4-oxadiazoles from amidoximes and organic nitriles. J. Org. Chem. 2009, 74, 5640–5643. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Kasibhatla, S.; Kuemmerle, J.; Kemnitzer, W.; Ollis-Mason, K.; Qiu, L.; Crogan-Grundy, C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery and structure− activity relationship of 3-aryl-5-aryl-1, 2, 4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J. Med. Chem. 2005, 48, 5215–5223. [Google Scholar] [CrossRef] [PubMed]
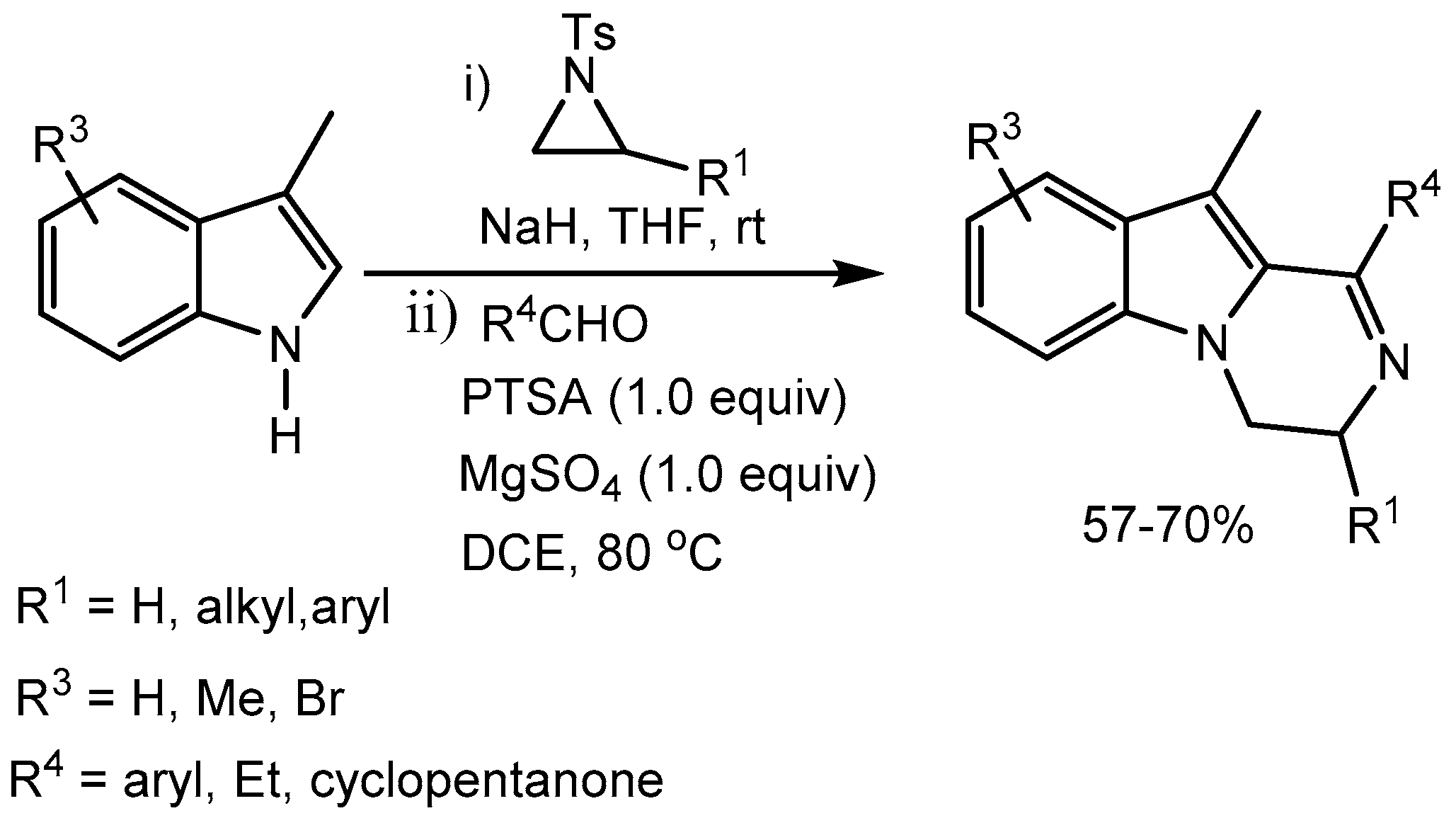
- Wani, I.A.; Das, S.; Mondal, S.; Ghorai, M.K. Stereoselective construction of pyrazinoindoles and oxazinoindoles via ring-opening/pictet-spengler reaction of aziridines and epoxides with 3-methylindoles and carbonyls. J. Org. Chem. 2018, 83, 14553–14567. [Google Scholar] [CrossRef]
- Zhou, W.; Long, Y.; Xiang, H.; Xu, B.; Zhou, X. Iron-catalyzed C–C bond cleavage of oximes for direct coupling of benzothiazole in water. J. Org. Chem. 2023, 88, 4875–4879. [Google Scholar] [CrossRef]
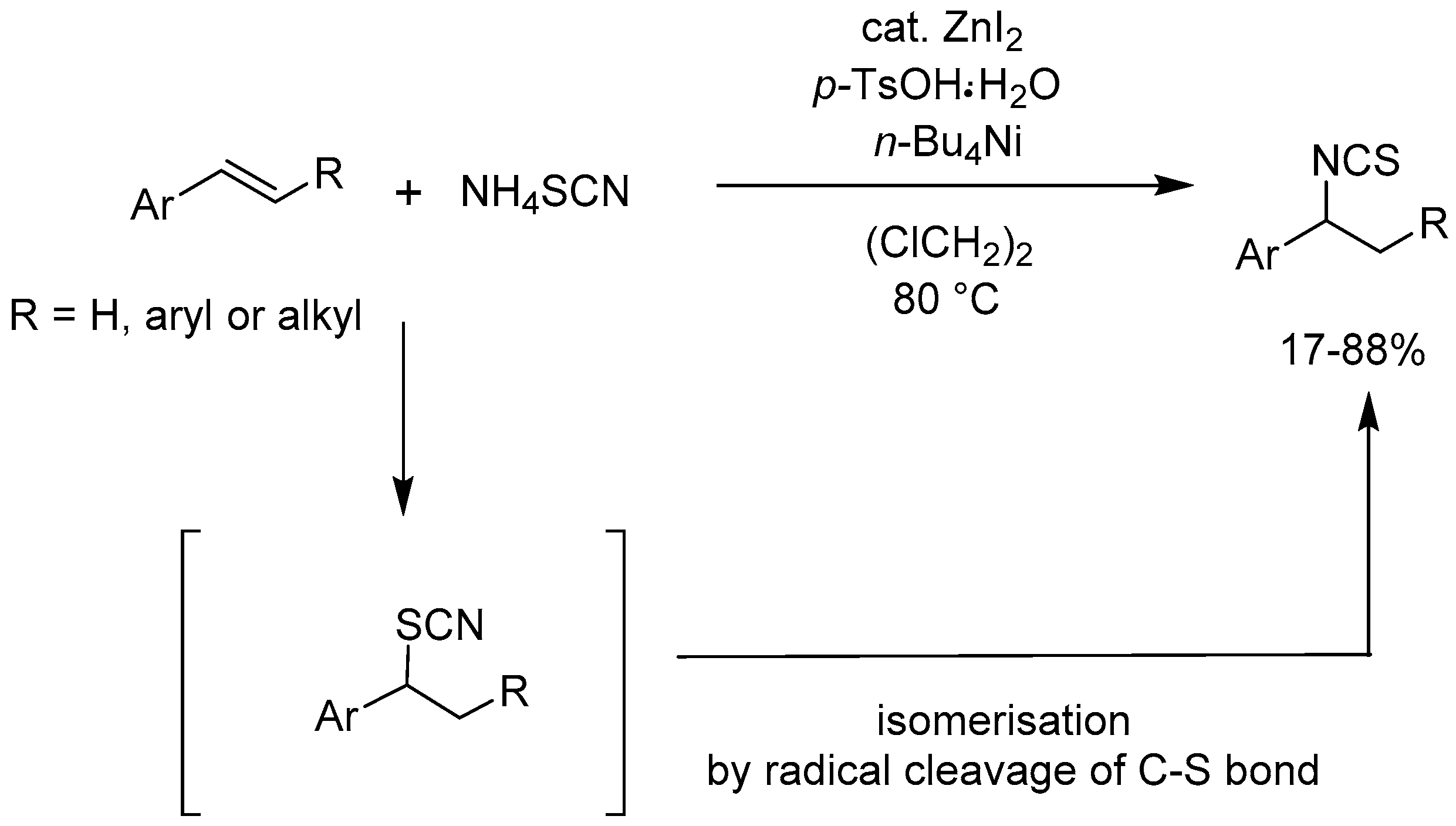
- Taniguchi, N. Zinc-Catalyzed Markovnikov-Type Hydroisothiocyanation of Alkenes with Ammonium Thiocyanate. Synlett 2023, 34, 73–76. [Google Scholar] [CrossRef]
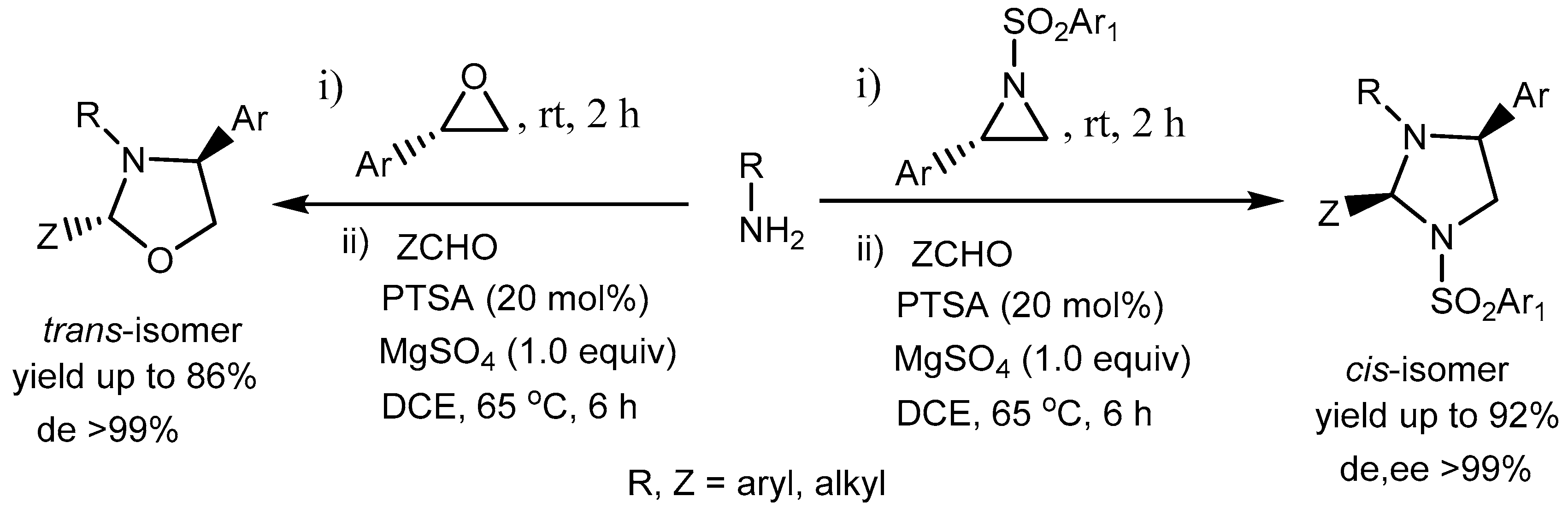
- Tarannum, S.; Sk, S.; Das, S.; Wani, I. A.; Ghorai, M. K. Stereoselective syntheses of highly functionalized imidazolidines and oxazolidines via ring-opening cyclization of activated aziridines and epoxides with amines and aldehydes. J. Org. Chem. 2019, 85, 367–379. [Google Scholar] [CrossRef] [PubMed]
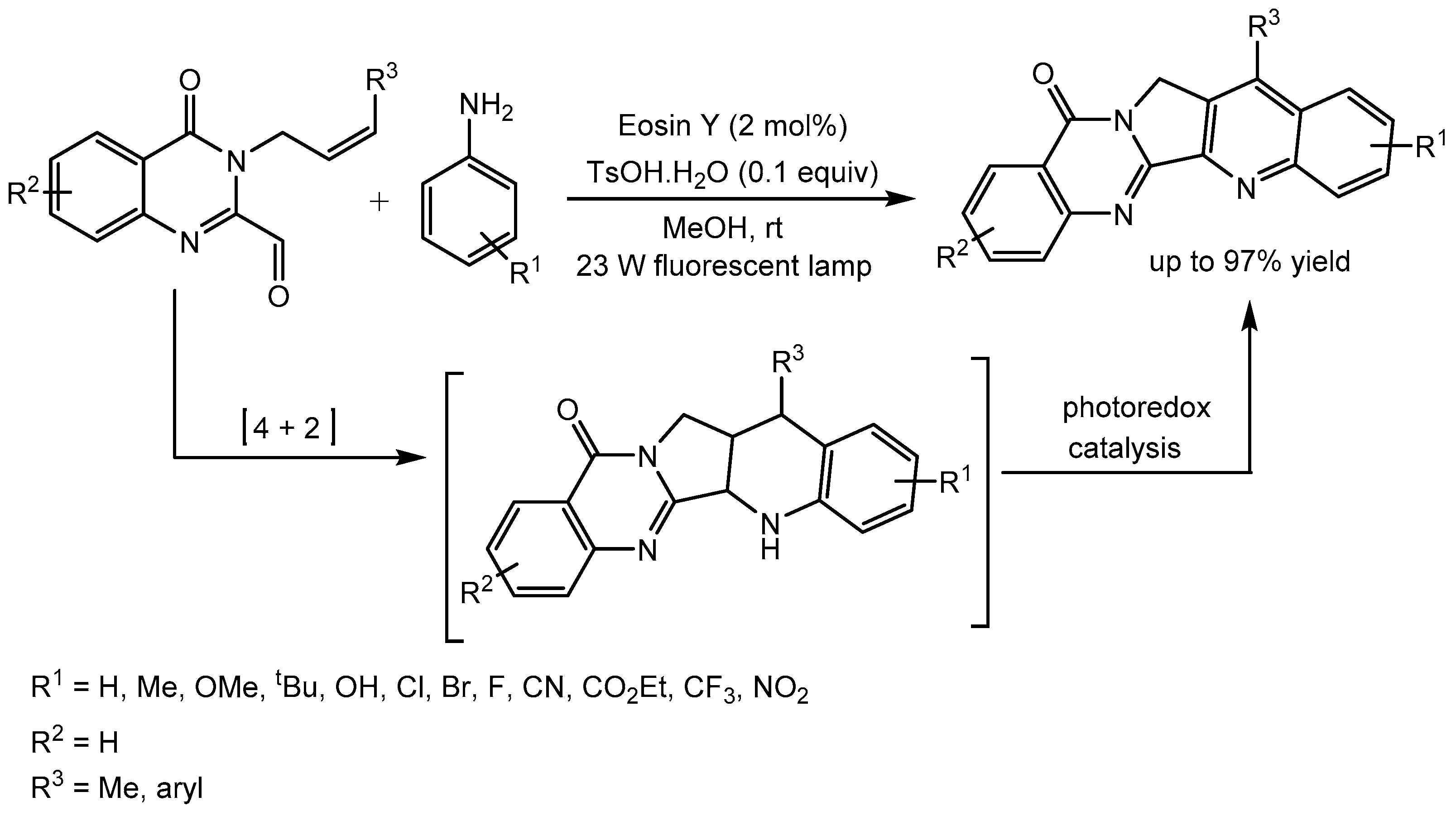
- Wu, F.; Dong, W.; Fan, S.; Yuan, Y.; Liang, C.; Chen, A.; Yin, Z.; Zhang, Z. Rapid Synthesis of Luotonin A Derivatives via Synergistic Visible-Light Photoredox and Acid Catalysis. J. Org. Chem. 2022, 87, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
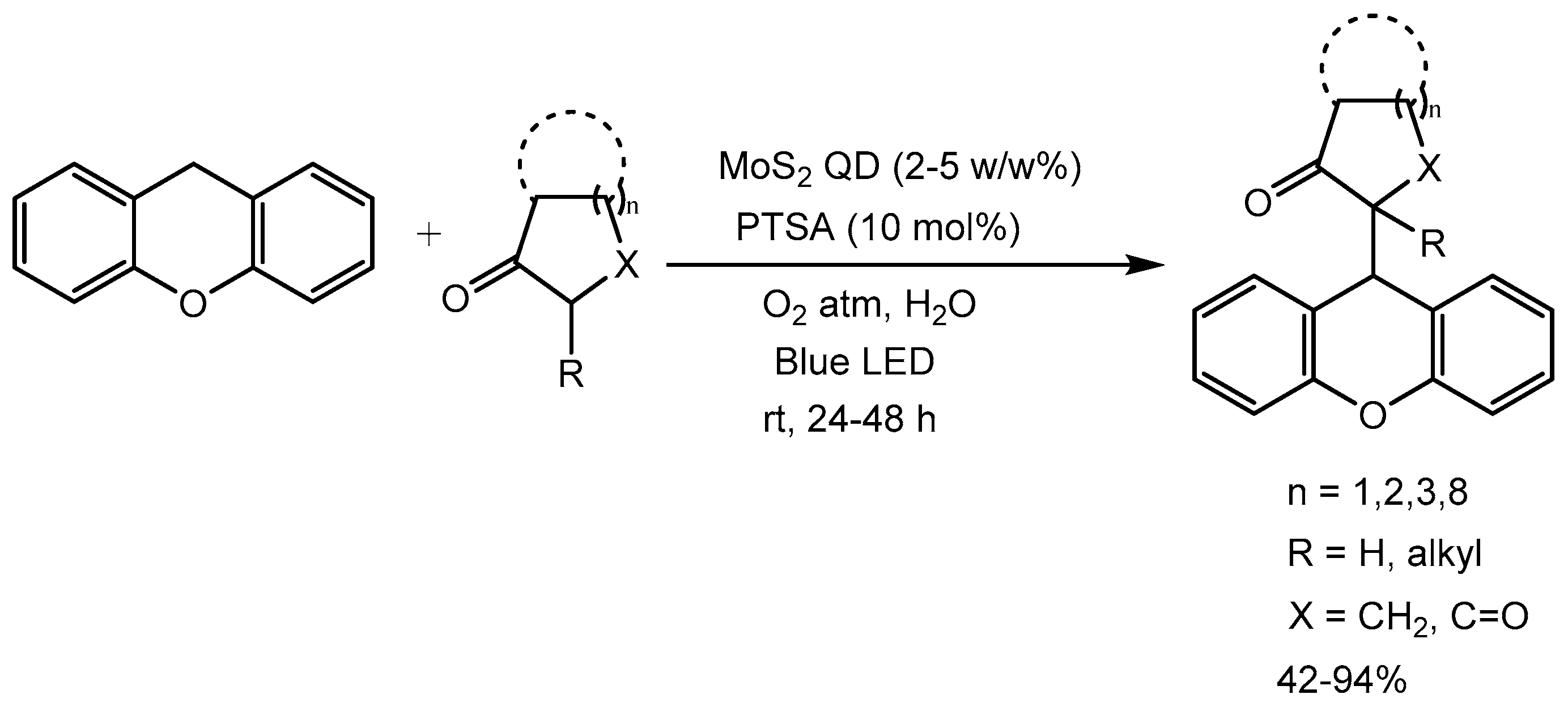
- Deore, J.P.; De, M. Photoredox C(sp3)−C(sp3) Cross-Dehydrogenative Coupling of Xanthene with β-keto Moiety using MoS2 Quantum Dot (QD) Catalyst. Adv. Synth. Catal. 2022, 364, 3049–3058. [Google Scholar] [CrossRef]
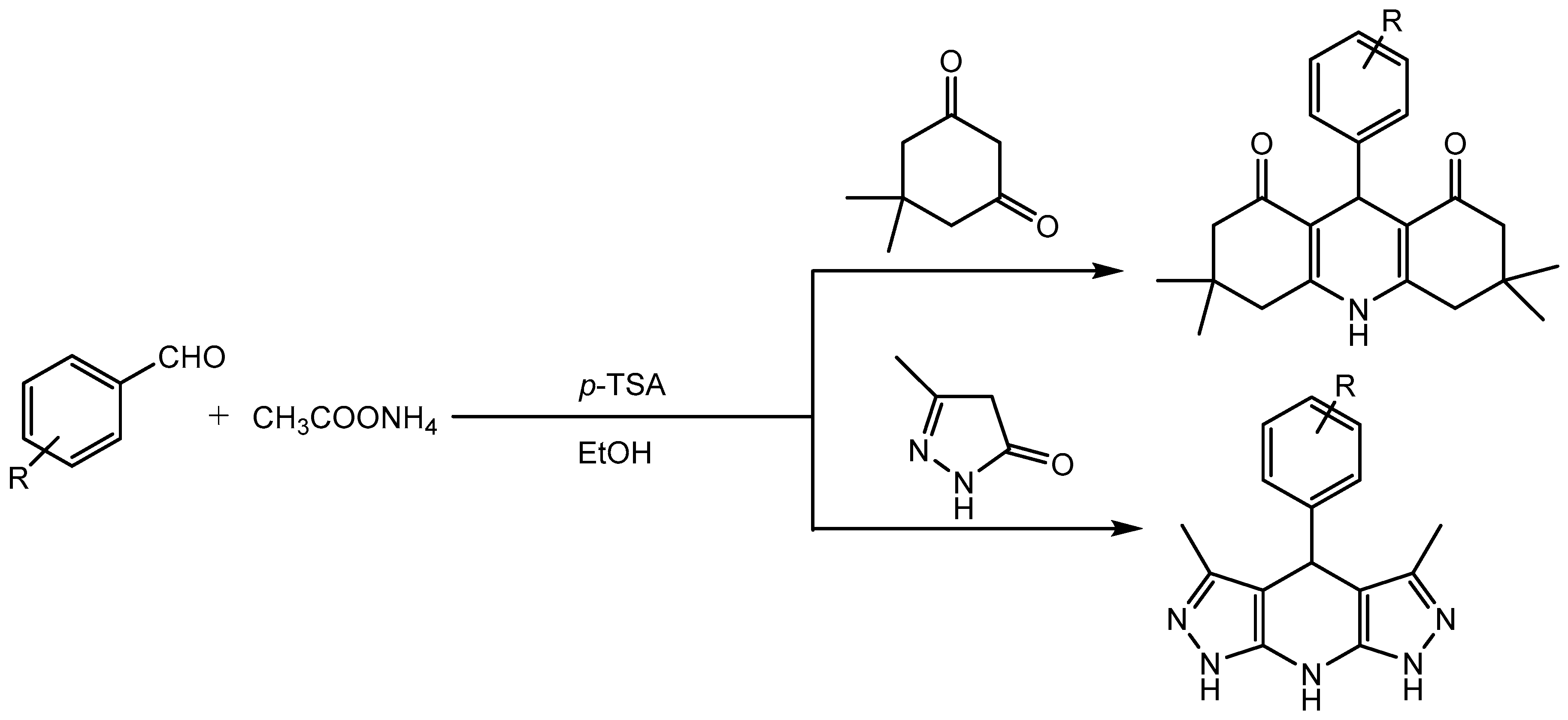
- Chaudhari, M.A.; Gujar, J.B.; Kawade, D.S.; Jogdand, N.R.; Shingare, M.S. A highly efficient and sustainable synthesis of dihydropyrano [2, 3-c] pyrazoles using polystyrene-supported p-toluenesulfonic acid as reusable catalyst. Cogent Chemistry 2015, 1, 1063830. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; Metz, P.; Metwally, N.H.; El-Mahrouky, S.F. Synthesis and molluscicidal activity of new cinnoline and pyrano [2, 3-c] pyrazole derivatives. Archiv der Pharmazie: An International Journal Pharmaceutical and Medicinal Chemistry, 2006; 339, 456–460. [Google Scholar]
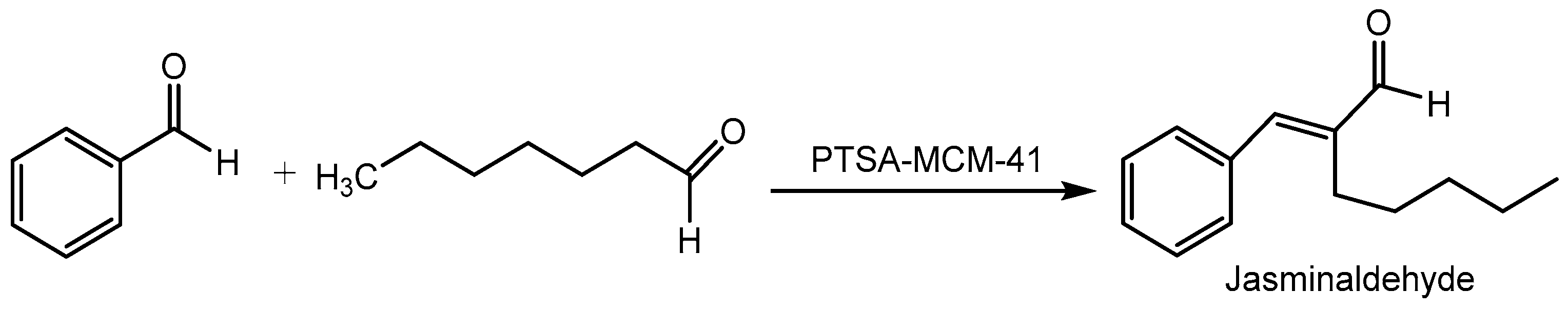
- Ganga, V.S.R.; Abdi, S.H.; Kureshy, R.I.; Noor-ul, H.K.; Bajaj, H.C. p-Toluene sulfonic acid (PTSA)-MCM-41 as a green, efficient and reusable heterogeneous catalyst for the synthesis of jasminaldehyde under solvent-free condition. Journal of Molecular Catalysis A: Chemical, 2016; 420, 262–271. [Google Scholar]
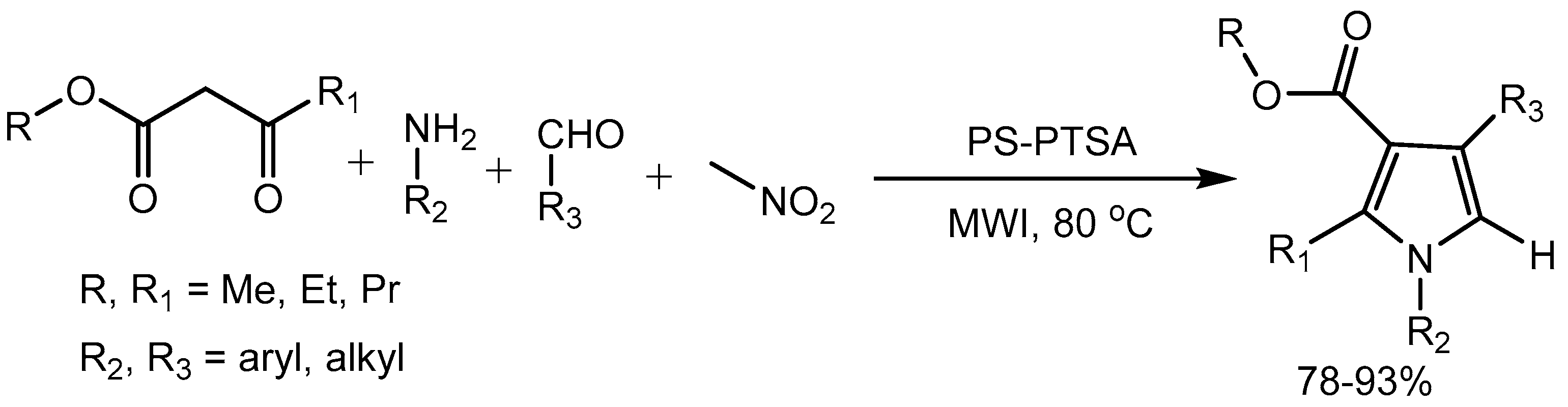
- Shinde, V.V.; Lee, S.D.; Jeong, Y.S.; Jeong, Y.T. p-Toluenesulfonic acid doped polystyrene (PS-PTSA): solvent-free microwave assisted cross-coupling-cyclization–oxidation to build one-pot diversely functionalized pyrrole from aldehyde, amine, active methylene, and nitroalkane. Tetrahedron Letters 2015, 56, 859–865. [Google Scholar] [CrossRef]
- Stopka, T.; Niggemann, M. Metal free carboamination of internal alkynes–an easy access to polysubstituted quinolines. Chem. Commun. 2016, 52, 5761–5764. [Google Scholar] [CrossRef]
- Tang, S.-Z.; Bian, H.-L.; Zhan, Z.-S.; Chen, M.-E.; Lv, J.-W.; Xie, S.; Zhang, F.-M. p-Toluenesulfonic acid catalysed fluorination of α-branched ketones for the construction of fluorinated quaternary carbon centres. Chem. Commun. 2018, 54, 12377–12380. [Google Scholar] [CrossRef]
- Sloan, N. L.; Luthra, S. K.; McRobbie, G.; Pimlott, S. L.; Sutherland, A. A one-pot radioiodination of aryl amines via stable diazonium salts: preparation of 125 I-imaging agents. Chem. Commun. 2017, 53, 11008–11011. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.-C.; Lu, W.-Q.; Zhou, D.-B.; Wang, G.-W. Unexpected Diels–Alder reaction of [60] fullerene with electron-deficient ferrocenes as cyclopentadiene surrogates. Chem. Commun. 2021, 57, 13389–13392. [Google Scholar] [CrossRef]
- Faggyas, R.k.J.; Grace, M.; Williams, L.; Sutherland, A. Multibond forming tandem reactions of anilines via stable aryl diazonium salts: one-pot synthesis of 3, 4-dihydroquinolin-2-ones. J. Org. Chem. 2018, 83, 12595–12608. [Google Scholar] [CrossRef] [PubMed]
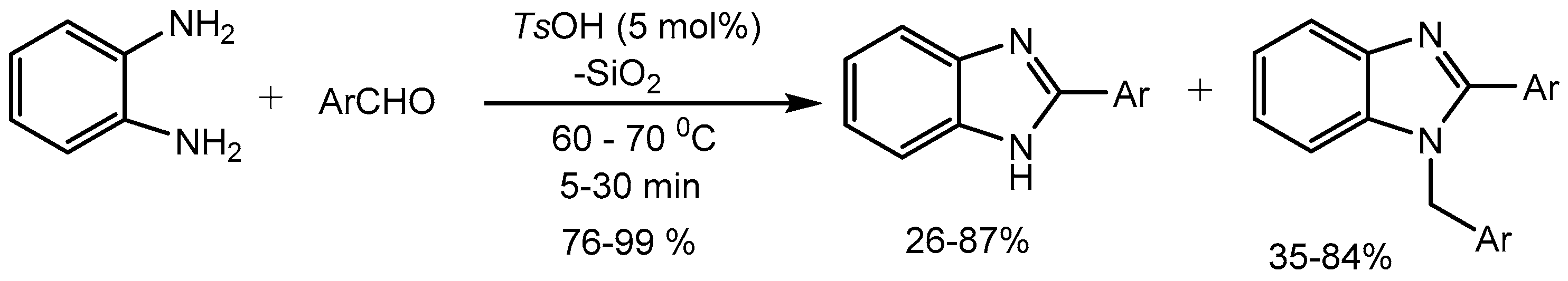
- Chakrabarty, M.; Mukherjee, R.; Karmakar, S.; Harigaya, Y. Tosic acid-on-silica gel: a cheap and eco-friendly catalyst for a convenient one-pot synthesis of substituted benzimidazoles. Monatsh Chem 2007, 138, 1279–1282. [Google Scholar] [CrossRef]
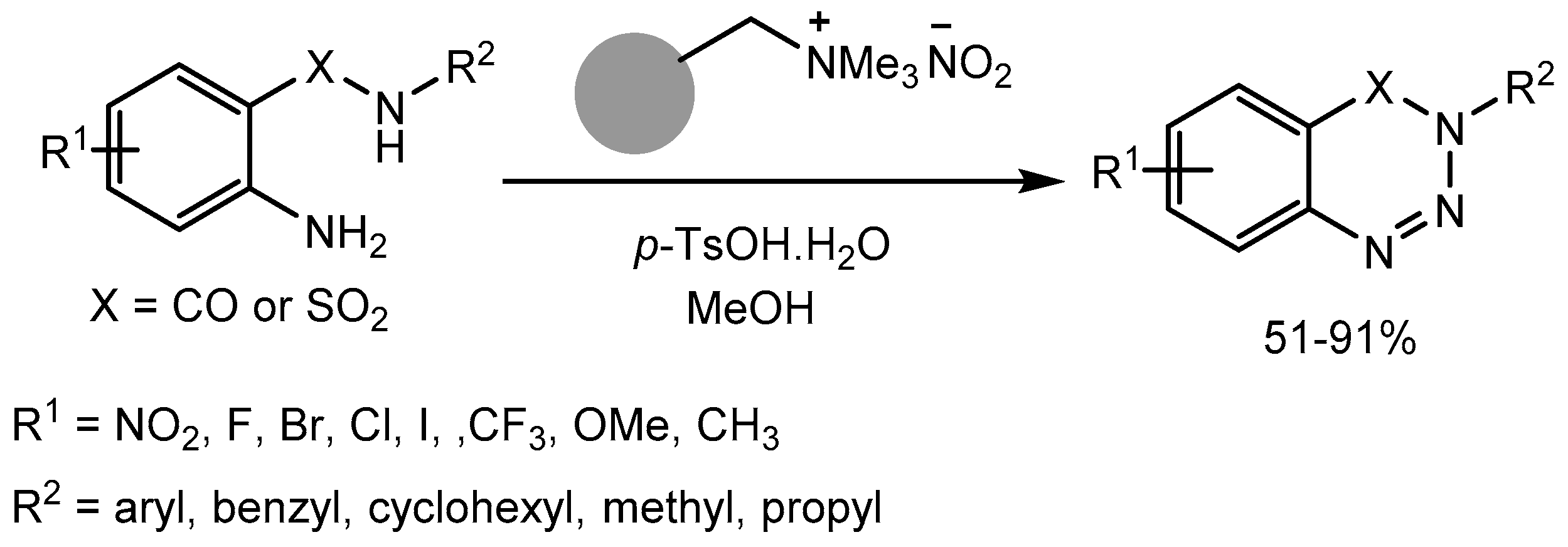
- McGrory, R.; Faggyas, R. J.; Sutherland, A. One-pot synthesis of N-substituted benzannulated triazoles via stable arene diazonium salts. Org. Biomol. Chem. 2021, 19, 6127–6140. [Google Scholar] [CrossRef] [PubMed]
- Saejong, P.; Somprasong, S.; Rujirasereesakul, C.; Luanphaisarnnont, T. Direct Synthesis of Coumarin Derivatives from Alkynoic Esters via Dual Organocatalysis. Synlett 2022, 33, 1399–1404. [Google Scholar]
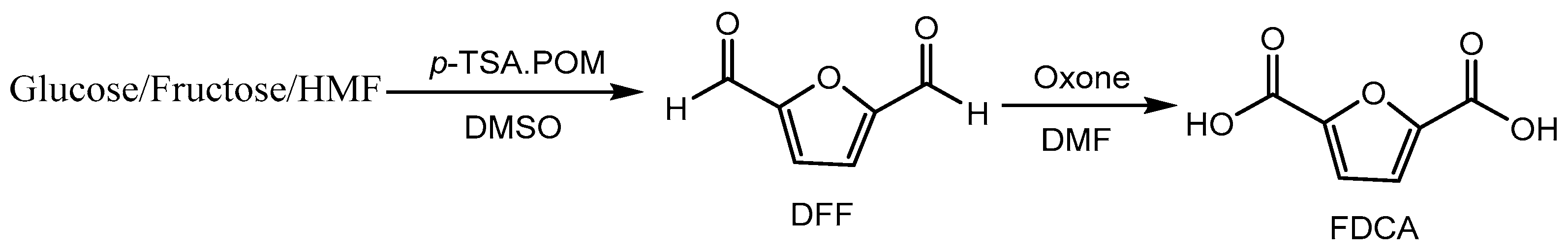
- Tamboli, A.T.B.; Kirdant, S.P.; Jadhav, V.H. Metal-free approach towards efficient synthesis of FDCA using a p-toluene sulfonic acid (p-TSA)-derived heterogeneous solid acid catalyst and oxone over two steps from HMF, fructose and glucose. New J. Chem. 2022, 46, 10272–10279. [Google Scholar] [CrossRef]
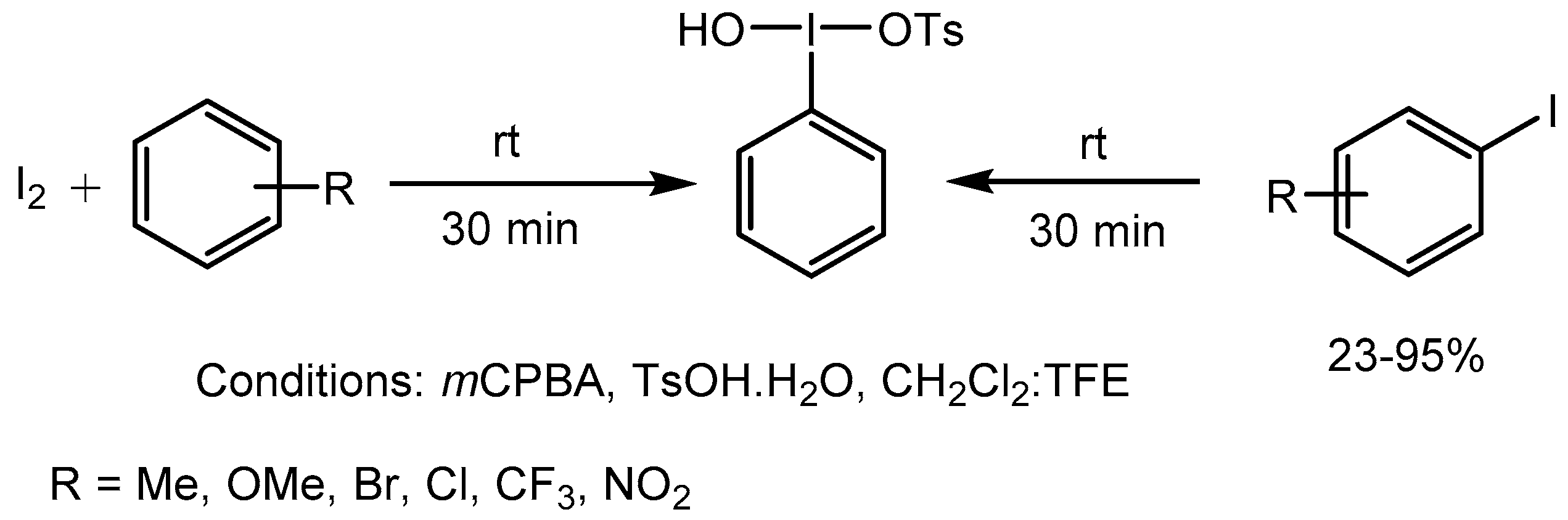
- Merritt, E.A.; Carneiro, V.M.; Silva Jr, L.F.; Olofsson, B. Facile synthesis of Koser’s reagent and derivatives from iodine or aryl iodides. J. Org. Chem. 2010, 75, 7416–7419. [Google Scholar] [CrossRef] [PubMed]
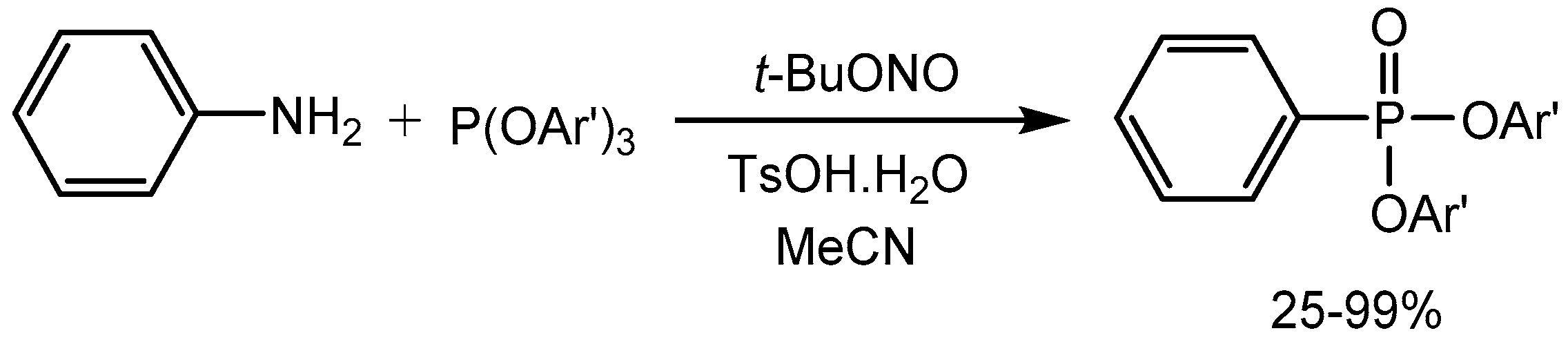
- Wang, S.; Qiu, D.; Mo, F.; Zhang, Y.; Wang, J. Metal-free aromatic carbon–phosphorus bond formation via a sandmeyer-type reaction. J. Org. Chem. 2016, 81, 11603–11611. [Google Scholar] [CrossRef]
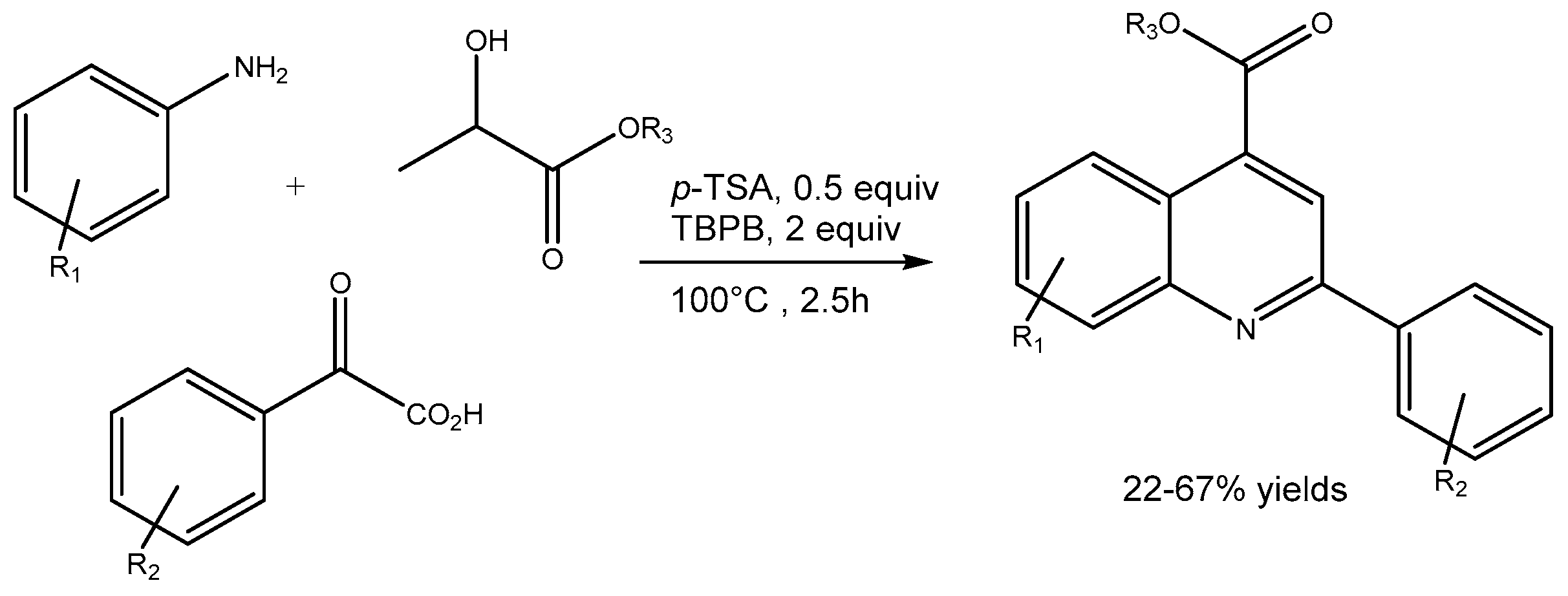
- Huang, L.; Yang, L.; Wan, J.-P.; Zhou, L.; Liu, Y.; Hao, G. Metal-free three-component assemblies of anilines, α-keto acids and alkyl lactates for quinoline synthesis and their anti-inflammatory activity. Org. Biomol. Chem. 2022, 20, 4385–4390. [Google Scholar] [CrossRef]
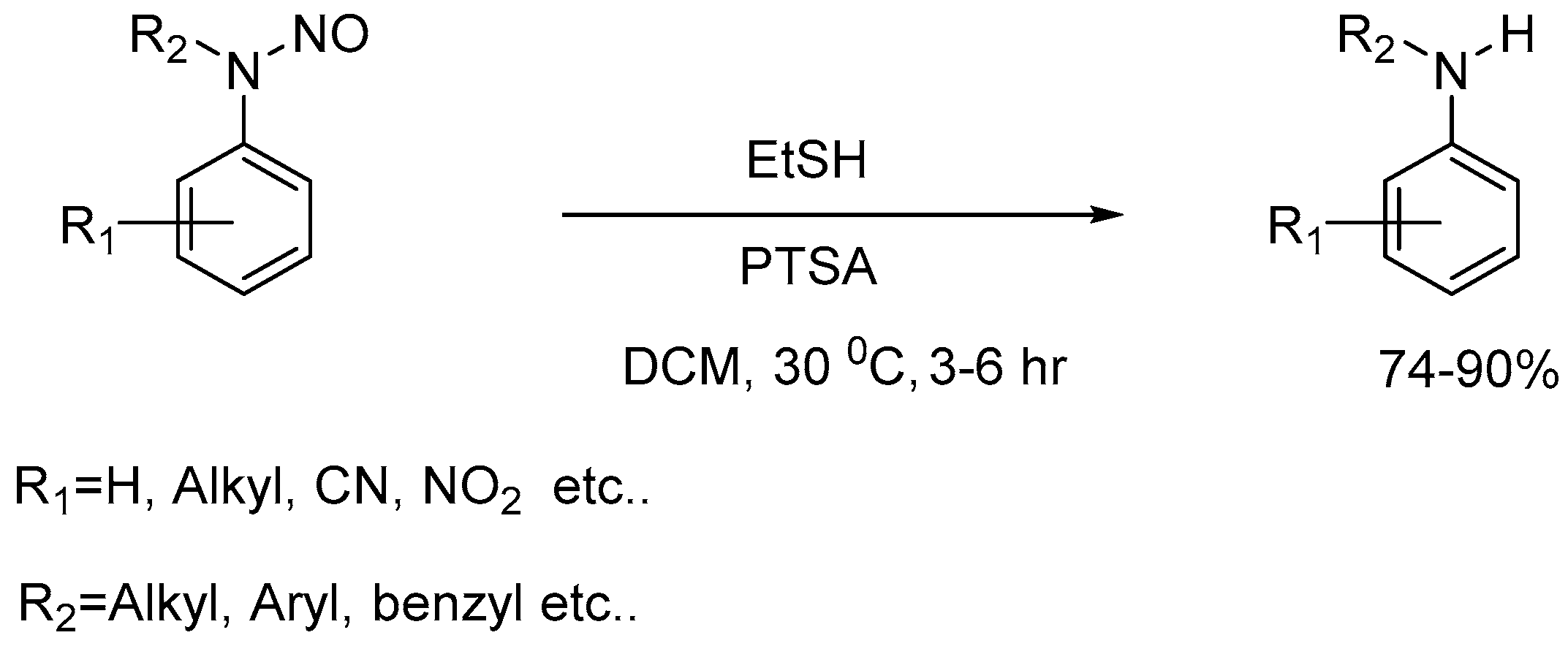
- Kanaujiya, V. K.; Tiwari, V.; Baranwal, S.; Srivastava, V.; Kandasamy, J. Denitrosation of Aryl-N-nitrosamines by a Transnitrosation Strategy Using Ethanethiol and p-Toluenesulfonic Acid under Mild Reaction Conditions. Synlett 2023, 34, 970–974. [Google Scholar]
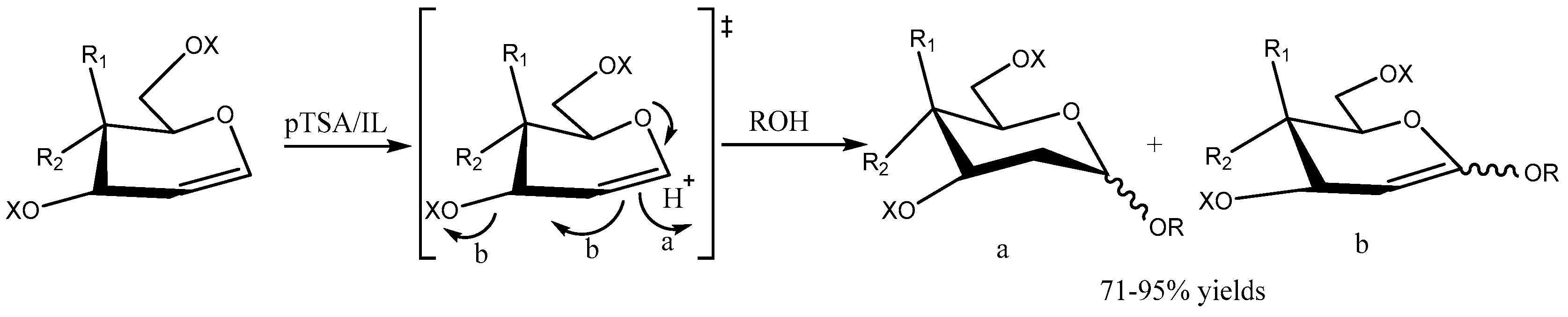
- Dı´az, G.; Ponzinibbio, A.; Bravo, R. D. pTSA/[bmim][BF4] Ionic Liquid: A Powerful Recyclable Catalytic System for the Synthesis of α-2-Deoxyglycosides. Top Catal, 2012, 55, 644–648. [Google Scholar] [CrossRef]
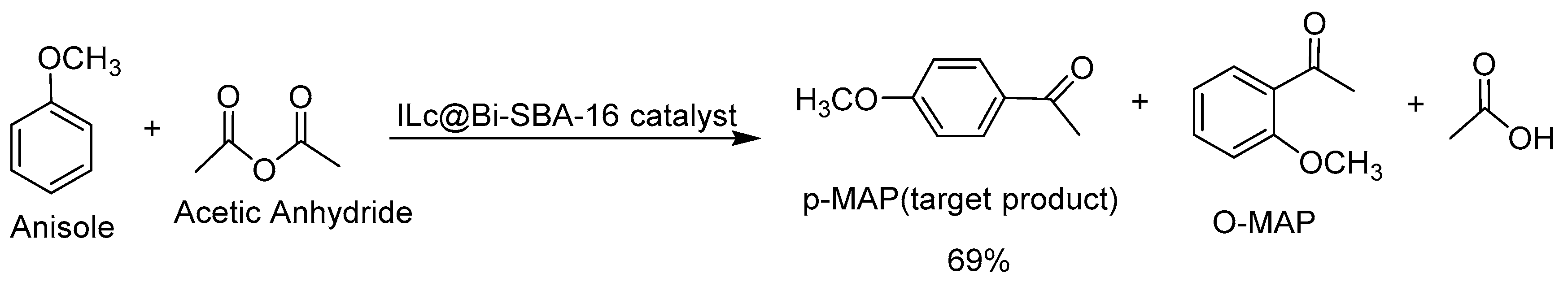
- Gao, G.; Zhao, Q.; Yang, C.; Jiang, T. p-Toluenesulfonic acid functionalized imidazole ionic liquids encapsulated into bismuth SBA-16 as high-efficiency catalysts for Friedel–Crafts acylation reaction. Dalton Trans. 2021, 50, 5871–5882. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-P.; Kirilov, P.; Matondo, H.; Baboulène, M. The reusable couple “PTSA/1-alkyl-3-methylimidazolium ionic liquids”: excellent reagents–catalysts for halogenation of fatty diols. Journal of Molecular Catalysis A: Chemical 2004, 218, 41–45. [Google Scholar] [CrossRef]
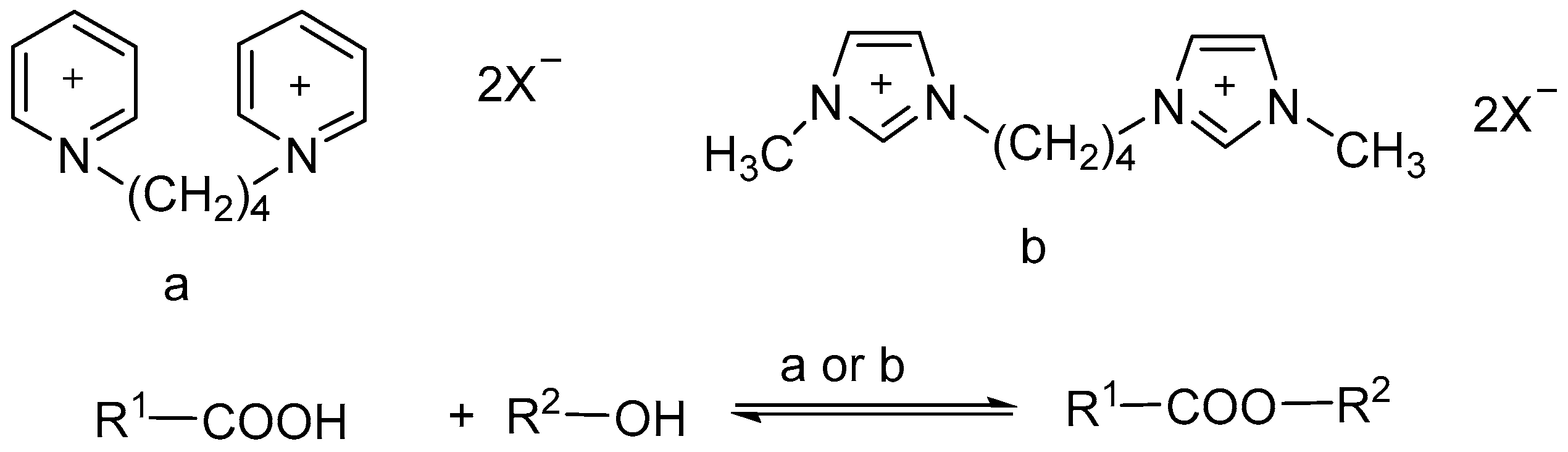
- Zhao, D.; Liu, M.; Ge, J.; Zhang, J.; Ren, P. Synthesis of binuclear ionic liquids and their catalytic activity for esterification. Chin. J. Org. Chem. 2012, 32, 2382. [Google Scholar] [CrossRef]
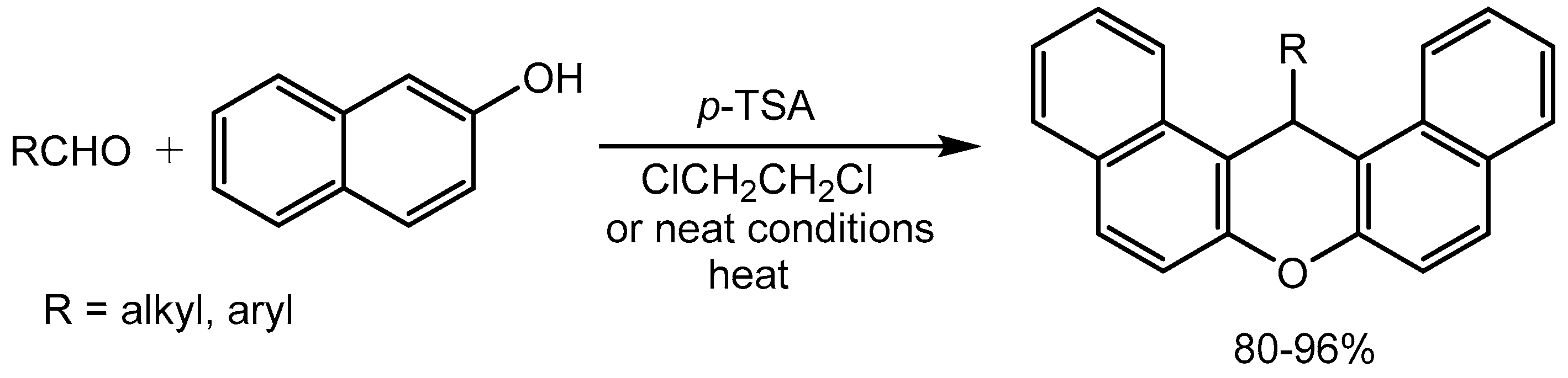
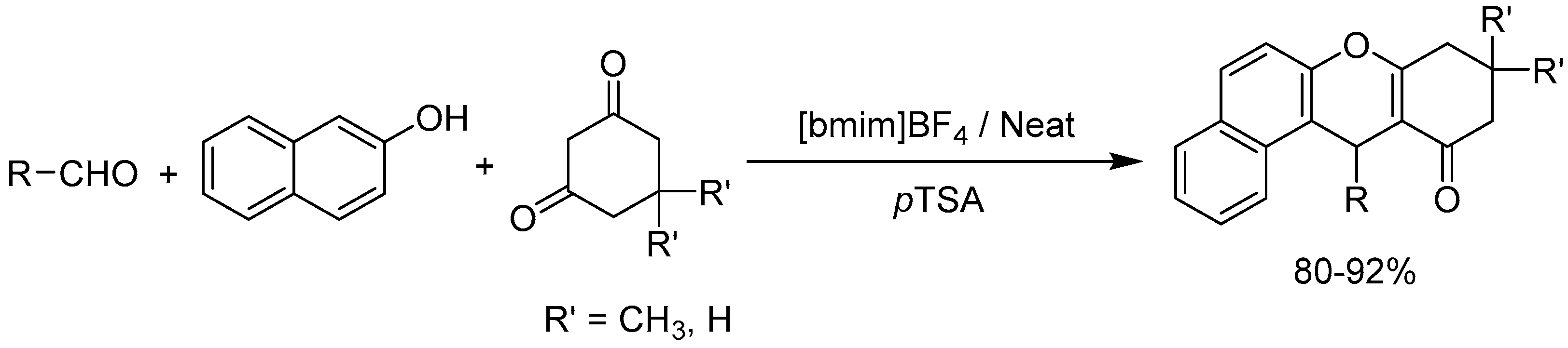
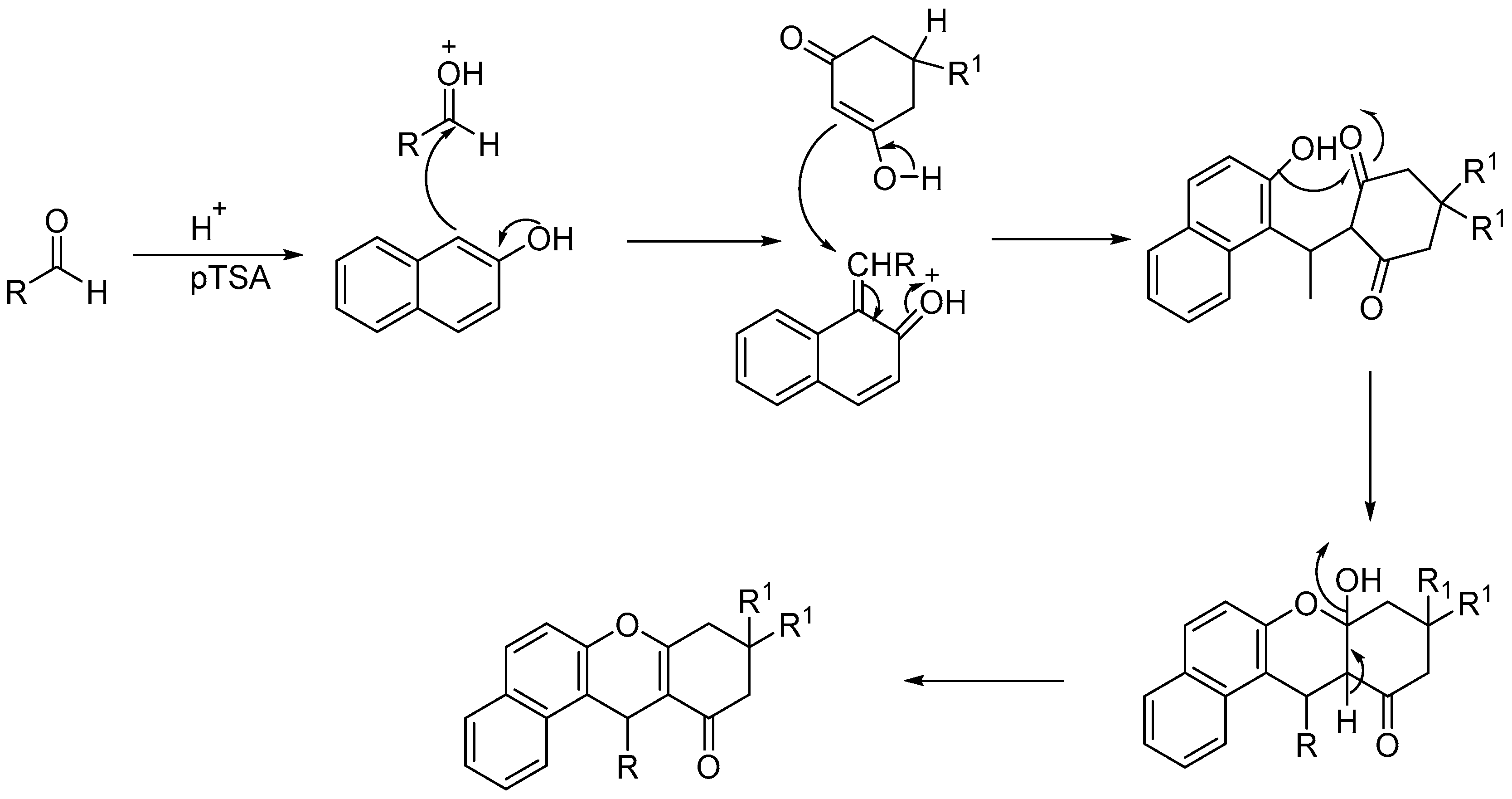
- Khurana, J. M.; Magoo, D. pTSA-catalyzed one-pot synthesis of 12-aryl-8, 9, 10, 12-tetrahydrobenzo [a] xanthen-11-ones in ionic liquid and neat conditions. Tetrahedron Letters 2009, 50, 4777–4780. [Google Scholar] [CrossRef]
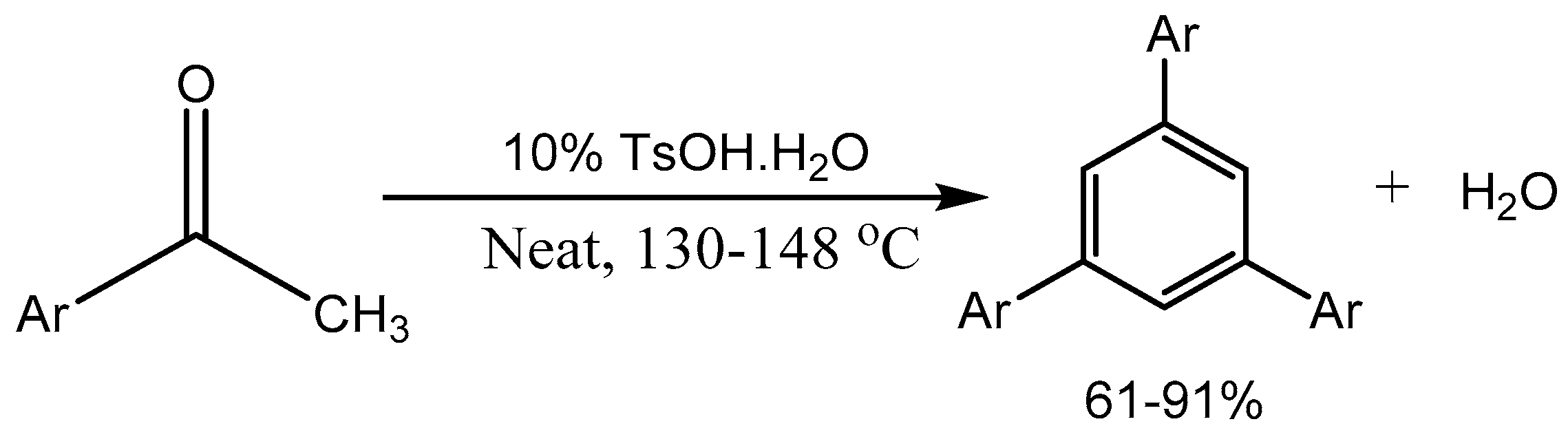

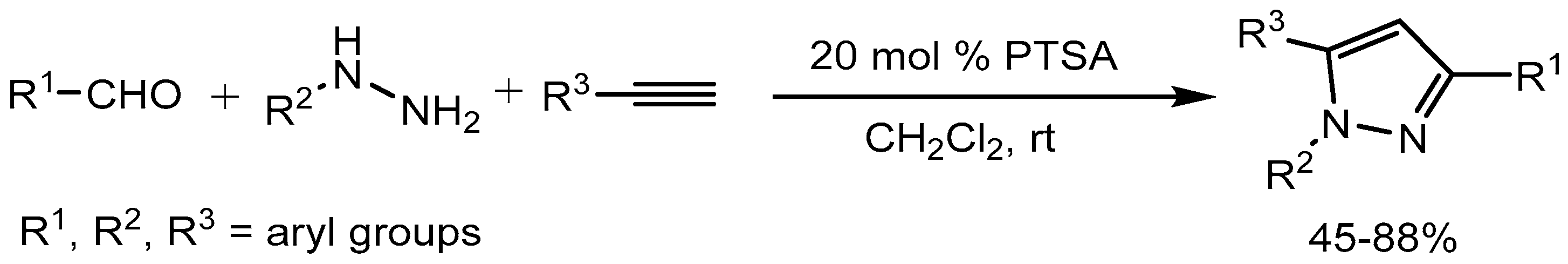
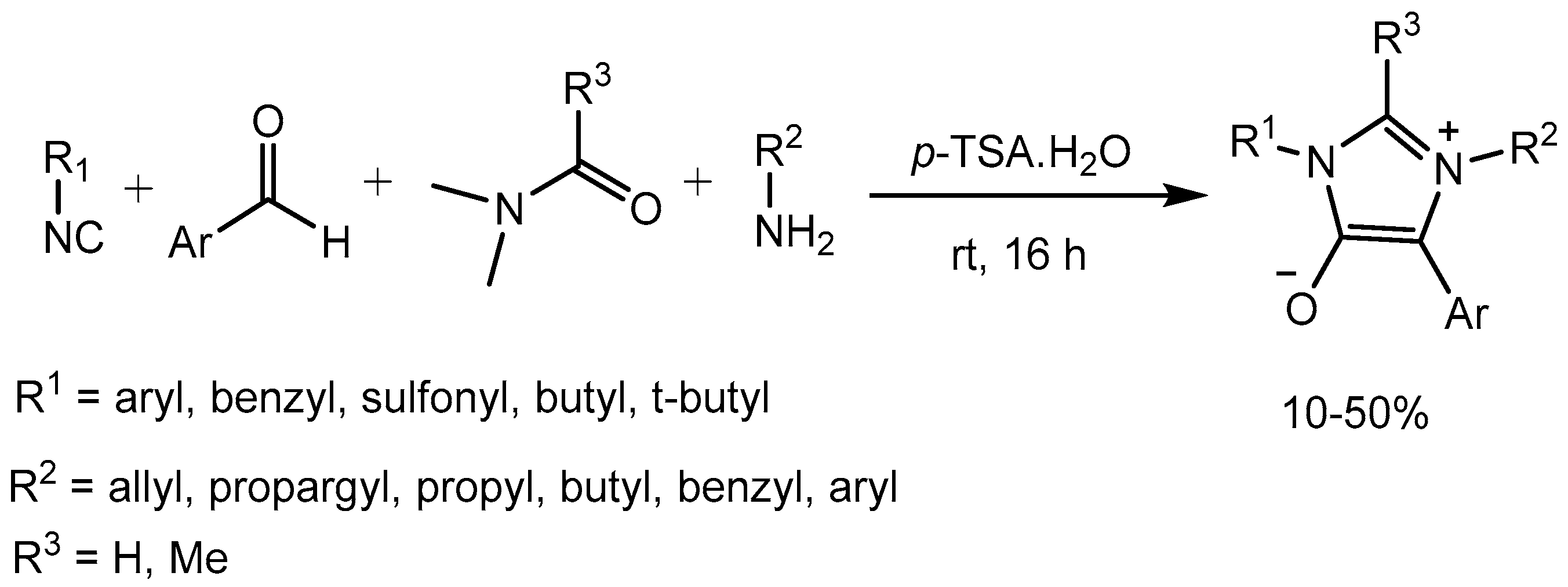
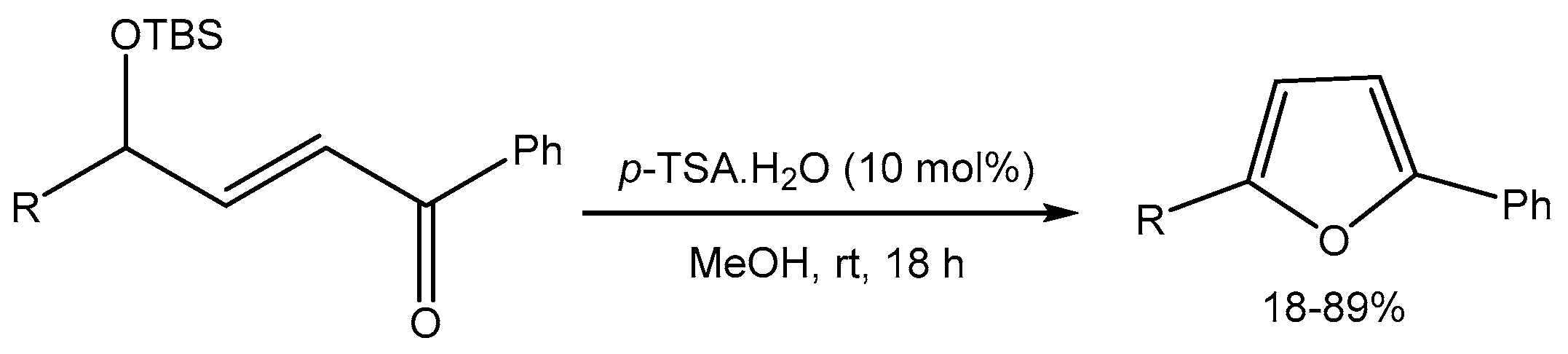
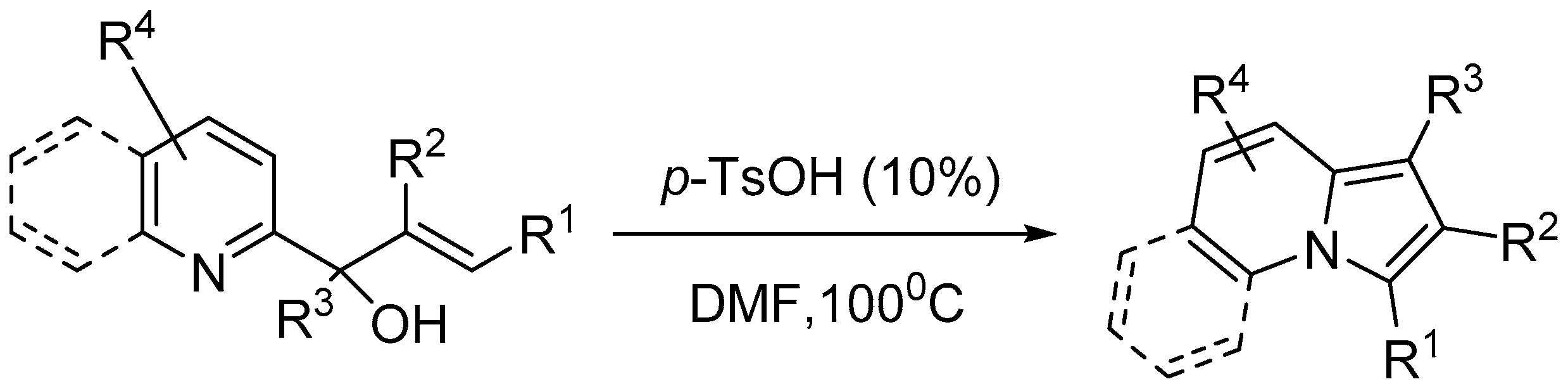
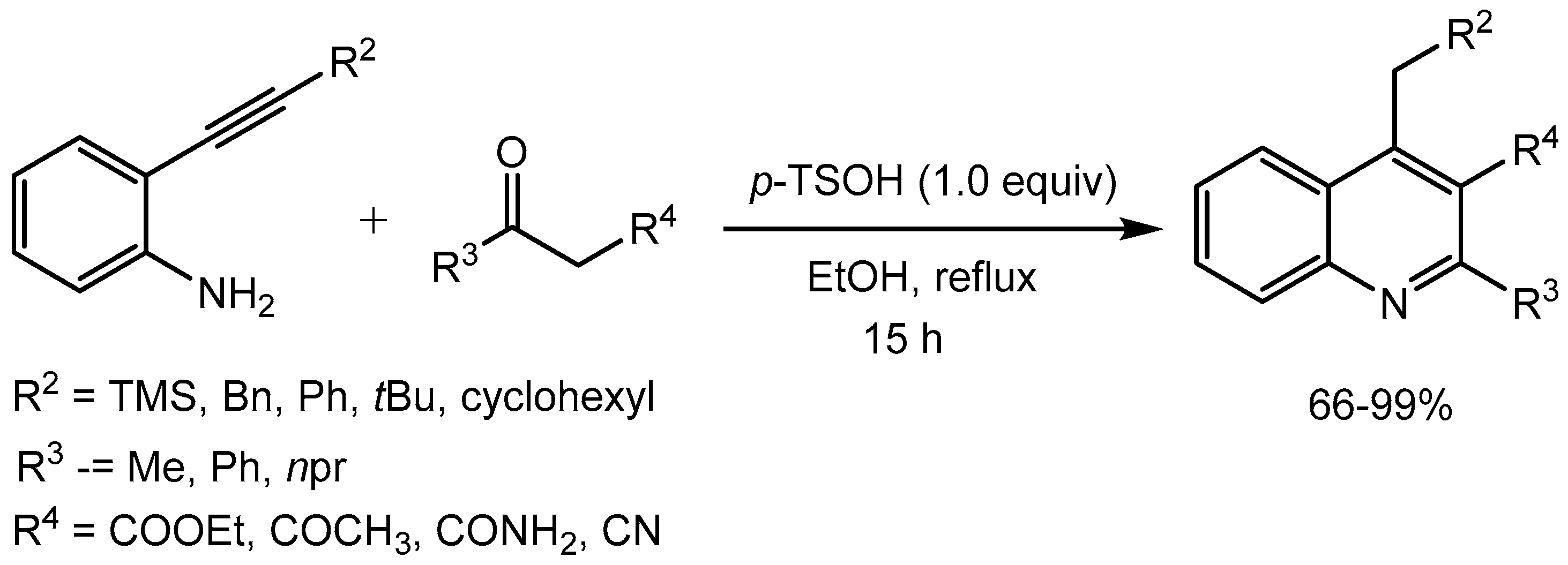
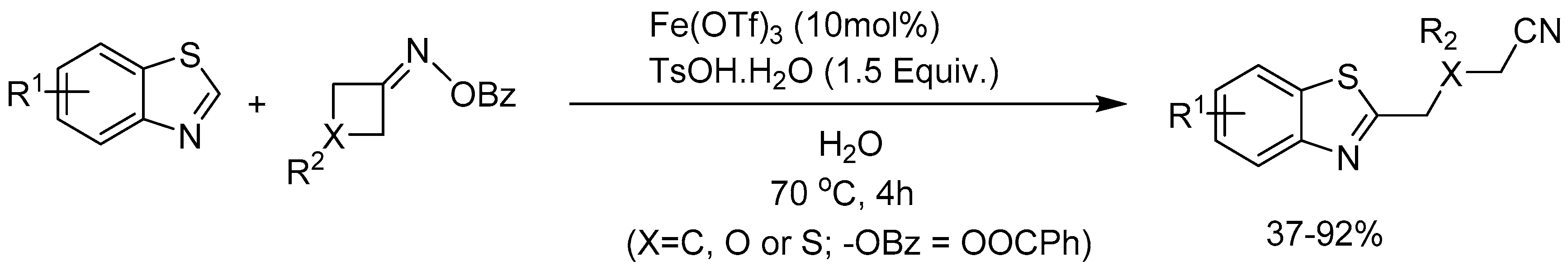
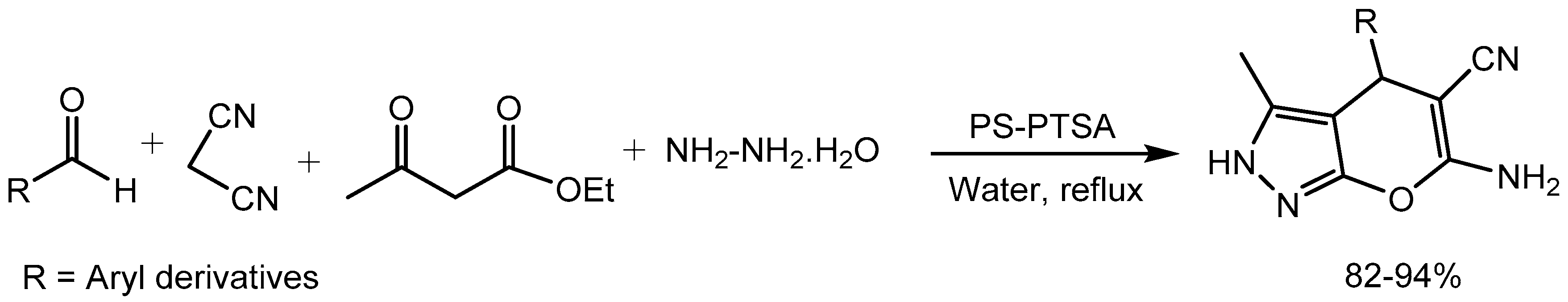


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).