1. Introduction
Genetic sequencing tools and bioinformatics platforms allowed scholars to isolate, characterize and observe the composition and function of microbiota and microbiomes from the intestine [
1]. The first characterization and investigation of the human microbiome was first initiated by the Human Microbiome Project sponsored by the National Institutes of Health [
2]. Microbiomes means that the gut microbiota and its whole genome; it provides certain benefits for human being. Thus, gut microbiota and host existed in symbiotic manner. This is evident from the truth that every anatomical location along the gastrointestinal tract (GIT) is characterized by its physicochemical conditions. The changing of these conditions exerts a selective pressure on the microbiota. Currently investigating intestinal microbiota and their metabolites has become an essential task which is required in clinical aspects for treating many diseases [
3,
4,
5].
During Eubiosis, the diversity of good and pathogenic bacteria was balanced. The cells of intestinal mucosa are working very well. For example; keeping its production of immunoglobulin A, mucus, defensins and keeping the tight junctions undamaged. After disturbance of intestinal microbiota, the condition is said to be dysbiosis. The number of pathogenic bacteria exceeds the good bacteria and affects the intestinal mucosa not to work properly. This caused disruption of intestinal mucosa and allowed the entering of bacteria, food antigens and other microbes into lamina propria. Finally, it results in over accumulation of proinflammatory cytokines [
6,
7,
8,
9].
Today probiotics, prebiotics and synbiotics are agents which are investigated for their modulatory effect on gut microbiota. Deep knowledge about symbiotic relation between host and microbiota will be baseline information for enhancing microbiota-based therapies such as modulation of intestinal microbiota [
10]. The good bacteria act in many ways to deliver an important benefit for us. They released metabolites such as bacteriocins, colcins and others. Probiotics ferment prebiotics such as nondigestible carbohydrates and create short-chain fatty acids (SCFAs) such as butyrate, propionate, lactate and acetate. For example; Butyrate aids in absorption of water and electrolytes, manages intestinal motility, allows repair of enterocytes and anti-inflammatory effect. Metabolic function, absorption of essential amino acids, folic acid, vitamins such as B12, K, thiamine and riboflavin are obtained through GIT microbiota [
6,
9].
Antibiotics are the cornerstone for treating various diseases. However, diversity of gut microbiota decreased due to side effects of antibiotics. Death due to antibiotic resistance will be expected to be ten million in 2025.Therefore, the rise of antibiotic resistant bacterial infection and the slowing rate of antibiotic discovery needs an alternative biological agent such as probiotics, prebiotics and synbiotics. For example, using probiotics is an effective way to tackle the problem of antibiotic resistant bacteria. Those alternative agents aimed to minimize side effects of antibiotics and excessive use of antibiotics [
2,
8,
11].In this review paper, we tried to look literatures on side effects of antibiotics, describing the probiotics, prebiotics and synbiotics as alternative to antibiotics in modulating GIT microbiota, challenges of using probiotics, prebiotics and synbiotics, methods to check safety of probiotics and we also tried to show some of clinical evidence on the need of probiotics, prebiotics and synbiotics.
2. Side effects of antibiotics and the need of an alternative antimicrobial agents
Antibiotic resistant infections are a critical concern across worldwide for economic loss. Mainly sub-Saharan Africa countries are severely affected due to poor sanitation, poor adherence to infection prevention and control and lack of sophisticated health care stations. The use of probiotics, prebiotics and synbiotics is a promising tool as an alternative approach to antibiotics. This is because they are easily available as dietary supplements, capacity to tackle AMR in a natural way, they are environmentally soundful, their ability to re-direct dysbiosis into eubiosis, their advantage as a prophylactic and therapeutic purpose, etc. [
12].
Antibiotics affect non-targeted GIT microflora and favored overgrowth of bacteria that induce diarrhea such as
Clostridium difficile (
C. difficile).
C. difficile is an opportunistic, gram positive, anaerobic bacteria having the capacity to cause a spectrum of disease ranging from antibiotic associated diarrhea (AAD) to pseudomembranous colitis.
C. difficile can survive during the time of antibiotic prescription because of their spores. AAD can also result from a decrease in metabolism of carbohydrates and bile acids. Probiotics such as
L. acidophilus,
L. rhamnosus GG,
L. delbruckii,
L. fermentum and
S. boulardii are very important to minimize the severity of AAD [
13,
14,
15].
During eubiosis, the population of the gut microbial community is diverse such as
Clostridium scindens (
C. scindens). Those bacteria convert primary bile acids into secondary bile acids, which in turn inhibit the growth of
C. difficile. However, prescription of antibiotics can affect
C. scindens and cause decreased production of secondary bile acids and increased proliferation of
C. difficile. The accumulation of primary bile acids facilitated the opportunity for spore germination of
C. difficile [
16]. Another commensal microflora affected by antibiotics are
Bacteroides taiotaomicron and
Bifidobacterium breve. They have capacity to induce the expression of C-type lectin and then they allow the regeneration of islet-derived protein III γ (REGIII γ), which have potential to tackle the growth of gram- positive bacteria. This overview implies that some gut microflora which have antagonistic activity against
C. difficile can be killed in antibiotic induced environments and subsequently their level of antimicrobial secretion will be reduced [
14].
2.1. Mechanisms of antibiotic resistance
During bacteria replication, there might be changes in base pairs. This caused alteration of amino acid in enzymes and other structural components. Then resistant strains were developed. Sometimes non-pathogenic bacteria became pathogenic after taking resistant genes from the other pathogenic bacteria. In appropriate antibiotic prescription, inadequate and over use of antibiotics may also facilitate the spread of AM resistance [
17]. Furthermore, modification of antibiotic target site, changes in the permeability of a bacterial cell, efflux of the antibiotics out of the cell and enzymatic degradation of the antibiotics are also some of the mechanisms which enhance the spread of antibiotic resistance [
18].
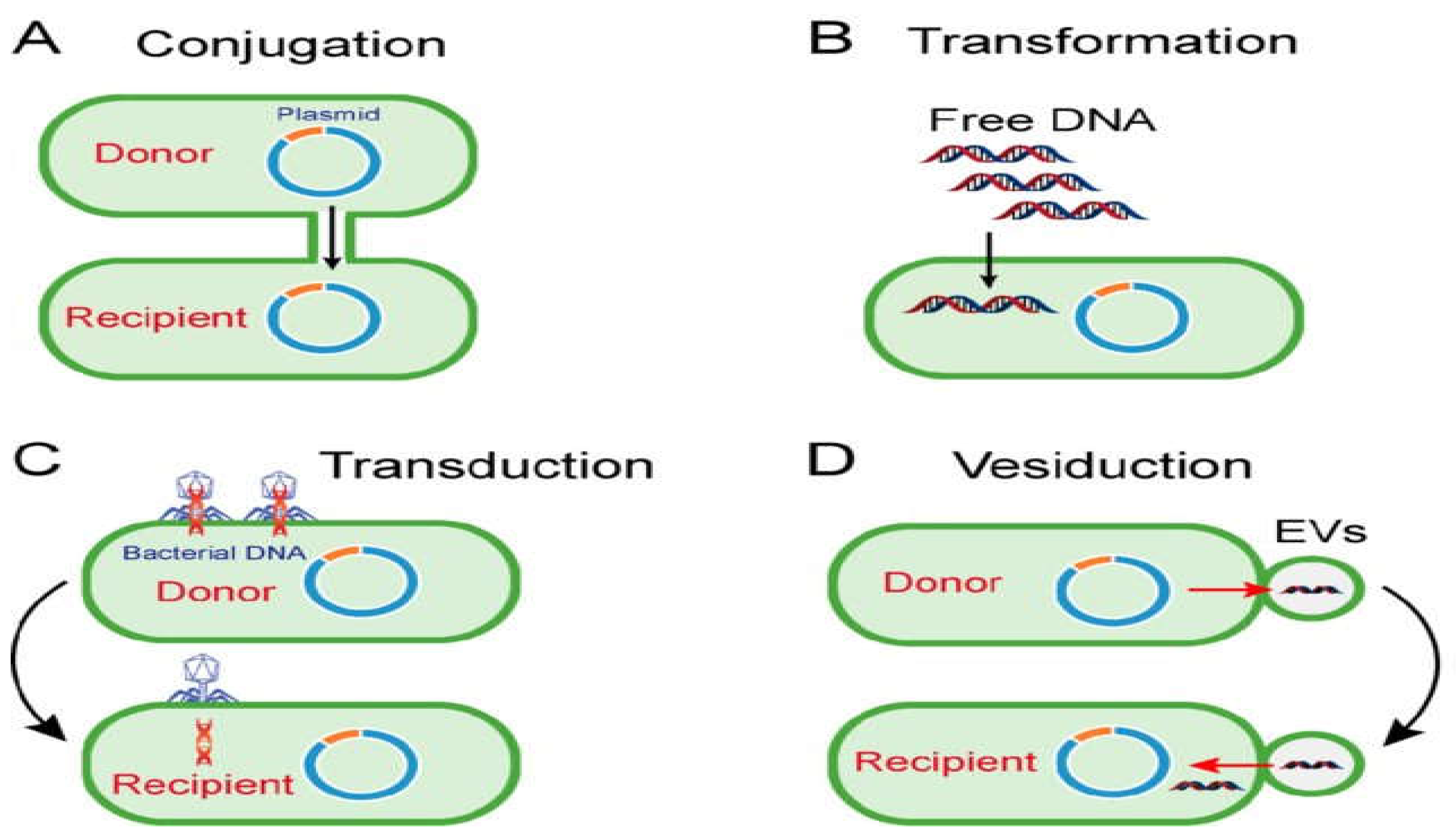
Among horizontal gene transfer; conjugation need a contact between donor and recipient bacteria while during transformation the environmental DNA integrated into the genome of recipient bacteria. However, the transduction is facilitated by a bacteriophage [
19]. For further clarification, look at below the figure 1.
Figure 1.
Mechanisms of horizontal gene transfer: (A) Conjugation; (B) Transformation; (C) Transduction and (D) Vesiduction [
20].
Figure 1.
Mechanisms of horizontal gene transfer: (A) Conjugation; (B) Transformation; (C) Transduction and (D) Vesiduction [
20].
3. Alternative methods of modulating disturbance of intestinal microbiota
3.1. Probiotics
Probiotics are live microorganisms, when administered in adequate amounts confer health benefits to the host [
21]. Probiotic healing approach involves introducing beneficial microorganisms into the host GIT through food influx.
Enterococcus faecium, Bifidobacterium,
Saccharomyces boulardii, Lactobacillus strains, Pediococcus are an important strain serving as probiotics and providing health benefits. Further,
Lactococcus lactis subsp.
Lactis, Streptococcus thermophilus, Leuconostoc mesenteroides, Bacillus subtilis, and
Escherichia coli Nissle 1917 are also another probiotic species which are commonly investigated [
12]. Among them, the probiotic effect of Lactobacilli has grown exponentially with an escalating number of researches. FAO and WHO jointly settled fundamental characteristics of probiotics. Those criteria are they must be generally recognized as safe for use, acid and bile tolerant, strong adhesion to intestinal mucosa, free from transferable genes and they must be lactic acid producers [
22]. Primarily probiotics are aimed at treating GIT problems. However, due to their communication with other organs, they are also important to treat oral infections, diarrhea, irritable bowel syndrome, inflammatory bowel infections, Helicobacter pylori infections, antitumor effects, others [
23,
24].
3.1.1. Mechanisms of probiotics in modulating disturbance of GIT microbiota
Probiotics use the mechanisms such as changing GIT environment through pH lowering, competition for nutrients, binding sites and killing the growth of pathogenic bacteria through producing and secreting antimicrobial compounds [
25,
26].
Enhancement of the epithelial barrier function
Intestinal epithelium contains enterocytes for absorbing molecules from the intestinal lumen, Paneth cells for secreting antimicrobial peptides (AMPs) at the time of interaction with enteric bacteria and also, intestinal epithelium contains mucus-secreting Goblet cells. While, lamina propria contains immune cells such as dendritic cells, macrophages, B (memory cells and plasma cells), T-lymphocytes such as CD4+ (helper or inducer) and CD8+ (suppressor or cytotoxic) cells. The structure of GIT epithelium is kept in a position by multi-protein complexes called tight junctions. Disintegration of tight junctions leads to a leaky gut. Then it allows the occurrence of various pathological conditions such as irritable bowel disease (IBD), irritable bowel syndrome (IBS) and celiac disease with inflammation and tissue damage. Therefore, probiotics promote barrier function through regulation of tight junction proteins [
8,
27,
28,
29].
The effect of probiotics on mucus layer
The intestinal mucus layer helps as a protective barrier against the harsh luminal environment by avoiding firm adhesion of bacteria to the epithelial cells and preventing their entry into the lamina propria. It is a highly hydrated gel containing glycoproteins such as mucin, defensins and immunoglobulin and thus, the mucus layer prevents inflammatory and infectious diseases. So, probiotics are able to regulate mucin gene expression (MUC2 and MUC3 genes) by goblet cells [
8,
27].
Competitive adherence to the mucosa and epithelium
For interacting with intestinal epithelial cells (IECs) and mucus, probiotics display different surface determinants. Because of this interaction, lactic acid bacteria (LAB) prevents competitors from being attached to the mucus. The main mucus-targeting LAB adhesins is mucus-binding (Mub) protein which is produced by
Lactobacillus reuteri. LAB such as
L. reuteri and
L. fermentum were used mucous adhesion-promoting protein (MapA) in order to attach them with mucus. The attachment of pathogens to GIT is prohibited through adherence of bacterial cells to each other, probiotics block essential binding sites and they also adhere with pathogens to avoid colonization of pathogens inside GIT [
25,
30]. Lactic acid bacteria can produce several antimicrobial compounds such as lactic acid, hydrogen peroxide, bacteriocins, carbon dioxide and Diacetyl [
31,
32]. For further classification, it is possible to see the table 1 below.
Table 1.
Antimicrobial compounds produced by LAB.
Table 1.
Antimicrobial compounds produced by LAB.
| S. No |
Antimicrobial compounds |
Their effect against pathogenic bacteria |
References |
| 1 |
Lactic acid |
It is more effective against gram positive bacteria. Acidification and subsequent pH reduction allowed inhibitory activity |
[33] |
| 2 |
Hydrogen peroxide(H2O2) |
It is due to its strong oxidizing effect on pathogens and molecular structures of the bacteria. |
[34] |
| 3 |
Bacteriocins |
Ribosomal synthesized antimicrobial peptides,
The showed enzyme activity modulation, inhibiting spore growth, formation of pores in pathogen cell membrane |
[34] |
| 4 |
Carbon dioxide (CO2) |
It creates anaerobic environment. Further it inhibits enzymatic decarboxylation. Its accumulation inside the cell membrane disturb the permeability the lipid bilayer |
[35] |
| 5 |
Diacetyl (2,3-butanedione) |
Diacetyl reacts with the arginine-binding protein of Gram-negative bacteria, hence, interfering with arginine utilization. |
[36] |
Avoiding the effect of pathogenic bacteria and their toxins
Pathogenic bacteria secrete toxins such as Shiga toxin, enterotoxin and others. Those toxins cause intestinal dysfunction such as disturbing tight junctions, loss of water and electrolytes. Among probiotics,
Lactobacillus zeae prevent enterotoxin production;
Lactobacillus kefir can minimize cytopathic effect of
C. difficile while Lactobacillus, Pediococcus, and Bifidobacterium strains down regulate the expression of Shiga toxin 2 gene [
30]. Probiotics are very important during elimination of vancomycin-resistant enterococcus (VRE), extended spectrum beta-lactamase (ESBL) Enterobacteriaceae and methicillin resistant
S. aureus [
37]. They are also important to control food poisoning bacteria such as
Clostridium perfringens,
Campylobacter jejuni,
Salmonella enteritidis,
Escherichia coli, various species of Shigella,
Staphylococcus and Yersinia [
38].
Modulation of immune system
Probiotics act on the luminal environment and intestinal mucosal barrier to regulate the mucosal immune system. They interact with innate and adaptive immune cells such as DCs, monocytes, Natural Killer (NK) cells, macrophages and lymphocytes. From host cells in the GIT, intestinal epithelial cells (IECs) and dendritic cells (DCs) were mainly interacted with probiotics. Both IECs and DCs can interact with and respond to gut microorganisms through their pattern recognition receptors (PRRs), which are responsible for the initial recognition of pathogen-associated molecular patterns (PAMPS). Later on, it results in the development of appropriate innate and acquired immune responses [
28].
Luminal gut contents were tasted by three mechanisms: (i) DCs extending dendrites through the tight junction and into the lumen; (ii) IEC pinocytosis of microbiota and (iii) Selective transfer of luminal contents via specialized epithelial cells, microfold (M) cells. Activation of DCs by probiotics allows them to be mature for antigen presentation. Then they are migrated into draining Mesenteric lymph nodes (MLN), where they present antigens to naive T and B cells. Naive T-helper cells (Th cells) were differentiated into Th1, Th2 and T-regulatory cells (Treg) immune cells. While B cells were shifted into plasma cells, which play an active role in humoral responses, and regulatory B (Breg) cells, which are involved in the production of tumor growth factor (TGF)-
β or IL-10 [
39,
40].
Probiotics can activate aryl hydrocarbon receptor which is very important in regulating inflammation. Some LABs are able to produce acetylcholine for avoiding GIT inflammation. In general probiotics can modulate the host immune system in three ways: (1). Initiation and keeping host immune reaction as well as its tolerance to environmental antigens; (2). Initiation and regulating immune response to pathogens ;( 3) Preventing allergic reactions [
38,
41].
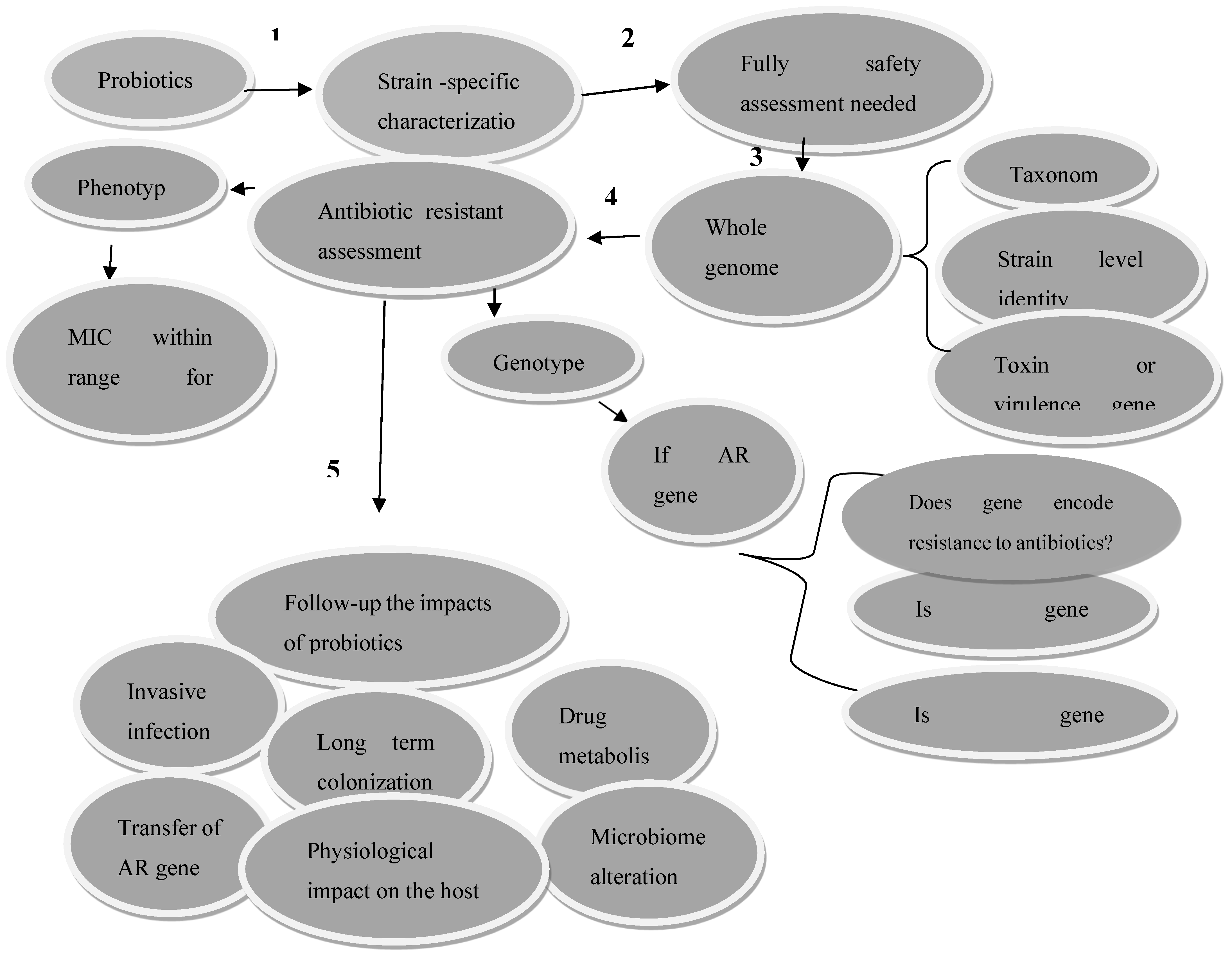
Figure 2.
Methods to check the safety of probiotics before disseminating them into the community. MIC-minimum inhibitory concentration, AR-antibiotic resistance [
42].
Figure 2.
Methods to check the safety of probiotics before disseminating them into the community. MIC-minimum inhibitory concentration, AR-antibiotic resistance [
42].
3.2. Prebiotics
Prebiotics are nondigestible carbohydrates but which are digested by colon resident microbiota and result in production of short chain fatty acids such as acetate, propionate and butyrate. Diets rich in nondigestible fiber increase microbial fermentation and the production of SCFAs in the colon promotes mucosal homeostasis [
43]. Prebiotics enhance the proper functioning of GIT through keeping intestinal epithelial cells healthy, increasing mucus production, adjusting pH in the intestine, blocking the adherence of pathogens to enterocytes, acetate helped as source of energy for muscle and colonic tissue; it also regulates fat storage, butyrate facilitate metabolism and modulating immune system while propionate is very important for gluconeogenesis in the liver. But prebiotics did not play a direct role; rather they provide indirect role [
9,
19].
Prebiotics are interesting to treat both local and distant organ systems. This is because SCFA has small size and they are easily diffused throughout the blood stream. Currently, the use of metabolites from intestinal microbiota is one of the important considerations for controlling GIT diseases in humans and they are a novel alternative therapeutic agent. Natural bioavailability, high amount, easy administration and tissue tolerability make those metabolites a candidate for therapeutic purpose [
5]. According to the panel conducted by International Scientific Association for Probiotics and Prebiotics (ISAPP) in 2016, prebiotics are substrates which are utilized selectively by probiotics and give health benefits for the host. Prebiotics are very important for the proliferation of probiotics such as
Lactobacillus and
Bifidobacterium [
44]
.
Prebiotics prevent colonization of pathogens which are known by producing toxic metabolites, increasing the production of SCFAs and helping in preventing the bacterial translocation. Some of the examples of prebiotics are inulin, fructo oligosaccharides (FOS), galacto-oligosaccharides (GOS), soya-oligosaccharides, xylo-oligosaccharides (XOS), pyrodextrins, human milk oligosaccharides, resistant starches and pectic oligosaccharides. Inulin and pectin are important to tackle the problem of diarrhea, IBS and colon cancer. Prebiotics must fulfill those criteria such as being tolerant to acid, bile salts and other hydrolyzing enzymes in the stomach, they must not be absorbed in the upper GIT and they must be easily digested by probiotics. The fermentation of prebiotics by probiotics is very important for the healthier composition of GIT microbiota and they will induce luminal or systemic beneficial effects for the host. They are also important in tackling the problems raised by pathogenic microbes. [
15,
45,
46,
47].
3.3. Synbiotics
The synergistic effect of probiotics and prebiotics is literally said to be synbiotics. In other words, prebiotics are consumed by probiotics and support their growth inside the host to give beneficial effects. Synergistic synbiotics mean that prebiotics are designed based on considering that they must be selectively digested by probiotics.
Synbiotics are mainly formulated to give solutions for survival difficulties of probiotics. If the prebiotic substrates are fermentable, it will enhance the existence of probiotics, it also protects them from gastric acidity and proteolytic effect. The combination of probiotics and prebiotics allowed the growth of essential microbiota and initiation of metabolism through them. An important probiotics species used in synbiotic preparations are
Lactobacillus spp.,
Bifidobacteria spp.,
S. boulardii and prebiotic substrates include FOS, GOS, and XOS. Clinical benefits of taking synbiotics include balancing gut microbiota, enhancing immunity and its modulation, preventing the leaking of bacteria and minimizing the risks of hospital acquired infections in surgical patients [
15,
23,
29,
48].
Table 2.
Clinical importance of probiotics, prebiotics and synbiotics.
Table 2.
Clinical importance of probiotics, prebiotics and synbiotics.
| Probiotic strain/prebiotcs/synbiotics |
Study finding |
References |
|
Lactobacillus fermentum, Leuconostoc dextranicum, Lactococcus casei, Lactococcus lactis 368, Lactobacillus curvatus MBSa3, Lactobacillus sakei MBSa1, combination of Lactobacillus amylovorus C94 and Lactobacillus salivarius C86 |
Inhibited S. aureus, complete killing and inhibition of Salmonella biofilm formation |
[49] |
|
Lactobacillus acidophilus, combination of L. acidophilus, Lactobacillus rhamnosus, L. casei, and Lactobacillus plantarum |
Showed AMA against E. coli, successfully inhibited the proliferation of E. coli O157:H7 |
[50] |
| Lactobacillus acidophilus |
Controlled the secretion of proinflammatory cytokines such as tumor necrosis factor-α, reversed GIT dysmotility, |
[48] |
| The combination of lactobacilli strains, bifidobacterial strains and Streptococcus salivarius sp. thermophilus
|
Successful evidence of reducing the incidence of IBD |
[51] |
|
L. acidophilus and Bifidobacterium spp, combination of L. casei PXN 37, L. rhamnosus PXN 54, S. thermophilus PXN 66, B. breve PXN 25, L. acidophilus PXN 35, B. longum PXN 30, L. bulgaricus PXN 39 with FOS |
Showed AMA against Helicobacter pylori, Eradication of H. pylori
|
[52,53] |
| Combination of Lactobacillus rhamnosus GG and Bifidobacteria subsp. Lactis with Oligofructose-enriched inulin |
Enhanced the growth of probiotics such as Bifidobacteria spp. and Lactobacillus spp., Prohibiting the growth of C. perfringens |
[54] |
| Combination of Bifidobacterium breve and L. casei Shirota with galactooligosaccharide |
Fecal SCFA increased, Bifidobacteria and lactobacillus concentration increased, body weight increased in patients with IBS |
[55] |
|
S. lactis, L. plantarum, S. cremoris, L. casei, S. diacetylactis, S. florentinus, L. cremoris, L. acidophilus, B. bifidum
|
Improved lactose digestion and tolerance, minimizing severity of diarrhea |
[38] |
|
L. casei, Lactobacillus rhamnosus GGSaccharomyces boulardii
|
Prevention of C. difficile associated diarrhea or AAD |
[56] |
4. How to replace antibiotics by probiotics, prebiotics and synbiotics? What are the challenges to use them?
Gut microbiota dysbiosis facilitates the severity of various diseases. Therefore, modulating GIT microbiota is crucial to minimize the spread of AR pathogens and the side effects of antibiotics on commensal flora. GIT microbiota dysbiosis is critical, this is because microbiota disturbance inside GIT not only affects GIT but also affects distant organs. For example; it affects central nervous system through gut-brain axis, it affects the gut-lung axis and also affects gut-liver axis. From this scenario, we clearly identify that there is an amazing interaction between intestinal microbiota and human health [
57,
58,
59]. An increasing number of researches confirmed the use of probiotics, prebiotics and synbiotics. But here the problem is a gap in understanding on definitions of the term 'probiotics’, their benefits to health, how they function, and where to find the best sources in food and healthcare products [
39].
Lack of sufficient LAB strain identification, safety and lack of knowledge about its dosage should be solved to expand the probiotic market. Probiotics which are administered through functional foods should be regulated like that of drugs. The probiotic researchers should be equipped with knowledge of gastroenterology, immunology and microbiology to do the clinical trials effectively. There is also a report about a fear to use probiotics;
S. boulardii and
Lactobacillus GG may cause some health problems in immune-compromised groups of peoples. Some Lactobacilli strains are resistant to vancomycin; this might be creating an opportunity for spreading of AR gene into the pathogens. Consuming prebiotics such as FOS may lead to bloating and flatulence [
15,
37]. Some probiotics cause invasive infection due to translocating out of GIT. Scholars also reported that some probiotics may affect drug function due to harboring drug modifying enzymes. Infections are also reported such as bacteremia, cholangitis and sepsis by
L. rhamnosus GG and
Bacillus subtilis. Additionally, sepsis caused by
S. boulardii also reported. During working with those biological agents; purity, potency and contaminants should be checked carefully. Sharing information about risks and benefits of using probiotic products is also mandatory. There is no consistent research finding with those biological agents. This is due to the presence of various probiotic species and strains, prebiotic structure and their dosage variation. Some factors inside GIT such as acidity, proteolytic enzymes and others may affect the working ability of probiotics and prebiotics [
9,
42,
51,
60].
5. Conclusion
Intestinal microbiota disturbed due to various factors. Among the factors, side effects of antibiotics are an urgent concern to be minimized. GIT microbiota and their metabolites make a bidirectional communication such as gut-lung axis, gut-brain axis, gut-liver axis and others. In this scenario, dysbiosis of GIT microbiota affects the occurrence of those bidirectional communication and leads to development of various diseases. Probiotics, prebiotics and synbiotics join multiple forces to modulate GIT microbiota; this might be creating an opportunity to utilize them instead of antibiotics. Therefore, targeting GIT microbiota might be a spot line of delivering a microbiota-based therapy such as modulation of intestinal microbiota through probiotics, prebiotics and synbiotics.
But more research work is required to identify/assess their benefits and risks. Careful screening and investigation of those biological agents should be well addressed. The products must be regulated in various levels of utilization. However, we are looking for a shade of bright light in the future, as microbiota-based therapy will be our hope to relief from diseases and illness. Finally, we would like to forward two main recommendations:(1).In spite of the fact that antibiotics are the front means of tackling bacterial diseases, currently there is a sharp increase of resistant bacterial infection, dysbiosis of gut microbiota and low rate of antibiotic discovery. Therefore; to solve the above problems utilization of probiotics, prebiotics, synbiotics and their metabolites might be safe; (2). Scientific findings acknowledged GIT microbiota since they play key roles in human health and ailment. Therefore, it is better if those microorganisms are manipulated with dietary supplements to improve and maintain health.
List of abbreviations
GIT: Gastrointestinal tract; AAD: Antibiotic associated diarrhea; TcdA and B: Clostridium difficile associated toxin A and B; CDI: Clostridium difficile infection; AMP’s: Antimicrobial peptides; IBD: Inflammatory bowel disease; IBS: Inflammatory bowel syndrome; IEC’S: Intestinal epithelial cells; ISAPP: International Scientific Association for Probiotics and Prebiotics; LAB: Lactic acid bacteria; Mub: Mucus binding protein; MapA: Mucus adhesion promoting protein; NK: Natural killer cells; DC’s: Dendritic cells; PRR’s: Pattern recognition receptors; PAMP’s: Pathogen associated molecular patterns; MLN: Mesenteric lymph nodes; SCFAs: short chain fatty acid
Author Contributions
Goa T and Sahilu E conducted reviewing the title and wrote the manuscript. Wolde T provided critical comment on the manuscript. Finally, the authors re-cheeked the manuscript and submitted the updated version of their work.
Funding
This study did not receive any funding in any form
Availability of Data and Materials
All reviewed information was present in this manuscript
Acknowledgment
Not applicable
Competing Interests
The authors declare that they have no competing interests.
Ethics approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable
References
- Chang, C.-J.; Lin, T.-L.; Tsai, Y.-L.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622, . [CrossRef]
- Tan, G.S.E.; Tay, H.L.; Tan, S.H.; Lee, T.H.; Ng, T.M.; Lye, D.C. Gut Microbiota Modulation: Implications for Infection Control and Antimicrobial Stewardship. Adv. Ther. 2020, 37, 4054–4067, . [CrossRef]
- Cammarota, G.; Ianiro, G.; Bibbò, S.; Gasbarrini, A. Gut microbiota modulation: probiotics, antibiotics or fecal microbiota transplantation?. Intern. Emerg. Med. 2014, 9, 365–373, . [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011, 6, 209–240, . [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: towards clinical revolution. J. Transl. Med. 2022, 20, 1–20, . [CrossRef]
- Barbuti, R.C.; Schiavon, L.L.; Oliveira, C.P.; Alvares-Da-Silva, M.R.; Sassaki, L.Y.; Passos, M.D.C.F.; Farias, A.Q.; Barros, L.L.; Barreto, B.P.; Albuquerque, G.B.d.M.L.d.; et al. Gut microbiota, prebiotics, probiotics, and synbiotics in gastrointestinal and liver diseases: proceedings of a joint meeting of the Brazilian society of hepatology (sbh), Brazilian nucleus for the study of helicobacter pylori and microbiota (nbehpm), and Brazilian Federation of Gastroenterology (FBG). Arq. de Gastroenterol. 2020, 57, 381–398, . [CrossRef]
- Mazhar, S.F.; Afzal, M.; Almatroudi, A.; Munir, S.; Ashfaq, U.A.; Rasool, M.; Raza, H.; Munir, H.M.W.; Rajoka, M.S.R.; Khurshid, M. The Prospects for the Therapeutic Implications of Genetically Engineered Probiotics. J. Food Qual. 2020, 2020, 1–11, . [CrossRef]
- Rabetafika, H.N.; Razafindralambo, A.; Ebenso, B.; Razafindralambo, H.L. Probiotics as Antibiotic Alternatives for Human and Animal Applications. Encyclopedia 2023, 3, 561–581, . [CrossRef]
- Mutalub, Y.B.; Abdulwahab, M.; Mohammed, A.; Yahkub, A.M.; Al-Mhanna, S.B.; Yusof, W.; Tang, S.P.; Rasool, A.H.G.; Mokhtar, S.S. Gut Microbiota Modulation as a Novel Therapeutic Strategy in Cardiometabolic Diseases. Foods 2022, 11, 2575, . [CrossRef]
- Airola, C.; Severino, A.; Porcari, S.; Fusco, W.; Mullish, B.H.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. Future Modulation of Gut Microbiota: From Eubiotics to FMT, Engineered Bacteria, and Phage Therapy. Antibiotics 2023, 12, 868, . [CrossRef]
- Goa, T.; Beyene, G.; Mekonnen, M.; Gorems, K. Isolation and Characterization of Lactic Acid Bacteria from Fermented Milk Produced in Jimma Town, Southwest Ethiopia, and Evaluation of their Antimicrobial Activity against Selected Pathogenic Bacteria. Int. J. Food Sci. 2022, 2022, 1–15, . [CrossRef]
- Habteweld, H.A.; Asfaw, T. Novel Dietary Approach with Probiotics, Prebiotics, and Synbiotics to Mitigate Antimicrobial Resistance and Subsequent Out Marketplace of Antimicrobial Agents: A Review. Infect. Drug Resist. 2023, ume 16, 3191–3211, . [CrossRef]
- FBarbut, J.L. Meynard, “Managing antibiotic associated diarrhea: Probiotics may help in prevention,” BMJ, vol .324, no.7350, pp. 1345-1346, 2002.
- Yang, J.; Yang, H. Non-antibiotic therapy for Clostridioides difficile infection: A review. Crit. Rev. Clin. Lab. Sci. 2019, 56, 493–509, . [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587, doi:10.1007/s13197-015-1921-1.
- Greathouse, K.L.; Harris, C.C.; Bultman, S.J. Dysfunctional Families: Clostridium scindens and Secondary Bile Acids Inhibit the Growth of Clostridium difficile. Cell Metab. 2015, 21, 9–10, . [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Heal. 2021, 14, 1750–1766, . [CrossRef]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777, . [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The spread of antibiotic resistance genes in vivo model. Canadian Journal of Infectious Diseases and Medical Microbiology. 2022,2022, 3348695.
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Yang, K.; Wang, Z. Correlation between Exogenous Compounds and the Horizontal Transfer of Plasmid-Borne Antibiotic Resistance Genes. Microorganisms 2020, 8, 1211, . [CrossRef]
- Silva, D.R.; Sardi, J.d.C.O.; Pitangui, N.d.S.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080, . [CrossRef]
- Martinez, R.C.R.; Bedani, R.; Saad, S.M.I. Scientific evidence for health effects attributed to the consumption of probiotics and prebiotics: an update for current perspectives and future challenges. Br. J. Nutr. 2015, 114, 1993–2015, . [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World Journal of Gastroenterology. 2023, 29, 2078.
- BBottari, V. Castellone, E. Neviani, “Probiotics and Covid-19,” International Journal of Food Sciences and Nutrition, vol.72, pp.293-299, 2021.
- MBermudez-Brito, J. Plaza-Díaz, S. Munoz-Quezada et al., “Probiotic mechanisms of action,” Ann Nutr. Metab, vol.61, no.2, pp.160-174, 2012.
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685, . [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the Gut Immune System: Indirect Regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21, . [CrossRef]
- Sanz, Y.; De Palma, G. Gut Microbiota and Probiotics in Modulation of Epithelium and Gut-Associated Lymphoid Tissue Function. Int. Rev. Immunol. 2009, 28, 397–413, . [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729, . [CrossRef]
- Kiernan, D.P.; O’doherty, J.V.; Sweeney, T. The Effect of Maternal Probiotic or Synbiotic Supplementation on Sow and Offspring Gastrointestinal Microbiota, Health, and Performance. Animals 2023, 13, 2996, . [CrossRef]
- RDenkova, B. Goranov, D. Teneva et al., “Antimicrobial activity of probiotic microorganisms: Mechanisms of interaction and methods of examination,” Antimicrobial research: Novel Bio Knowledge and Educational Programs, pp.201-212, Id.85454328, 2017.
- E. Assefa. Antimicrobial Activity of Lactic Acid Bacteria Isolated from ‘Ergo’, Ethiopian Traditional Fermented Milk, On Some Food borne Pathogens. Master thesis, Addis Ababa University. Accessed June 10, 2022. http://etd.aau.edu.et/xmlui/handle/123456789/20485.
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283, . [CrossRef]
- Suskovic, J.; Kos, B.; Beganovic, J.; Lebos Pavunc, A.; Habjanic, K.; Matosic, S. Antimicrobial activity–the most important property of probiotic and starter lactic acid bacteria. Food Technology and Biotechnology. 2010,48, 296-307.
- Sharma, M.; Modi, D.R.; Saxena, M. Role of lactic acid bacteria as probiotics in health and disease. Prevention. 2014, 82, 84.
- Wang, S.; Chen, P.; Dang, H. Lactic acid bacteria and γ-aminobutyric acid and diacetyl. Lactic Acid Bacteria: Bioengineering and Industrial Applications. 2019, 1-9.
- Newman, A.M.; Arshad, M. The Role of Probiotics, Prebiotics and Synbiotics in Combating Multidrug-Resistant Organisms. Clin. Ther. 2020, 42, 1637–1648, . [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [CrossRef]
- HHardy, J. Harris, E. Lyon et al., “Probiotics, prebiotics and immunomodulation of gut mucosal defenses: homeostasis and immunopathology,” Nutrients, vol.5, no.6, pp.1869-912, 2013.
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386, . [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112, . [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034, . [CrossRef]
- Bvan der Hee, J.M. Wells, “Microbial regulation of host physiology by short-chain fatty acids,” Trends in Microbiology, vol.29, no.8, pp.700-712, 2021.
- Quigley, E.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2018, 17, 333–344, . [CrossRef]
- Megur, A.; Daliri, E.B.-M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097, . [CrossRef]
- S. Macfarlane, “Prebiotics in the gastrointestinal tract,” Bioactive Foods in Promoting Health, pp.145-156, 2010.
- Steer, T.; Carpenter, H.; Tuohy, K.; Gibson, G.R. Perspectives on the role of the human gut microbiota and its modulation by pro- and prebiotics. Nutr. Res. Rev. 2000, 13, 229–254, . [CrossRef]
- Batista VL, Da Silva TF, de Jesus LC, et al. Probiotics, prebiotics, synbiotics, and paraprobiotics as a therapeutic alternative for intestinal mucositis. Frontiers in Microbiology. 2020, 11, 544490.
- FDaliri, A.A. Aboagye, EBM. Daliri, “Inactivation of Food borne Pathogens by Lactic Acid.
- Bacteria,” Journal of Food Hygiene and Safety, vol.35, no.5, pp.419 - 429, 2020.
- ZGao, E.B. Daliri, JU. Wang et al., “Inhibitory effect of lactic acid bacteria on food borne pathogens: a review,” Journal of Food Protection, vol.82, no.3, pp.441-453, 2019.
- Olveira, G.; González-Molero, I. An update on probiotics, prebiotics and symbiotics in clinical nutrition. 2016, 63, 482–494, . [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521, . [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701, . [CrossRef]
- Shafi, A.; Farooq, U.; Akram, K.; Hayat, Z.; Murtaza, M.A. Prevention and Control of Diseases by Use of Pro- and Prebiotics (Synbiotics). Food Rev. Int. 2014, 30, 291–316, . [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Inflammatory bowel disease: Current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int. J. Food Microbiol. 2007, 115, 1–11, . [CrossRef]
- Jenkins, G.; Mason, P. The Role of Prebiotics and Probiotics in Human Health: A Systematic Review with a Focus on Gut and Immune Health. Food Nutr. J. 2022, 6, 245.
- Thilagavathi, T. Probiotics, Prebiotics, Synbiotics and its Health Benefits. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 497–511, . [CrossRef]
- Li C, Niu Z, Zou M, et al. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. Journal of dairy science. 2020, 103, 5816-29.
- Rajput, S.; Paliwal, D.; Naithani, M.; Kothari, A.; Meena, K.; Rana, S. COVID-19 and Gut Microbiota: A Potential Connection. Indian J. Clin. Biochem. 2021, 36, 266–277, . [CrossRef]
- Quintero, D.F.G.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725, . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).