1. Introduction
Extracranial Pediatric Germ Cell tumors (eGCTs), a model of developmental tumorgenesis, are rare neoplasms, occurring in different sites and ages [
1]. According to data from the DKKR (Deutsches Kinderkrebsregister) from 1987 to 2011, the incidence rate is 4.8 per million (children aged < 15 years). For girls, the incidence is 5.3, and for boys, 4.4 per million [
2]. These tumors arise from primordial germ cells and are localized in the gonads or in extragonadal tissues along the body midline, both extra- and intracranially [
3,
4]. The preferred region of origin of eGCTs are the gonads (testis, ovary), sacrococcygeal region and mediastinum [
5]. eGCTs are classified according to Teilum in respect to their histological degree of embryonic differentiation (teratoma, embryonal carcinoma), extraembryonic differentiation (yolk sac tumor, chorionic carcinoma) and germinal differentiation (seminoma in male patients, dysgerminoma in female patients, and germinoma when occurring in the CNS) [
3]. Mixed forms with more than one histological subtype are possible, especially beyond childhood (ICD-11 - Mortality and Morbidity Statistics (who.int)).
Some risk factors have been suggested including genetic syndromes such as Swyer syndrome [
6,
7], Down syndrome [
8,
9,
10], Currarino syndrome defined by the triad of a presacral mass, sacral and anorectal congenital anomalies [
11], or Klinefelter syndrome [
12,
13]. Furthermore, the occurrence of eGCTs has been associated with non-syndromic defects as e.g. cryptorchidism, inguinal hernias, congenital heart defects and skeletal congenital anomalies [
14,
15]. A recent publication suggested that birth defects might only be related to eGCTs if related to syndromes (syndromic defects), but not for non-syndromic defects.
We analyzed cases of the German MAKEI 96/MAHO 98 registry providing data on eGCT. To identify potentially related risk on a broader range of syndromes we identified all cases with any reported syndrome in MAKEI/MAHO and compared their prevalence to that reported in Orphanet. Additionally, we identified cases with reported non-syndromic defects and addressed their overall potential risk for any eGCT and by specific tumor site using a German birth cohort. We performed an aggregated data analysis. The objective was to confirm or refuse already known risk factors regarding syndromes with or without associated congenital anomalies, to identify potentially new syndromes associated with eGCTs and to assess a potential role for non-syndromic defects.
2. Materials and Methods
The MAKEI 96 registry was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Heinrich-Heine-University, Düsseldorf (study number 837, decision of 22/08/1995). All participants provided their written informed consent. Informed consent was given by the respective legal guardians for affected children. All patients had an eGCT. In the patient data evaluated here, cases with an additional non-oncologic diagnosis (genetic syndromes or congenital anomalies) were assessed in detail.
2.1. Study Population
The MAKEI 96/MAHO 98 registry comprises a large data collection of children and adolescents with an eGCT (n = 2610). Patients were prospectively enrolled in the registry over a 20-year period. Data were collected in collaboration with centers of the Society for Pediatric Oncology and Hematology (GPOH). Between January 1996 and October 2017, a total of 2,610 patients with a newly diagnosed eGCT were prospectively registered by 62 pediatric oncology units in Germany, Austria and Switzerland. Presacral, sacral and coccygeal tumors were grouped together in the sacrococcygeal group. The presacral mass occurred as part of the Currarino syndrome.
In the current study all patients with documented associated genetic syndromes and congenital anomalies of the genitourinary tract, the heart, the digestive system, the musculoskeletal system and central nervous system were identified. During the initial survey following registration of patients, epidemiologic data were collected by the MAKEI 96/MAHO 98 registry. In case of incomplete reporting the hospitals were reminded until presumed complete reporting was achieved. Basic demographic data were also documented. The CRF included information on anatomical site of origin, symptoms, diagnosis, tumor markers, and histology. Furthermore, non-oncologic diagnoses such as underlying genetic syndromes, chronic diseases and congenital anomalies were documented.
2.2. Case Definitions
Syndromes categorized according to Orphanet in MAKEI/MAHO were identified. Verification of the diagnoses by molecular genetical analysis was beyond the scope of the MAKEI/MAHO registry. For analysis we used any syndrome irrespective of any potential additional congenital anomaly.
Diagnosis of congenital anomalies was based on clinical aspect, ultrasound, or CT/MRI imaging (in cases). In accordance with EUROCAT non-syndromic defects were grouped according to their localization [
16,
17,
18]. We additionally considered the associated type of tumor and potential syndrome.
2.3. Definition of Exposure
In accordance with Schraw et al. [
19] we distinguished effects of syndromes and isolated non-syndromic defects. In cases with an associated syndrome, we could not distinguish those potential additional non-syndromic defects because of the aggregated data structure regarding controls.
2.4. Reference Population
To assess the association with syndromes we used the respective prevalence of genetic syndromes in the general population as documented in Orphanet. The prevalence of genetic syndromes in the general population was extracted from the Orphanet database (
https://www.orpha.net/).
The prevalence of non-syndromic defects in the general population was estimated from the Mainz malformation registry with complete, extensive and standardized assessment of birth defects in the first week in of life including ultrasound of the urogenital system at birth. The data were extracted from the EUROCAT registry for children born between 1996 and 2017 (
http://www.eurocat-network.eu/accessprevalencedata/prevalencetables).
2.5. Statistical Analysis
Because the registries provided only aggregated data, case data were aggregated accordingly using excel files. These aggregated data were entered into stat calc to calculate the odds ratios and 95% confidence intervals. Stat calc is a public domain app sponsored and provided by CDC (
https://www.cdc.gov/epiinfo/user-guide/statcalc/statcalcintro.html). Stat calc also provides formal statistical testing using different methods. We used the p-values for Fisher’s exact test with additional Bonferroni correction for multiple testing regarding potential new syndromes associated with eGCTs. To assess whether the number of cases was sufficient to detect the observed effect size in previously reported associations with a power of at least 80%, we used the calculation of power with the power and simple size program (
https://vbiostatps.app.vumc.org/ps/) in a posthoc analysis.
3. Results
3.1. Overall Frequency of Registered eGCTs
The most frequent tumors in the total MAKEI 96/MAHO 98 registry sample of 2,610 patients at the time of analysis were tumors of the ovaries (47 %, n = 1,232), followed by those of the sacrococcygeal region (21 %, n = 540), the testis (17 %, n = 447) and the mediastinum (4 %, n = 114). A total of 277 tumors were found in other anatomical structures of the body midline (11 %), neck, eye or retroperitoneum. Of the 2,610 patients with an eGCT, 754 were male (28.9 %) and 1856 were female (71.1 %). In comparison, in the group of patients with an eGCT and an additional non-oncologic diagnosis, 44 were male (45.9 %) and 51 were female (54.1 %).
3.2. Location and Histology of eGCTs in Patients with Genetic Syndromes
Gonadal GCTs: 34 patients with genetic syndromes were found in combination with a gonadal GCT (26 of 1,232 ovarian GCTs, 7 of 447 testicular GCTs). 62 % of these 26 ovarian GCT were dysgerminomas, followed by gonadoblastomas (15 %). When analyzing the patients with a genetic syndrome and a testicular GCT, four (57 %) presented with mixed eGCTs. In addition, one case each of seminoma, embryonal carcinoma and embryonal carcinoma with a yolk sac tumor was detected.
Extragonadal GCTs: Nine patients with a tumor in the sacrococcygeal region (9 of 540 tumors of the sacrococcygeal region) have been documented. In the group of sacrococcygeal tumors, only teratomas were found, and one of them was combined with a yolk sac tumor. Three patients with a genetic syndrome had a mediastinal GCT (3 of 114) comprising one teratoma, one chorionic carcinoma and one mixed eGCT. Among patients with a tumor of the retroperitoneum, 3 patients had a genetic syndromic background (3 of 277) comprising Down syndrome, neurofibromatosis type 1, and one patient with intellectual disability, thoraco-lumbar scoliosis, and tethered cord. Among these, two patients had teratomas and one a mixed eGCT.
3.3. eGCT and Associated Genetic Syndromes
Of the 95 patients with an additional non-oncologic diagnosis, 48 had a syndrome. Most syndromes recorded in the MAKEI/MAHO registry could be linked to a specific tumor (
Table 1a), except for Down syndrome, which was found in connection with eGCT of different localizations. Most underlying genetic syndromes were found in the group of patients with an ovarian eGCT (n = 26). In terms of age, most germ cell tumors in patients with an additional syndrome were diagnosed in the prepubertal or pubertal period. Only in the group of sacrococcygeal GCTs the peak incidence was in the first year of life. The majority of eGCTs were malignant; only in patients with Currarino syndrome and eGCTs benign tumors predominated.
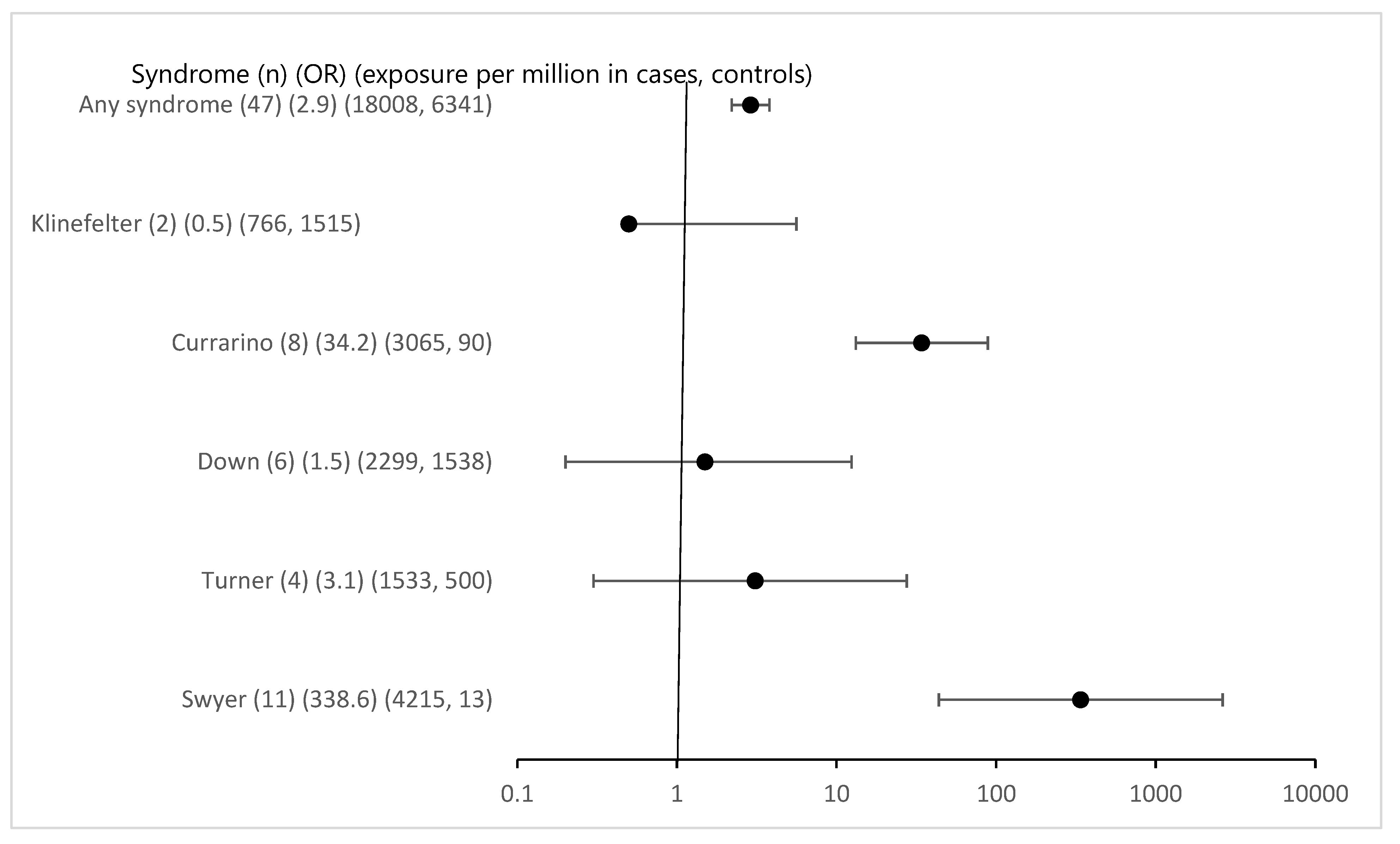
Overall associated syndromes were only rarely detected in the study group with eGCTs (1.8%). Regarding any syndrome registered in MAKEI 96/MAHO 98 the odds ratio was 2.9 (95% CI 2.2-3.8) (
Figure 1). The previously described correlations between Swyer and Currarino syndromes and the occurrence of eGCTs were confirmed. The strength of the effect was substantial with an OR for Swyer syndrome above 100 and 34 for Currarino syndrome respectively with 95% CI clearly excluding 1. For Down syndrome, the OR was 1.5 with a 95% CI including 1 (95% CI 0.2-12.4) despite a considerable number of cases. The power calculation in a posthoc analysis showed that the power to detect the observed effect with an alpha of < 0.05 was only 0.08. For Klinefelter and Turner syndromes [
20] we failed to identify a significant association [
21]. The effect estimates for the known syndromes did not require Bonferroni correction because of a priori hypothesis (
Figure 1).
Interestingly 17 new syndromes were found in children with eGCTs and all were female. However, these were only isolated cases, never more than one. Compared to the registry these yielded low p-values for Fisher’s exact and for some high OR which were driven by the extreme paucity of such syndromes in the general population. Following Bonferroni correction for multiple testing the respective p-values were clearly above the Bonferroni adjusted p-value of 0.00238 however (see
Supplementary File S1). A remarkable finding however is the fact that cases were confined to girls. While this is evident for the Triple X syndrome, the sex distribution of the other syndromes was either equal or slightly shifted to male or female patients (
https://www.orpha.net/).
3.4. eGCT and Associated Malformations
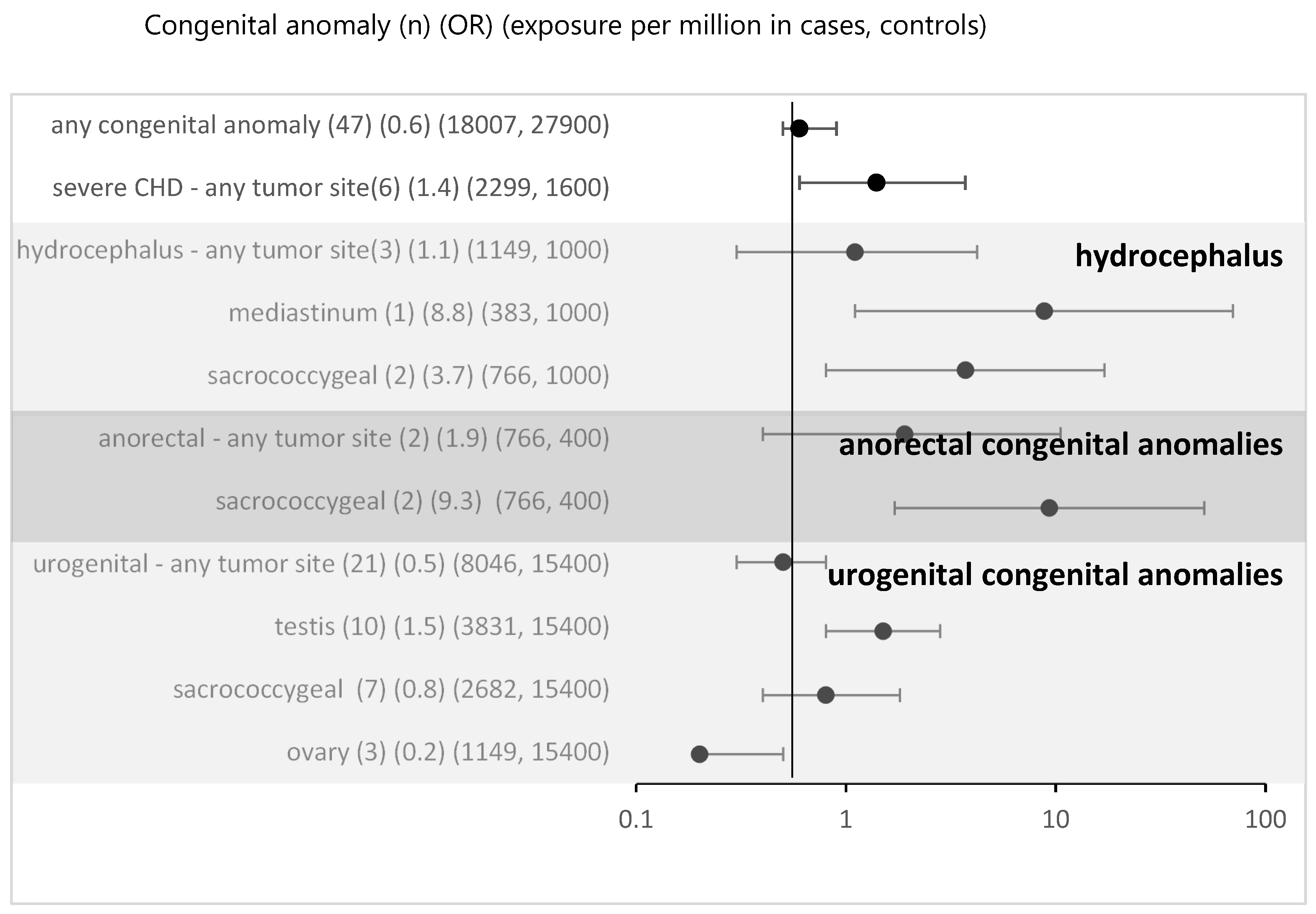
In the MAKEI 96/MAHO 98 registry 47 patients with a non-syndromic defect were recorded. Non-syndromic defects occurred in connection with eGCTs of all localizations. Most were described in the group of sacrococcygeal GCTs (n = 18) (
Table 1b). Urogenital malformations accounted for the largest proportion. The age distribution at the time of tumor diagnosis also showed a peak postnatally for the sacrococcygeal GCTs and prepubertal or pubertal for the other tumor localizations.
For all non-syndromic defects in the MAKEI/MAHO registry compared to the data from Mainz registry the OR was 0.6 (95% CI 0.5-0.9) (
Figure 2). Thus, an overall risk related to isolated congenital anomalies appears highly unlikely. To assess whether local vicinity of tumors and congenital anomalies might be related to a higher risk, we performed a subgroup analysis. For most subgroups there was no evidence and following Bonferroni correction (Bonferroni corrected p-value 0.005) none of the associations reached significance. Overall, no strong effect sizes were found.
4. Discussion
A main finding of our paper was the confirmation of the role of several syndromes accounting for the risk of an eGCT. We observed high OR both for Swyer and Currarino syndrome. Regarding non-syndromic defects no overall association was found. Subgroup analysis might suggest a hint to a potential role for non-syndromic defects close to the tumor site.
The identification of risk factors requires a control population and cautious interpretation of statistical findings. In the literature few papers used a control group describing potential associations of syndromes and later emergence of eGCTs [
39,
40,
41,
42,
43]. Whereas some previous papers only reported associations between tumor and syndrome in cases [
44,
45,
46,
47], a recent paper with a control group allowing for individual case control analysis with adjustment confirmed a strong statistical association for Klinefelter with comparison to controls, but not for Turner and Down syndrome [
19]. Our data confirmed the latter. Regarding our data the estimate for Klinefelter syndrome was likely, however, to reflect diagnostic bias. Unlike in other registers [
19] no systematic screening for Klinefelter was performed in our MAKEI/MAHO patients. Similarly, no systematic screening for Turner syndrome was performed in eGCT cases in MAKEI/MAHO. As many of the eGCTs occur prepubertal or in early puberty such cases may well be missed because of the later occurrence of the lead symptoms. Down syndrome in contrast is most likely to be diagnosed early in life. Additionally, some biological probability for causality was described in literature. The result of a study by Cools et al. (2006) was a longer expression of OCT3/4, PLAP, and c-KIT, biomarkers for eGCT, in male fetuses with trisomy 21 explaining the connection between testicular eGCT and Down syndrome [
48]. The estimate for the strength of the effect, however, was low (OR 1.5) with wide 95% CI clearly including one. In a posthoc power analysis we found that with the observed case numbers the power to detect such a small effect size was only very low (0.08).
Regarding the presumed new syndromes our data point to the need for a very careful interpretation of statistics in case of isolated findings of syndromes in eGCT cases. Although effect estimates based on comparison with control groups may appear huge, the risk is far from proven. In case of many “new associations” it is important to perform at least a Bonferroni correction. On the other hand, a causative association is difficult to rule out, especially when only isolated cases are identified. Although a role for the individual syndromes could not be established, confinement to girls is striking. Among patients with gonadal eGCT, associations with genetic syndromes based on disorders of sexual development have been described previously. About one in 4,500 births (0.02 %) is affected by a disorder of sexual development (17). Several publications describe an increased risk for developing an eGCT in individuals with gonadal dysgenesis or in patients with Turner syndrome and Y chromosomal material [
6,
49,
50,
51,
52,
53,
54]. The MAKEI 96/MAHO 98 registry comprised 18 patients with gonadal dysgenesis who had an underlying genetic syndrome (0.69 %). Among published patients with disorders of sexual development, gonadoblastoma are diagnosed in 4.7-25 % of cases. Of these, 50 % develop a dysgerminoma [
14,
55,
56,
57]. These histology types were prominent in the 18 patients of the MAKEI 96/MAHO 98 registry (15 of 18 patients with a pure gonadoblastoma, 11 of 18 patients with gonadoblastoma and dysgerminoma). eGCTs most commonly occur in gonadal dysgenesis in association with Swyer syndrome [
58]. Various molecular biomarkers such as miR-371a-3p were discovered in patients with germ cell tumors and disorders of sexual development [
59].
Mutations of the MNX1 gene have been found in patients with Currarino syndrome, and further molecular genetic correlations are being sought [
60].
For non-syndromic defects we failed to identify an increased risk for eGCTs in accordance with Schraw et al [
19]. A subgroup analysis linking tumor site and congenital anomaly did not yield significant results either.
Confirmation of high risks for Currarino and Swyer syndrome might suggest a cause for screening in these syndromes, because in children with these syndromes the a priori probability of GCTs might be high enough to allow for screening with acceptable positive predictive values. Unfortunately, however most cases of Currarino syndrome occur in the first year of life, when the test is not specific. For Swyer syndrome with pure dysgerminomas a screening is not useful, because of the absence of known tumor markers such as AFP and beta-HCG.
4.1. Strengths and Limitations
The MAKEI 96/MAHO 98 registry is very complete regarding the recording of eGCT.
Beyond the presumed complete eGCT registration for Germany in MAKEI 96/MAHO 98 and comparison to a standard control group (Orphanet) the strength of our paper pertains to statistical analysis. We provide the mandatory Bonferroni correction, if needed, and show that an apparently strong effect suggested for one case only in extremely rare conditions may well be spurious. Such associations should only be discussed in case of a possible causative pathways. Regarding the congenital anomalies the Mainz registry, like EUROCAT aims to provide essential epidemiological information on the birth prevalence of congenital malformations and anomalies in Europe [
18,
19]. The strength of the Mainz registry, however, is a complete case ascertainment and a structured clinical assessment and ultrasound screening for the entire cohort. Therefore, the Mainz registry provides a unique, valid, comparator to presumed complete congenital anomaly assessment in the cases. The latter is likely because all the children with eGCT undergo extensive screening for metastasis.
As with all registries there is an issue about diagnostic and an ascertainment bias. Regarding the cases this would account for underestimation of the effect sizes. As outlined for specific syndromes as Turner and Klinefelter syndrome may be very plausible reasons for diagnostic bias. For other syndromes and non-syndromic defects this may be less evident, however, were possible.
Previous articles on the association of an eGCT and an additional non-oncologic diagnosis were based on small data sets or case reports. As the proportion of diagnosed eGCTs increased over the observed period, we assume that this could be due to the improved diagnostic capabilities that developed over time. Still, our study has several limitations. Compared to today, fewer predispositions to tumors were known at the beginning of data collection. Not all co-occurring phenotypic features among other patients registered in the MAKEI 96/MAHO 98 registry have led to the diagnosis of a genetic syndrome. Any errors in diagnosis or coding may adversely affect the accuracy of the presented results. Furthermore, underreporting may be an issue as patients with sacrococcygeal eGCTs in particular those who undergo postnatal surgery in smaller hospitals might not been registered in the MAKEI 96/MAHO 98 registry. In addition, potential candidates for the registry may have died from pathologies due to the genetic syndrome or congenital anomalies before the eGCT could be diagnosed and registered. An evaluation of the success of the therapy in the reported cases was not part of this paper. In the eGCTs of the sacrococcygeal region, tumors with different relationships to the os sacrum and os coccyx were combined without considering the migration of the primordial germ cells. Further, only one leading syndromic abnormality has been documented per case. Regarding congenital anomalies, one case may be recorded for different anomalies, also affecting the statistical results. Patients who were diagnosed with eGCT and congenital anomaly shortly after birth were also included. No data was collected on premature births as a possible cause of the anomaly. Possible overlaps between the data sets of MAKEI 96/MAHO 98 registry, EUROCAT and the Mainz registry cannot be ruled out with certainty.
Unfortunately, we could not access the entire Mainz data to allow for individual case statistic analysis, because the registration in the Mainz registry has been closed and the involved persons have retired. This is why we could only use the Mainz registry as reported in EUROCAT.
5. Conclusions
In our study we confirmed an association between Swyer and Currarino syndrome and eGCTs with high effect sizes. There is biological plausibility for these associations. Our failure to confirm an association between Klinefelter and Turner syndrome and eGCTs might be due to probable diagnostic bias of these conditions in cases. The presumed size of the association of Down syndrome with eGCT appeared to be small and thus could not be identified with the available case numbers. Case reports of associations of rare syndromes in children with eGCT are most likely to be caused by chance. Considering non-syndromic defects, no association was shown in relation to eGCTs.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Odds ratio and 95% confidence intervals for eGCT in patients with syndromes.
Author Contributions
Conceptualization, Judit Schultewolter, Rüdiger Von Kries, Heiko Reutter and Gabriele Calaminus; Data curation, Anke K. Rißmann, Dietrich Von Schweinitz, Michael Frühwald, Claudia Blattmann, Lars Fischer, Bjoern Lange, Rüdiger Wessalowski, Birgit Fröhlich, Wolfgang Behnisch, Irene Schmid, Harald Reinhard, Matthias Duerken, Patrick Hundsdoerfer, Martin Heimbrodt, Christian Vokuhl, Stefan Schönberger, Dominik T. Schneider, Guido Seitz, Ulrich Göbel and Gabriele Calaminus; Formal analysis, Judit Schultewolter and Rüdiger Von Kries; Investigation, Judit Schultewolter; Writing – original draft, Judit Schultewolter and Rüdiger Von Kries; Writing – review & editing, Judit Schultewolter, Anke K. Rißmann, Dietrich Von Schweinitz, Michael Frühwald, Claudia Blattmann, Rüdiger Wessalowski, Wolfgang Behnisch, Irene Schmid, Harald Reinhard, Martin Heimbrodt, Stefan Schönberger, Dominik T. Schneider, Guido Seitz, Ulrich Göbel, Rüdiger Von Kries, Heiko Reutter and Gabriele Calaminus.
Funding
This research received no external funding.
Institutional Review Board Statement
The MAKEI 96 registry was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Heinrich-Heine-University, Düsseldorf (study number 837, decision of 22/08/1995).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper. Informed consent was given by the respective legal guardians for affected children.
Data Availability Statement
Acknowledgments
MAKEI 96 was supported by the German Cancer Aid. We acknowledge the Deutsche Forschungsgemeinschaft and the Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding program “Open Access Publication Funding”. Open Access funding enabled and organized by Projekt DEAL. We thank all the patients and their families who participated in the study, as well as all teams of all participating centers for their dedicated effort. We would particularly like to thank A. Wiesel for providing the data of Mainz registry and the data managers Carmen Teske and Jans-Enno Müller of the MAKEI group in Bonn.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lobo J, Gillis AJM, Jerónimo C, Henrique R, Looijenga LHJ. Human Germ Cell Tumors are Developmental Cancers: Impact of Epigenetics on Pathobiology and Clinic. International journal of molecular sciences 2019; 20 (2). [CrossRef]
- Kaatsch P, Häfner C, Calaminus G, Blettner M, Tulla M. Pediatric germ cell tumors from 1987 to 2011: Incidence rates, time trends, and survival. Pediatrics 2015;135 (1), e136-43. [CrossRef]
- Teilum G. Classification of endodermal sinus tumour (mesoblastoma vitellinum) and so-called “embryonal carcinoma” of the ovary. Acta Pathologica Microbiologica Scandinavica 1965. 64(4), 407-29. [CrossRef]
- Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: Old concepts, new perspectives. International Journal of Andrology 2007;30(4):256–64. [CrossRef]
- Schneider DT, Calaminus G, Koch S, Teske C, Schmidt P, Haas RJ et al. Epidemiologic analysis of 1,442 children and adolescents registered in the German germ cell tumor protocols. Pediatric Blood & Cancer 2004;42(2):169-75. [CrossRef]
- Swyer GIM. Male Pseudohermaphroditism: A Hitherto Undescribed Form. British Medical Journal 1955; 2(4941): 709-12. [CrossRef]
- Matsumoto F, Shimada K, Ida, S. Tumors of Bilateral Streak Gonads in Patients with Disorders of Sex Development Containing Y Chromosome Material. Clinical Pediatric Endocrinology 2014;23(3), 93–7. [CrossRef]
- Down JL. Observations on an ethnic classification of idiots. 1866. Mental Retardation 1995; 33: 54–6.
- Hasle H. Pattern of malignant disorders in individuals with Down’s syndrome. The Lancet Oncology 2001;2 (7), 429–36. [CrossRef]
- Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet 2000;355: 165–69. [CrossRef]
- Currarino G, Coln D, Votteler T. Triad of anorectal, sacral, and presacral anomalies. AJR. American journal of roentgenology 1981;137 (2), 395–8. [CrossRef]
- Klinefelter HF, Reifenstein EC, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without a-Leydigism, and increased excretion of follicle-stimulating hormone. Journal of Clinical Endocrinology and Metabolism 1942;2:615–27. [CrossRef]
- Sogge MR, McDonald SD, Cofold B. The malignant potential of the dysgenetic germ cell in Klinefelter’s syndrome. The American journal of medicine 1979;66 (3), 515-8. [CrossRef]
- Verp MS, Simpson JL. Abnormal Sexual Differentiation and Neoplasia. Cancer Genetics and Cytogenetics 1987;25:191-218. [CrossRef]
- Nishi M, Miyake H, Takeda T, Hatae Y. Congenital Malformations and Childhood Cancer. Medical and pediatric oncology 2000;34:250–4. [CrossRef]
- Greenlees R, Neville A, Addor MC, Amar E, Arriola L, Bakker M et al. Paper 6: EUROCAT Member Registries: Organization and Activities. Birth Defects Research (Part A): Clinical and Molecular Teratology 2011;91:S51-S100. [CrossRef]
- Tucker FD, Morris JK, on behalf of the JRC Management Committee; Neville A, Garne E, Kinsner-Ovaskainen A, Lanzoni, M et al. EUROCAT: An update on its functions and activities. Journal of Community Genetics 2018;Oct;9(4):407-10. [CrossRef]
- EUROCAT (2013). EUROCAT Guide 1.4: Instruction for the registration of congenital anomalies. EUROCAT Central Registry, University of Ulster.
- Schraw JM, Sok P, Desrosiers TA, Janitz AE, Langlois PH, Canfield MA et al. Associations between birth defects and childhood and adolescent germ cell tumors according to sex, histologic subtype, and site. Cancer 2023;129(20):3300-8. Epub 2023 Jun 27. [CrossRef]
- Turner HH. A syndrome of infantilism, congenital webbed neck and cubitus valgus. Endocrinology 1938;23:566-74. [CrossRef]
- Schneider DT, Schuster AE, Fritsch MK, Calaminus G, Göbel U, Harms D et al. Genetic analysis of mediastinal nonseminomatous germ cell tumors in children and adolescents. Genes, chromosomes and cancer 2002;34:115-25. [CrossRef]
- Hirschhorn K, Cooper HL, Firschein IL. Deletion of short arms of chromosome 4-5 in a child with defects of midline fusion. Humangenetik 1965;1(5):479-82. [CrossRef]
- Caffey J. Prenatal bowing and thickening of tubular bones, with multiple cutaneous dimples in arms and legs; a congenital syndrome of mechanical origin. American journal of diseases of children 1947;74(5):543-62. [CrossRef]
- Capito C, Leclair MD, Arnaud A, David A, Baron S, Corradini N, Héloury Y. 46, XY pure gonadal dysgenesis: Clinical presentations and management of the tumor risk. Journal of pediatric urologiy 2011; 7(1):72-5. [CrossRef]
- Cost NG, Ludwig AT, Wilcox DT, Rakheja D, Steinberg SJ, Baker LA. A novel SOX9 mutation, 972delC, causes 46, XY sex-reversed campomelic dysplasia with nephrocalcinosis, urolithiasis, and dysgerminoma. Journal of pediatric surgery 2009;44(2):451-4. [CrossRef]
- Lloyd KM, Dennis M. Cowden’s disease. A possible new symptom complex with multiple system involvement. Annals of internal medicine 1963;58:136-42. [CrossRef]
- Denys P, Malvaux P, Van Den Berghe H, Tanghe W, Proesmans W. Association of an anatomo-pathological syndrome of male pseudohermaphroditism, Wilms' tumor, parenchymatous nephropathy and XX/XY mosaicism. Archives francaises de pediatrie 1967;24:729–39.
- Hersmus R, van Bever Y, Wolffenbuttel KP, Biermann K, Cools M, Looijenga LH. The biology of germ cell tumors in disorders of sex development. Clinical genetics 2017;91 (2), 292–301. [CrossRef]
- Frasier SD, Bashore RA, Mosier HD. Gonadoblastoma associated with pure gonadal dysgenesis in monozygous twins. Journal of Pediatrics 1964;64, 740–5. [CrossRef]
- Imerslund O. Idiopathic chronic megaloblastic anemia in children. Acta Paediatrica. Supplementum 1960;49:1–115.
- Lennox WG, Davis JP. Clinical correlates of the fast and the slow spike-wave electroencephalogram. Pediatrics 1950;5(4):626-44.
- Noonan JA, Ehmke DA. Associated non-cardiac malformations in children with congenital heart disease. Journal of pediatrics 1963; 63:469.
- Poland A. Deficiency of the pectoral muscles. Guy’s hospital reports 1841;6:191-3.
- Rett A. Über ein eigenartiges hirnatrophisches Syndrom bei Hyperammonämie im Kindesalter. Wiener medizinische Wochenschrift 1966;116(37):723–6.
- Sturge WA. A case of partial epilepsy apparently due to lesion of one of the vasomotor centres of the brain. Transactions of the clinical society of London 1979;12:162.
- West, WJ. On a peculiar form of infantile convulsions. The Lancet 1841;724-5. [CrossRef]
- De Lange C. Sur une type nouveau de dégénération (typus Amstelodamensis). Archives de médecine des enfants 1933;713-9.
- Cohen MM, Hayden PW. A newly recognized hamartomatous syndrome. Birth defects original article series 1979;15(5B):291-6.
- Johnson KJ, Ross JA, Poynter JN, Linabery AM, Robison LL, Shu XO. Paediatric germ cell tumours and congenital abnormalities: A Children’s Oncology Group study. British Journal of Cancer 2009 Aug 4;101(3):518-21. [CrossRef]
- Johnson KJ, Lee JM, Ahsan K, Padda H, Feng Q, Partap S et al. Pediatric cancer risk in association with birth defects: A systematic review. PLoS ONE 2017 Jul 27;12(7):e0181246. [CrossRef]
- Botto LD, Flood T, Little J, Fluchel MN, Krikov S, Feldkamp ML, Wu Y, Goedken R, Puzhankara S, Romitti PA. Cancer risk in children and adolescents with birth defects: A population-based cohort study. PLoS ONE. 2013 Jul 17;8(7):e69077. [CrossRef]
- Altmann AE, Halliday JL, Giles GG. Associations between congenital malformations and childhood cancer. A register-based case-control study. British Journal of Cancer 1998 Nov;78(9):1244-9. [CrossRef]
- Carozza SE, Langlois PH, Miller EA, Canfield M. Are children with birth defects at higher risk of childhood cancers? American Journal of Epidemiology 2012;175(12):1217-24. [CrossRef]
- Zhu HL, Bao DM, Wang Y, Shen DH, Li Y, Cui H. Swyer's Syndrome with Mixed Ovarian Malignant Germ Cell Tumor and Ovarian Gonadoblastoma. Chinese Medical Journal (English) 2016 Jul 20;129(14):1752-4. [CrossRef]
- Satgé D, Sasco AJ, Curé H, Leduc B, Sommelet D, Vekemans MJ. An excess of testicular germ cell tumors in Down's syndrome: Three case reports and a review of the literature. Cancer 1997 Sep 1;80(5):929-35.
- Rod J, Cretolle C, Faivre L, Jacquot C, Yacoub O, Ravasse P et al. Malignant transformation of presacral mass in Currarino syndrome. Pediatric Blood & Cancer 2019 Jun;66(6):e27659. [CrossRef]
- Bonouvrie K, van der Werff Ten Bosch J, van den Akker M. Klinefelter syndrome and germ cell tumors: Review of the literature. International Journal of Pediatric Endocrinology 2020;2020:18. [CrossRef]
- Cools M, Honecker F, Stoop H, Veltman JD, De Krijger RR, Steyerberg E et al. Maturation delay of germ cells in fetuses with trisomy 21 results in increased risk for the development of testicular germ cell tumors. Human Pathology 2006;37(1): 101-11. [CrossRef]
- Hughes IA, Houk C, Ahmed SF, Lee PA. Consensus statement on management of intersex disorders. Archives of disease in childhood 2006;91(7): 554-63. [CrossRef]
- Abel R. Ein Fall von Pseudohermaphroditismus masculinus mit sarcomatöser Cryptorchis sinistra. Archiv f. pathol. Anat. 1891;126 (3), 420–37.
- Bispo AVS, Burégio-Frota P, dos Santos LO, Ferraz Leal G, Rezende Duarte A, Araújo J et al. Y chromosome in Turner syndrome: Detection of hidden mosaicism and the report of a rare X;Y translocation case. Reproduction, Fertility and Development 2014;26, 1176–82. [CrossRef]
- Matsumoto F, Matsuyama S, Matsui F, Yazawa K, Matsuoka K. Variation of Gonadal Dysgenesis and Tumor Risk in Patients With 45,X/46, XY Mosaicism. Pediatric Urology 2020;137:157-60. [CrossRef]
- Piazza MJ, Urbanetz AA. Germ Cell Tumors in Dysgenetic Gonads. Clinics 2019;74: e408. [CrossRef]
- Hennes E, Zahn S, Lopes LF, Schönberger S, Leuschner I, Göbel U et al. Molecular genetic analysis of bilateral ovarian germ cell tumors. Klinische Pädiatrie 2012. 224(6): 359-65. [CrossRef]
- Kathrins M, Kolon TF. Malignancy in disorders of sex development. Translational Andrology and Urology 2016;5(5): 794-8. [CrossRef]
- Pleskacova J, Hersmus R, Oosterhuis JW, Setyawati BA, Faradz SM, Cools M. et al. Tumor risk in disorders of sex development. Sexual development: Genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation 2010;4 (4-5), 259–69. [CrossRef]
- Cools M, Drop SLS, Wolffenbuttel KP, Oosterhuis JW, Looijenga LH. Germ Cell Tumors in the Intersex Gonad: Old Paths, New Directions, Moving Frontiers. Endocrine Reviews 2006;27(5):468–84. [CrossRef]
- Pyle LC, Nathanson KL. A practical guide for evaluating gonadal germ cell tumor predisposition in differences of sex development. American Journal of Medical Genetics. Part C Seminars in Medical Genetics 2017;175(2): 304–14. [CrossRef]
- Looijenga LHJ, Kao C-S, Idrees MT. Predicting gonadal germ cell cancer in people with disorders of sex development; insights from developmental biology. International Journal of Molecular Sciences 2019;10; 20(20): 5017. [CrossRef]
- Dworschak GC, Reutter HM, Ludwig M. Currarino syndrome: A comprehensive genetic review of a rare congenital disorder. Orphanet journal of rare diseases 2021;16(1):167. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).