1. The Kirkhouse Trust
The Kirkhouse Trust (KT) is a UK-registered charity founded by Sir Ed Southern to fund the improvement of legume crops important for food and nutrition security in African countries and India and to promote scientific education. The origins of KT are entwined with the development of Sir Ed’s molecular biology company, Oxford Gene Technology (OGT). In 1997 Oxford University assigned Sir Ed’s microarray patents to OGT. In 2000, OGT’s income had begun to grow, and KT was registered as a charity and endowed by an initial donation from the company.
Sir Ed Southern was persuaded to focus on plant sciences because this field is typically neglected both by governments and the UK charity sector. KT was to concentrate on crop improvement in developing countries with respect to a small number of crops and spend its resources proactively over a period of ~15 years. KT was also to promote the use of molecular biology tools to accelerate the development of improved crop varieties.
The strategy for KT was designed to build a network of scientists to generate a research community. Since sustained, long term funding is needed to ensure that any investment in facilities is not wasted due to lack of continued support, KT has maintained the essential laboratory and operational infrastructure initially provided to projects and enlists the co-operation of the ‘host’ institute. KT has inevitably taken a risk investing in those of its projects sited in countries with limited resources and volatile politics. However, if scientific capacity is to be built in a region, it needs to be driven by indigenous scientists. In 2020, KT became a charity in perpetuity, spending the income of its assets to fund its operations.
Legumes important in the target countries were selected as the focus for several reasons: 1) tropical legumes can produce a good harvest in very harsh conditions with relatively limited agricultural inputs, making them suitable for climate change adaptation; 2) their grains are a good source of dietary protein and essential micronutrients, and they can also be traded in local markets to provide a source of rural income; 3) legumes have the ability to establish symbiotic relationships with nitrogen-fixing soil bacteria, improving soil fertility and reducing the farmers’ dependency on synthetic inorganic fertilisers; and 4) their leftover biomass provides a good quality feed for livestock in the dry season.
2. KT’s Funding Model
This KT’s funding model aims to address its twin objectives of improving legume crops important for smallholder farming systems in target countries and raising national scientific capacity. KT has a hands-on strategy, with a team of international scientific consultants working closely with the PIs and students they mentor, providing technological backup as needed, and hosting PIs and students for study visits in their laboratories. Since the recipients of awards must be able to do all their work in their host institution, KT funds the establishment and maintenance of essential infrastructure (including laboratories, greenhouses, and screening facilities). The support provided is intended to be long-term after an initial one-year pilot project, awarding three-year rolling grants to successful projects. To promote the establishment of international scientific communities and to encourage collaboration and the sharing of tools and resources, KT-funded projects are grouped into consortia focusing on the same crop and similar or complementary problems. Once a year, KT convenes an in-person meeting (online during the pandemic years) for KT grantees to share progress and interact with each other.
This article reviews the successes of the first two decades of KT-funded projects. The individual sections focus on the achievements of the early breeding projects funded in India, the cowpea (Vigna unguiculata) and common bean (Phaseolus vulgaris) breeding consortia in Africa, and a collaborative Indo-African initiative set of projects aiming to establish the potential of a selected group of orphan legumes for climate change adaptation. The paper concludes with some reflections regarding the main challenges encountered and the lessons learned.
3. The Dolichos Breeding Programme at the University of Agricultural Sciences, Bangalore, India
The University of Agricultural Sciences in Bangalore (UAS-B) was the first recipient of KT’s funding, which supported a series of research projects, between 2002 and 2019, focusing on an array of legume crops, along with assorted infrastructure projects. The dolichos lablab (Lablab purpureus) breeding programme led by M. Byre Gowda has had a major impact on farmer livelihoods in South India, thanks largely to his recognition that converting what was a photoperiod-sensitive crop into an insensitive one would deliver flexibility with respect to the crop’s planting time, to the extent that more than one crop could be grown in a given year. Until the release of the HA series of photoinsensitive varieties bred by the UAS-B programme, local farmers grew a single crop per year of photoperiod sensitive, indeterminate types. Indeterminacy has traditionally been favoured by subsistence farmers as it enables pods to be harvested over a lengthy period, but the increasingly market-led economy of India requires year-round production, which can only be achieved using photoperiod insensitive cultivars. The HA varieties, in addition to their photoinsensitivity, produced determinate plants, thereby offering an opportunity to switch from manual to mechanical harvesting.
Alongside the breeding programme, KT also supported the establishment at UAS-B of an
ex situ dolichos germplasm collection. By 2016, this collection had grown to include about 650 accessions, representing the largest ex situ collection of this species in the world [
1]. A substantial effort has been dedicated to characterising and evaluating this germplasm with respect to a wide range of vegetative, inflorescence, pod and seed traits [
2]. A core set of 64 accessions has since been assembled [
3] to simplify the analysis of the species’ diversity.
4. The West African Cowpea Consortium (WACC): from Conception to Improved Crops in the Field
4.1. The Origins of the WACC
Cowpea (
Vigna unguiculata) is a widely cultivated legume species in over 65 countries covering Africa, Asia, the Middle East and Southern Europe, and the Americas [
4]. It has a high content of protein in its seeds (25% to 30% by weight), mineral rich leaves, and abundant straw, making it a major source of food for humans as well as livestock in resource challenged environments. Because of its ability to fix atmospheric nitrogen it requires less fertilizers and can contribute to soil fertility. Moreover, it generally withstands high temperatures and low water availability making it a climate resilient crop in drought prone areas [
5]. Today it is estimated that cowpea is cultivated on over 10.6 million hectares worldwide with an annual production of over 7.4 million tonnes with Africa producing nearly 5.2 million tonnes. Cowpea is especially important in Sub Saharan West Africa which accounts for over 80% of its production, although with climate change constraining legume production across the continent, increased production of cowpea can now be seen in other regions, especially East and Southern Africa. Although cowpea is an African crop in origin, it has been introduced across the globe. It is assumed to have been introduced in Brazil in the 16th century, and its cultivation has expanded rapidly. Cowpea is now the second most consumed type of bean in Brazil being cultivated in the North, Northeast, and Centre-West regions, and Brazil is now the second most important producer of cowpea grain.
Historically, the average yield of cowpea was relatively low, with traditional land races and cultivated forms yielding 400-500 kg/ha or less. This was due to a lack of genetic improvement since domestication and the large number of diseases, pests and parasites that constrained cowpea growth and productivity. Although genetic improvement programs began with the Green Revolution in the late 1960s, little progress was realised until cowpea was listed among the mandated target crop for improvement by the International Institute of Tropical Agriculture (IITA). Under the direction of Professor B.B. Singh and others, the IITA was able to collect and evaluate cowpea germplasm from across Africa and begin systematically breeding for disease and pest resistance as well as farmer desired agronomic traits such as larger seed size, lack of photoperiodism, and shorter times to maturity. Breeding activities focused on stacking these various farmer preferred traits, which using conventional selection and breeding techniques available at the time, was a long and tedious process taking years to decades to realise release of improved varieties.
With the advent of molecular genetics in agriculture, emphasis was placed on leveraging genomic information for rapid improvement in crops through molecular based breeding. Initial applications of marker assisted selection and breeding platforms were targeted at high value crops like maize, rice, wheat, and soybean. Consequently, few resources were allocated to developing genomic scale resources necessary for improvement of cowpea, a crop thought to have little socioeconomic importance outside of West Africa. With a greater appreciation of cowpea’s value as a climate resilient grain legume, and the recognition of the broader importance of the crop to the lives and livelihoods of small shareholder farmers in resource limited Sub Saharan Africa, from the early 2000’s onward an intensive effort was undertaken to improve genomic resources for cowpea leading to greater ability to speed the delivery of improved cultivars for use in low input agricultural production in Africa. This switch coincided with decreased costs for genomic scale sequencing that would allow for the development of molecular tools needed for improving crops such as cowpea.
Among the first attempts at characterising the gene content and complexity of the cowpea genome was a KT supported project initiated in early 2006 with the University of Virginia aimed at obtaining the hypomethylated, gene-rich coding sequences in the genome of the African cowpea cultivar IT97K-499-35 using methylation filtration (MF), a reduced representational sequencing approach known to overcome the presence of ubiquitous repetitive DNA. Using MF, Timko et al [
6] were able to achieve a 4.1-fold enrichment for the gene-rich space of cowpea and generated 263,425 gene-space sequence reads (GSRs) that could be assembled into 41,260 unigenes representing 19,786 unique GenBank accession numbers [
7]. Initially they were able to predict and confirm over 1000 simple sequence repeats (SSR) that were associated with genic regions for annotated resistance genes. These were used in a KT funded program dubbed the West African Cowpea Consortium (WACC) which sought to integrate comparative genomics for molecular marker discovery, marker validation through coordinated participatory field testing of markers by members of the consortium, and capacity building through training in molecular biology, breeding, and bioinformatics. At its establishment in March 2007 the WACC included research partners from Nigeria, Burkina Faso, and Cameroon and later expanded to include breeders from Mali, Niger, Senegal, Ghana, Togo, and Benin.
At its core, the WACC was dedicated to the development of tools and techniques for marker assisted breeding (MAB) that are affordable, readily available and easily applicable in African laboratory settings. Rather than tackle the many biotic and abiotic constraints that limited cowpea productivity, a conscious decision was made to first focus on a small set of major constraints under simple genetic control to accelerate the improvement of local germplasm and train local breeders.
4.2. Achievements of the WACC
Among the early successes of the WACC was the capacity to improve local varieties for resistance to Striga gesnerioides, a parasitic plant which attaches itself to the cowpea root, and severely constrains yields throughout the Sahel. Studies carried out in the early 2000’s identified AFLP markers associated with genes conferring resistance to
S. gesnerioides SG1 and SG3 resistance [
8,
9,
10,
11]. These AFLP markers were used to generate SCAR markers 61R and 61RM2 (MahSE2) linked to SG1 and SG3 resistance genes [
10] as well as C42-2B that was linked to SG5 resistance. At first, the use of these markers allowed for initial attempts at marker selection based breeding of Striga resistant cultivars [
4].
The development of a rich panel of SSR markers (see above) and the identification of SSR-1, a microsatellite embedded in the gene conferring resistance to SG3, the dominant race of
S. gesnerioides found in West Africa [
12] proved pivotal in the implementation of broad scale MAS. The ability to use SSR-1 in molecular breeding based screening of germplasm using simple PCR and gel electrophoretic technologies allowed researchers within the WACC to rapidly improve Striga-susceptible local farmer preferred cultivar for resistance. Subsequent studies identifying germplasm resistant to the various distinct races (pathotypes) of
S. gesnerioides present across West Africa [
13] and the development of SSR molecular markers able to select for these resistances [
14] resulted in the development of Striga-resistant cultivars useful throughout the WACC footprint. The use of SSR-1 (race RSG3), and the two SCAR markers C42B (race RSG 5) and 61R/Mahse2 (RSG1 and 2) have been used to breed for Striga resistance in Burkina Faso, Nigeria, Cameroon, Mali and Ghana. Among the successes are the new varieties FUAMPEA 1 to FUAMPEA 4 (developed by Lucky Omoigui and his team at UAM in Nigeria), ‘Zaayura Pali’, ’Soo-Sima’, ’Diffeele’, ’Wang Kae’ and ‘Kirkhouse Benga’ developed by Francis Kusi and his colleagues at Savanna Agricultural Research Institute (SARI) in Ghana, Komcallé, Tiligré, Nafi, and Gourgou developed by Benoit Batieno and his colleagues at INERA, Burkina Faso, IR15-MA-02 and IR15-MA33 developed by Sobda Gonné at IRAD, Cameroon and CZ06-3-1 (Acar 1), CZ06-2-17 (Simbo), CZ06-1-12, CZ06-4-16, and CZ06-1-05 developed by Mamadou Touré, Sory Diallo and their coworkers at IER, Mali.
Since these first initial studies, the continued development and use of molecular marker technologies for molecular breeding activities within the WACC permitted an expansion of the types and number of constraints that could be selected for improvement. This led to the identification of new resistance linked markers and the breeding of new cowpea varieties with stacked resistances to Striga [
15,
16], Alectra [
17], aphids [
18], brown blotch [
19] and Fusarium [
20].
Coincident with this was application of emerging platforms for high throughput DNA and cDNA sequencing and single nucleotide polymorphism (SNP) detection in cowpea by Muchero et al. [
21]. These researchers were able to map 928 expressed sequence tag (EST)-derived SNPs using an Illumina 1536 GoldenGate platform. Building upon this work, Lucas et al. [
22] developed a new consensus map containing 1107 EST-derived SNP markers developed by integrating 13 population specific maps. The use of SNP technologies changed how molecular breeding in cowpea could be accomplished and demonstrated the effectiveness of integrating SSR and SNP markers for trait mapping and marker assisted breeding.
With the emergence of resistance to aphids (
Aphis craccivora) as a priority among WACC partners Fransis Kusi and his colleagues at the SARI, Ghana began studies aimed at the development of robust screening techniques useful in the identification of new sources of resistance. Using improved screenhouse and field phenotyping, Kusi and his group [
23] identified SARC 1-57-2 as a cowpea breeding line containing a novel aphid resistance locus. Using a panel of SSRs developed within the WACC, they were able to identify a co-dominant SSR marker (CP 171F/172R) that segregated with aphid resistance with a recombination fraction of 5.91% in an F2 population derived from crossing Apagbaala x SARC 1-57-2. Using CP 171F/172R for foreground selection and a KASP-SNP marker panel for background selection in marker assisted backcrossing, the resistance from SARC 1-57-2 was introduced into elite susceptible cultivar Zaayura.
Subsequent genomic scale sequence characterisation and transcriptomic profiling resulted in the development of high quality genetic maps and genome sequence assemblies that permitted the development of effective molecular markers for a broader set of biotic constraints within the WACC. A genetic linkage map based on segregation of simple sequence repeat (SSR) markers developed using a recombinant inbred (RI) population derived from a cross between the California Blackeye type 524B and 219-01, a perennial wild cowpea from Kenya, allowed Andargie et al. [
24] to map agronomic traits related to domestication such as seed weight and pod shattering, as well as floral scent characteristics.
With the advent of new and improved technologies came the development of platforms for high throughput single nucleotide polymorphism (SNP) detection beginning with the Illumina 1536 GoldenGate platform developed by Muchero et al. [
21] with 928 expressed sequence tag (EST)-derived SNPs and culminating with the 51,128-SNP Cowpea iSelect Consortium Array [
25]. This timeframe also saw the refinement of resources for accurate genome annotation, identification of candidate genes, and comparative genomic analysis, pushing cowpea functional genomics to new levels of utility [
26,
27,
28,
29]. Using these improved SNP data, Dr. Erik Ohlson at UVA created a panel of ~200 well-spaced allele-specific SNPs for use within the WACC that could provide laboratories a quick way of locating the approximate chromosomal location of resistance genes using PCR and gel electrophoresis. One could then identify additional SNPs in that region to identify more tightly linked markers as needed. Most recently, a mid-density marker platform for cowpea genotyping has been developed that contains 2,602 SNP markers with an average density of about 4 SNPs / Mbp throughout the 11 cowpea chromosomes [
30]. This genotyping platform, based on diversity array technology (DArT), is currently the most useful low-cost, high throughput method for rapid identification of new markers for key constraints.
In 2023, the WACC expanded its geographic focus engaging researchers in Malawi, Zambia, and Zimbabwe and other locations in Southeastern Africa, and was renamed as the African Cowpea Programme (ACP). This expansion was done with the hope of transferring molecular tools, breeding materials, and technical know how from the WACC to speed the development of improved genotypes for regions across the African continent.
4.3. Future Priorities for the African Cowpea Programme (ACP; formerly WACC)
The ACP will continue to support the deployment of marker-trait associations in the selection of breeding lines, speeding up the development of improved germplasm for local markets. A priority will be to facilitate the transmission of knowledge between programmes, with markers and germplasm already developed by prior WACC projects being transferred to newly funded teams in Southeastern Africa. This includes markers and donor lines carrying resistance alleles to several biotic stressors such as aphid, Fusarium and Striga. The newly developed genomic resources including the cowpea reference genome and pan-genome [
29,
31] will be useful in developing new markers in cases where the linkage between the available marker and the causative locus is broken. At the same time, the ACP will support the identification of new sources of resistance for similar or new biotic threats, facilitating the discovery of novel marker-trait associations whenever needed.
An expansion in the number of traits targeted by the ACP is among KT´s objectives. We are aware of the importance of breeding for consumer-related traits in order to develop varieties that are accepted by the public. The genetics of many traits related to seed coat colour and patterning are better understood now (e.g. [
32,
33]), and the findings can be used by cowpea breeding programmes to select lines with locally preferred seed coat characteristics at an early point in the breeding cycle. The feasibility of using marker-assisted selection to increase seed size has been demonstrated by the success of Lo [
34] in introgressing multiple loci into a popular Senegalese cultivar. Seed coat texture, an important quality trait influencing the end use of the seed, is expected to be under a relatively simple genetic control [
35]. Seed coat roughness is known to be negatively correlated with cooking time, a trait of major importance in sub-Saharan Africa, not just because firewood and charcoal are in short supply, but also because of the importance of reducing exposure to smoke. Investments that enable the identification and deployment of markers tightly linked to loci controlling consumer traits will help ensure that improved varieties are adopted by farmers and consumers.
Abiotic stresses, especially heat and drought, are major threats to cowpea productivity, and the frequency of their occurrence is rising as a result of climate change. Even though cowpea is well adapted to a semi-arid hot environment, extreme events including heatwaves and long drought periods are negatively impacting cowpea yields [
36,
37]. While improving adaptation to these abiotic stresses is urgently needed, their complex inheritance makes them more difficult to tackle using a conventional breeding approach. In this sense, KT´s strategy of promoting the use of marker assisted selection to identify desirable segregants is the only effective breeding strategy for developing varieties with an increased level of stress tolerance. First, however, it will be necessary to improve our understanding of the genetic architecture of the stress response. To date, no major loci have been validated for traits related to these abiotic stresses, and the only strategy implemented by the cowpea programmes so far has been to target early flowering individuals that can escape droughts occurring at the late growth stage. The ACP is determined to take steps towards making marker assisted breeding of heat and drought tolerant cowpea varieties a reality in the near future. These steps include: 1) connecting with funding agencies, institutions and researchers conducting work on abiotic stresses to foster discoveries through collaboration; and 2) improving data collection related to field trials at many different locations in Africa (both agronomic and climate data from weather stations), which can be used to gain a better understanding of drought and heat-related traits and to help develop model based predictions.
Capacity building and training will continue to be a priority for the ACP. The experience gained throughout the years has helped define our training priorities. Special emphasis will be put on concepts behind marker-assisted selection (e.g. linkage disequilibrium), which are often incomplete. The use of markers in the selection process is an important component that all KT-funded programmes include, and gaining a deeper understanding of marker-assisted selection technology would empower African breeders and scientists to fully utilise the method in their breeding programmes and to troubleshoot any problem quickly and in an independent way. Another issue that is becoming increasingly challenging is the maintenance of the identity and purity of the breeding materials. The successful use of markers to guide breeding decisions depends heavily on maintaining the genetic purity of seed. Therefore, the ACP will provide sustained support to increase awareness and to endorse better practices during the process of seed increase, whether in a screenhouse or in the field.
The number and quality of the genetic and genomic resources available for this crop are expanding and will continue to be exploited by the ACP to maximize the impact of investment on cowpea improvement programmes. Multiple populations genotyped at a high-density are available, including several biparental RIL populations [
25], a multi-parent MAGIC population [
38], and core (IITA Core, [
39]) and mini-core (UCR Minicore, [
40]) collections. The use of these resources, coupled with the germplasm developed by prior WACC projects, will surely broaden the achievements of the ACP.
5. The African Bean Consortium (ABC): Developing Improved bean Varieties in the World’s Highest per Capita Bean-Consuming Region
5.1. The Origins of the ABC
The ABC project sought to accomplish three complementary goals. First, it aimed to empower African bean breeders in developing their cultivars from the initial varietal concept to advanced lines ready to be submitted to national trials. Second, it sought to upgrade the capabilities of the breeding programmes so they could take advantage of genomic information for the common bean and apply marker-assisted selection in the breeding pipeline [
41]. Third, it allowed a more decentralised pursuit of plant breeding to take advantage of specific or local, rather than general, adaptation [
42,
43]. Accomplishing these goals required a careful choice of each project's specific objectives. These were discussed at the first meeting of the ABC project, which took place in Nairobi in 2006. Participating at this meeting were representatives from bean breeding programmes in East Africa, namely from Kenya (Kenya Agricultural and Livestock Research Organization and the University of Nairobi), Malawi (Bunda College of Agriculture), Rwanda (Rwanda Agricultural Board), Tanzania (Sokoine University of Agriculture) and Uganda (National Agricultural Research Organization). Later additions to the consortium were breeders from the Southern Agricultural Research Institute (Hawassa, Ethiopia), the University of Zambia, the University of Embu (Kenya), and the Instituto de Investigação Agrária de Moçambique (Mozambique).
At the 2006 meeting, the attendees decided that the ABC would focus on introducing multiple disease resistances for several reasons. First, diseases are a nearly general production constraint worldwide where beans are produced. Introducing resistance to these multiple diseases would represent a significant improvement as each of the pathogens causes substantial yield losses [
44,
45]. Second, bean breeders and geneticists, mainly in the USA and Brazil, had already developed molecular markers tagging major resistance genes for several diseases (e.g. [
46,
47,
48,
49,
50,
51,
52,
53,
54]). Third, for several diseases or the Rhizobium symbiont, a process of parallel geographic diversity, possibly due to bean-microbe or virus coevolution, had been observed [
55,
56,
57,
58,
59] prior to the initiation of the project. Alongside the backcrossing of resistance genes into preferred cultivars, the ABC projects included the collection of diseased plant tissue and seed, the isolation of pathogen strains and the characterisation of the genetic diversity of both the host and the pathogen. These constituted research topics for KT-supported students pursuing a graduate degree (e.g. [
60,
61,
62,
63,
64,
65]) and would have important implications for resistance breeding, including information about pathogen and host diversity and choice of host resistance sources.
Five diseases, based on the significant losses they cause in East Africa, and on the availability of DNA markers, would be prioritised. The five diseases were BCMV/BCMNV (bean common mosaic virus/bean common mosaic necrosis virus), CBB (common bacterial blight, caused by Xanthomonas campestris), anthracnose (caused by the fungus Colletotrichum lindemuthianum), ALS (angular leaf spot, caused by the fungus Pseudocercospora griseola) and Pythium root rot. Other constraints were considered but were ultimately not adopted in order to streamline the multi-project consortium. These constraints were rust (Uromyces appendiculatus), the insect herbivores bean stem maggot (Ophiomyia sp.), bruchids (Acanthoscelides obtectus) and aphids (Aphis sp.), drought stress, low soil fertility, and consumer traits such as cooking time and biofortification. Insufficient information about the genetic control of these traits and absence of marker tags were the predominant reasons to defer breeding for these traits.
The markers available in 2006 at the initiation of the ABC project were mainly sequence characterised amplified region (SCAR) markers [
66]. The conversion to SCAR markers from the original RAPD markers maintained the dominant nature of the marker. When in the cis phase with the resistance gene, dominant markers are useful to identify individuals carrying the resistance allele in successive backcross progenies. However, co-dominant markers are preferred, since these allow for identification of homozygous and heterozygous carriers. A successful ABC-supported programme to replace a dominant by a co-dominant marker was the development of an insertion-deletion (InDel) marker tagging the
Phg-2 gene for resistance to ALS [
67].
5.2. Phaseolus Genes, the “Bean Breeders’ Marker Toolbox” and the Research It Supported
The search for molecular markers was facilitated by the development of the PhaseolusGenes marker database as a collaboration between the Gepts group in the Department of Plant Sciences at UC Davis and the Bioinformatics Unit of the UC Davis Genome Center starting in 2008, with the assistance of D. Lin, J. Boveda, M. Briton, J. Fass, N. Joshi, Z. Lu, and A. Schaal [
68]. PhaseolusGenes provided information on legacy markers [e.g. Sequence Characterised Amplified Region (SCAR) and Sequence-Tagged SITES (STS) markers] and newly developed markers [Simple Sequence Repeats (SSRs) and Insertion-Deletion (InDels)], all anchored on a reference whole-genome sequence. Initially, this sequence was the soybean sequence (
Glycine max), a phylogenetically relatively close taxon but with a tetraploid genome; [
69], but it was later replaced by the first common bean reference sequence (version 1.0, developed in the Andean landrace G19833; [
70].
The initial design of the database included three elements: 1) a database proper, with information on individual markers (including sequence, PCR primers, description of potential usefulness as markers, linkage map and genome locations), and source; 2) a genome browser, showing the genome location of several marker categories aligned initially against the soybean genome and, more recently, the Andean reference sequence for common bean (version 1.0, [
70]); and 3) a comparative mapping instance (CMap) based on shared markers among several molecular genetic maps, useful to determine linkage relationships among widely different traits.
The genome browser included a broad range of genomic sequence types from various sources that had been collected over the years PhaseolusGenes existed. In addition to individual tracks devoted to previously established markers, such as SCAR and STS, the browser included tracks for ESTs, BAC-end sequences BAT93 library; [
71], STSs of P. vulgaris [
72], ESTs of other species (developed by the J. Craig Venter Institute: runner bean,
Phaseolus coccineus; cowpea,
Vigna unguiculata, etc.), and a track for alignments of common bean sequence reads containing predicted SSR motifs.
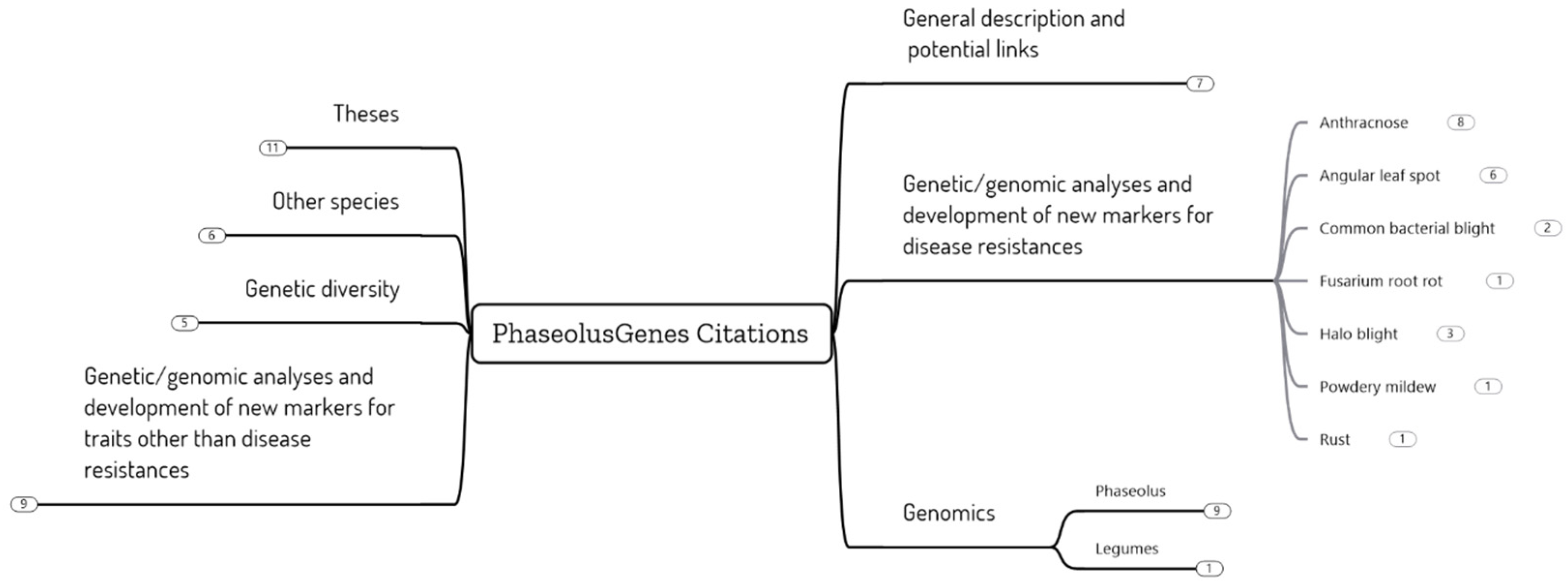
A survey of the scientific literature over the last 9 years, since the inception of the PhaseolusGenes database, provides an assessment of the different uses of the database (
Figure 1). As expected, based on its design [
67,
68], the PhaseolusGenes database was used repeatedly to identify markers linked to agronomic traits, mainly disease resistance genes, with some 65 studies citing the database. The markers developed included three for the diseases that are objectives of the ABC anthracnose (ANT), angular leaf spot (ALS), and common bacterial blight (CBB).
Gonçalves-Vidigal et al. [
73] identified two sequence-tagged site (STS) markers, CV542014450 and TGA1.1570, linked to a disease resistance cluster containing the Co-14 and Phg-1 loci on chromosome Pv01. Dr. Celeste Gonçalves-Vidigal was a visiting scientist from Brazil in the Gepts group at that time. With the assistance of Dr. James Kami, she identified and validated these two markers in approximately two weeks. Prior to the existence of PhaseolusGenes, such identification would have taken several months on average. More recently, Murube et al. [
74] integrated the genetic and physical map positions of ANT resistance genes on chromosomes Pv01 and Pv04.
The PhaseolusGenes database was also useful to identify markers of other genes for resistance to ALS. Miller et al. [
75] identified several markers closely linked to the
Phg-2 locus on chromosome Pv08. Of these, the indel g796 provided the most reliable and reproducible indel marker, located at 3 cM from
Phg-2. More recent research by Gil et al. [
76], based on data of Lobaton et al. [
75], has further narrowed down the chromosome fragment containing
Phg-2 to ~400 Kbp. More recently, Murube et al. [
74] integrated the genetic and physical map positions of ANT resistance genes on chromosomes Pv01 and Pv04.
In research, wholly or partially funded by KT, Okii et al. [
77] studied the population structure and genetic diversity of the Ugandan common bean collection. They observed that in this collection, Andean and Mesoamerican genotypes were about equally represented. A study combining socio-economic surveys and SNP marker analysis by Wilkus et al. [
78] found that farmers who had participated in regional participatory research were more likely to adopt new varieties and diversify their seed stocks. There were differences among farmers for their propensity to adopt Mesoamerican varieties, the group they were least familiar with, but which represented the largest change in bean diversity.
For a variety of reasons, including scientific (comparative genomics), organisational, and streamlining purposes [
79,
80,
81], PhaseolusGenes was transferred into PhaseolusMine in the Legume Information System (LIS) [
82] to maximise the utility of the information for the legume community and to make available all online comparative genomics tools available within LIS.
5.3. Achievements of the ABC
The principal accomplishment of the ABC project has been the development of improved varieties and advanced breeding lines that have reached the stage where they have been tested, or will be shortly, by the respective national authorities for release as commercial varieties in their respective countries. They include: Redwoylata and Ibado improved with resistance to angular leaf spot (ALS) and common bacterial blight (CBB) released in Ethiopia in 2023 by Yayis Rezene Tedla; NABE 12CR improved with resistance to anthracnose and NABE 14R improved with resistance to root rot released in Uganda in 2024 by Stanley Nkalubo; and Zerengeti (resistant to anthracnose) and Kundadila (a faster cooking, high iron bioavailability) developed by Kelvin Kamfwa and candidates for release in Zambia after completing the last year of testing required for registration.
These high yielding, multiple disease resistant varieties have progressed through the KT-funded breeding pipeline of each project from the initial cross-hybridisation through the early-generation marker tests to end with the later generation field testing.
5.4. Future Priorities for the ABC
Moving forward, the ABC will continue to prioritise two main focal activities: First, the breeding of new bean varieties for African smallholder farmers; and second, the training of new generations of African breeders to efficiently develop such novel varieties in the future. The African Bean Consortium’s strategy focuses on giving local breeders the tools needed to conduct all elements of the breeding pipeline, from parent selection, crossing, and evaluation, through variety release. This approach strives to ensure that all rounds of selection are conducted in response to local demands and environmental conditions. It also empowers the local community to be able to deploy a broad range of strategies to address complex challenges in their areas. Ultimately, this investment is aimed to maximise nutritional security and livelihoods for smallholder farming communities across Africa.
Future breeding work will include a renewed focus on maximising yield, key disease resistances, and a small number of the most important consumer traits. This will be accomplished through hybridisation between existing preferred varieties, with the introduction of valued alleles (such as key disease resistances) from exotic sources through recurrent backcrossing. In general, early-generation selection will focus on traits that are highly heritable and/or inexpensive to screen, including seed type (e.g. seed colour, size, and shape), and disease resistances. Marker-assisted selection will focus primarily on early generations and allele introgression through recurrent backcrossing. Selection in later generations will focus on traits with lower heritability and/or higher phenotyping costs, such as yield. In all cases, breeding priorities will continue to be set by African breeders based on their local demands, with input from international consultants on methods and planning. Education of ABC teams will continue to take several forms, including training visits to international labs (e.g. UC Davis), training sessions at annual meetings for projects, Zoom trainings, and cross-training between teams, particularly following visits to international labs. Educational objectives include training with the latest genotyping methods, phenotyping methods, and data analysis software tools.
Several new technological opportunities for growth exist for ABC projects. Advances in genotyping methods offer the chance for funded projects to use marker types with improved linkage to variation of interest, codominance for identifying heterozygotes, and higher-throughput screening. Historically, ABC teams have primarily conducted marker-assisted selection using dominant SCAR markers, which face limitations that are overcome by newer technology. A variety of codominant marker types exist and have begun to be explored by programmes, these include simple indel length polymorphisms, Cleaved Amplified Polymorphic Sequence (CAPS) markers, and allele-specific competitive-extension PCR-based assays (e.g. KASP, PACE). These latter marker types are derived from SNPs, which have become the predominant marker type used in common bean genetic studies [
79], thus offering great flexibility in design and deployment. These markers can be designed in extremely tight linkage with causal genetic variation, and can be rapidly developed for virtually any trait of interest that has been genetically mapped in a diverse population [
80]. Importantly, these assays are also co-dominant, allowing for the distinction between heterozygotes and homozygotes of each allele state. This greatly facilitates the stabilization of resistances, especially when pyramiding multiple genes simultaneously. KASP and PACE are also extremely high throughput, with data managed electronically, and bypass gel electrophoresis altogether, enabling the screening of hundreds of samples at a time depending on the equipment used. Perhaps most importantly, SNP-based marker assays have become the favoured technology for many genetics and breeding programs internationally [
81], which often no longer develop SCAR markers linked to alleles of interest. To keep up with the leading advances in dry bean genetics and breeding, it will be important for ABC-funded teams to have the capacity to screen markers of these important and growing modern marker classes. Adopting the new technologies will encounter challenges such as the shipment of specialised equipment, its maintenance, and project teams training in new techniques. The application of optimal marker technologies by ABC research teams across Africa is a priority for the consortium.
Further opportunities exist for ABC teams related to materials sharing. The development and release of promising breeding materials by ABC programmes offers further opportunities to expand collaborations between funded projects and between countries. Certain market classes are broadly valued by populations of several African countries (e.g. Kablanketi types in Malawi, Tanzania, Zambia, and Kenya: seeds with dense purple stippling yellow beans [
82]; Kalima types; sugar beans; etc.). These regions also often value similar consumer traits and suffer from similar limitations in pathogens, pests, and abiotic stress. The continued cross-sharing of materials between neighbouring countries, both as finished cultivar releases and parents for future breeding, will be another promising area for future development.
ABC projects continue to make considerable breeding progress across numerous bean market classes, traits of interest, and countries. Continued investment in the ABC programmes will include a significant training component, ensuring that funded projects have access to the most effective genotyping, phenotyping, and data analysis methods for their respective goals. Meanwhile, as new breeding materials continue to be released, the consortium will seek to maximise its impact through the sharing of materials, through cross-release into neighbouring countries where applicable and appropriate, as well as sharing of improved lines for use as parents in continued breeding. Through this collaborative investment, the ABC seeks to continually improve the nutritional security and quality of life for smallholder farmers and their communities across the African continent.
6. The Stress Tolerant Orphan Legumes (STOL) Consortium
6.1. The Origins of the STOL Consortium
Roughly 2.5 billion people (30% of the world’s population) live in semi-arid regions, and approximately a third of these people depend on agriculture for their food security and livelihood. Crop production in these regions has always faced challenges associated with excess heat, drought, a highly variable climate, land degradation, and a loss of biodiversity, which has been exacerbated in recent times by climate change, limited access to technology, poor market linkages, weak institutions, and lack of national and international partnerships. A possible strategy to cope with climate change is to switch from the cultivation of current crops to ones which are more drought hardy [
83,
84]. These include a number of minor legume crops, commonly known as orphan legumes, currently being grown to a limited extent in the drier regions of both Africa and Asia, to provide a measure of food and nutritional security to households, as well as some income to farmers. These species have remained relatively neglected by both researchers and industry because of their limited economic importance in the global market.
The STOL consortium was established in 2018 under the Promoting India-Africa Framework for Strategic Cooperation Initiative in partnership with the Indian Council of Agricultural Research (ICAR). The STOL programme aims to facilitate the introduction and exchange of stress tolerant orphan legume varieties among partnering Indian and African institutions and assess the relative response of selected species to the higher levels of abiotic stresses expected because of climate change. The species chosen were moth bean (Vigna aconitifolia), mung bean (V. radiata), horsegram (Macrotyloma uniflorum), dolichos (Lablab purpureus), Bambara groundnut (V. subterranea), marama bean (Tylosema esculentum), tepary bean (Phaseolus acutifolius), rice bean (V. umbellata), pigeon pea (Cajanus cajan), lima bean (P. lunatus) and adzuki bean (V. angularis), with cowpea included as the reference crop. The selection of the varieties and germplasm of the crops identified from India was based on their performance in the drier parts of the country, especially in western Rajasthan, where the climatic conditions closely match those of the target sites in Africa (in the Sahel). It was agreed that following an initial trial of two consecutive years, only those sites where the performance of the STOL crops was promising (compared to cowpea) would be selected for the subsequent testing and identification of promising varieties to be submitted for release for wider cultivation.
To resolve the institutional difficulty associated with seed exchange between India and African countries and vice versa, the project took advantage of a recently established India-Africa Strategic Partnership, a multi-dimensional South-South cooperation arrangement. Under this agreement, the Indian Government and the African Union signed a memorandum of understanding (MoU) agreeing to increase cooperation for improving the productivity, nutritious quality and resilience of local and traditional food systems while preserving biodiversity. In 2018, a MoU was also concluded between KT and the Indian Council of Agricultural Research-National Bureau of Plant Genetic Resources (ICAR-NBPGR). Under this agreement, nine African countries (Burkina Faso, Ghana, Kenya, Mali, Namibia, Niger, Nigeria, Tanzania and Uganda) and India agreed to collaborate in a number of activities, namely to: 1) identify a panel of fifty accessions of each STOL species; 2) multiply and distribute seed to the various project partners for evaluation; 3) carry out field trials of the best performing species and accessions to submit proposal for release of varieties; and 4) train extension workers and farmers in the management of these crops.
6.2. The Achievements of the STOL Programme
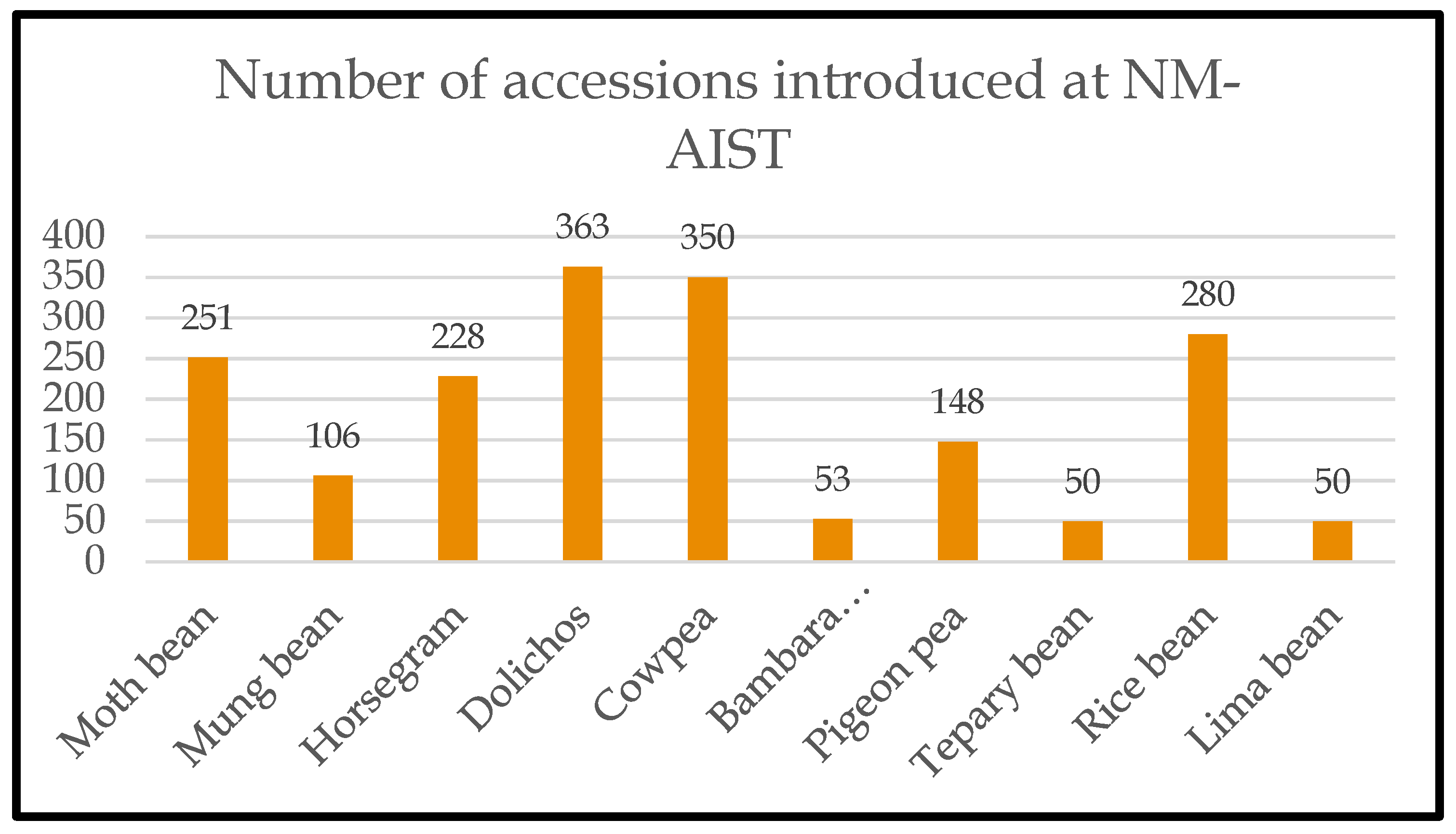
A collection comprising 1,879 accessions (
Figure 2) was assembled through the signing of a material transfer agreement (MTA) with the Nelson Mandela African Institute of Science and Technology (NM-AIST, Tanzania). Selected accessions were planted at NM-AIST, Moshi, for field evaluation during the 2017-18 growing season and early and late maturing accessions were selected for subsequent studies.
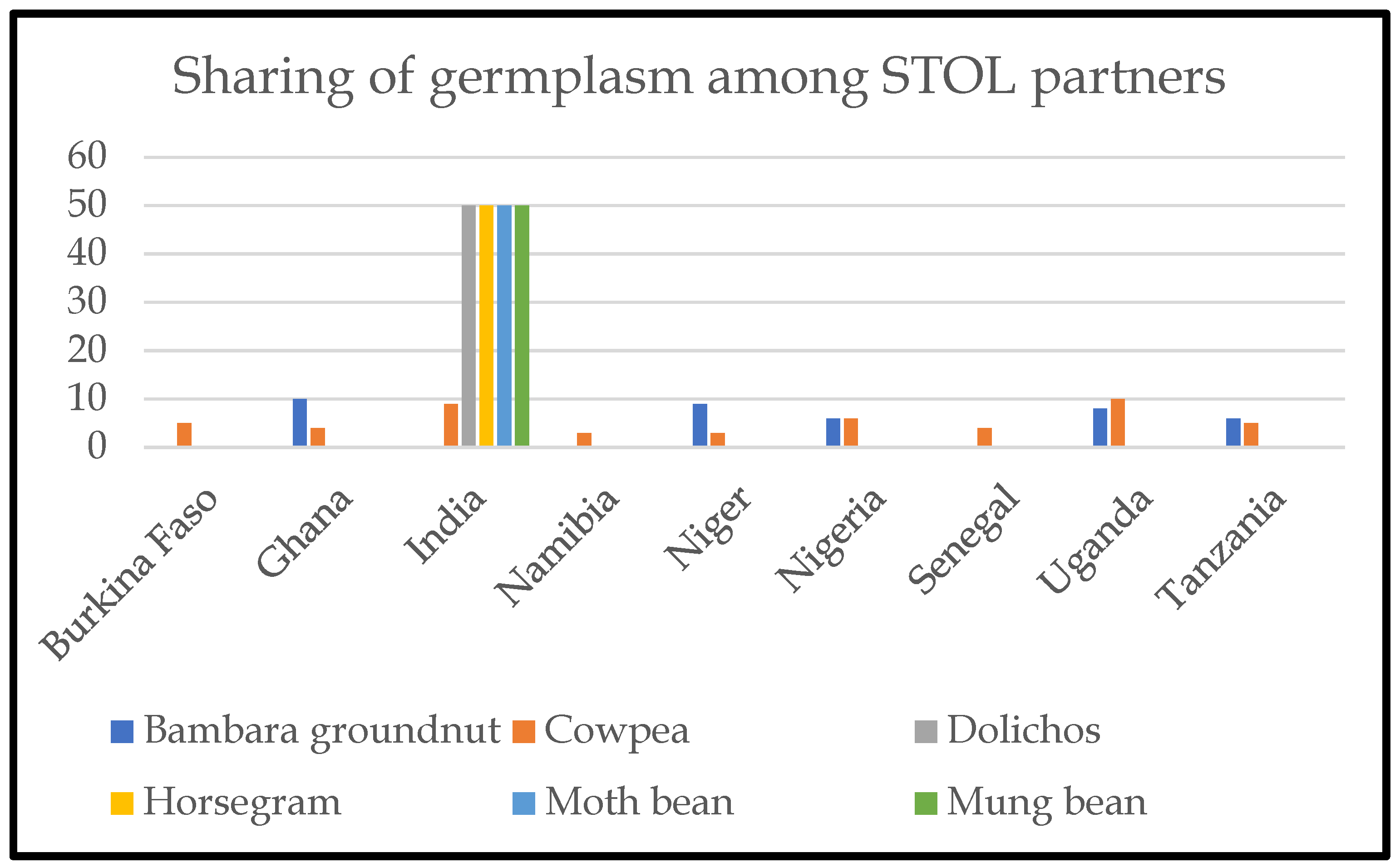
Under the KT-ICAR agreement, systematic selection and exchange of promising germplasm from India were identified and shared under the agreed MTA (
Figure 3).
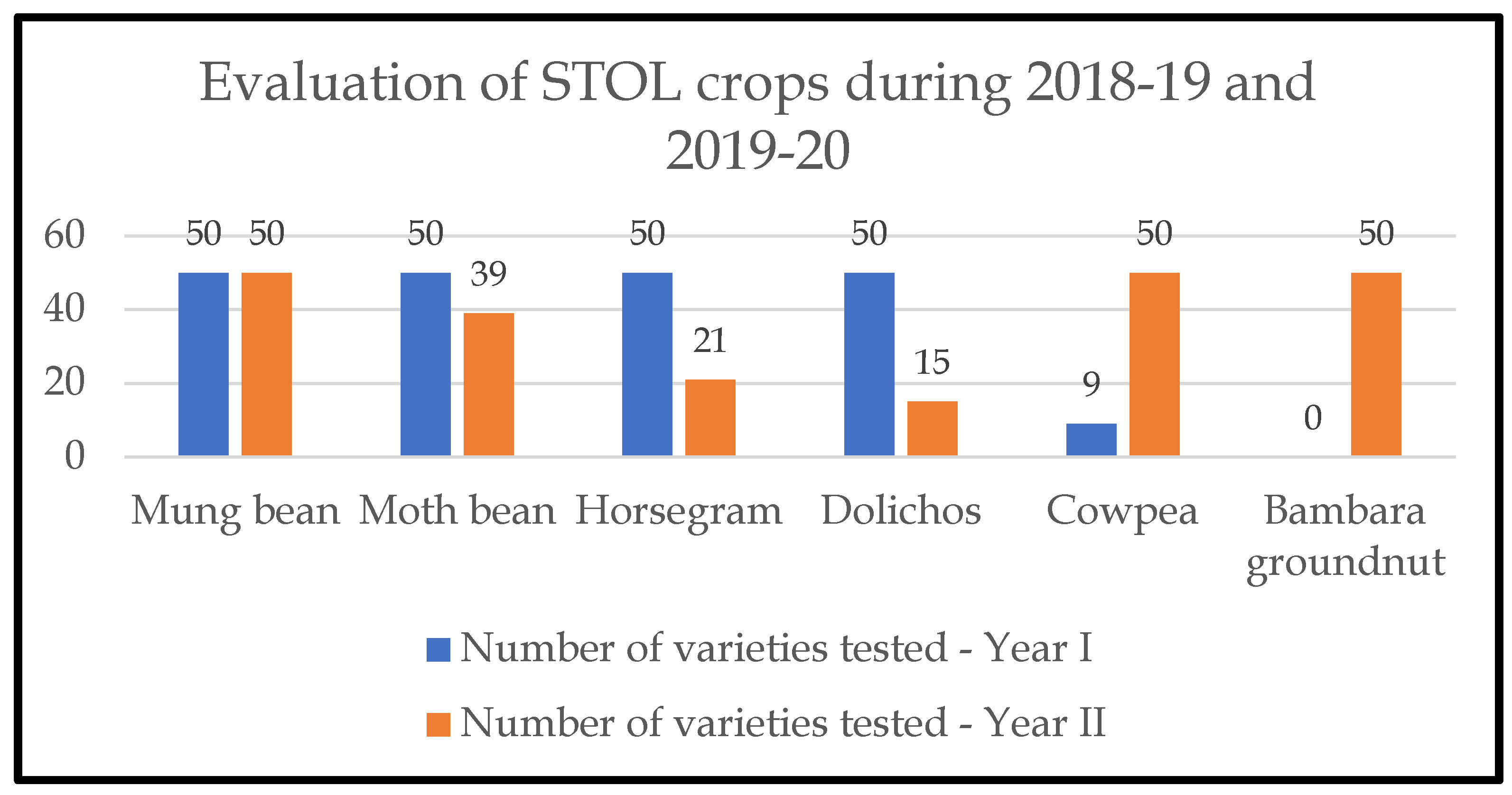
Following this exchange, germplasm was planted for field evaluation using an augmented block design, applying standard practices and including four local cowpea varieties as a check to compare the performance of the new crops across all locations. The field evaluation was continued for two consecutive years (2018-19 and 2019-2020). During each cycle of field evaluation the best performing accessions were selected, and poorly performing accessions were dropped, as shown in
Figure 4.
A participatory selection approach was followed for the selection of promising varieties by organising farmers’ field days (
Figure 5).
Most of the farmers who participated in field days showed interest in trying these new crops, either for grain purpose or as fodder crop. Mung bean was considered the best grain crop by all farmers across all countries, followed by moth bean in selected sites where the average yearly rainfall lay below 500 mm. Horsegram and dolichos were identified as a good source of fodder crop. Based on the field evaluation, 4-10 of the best performing varieties of each crop were identified to be tested at farmers’ field and research stations, across diverse agroclimatic zones to make recommendations for the release of varieties following national variety release guidelines.
Based on the sustained performance over years and across sites, two countries (Burkina Faso and Senegal) were considered most suitable for the cultivation of the STOL crops among the ones initially selected. Further support was provided aiming to formally release varieties of mungbean, moth bean and dolichos at the national and province levels. The other former STOL country partners are conserving the germplasm introduced from India in their respective genebanks and will look for opportunities for their use in national crop improvement programmes as and when necessary. Promising germplasm identified in Burkina Faso based on agro-morphological traits and genotype x environment interactions was further tested on farmers’ fields. Two varieties of dolichos (IC-0623093, HA-3) and three varieties of mung bean (Ganga-1 and IC-103245 received from NBPGR and AVMU-1621 received from the World Vegetable Center) have been established as suitable for formal registration and their release is expected in 2025. Further trials are under way for selected moth bean accessions aiming to release suitable varieties in the coming years. In Senegal, four varieties of mungbean (Ganga-8, GAM-5, MH-421 and IC-39383 received from NBPGR) and three varieties of moth bean (RMO-4-1-6-9, RMO-25 and GMO-2 received from NBPGR) were identified as promising varieties based on their performance across five locations and are considered as suitable for formal registration and their release in 2025.
In a parallel activity, 521 marama bean accessions were collected from across 40 target collecting sites in Namibia. Marama bean is a drought tolerant legume native to Southern Africa that produces both seed and tubers. In addition, since a plant produces numerous prostrate stems that grow up to 3 m in length its cultivation in arid areas may contribute to preserve soil structure. The accessions collected were planted in the field for maintenance and characterisation. A set of 50 promising accessions were identified based on their performance with respect to various agro-morphological traits. All these accessions are being maintained in the field and seed samples are also being stored at NUST. Of these, 18 promising accessions have been identified and shared with STOL partners, with trials in India currently underway.
In addition to the above crops, a programme of introduction and testing for the adaptability of tepary bean (germplasm provided in 2017 by the Phaseolus gene bank at the Centro Internacional de Agricultura Tropical (CIAT) in Cali, Colombia) was initiated in both India and Burkina Faso [
85], in partnership with UC Davis, USA. Under this programme, 26 selected tepary germplasm and improved lines were shared with the STOL partners and are currently under field evaluation.
STOL researchers complied monographs, commissioned by KT, focusing on dolichos, horsegram, marama bean, moth bean and tepary bean, which are in the final stage of publication.
7. The Bambara Breeding Initiative (BBI)
One of the intended outcomes of the STOL consortium was to identify a crop to become the focus of breeding projects funded by KT, and the choice fell on Bambara groundnut, an African species which has been cultivated across the continent for centuries in low input agricultural settings; the crop is currently also being grown in Southeast Asia.
The plant is adapted to a wide range of agroecological conditions, and since it is both highly tolerant of drought and high temperatures and its grain is very nutritious, the species provides very good opportunities for climate change adaptation [
86,
87,
88]. In view of the global expansion of semi-arid climate, promoting the use of this species would also help conserve important genetic resources for the continent which are very valuable for other regions of the world [
89,
90]. Its wide adoption is limited largely by its low productivity. While the crop can yield in the region of 4 t/ha when grown in a research station environment, farmers working under marginal conditions typically attain only a tenth of this production level [
91]. Other constraints include prolonged cooking time, susceptibility to pests and diseases and photoperiod sensitivity. In addition, as is the case for all orphan crops, seed systems are nearly non-existent and value chains need to be developed.
Bambara groundnut has been the focus of numerous studies over the last few decades. As a result, genetic resources have been characterised to varying degrees, and a range of molecular genetic tools have been developed, including DNA-based markers for some key traits [
87,
88,
92,
93,
94,
95,
96,
97]. Thus, the elements needed to develop an effective marker assisted breeding programme are already in place. KT currently funds two projects focused on Bambara groundnut, the first members of the Bambara Breeding Initiative: 1) a project in South Africa aiming to identify genomic regions associated with desirable agronomic traits and cooking quality as a prelude to marker assisted selection to combine traits in adapted materials for southern African conditions; 2) and a project focused on fungal pathogens in Nigeria. Since this is a very new field for KT, it has been important to reach out to the existing key players and learn about ongoing research initiatives focused on Bambara groundnut, to avoid duplications and capitalise on potential synergies and complementarities. To this aim KT convened a two-day online symposium, which aimed to establish collective research priorities on this crop, and to discuss the most appropriate way of sharing breeding resources among partners, who committed to devote resources as a research community.
8. Capacity Building
Providing opportunities for scientific training is a key objective. KT has awarded to date 49 PhD and 58 MSc scholarships among the different research consortia. Additional training opportunities are actively sought, in the form of study visits for PIs and PhD students hosted by the consultants and more experienced African PIs, independent short trainings throughout the year (some online), and in sessions during the yearly in person meeting for all KT breeding PIs and PhD students. These included a course for 36 ABC project members delivered by highly qualified personnel of CIAT (Uganda) on topics related to the use of molecular markers and plant pathology techniques. The most experienced PIs funded by KT have also delivered training sessions for their colleagues, namely a course on plant breeding in the ABC held in Kenya, and a plant pathology course in the WACC hosted by the Environmental Institute for Agricultural Research (INERA) in Burkina Faso.
KT also funded two teaching projects that are independent of the breeding consortia: the KT Mobile Lab in Ghana, which ran until from 2006 to 2022, and the molecular biology teaching lab at the University of Zimbabwe, which is still operational. The Mobile Lab was an ex-military vehicle purchased by KT in 2006 and refitted as a molecular biology laboratory (
Figure 6).
The Mobile Lab was shipped to Ghana and donated to the Cocoa Research Institute, Ghana (CRIG) to operate it as a training facility. Under the direction initially of Jemmy Takrama and subsequently of Margaret Acheampong, the KT Mobile Lab visited research stations, universities and secondary level institutions (to a lesser extent) across the country to provide training to scientists and students in molecular breeding and basic molecular biology techniques. Since the refitted vehicle had standing room for at most 12 persons, to enable use by a larger number of students the equipment was moved into the main labs of the institutions visited for the training sessions to be conducted there. Over 9,000 people benefited from the training opportunities it provided.
The molecular biology teaching lab at the University of Zimbabwe, under the direction of Idah Sithole-Niang, aims to increase the capacity of students and staff in the application of basic molecular biology methods applicable to many scientific disciplines. To date close to 2,800 students have received training.
9. Challenges and Lessons Learned
The previous sections have outlined the main successes of the KT-funded projects. There have also been many challenges. By including the building of scientific capacity in countries with limited opportunities for training as one of the key objectives, KT had to accept that progress would at times be slower, and that there would be setbacks. Since the projects are managed remotely and some are in countries where KT’s staff and consultants cannot visit because of civil unrest, it is more difficult to provide support. KT continues to try to improve its processes for remote project monitoring and for the provision of scientific mentorship remotely. This was particularly important during the COVID-19 pandemic years when all travel stopped.
Key factors for the success of projects are the correct management of germplasm, including maintaining the purity of the recipient parents, ensuring that the target traits for introgression are not lost during the backcross process, and the good management of field trials. In some projects led by PIs who were already experienced conventional breeders, a change in approach was needed for the team to use molecular markers to aid selection, as opposed to using them afterwards to verify the lines selected phenotypically.
KT invests in countries with limited economic resources, including countries experiencing political instability and comparatively high investment risk. However, since it is critical for researchers to be able to do most of the work in their home institutions this risk has to be factored in. As part of the agreement with the host institution, the latter takes responsibility for providing a suitable space and the utilities, and KT provides the scientific equipment and a regular supply of consumables. Since its inception, KT has established or contributed equipment to 33 molecular laboratories in 17 countries (16 in Africa), of which at least 13 remain operational, and 5 operate independent of KT’s support. It also funded nine screenhouses and glasshouses.
The procurement and dispatch of scientific equipment and consumables come with their own challenges, handled by a full-time staff at KT’s offices in the UK. Since most of the goods need to be imported, the challenges include navigating ever-changing customs regulations; establishing the administrative requirements of individual institutions; identifying the best suppliers either in the UK or EU or in another African country (usually South Africa), or in a combination of these; and selecting the most appropriate transport option which needs to balance cost and speed, since some of the consumables are perishable. It can take just as long and be as expensive for consumables and equipment to be delivered by African suppliers as it does when the goods are sent from the UK because African companies must also comply with import requirements. Even careful planning does not guarantee a smooth process: the delivery of a consignment of consumables can take from two to eight months (and longer in some cases). A successful consignment to a country also does not guarantee that the next one will not be problematic. Also challenging is the maintenance of equipment, since not many African countries have good repair centres. Faulty or broken equipment has been brought back to the UK by KT for repair, but having previously exported the merchandise usually means that the warranty has been voided. Therefore, fixing equipment used in African countries is also much more expensive and takes longer than if the same equipment was used in the UK. Overall, the actual cost of scientific goods used in African countries is between two to four times higher than in the UK once customs and transport costs (but not repair costs) are factored in.
The difficulty in establishing advanced molecular laboratories in African countries limits the choice to relatively uncomplicated and inexpensive technologies. While molecular markers can be used very effectively to aid in the selection of traits under relatively simple genetic control, they are not as suited to aid the introgression of complex traits.
Challenges of a different nature include severe climatic conditions, with both droughts and floods compromising field experiments. The increased frequency and severity of these events renders the correct management of genetic resources essential to avoid losing an entire breeding programme during extreme weather conditions.
As expected, not all the projects funded have been successful in developing and releasing improved varieties, and funding has been discontinued when it became apparent that this objective would not be met. However, this is not considered a failure since all projects generated useful data and breeding lines and contributed to the training of graduate students. With hindsight, the best approach has arguably been to initially award grants for projects with few key objectives, allowing them to naturally increase in complexity as the research team gains experience in the use of molecular biology techniques and can build on earlier achievements. It was also important for KT to engage with other organisations to understand the national and international landscape for crop improvement programmes to ensure the projects funded add value to other breeding initiatives, increasing synergies instead of duplicating efforts. Promoting the integration of the breeding projects funded in the national programmes also helps to “institutionalise” them hoping that with time they become less dependent on development aid and are more financed by the national, regional, or local authorities.
Ultimately, the biggest challenges faced by KT projects is making sure the improved varieties released reach the hands of farmers, the intended beneficiaries of KT’s support. Seed systems in African countries are poorly developed, suffer from poor governance, focus on formal seed systems while neglecting informal seed systems [
78,
84], and are mostly focused on key traded commodities such as maize. KT funded seed dissemination projects between 2016 to 2020, notably in Nigeria, Niger, Benin, Ghana, and Togo. However, these projects were discontinued since KT does not have enough resources to attain the sustainability and scale needed for impact. A decision was therefore made to actively seek partnerships for seed dissemination.
Increased cooperation is an overall objective for KT going forward. This includes fostering collaboration and cross-learning among projects of the same and different KT consortia. Engaging other actors in the field is also critical. Since the overall funding available for the improvement of tropical legumes is limited, a collaborative effort is needed to maximise its impact.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Reference list citing the PhaseolusGenes Molecular Marker Database used to develop
Figure 1.
Acknowledgments
We would like to thank first of all the researchers and students funded by KT over the last 20 years who take the credit for the work described in the manuscript. We regret that due to length constraints not all the research undertaken could be reviewed. We thank Clare Mukankusi (CIAT) for her support of the ABC over the years, and Eugene Terry and Joseph DeVries for their advice on the seed dissemination activities. We are grateful to Paul Nurse for recommending that KT focuses on plant breeding activities and would like to thank all current and former KT trustees for their guidance: Paul Nurse, Adrian Bird, Daniel Hulls, Appolinaire Djikeng, Gordon Conway and Keith Palmer. Many people were instrumental during the early years of KT. These include Noel Ellis, Maggie Knox, Sharat Chandra, Iain Mackay, Brigitte Maass, Bruce Pengelly, Jacob Hodeba Mingouna, Robin Buruchara, Jeremy Ouédraogo, Susan Nchimbi Msolla, Sean Mayes and Merion M Liebenberg. Last but not least, KT funded research projects are supported by a small, dedicated team based near Oxford, UK. Current members are Emma Graham, Fleur Geoghegan, Philip Pinheiro, Cynthia Sam, Mark-Sharbel Asman, Ann Pearce, Helen Holt-Davies and Gordon Spankie. We thank them for their comments and contributions to this manuscript. We would also like to acknowledge the work of former KT employees: Colin Dexter, Antony Bowes, Becky Lockyer, Kerry du Plessis, Janice Henderson, Tumie Akintewe, Luisa Doughty, Chris Edwards and Pat Waters.
Conflicts of Interest
The authors declare no conflicts of interest.
About the authors
| Dr Claudia Canales-Holzeis, former plant geneticist, and KT Chief Executive (2019 to present) (lead author) |
| Prof. Paul Gepts, Distinguished Professor Emeritus University of California, Davis, USA, plant geneticist and bean breeder, and former KT ABC Coordinator (2006 to 2022) |
| Dr Robert Koebner, former wheat geneticist Plant Breeding Institute in Cambridge, UK, and John Innes Centre, Norwich, UK, and current KT consultant (2006 to present) |
| Dr Prem N. Mathur, former Indian Council of Agricultural Research Principal Scientist and former Bioversity International Regional Representative for Central and South Asia, and current KT STOL Leader and Coordinator (2018 to present) |
| Dr Sonia Morgan, former geneticist, former KT Trust Secretary (2000 to 2019) and KT Financial Oversight (2000 to present) |
| Dr María Muñoz Amatriaín, Beatriz Galindo' distinguished researcher at the University of León, Spain, current KT ACP Coordinator (2022 to present) |
| Dr Travis Parker, Professional Researcher, University California Davis, USA and KT ABC Coordinator (2022 to present) |
| Prof. Sir Edwin Southern, Emeritus Professor of Biochemistry at the University of Oxford, UK, KT Founder and Chair |
| Prof. Michael Timko, Lewis & Clark Professor of Biology, Professor of Public Health Science, University of Virgina USA and KT consultant (2006 to present) and WACC Coordinator (2006 to 2022) |
References
- Ramesh, S.; Byregowda, M. Dolichos bean (Lablab purpureus L. Sweet, var. lignosus) genetics and breeding-present status and future prospects. 2016. [Google Scholar]
- Vaijayanthi, P.; Ramesh, S.; Gowda, M.B.; Rao, A.M.; Keerthi, C. Development of core sets of Dolichos bean (Lablab purpureus L. Sweet) germplasm. Journal of Crop Improvement 2015, 29, 405–419. [Google Scholar] [CrossRef]
- Vaijayanthi, P.; Ramesh, S.; Gowda, M.; Rao, A.; Keerthi, C. Genome-wide marker-trait association analysis in a core set of Dolichos bean germplasm. Plant genetic resources 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Timko, M.P.; Gowda, B.S.; Ouedraogo, J.; Ousmane, B. Molecular markers for analysis of resistance to Striga gesnerioides in cowpea. In Integrating new technologies for Striga control: towards ending the witch-hunt; World Scientific, 2007; pp. 115–128. [Google Scholar]
- Timko, M.P.; Singh, B. Cowpea, a multifunctional legume. In Genomics of tropical crop plants; Springer, 2008; pp. 227–258. [Google Scholar]
- Timko, M.P.; Rushton, P.J.; Laudeman, T.W.; Bokowiec, M.T.; Chipumuro, E.; Cheung, F.; Town, C.D.; Chen, X. Sequencing and analysis of the gene-rich space of cowpea. BMC genomics 2008, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Laudeman, T.W.; Rushton, P.J.; Spraggins, T.A.; Timko, M.P. CGKB: an annotation knowledge base for cowpea (Vigna unguiculata L.) methylation filtered genomic genespace sequences. BMC bioinformatics 2007, 8, 1–9. [Google Scholar] [CrossRef]
- Ouédraogo, J.; Maheshwari, V.; Berner, D.; St-Pierre, C.-A.; Belzile, F.; Timko, M. Identification of AFLP markers linked to resistance of cowpea (Vigna unguiculata L.) to parasitism by Striga gesnerioides. Theoretical and Applied Genetics 2001, 102, 1029–1036. [Google Scholar] [CrossRef]
- Ouédraogo, J.; Gowda, B.; Jean, M.; Close, T.; Ehlers, J.; Hall, A.; Gillaspie, A.; Roberts, P.; Ismail, A.; Bruening, G. An improved genetic linkage map for cowpea (Vigna unguiculata L.) combining AFLP, RFLP, RAPD, biochemical markers, and biological resistance traits. Genome 2002, 45, 175–188. [Google Scholar] [CrossRef]
- Ouédraogo, J.T.; Tignegre, J.-B.; Timko, M.P.; Belzile, F.J. AFLP markers linked to resistance against Striga gesnerioides race 1 in cowpea (Vigna unguiculata). Genome 2002, 45, 787–793. [Google Scholar] [CrossRef]
- Boukar, O.; Kong, L.; Singh, B.; Murdock, L.; Ohm, H. AFLP and AFLP-derived SCAR markers associated with Striga gesnerioides resistance in cowpea. Crop Science 2004, 44, 1259–1264. [Google Scholar] [CrossRef]
- Li, J.; Timko, M.P. Gene-for-gene resistance in Striga-cowpea associations. Science 2009, 325, 1094–1094. [Google Scholar] [CrossRef]
- Botanga, C.J.; Timko, M.P. Phenetic relationships among different races of Striga gesnerioides (Willd.) Vatke from West Africa. Genome 2006, 49, 1351–1365. [Google Scholar] [CrossRef]
- Li, J.; Lis, K.E.; Timko, M.P. Molecular genetics of race-specific resistance of cowpea to Striga gesnerioides (Willd.). Pest Management Science: formerly Pesticide Science 2009, 65, 520–527. [Google Scholar] [CrossRef]
- Omoigui, L.; Kamara, A.; Alunyo, G.; Bello, L.; Oluoch, M.; Timko, M.; Boukar, O. Identification of new sources of resistance to Striga gesnerioides in cowpea Vigna unguiculata accessions. Genetic Resources and crop evolution 2017, 64, 901–911. [Google Scholar] [CrossRef]
- Omoigui, L.O.; Kamara, A.Y.; Moukoumbi, Y.D.; Ogunkanmi, L.A.; Timko, M.P. Breeding cowpea for resistance to Striga gesnerioides in the Nigerian dry savannas using marker-assisted selection. Plant breeding 2017, 136, 393–399. [Google Scholar] [CrossRef]
- Ohlson, E.W.; Timko, M.P. Mapping and Validation of Alectra vogelii Resistance in the Cowpea Landrace B301. Agronomy 2022, 12, 2654. [Google Scholar] [CrossRef]
- Omoigui, L.; Ekeuro, G.; Kamara, A.; Bello, L.; Timko, M.; Ogunwolu, G. New sources of aphids [Aphis craccivora (Koch)] resistance in cowpea germplasm using phenotypic and molecular marker approaches. Euphytica 2017, 213, 1–15. [Google Scholar] [CrossRef]
- Ohlson, E.W.; Thio, G.I.; Sawadogo, M.; Sérémé, P.; Timko, M.P. Quantitative trait loci analysis of brown blotch resistance in cowpea variety KN1. Molecular breeding 2018, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Omoigui, L.O.; Danmaigona, C.C.; Kamara, A.Y.; Ekefan, E.J.; Timko, M.P. Genetic analysis of Fusarium wilt resistance in cowpea (Vigna unguiculata Walp.). Plant breeding 2018, 137, 773–781. [Google Scholar] [CrossRef]
- Muchero, W.; Diop, N.N.; Bhat, P.R.; Fenton, R.D.; Wanamaker, S.; Pottorff, M.; Hearne, S.; Cisse, N.; Fatokun, C.; Ehlers, J.D. A consensus genetic map of cowpea [Vigna unguiculata (L) Walp.] and synteny based on EST-derived SNPs. Proceedings of the national academy of sciences 2009, 106, 18159–18164. [Google Scholar] [CrossRef]
- Lucas, M.R.; Diop, N.N.; Wanamaker, S.; Ehlers, J.D.; Roberts, P.A.; Close, T.J. Cowpea–soybean synteny clarified through an improved genetic map. The Plant Genome 2011, 4. [Google Scholar] [CrossRef]
- Kusi, F.; Padi, F.K.; Obeng-Ofori, D.; Asante, S.K.; Agyare, R.Y.; Sugri, I.; Timko, M.P.; Koebner, R.; Huynh, B.L.; Santos, J.R. A novel aphid resistance locus in cowpea identified by combining SSR and SNP markers. Plant breeding 2018, 137, 203–209. [Google Scholar] [CrossRef]
- Andargie, M.; Pasquet, R.S.; Gowda, B.S.; Muluvi, G.M.; Timko, M.P. Construction of a SSR-based genetic map and identification of QTL for domestication traits using recombinant inbred lines from a cross between wild and cultivated cowpea (V. unguiculata (L.) Walp.). Molecular Breeding 2011, 28, 413–420. [Google Scholar] [CrossRef]
- Muñoz-Amatriaín, M.; Mirebrahim, H.; Xu, P.; Wanamaker, S.I.; Luo, M.; Alhakami, H.; Alpert, M.; Atokple, I.; Batieno, B.J.; Boukar, O. Genome resources for climate-resilient cowpea, an essential crop for food security. The Plant Journal 2017, 89, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Jiang, C.; Huang, Z.; Torres-Jerez, I.; Chang, J.; Zhang, H.; Udvardi, M.; Liu, R.; Verdier, J. The Vigna unguiculata Gene Expression Atlas (Vu GEA) from de novo assembly and quantification of RNA-seq data provides insights into seed maturation mechanisms. The Plant Journal 2016, 88, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Muñoz-Amatriaín, M.; Castro, I.; Lino-Neto, T.; Matos, M.; Egea-Cortines, M.; Rosa, E.; Close, T.; Carnide, V. Genetic diversity and structure of Iberian Peninsula cowpeas compared to world-wide cowpea accessions using high density SNP markers. BMC genomics 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.A.; Wang, Y.; Timko, M.P. A compendium of transcription factor and transcriptionally active protein coding gene families in cowpea (Vigna unguiculata L.). BMC genomics 2017, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.C. The genome of cowpea (Vigna unguiculata [L.] Walp.). The Plant Journal 2019, 98, 767–782. [Google Scholar] [CrossRef]
- Ongom, P.O.; Fatokun, C.; Togola, A.; Garcia-Oliveira, A.L.; Ng, E.H.; Kilian, A.; Lonardi, S.; Close, T.J.; Boukar, O. A mid-density single-nucleotide polymorphism panel for molecular applications in cowpea (Vigna unguiculata (L.) Walp). International Journal of Genomics 2022, 2024. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Muñoz-Amatriaín, M.; Shu, S.; Lo, S.; Wu, X.; Carlson, J.W.; Davidson, P.; Goodstein, D.M.; Phillips, J.; Janis, N.M. A view of the pan-genome of domesticated Cowpea (Vigna unguiculata [L.] Walp.). The Plant Genome 2023, e20319. [Google Scholar] [CrossRef]
- Herniter, I.A.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Close, T.J. Identification of candidate genes controlling black seed coat and pod tip color in cowpea (Vigna unguiculata [L.] Walp). G3: Genes, Genomes, Genetics 2018, 8, 3347–3355. [Google Scholar] [CrossRef]
- Herniter, I.A.; Lo, R.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Huynh, B.-L.; Lucas, M.; Jia, Z.; Roberts, P.A.; Lonardi, S. Seed coat pattern QTL and development in cowpea (Vigna unguiculata [L.] Walp.). Frontiers in Plant Science 2019, 10, 461982. [Google Scholar] [CrossRef]
- Lo, S. Mapping Domestication-Related Traits and QTL Pyramiding in Cowpea [Vigna unguiculata (L.) Walp]; University of California, Riverside, 2019. [Google Scholar]
- Singh, B.; Ishiyaku, M. Brief communication. Genetics of rough seed coat texture in cowpea. Journal of Heredity 2000, 91, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Porch, T.G.; Hall, A.E. Heat tolerance. Genomics and Breeding for Climate-Resilient Crops: Vol. 2 Target Traits 2013, 167–202. [Google Scholar]
- Omomowo, O.I.; Babalola, O.O. Constraints and prospects of improving cowpea productivity to ensure food, nutritional security and environmental sustainability. Frontiers in Plant Science 2021, 12, 751731. [Google Scholar] [CrossRef] [PubMed]
- Huynh, B.-L.; Ehlers, J.D.; Munoz-Amatriain, M.; Lonardi, S.; Santos, J.R.; Ndeve, A.; Batieno, B.J.; Boukar, O.; Cisse, N.; Drabo, I. A multi-parent advanced generation inter-cross population for genetic analysis of multiple traits in cowpea (Vigna unguiculata L. Walp.). bioRxiv 2017, 149476. [Google Scholar] [CrossRef]
- Fiscus, C.J.; Herniter, I.A.; Tchamba, M.; Paliwal, R.; Muñoz-Amatriaín, M.; Roberts, P.A.; Abberton, M.; Alaba, O.; Close, T.J.; Oyatomi, O.; Koenig, D. The pattern of genetic variability in a core collection of 2,021 cowpea accessions. bioRxiv 2023. [Google Scholar] [CrossRef]
- Muñoz-Amatriaín, M.; Lo, S.; Herniter, I.A.; Boukar, O.; Fatokun, C.; Carvalho, M.; Castro, I.; Guo, Y.N.; Huynh, B.L.; Roberts, P.A. The UCR Minicore: A resource for cowpea research and breeding. Legume Science 2021, 3, e95. [Google Scholar] [CrossRef]
- Covarrubias-Pazaran, G.; Gebeyehu, Z.; Gemenet, D.; Werner, C.; Labroo, M.; Sirak, S.; Coaldrake, P.; Rabbi, I.; Kayondo, S.I.; Parkes, E. Breeding schemes: what are they, how to formalize them, and how to improve them? Frontiers in Plant Science 2022, 12, 791859. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, N. Selection for local adaptation in a plant breeding programme. Theoretical and Applied Genetics 1991, 82, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S. Specific adaptation and breeding for marginal conditions. Euphytica 1994, 77, 205–219. [Google Scholar] [CrossRef]
- Schwartz, H.F.; Corrales, M.A.P. Bean production problems in the tropics; CIAT, 1989. [Google Scholar]
- Wortmann, C.S. Atlas of common bean (Phaseolus vulgaris L.) production in Africa; CIAT, 1998. [Google Scholar]
- Melotto, M.; Afanador, L.; Kelly, J. Development of a SCAR marker linked to the I gene in common bean. Genome 1996, 39, 1216–1219. [Google Scholar] [CrossRef]
- Carvalho, G.d.; Paula Júnior, T.d.; Alzate-Marin, A.; Nietsche, S.; Barros, E.d.; Moreira, M. Inheritance of resistance of the Andean bean line AND-277 to race 63-23 of Phaeoisariopsis griseola and identification of a RAPD marker linked to the resistance gene. 1998. [Google Scholar]
- Johnson, W.C.; Guzmán, P.; Mandala, D.; Mkandawire, A.B.; Temple, S.; Gilbertson, R.; Gepts, P. Molecular tagging of the bc-3 gene for introgression into Andean common bean. Crop Science 1997, 37, 248–254. [Google Scholar] [CrossRef]
- Kelly, J.D.; Miklas, P.N. The role of RAPD markers in breeding for disease resistance in common bean. Molecular breeding 1998, 4, 1–11. [Google Scholar] [CrossRef]
- Nietsche, S.; Borém, A.; Rocha, R.; Caixeta, E.; de Barros, E.; Moreira, M. Angular leaf spot resistance sources in common bean in Brazil. Revista Ceres 2000, 47, 567–571. [Google Scholar]
- Kelly, J.; Gepts, P.; Miklas, P.; Coyne, D. Tagging and mapping of genes and QTL and molecular marker-assisted selection for traits of economic importance in bean and cowpea. Field Crops Research 2003, 82, 135–154. [Google Scholar] [CrossRef]
- Alzate-Marin, A.L.; Arruda, K.M.; de Souza, K.A.; de Barros, E.G.; Moreira, M.A. Introgression of Co-42 and Co-5 anthracnose resistance genes into ‘carioca’common bean cultivars. Crop Breeding and Applied Biotechnology 2004, 4. [Google Scholar]
- Caixeta, E.T.; Borém, A.; Alzate-Marin, A.L.; Fagundes, S.d.A.; Silva, M.G.d.M.e.; de Barros, E.G.; Moreira, M.A. Allelic relationships for genes that confer resistance to angular leaf spot in common bean. Euphytica 2005, 145, 237–245. [Google Scholar] [CrossRef]
- Mukeshimana, G.; Paneda, A.; Rodríguez-Suárez, C.; Ferreira, J.J.; Giraldez, R.; Kelly, J.D. Markers linked to the bc-3 gene conditioning resistance to bean common mosaic potyviruses in common bean. Euphytica 2005, 144, 291–299. [Google Scholar] [CrossRef]
- Guzmán, P.; Gilbertson, R.; Nodari, R.; Johnson, W.; Temple, S.; Mandala, D.; Mkandawire, A.; Gepts, P. Characterization of variability in the fungus Phaeoisariopsis griseola suggests coevolution with the common bean (Phaseolus vulgaris). Phytopathology 1995, 85, 600–607. [Google Scholar] [CrossRef]
- Geffroy, V.; Sicard, D.; de Oliveira, J.C.; Sévignac, M.; Cohen, S.; Gepts, P.; Neema, C.; Langin, T.; Dron, M. Identification of an ancestral resistance gene cluster involved in the coevolution process between Phaseolus vulgaris and its fungal pathogen Colletotrichum lindemuthianum. Molecular Plant-Microbe Interactions 1999, 12, 774–784. [Google Scholar] [CrossRef]
- Aguilar, O.M.; Riva, O.; Peltzer, E. Analysis of Rhizobium etli and of its symbiosis with wild Phaseolus vulgaris supports coevolution in centers of host diversification. Proc Natl Acad Sci USA 2004, 101, 13548–13553. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-S.; Gepts, P.; Gilbertson, R. Genetics of resistance to the geminivirus, Bean dwarf mosaic virus, and the role of the hypersensitive response in common bean. Theoretical and Applied Genetics 2004, 108, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Capelle, J.; Neema, C. Local adaptation and population structure at a micro-geographical scale of a fungal parasite on its host plant. Journal of Evolutionary Biology 2005, 18, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Ddamulira, G.; Mukankusi, C.; Ochwo-Ssemakula, M.; Edema, R.; Sseruwagi, P.; Gepts, P. Distribution and variability of Pseudocercospora griseola in Uganda. 2012. [Google Scholar] [CrossRef]
- Ddamulira, G.; Mukankusi, C.M.; Ochwo-Ssemakula, M.; Edema, R.; Sseruwagi, P.; Gepts, P.L. Identification of new sources of resistance to angular leaf spot among Uganda common bean landraces. Canadian Journal of Plant Breeding 2014. [Google Scholar]
- Chilagane, L.A.; Nchimbi-Msolla, S.; Kusolwa, P.M.; Porch, T.G.; Diaz, L.M.S.; Tryphone, G.M. Characterization of the common bean host and Pseudocercospora griseola, the causative agent of angular leaf spot disease in Tanzania. African Journal of Plant Science 2016, 10, 238–245. [Google Scholar]
- Rezene, Y.; Mitiku, M.; Tesfaye, K.; Male, A.; Gepts, P. Analysis of the molecular diversity of common bacterial blight. Xanthomonas campestris 2018, 2. [Google Scholar] [CrossRef]
- Rezene, Y.; Tesfaye, K.; Clare, M.; Gepts, P. Pathotypes characterization and virulence diversity of Pseudocercospora griseola the causal agent of angular leaf spot disease collected from major common bean (Phaseolus vulgaris L.) growing areas of Ethiopia. J. Plant Pathol. Microbiol 2018, 9. [Google Scholar] [CrossRef]
- Uwera, A.; Rusagara, J.N.; Msolla, S.N.; Musoni, A.; Assefa, T. Molecular marker-assisted backcrossing of anthracnose resistance genes into common beans (Phaseolus vulgaris L.) varieties. American Journal of Plant Sciences 2021, 12, 771–781. [Google Scholar] [CrossRef]
- Tryphone, G.M.; Chilagane, L.A.; Protas, D.; Kusolwa, P.M.; Nchimbi-Msolla, S. Marker assisted selection for common bean diseases improvements in Tanzania: prospects and future needs. Plant breeding from laboratories to fields. Intech 2013, 22, 121–147. [Google Scholar]
- Miller, T.; Gepts, P.; Kimno, S.; Arunga, E.; Chilagane, L.A.; Nchimbi-Msolla, S.; Namusoke, A.; Namayanja, A.; Tedla, Y.R. Alternative markers linked to the Phg-2 angular leaf spot resistance locus in common bean using the Phaseolus genes marker database. African Journal of Biotechnology 2018, 17, 818–828. [Google Scholar]
- Gepts, P.; Lin, D. Development of phaseolusgenes, a genome database for marker discovery and candidate gene identification in common. Cooperative 2010, 30. [Google Scholar]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, J.; McClean, P.E.; Mamidi, S.; Wu, G.A.; Cannon, S.B.; Grimwood, J.; Jenkins, J.; Shu, S.; Song, Q.; Chavarro, C. A reference genome for common bean and genome-wide analysis of dual domestications. Nature genetics 2014, 46, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Kami, J.; Poncet, V.; Geffroy, V.; Gepts, P. Development of four phylogenetically-arrayed BAC libraries and sequence of the APA locus in Phaseolus vulgaris. Theoretical and applied genetics 2006, 112, 987–998. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.; Mamidi, S.; Lee, R.; Chikara, S.; Rossi, M.; Papa, R.; McClean, P. Syntenic relationships among legumes revealed using a gene-based genetic linkage map of common bean (Phaseolus vulgaris L.). Theoretical and Applied Genetics 2010, 121, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Vidigal, M.; Cruz, A.; Lacanallo, G.; Vidigal Filho, P.; Sousa, L.; Pacheco, C.; McClean, P.; Gepts, P.; Pastor-Corrales, M. Co-segregation analysis and mapping of the anthracnose Co-10 and angular leaf spot Phg-ON disease-resistance genes in the common bean cultivar Ouro Negro. Theoretical and Applied Genetics 2013, 126, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Murube, E.; Campa, A.; Ferreira, J.J. Integrating genetic and physical positions of the anthracnose resistance genes described in bean chromosomes Pv01 and Pv04. PLoS One 2019, 14, e0212298. [Google Scholar] [CrossRef]
- Lobaton, J.D.; Miller, T.; Gil, J.; Ariza, D.; de la Hoz, J.F.; Soler, A.; Beebe, S.; Duitama, J.; Gepts, P.; Raatz, B. Resequencing of common bean identifies regions of inter–gene pool introgression and provides comprehensive resources for molecular breeding. The plant genome 2018, 11, 170068. [Google Scholar] [CrossRef]
- Gil, J.; Solarte, D.; Lobaton, J.D.; Mayor, V.; Barrera, S.; Jara, C.; Beebe, S.; Raatz, B. Fine-mapping of angular leaf spot resistance gene Phg-2 in common bean and development of molecular breeding tools. Theoretical and applied genetics 2019, 132, 2003–2016. [Google Scholar] [CrossRef]
- Okii, D.; Tukamuhabwa, P.; Kami, J.; Namayanja, A.; Paparu, P.; Ugen, M.; Gepts, P. The genetic diversity and population structure of common bean (Phaseolus vulgaris L) germplasm in Uganda. African Journal of Biotechnology 2014, 13. [Google Scholar] [CrossRef]
- Wilkus, E.L.; Berny Mier y Teran, J.C.; Mukankusi, C.M.; Gepts, P. Genetic patterns of common-bean seed acquisition and early-stage adoption among farmer groups in western Uganda. Frontiers in Plant Science 2018, 9, 301055. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.A.; Gepts, P. Population genomics of Phaseolus spp.: A domestication hotspot. Springer, 2021. [Google Scholar] [CrossRef]
- Kalendar, R.; Shustov, A.V.; Akhmetollayev, I.; Kairov, U. Designing allele-specific competitive-extension PCR-based assays for high-throughput genotyping and gene characterization. Frontiers in Molecular Biosciences 2022, 9, 773956. [Google Scholar] [CrossRef] [PubMed]
- Cooperative, B.I. Annual Report of the Bean Improvement Cooperative; Bean Improvement Cooperative, 2016. [Google Scholar]
- Sadohara, R.; Izquierdo, P.; Couto Alves, F.; Porch, T.; Beaver, J.; Urrea, C.A.; Cichy, K. The Phaseolus vulgaris L. Yellow Bean Collection: genetic diversity and characterization for cooking time. Genetic Resources and Crop Evolution 2022, 69, 1627–1648. [Google Scholar] [CrossRef]
- Mayes, S.; Massawe, F.; Alderson, P.; Roberts, J.; Azam-Ali, S.; Hermann, M. The potential for underutilized crops to improve security of food production. Journal of experimental botany 2012, 63, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Gepts, P. Biocultural diversity and crop improvement. Emerging Topics in Life Sciences 2023, 7, 151–196. [Google Scholar] [CrossRef] [PubMed]
- Zida, W.-P.F.M.S.; Batieno, T.B.J.; Ouedraogo, M.H.; Ouedraogo, T.J.; Sawadogo, M. Agro-morphological characterization and genetic diversity study on tepary bean (Phaseolus acutifolius a. gray) collection introduced in Burkina Faso. 2023. [Google Scholar] [CrossRef]
- Mayes, S.; Ho, W.K.; Chai, H.H.; Gao, X.; Kundy, A.C.; Mateva, K.I.; Zahrulakmal, M.; Hahiree, M.K.I.M.; Kendabie, P.; Licea, L.C. Bambara groundnut: an exemplar underutilised legume for resilience under climate change. Planta 2019, 250, 803–820. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Oyatomi, O.; Babalola, O.O.; Abberton, M. Breeding potentials of Bambara groundnut for food and nutrition security in the face of climate change. Frontiers in Plant Science 2022, 12, 798993. [Google Scholar] [CrossRef]
- Pasipanodya, J.T.; Horn, L.N.; Achigan-Dako, E.G.; Musango, R.; Sibiya, J. Utilization of plant genetic resources of Bambara groundnut conserved ex situ and genetic diversification of its primary genepool for semi-arid production. Agriculture 2022, 12, 492. [Google Scholar] [CrossRef]
- Huang, J.; Ji, M.; Xie, Y.; Wang, S.; He, Y.; Ran, J. Global semi-arid climate change over last 60 years. Climate Dynamics 2016, 46, 1131–1150. [Google Scholar] [CrossRef]
- Kundy, A.C.; Mayes, S.; Msanya, B.; Ndakidemi, P.; Massawe, F. Building Resilient Crop Production Systems for Drought-Prone Areas—A Case for Bambara Groundnut (Vigna subterranea L. Verdc) and Groundnut (Arachis hypogaea L.). Agronomy 2023, 13, 383. [Google Scholar] [CrossRef]
- Heller, J.; Begemann, F.; Mushonga, J. Bambara groundnut, Vigna subterranea (L.) Verdc; 1997. [Google Scholar]
- Ahmad, N.S.; Redjeki, E.S.; Ho, W.K.; Aliyu, S.; Mayes, K.; Massawe, F.; Kilian, A.; Mayes, S. Construction of a genetic linkage map and QTL analysis in bambara groundnut. Genome 2016, 59, 459–472. [Google Scholar] [CrossRef]
- Ho, W.K.; Chai, H.H.; Kendabie, P.; Ahmad, N.S.; Jani, J.; Massawe, F.; Kilian, A.; Mayes, S. Integrating genetic maps in bambara groundnut [Vigna subterranea (L) Verdc.] and their syntenic relationships among closely related legumes. BMC genomics 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chai, H.H.; Ho, W.K.; Kundy, A.C.; Mateva, K.I.; Mayes, S.; Massawe, F. Genetic linkage map construction and identification of QTLs associated with agronomic traits in bambara groundnut (Vigna subterranea (L.) Verdc.) using DArTseq-based SNP markers. Food and Energy Security 2023, 12, e353. [Google Scholar] [CrossRef]
- Gao, X.; Chai, H.H.; Ho, W.K.; Mayes, S.; Massawe, F. Deciphering the molecular basis for photosynthetic parameters in Bambara groundnut (Vigna subterranea L. Verdc) under drought stress. BMC plant biology 2023, 23, 287. [Google Scholar] [CrossRef]
- Uba, C.U.; Oselebe, H.O.; Tesfaye, A.A.; Agbo, C.U.; Abtew, W.G. Comparative analysis of variation in African Bambara groundnut [Vigna subterranea (L) Verdc.] landraces assessed through seed traits. South African Journal of Botany 2022, 150, 48–57. [Google Scholar] [CrossRef]
- Odesola, K.A.; Olawuyi, O.J.; Paliwal, R.; Oyatomi, O.A.; Abberton, M.T. Genome-wide association analysis of phenotypic traits in Bambara groundnut under drought-stressed and non-stressed conditions based on DArTseq SNP. Frontiers in Plant Science 2023, 14, 1104417. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).