1. Introduction
The formation of the secondary palate involves extensive growth and remodeling of the palatine shelves. The palatine shelves initially grow vertically, then reorientate horizontally as the tongue descends, allowing them to fuse at the midline and separate the oral and nasal cavities [
1,
2,
3]. The extracellular matrix protein (EMB) is critical in palatal development as it forms a framework to which cells adhere via integrin receptors. This interaction is crucial for the palatal shelves to change to a horizontal position and grow until they fuse to form the midline epithelial seam [
4,
5]. The fate of the midline epithelial seam in palatal development is debated. Theories suggest it may undergo apoptosis, transform into mesenchymal cells, or migrate toward nasal and oral epithelium. Some propose a combination of these events may occur, depending on the developmental stage (
Figure 1) [
6,
7].
Craniofacial anomalies are a significant public health concern, accounting for over 30% of all developmental defects globally. Among these anomalies, cleft lip with or without cleft palate is the most common, representing about 46% of cases, followed by isolated palatal cleft at 33%. Cleft palate can manifest unilaterally, bilaterally, cleft uvula, or as a submucous cleft and may be syndromic, associated with other genetic or environmental factors, or non-syndromic, occurring in isolation. [
6,
7,
8,
9,
10]. According to Bishop et al. (2020), approximately 50% of cleft palate cases are non-syndromic type, indicating that there are no obvious abnormalities in other organs or systems [
11]. Cleft palate can arise from a variety of causes, reflecting the complexity of embryonic development. Insufficient growth of the palatal shelves or their failure to move from the vertical to the horizontal direction can disrupt the fusion process, resulting in a cleft. Defects in the fusion mechanisms or retraction of initially fused shelves can also lead to palate deformity. In addition, insufficient accumulation of extracellular matrix represents another potential contributing factor. Often, cleft palate arises from a combination of these factors, indicating its multifactorial nature [
12,
13]. Genetic predisposition and environmental factors can stunt growth and cause deformities. Several specific genes may be involved in the development of cleft palate, including MTHFR on chromosome 1, TGF-α on chromosome 2, MSX-1 on chromosome 4, TGF-β3 on chromosome 14, and RAR-α on chromosome [
13,
14]. Several environmental factors are linked to cleft palate, including alcohol abuse, smoking, nutritional deficiencies (like folic acid), use of corticosteroids, and anticonvulsants such as phenobarbital. These factors can increase the risk of palate malformation during fetal development [
15,
16].
Hydrocortisone is a crucial glucocorticoid hormone produced by the zona fasciculata and zona reticularis of the adrenal gland. It plays a vital role in various physiological processes, including metabolism and regulation of the immune response [
17]. Therapeutic corticosteroids are commonly used to reduce inflammation and modulate immune responses in various conditions such as asthma, eczema, rheumatoid arthritis, and other inflammatory disorders [
18]. Long-term use of corticosteroids demonstrates various side effects on different body tissues and organs, including impacts on palatogenesis. These effects include delayed wound healing, loss of bone density (osteoporosis), and metabolic changes [
19,
20]. Hviid & Molgaard-Nielsen (2011) conducted a study using nationwide health records in Denmark over 12 years, focusing on newborns whose mothers were treated with corticosteroids during pregnancy. This group constituted more than 6% of the total sample. The study found that out of 1232 orofacial cleft cases, 84 were isolated cases, representing 6.81% of those exposed to corticosteroids during the first trimester of pregnancy. This suggests a potential association between corticosteroid use in early pregnancy and the risk of orofacial clefts, although further research is needed to establish causality [
21]. Transmission electron microscopy of dexamethasone-exposed mouse palates showed cellular swelling and loose cell-cell contacts. Additionally, there were decreased numbers of mitochondria and extracellular matrix. These findings suggest that dexamethasone exposure may disrupt normal cellular structure and function in the developing palate, potentially contributing to the development of cleft [
22]. Hydrocortisone has been experimentally implicated in the production of secondary cleft palate in mice strains sensitive to steroids. Researchers have conducted various studies on this topic to understand the mechanisms behind cleft palate development and how to manage it. These studies aim to improve our understanding of the effects of steroids on palate development and potentially find ways to prevent or treat cleft palate.
Vitamins are organic compounds essential for various physiological functions, including metabolism, immune system support, and tissue repair. They must be obtained in small quantities through the diet to maintain overall health and well-being. They are classified into two categories: water-soluble vitamins (such as vitamin C and B vitamins) and fat-soluble vitamins (such as vitamins A and D) [
23]. Vitamin B6, an essential member of the vitamin B complex group, was discovered in 1934 [
24]. It exists in three forms in nature: pyridoxine, pyridoxal, and pyridoxamine. These forms can be interconverted in the liver to pyridoxal-5-phosphate by the enzyme pyridoxal kinase using adenosine triphosphate [
25]. Vitamin B6 play vital roles in various biochemical reactions, including amino acid metabolism, neurotransmitter synthesis, and glycogen breakdown [
24]. Vitamin B6 is found in a variety of foods, including meat, fish, poultry, nuts, and whole grains Deficiency in vitamin B6 can lead to symptoms such as anaemia, dermatitis, and neurological disorders. Vitamin B6 plays a crucial role as a cofactor in more than 150 biochemical reactions within cells [
26]. These include reactions involved in the biosynthesis and catabolism of amino acids, such as the conversion of tryptophan to niacin and serotonin. Vitamin B6 is also involved in the synthesis of neurotransmitters such as dopamine, norepinephrine, and gamma-aminobutyric acid. Additionally, vitamin B6 is important for the breakdown of glycogen and the synthesis of haemoglobin [
27]. Vitamin B6 also, modulates the action of steroid hormones in vivo by interacting with steroid receptor complex [
28]. Pyridoxine is widespread in foods; good sources include whole-grain breads, meat, liver, kidney, soybean, and cereals [
29]. Shaw et al. (2004) investigated the association between maternal vitamin B6 intake and the risk of neural tube defects in offspring. The study found that higher maternal intake of vitamin B6 was associated with a reduced risk of neural tube defects. This suggests that adequate intake of vitamin B6 during pregnancy may play a protective role against the development of neural tube defects in newborns [
30]. The current study aimed to evaluate whether administering vitamin B6 from day zero of pregnancy until sacrifice could reduce the incidence of corticosteroid-induced cleft palate in a mouse model.
2. Material and Methods
2.1. Experimental Animals
Twenty-seven C57BL/6J mice (18 females and 9 males) aged 6-8 weeks and weighing approximately 40-45 grams were used in the experiment. The mice were obtained from the Egyptian Organization for Biological Products and Vaccines in Cairo, Egypt. All mice were housed in a controlled environment with a 16/8 light/dark cycle and were provided with ordinary hard and soft food ad-libitum, along with unrestricted access to water. Throughout the study, animals were maintained in an animal care facility under the supervision of ethical committee protocol. This ensures that animal welfare is carefully monitored and that all procedures follow ethical guidelines and regulations for animal research.
2.2. Induction of Pregnancy
In this experimental setup, two virgin female and one male were placed in a polycarbonate cage covered with a stainless-steel wire cover. The animals were allowed to mate together from 17:00 to 19:00 pm, after which the male was separated from the females. Pregnancy was determined based on a vaginal swab, and this was considered day 0 of pregnancy.
2.3. Grouping and Medications
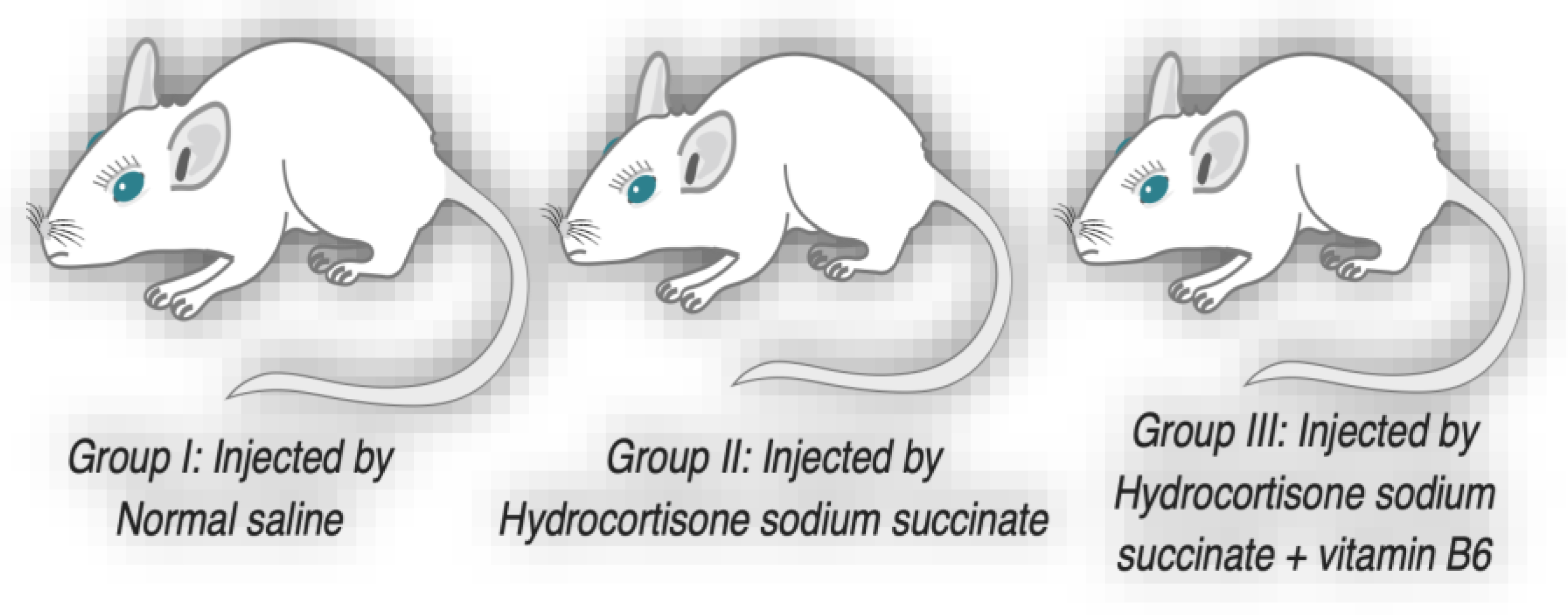
Eighteen pregnant mice were divided into three equal groups. Group I served as the control group, Group II received injections of hydrocortisone, and Group III received injections of hydrocortisone along with vitamin B6 in their drinking water. Animals of group II were injected intramuscularly by a dose of 125 mg/kg hydrocortisone sodium succinate (Solu-Cortif, manufactured by Egyptian, EIPICO, ARE, under license of Upjohn Pfizer, USA) dissolved in benzyl alcohol 0.9% daily on 11th through 14th days of gestation. Animals of group III were injected by hydrocortisone sodium succinate as in group II and drink water containing 500 µg/ml vitamin B6 on zero day of gestation until delivery with minimal drinking 5 ml daily. Animals of control group were injected with saline instead of hydrocortisone using the same procedure as the other groups (
Figure 2). The pregnant mice were allowed to carry their pregnancies to term, and after delivery, their offspring were counted, and their head circumferences were measured. Two days after delivery, the offspring were sacrificed for further analysis.
2.4. Tissue Preparation
In this study, the heads of all foetuses were fixed in Bouin's solution for three days. After fixation, the head tissues of the neonates were embedded in paraffin and sectioned into slices of 5-6 µm thickness. These tissue sections were then stained with Haematoxylin and Eosin (H&E). In this study, a histological examination was conducted by preparing serial coronal sections of the fetal mice heads. These sections were cut in a plane that was positioned perpendicular to the mid-sagittal plane. In this study, each fetal head was carefully selected to evaluate the development of the secondary palate in an antero-posterior order. This approach likely involved examining the palatal structures from front to back to assess their developmental progression and any potential abnormalities. In the case of cleft palate foetuses, special attention was given to the palatal shelves to determine the type of cleft, whether unilateral or bilateral. This observation helped classify the nature and extent of the palate defect in the affected fetuses. For fetuses with complete closure of the palate, the epithelial seam was examined to assess its persistence or signs of degeneration.
2.5. Statistical Analysis
The study conducted morphometric measurements of mouse length and head circumference to assess growth parameters. Additionally, histological examinations were performed to determine the frequencies of cleft palate in the offspring of both experimental and control groups. the results of these analyses are crucial for understanding the potential impact of these interventions on palate development. Statistical analysis of the recorded data of all groups was done using one-way analysis of variance (ANOVA), Tukey's test, and normality tests (Kolmogorov-Smirnov and Shapiro-Wilk) to determine the significant relationship (P<0.05) For each variable separately.
4. Discussion
The palate forms as bilateral shelves emerge from the maxillary processes, initially growing vertically. As development progresses, these shelves reposition horizontally, moving toward each other for fusion. Disruptions in this process due to genetic and environmental factors can lead to cleft palate in mice. Corticosteroids, potent anti-inflammatory agents, are commonly used to treat certain conditions in reproductive-age females due to their immune-modulating properties [
12,
15]. However, their safety during early pregnancy remains uncertain [
31,
32].
The current findings suggest a significant association between therapeutic hydrocortisone injection and the development of cleft palate in offspring, with around 58% of the offspring affected. These results are in line with findings from other researchers who have also reported varying rates of cleft palate over the past decade following the administration of therapeutic hydrocortisone [
21,
22,
32]. However, the rates observed in the current research are higher than those reported in other studies. This discrepancy may be attributed to the time factor between injection and sacrifice, suggesting that the effects of cortisone on palate development may worsen over time. The increase in the rate of cleft palate observed after birth further supports this notion, indicating the persistent and progressive nature of the pathological effects of cortisone.
Cleft palate resulting from hydrocortisone exposure is categorized into unilateral and bilateral types, with the bilateral form being more prevalent. Interestingly, both unilateral and bilateral cleft palates were observed in offsprings from the same pregnant mouse, indicating variability in the outcomes within the same experimental context. This suggests that there may be distinct teratogenic mechanisms underlying bilateral cleft palate compared to unilateral cases, despite both resulting from hydrocortisone exposure during pregnancy. The midline epithelial seam is crucial for the fusion of the palatal shelf during embryonic development, with apoptosis and subsequent removal being essential for proper closure and calcification. However, in hydrocortisone-exposed offspring, where palatal closure appeared morphologically complete, epithelial seam cells did not undergo apoptosis. This finding suggests a disruption in the normal palate development process, despite external closure appearing intact. The incomplete degeneration of the epithelial seam reveals underlying abnormalities at the cellular or tissue level, indicating a potential effect of hydrocortisone on molecular pathways involved in epithelial seam. This suggests dysfunction within the complex mechanisms controlling palate development. The findings regarding the potential effect of hydrocortisone on palate development highlight the urgent need to evaluate the risk-benefit balance of corticosteroid therapy in pregnant females. While corticosteroids are an efficient treatment for various conditions, including those occurring during pregnancy, their teratogenic effects on fetal development must be carefully studied. Therefore, health care providers must carefully balance the benefits of corticosteroid therapy with the health risks to the fetus. Close monitoring through prenatal examinations of pregnant females treated with hydrocortisone is vital for early detection of any abnormalities, allowing timely intervention if necessary.
This study provides compelling evidence of the effectiveness of treatment with vitamin B6 in cases where teratogenic therapy is required to reduce the incidence of cleft palate. The results reveal a significant reduction in the incidence of cleft palate, decreasing from 57.58% in the hydrocortisone group to 27.59% in the group receiving the combination treatment. This significant reduction suggest that vitamin B6 may have a protective effect against the adverse impacts of hydrocortisone on embryonic palate development. Parra et al, (2018) noted that Vitamin B6 plays crucial roles in various cellular processes, including amino acid metabolism, neurotransmitter synthesis, and gene expression regulation [
24]. Interestingly, Yoneda and Pratt (1982) noted that vitamin B6 can reduce the incidence of cortisone-induced cleft palate by altering the binding of glucocorticoids to their cytoplasmic receptors, which in turn inhibits the release of lysosomal enzymes necessary for programmed cell death [
20]. The findings by Oh et al. (2020) suggesting that vitamin B6 supplementation during pregnancy is associated with a reduced risk of developmental abnormalities in tissues beyond cleft palate highlight the broad-ranging reparative effects of this vitamin. [
33]. Clinically, these results suggest a potential strategy to reduce the risk of cleft palate formation in women receiving emergency corticosteroid therapy during pregnancy through balanced doses of vitamin B6. The observation that the majority of cleft palates in the vitamin B6 group were unilateral compared to bilateral hydrocortisone clefts suggests possible implications regarding the effect of vitamin B6 on the severity or elasticity of cleft palate formation. This finding suggests that vitamin B6 may play a role in modifying the development or expression of cleft palates, which may influence the type or extent of cleft that occurs. These findings suggest that vitamin B6 supplementation may not only reduce the overall incidence of cleft palate but also influence the specific characteristics or severity of clefts that occur.
The disappearance of the midline epithelium seam in most offspring with complete palate closure suggests a significant therapeutic effect of vitamin B6 on prevention of cleft palate and enhance its development. This observation underscores the potential of vitamin B6 to counteract the adverse effects of hydrocortisone exposure on palate development, likely through its role in supporting normal cellular processes and enhancing cellular repair mechanisms. Although the protective role of vitamin B6 is in modifying the cellular processes associated with the development of the palate, which contributes to the prevention of cleft palate, but there was an opposing opinion, as Atikala et al, (2001) reported that the intake of multivitamin supplements during early pregnancy may increase the risk of orofacial clefts in offspring, micronutrient intake from food does not appear to be associated with this occurrence [
34].
Acknowledgments
"I extend our sincere thanks and appreciation to the Faculty of Dentistry, New Giza University, Egypt, under the leadership of the Dean of the College, Professor Dr. Randa Al-Salawi, for their strenuous efforts and hard work in making the College of Dentistry, New Giza University, one of the leading colleges. Also, I extend our gratitude to all the faculty members for achieving a distinguished level of excellence".
Ethics Approval: This study was approved by the ethical committee of Faculty of Dentistry Azhar University, Egypt under the number (AUAREC20230001-2). It was ensured that animal care was carefully cared for and that all research procedures followed the guidelines and ethical regulations for animal research according to the principles of the Helsinki Declaration.
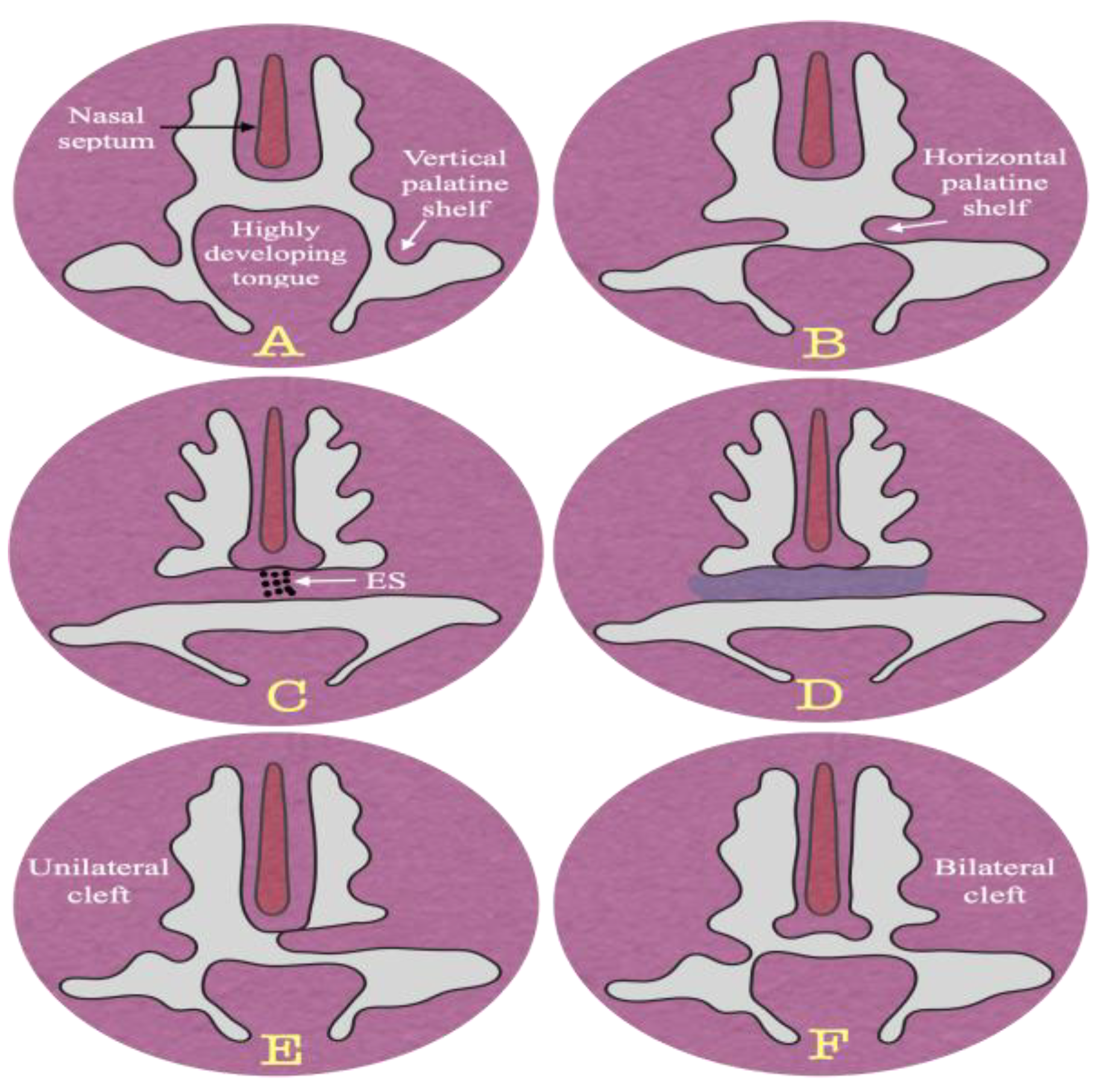
Figure 1.
Stages of palatal development. Vertical positioning shelves (A), horizontal positioning shelves (B), fused shelves with degeneration of epithelial seam (C), and calcification (D), unilateral cleft (E), and bilateral cleft palate (F).
Figure 1.
Stages of palatal development. Vertical positioning shelves (A), horizontal positioning shelves (B), fused shelves with degeneration of epithelial seam (C), and calcification (D), unilateral cleft (E), and bilateral cleft palate (F).
Figure 2.
Grouping and medications of pregnant mice.
Figure 2.
Grouping and medications of pregnant mice.
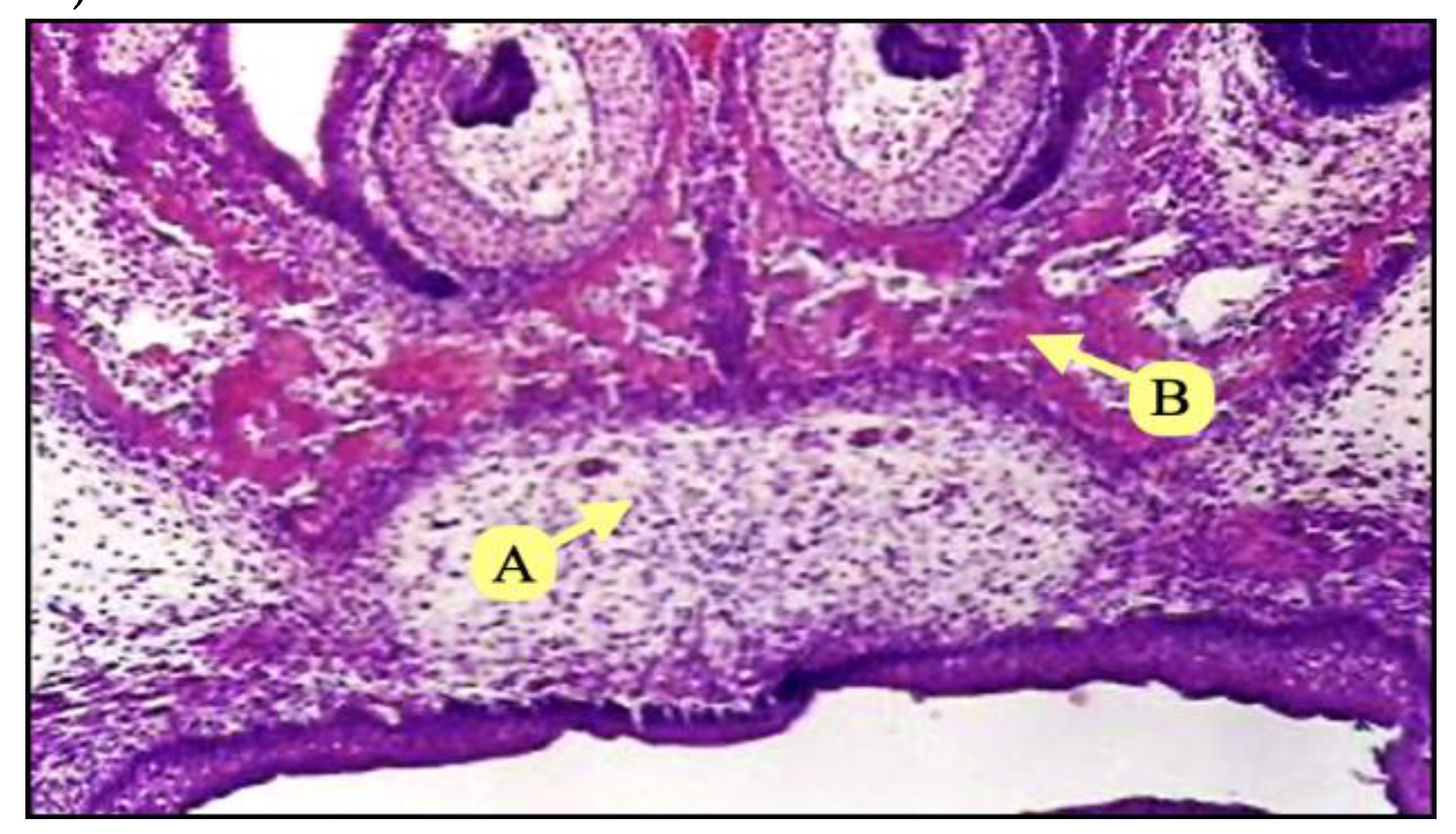
Figure 3.
Photomicrograph of coronal section of control group showing complete fusion of palatine shelves (A), with ossification (B) (H&Ex100).
Figure 3.
Photomicrograph of coronal section of control group showing complete fusion of palatine shelves (A), with ossification (B) (H&Ex100).
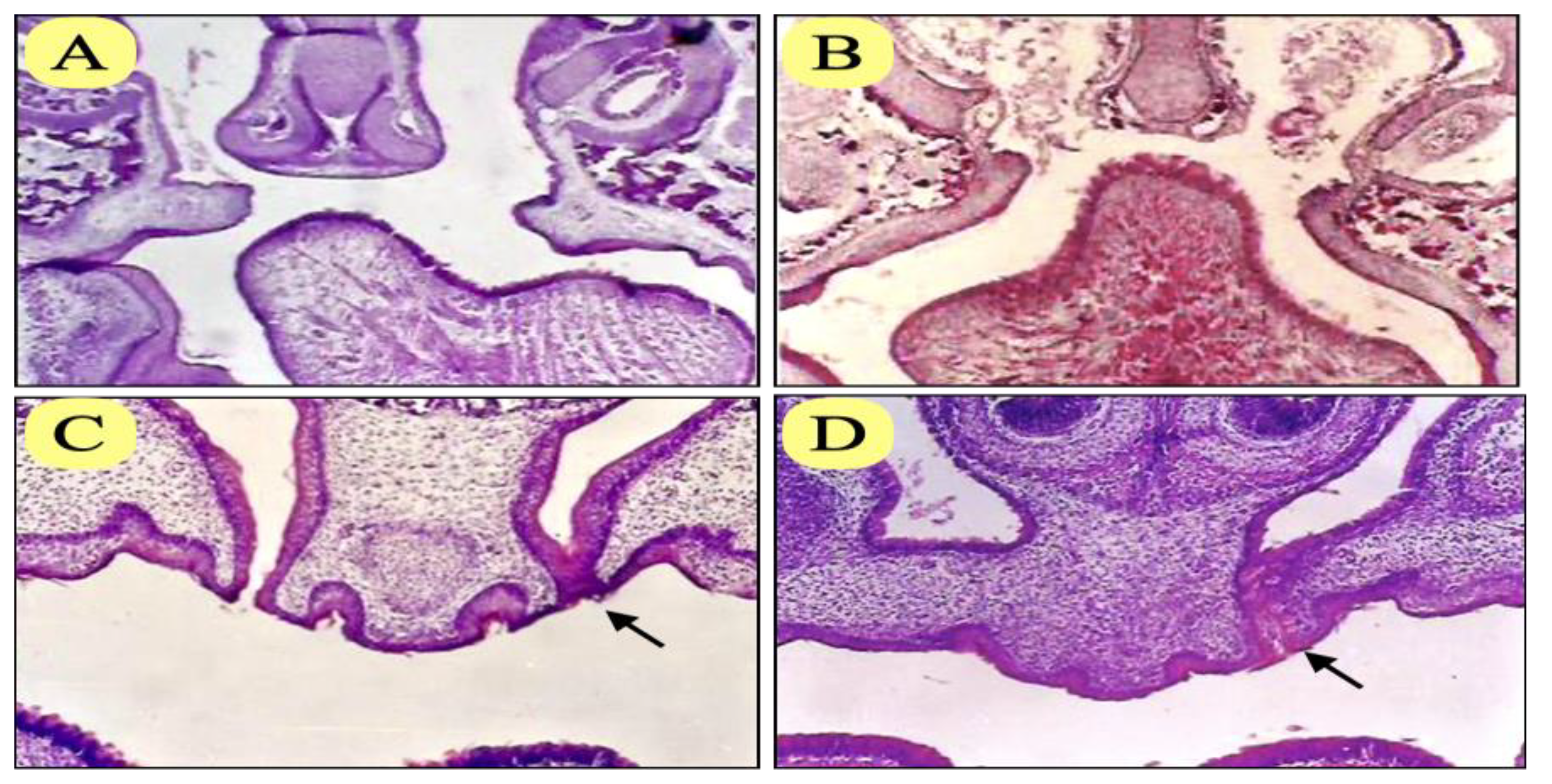
Figure 4.
Photomicrograph of coronal section of group II injected with hydrocortisone showed bilateral cleft palate of anterior region (A), bilateral cleft palate of posterior region (B), unilateral cleft palate (C), with persistent epithelial seam of the fused shelf (arrow) and complete palate closure (D), with persistent epithelial seam at one side (arrow) (H&E x100).
Figure 4.
Photomicrograph of coronal section of group II injected with hydrocortisone showed bilateral cleft palate of anterior region (A), bilateral cleft palate of posterior region (B), unilateral cleft palate (C), with persistent epithelial seam of the fused shelf (arrow) and complete palate closure (D), with persistent epithelial seam at one side (arrow) (H&E x100).
Figure 5.
Photomicrograph of coronal section of group III injected with hydrocortisone and administered vitamin B6 showed bilateral cleft palate (A), unilateral cleft palate (B), with persistent epithelial seam of the fused shelf (arrow), complete palate closure (C), with persistent epithelial seam at one side (arrow), and complete palate closure with degeneration of epithelial seam (D) (H&E x100).
Figure 5.
Photomicrograph of coronal section of group III injected with hydrocortisone and administered vitamin B6 showed bilateral cleft palate (A), unilateral cleft palate (B), with persistent epithelial seam of the fused shelf (arrow), complete palate closure (C), with persistent epithelial seam at one side (arrow), and complete palate closure with degeneration of epithelial seam (D) (H&E x100).
Figure 6.
Frequency of cleft palate chart.
Figure 6.
Frequency of cleft palate chart.
Table 1.
Frequencies of cleft palate and persistence of epithelial seam in all groups.
Table 1.
Frequencies of cleft palate and persistence of epithelial seam in all groups.
| Groups |
Number of offsprings |
Cleft palate foetuses |
Palatine closure with persistence of epithelial seam |
Complete palatine closure foetuses |
| Group I |
31 |
1/31 (3.22%) |
0/31 (0%) |
30/31 (96.78%) |
| Group II |
33 |
19/33 (57.58%) |
7/33 (21.21%) |
7/33 (21.21%) |
| Group III |
29 |
8/29 (27.59%) |
8/29 (27.59%) |
13/29 (44.83%) |
Table 2.
Tukey HSD test on cleft palate.
Table 2.
Tukey HSD test on cleft palate.
| (I) Groups |
(J) Groups |
Mean Difference (I-J) |
Std. Error |
Sig. |
95% Confidence Interval |
| Lower Bound |
Upper Bound |
| 1 |
2 |
-.57380* |
.10088 |
.000 |
-.8142 |
-.3334 |
| 3 |
-.24360 |
.10419 |
.056 |
-.4919 |
.0047 |
| 2 |
1 |
.57380* |
.10088 |
.000 |
.3334 |
.8142 |
| 3 |
.33020* |
.10266 |
.005 |
.0856 |
.5748 |
| 3 |
1 |
.24360 |
.10419 |
.056 |
-.0047 |
.4919 |
| 2 |
-.33020* |
.10266 |
.005 |
-.5748 |
-.0856 |
Table 3.
Tukey HSD test on head circumferences.
Table 3.
Tukey HSD test on head circumferences.
| (I) Groups |
(J) Groups |
Mean Difference (I-J) |
Std. Error |
Sig. |
95% Confidence Interval |
| Lower Bound |
Upper Bound |
| 1 |
2 |
3.80762* |
.39854 |
.000 |
2.8579 |
4.7574 |
| 3 |
2.30512* |
.41164 |
.000 |
1.3241 |
3.2861 |
| 2 |
1 |
-3.80762* |
.39854 |
.000 |
-4.7574 |
-2.8579 |
| 3 |
-1.50251* |
.40556 |
.001 |
-2.4690 |
-.5360 |
| 3 |
1 |
-2.30512* |
.41164 |
.000 |
-3.2861 |
-1.3241 |
| 2 |
1.50251* |
.40556 |
.001 |
.5360 |
2.4690 |
Table 4.
Normality tests (Kolmogorov-Smirnov and Shapiro-Wilk) for all variables.
Table 4.
Normality tests (Kolmogorov-Smirnov and Shapiro-Wilk) for all variables.
| |
Groups |
Kolmogorov-Smirnova
|
Shapiro-Wilk |
| |
Statistic |
df |
Sig. |
Statistic |
df |
Sig. |
| Cleft palate |
1 |
.539 |
31 |
.000 |
.176 |
31 |
.000 |
| 2 |
.392 |
33 |
.000 |
.621 |
33 |
.000 |
| 3 |
.452 |
29 |
.000 |
.561 |
29 |
.000 |
| Head circumference |
1 |
.107 |
31 |
.200*
|
.914 |
31 |
.017 |
| 2 |
.172 |
33 |
.015 |
.914 |
33 |
.012 |
| 3 |
.187 |
29 |
.011 |
.921 |
29 |
.033 |
Table 5.
One-way ANOVA test for all variables.
Table 5.
One-way ANOVA test for all variables.
| |
Sum of Squares |
df |
Mean Square |
F |
Sig. |
| Cleft palate frequency |
|---|
| Between Groups |
5.317 |
2 |
2.659 |
16.345 |
.000 |
| Within Groups |
14.640 |
90 |
.163 |
|
|
| Total |
19.957 |
92 |
|
|
|
| Head circumference |
| Between Groups |
234.073 |
2 |
117.037 |
46.098 |
.000 |
| Within Groups |
228.496 |
90 |
2.539 |
|
|
| Total |
462.569 |
92 |
|
|
|