1. Introduction
In today’s world one crucial problem is the recycling of various wastes in tandem with energy production. In general, aluminium (Al) recycling is well established industry with somewhat known parameters, such as packaging and beverage can recycling. The classification of aluminium scraps has been created and currently dictated by the standards EN12258 and EN13980 [
1]. Moreover, aluminium-rich solid waste materials are non-compliant to disposal in a non-hazardous waste landfill, as defined by the EU criteria. As a matter of fact, the EU regulations classify aluminium-rich by-products as special hazardous wastes capable to create flammable gases and to form explosive mixtures with ambient air (hazard class codes: HP10; HP11; HP12; HP13; European Waste Code, EWC: 100323*). Aluminium recycling is not only energy intense endeavor but also there is a limit on the recycling extent. There are Al wastes that have high recyclability such as cans (containing ca. 94 wt.% Al, 1 wt.% oxides and 5 wt.% other inclusions), and there are also large amounts of unrecyclable or hard to recycle wastes, i.e., dross. The recycled aluminium nowadays amounts to ca. 35% of the total primary Al production and requires 10-15 times less energy consumption [
2,
3,
4]. In industrial production scale the first step in aluminium recycling is the separation of the metallic components from the non-metallic ones [
2,
5,
6] by applying various screening techniques or even their combination, such as magnetic separation, Eddy current separation, density separation, and others [
5,
6,
7,
8,
9,
10,
11]. It is well known that aluminium water reaction can produce hydrogen, thus the hazardous nature of Al waste in landfill; it can be used for electricity production where a by-product is aluminium hydroxide. One of crucial components of use of Al-H
2O reaction and utilization of waste Al is the mitigation of CO
2 emissions, as proven by Hiraki et al. Life Cycle analysis, where this reaction decreased energy requirement to only 2% of the conventional method which leads to 4% of CO
2 emissions [
12]. Known literature has concluded that impurities largely influence the production yield as well as amount of by-product created in Al-Water reaction.
Al-Water reaction is distinguished by the absence or presence of the catalyst. As a fact, aluminium reacts with ambient oxygen to create a protective surface layer. A piece of aluminium, placed into water, already has a surface layer of aluminium oxide or alumina, Al
2O
3, that reacts with water even at moderate temperature to produce a boehmite AlOOH layer. This is so called induction step:
At the induction step the boehmite film is growing, and in the meantime the diffusion of OH
- ions through the AlOOH layer occurs. As a result, hydrogen bubbles appear at the Al:Al
2O
3 interface:
The aim of catalyst usage is to eliminate the protective layer of aluminium oxide that hinders to proceed the reaction with water. In a large scale hydrogen producing, the most common are alkaline catalysts, namely, sodium or potassium hydroxide. In the case of sodium hydroxide as the catalyst, aluminium oxide is being dissolved:
Further, exposed aluminium surface is capable to react with water to form hydrogen:
The surface layer of aluminium hydroxide is being dissolved by sodium:
Excluding all dissociated ions, equations (4, 5) can be composed as:
Meanwhile, the regeneration of sodium hydroxide takes place by decomposition of aqueous NaAl(OH)
4 that results in aluminium hydroxide residue:
Necessary to note that several parameters affect given above reaction chain: purity of aluminium material as well as its morphology, temperature, alkaline concentration and alkaline stirring rate. Thus, it is expected to have variations of hydrogen production depending on the sample content.
The goal of this study is to investigate set of industrial samples (White dross) identifying the composition and elemental content, and estimate its use in Al-Water reaction with alkali catalysts for hydrogen production; taking into account the produced gas purity and estimate it’s potential to be part of green hydrogen production.
Industrial samples were provided by Alcoa, those were investigated using XRD, EDS, SEM, reaction kinetics and analysis of gases as well as reaction efficiency analysis was done to estimate the potential use.
2. Materials and Methods
The Materials used and tested in this study are industrial aluminium production by-products from various stages of smelting. The expected composition of these samples are as follows:
SOW: dross from pure aluminium (expected approx. 80% aluminium);
HDC: dross from 7% aluminium alloys (expected approx. 40% aluminium and approx. impurity content: Si 7%, Mg 2%, Ti 0,5% Sr 1%);
RM: dross from 1xxx series aluminium (approx. 40% aluminium), (approx. other impurity content: B 1%, Ti 2% V 5%).
Samples were analyzed in various methods to determine the elemental, crystalline composition, as well as hydrogen production potential was estimated via water-sample reaction and kinetics evaluation. Decomposition with increased temperature was analyzed using thermogravimetric analysis (Shimadzu Labsys Evo TGA) with heating rate of 20K⸳min-1 in the first section of analysis and then 10 K⸳min-1.
Morphological and structural analysis was carried out in scanning electron microscope (SEM, Hitachi S3400 N, Tokyo, Japan) with elemental composition and elemental mapping carried out by energy dispersive X-ray spectroscopy (EDS, Bruker Quad 5040, Hamburg, Germany). Crystalline structure was analyzed using X-ray diffractometer (XRD, Brucker D8, Hamburg, Germany) using a Cu Kα radiation and Lynx Eye linear position sensitive detector at 2 theta angles in the range 20–70°.
Hydrogen production from water -dross reaction was carried out in a reactor reported in our previous work (see
Figure 3) and produced gasses analyzed in Mass Spectrometer (RGA100 MS, Stanford Research Systems) [
13]. 0.3 g of each sample was immersed in 100 ml of NaOH solution in deionized water at 1 M concentration; temperature of water was kept at 40 °C. Experiments were done without stirring.
O/N gas analysis was carried out (Horiba EMGA), where samples are heated by the impulse furnace to extract the gas enclosed within the samples, and directly analyzed by the detectors.
3. Results
3.1. Thermogravimetric Analysis
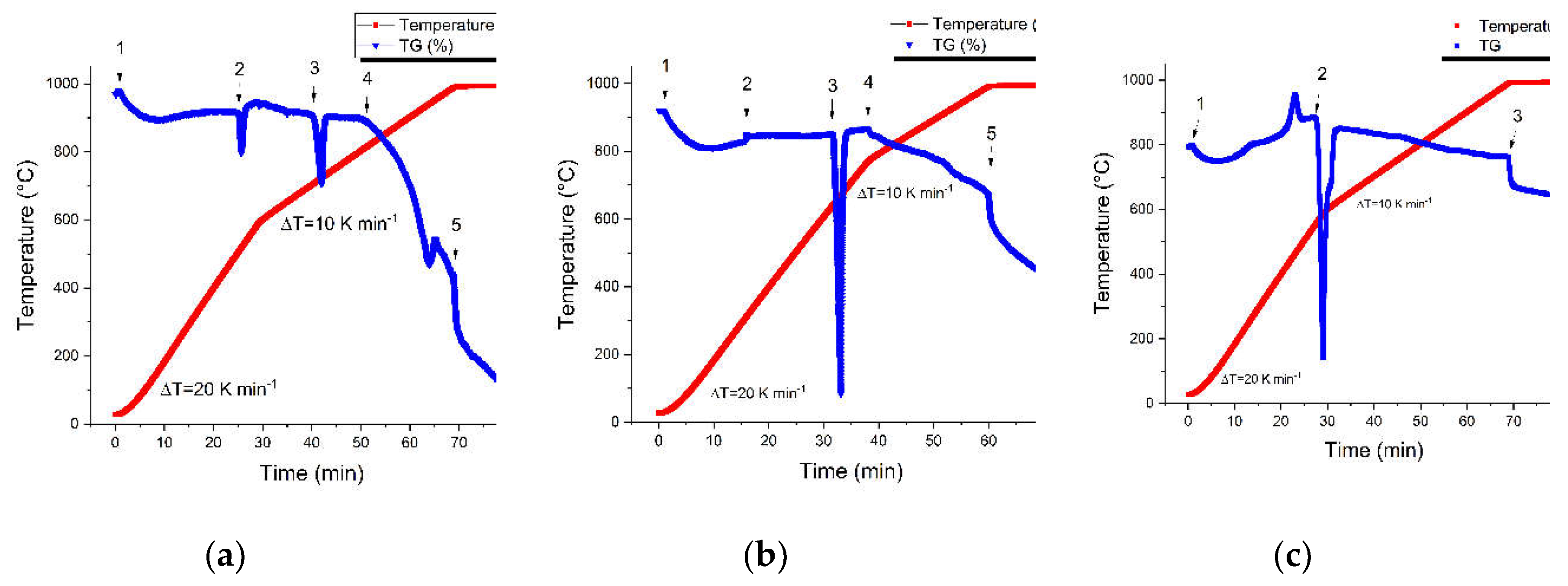
To identify decomposition of samples with increased temperature, heating rate was set to 10 K per minute. Thermogravimetric investigation shows that there are couple of decomposition processes happening in all investigated samples, which is expected as the dross is a complicated sample with many possible components. Most likely those points arise from hydroxide, carbonate and possibly from metallic Al melting. SOW has 5 points, RM also has 5 points whereas HDC has only 3 points. The initial mass loss (starting from point 1) is related to evaporation of moisture and water from the samples.
Figure 1.
(a) SOW sample have 5 distinct changing points, (b) RM has 5 distinct point (c) on the other hand HDC only 3 distinct points.
Figure 1.
(a) SOW sample have 5 distinct changing points, (b) RM has 5 distinct point (c) on the other hand HDC only 3 distinct points.
3.2. Elemental and Structural Analysis
3.2.1. SOW Sample
SEM and EA for sample SOW have been carried out in (Device)
The results are as follows;
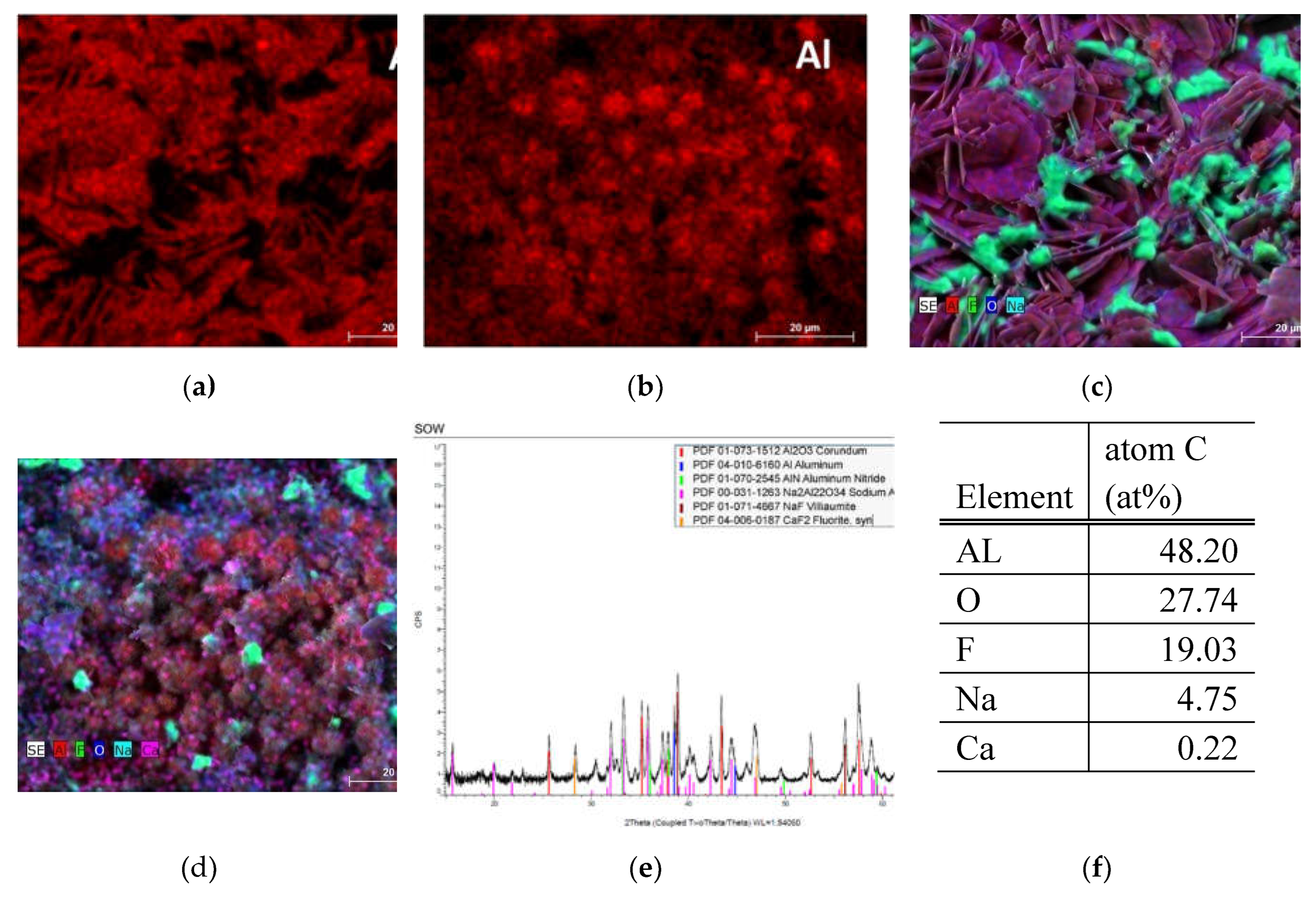
As we can see in the
Figure 2 the SOW sample consists of majority aluminium and alumina, but with substantial amount of impurities, mostly fluoride and sodium that comes from the material processing reactants in addition to Ca and Fe as regular additives to aluminium alloys. XRD analysis shows that crystalline structures are present from corundum to calcium fluoride, in addition to other components. It has amounts of nitride which changes the thermal properties of the material substantially and makes it hard to physically process. Even in our processing experience preparation of samples was very complicated as separation and grinding of samples took a lot of tools and time. Thus, especially this step material is very complicated to recycle in a conventional way.
Figure 2.
SEM, EDS and XRD analysis of sample SOW, (a) EDS analysis of Al composition in one point (b) EDS measurement of Al composition in 2. point (c) Overall composition of elements in point 1 (d) overall EDS measured composition in point 2 (e) XRD diffractogram of sample (f) average value of atomic concentration percentage as measured by EDS.
Figure 2.
SEM, EDS and XRD analysis of sample SOW, (a) EDS analysis of Al composition in one point (b) EDS measurement of Al composition in 2. point (c) Overall composition of elements in point 1 (d) overall EDS measured composition in point 2 (e) XRD diffractogram of sample (f) average value of atomic concentration percentage as measured by EDS.
3.2.1. RM Sample
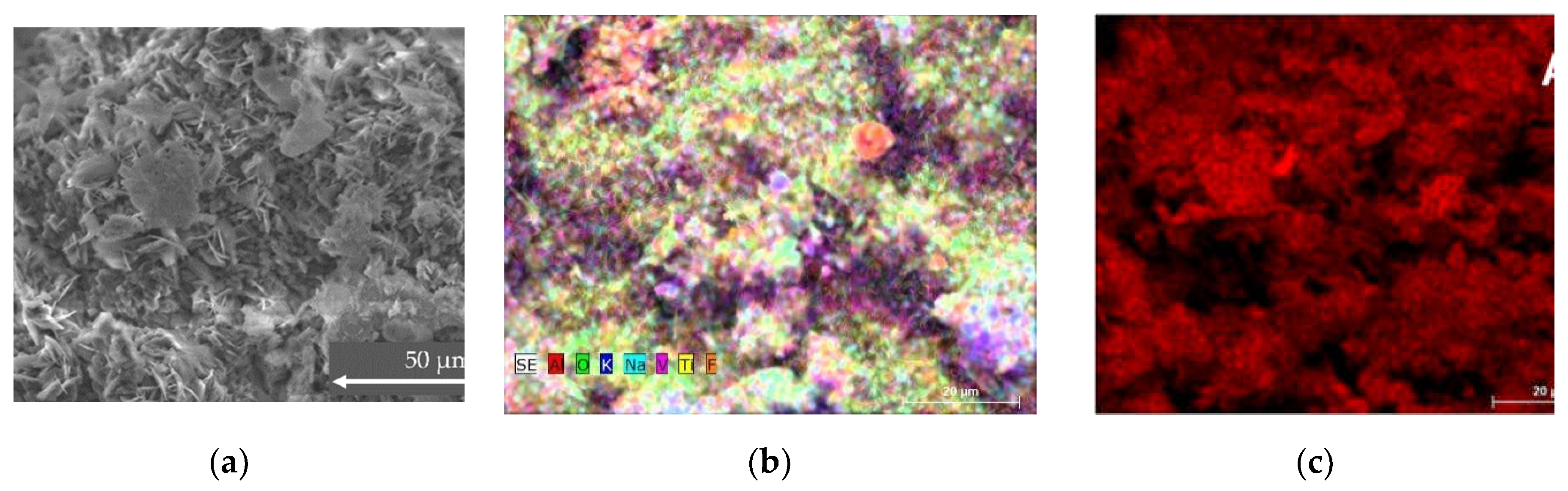
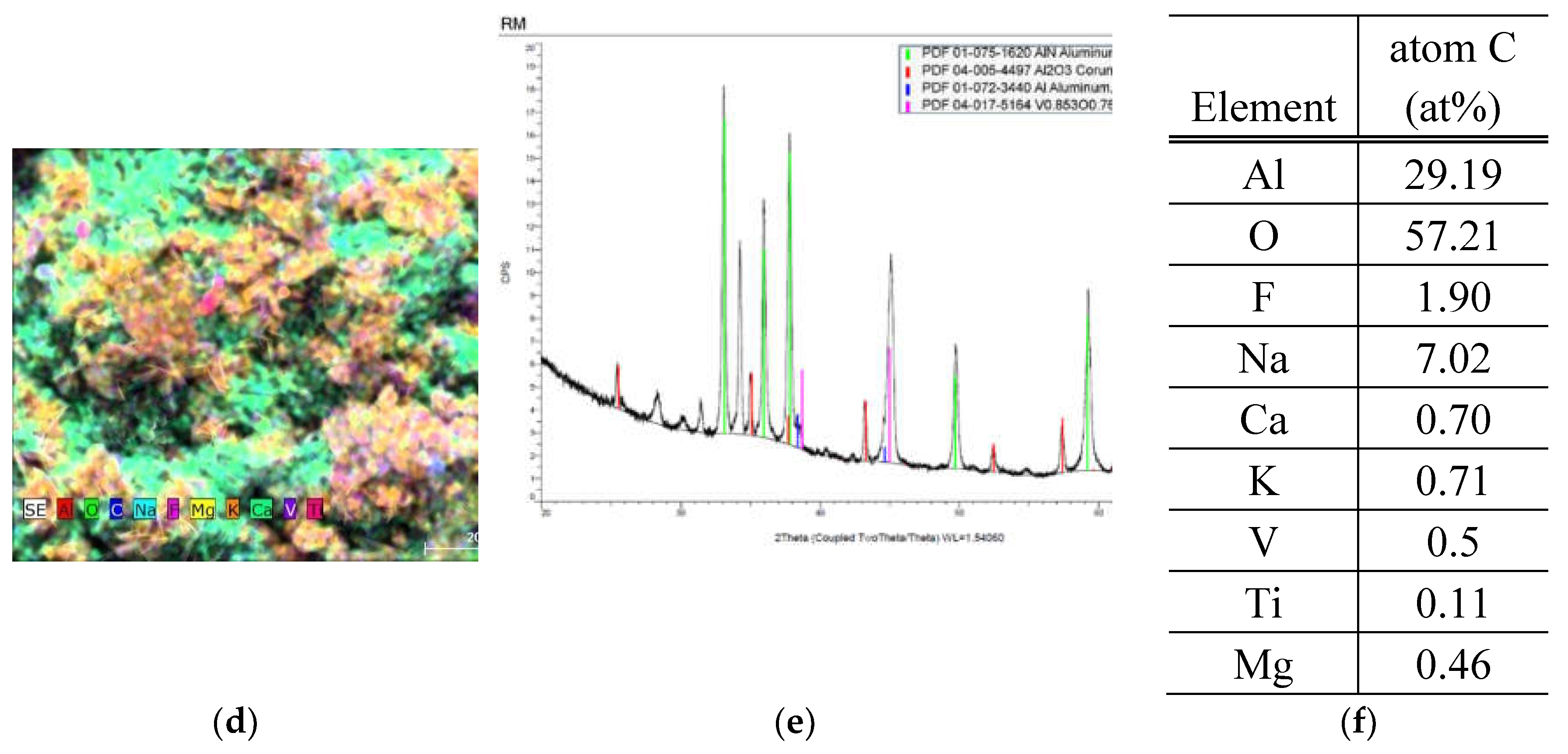
SEM and EDS analysis of RM sample
Sample consists of Al, O, K, Mg, Ca, Na, F, V, Fe and Ti. The impurities are not so evenly distributed and the morphology differs a little bit as well depending on the measuring point. Although the impurities are a small part of the total concentration, some parts show a higher amount of fluorine. The Al content changes from 30% to 45 % and oxygen changes from 43 to 59%, other trace elements also change substantially. It is known that composition of samples will have substantial effect on the hydrogen production. On the other hand, crystalline structure shows lower number of components, such as Al nitride, oxide, and vanadium oxide. In comparison to SOW sample, the amount of crystalline components is lower, and it is mostly composed of oxides.
Figure 3.
SEM, EDS and XRD analysis of sample RM. (a) SEM of the sample (b) ESD analysis with all element composition at once (c) Al composition of the sample (d) second overall view of all present element distribution (e) XRD of the sample (f) summary of average atomic concentration of the sample.
Figure 3.
SEM, EDS and XRD analysis of sample RM. (a) SEM of the sample (b) ESD analysis with all element composition at once (c) Al composition of the sample (d) second overall view of all present element distribution (e) XRD of the sample (f) summary of average atomic concentration of the sample.
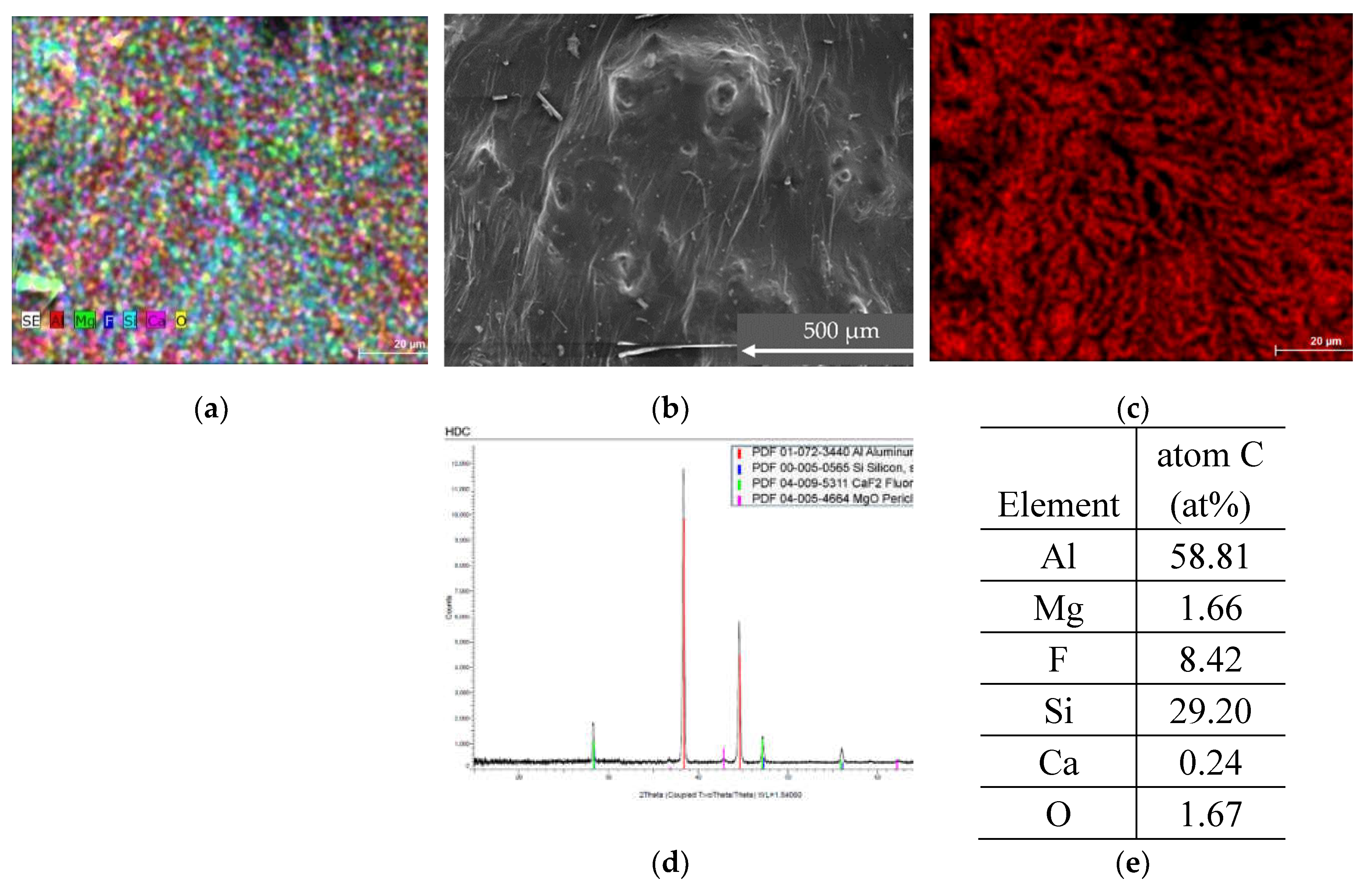
3.2.2. HDC Sample
The sample consists of following elements: Al, Mg, F, Si, Ca and O are evenly distributed across the sample area.
Figure 4.
SEM, EDS and XRD analysis of sample HDC (a) ESD mapping of the sample (b) SEM image of the sample (c) ESD analysis with Al element composition (d) XRD of the sample (e) summary of average atomic concentration of the sample.
Figure 4.
SEM, EDS and XRD analysis of sample HDC (a) ESD mapping of the sample (b) SEM image of the sample (c) ESD analysis with Al element composition (d) XRD of the sample (e) summary of average atomic concentration of the sample.
From EDS and SEM we see that HDC sample is somewhat homogeneous without visible crystalline structures, similarly that is visible in XRD results, as the crystalline forms Al metal, also Si, CaF2 and MgO visible from found strong peaks in the diffractogram pattern.
From elemental analysis we see that depending on the processing stage of aluminium, dross has variations of compositions and structures, which in turn complicates recycling and reusing these materials. The simplified estimated energy consumption of 1t of dross is 454 kWh/t in oil and 72 kWh/t in electricity with yield of 0.45 t of metallic Al [
14]. It is noteworthy that often the exact composition of used dross is not revealed, nonetheless it is quite complicated to compare results and composition as depending on the stage of sample collection, moreover various producers will most likely have variations of compositions that will depend on the individual processes, raw materials and treatments.
3.3. Hydrogen Production from Samples
The water aluminium reaction produces gases at a various rate, and as it turns out we have to see what gasses are being produced during the reaction with the electrolyte. Following are separation into each sample.
3.3.1. SOW Sample
SOW sample measuring the gas composition at the beginning of the reaction and at a later time, composition of the analyzed gases is visible in
Table 1. We can see that 0.2% of methane was detected at the beginning of the reaction with, 97.4% hydrogen, 0.3% O
2, 1.2% N
2 and 0.1 % CO
2. At the end of the reaction there was still methane present 0.2%, water vapor 0.1% and 99.6% of hydrogen.
3.3.2. RM Sample
In the beginning of the experiment 98.3% of hydrogen was detected and 0.0% methane, in addition to N
2 0.4%, O
2 0.1%, and water 1.9%. Then the reaction was let to run till completion. From gas analysis one can see that methane is present in the first hour – however, it is impossible to find more than 0.09 wt% methane in the following reaction hours. Hydrogen composes the majority of the collected and analyzed gas as shown in the
Table 2.
3.3.3. HDC Sample
H2 production of HDC sample.
Pressure-temperature measurement shows steady production of gas and heat during sample - water reaction. Reaction starts with release of CO
2 and expected H
2 as seen in
Table 3, then Sample was left in the reactor overnight to detect the maximum gas generation and allow full reaction with electrolyte. During the night Methane evolution was detected as well as nitrogen which decreases the gas ration, thus amount of H
2 decreases to 92.5%.
Methane, Ethane and CO2 are the most present impurities from reaction, but CO2 might be also introduced from the leaks of the flow reactor as N2 is greatly introduced into the reactor. However, Methane is the strongest hazardous impurity of 0.4% of the gas. Gas was collected in 400 cc collection volume. This means that within 1st day only 1bar of H2 was generated. The last MS spectra is after additional sample addition which might introduce air from vale opening – this also explains the increase in methane as much more “pristine” HDC surfaces are exposed to the electrolyte.
3.3.4. Comparison of Hydrogen Generation and Reaction Efficiency
O/N gas analysis was measured several times for each sample, average results are depicted in
Table 4. The obtained results seem quite scattered due to inhomogeneous composition of the samples. Some particles are with high purity, on the other hand many parts of samples show high amount of oxygen and increase amount of nitrogen (as XRD patterns).
From the sample-water reaction, it was identified that AlN (as XRD detected) containing samples would result in the formation of ammonia. Thus, the application of PEM fuel cell is not viable for these types of materials as ammonia would degrade and poison the FC. Any contamination must be avoided if generated gas is to be used in a fuel cell. Ammonia (even at ppm level) is a real contaminant to the PEM hydrogen fuel cell [
15]. 15 h exposure to 30 ppm NH
3 in the anode fuel caused a rapid drop in cell performance.
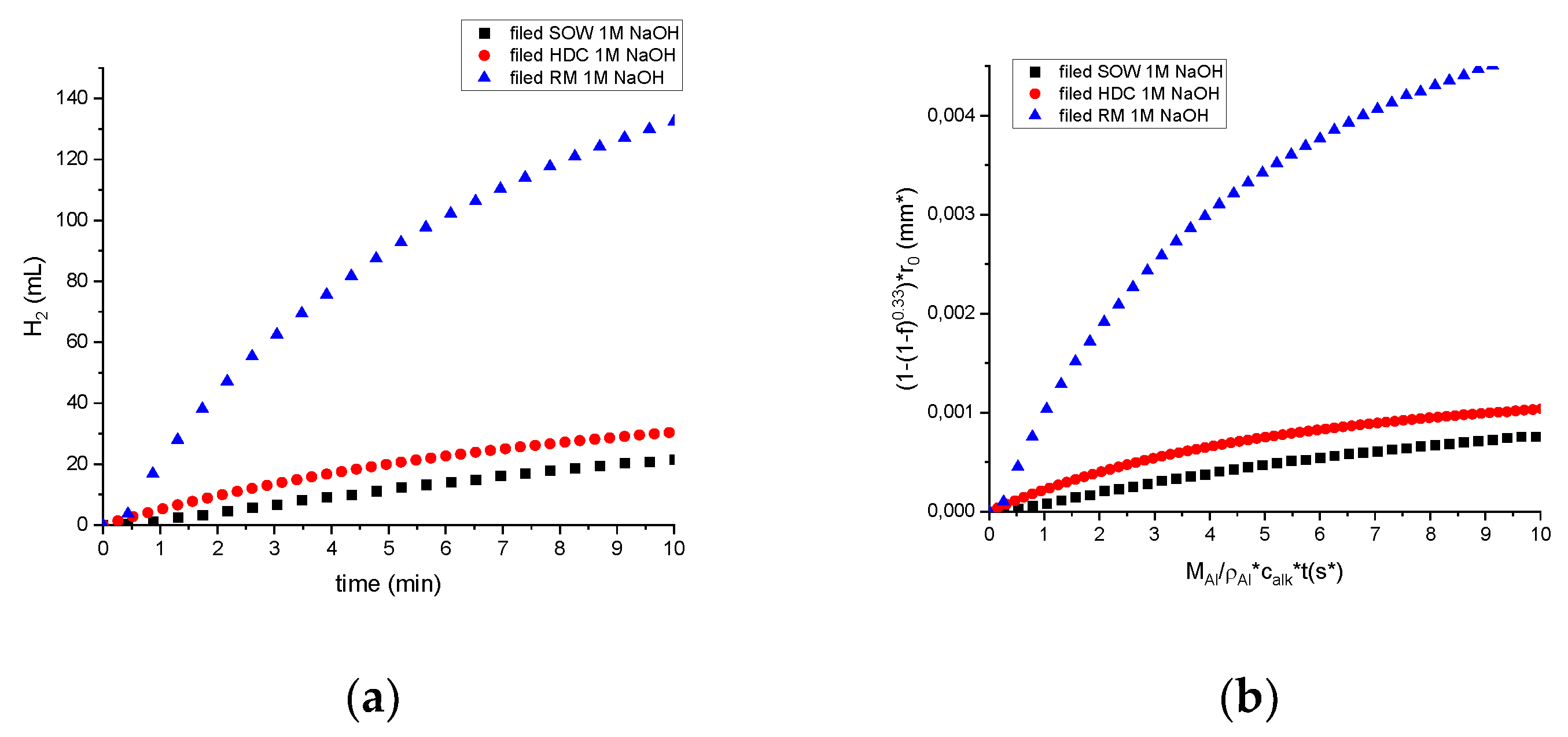
For initial experiments with 1M alkaline and elevated temperature (40 °C), the reaction was not as fast as expected. The slowest H2 generation reaction was recorded from HDC sample, while RM showed the highest generation velocity/rate. However, we observed that some particles of SOW and RM samples did not react at all.
From the investigation of sample reaction efficiency, it seems that RM shows highest efficiency, even though the initial expectation is that this samples has roughly 40% Al, from composition we see that it is mostly aluminium oxide, XRD doesn’t show much of hydroxide. Other samples have impurities as Si or N, C which hinder the reaction. To increase the reactivity of samples and compare gas generation. After filing down the samples to comparable particle size the reaction efficiency is shown in the
Figure 6a, where reaction efficiencies are depicted in
Figure 6b and comparison of dross samples with Al waste from construction material in
Figure 6c.
As the samples are pre-treated with the file, the particles are more like spheric as cylindric, plates or films. It means, in the first degree of monodisperse approximation, the geometry of particles is described only by the single radius. With respect to volumetric bodies, at the chemical reaction the shrinking of the solid reactant occurs, therefore the radius is time-dependent, r(t). The base of the shrinking core model for a spherical particle is the expression that links the molar aluminium reaction rate
dnAl/
dt on the surface with diminishing the size of a particular aluminium particle:
where
ρAl ans
MAl are density and molar mass of the solid (aluminium). Eq. (8) is correct for the first and more intensive, so-called surface reaction rate step, being analyzed in the present work. This equation is widely solved out in literature [
16], where it is shown that at a sufficient stirring:
where
r0 is the initial radius of the solid particle, and
f is normalized non-dimensional hydrogen yield (0 <
f < 1), where
f=1 means the maximal theoretical yield, calculated by ideal (or real) gas equation of state. At the experimental case of gaining the pressure
p of yielded hydrogen in the constant volume
V:
Here
WAl is reacted aluminium mass,
p0 and
Ta are the reference pressure and temperature, respectively. The coefficient 2/3 comes from the stoichiometry in reaction between solid and liquid, eq. (6). Summarizing Hiraki et al. [
16], the reaction rate constant at the surface reaction step,
ks, has to be derived from the equation:
The coordinates in
Figure 6b,c are chosen so that the slope of each curve at each point means the reaction rate constant
ks.
Table 5 displays the best possible detected constant values of
ks before the surface reaction step is affected by the second – mass transfer step of the chemical reaction, [
13]. We prefer to sign units on
x,
y axes as mm*, s* because of non-dimensional multipliers.
Figure 6.
(a) Hydrogen reaction efficiency for non-uniform samples, (b) Reaction efficiency for uniform size samples.
Figure 6.
(a) Hydrogen reaction efficiency for non-uniform samples, (b) Reaction efficiency for uniform size samples.
In the
Table 5 we are comparing industrial white dross H
2 generated efficiency compared to pure Al reaction with water. We have reported efficiency close to 100% of aluminium waste from window frame scrap[
17]. Here for calculations, we have used Al density as 2700 g⸳cm
-3, initial particle radius is 0.06 mm, Al molar mass 27 g⸳mol
-1 and 1M NaOH electrolyte. As we can see the investigated samples as HDC barely reaches over 12%, which is consistent with the reaction rate and sample composition.
4. Discussion
Comparison to previously investigated samples indicates that these samples (SOW, RM, HDC) are with much lower efficiency in comparison to previously used and plasma treated alumina scrap samples. There are some experimental results on the composition of dross such as in works by [
18], where they reported dross content.
Their work was focused on electrochemical and various heating methods to produce Al-Si alloy and Brown Fused alumina, where electrolytic process seemed to be much more efficient than the conventional method. It is noteworthy that reported was not only the elemental composition but also crystalline such alumina, silica, titania, and other oxides [
18]. On the other hand, David and Kopac work focused on milling dross for hydrogen production via Al-water reaction, where they reported only elemental composition of dross. They reported higher hydrogen generation values after ball milling the material and ascribed the increase to elements such as Ni, Zn, Mg as reason as those can also release hydrogen via reaction with NaOH and water [
19]. A in-depth summary of various hydrogen producing materials such as Mg, MgCo, Ni
50Al
50 and others produced hydrogen, this work encompasses various use-cases for Al-water reaction [
20]. Contrary to results reported by Zhao et al. where Ca presence was correlated to lower hydrogen yield [
21], though Kup Aylikci et al. investigated shredded cans and compared to Al foam with impurities as Mg, Ti and others, investigated 1N and 2N NaOH electrolytes with added voltage to promote hydrogen production; contrary to our previous findings [
17] they didn’t see temperature influence on the production rate. In addition, the maximum yield was reached at 5V and 2N NaOH, 750 mL from 2 g of aluminium. They noted that impurities as Mg
2+ and Ca
2+ ions hinder hydrogen generation due to the affinity to OH
- ions [
22]. Thus, the influence of Mg and Ca ions is not clear. At the same time we have seen that other Mg and Ni containing materials can generate hydrogen via water reaction [
23]. Alviani et al. investigated hydrogen production in acidic environment pH ~1 and elevated temperature, the environment of hot spring contains majority Cl
- ions. In this work Al cutting chips (pure Al, size 5-30 mm length and 0.05 – 0.15 mm thickness) and Al black dross (Al content up to 23.5 wt% with particle size around 63 μm) was used. Reaction times from 6 to 144 hours, respectively. They reported efficiency up to 100% of theoretical hydrogen generation within the experiment time [
24]. Thus, it is clear that the generated amount of hydrogen will depend on the type of dross, as similarly was shown by Elsarrag et al. where landfilled dross is compared to secondary aluminium production dross, respectively produced 30% and 40% of theoretical possible value considering Al content. They also compared to other works, though, taking into consideration various primary aluminium scrap as cans. The by-product of reaction included crystalline structures as Na
5Al
3F
14, Na
3AlF
6, Na
2Ca
3Al
2F
14 Al
2O
3 for both materials at different ratios. After the Al-water reaction by-product of reaction this dross produced α and γ aluminium hydroxide as well as some leftover Na
3AlF
6 and alumina [
25]. A summary of elemental composition of investigated dross is seen in
Table 6 compared to other works.
Composition of dross is directly dependent on the production cycle step that dross is created as shown by Raabe et al. in an extensive investigation of alloy recycling pointing out there can be a decreasing Al content from 90% to under 20% depending on the location of dross collection, as well as extensive explanation on the sources of various elements [
26]. It is important to reiterate that as the dross can be collected from various processes and industries, primary and secondary aluminium production, it will have substantial differences in elemental and crystalline composition; thus, potential of generated H
2 will vary. Even though there is an alternative use for dross such as shown by Dangtungee et al. .investigating dross as plant fertilizers it still involved treatment with acids [
27].
We see that investigation of hydrogen generation from dross as well as in-depth analysis of dross has not been done as widely as pure aluminium investigation, even though there is a technical and economic incentive to pursue novel options of dross utilization.
The activity of reaction stems from the composition and content of investigated samples in addition to final use of generated gas; some dross produced pure hydrogen, on the other hand this work identified other gasses as methane, nitrogen, CO2 which points to different end use. During TG analysis we see multiple melting peaks indicating various components that are present in the sample; no methane was identified /detected during the heating and TG analysis. On the other hand, samples produce methane in the beginning of the hydrolysis reaction.
The hydrogen produced is classified as green hydrogen and depending on the dross type it could be used for electricity production or can find other use cases. But the reaction parameters have to be adjusted for the final goal, such as alkali concentration, as our previous investigation shows concentration playing a crucial role in the reaction rate [
17] as well as temperature.
5. Conclusions
In this work three dross samples from primary aluminium production were investigated and tested for hydrogen production via direct hydrolysis with low concentration alkali solution. Produced gas analysis was performed and reaction kinetics estimated. The results are compared to literature data and industry expected content of samples. It was shown that majority of composition is somewhat coinciding with literature, but it is noteworthy that the composition of dross will strongly depend on the industry, process and stage of production. It was identified that hydrolysis reaction generated methane, thus, dismissing the use of dross for electricity generation via PEM FC, but could be used with other FC, for example SOFC which requires large amount of heat, but that is not a problem for aluminium industry and smelters.
Even though investigated dross samples have lower reaction efficiency compared to high Al content waste from window frame - the option to produce green hydrogen show promise for material that conventional utilization is highly complicated and energy demanding to recycle.
It’s important to further investigate the use of aluminium dross for water-dross reaction as it is not fully clear what are the yields of such interactions or the impurity influence as literature provides not only contrary models but the composition vary.
Supplementary Materials
Not applicable.
Author Contributions
Conceptualization, J.K. and A.K.; methodology, S.V. and G.G.; validation, D.M., J.K. and C.R.; formal analysis, A.M. and A.K.; investigation, S.V. and M.U; resources, R.M.; writing—original draft preparation, A.K.; writing—review and editing, A.M., S.V; visualization, A.K.; supervision, J.K.; project administration, A.K.; funding acquisition, A.K., J.K., S.V., G.G. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
Please add: “The authors acknowledge financial support from the Baltic Research Programme project No. EEA-RESEARCH-92 “Aluminium in circle economy - from waste through hydrogen energy to alumina” – AliCE-Why” under the EEA Grant of Iceland, Liechtenstein and Norway (No. EEZ/BPP/VIAA/2021/5)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data reported in this article is available upon request.
Acknowledgments
The authors acknowledge experimental involvement of students and technicians by Peteris Lesnicenoks, Roberts Palmbahs, Raitis Kaspars Sika, Laimonis Jekabsons.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Supporting S, Resource M U.S. Geological Survey Mineral Resources Program— 2007,. Program.
- Capuzzi S, Timelli G Preparation and melting of scrap in aluminum recycling: A review 2018,. Metals (Basel). 8:249.
- Blomberg J, Söderholm P The economics of secondary aluminium supply: An econometric analysis based on European data 2009,. Resour Conserv Recycl Volume 53, pp. 455–463. [CrossRef]
- John A. S. Green Aluminum Recycling and Processing for Energy Conservation and Sustainability 2007,.
- Padamata SK, Yasinskiy A, Polyakov P A Review of Secondary Aluminum Production and Its Byproducts 2021,. JOM 73:2603–2614.
- Bell S, Davis B, Javaid A, Essadiqi E Final Report on Refining Technologies of Aluminum Enhanced Recycling, Action Plan 2000 on Climate Change, Minerals and Metals Program-The Government of Canada Action Plan 2000 on Climate Change Minerals and Metals Program, managed by the Minerals and Meta 2003.
- Smith YR, Nagel JR, Rajamani RK Eddy current separation for recovery of non-ferrous metallic particles: A comprehensive review 2019,. Miner. Eng. 133:149–159.
- Schlesinger ME Aluminum Recycling 2006,. CRC Press.
- Mesina MB, De Jong TPR, Dalmijn WL Improvements in separation of non-ferrous scrap metals using an electromagnetic sensor 2003,. Phys Sep Sci Eng Volume 12, pp. 87–101. [CrossRef]
- Coates G, Rahimifard S Modelling of post-fragmentation waste stream processing within UK shredder facilities 2009,. Waste Manag Volume 29, pp. 44–53. [CrossRef]
- Venkoba Rao B, Kapur PC, Konnur R Modeling the size-density partition surface of dense-medium separators 2003,. Int J Miner Process Volume 72, pp. 443–453. [CrossRef]
- Hiraki T, Takeuchi M, Hisa M, Akiyama T Hydrogen production from waste aluminum at different temperatures, with LCA 2005,. Mater Trans Volume 46, pp. 1052–1057. 1057. [CrossRef]
- Mezulis A, Richter C, Lesnicenoks P, et al. Studies on Water–Aluminum Scrap Reaction Kinetics in Two Steps and the Efficiency of Green Hydrogen Production 2023,. Energies Volume 16, pp. 5554. [CrossRef]
- Ingason HT, Sigfusson TI Processing of Aluminum Dross: The Birth of a Closed Industrial Process 2014,. Jom Volume 66, pp. 2235–2242. [CrossRef]
- Cheng X, Shi Z, Glass N, et al. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation 2007,. J. Power Sources 165:739–756.
- Hiraki T, Takeuchi M, Hisa M, Akiyama T Hydrogen production from waste aluminum at different temperatures, with LCA 2005,. Mater Trans Volume 46, pp. 1052–1057. [CrossRef]
- Urbonavicius M, Varnagiris S, Mezulis A, et al. Hydrogen from industrial aluminium scraps: Hydrolysis under various conditions, modelling of pH behaviour and analysis of reaction by-product 2024,. Int J Hydrogen Energy Volume 50, pp. 431–446. [CrossRef]
- Hong JP, Wang J, Chen HY, et al. Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina 2010,. Trans Nonferrous Met Soc China (English Ed Volume 20, pp. 2155–2161. [CrossRef]
- David E, Kopac J Hydrolysis of aluminum dross material to achieve zero hazardous waste 2012,. J Hazard Mater Volume 209–210, pp. 501–509. [CrossRef]
- Olivares-Ramirez JM, de Jesus AM, Jimenez-Sandoval O, Pless RC Hydrogen Generation by Treatment of Aluminium Metal with Aqueous Solutions: Procedures and Uses 2012,. In: Hydrogen Energy - Challenges and Perspectives. IntechOpen.
- Zhao Z, Chen X, Hao M Hydrogen generation by splitting water with Al-Ca alloy 2011,. Energy Volume 36, pp. 2782–2787. [CrossRef]
- Küp Aylikci N, Mert SO, Aylikci V, et al. Microhydrogen production with water splitting from daily used waste aluminum 2021,. Int J Hydrogen Energy Volume 46, pp. 28912–28924. [CrossRef]
- Varnagiris S, Urbonavicius M Hydrogen generation kinetics via hydrolysis of Mg2Ni and Mg2NiH4 powders 2021,. Int J Hydrogen Energy Volume 46, pp. 36323–36335. [CrossRef]
- Alviani VN, Hirano N, Watanabe N, et al. Local initiative hydrogen production by utilization of aluminum waste materials and natural acidic hot-spring water 2021,. Appl Energy Volume 293, pp. 116909. [CrossRef]
- Elsarrag E, Elhoweris A, Alhorr Y The production of hydrogen as an alternative energy carrier from aluminium waste 2017,. Energy Sustain Soc Volume 7, pp. 1–14. [CrossRef]
- Raabe D, Ponge D, Uggowitzer P, et al. Making sustainable aluminum by recycling scrap: The science of “dirty” alloys 2022,. Prog Mater Sci Volume 128, pp. 100947. [CrossRef]
- Dangtungee R, Vatcharakajon P, Techawinyutham L Aluminium dross neutralization and its application as plant fertilizer 2021,. In: Materials Today: Proceedings. Elsevier, pp 2420–2426.
Table 1.
Generated gas analysis from sample SOW, in the beginning of the reaction and at the end of the reaction.
Table 1.
Generated gas analysis from sample SOW, in the beginning of the reaction and at the end of the reaction.
| SOW gas analysis |
Beginning of reaction |
End of reaction |
| Argon |
0.0 |
0.0 |
| Carbon Dioxide |
0.1 |
0.0 |
| Ethane |
0.0 |
0.0 |
| Hydrogen |
97.4 |
99.6 |
| Methane |
0.2 |
0.2 |
| Nitrogen |
1.2 |
0.0 |
| Oxygen |
0.3 |
0.0 |
| Water |
0.7 |
0.1 |
Table 2.
Generated gas analysis from sample RM, in the beginning of the reaction and at the end of the reaction.
Table 2.
Generated gas analysis from sample RM, in the beginning of the reaction and at the end of the reaction.
| RM gas analysis |
Beginning of reaction |
End of reaction |
| Argon |
0.0 |
0.0 |
| Carbon Dioxide |
0.2 |
0.2 |
| Ethane |
0.0 |
0.0 |
| Hydrogen |
98.3 |
97.2 |
| Methane |
0.0 |
0.0 |
| Nitrogen |
0.4 |
0.5 |
| Oxygen |
0.1 |
0.2 |
| Water |
1.9 |
1.9 |
Table 3.
Generated gas analysis from sample RM, in the beginning of the reaction and at the end of the reaction.
Table 3.
Generated gas analysis from sample RM, in the beginning of the reaction and at the end of the reaction.
| HDC gas analysis |
Beginning of reaction |
End of reaction |
| Argon |
0.0 |
0.0 |
| Carbon Dioxide |
0.2 |
0.2 |
| Ethane |
0.0 |
0.1 |
| Hydrogen |
98.3 |
92.5 |
| Methane |
0.0 |
0.4 |
| Nitrogen |
0.4 |
5.2 |
| Oxygen |
0.1 |
1.0 |
| Water |
0.9 |
0.5 |
Table 4.
O/N gas analysis.
Table 4.
O/N gas analysis.
| Sample and measurement |
Oxygen |
Nitrogen |
| RM |
|
|
| 1 |
5.31 |
0.009 |
| 2 |
6.87 |
2.595 |
| 3 |
0.58 |
0.013 |
| SOW |
|
|
| 1 |
0.330 |
0.007 |
| 2 |
0.067 |
0.004 |
| 3 |
4.86 |
0.530 |
| HDC |
|
|
| 1 |
1.329 |
0.044 |
| 2 |
2.714 |
0.070 |
Table 5.
Rection rate coefficient as calculated from the activity graphs.
Table 5.
Rection rate coefficient as calculated from the activity graphs.
| Sample |
H2 generation (mL) |
Yield of the theoretical value (%) |
Reaction rate constant ks (mm/s) |
| RM |
224 |
65.7 |
0.001 |
| SOW |
61 |
24.9 |
0.000107 |
| HDC |
56 |
12.7 |
0.000229 |
Table 6.
Dross and Al composition comparison, quantitative and qualitative content analysis.
Table 6.
Dross and Al composition comparison, quantitative and qualitative content analysis.
| Source |
Al |
Cr |
Fe |
Ca |
Si |
Na |
K |
Cu |
Zn |
Ni |
Mg |
Ti |
Pb |
Sn |
Mn |
B |
C |
F |
O |
| [19] |
43.3 |
0.088 |
4.32 |
0.45 |
10.9 |
0.8 |
0.21 |
1.17 |
0.9 |
0.87 |
1.85 |
0.27 |
0.053 |
- |
0.2 |
- |
|
|
|
| [18] |
73.05 |
- |
0.91 |
2.79 |
7.13 |
5.78 |
|
|
|
|
9.26 |
1.08 |
|
|
|
|
|
|
|
| [24] |
56 |
12.7 |
0.000229 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| [25] |
11.65 |
|
0.18 |
1.51 |
0.08 |
17.96 |
0.05 |
- |
- |
- |
0.02 |
|
|
|
|
|
|
|
|
| [25] |
40.02 |
|
0.08 |
0.34 |
0.07 |
0.48 |
0.02 |
- |
- |
- |
0.86 |
|
|
|
|
|
|
|
|
| Al_w1 [17] |
94.3 |
- |
- |
|
|
|
|
|
|
|
0.6 |
|
|
|
|
|
5.0 |
|
0.1 |
| RM |
29.19 - 32.46 |
- |
- |
- |
- |
- |
Trace - 0.71 |
Trace - 0.1 |
- |
- |
- |
Trace - 0.28 |
- |
- |
- |
- |
Trace - 5.70 |
Trace - 1.98 |
13.19 - 57.76 |
| SOW |
19.11-48.20 |
- |
Trace - 0.29 |
Trace - 0.29 |
- |
4.75 - 18.96 |
- |
- |
- |
- |
- |
- |
- |
- |
|
|
|
19.03 - 53.76 |
7.58 – 27.74 |
| HDC |
58.81 |
|
trace |
0.24 |
29.20 |
|
|
trace |
|
|
1.66 |
trace |
|
- |
- |
- |
Trace - 28.32 |
8.42 - 16.60 |
1.67 - 4.23 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).