Submitted:
18 April 2024
Posted:
19 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Supramolecular Chemistry in Confined Space
3. Supramolecular Assemblies
4. Significant Advances in MOFs Architecture and Its Analogues Assembled by Novel Strategies
5. Electrochemical Synthesis of MOFs and Their Relating Specific Architecture
6. Switchable MOFs
7. Summary, conclusions, and Outlook
Author Contributions
Conflicts of Interest
References
- Wang, H.; Tian, J.; Xu, Z. Y.; Zhang, D. W.; Wang, H.; Xie, S. H.; Xu, D. W.; Ren, Y. H.; Liu, Y.; Li, Z. T. Supramolecular Metal-Organic Frameworks That Display High Homogeneous and Heterogeneous Photocatalytic Activity for H2 Production. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Mon, M.; Greco, R.; Kalinke, L. H. G.; Vidal-Moya, A.; Fernandez, A.; Winpenny, R. E. P.; Domenech-Carbo, A.; Leyva-Perez, A.; Armentano, D.; Pardo, E.; Ferrando-Soria, J. Self-Assembly of Catalytically Active Supramolecular Coordination Compounds within Metal−organic Frameworks. J. Am. Chem. Soc. 2019, 141, 10350–10360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. T.; Qian, B. Bin; Zhang, D. S.; Yu, M. ;Hui; Chang, Z.; Bu, X. H. Recent Progress in Host–Guest Metal–Organic Frameworks: Construction and Emergent Properties. Coord. Chem. Rev. 2023, 476, 214921. [Google Scholar] [CrossRef]
- Noor, A. Recent Developments in Two Coordinate Transition Metal Chemistry. Coord. Chem. Rev. 2023, 476, 214941. [Google Scholar] [CrossRef]
- Horiuchi, S.; Umakoshi, K. Recent Advances in Pyrazolato-Bridged Homo- and Heterometallic Polynuclear Platinum and Palladium Complexes. Coord. Chem. Rev. 2023, 476, 214924. [Google Scholar] [CrossRef]
- Wang, H.; Chen, B. H.; Liu, D. J. Metal–Organic Frameworks and Metal–Organic Gels for Oxygen Electrocatalysis: Structural and Compositional Considerations. Adv. Mater. 2021, 33, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.; Meng, L.; Imaz, I.; Maspoch, D. Self-Assembly of Colloidal Metal-Organic Framework (MOF) Particles. Chem. Soc. Rev. 2023, 52, 2528–2543. [Google Scholar] [CrossRef] [PubMed]
- Kuosmanen, R.; Rissanen, K.; Sievänen, E. Steroidal Supramolecular Metallogels. Chem. Soc. Rev. 2020, 49, 1977–1998. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gates, B. C. Characterization, Structure, and Reactivity of Hydroxyl Groups on Metal-Oxide Cluster Nodes of Metal–Organic Frameworks: Structural Diversity and Keys to Reactivity and Catalysis. Adv. Mater. 2024, 36, 2305611. [Google Scholar] [CrossRef]
- Ji, P.; Manna, K.; Lin, Z.; Urban, A.; Greene, F. X.; Lan, G.; Lin, W. Single-Site Cobalt Catalysts at New Zr8(Μ2-O)8(Μ2-OH)4 Metal-Organic Framework Nodes for Highly Active Hydrogenation of Alkenes, Imines, Carbonyls, and Heterocycles. J. Am. Chem. Soc. 2016, 138, 12234–12242. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, L.; Zhang, L.; Gates, B. C.; Yang, D. Pair Sites on Nodes of Metal-Organic Framework Hcp UiO-66 Catalyzetert-Butyl Alcohol Dehydration. J. Phys. Chem. Lett. 2021, 12, 6085–6089. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Hassan, Z.; Bräse, S.; Tsotsalas, M. Polymerization in MOF-Confined Nanospaces: Tailored Architectures, Functions, and Applications. Langmuir 2020, 36, 10657–10673. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, V.; Della Sala, P.; Talotta, C.; De Rosa, M.; Soriente, A.; Gaeta, C.; Neri, P. Supramolecular Control on Reactivity and Selectivity inside the Confined Space of H-Bonded Hexameric Capsules. Curr. Opin. Colloid Interface Sci. 2023, 65, 101692. [Google Scholar] [CrossRef]
- Suremann, N. F.; McCarthy, B. D.; Gschwind, W.; Kumar, A.; Johnson, B. A.; Hammarström, L.; Ott, S. Molecular Catalysis of Energy Relevance in Metal-Organic Frameworks: From Higher Coordination Sphere to System Effects. Chem. Rev. 2023, 123, 6545–6611. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, A.; Oppenheim, J. J.; Sun, C.; Dincǎ, M. Conceptual and Practical Aspects of Metal-Organic Frameworks for Solid-Gas Reactions. Chem. Rev. 2023, 123, 6197–6232. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. E. M.; Crowley, J. D. Metallo-Supramolecular Self-Assembly with Reduced-Symmetry Ligands. Chempluschem 2020, 85, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Hosono, N.; Kitagawa, S. Modular Design of Porous Soft Materials via Self-Organization of Metal-Organic Cages. Acc. Chem. Res. 2018, 51, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Ferrando-Soria, J.; Pardo, E.; Armentano, D. Controlled Supramolecular Self-Assembly in MOF Confined Spaces. Supramol. Nanotechnol. Adv. Des. Self-Assembled Funct. Mater. 2023, 1, 151–174. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Zhao, L.; Duan, C. Photochemical Properties of Host-Guest Supramolecular Systems with Structurally Confined Metal-Organic Capsules. Acc. Chem. Res. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Sinha, I.; Mukherjee, P. S. Chemical Transformations in Confined Space of Coordination Architectures. Inorg. Chem. 2018, 57, 4205–4221. [Google Scholar] [CrossRef]
- Kishida, N.; Tanaka, Y.; Yoshizawa, M. CH-π Multi-Interaction-Driven Recognition and Isolation of Planar Compounds in a Spheroidal Polyaromatic Cavity. Chem. - A Eur. J. 2022, 28, e202202075. [Google Scholar] [CrossRef] [PubMed]
- Lutz, N.; Bicknell, J.; Loya, J. D.; Reinheimer, E. W.; Campillo-Alvarado, G. Channel Confinement and Separation Properties in an Adaptive Supramolecular Framework Using an Adamantane Tecton. CrystEngComm 2024, 26, 1067–1070. [Google Scholar] [CrossRef]
- Tan, C.; Chu, D.; Tang, X.; Liu, Y.; Xuan, W.; Cui, Y. Supramolecular Coordination Cages for Asymmetric Catalysis. Chem. - A Eur. J. 2019, 25, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Doud, E. A.; Voevodin, A.; Hochuli, T. J.; Champsaur, A. M.; Nuckolls, C.; Roy, X. Superatoms in Materials Science. Nat. Rev. Mater. 2020, 5, 371–387. [Google Scholar] [CrossRef]
- Guerin, G.; Cruz, M.; Yu, Q. Formation of 2D and 3D Multi-Tori Mesostructures via Crystallization-Driven Self-Assembly. Sci. Adv. 2020, 6. [Google Scholar] [CrossRef]
- Tu, Y.; Peng, F.; Adawy, A.; Men, Y.; Abdelmohsen, L. K. E. A.; Wilson, D. A. Mimicking the Cell: Bio-Inspired Functions of Supramolecular Assemblies. Chem. Rev. 2016, 116, 2023–2078. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, L. J.; Zhang, Y.; Tan, H. W.; Xu, L.; Yang, H. B. Hierarchical Self-Assembly of Triangular Metallodendrimers into the Ordered Nanostructures. Chinese Chem. Lett. 2016, 27, 607–612. [Google Scholar] [CrossRef]
- Wu, G. Y.; Chen, L. J.; Xu, L.; Zhao, X. L.; Yang, H. B. Construction of Supramolecular Hexagonal Metallacycles via Coordination-Driven Self-Assembly: Structure, Properties and Application. Coord. Chem. Rev. 2018, 369, 39–75. [Google Scholar] [CrossRef]
- McTernan, C. T.; Davies, J. A.; Nitschke, J. R. Beyond Platonic: How to Build Metal-Organic Polyhedra Capable of Binding Low-Symmetry, Information-Rich Molecular Cargoes. Chem. Rev. 2022, 122, 10393–10437. [Google Scholar] [CrossRef]
- Ji, Z.; Li, T.; Yaghi, O. M. Sequencing of Metals in Multivariate Metal-Organic Frameworks. Science. 2020, 369, 674–680. [Google Scholar] [CrossRef]
- Insua, I.; Bergueiro, J.; Méndez-Ardoy, A.; Lostalé-Seijo, I.; Montenegro, J. Bottom-up Supramolecular Assembly in Two Dimensions. Chem. Sci. 2022, 13, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ma, X. Tailoring Tunable Luminescence via Supramolecular Assembly Strategies. Cell Reports Phys. Sci. 2020, 1, 1–26. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Symons, H. E.; Xu, J. F.; Faul, C. F. J.; Zhang, X. Molecular Engineering of Polymeric Supra-Amphiphiles. Chem. Soc. Rev. 2019, 48, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. M.; Chen, X.; Hou, X. F.; Zhang, S.; Chen, D.; Li, Q. Self-Assembled Supramolecular Artificial Light-Harvesting Nanosystems: Construction, Modulation, and Applications. Nanoscale Adv. 2023, 5, 1830–1852. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Z.; Wang, Y. X.; Li, D.; Coen, C. T.; Hwaun, E.; Chen, G.; Wu, H. C.; Zhong, D.; Niu, S.; Wang, W.; Saberi, A.; Lai, J. C.; Wu, Y.; Wang, Y.; Trotsyuk, A. A.; Loh, K. Y.; Shih, C. C.; Xu, W.; Liang, K.; Zhang, K.; Bai, Y.; Gurusankar, G.; Hu, W.; Jia, W.; Cheng, Z.; Dauskardt, R. H.; Gurtner, G. C.; Tok, J. B. H.; Deisseroth, K.; Soltesz, I.; Bao, Z. Topological Supramolecular Network Enabled High-Conductivity, Stretchable Organic Bioelectronics. Science (80-. ). 2022, 375, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S. J. K.; Huang, Z.; Ahmed, M. H.; Boys, A. J.; Velasco-Bosom, S.; Li, J.; Owens, R. M.; McCune, J. A.; Malliaras, G. G.; Scherman, O. A. Tissue-Mimetic Supramolecular Polymer Networks for Bioelectronics. Adv. Mater. 2023, 35, 2207634. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Cayer-Barrioz, J.; Mazuyer, D. Elastohydrodynamic Film Formation and Sol/Gel Transition of Aqueous Fluids. Tribol. Lett. 2022, 70, 1–10. [Google Scholar] [CrossRef]
- Ahmadi, M.; Löser, L.; Pareras, G.; Poater, A.; Saalwächter, K.; Seiffert, S. Connectivity Defects in Metallo-Supramolecular Polymer Networks at Different Self-Sorting Regimes. Chem. Mater. 2023, 35, 4026–4037. [Google Scholar] [CrossRef]
- Alfonso Herrera, L. Á.; Beltrán, H. I. Infiltration as a Frontier Bandgap Engineering Strategy in MOFs: A Critical Review. Coord. Chem. Rev. 2024, 505, 215658. [Google Scholar] [CrossRef]
- Choudhari, M.; McDonald, P. W.; Ritchie, C. Polyoxometalate-Induced Preorganization of Halogenated Ligands in Supramolecular Assemblies. Cryst. Growth Des. 2024, 24, 932–937. [Google Scholar] [CrossRef]
- Ranscht, A.; Rigodanza, F.; Gobbato, T.; Crea, I.; Quadrelli, E. A.; Canivet, J.; Bonchio, M. Combined Covalent and Supramolecular Polymerization to Reinforce Perylenebisimide Photosynthetic “Quantasomes.” Chem. - A Eur. J. 2024, e202303784. [CrossRef]
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the Stability of Metal-Organic Frameworks. Adv. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Ravon, U.; Savonnet, M.; Aguado, S.; Domine, M. E.; Janneau, E.; Farrusseng, D. Engineering of Coordination Polymers for Shape Selective Alkylation of Large Aromatics and the Role of Defects. Microporous Mesoporous Mater. 2010, 129, 319–329. [Google Scholar] [CrossRef]
- Furukawa, Y.; Ishiwata, T.; Sugikawa, K.; Kokado, K.; Sada, K. Nano- and Microsized Cubic Gel Particles from Cyclodextrin Metal-Organic Frameworks. Angew. Chemie - Int. Ed. 2012, 51, 10566–10569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bosch, M.; Gentle, T.; Zhou, H. C. Rational Design of Metal-Organic Frameworks with Anticipated Porosities and Functionalities. CrystEngComm 2014, 16, 4069–4083. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C. J.; Nidetzky, B.; Tsung, C. K.; Falcaro, P.; Doonan, C. J. Metal-Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Holten-Andersen, N.; Buehler, M. J. Transition-Metal Coordinate Bonds for Bioinspired Macromolecules with Tunable Mechanical Properties. Nat. Rev. Mater. 2021, 6, 421–436. [Google Scholar] [CrossRef]
- Cazzell, S. A.; Holten-Andersen, N. Expanding the Stoichiometric Window for Metal Cross-Linked Gel Assembly Using Competition. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 21369–21374. [Google Scholar] [CrossRef]
- Ikezoe, Y.; Washino, G.; Uemura, T.; Kitagawa, S.; Matsui, H. Autonomous Motors of a Metal-Organic Framework Powered by Reorganization of Self-Assembled Peptides at Interfaces. Nat. Mater. 2012, 11, 1081–1085. [Google Scholar] [CrossRef]
- Riccò, R.; Liang, W.; Li, S.; Gassensmith, J. J.; Caruso, F.; Doonan, C.; Falcaro, P. Metal-Organic Frameworks for Cell and Virus Biology: A Perspective. ACS Nano 2018, 12, 13–23. [Google Scholar] [CrossRef]
- Grindy, S. C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D. G.; Guan, Z.; Messersmith, P. B.; Holten-Andersen, N. Control of Hierarchical Polymer Mechanics with Bioinspired Metal-Coordination Dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef]
- Fu, L. Q.; Chen, X. Y.; Cai, M. H.; Tao, X. H.; Fan, Y. Bin; Mou, X. Z. Surface Engineered Metal-Organic Frameworks (MOFs) Based Novel Hybrid Systems for Effective Wound Healing: A Review of Recent Developments. Front. Bioeng. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Bagherzadeh, M.; Jouyandeh, M.; Zarrintaj, P.; Saeb, M. R.; Mozafari, M.; Shokouhimehr, M.; Varma, R. S. Natural Polymers Decorated MOF-MXene Nanocarriers for Co-Delivery of Doxorubicin/PCRISPR. ACS Appl. Bio Mater. 2021, 4, 5106–5121. [Google Scholar] [CrossRef] [PubMed]
- Kashnik, I. V.; Yang, B.; Yarovoi, S. S.; Sukhikh, T. S.; Cordier, M.; Taupier, G.; Brylev, K. A.; Bouit, P. A.; Molard, Y. Luminescent Supramolecular Ionic Frameworks Based on Organic Fluorescent Polycations and Polyanionic Phosphorescent Metal Clusters. Chem. - A Eur. J. 2024. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Ko, Y. G. Nature-Inspired Architecture Combining Organic–Inorganic Frameworks: Unique Structure and Active Sites toward a Stable Anti-Corrosion Coating. Appl. Mater. Today 2023, 32, 101852. [Google Scholar] [CrossRef]
- Gloag, L.; Somerville, S. V.; Gooding, J. J.; Tilley, R. D. Co-Catalytic Metal–Support Interactions in Single-Atom Electrocatalysts. Nat. Rev. Mater. 2024, 9, 173–189. [Google Scholar] [CrossRef]
- Batten, S. R.; Chen, B.; Vittal, J. J. Coordination Polymers/MOFs: Structures, Properties and Applications. Chempluschem 2016, 81, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Horike, S. Metal-Organic Network-Forming Glasses. Chem. Rev. 2022, 122, 4163–4203. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, N.; Shen, K.; Xie, Y.; Tan, Y.; Li, Y. MOF-Derived Isolated Fe Atoms Implanted in N-Doped 3D Hierarchical Carbon as an Efficient ORR Electrocatalyst in Both Alkaline and Acidic Media. ACS Appl. Mater. Interfaces 2019, 11, 25976–25985. [Google Scholar] [CrossRef]
- Gopi, S.; Kathiresan, M.; Yun, K. Metal-Organic and Porous Organic Framework in Electrocatalytic Water Splitting. J. Ind. Eng. Chem. 2023, 126, 127–136. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I. H.; Medishetty, R.; Vittal, J. J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T. Fabrication of Metal-Organic Framework Architectures with Macroscopic Size: A Review. Coord. Chem. Rev. 2022, 462, 214520. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S. Incorporation of UiO-66-NH2 MOF into the PAN / Chitosan Nanofibers for Adsorption and Membrane Filtration of Pb ( II ), Cd ( II ) and Cr ( VI ) Ions from Aqueous Solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jin, H.; Li, Y.; Ni, B.; Li, Y.; Huang, S.; Lin, W.; Zhang, Y. Tuning of Second-Harmonic Generation in Zn-Based Metal-Organic Frameworks by Controlling the Structural Interpenetrations: A First-Principles Investigation. J. Phys. Chem. C 2023, 127, 2058–2068. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H. C.; Jiang, H. L. Metal-Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of Biomolecules in Metal-Organic Frameworks for Advanced Applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Doonan, C.; Riccò, R.; Liang, K.; Bradshaw, D.; Falcaro, P. Metal-Organic Frameworks at the Biointerface: Synthetic Strategies and Applications. Acc. Chem. Res. 2017, 50, 1423–1432. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, C.; Kaneti, Y. V.; Eguchi, M.; Lin, J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231–4249. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y.; Chen, B. Ordered Macro-Microporous Metal-Organic Framework Single Crystals. Science. 2018, 359, 206–210. [Google Scholar] [CrossRef] [PubMed]
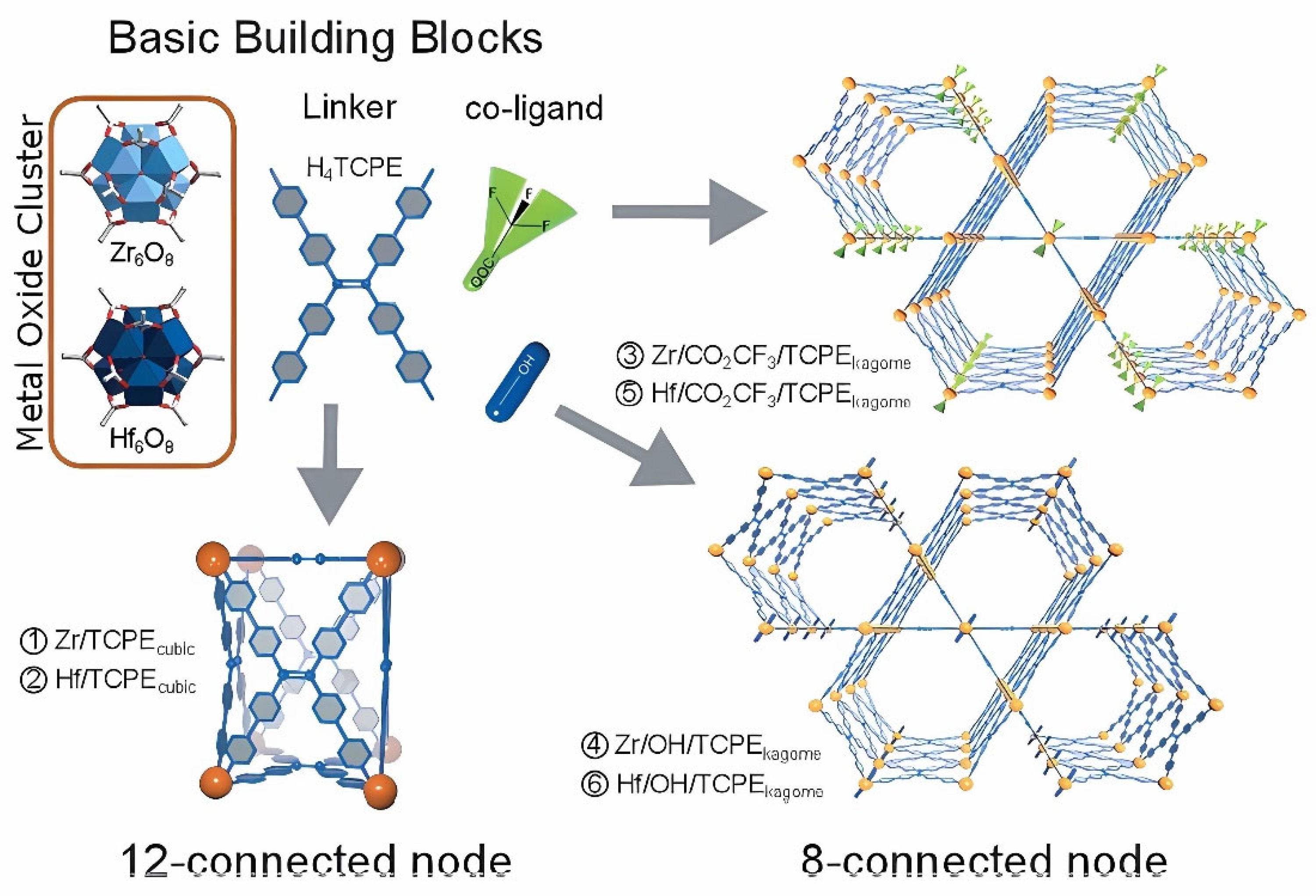
- Rosi, N. L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O. M. Rod Packings and Metal-Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef]
- Kim, H.; Hong, C. S. MOF-74-Type Frameworks: Tunable Pore Environment and Functionality through Metal and Ligand Modification. CrystEngComm 2021, 23, 1377–1387. [Google Scholar] [CrossRef]
- Arun Kumar, S.; Balasubramaniam, B.; Bhunia, S.; Jaiswal, M. K.; Verma, K.; Prateek; Khademhosseini, A. ; Gupta, R. K.; Gaharwar, A. K. Two-Dimensional Metal Organic Frameworks for Biomedical Applications. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Li, R.; Chen, T.; Pan, X. Metal-Organic-Framework-Based Materials for Antimicrobial Applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I. S.; Bau, J. A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed]
- Shekurov, R. P.; Khrizanforov, M. N.; Bezkishko, I. A.; Ivshin, K. A.; Zagidullin, A. A.; Lazareva, A. A.; Kataeva, O. N.; Miluykov, V. A. Influence of the Substituent’s Size in the Phosphinate Group on the Conformational Possibilities of Ferrocenylbisphosphinic Acids in the Design of Coordination Polymers and Metal–Organic Frameworks. Int. J. Mol. Sci. 2023, 24, 14087. [Google Scholar] [CrossRef] [PubMed]
- Djalali, S.; Yadav, N.; Delbianco, M. Towards Glycan Foldamers and Programmable Assemblies. Nat. Rev. Mater. 2024, 9, 190–201. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sánchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, Secondary, Tertiary and Quaternary Structure Levels in Linear Polysaccharides: From Random Coil, to Single Helix to Supramolecular Assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Al-Kutubi, H.; Gascon, J.; Sudhölter, E. J. R.; Rassaei, L. Electrosynthesis of Metal-Organic Frameworks: Challenges and Opportunities. ChemElectroChem 2015, 2, 462–474. [Google Scholar] [CrossRef]
- Versha, M. V.; Nageswaran, G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar] [CrossRef]
- Udourioh, G. A.; Solomon, M. M.; Matthews-Amune, C. O.; Epelle, E. I.; Okolie, J. A.; Agbazue, V. E.; Onyenze, U. Current Trends in the Synthesis, Characterization and Application of Metal-Organic Frameworks. React. Chem. Eng. 2022, 8, 278–310. [Google Scholar] [CrossRef]
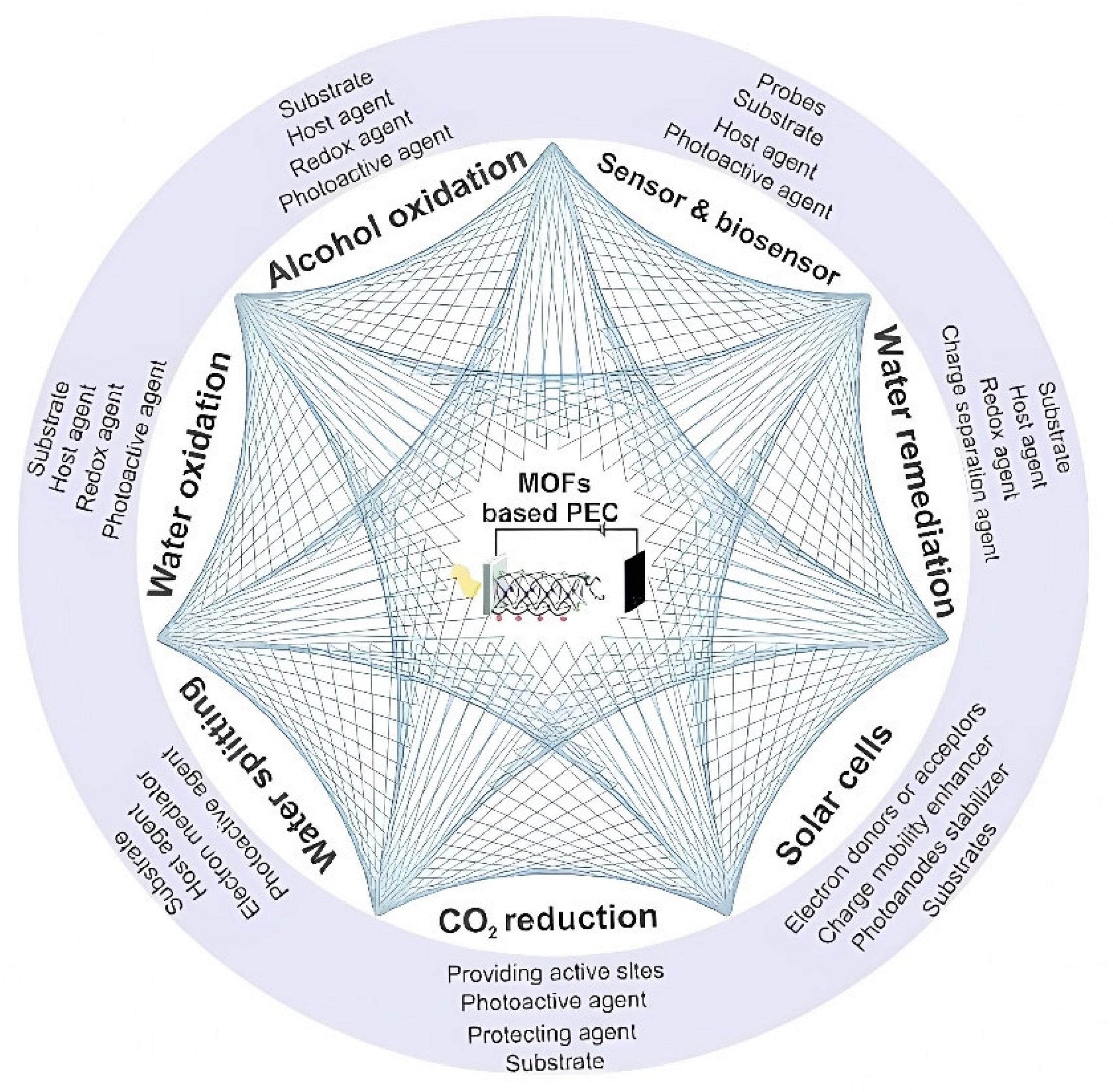
- Dashtian, K.; Shahbazi, S.; Tayebi, M.; Masoumi, Z. A Review on Metal-Organic Frameworks Photoelectrochemistry : A Headlight for Future Applications. Coord. Chem. Rev. 2021, 445, 214097. [Google Scholar] [CrossRef]
- Chen, H.; Fraser Stoddart, J. From Molecular to Supramolecular Electronics. Nat. Rev. Mater. 2021, 6, 804–828. [Google Scholar] [CrossRef]
- Bai, J.; Wu, M.; He, Q.; Wang, H.; Liao, Y.; Chen, L.; Chen, S. Emerging Doped Metal – Organic Frameworks : Recent Progress in Synthesis, Applications, and First-Principles Calculations. Small 2024, 2306616, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Jabarian, S.; Ghaffarinejad, A. Electrochemical Synthesis of NiBTC Metal Organic Framework Thin Layer on Nickel Foam: An Efficient Electrocatalyst for the Hydrogen Evolution Reaction. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1565–1574. [Google Scholar] [CrossRef]
- Vignesh, A.; Vajeeston, P.; Pannipara, M.; Al-Sehemi, A. G.; Xia, Y.; kumar, G. G. Bimetallic Metal-Organic Framework Derived 3D Hierarchical NiO/Co3O4/C Hollow Microspheres on Biodegradable Garbage Bag for Sensitive, Selective, and Flexible Enzyme-Free Electrochemical Glucose Detection. Chem. Eng. J. 2022, 430. [Google Scholar] [CrossRef]
- Bhindi, M.; Massengo, L.; Hammerton, J.; Derry, M. J.; Worrall, S. D. Structure Control Using Bioderived Solvents in Electrochemical Metal-Organic Framework Synthesis. Appl. Sci. 2023, 13, 720. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, W.; Han, N.; Xie, S.; Zhou, Z.; Monnens, W.; Martinez Mora, O.; Xue, Z.; Zhang, X.; Zhang, X.; Fransaer, J. Electrosynthesis of Metal–Organic Framework Films with Well-Defined Facets. Chem. - A Eur. J. 2023, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Chen, Y.; Wang, Q.; Yao, Z.; Wang, L. Spectrochimica Acta Part A : Molecular and Biomolecular Spectroscopy Electrochemical Synthesis of Co / Ni Bimetal-Organic Frameworks : A High-Performance SERS Platform for Detection of Tetracycline. 2023, 285, 121843. [CrossRef]
- Hirohata, T.; Shida, N.; Villani, E.; Ogoshi, T.; Tomita, I.; Inagi, S. Morphologically Controlled Electrochemical Assembly of Pillar[6]Quinone Crystals through the Interaction with Electrolytes. ChemElectroChem 2024, 11, e202300480. [Google Scholar] [CrossRef]
- Araújo-Cordero, A. M.; Caddeo, F.; Mahmoudi, B.; Bron, M.; Wouter Maijenburg, A. Direct Electrochemical Synthesis of Metal-Organic Frameworks: Cu3(BTC)2 and Cu(TCPP) on Copper Thin Films and Copper-Based Microstructures. Chempluschem 2024, 89, e202300378. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Bon, V.; Senkovska, I.; Ehrling, S.; Bönisch, N.; Mäder, G.; Grünzner, S.; Khadiev, A.; Novikov, D.; Maity, K.; Richter, A.; Kaskel, S. Spatiotemporal Design of the Metal–Organic Framework DUT-8(M). Adv. Mater. 2023, 35, 1–10. [Google Scholar] [CrossRef]
- Sterin, I.; Hadynski, J.; Tverdokhlebova, A.; Masi, M.; Katz, E.; Wriedt, M.; Smutok, O. Electrochemical and Biocatalytic Signal-Controlled Payload Release from a Metal–Organic Framework. Adv. Mater. 2024, 36, 1–7. [Google Scholar] [CrossRef]
- Bigdeli, F.; Lollar, C. T.; Morsali, A.; Zhou, H. C. Switching in Metal–Organic Frameworks. Angew. Chemie - Int. Ed. 2020, 59, 4652–4669. [Google Scholar] [CrossRef]
- Medishetty, R.; Nemec, L.; Nalla, V.; Henke, S.; Samoć, M.; Reuter, K.; Fischer, R. A. Multi-Photon Absorption in Metal–Organic Frameworks. Angew. Chemie - Int. Ed. 2017, 56, 14743–14748. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, I. The Future of Molecular Machines. ACS Cent. Sci. 2020, 6, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Ren, J.; Wei, Y.; Yan, T.; Wang, J. X.; Liu, D.; Chen, J. F. Discovery of a Scalable Metal-Organic Framework with a Switchable Structure for Efficient CH4/N2 Separation. Chem. Mater. 2023, 35, 4286–4296. [Google Scholar] [CrossRef]
- Walenszus, F.; Bon, V.; Evans, J. D.; Krause, S.; Getzschmann, J.; Kaskel, S.; Dvoyashkin, M. On the Role of History-Dependent Adsorbate Distribution and Metastable States in Switchable Mesoporous Metal-Organic Frameworks. Nat. Commun. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Flinn, B. T.; Rance, G. A.; Cull, W. J.; Cardillo-zallo, I.; Pitcairn, J.; Cliffe, M. J.; Fay, M. W.; Tyler, A. J.; Weare, B. L.; Stoppiello, C. T.; Davies, E. S.; Mather, M. L.; Khlobystov, A. N. Sensing the Spin State of Room-Temperature Switchable Cyanometallate Frameworks with Nitrogen-Vacancy Centers in Nanodiamonds. ACS Nano 2024, 18, 7148–7160. [Google Scholar] [CrossRef] [PubMed]
- Askalany, A.; Alsaman, A. S.; Ghazy, M.; Mohammed, R. H.; AL-Dadah, R.; Mahmoud, S. Experimental Optimization of the Cycle Time and Switching Time of a Metal Organic Framework Adsorption Desalination Cycle. Energy Convers. Manag. 2021, 245, 114558. [Google Scholar] [CrossRef]
- Nangare, S.; Chaudhari, K.; Patil, P. Poly-L-Lysine Functionalized Graphene Quantum Dots Embedded Zirconium Metal–Organic Framework-Based Fluorescence Switch on-off-on Nanoprobe for Highly Sensitive and Selective Detection of Taurine. J. Photochem. Photobiol. A Chem. 2024, 446, 115158. [Google Scholar] [CrossRef]
- Newacheck, S.; Uyen Huynh, N.; Youssef, G. Tunable and Switchable Magnetoresistance of P3HT:PCBM Organic Framework. Mater. Lett. 2024, 363, 136258. [Google Scholar] [CrossRef]
- Perego, J.; Daolio, A.; Bezuidenhout, C. X.; Piva, S.; Prando, G.; Costarella, B.; Carretta, P.; Marchiò, L.; Kubicki, D.; Sozzani, P.; Bracco, S.; Comotti, A. Solid State Machinery of Multiple Dynamic Elements in a Metal–Organic Framework. Angew. Chemie - Int. Ed. 2024, 63, e202317094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




