Submitted:
20 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
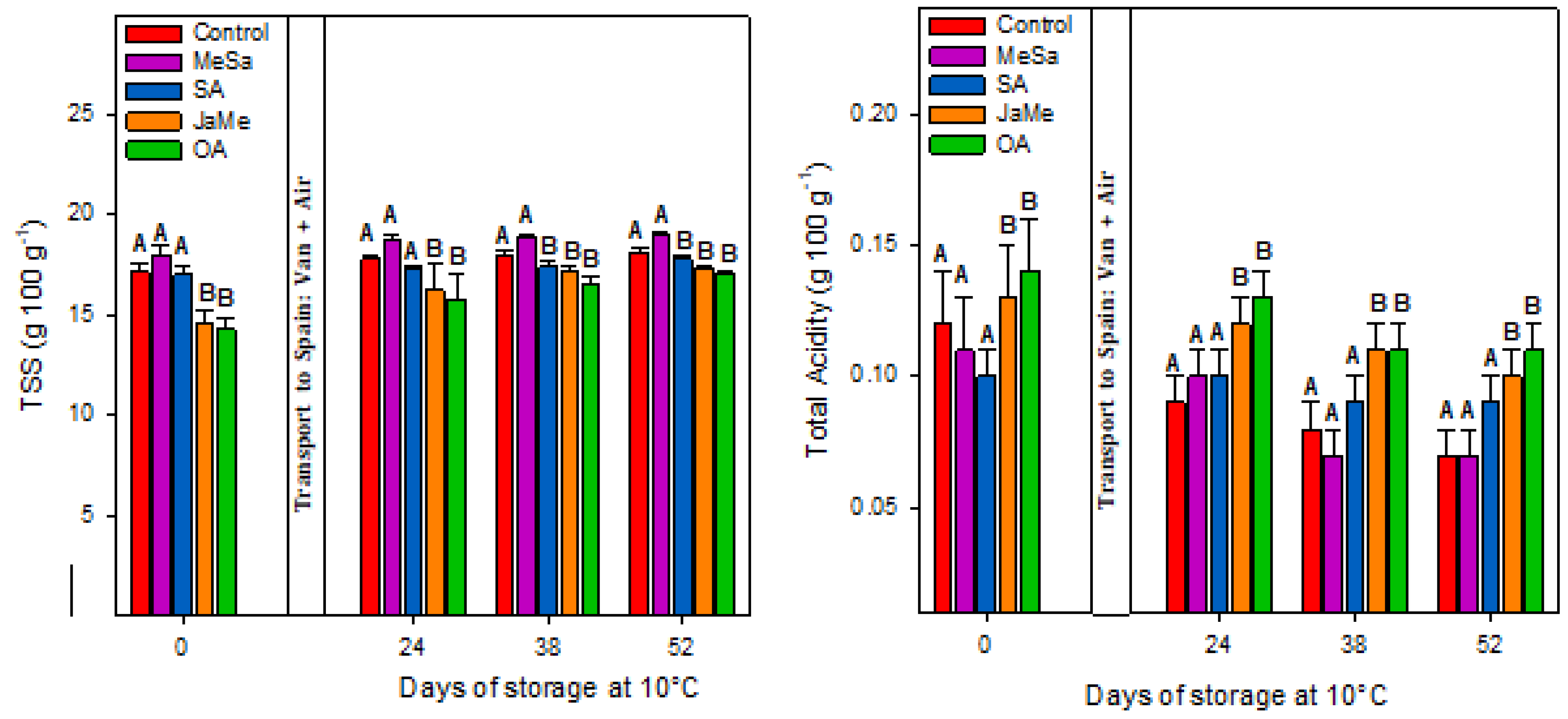
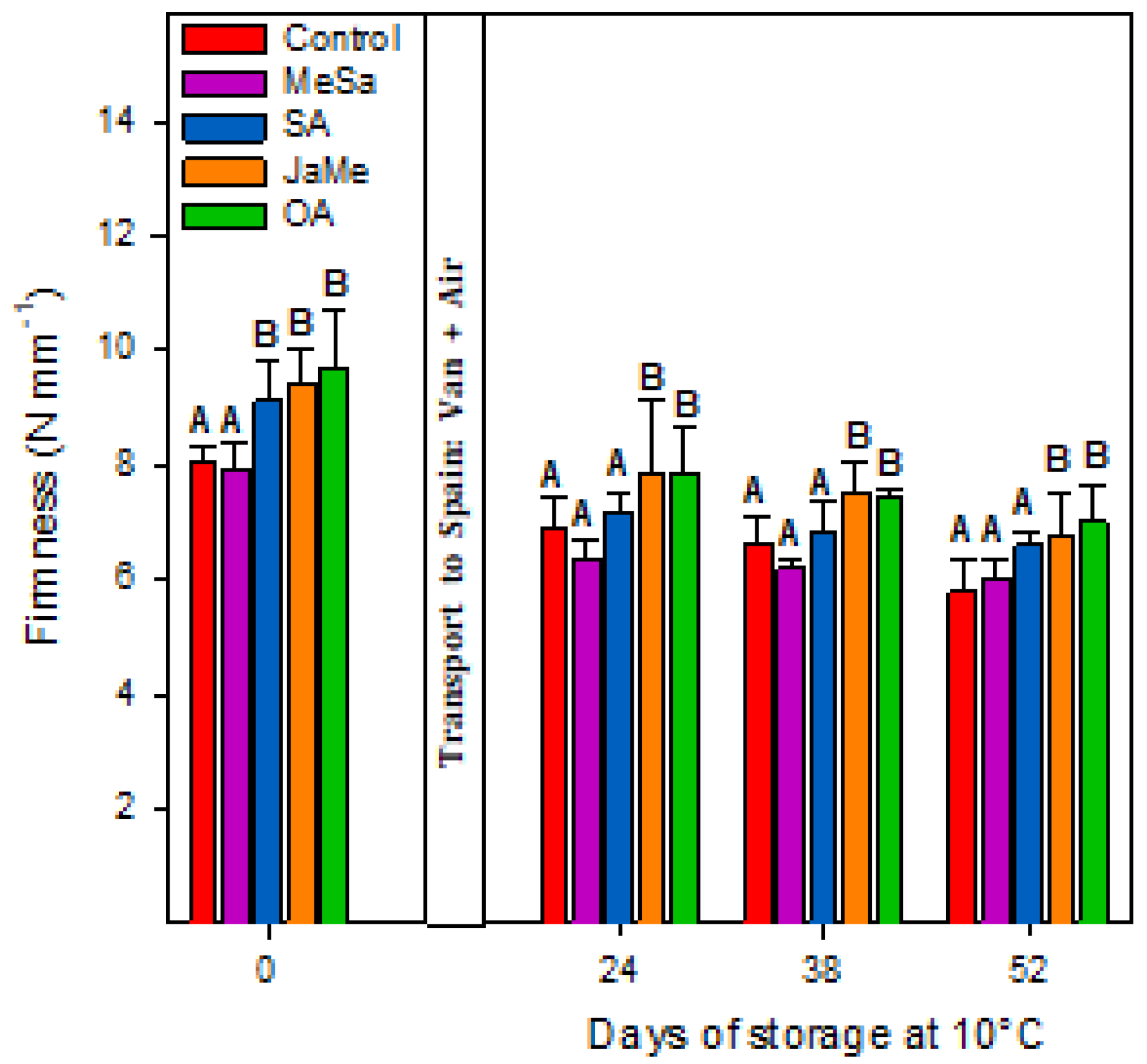
2.1. Fruit Quality at Harvest and during Postharvest Storage
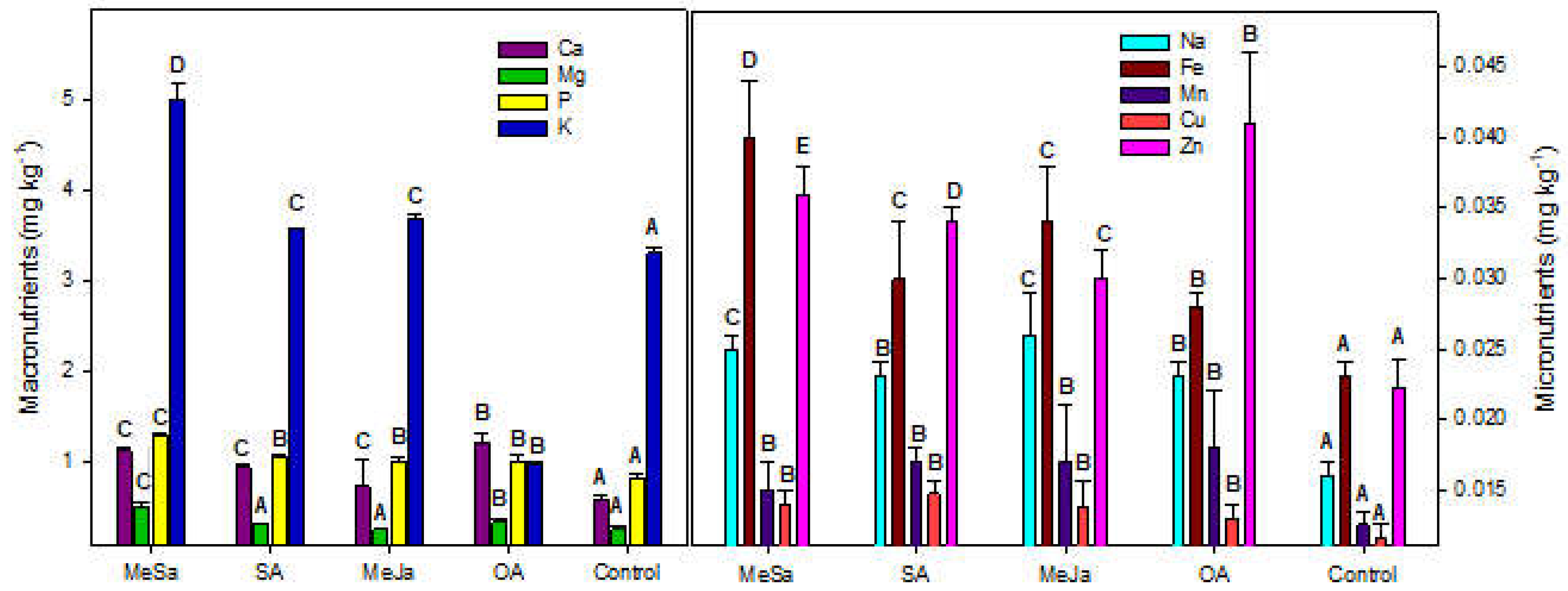
2.2. Mineral Composition and Bioactivos Content of the Peel
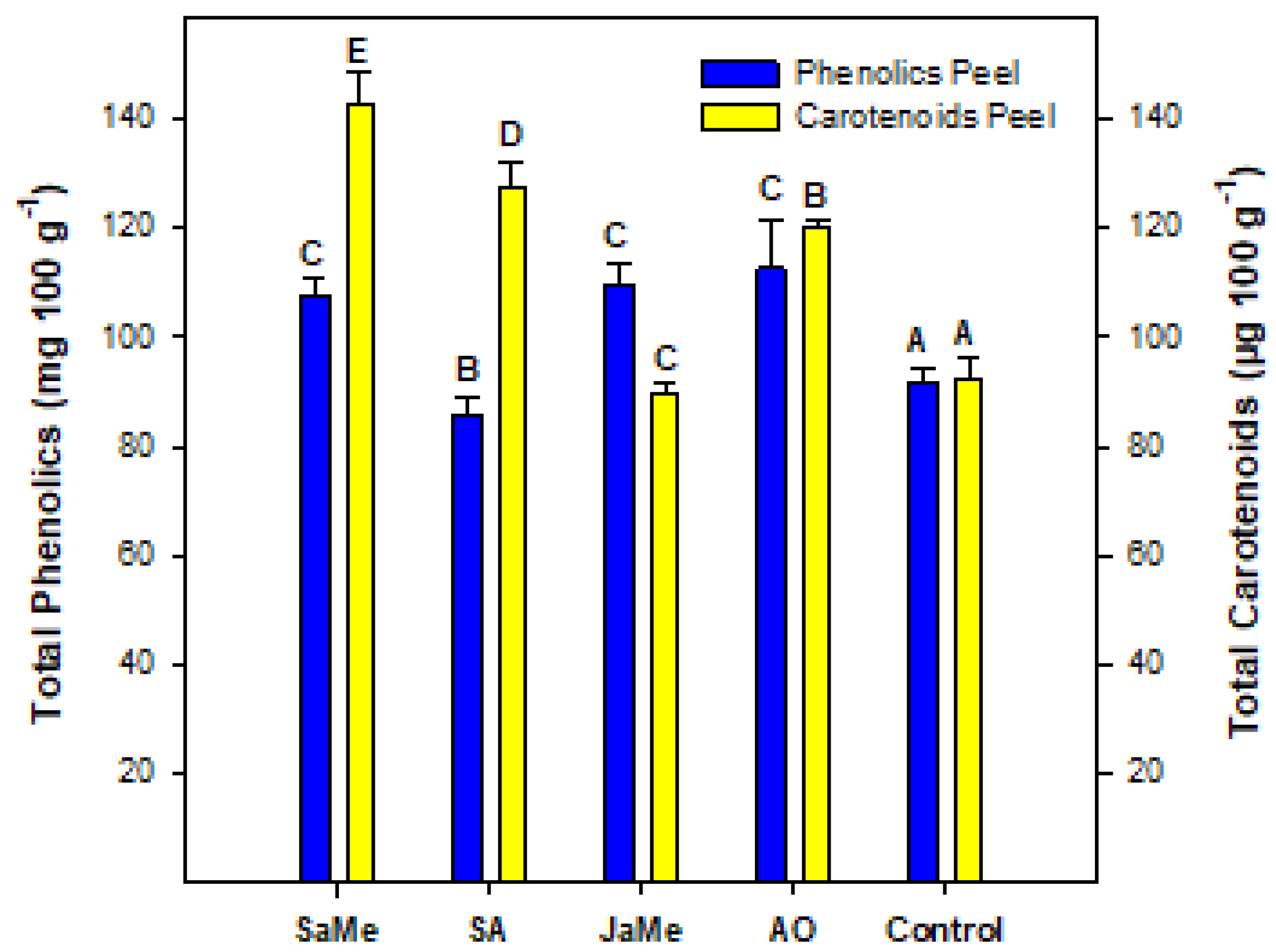
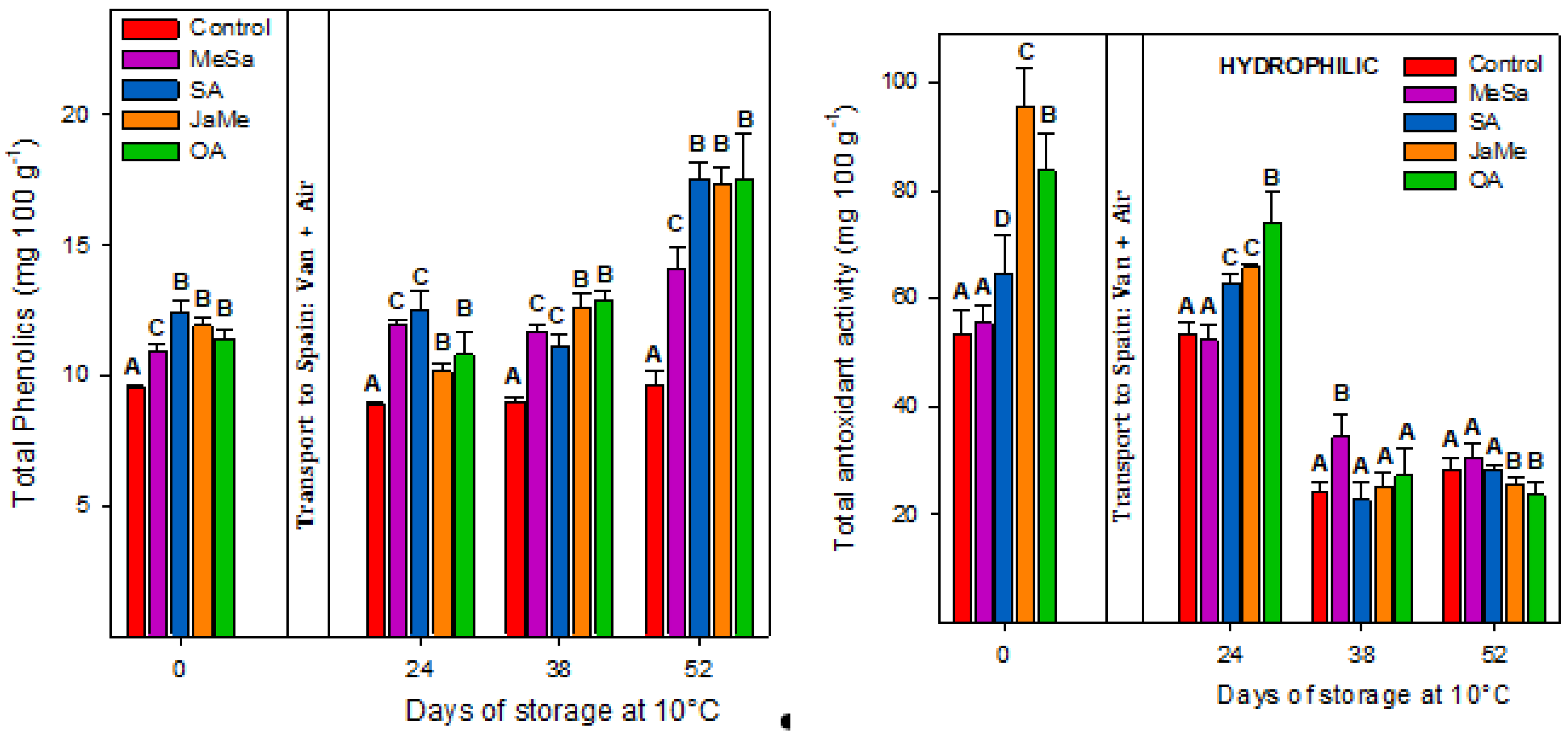
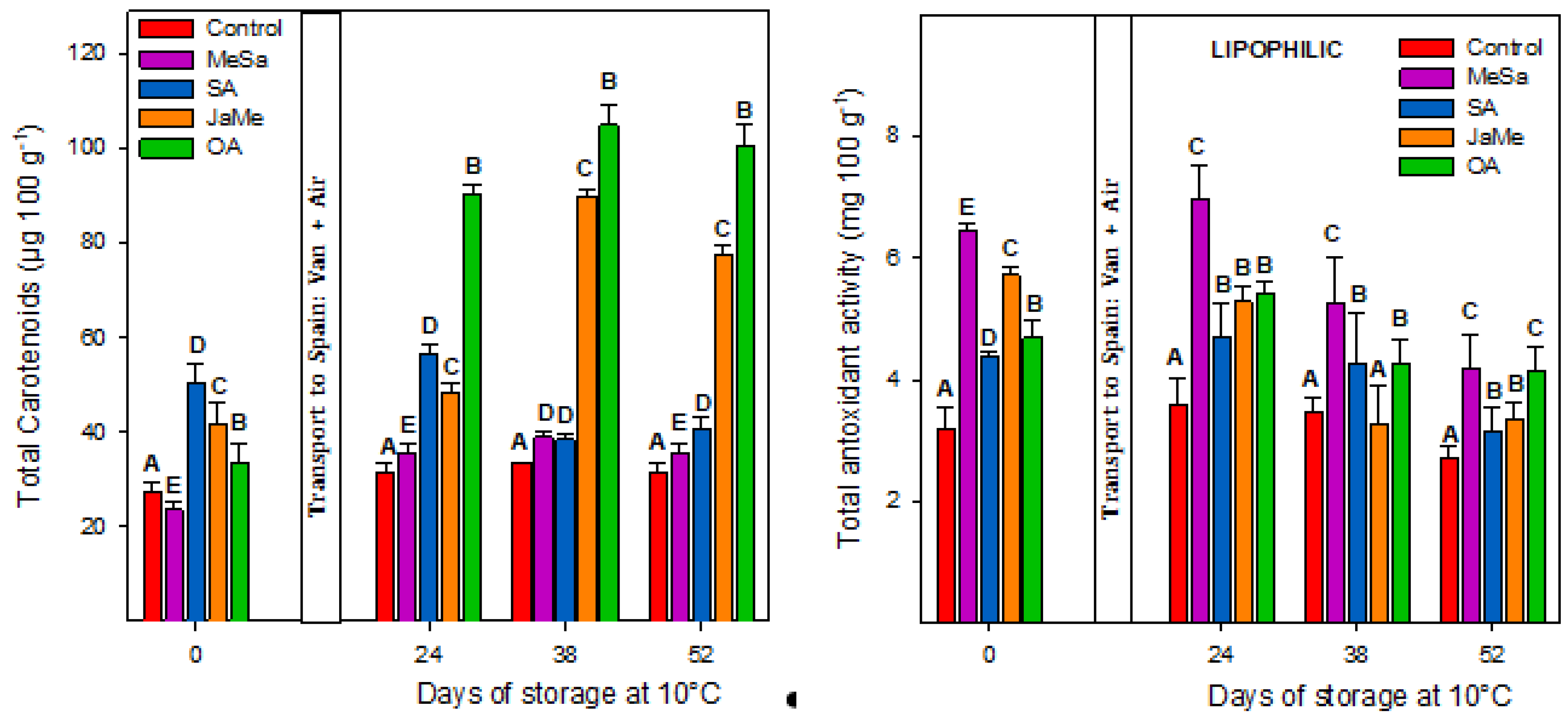
2.3. Bioactive Compounds and Antioxidant Activity at Harvest and during Postharvest Storage
3. Discussion
4. Materials and Methods
4.1. Plant Material, Treatments and Experimental Design
4.2. Postharvest Storage
4.3. Mineral Composition of the Peel
4.4. Measurement of Quality Traits
4.5. Bioactive Compounds and Antioxidant Activity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez, L.; González, M.; Cruz, S.; Santana, R.; Villacís, L. Análisis poscosecha de frutos de pitahaya amarilla (Cereus triangularis Haw. ) a distintos niveles de madurez y temperatura. J. Selva Andina Biosphere. 2017, 5, 107–115. [Google Scholar] [CrossRef]
- Vargas, Y.; Pico, J.; Díaz, A.; Sotomayor, D.; Burbano, A.; Caicedo, C.; Paredes, N.; Congo, C.; Tinoco, L.; Bastidas, S.; Chuquimarca, J.; Macas, J.; Viera, W. Manual Técnico del cultivo de pitahaya. INIAP: Quito, Ecuador, 2020; 39 pp.
- Verona-Ruiz, A.; Urcia-Cerna, J.; Paucar-Menacho, L. Pitahaya (Hylocereus spp. ): Culture, physicochemical characteristics, nutritional composition, and bioactive compounds. Sci. Agropecu. 2020, 11, 439–453. [Google Scholar]
- Morillo-Coronado, A.C.; Manjarres Hernández, E.H.; Forero-Mancipe, L. Phenotypic diversity of morphological characteristics of pitahaya (Selenicereus megalanthus Haw. ) germplasm in Colombia. Plants 2021, 10, 2255. [Google Scholar] [PubMed]
- Al-Mekhlafi, N.A.; Mediani, A.; Ismail, N.H.; Abas, F.; Dymerski, T.; Lubinska-Szczygeł, M.; Vearasilp, S.; Gorinstein, S. Metabolomic and antioxidant properties of different varieties and origins of Dragon fruit. Microchem. J. 2020, 160, 105687. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Ku, Y.G.; Luksirikul, P.; Weisz, M.; Gorinstein, S. Bioactivity and cytotoxicity of different species of pitaya fruits – A comparative study with advanced chemometric analysis. Food Biosci. 2021, 40. [Google Scholar] [CrossRef]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp): A new fruit crop, a market with a future. Fruits 2006, 61, 237. [Google Scholar] [CrossRef]
- Caicedo, L.H.; Villagómez, A.L.; Sáenz, D.; Zavala, C.E.; Espinoza, E.; Romero, H. Elicitors: bioethical implications for agriculture and. human health. Rev. Bioet. 2021, 29, 76–86. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Farooq, M.S.; Uzair, M.; Raza, A.; Habib, M.; Xu, Y.; Yousuf, M.; Yang, S.H.; Khan, M.R. Uncovering the Research Gaps to Alleviate the Negative Impacts of Climate Change on Food Security: A Review. Front. Plant Sci. 2022, 13, 927535. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Zapata, P.J.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M.; Guillén, F. Preharvest Salicylate Treatments Enhance Antioxidant Compounds, Color and Crop Yield in Low Pigmented-Table Grape Cultivars and Preserve Quality Traits during Storage. Antioxidants 2020, 9, 832. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest Application of Oxalic Acid Improved Pomegranate Fruit Yield, Quality, and Bioactive Compounds at Harvest in a Concentration-Dependent Manner. Agronomy 2020, 10, 1522. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; García-Viguera, C.; Castillo, S.; Serrano, M. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L. ). J. Agric. Food Chem. 2014, 62, 3432–3437. [Google Scholar] [CrossRef] [PubMed]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 2018, 247, 380–389. [Google Scholar] [CrossRef]
- Gong, D.; Bi, Y.; Li, Y.; Wang, Y.; Prusky, D.; Alkan, N. Preharvest Elicitors Spray Improves Antioxidant Activity, Alleviates Chilling Injury, and Maintains Quality in Harvested Fruit. Horticulturae 2022, 8, 1208. [Google Scholar] [CrossRef]
- Chen, C.; Sun, C.; Wang, Y.; Gong, H.; Zhang, A.; Yang, Y.; Guo, F.; Cui, K.; Fan, X.; Li, X. The preharvest and postharvest application of salicylic acid and its derivatives on storage of fruit and vegetables: A review. Sci. Hortic. 2023, 312. [Google Scholar] [CrossRef]
- Le, N.L. Functional compounds in dragon fruit peels and their potential health benefits: a review. Int. J. Food Sci. Technol. 2022, 57, 2571–2580. [Google Scholar] [CrossRef]
- Ferreira, V.C.; Ampese, L.C.; Sganzerla, W.G.; Colpini, L.M.S.; Forster-Carneiro, T. An updated review of recent applications and future perspectives on the sustainable valorization of pitaya (Hylocereus spp.) by-products. Sustain. Chem. Pharm. 2023, 33. [Google Scholar] [CrossRef]
- Tripathi, M.; Diwan, D.; Shukla, A.C.; Gaffey, J.; Pathak, N.; Dashora, K.; Pandey, A.; Sharma, M.; Guleria, S.; Varjani, S.; et al. Valorization of dragon fruit waste to value-added bioproducts and formulations: A review. Crit. Rev. Biotechnol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic Acid as a Safe Plant Protector and Growth Regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A. , A. Salicylic Acid–Mediated Defense Mechanisms to Abiotic Stress Tolerance. In Plant Signaling Molecules: Role and Regulation Under Stressful Environments; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, M.A., Eds.; Eds.; Elsevier: Oxford, UK, 2019; pp. 355–369. [Google Scholar]
- Hazarika, T.; Marak, T. Salicylic acid and oxalic acid in enhancing the quality and extending the shelf life of grape cv. Thompson seedless. Food Sci. Technol. Int. 2021, 28, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Samaradiwakara, S.D.; Champa, W.A.H.; Eeswara, J.P. Preharvest foliar spray of plant growth regulators expand the harvest season and improve fruit quality of acid lime (Citrus aurantifolia (Christm) Swingle). J. Hort. Postharvest Res. 2023, 6, 207–220. [Google Scholar]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Technol. 2014, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Xiukang, W.; Mohammad, J.T.; Fahad, M.W.; Mahmood, U.l.H.; Muhammad, S.; Mehwish, L.; Sana, A.; Tanveer, H.; Sajid, F.; Waseem, A. Foliar application of salicylic acid at different phenological stages of peach fruit cv. ‘Florda King’ improves harvest quality and reduce chilling injury during low temperature storage. Plants 2021, 10, 1–20. [Google Scholar]
- García-Pastor, M.E.; Giménez, M.J.; Zapata, P.J.; Guillén, F.; Valverde, J.M.; Serrano, M.; Valero, D. Preharvest application of methyl salicylate, acetyl salicylic acid and salicylic acid alleviated disease caused by Botrytis cinerea through stimulation of antioxidant system in table grapes. Int. J. Food Microbiol. 2020, 334, 108807. [Google Scholar] [CrossRef]
- Erazo-Lara, A.; García-Pastor, M.E.; Padilla-González, P.A.; Serrano, M.; Valero, D. Yellow pitahaya (Selenicereus megalanthus Haw.) growth and ripening as affected by preharvest elicitors: Enhancement of yield and quality at harvest. Horticulturae (In presss).
- Hubbard, N.L.; Pharr, D.M.; Huber, S.C. Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruit of various species. Physiol. Plant. 1991, 82, 191–196. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional Roles in Stress Tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Cai, X.; Han, Z.; Si, J.; Chen, D. Jasmonate Signaling Pathway Modulates Plant Defense, Growth, and Their Trade-Offs. Int. J. Mol. Sci. 2022, 23, 3945. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Yastreb, T.O.; Dmitriev, A.P. Signal Mediators in the Implementation of Jasmonic Acid’s Protective Effect on Plants under Abiotic Stresses. Plants 2023, 12, 2631. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Effects of exogenous methyl jasmonate on quality and preservation of postharvest fruits: A review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef] [PubMed]
- Dhami, K.; Asrey, R.; Awasthi, O.; Bhowmik, A. Pre and postharvest treatments of methyl jasmonate: Maintain quality and shelf-life of Kinnow mandarin fruit during cold storage. South Afr. J. Bot. 2022, 151, 808–815. [Google Scholar] [CrossRef]
- Ruiz-Aracil, M.C.; Valverde, J.M.; Lorente-Mento, J.M.; Carrión-Antolí, A.; Castillo, S.; Martínez-Romero, D.; Guillén, F. Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons. Agriculture 2023, 13, 1244. [Google Scholar] [CrossRef]
- Chen, M.; Guo, H.; Chen, S.; Li, T.; Li, M.; Rashid, A.; Xu, C.; Wang, K. Methyl Jasmonate Promotes Phospholipid Remodeling and Jasmonic Acid Signaling To Alleviate Chilling Injury in Peach Fruit. J. Agric. Food Chem. 2019, 67, 9958–9966. [Google Scholar] [CrossRef] [PubMed]
- Trejo, E.O.; Brizzolara, S.; Cardillo, V.; Ruperti, B.; Bonghi, C.; Tonutti, P. The impact of PGRs applied in the field on the postharvest behavior of fruit crops. Sci. Hortic. 2023, 318. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic acids in fruits: metabolism, functions and contents. Hortic. Rev. 2018, 45, 371–430. [Google Scholar]
- Hasan, M.U.; Singh, Z.; Shoaib, S.; Kaur, H.M. , Woodward, J. , Afrifa-Yamoah, A.; Malik, A.U.E. Oxalic acid: A blooming organic acid for postharvest quality preservation of fresh fruit and vegetables. Postharvest Biol. Technol. 2023, 206, 112574. [Google Scholar]
- Razavi, F.; Hajilou, J. Enhancement of postharvest nutritional quality and antioxidant capacity of peach fruits by preharvest oxalic acid treatment. Sci. Hortic. 2016, 200, 95–101. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest Treatment with Oxalic Acid Improves Postharvest Storage of Lemon Fruit by Stimulation of the Antioxidant System and Phenolic Content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef]
- Huang, Y.; Brennan, M.A.; Kasapis, S.; Richardson, S.J.; Brennan, C.S. Maturation Process, Nutritional Profile, Bioactivities and Utilisation in Food Products of Red Pitaya Fruits: A Review. Foods 2021, 10, 2862. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: a review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Jamilah, B.; Shu, C.E.; Kharidah, M.; Dzulkifly, M.A.; Noranizan, A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J 2011, 18, 279–286. [Google Scholar]
- Nishikito, D.F.; Borges, A.C.A.; Laurindo, L.F.; Otoboni, A.M.M.B.; Direito, R.; Goulart, R.d.A.; Nicolau, C.C.T.; Fiorini, A.M.R.; Sinatora, R.V.; Barbalho, S.M. Anti-Inflammatory, Antioxidant, and Other Health Effects of Dragon Fruit and Potential Delivery Systems for Its Bioactive Compounds. Pharmaceutics 2023, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.M.; Numan, S.M.N.; Akhtar, S. Cultivation, nutritional value, and health benefits of dragon fruit (Hylocereus spp. ): A Review. Int. J. Hortic. Sci. Technol. 2021, 8, 259–269. [Google Scholar]
- Shah, K.; Chen, J.; Chen, J.; Qin, Y. Pitaya Nutrition, Biology, and Biotechnology: A Review. Int. J. Mol. Sci. 2023, 24, 13986. [Google Scholar] [CrossRef] [PubMed]
- de Faria, R.C.; Morgado, C.M.A.; Vespucci, I.L.; de Campos, A.J. UV-C radiation in the post-harvest quality of red pitaia. Comunicata Scientiae 2022, 13, e3857. [Google Scholar]
- Abirami, K.; Swain, S.; Baskaran, V.; Venkatesan, K.; Sakthivel, K.; Bommayasamy, N. Distinguishing three Dragon fruit (Hylocereus spp.) species grown in Andaman and Nicobar Islands of India using morphological, biochemical and molecular traits. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Xie, F.; Chen, C.; Chen, J.; Yuan, Y.; Hua, Q.; Zhang, Z.; Zhao, J.; Hu, G.; Chen, J.; Qin, Y. Metabolic Profiling of Sugars and Organic Acids, and Expression Analyses of Metabolism-Associated Genes in Two Yellow-Peel Pitaya Species. Plants 2022, 11, 694. [Google Scholar] [CrossRef]
- Arivalagan, M.; Karunakaran, G.; Roy, T.; Dinsha, M.; Sindhu, B.; Shilpashree, V.; Satisha, G.; Shivashankara, K. Biochemical and nutritional characterization of dragon fruit (Hylocereus species). Food Chem. 2021, 353, 129426. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Chen, C.; Zur, N.T.; Wang, H.; Wu, J.; Chen, J.; Zhang, Z.; Zhao, J.; Hu, G.; Qin, Y. Metabolomic characterization of pitaya fruit from three red-skinned cultivars with different pulp colors. Plant Physiol. Biochem. 2018, 126, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lupuche, E.Q.; Pérez, J.A.C.; Medina-Pizzali, M.L.; Gutiérrez, L.L.; Suárez, E.A. Chemical characterization, polyphenol content and antioxidant capacity of two pitahaya ecotypes (Hylocereus spp. ). 2021, 74, 9723–9734. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Mucilage from Yellow Pitahaya (Selenicereus megalanthus) Fruit Peel: Extraction, Proximal Analysis, a Molecular Characterization. Molecules 2023, 28, 786. [Google Scholar] [CrossRef] [PubMed]
- Pawde, S.; I Talib, M.; Parate, V.R. Development of Fiber-Rich Biscuit by Incorporating Dragon Fruit Powder. Int. J. Fruit Sci. 2020, 20, S1620–S1628. [Google Scholar] [CrossRef]
- Faizy, A.H.; Ozturk, B.; Aglar, E.; Yıldız, K. Role of methyl jasmonate application regime on fruit quality and bioactive compounds of sweet cherry at harvest and during cold storage. J. Food Process. Preserv. 2021, 45, e15882. [Google Scholar] [CrossRef]
- Constantino, L.V.; Zeffa, D.M.; Ventorim, M.F.; Gonçalves, L.S.A.; Marcos, A.W.; Sanzovo, A.W. dos S. , Rossetto, L.M.; Alves, S.M.; Resende, J.T.V.; Takahashi, L.S.A. Qualidade nutricional e potencial tecnológico de espécies de pitaya. Semina: Ciências Agrárias 2021, 42, 2023–2030. [Google Scholar]
- Attar, H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional Analysis of Red-Purple and White-Fleshed Pitaya (Hylocereus) Species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef] [PubMed]
- Corea, R.C.G.; Garcia, J.A.A.; Correa, V.G.; Vieira, T.F.; Bracht, A.; Peralta, R.M. Pigments and vitamins from plants as functional ingredients: Current trend and perspectives. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L, Eds.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- da Silveira Agostini-Costa, T. Bioactive compounds and health benefits of Pereskioideae and Cactoideae: A review. Food Chem. 2020, 327, 126961–12975. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates. Int. J. Mol. Sci. 2017, 18, 1911. [Google Scholar] [CrossRef] [PubMed]
- Trejo, E.O.; Brizzolara, S.; Cardillo, V.; Ruperti, B.; Bonghi, C.; Tonutti, P. The impact of PGRs applied in the field on the postharvest behavior of fruit crops. Sci. Hortic. 2023, 318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




