Submitted:
21 April 2024
Posted:
23 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Discussion
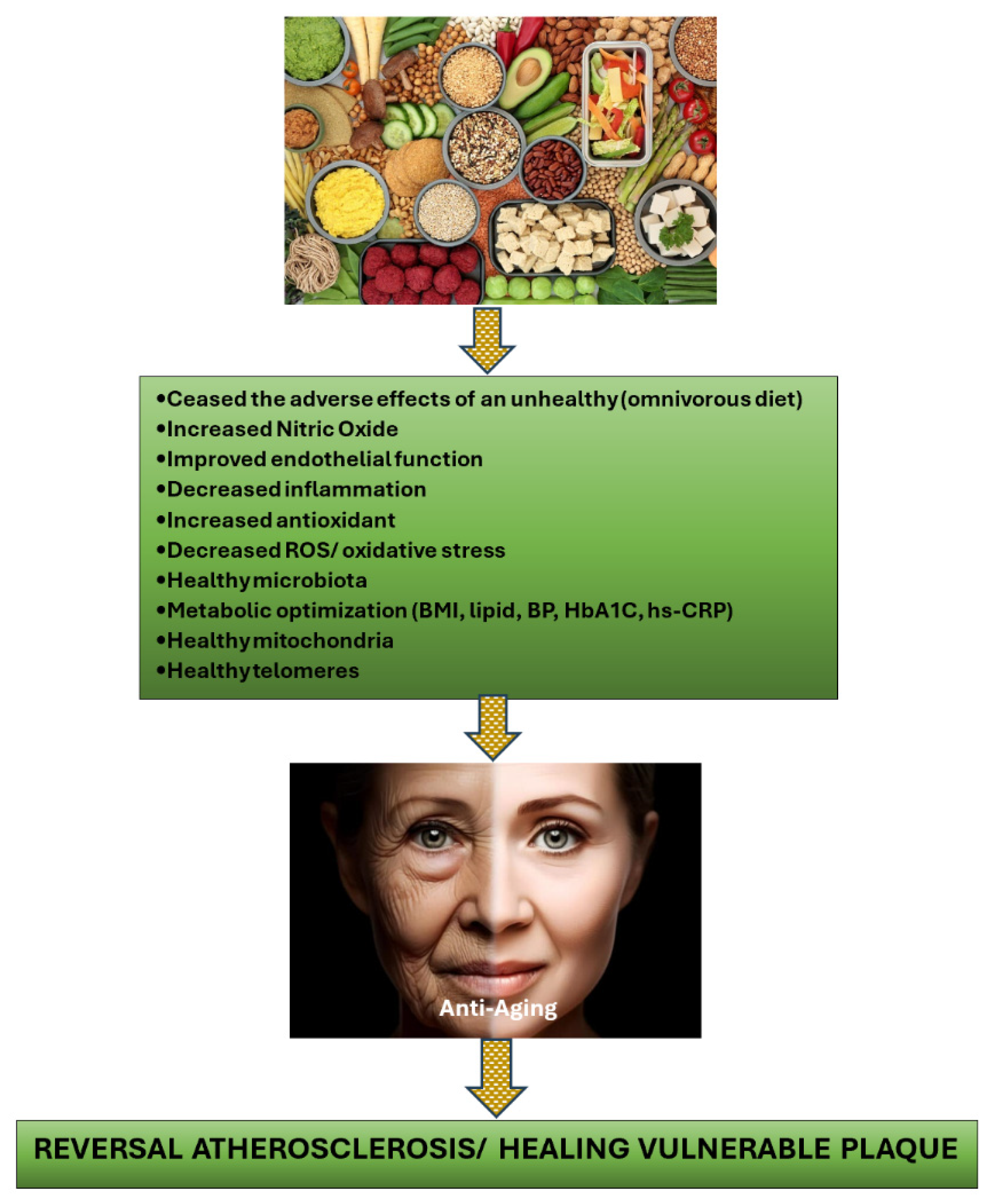
2.1. Plant-Based Diets as the Solution to Treat Vulnerable Plaque in CCS Patients
2.2. Supplements Are Compulsory for PBD Followers
3. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Muharram FR, Multazam CECZ, Mustofa A, et al. The 30 Years of Shifting in The Indonesian Cardiovascular Burden- Analysis of The Global Burden of Disease Study. Journal of Epidemiology and Global Health. 2024. [CrossRef]
- Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Aug 29;148(9):e9-e119. doi: 10.1161/CIR.0000000000001168. Epub 2023 Jul 20. Erratum in: Circulation. 2023 Sep 26;148(13):e148. Erratum in: Circulation. 2023 Dec 5;148(23):e186. PMID: 37471501.
- Boden WE, O’Rourke RA, Teo KK, et al. COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007 Apr 12;356(15):1503-16. [CrossRef] [PubMed]
- Al-Lamee R, Thompson D, Dehbi HM, et al. ORBITA investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomized controlled trial. Lancet. 2018 Jan 6;391(10115):31-40. doi: 10.1016/S0140-6736(17)32714-9. Epub 2017 Nov 2. Erratum in: Lancet. 2018 Jan 6;391(10115):30. PMID: 29103656.
- Maron DJ, Hochman JS, Reynolds HR, et al. ISCHEMIA Research Group. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020 Apr 9;382(15):1395-1407. [CrossRef] [PubMed]
- Kurup R, Wijeysundera HC, Bagur R, et al. Complete Versus Incomplete Percutaneous Coronary Intervention-Mediated Revascularization in Patients With Chronic Coronary Syndromes. Cardiovasc Revasc Med. 2023 Feb;47:86-92. [CrossRef] [PubMed]
- Stone GW, Ali ZA, O’Brien SM, Rhodes G, et al. ISCHEMIA Research Group. Impact of Complete Revascularization in the ISCHEMIA Trial. J Am Coll Cardiol. 2023 Sep 19;82(12):1175-1188. [CrossRef] [PubMed]
- Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol. 2021 Mar;18(3):155-168. [CrossRef] [PubMed]
- Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V, Velazquez E, Diegeler A, Sigusch H. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 Mar 5;73(8):964-976. [CrossRef] [PubMed]
- van Veelen A, van der Sangen NMR, Delewi R, et al. Detection of Vulnerable Coronary Plaques Using Invasive and Non-Invasive Imaging Modalities. J Clin Med. 2022 Mar 1;11(5):1361. [CrossRef] [PubMed]
- Tomaniak M, Katagiri Y, Modolo R, et al. Vulnerable plaques and patients: state-of-the-art. Eur Heart J. 2020 Aug 14;41(31):2997-3004. [CrossRef] [PubMed]
- Virani SS, Newby LK, Arnold SV, et al. Peer Review Committee Members. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023 Aug 29;148(9):e9-e119. doi: 10.1161/CIR.0000000000001168. Epub 2023 Jul 20. Erratum in: Circulation. 2023 Sep 26;148(13):e148. Erratum in: Circulation. 2023 Dec 5;148(23):e186. PMID: 37471501.
- Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011 Jan 20;364(3):226-35. doi: 10.1056/NEJMoa1002358. Erratum in: N Engl J Med. 2011 Nov 24;365(21):2040. PMID: 21247313.
- Stone GW, Maehara A, Ali ZA, et al. PROSPECT ABSORB Investigators. Percutaneous Coronary Intervention for Vulnerable Coronary Atherosclerotic Plaque. J Am Coll Cardiol. 2020 Nov 17;76(20):2289-2301. [CrossRef] [PubMed]
- Park SJ, Ahn JM, Kang DY, et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): a multicentre, open-label, randomized controlled trial. www.thelancet.com. 2024. [CrossRef]
- van Veelen A, Küçük IT, Fuentes FH, et al. First-in-Human Drug-Eluting Balloon Treatment of Vulnerable Lipid-Rich Plaques: Rationale and Design of the DEBuT-LRP Study. J Clin Med. 2023 Sep 6;12(18):5807. doi: 10.3390/jcm12185807. Erratum in: J Clin Med. 2024 Mar 04;13(5): PMID: 37762747; PMCID: PMC10531515.
- Mulijono, D. Mechanism of How Plant-Based Diet (PBD) Reduces the Risk of In-Stent Restenosis (ISR) and Stent Thrombosis (ST). Preprints 2024, 2024040628. [Google Scholar] [CrossRef]
- Mortensen MB, Dzaye O, Steffensen FH, et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. J Am Coll Cardiol. 2020 Dec 15;76(24):2803-2813. [CrossRef] [PubMed]
- Peña-Jorquera H, Cid-Jofré V, Landaeta-Díaz L, et al. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome—A Comprehensive Review. Nutrients 2023, 15, 3244. [Google Scholar] [CrossRef] [PubMed]
- Salehin S, Rasmussen P, Mai S, et al. Plant Based Diet and Its Effect on Cardiovascular Disease. Int J Environ Res Public Health. 2023 Feb 14;20(4):3337. [CrossRef] [PubMed]
- Koutentakis M, Surma S, Rogula S, et al. The Effect of a Vegan Diet on the Cardiovascular System. J Cardiovasc Dev Dis. 2023 Feb 22;10(3):94. [CrossRef] [PubMed]
- Tucci M, Marino M, Martini D, et al. Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients. 2022 Jun 24;14(13):2615. [CrossRef] [PubMed]
- Islam SU, Ahmed MB, Ahsan H, et al. Recent Molecular Mechanisms and Beneficial Effects of Phytochemicals and Plant-Based Whole Foods in Reducing LDL-C and Preventing Cardiovascular Disease. Antioxidants (Basel). 2021 May 15;10(5):784. doi: 10.3390/antiox10050784. PMID: 34063371; PMCID: PMC8157003.
- Mehta P, Tawfeeq S, Padte S, et al. Plant-based diet and its effect on coronary artery disease: A narrative review. World J Clin Cases. 2023 Jul 16;11(20):4752-4762. [CrossRef] [PubMed]
- Bruns A, Greupner T, Nebl J, et al. Plant-based diets and cardiovascular risk factors: a comparison of flexitarians, vegans and omnivores in a cross-sectional study. BMC Nutr 10, 29 (2024). [CrossRef]
- Di Sotto A, Vitalone A, Di Giacomo S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines (Basel). 2020 Aug 22;8(3):468. [CrossRef] [PubMed]
- Xu K, Kh Al-ani M, Pan X, et al. Plant-Derived Products for Treatment of Vascular Intima Hyperplasia Selectively Inhibit Vascular Smooth Muscle Cell Functions, Evidence-Based Complementary and Alternative Medicine, vol. 2018, Article ID 3549312, 17 pages, 2018. [CrossRef]
- Monsalve B, Concha-Meyer A, Palomo I, et al. Mechanisms of Endothelial Protection by Natural Bioactive Compounds from Fruit and Vegetables. An Acad Bras Cienc. 2017 May;89(1 Suppl 0):615-633. [CrossRef] [PubMed]
- Aquila G, Marracino L, Martino V, et al. The Use of Nutraceuticals to Counteract Atherosclerosis: The Role of the Notch Pathway. Oxid Med Cell Longev. 2019 May 2;2019:5470470. [CrossRef] [PubMed]
- Moss J, Dipak R. (2016). Nutraceutical therapies for atherosclerosis. Nature Reviews Cardiology. 2016; 13(9):513-532. [CrossRef]
- Wei T, Liu J, Zhang D, et al. The Relationship Between Nutrition and Atherosclerosis. Front Bioeng Biotechnol. 2021 Apr 19;9:635504. [CrossRef] [PubMed]
- Mitu O, Cirneala IA, Lupsan AI, et al. The Effect of Vitamin Supplementation on Subclinical Atherosclerosis in Patients without Manifest Cardiovascular Diseases: Never-ending Hope or Underestimated Effect? Molecules 2020, 25, 1717. [Google Scholar] [CrossRef] [PubMed]
- Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013 Oct 15;62(16):1421-31. [CrossRef] [PubMed]
- Sandoval Y, Brilakis ES, Garcia S. Completeness of revascularization in multivessel coronary artery disease. J Thorac Dis. 2016 Nov;8(11):E1493-E1496. [CrossRef] [PubMed]
- Hwang D, Kang J, Yang HM, et al. Better Prognosis After Complete Revascularization Using Contemporary Coronary Stents in Patients With Chronic Kidney Disease. Circ Cardiovasc Interv. 2019 Aug;12(8):e007907. [CrossRef] [PubMed]
- Iqbal MB, Moore PT, Nadra IJ, et al. British Columbia Cardiac Registry Investigators. Complete revascularization in stable multivessel coronary artery disease: A real world analysis from the British Columbia Cardiac Registry. Catheter Cardiovasc Interv. 2022 Feb;99(3):627-638. [CrossRef] [PubMed]
- Williams T, Mittal A, Karageorgiev D, et al. e-Ultimaster investigators. Complete revascularization optimizes patient outcomes in multivessel coronary artery disease: Data from the e-Ultimaster registry. Catheter Cardiovasc Interv. 2022 Mar;99(4):961-967. [CrossRef] [PubMed]
- Pham V, Moroni A, Gall E, et al. Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review. J Clin Med. 2023 Apr 12;12(8):2833. [CrossRef] [PubMed]
- Giubilato S, Lucà F, Abrignani MG, et al. Management of Residual Risk in Chronic Coronary Syndromes. Clinical Pathways for a Quality-Based Secondary Prevention. J Clin Med. 2023 Sep 15;12(18):5989. [CrossRef] [PubMed]
- Rose, Stewart D. “A Comprehensive Review of the Prevention and Treatment of Heart Disease with a Plant-Based Diet.” Journal of Cardiology & Cardiovascular Therapy (2018): n. pag.
- Bilal M, Ashraf S, Zhao X. Dietary Component-Induced Inflammation and Its Amelioration by Prebiotics, Probiotics, and Synbiotics. Front Nutr. 2022 Jul 22;9:931458. [CrossRef] [PubMed]
- Upadhyay S, Dixit M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid Med Cell Longev. 2015;2015:504253. [CrossRef] [PubMed]
- Wu YT, Chen L, Tan ZB, et al. Luteolin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration by Inhibiting TGFBR1 Signaling. Front Pharmacol. 2018 Sep 21;9:1059. [CrossRef] [PubMed]
- Hong CG, Florida E, Li H, Parel PM, Mehta NN, Sorokin AV. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front Cardiovasc Med. 2023 Jan 16;9:1023651. [CrossRef] [PubMed]
- Marchio P, Guerra-Ojeda S, Vila JM, et al. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2019 Jul 1;2019:8563845. [CrossRef] [PubMed]
- Adarsh Ray, Krushna Ch. Maharana, et al. Endothelial dysfunction and its relation in different disorders: Recent update, Health Sciences Review, Volume 7, 2023, 100084, ISSN 2772-6320. [CrossRef]
- Almeida C, Barata P, Fernandes R. The influence of gut microbiota in cardiovascular diseases-a brief review. Porto Biomed J. 2021 Jan 18;6(1):e106. [CrossRef] [PubMed]
- Pollicino F, Veronese N, Dominguez LJ, et al. Mediterranean diet and mitochondria: New findings. Exp Gerontol. 2023 Jun 1;176:112165. [CrossRef] [PubMed]
- Cinegaglia N, Antoniazzi L, Rosa D, et al. Shortening telomere is associated with subclinical atherosclerosis biomarker in omnivorous but not in vegetarian healthy men. Aging (Albany NY). 2019 Jul 19;11(14):5070-5080. [CrossRef] [PubMed]
- Plotnikoff GA, Dobberstein L, Raatz S. Nutritional Assessment of the Symptomatic Patient on a Plant-Based Diet: Seven Key Questions. Nutrients. 2023 Mar 13;15(6):1387. [CrossRef] [PubMed]
- Neufingerl N, Eilander A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients. 2021 Dec 23;14(1):29. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




