Submitted:
23 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
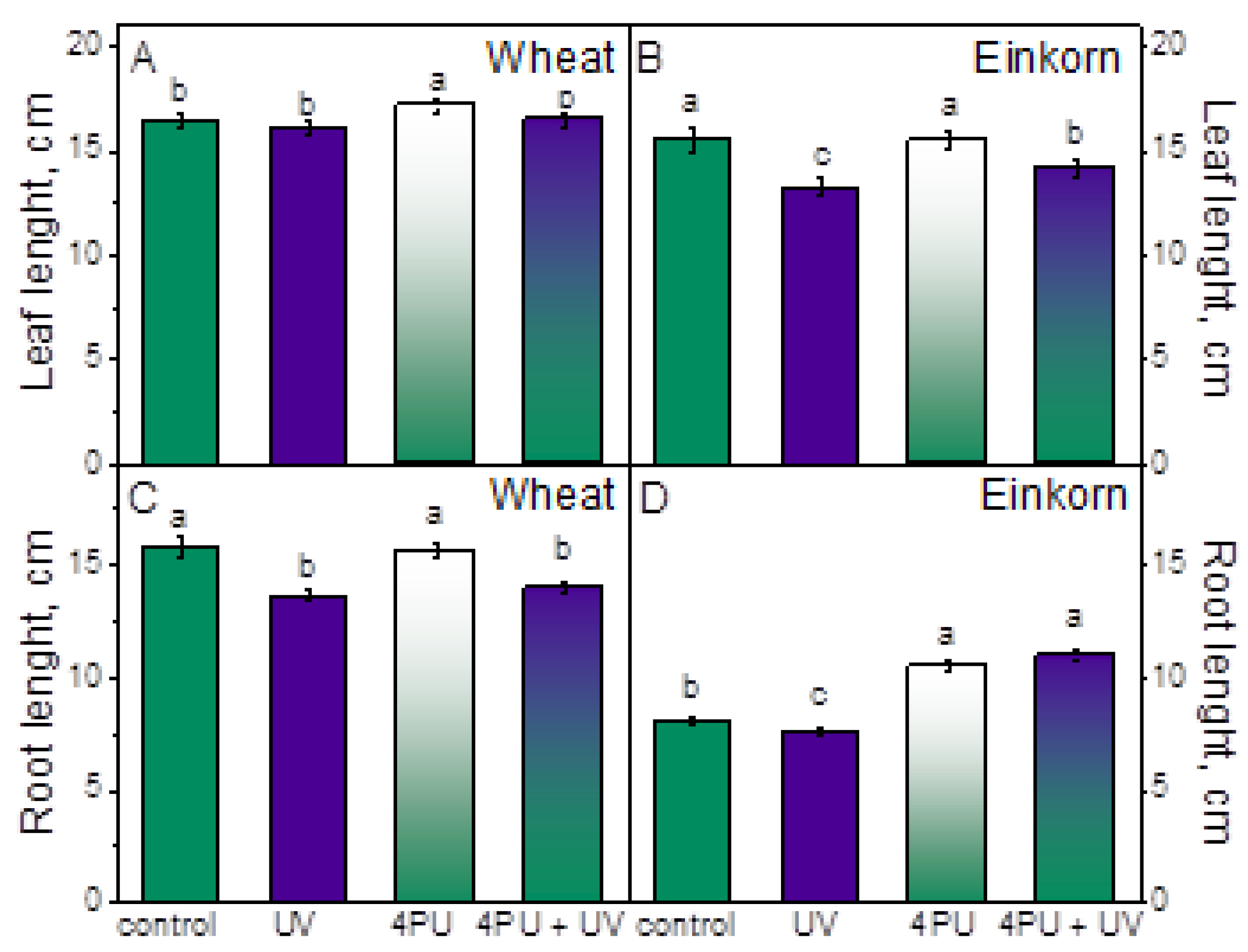
2.1. Effect of Cytokinin Priming and Subsequent UV-B Treatment on Growth Parameters in Wheat and Einkorn Seedlings
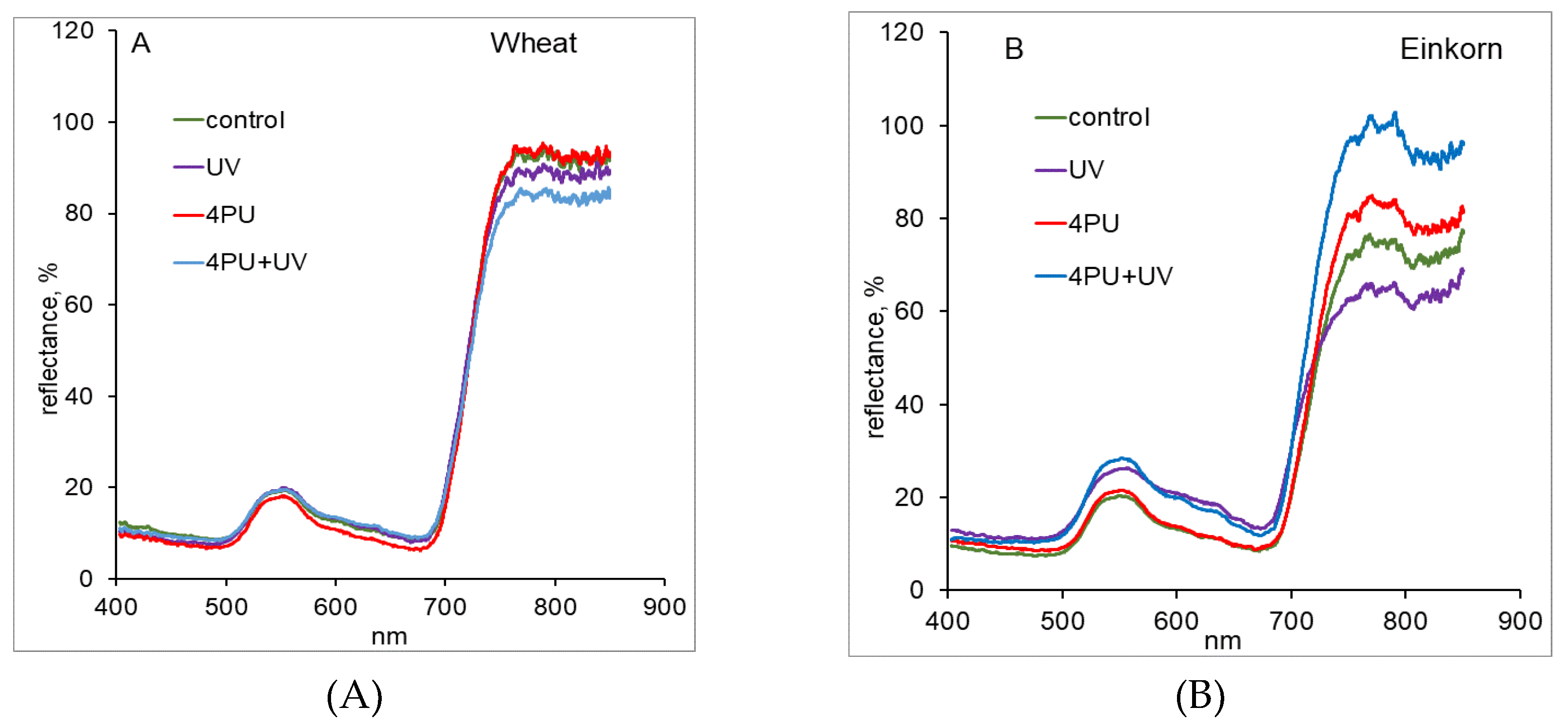
2.2. Effect of 4PU-30 and UV-B treatment on reflectance spectra of wheat and einkorn seedlings
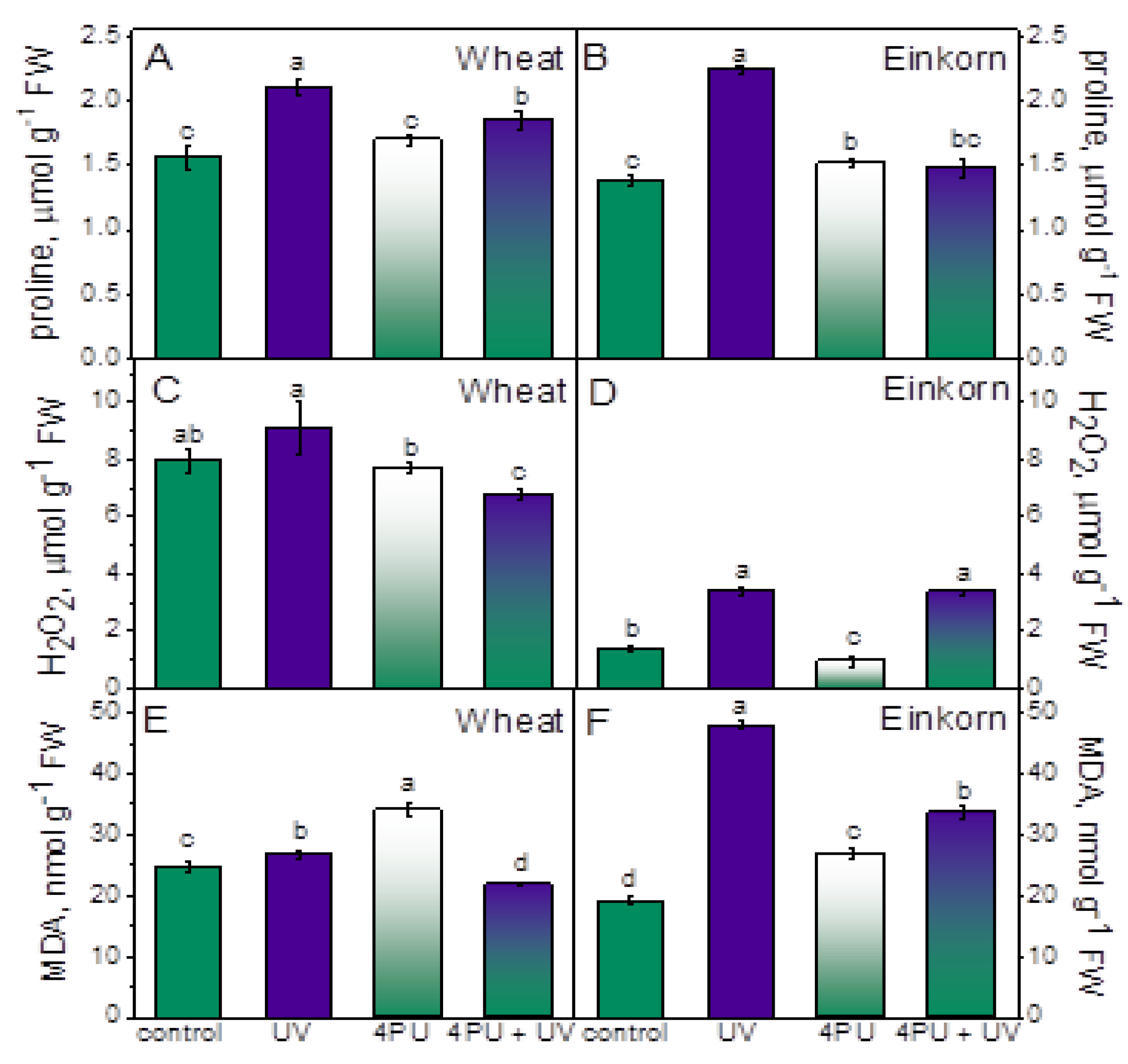
2.3. Effect of 4PU-30 and UV-B treatment on stress markers content in the leaves of wheat and einkorn seedlings
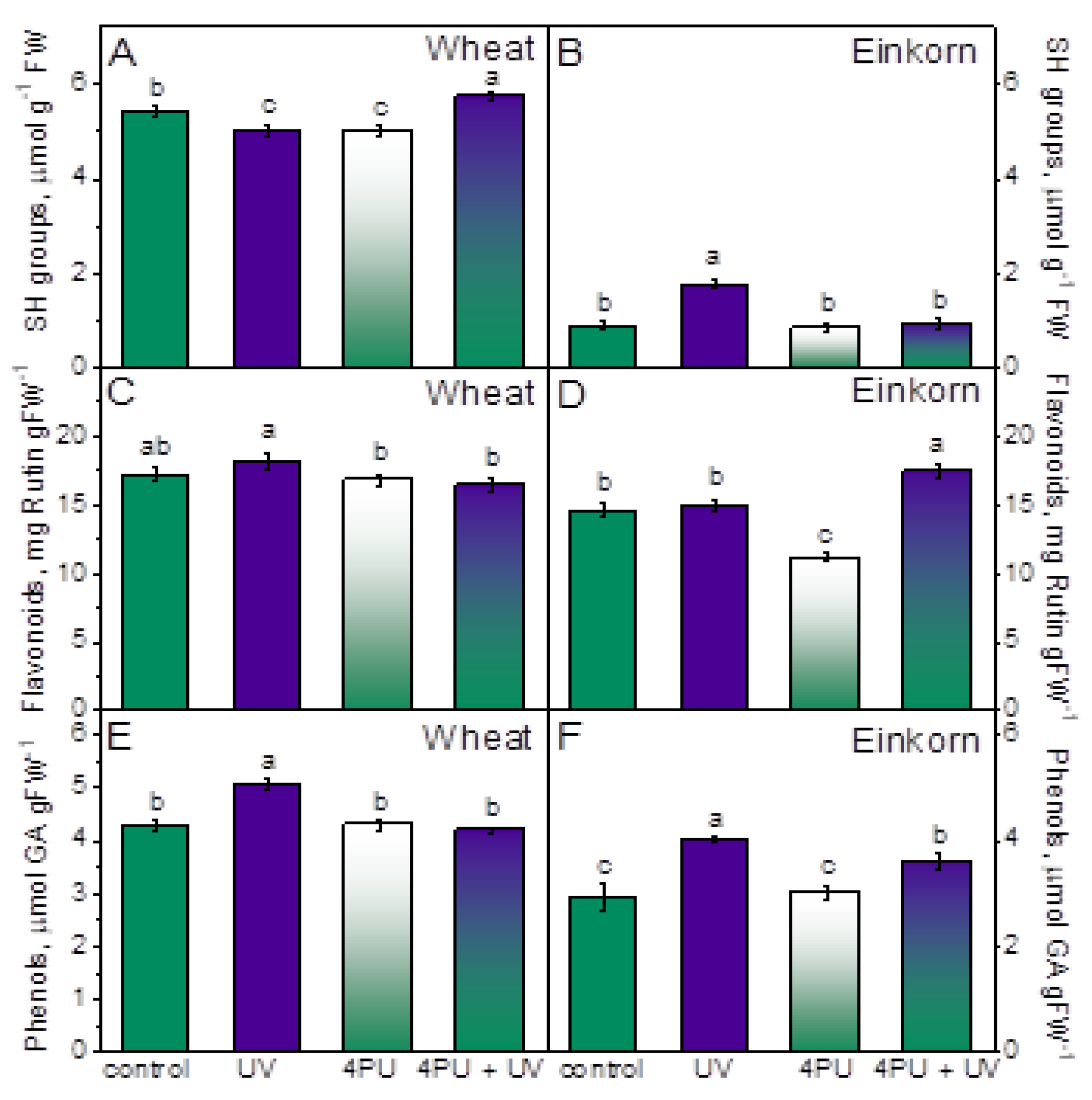
2.4. Non-enzymatic antioxidants (free thiols, flavonoids, and total phenolic compounds)
2.5. Free radical scavenging and antioxidant activity
2.6. Activity of ROS-scavenging enzymes (SOD, CAT, POX, GST)
2.7. Gene expression analysis
3. Discussion
4. Materials and Methods
4.1. Plant material, growth conditions and experimental design
4.2. Plant biometry and estimation of leaf pigment content
4.3. Remote sensing spectrometric data
4.4. Biochemical analyses
4.4.1. Oxidative stress markers analysis (free proline, hydrogen peroxide, malondialdehyde)
4.4.2. Non-enzymatic antioxidants (total phenolic compounds, flavonoids and free thiol groups)
4.4.3. Free radical scavenging activity and antioxidant capacity
4.4.4. Preparation of crude extracts and assays for antioxidant (ROS-scavenging) enzymes (CAT, SOD, POX, GST) activities.
4.5. Gene expression analysis
| Primer name | Primer sequence | Reference | Amplicon length | Target gene Tm/Ta | Acc. No & Chromosome localization (in wheat) |
|---|---|---|---|---|---|
| MnSOD-F MnSOD-R |
TCCGCCGTCGTCCACCTC CCACCACCCTCGCTGATG CCACCACCCTCGCTAATA (this study) |
Karimi et al., 2017 [99] | 104 bp | TmMnSOD TaMnSOD3.1 |
MK091461.1 - Tm XM_044603645.1 – Ta 2A |
| TaFe-SOD-F TaFe-SOD-R |
CCTACTGGATGAGACGGAGAG GGACGAGGACAACGACGAA |
Luo et al., 2019 [100] | 124 bp | TaFeSOD | AK453889.1 – Ta 7D JX398977.1 – Ta 7D XM_044510132.1 – Ta 4A |
| TaCu/Zn-SOD-F TaCu/Zn-SOD-R |
TGGGAGAGCGTTTGTTGTTC GTCTTCCACCAGCATTTCCA |
Luo et al., 2019 [100] | 92 bp | TaCu/Zn-SOD, SOD1.2 |
XM_044573537.1 - Ta 7A, X1 XM_044573538.1 - Ta 7A, X2 XM_044573539.1 - Ta 7A, X3 XM_044578041.1 - Ta 7B, X1 XM_044578042.1 - Ta 7B, X2 XM_044587054.1 - Ta 7D |
| qTmCAT1-F qTmCAT1-R |
CGAGAAGATGGTGATCGAGAA TGTTGATGAATCGCTCTTGC |
Tounsi et al., 2019b [73] | 95 bp | TmCAT1 TaCAT1 |
MK091459.1 - Tm NM_001405704.1 – Ta 4B XM_044520955.1 – Ta 4D XM_044527958.1 – Ta 5A |
| TGST2-F TGST2-R |
TACGAGGACGTGGAGGAGAA TGTGGATGAGCACGGGTATC |
This study | 91 bp | TmGSTU TaGSTU61 |
Tm EF044232.1 XM_044552184.1 - 6A XM_044550330.1 – 6A |
| TaGSTU56-F TaGSTU56-R |
TTAAAGATCTCGTCGTTCCAC AACAGCTACTCACAAGGCAGA |
Wang et al., 2019 [71] | 92 bp | TaGSTU62 | XM_044570870.1 - Ta 7A XM_044581608.1 - Ta 7D |
| TaGSTL10-F TaGSTL10-R |
ATGTGCCATTTATCGAAAGGT TCCATGCTGCAGTAGTTCCC |
Wang et al., 2019 [71] | 234 bp | TaIN2-1 homolog B | XM_044507158.1 - Ta 4A XM_044514325.1 - Ta 4B XM_044519678.1 - Ta 4D, X1 XM_044519679.1 - Ta 4D, X2 |
| POX1u-F POX1u-R |
CTCCAGGGTGAACTCGTGAT GCCTTTGCATGAGAAAGTGGG |
This study | 219 bp | TmPOX1 TaPOX1 |
AY857755.1 - Tm XM_044599368.1 - Ta 2A XM_044466236.1 - Ta 2B XM_044474478.1 - Ta 2D |
| POX7u1-F POX7u1-R |
GTCGTGGACGAGGTCAAGAG TGGGTCCACCAGTCAGCA |
This study | 112 bp | TmPOX7 TaPOX17-like |
AY857761.1 – Tm XM_044525767.1 - Ta 5A XM_044533224.1 - Ta 5B XM_044541856.1 - Ta 5D |
4.6. Statistical analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feldman, M.; Levy, A.A. Wheat Evolution and Domestication; Springer: Switzerland, 2023; p. 673. [Google Scholar] [CrossRef]
- Benincasa, P.; Tosti, G.; Farneselli, M.; Maranghi, S.; Bravi, E.; Marconi, O.; Falcinelli, B.; Guidicci, M. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann. Agric. Sci. 2020, 65, 68–76. [Google Scholar] [CrossRef]
- Keçeli, A. A review on the bioactive, antioxidant properties of einkorn (Triticum monococcum L. ssp. monococcum) populations and using in organic agriculture. Turk J. Agric. – Food Sci. Technol. 2019, 7, 2111–2120. [Google Scholar] [CrossRef]
- Desheva, G.; Valchinova, E.; Chipilski, R.; Uzundzhieva, K.; Kyosev, B. Morphophyziological and anatomical characteristics of leaves in accessions of wild einkorn (Triticum boeoticum Boiss.). Int. J. Env. Agric. Biotechnol. 2018, 3, 1391–1400. [Google Scholar] [CrossRef]
- Guzman, C.; Alvarez, J.B. Ancient wheats role in sustainable wheat cultivation. In Trends in Wheat and Bread Making. Galanakis, C.M., Ed.; Charlotte Cockle: Academic Press, 2021; pp. 29–66. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, Y.; Huang, X. Plant responses to UV-B radiation: Signaling, acclimation and stress tolerance. Stress Biol. 2022, 2, 51. [Google Scholar] [CrossRef]
- Manova, V.; Gruszka, D. DNA damage and repair in plants–from models to crops. Front. Plant Sci. 2015, 6, 885. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Jankû, M.; Luhová, L.; Petřivalský, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Song, S.; Abdulrashid, K.; Chai, Y.; Yue, M.; Liu, X. Separate and combined response to UV-B radiation and jasmonic acid on photosynthesis and growth characteristics of Scutellaria baicalensis. Int. J. Mol. Sci. 2018, 19, 1194. [Google Scholar] [CrossRef]
- Shi, C.; Liu, H. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021, 187, 1096–1103. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, S.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voyenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous phytohormones in the regulation of growth and development of cereals under abiotic stresses. Mol. Biol. Rep. 2022, 49, 617–628. [Google Scholar] [CrossRef]
- Veselov, D.S.; Kudoyarova, G.R.; Kudryakova, N.V.; Kusnetsov, V.V. Role of cytokinins in stress resistance of plants. Russ. J. Plant. Physiol. 2017, 64, 15–27. [Google Scholar] [CrossRef]
- Pavlů, J.; Novák, J.; Koukalová, V.; Luklová, M.; Brzobohatý, B.; Černý, M. Cytokinin at the crossroads of abiotic stress signalling pathways. Int. J. Mol. Sci. 2018, 19, 2450. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Cui, X.; Wang, K.; Wang, Y.; He, Y. Phytohormones regulate the abiotic stress: An overview of physiological, biochemical, and molecular responses in horticultural crops. Front. Plant Sci. 2023, 13, 1095363. [Google Scholar] [CrossRef] [PubMed]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell. Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Meng, Z.; Wang, B.; Chen, M. Research progress on the roles of cytokinin in plant response to stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef]
- Todorova, D.; Moskova, I.; Sergiev, I.; Alexieva, V.; Mapelli, S. Changes in endogenous polyamines and some stress markers content induced by drought, 4PU-30 and abscisic acid in wheat plants. In Abiotic Stress and Plant Responses; Khan, N., Singh, S.I.K., Eds.; International Publishing House Pvt. Ltd.: New Delhi, India, 2008; pp. 205–215. [Google Scholar]
- Yonova, P. Design, synthesis and properties of synthetic cytokinins. Recent advances on their application. Gen. App. Plant Physiol. 2010, 36, 124–147. [Google Scholar]
- Ellouzi, H.; Zorrig, W.; Amraoui, S.; Oueslati, S.; Abdelly, C.; Rabhi, M.; Siddique, K.H.M.; Hessini, K. Seed priming with salicylic acid alleviates salt stress toxicity in barley by suppressing ROS accumulation and improving antioxidant defense systems, compared to halo- and gibberellin priming. Antioxidants 2023, 12, 1779. [Google Scholar] [CrossRef]
- Moskova, I.; Kocheva, K. Phenylurea-type cytokinin ameliorates the performance of young pea plants under salt stress. Botanica 2021, 27, 141–148. [Google Scholar] [CrossRef]
- Sergiev, I.G.; Alexieva, V.S.; Ivanov, S.V.; Moskova, I.I.; Karanov, E.N. The phenylurea cytokinin 4PU-30 protects maize plants against glyphosate action. Pestic. Biochem. Physiol. 2006, 85, 139–146. [Google Scholar] [CrossRef]
- Shopova, E.; Katerova, Z.; Brankova, L.; Dimitrova, L.; Sergiev, I.; Todorova, D.; Talaat, N.B. Modulation of physiological stress response of Triticum aestivum L. to glyphosate by brassinosteroid application. Life 2021, 11, 1156. [Google Scholar] [CrossRef]
- Moskova, I.; Dikova, B.; Balacheva, E.; Sergiev, I. Protective effect of plant growth regulators MEIA and 4PU-30 against tomato spotted wilt virus (TSWV) on two tomato genotypes. Compt. Rend. Acad. Bulg. Sci. 2020, 73, 1538–1544. [Google Scholar] [CrossRef]
- Mandal, S.; Ghorai, M.; Anand, U.; Samanta, D.; Kant, N.; Mishra, T.; Rahman, M.H.; Jha, N.K.; Jha, S.K.; Lal, M.K.; Tiwari, R.K.; Kumar, M.; Radha; Prasanth, D. A.; Mane, A.B.; Gopalakrishnan, A.V.; Biswas, P.; Proćków, J.; Dey, A. Cytokinin and abiotic stress tolerance -What has been accomplished and the way forward? Front. Genet. 2022, 13, 943025. [Google Scholar] [CrossRef]
- Hudeček, M.; Nožková, V.; Plíhalová, L.; Plíhal, O. Plant hormone cytokinin at the crossroads of stress priming and control of photosynthesis. Front. Plant Sci. 2023, 13, 1103088. [Google Scholar] [CrossRef] [PubMed]
- Kolupaev, Y.E.; Karpets, Y.V.; Kabashnikova, L.F. Antioxidative system of plants: Cellular compartmentalization, protective and signaling functions, mechanisms of regulation. Appl. Biochem. Microbiol. 2019, 55, 441–459. [Google Scholar] [CrossRef]
- Cantarello, C.; Volpe, V.; Azzolin, C.; Bertea, C. Modulation of enzyme activities and expression of genes related to primary and secondary metabolism in response to UV-B stress in cucumber (Cucumis sativus L.). J. Plant Interact. 2005, 1, 151–161. [Google Scholar] [CrossRef]
- Gaafar, R.M.; Osman, M.E.-A.H.; Abo-Shady, A.M.; Almohisen, I.A.A.; Badawy, G.A.; El-Nagar, M.M.F.; Ismail, G.A. Role of antioxidant enzymes and glutathione S-transferase in bromoxynil herbicide stress tolerance in wheat plants. Plants 2022, 11, 2679. [Google Scholar] [CrossRef]
- Navabpour, S.; Yamchi, A.; Bagherikia, S.; Kafi, H. Lead-induced oxidative stress and role of antioxidant defense in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plant. 2020, 26, 793–802. [Google Scholar] [CrossRef]
- Nasirzadeh, L.; Sorkhilaleloo, B.; Majidi Hervan, E.; Fatehi, F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res. Comm. 2021, 49, 83–89. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—Mentor of abiotic stress tolerance in crop plants. Environ. Sci. Poll. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Zameer, R.; Fatima, K.; Azeem, F.; ALgwaiz, H.I.; Sadaqat, M.; Rasheed, A.; Batool, R.; Shah, A.N.; Zaynab, M.; Shah, A.A.; Attia, K.A. Genome-wide characterization of superoxide dismutase (SOD) genes in Daucus carota: Novel insights into structure, expression, and binding interaction with hydrogen peroxide (H2O2) under abiotic stress condition. Front. Plant Sci. 2022, 13, 870241. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, L.; He, Y.; Zhang, H.; Li, W.; Chen, H.; Ma, D.; Yin, J. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). Peer J 2019, 7, e8062. [Google Scholar] [CrossRef]
- Tounsi, S.; Feki, K.; Kamoun, Y.; Saïdi, M.N.; Jemli, S.; Ghorbel, M.; Alcon, C.; Brini, F. Highlight on the expression and the function of a novel MnSOD from diploid wheat (T. monococcum) in response to abiotic stress and heavy metal toxicity. Plant Physiol. Biochem. 2019, 142, 384–394. [Google Scholar] [CrossRef]
- Boldt, R.; Scandalios, J.G. Influence of UV-light on the expression of the Cat2 and Cat3 catalase genes in maize. Free Radic. Biol. Med. 1997, 23, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Zribi, I.; Haddaji, N.; Siddiqui, A.J.; Bouali, N.; Brini, F. Genome-wide identification and expression analysis of catalase gene families in Triticeae. Plants 2024, 13, 11. [Google Scholar] [CrossRef]
- Sharma, I.; Ahmad, P. Catalase: A versatile antioxidant in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Elsevier, 2014; pp. 131–148. [Google Scholar] [CrossRef]
- Kancheva, R. Main principles in vegetation spectrometric studies. Annual of University of Mining and Geology 2003, 46, 351–353. [Google Scholar]
- Kancheva, R.; Iliev, I.; Borisova, D.; Chankova, S.; Kapchina, V. Detection of plant physiological stress using spectral data. Ecol. Engin. Environ. Prot. 2005, 1, 4–9. [Google Scholar]
- Kancheva, R.; Georgiev, G. Seasonal spectral response patterns of winter wheat canopy for crop performance monitoring. Proc. SPIE 8887, Remote Sens. Agric. Ecosyst. Hydrol. 2013, 15, 8887, 88871V. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Changing scenario in plant UV-B research: UV-B from a generic stressor to a specific regulator. J. Photochem. Photobiol. B: Biology 2015, 153, 334–343. [Google Scholar] [CrossRef]
- Lima, M.P.; Soares, A.M.; Loureiro, S. Responses of wheat (Triticum aestivum) and turnip (Brassica rapa) to the combined exposure of carbaryl and ultraviolet radiation. Environ. Toxicol. Chem. 2015, 34, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Exogenous kinetin promotes the nonenzymatic antioxidant system and photosynthetic activity of coffee (Coffea arabica L.) plants under cold stress conditions. Plants 2020, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Prerostova, S.; Dobrev, P.I.; Gaudinova, A.; Knirsch, V.; Korber, N.; Pieruschka, R.; Fiorani, F.; Brzobohaty, B.; Cerny, M.; Spichal, L.; Humplik, J.; Vanek, T.; Schurr, U.; Vankova, R. Cytokinins: Their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Xiaotao, D.; Yuping, J.; Hong, W.; Haijun, J.; Hongmei, Z.; Chunhong, C.; Jizhu, Y. Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters, antioxidative system and carbohydrate accumulation in cucumber (Cucumis sativus L.) under low light. Acta Physiol. Plant. 2013, 35, 1427–1438. [Google Scholar] [CrossRef]
- Katerova, Z.; Shopova, E.; Georgieva, N.; Nikolova, A.; Sergiev, I.; Todorova, D. MEIA acts as protector against UV-C irradiation in young wheat plants. Compt. Rend. Acad. Bulg. Sci. 2012, 65, 1373–1378. [Google Scholar]
- Ivanov, V.B.; Filin, A.N. Cytokinins regulate root growth through its action on meristematic cell proliferation but not on the transition to differentiation. Funct. Plant Biol. 2017, 45, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R. Cytokinin and its key role to enrich the plant nutrients and growth under adverse conditions - an update. Front. Genet. 2022, 13, 883924. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Ustin, S.L.; Jacquemoud, S. How the optical properties of leaves modify the absorption and scattering of energy and enhance leaf functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer: Cham, 2020; pp. 349–384. [Google Scholar]
- Mohd Asaari, M. S.; Mertens, S.; Verbraeken, L.; Dhondt, S.; Inzé, D.; Bikram, K.; Scheunders, P. Non-destructive analysis of plant physiological traits using hyperspectral imaging: A case study on drought stress. Comp. Electron. Agric. 2022, 195, e106806. [Google Scholar] [CrossRef]
- Williams, D.; Karley, A.; McCallum, S.; Graham, J. Raspberry plant stress detection using hyperspectral imaging. Plant Direct. 2023, 7, e490. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2020, 105, 459–476. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Kumar, R.; Altaf, M.M.; Kumar, A.; Khan, L.U.; Saqib, M.; Nawaz, M.A.; Saddiq, B.; Bahadur, S.; Tiwari, R.K.; Lal, M.K; Naz, S. Phytohormones mediated modulation of abiotic stress tolerance and potential crosstalk in horticultural crops. J. Plant Growth Regul. 2023, 42, 4724–4750. [Google Scholar] [CrossRef]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M.A. Central role for thiols in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Fut. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.; Azevedo, R.A.; Fidalgo, F. Plants facing oxidative challenges – A little help from the antioxidant networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Gallé, Á.; Csiszár, J.; Secenji, M.; Guóth, A.; Cseuz, L.; Tari, I.; Györgyey, J.; Erdei, L. Glutathione transferase activity and expression patterns during grain filling in flag leaves of wheat genotypes differing in drought tolerance: Response to water deficit. J. Plant Physiol. 2009, 166, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xu, S.; Lyu, Z.; Wang, H.; Kong, L.; Sun, S. Comparative analysis of the glutathione s-transferase gene family of four Triticeae species and transcriptome analysis of GST genes in common wheat responding to salt stress. Int. J. Genom. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sheng, X.; Greenshields, D.L.; Ogieglo, A.; Kaminskyj, S.; Selvaraj, G.; Wei, Y. Profiling of wheat class III peroxidase genes derived from powdery mildew-attacked epidermis reveals distinct sequence-associated expression patterns. Mol. Plant - Microbe Interact. 2005, 18, 730–741. [Google Scholar] [CrossRef]
- Wang, M.; Leng, C.; Zhu, Y.; Wang, P.; Gu, Z.; Yang, R. UV-B treatment enhances phenolic acids accumulation and antioxidant capacity of barley seedlings. LWT 2022, 153, 112445. [Google Scholar] [CrossRef]
- Wang, R.; Ma, J.; Zhang, Q.; Wu, C.; Zhao, H.; Wu, Y.; Yang, G.; He, G. Genome-wide identification and expression profiling of glutathione transferase gene family under multiple stresses and hormone treatments in wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vásquez, A.; Fonseca, A.; Ugalde, J.M.; Lamig, L.; Seguel, A.; Moyano, T.C.; Gutiérrez, R.A.; Salinas, P.; Vidal, E.A.; Holuigue, L. TGA class II transcription factors are essential to restrict oxidative stress in response to UV-B stress in Arabidopsis. J. Exp. Bot. 2021, 72, 1891–1905. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, S.; Kamoun, Y.; Feki, K.; Jemli, S.; Saïdi, M.N.; Ziadi, H.; Alcon, C.; Brini, F. Localization and expression analysis of a novel catalase from Triticum monococcum TmCAT1 involved in response to different environmental stresses. Plant Physiol. Biochem. 2019, 139, 366–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Yun, L.; Ji, L.; Li, G.; Ji, M.; Shi, Y.; Zheng, X. Catalase (CAT) gene family in wheat (Triticum aestivum L.): Evolution, expression pattern and function analysis. Int. J.Mol. Sci. 2022, 23, 542. [Google Scholar] [CrossRef]
- Ermakov, A.; Bobrovskikh, A.; Zubairova, U.; Konstantinov, D.; Doroshkov, A. Stress-induced changes in the expression of antioxidant system genes for rice (Oryza sativa L.) and bread wheat (Triticum aestivum L.). Peer J 2019, 7, e7791. [Google Scholar] [CrossRef]
- Ambasht, N.K; Agrawal, M. Effects of enhanced UV-B radiation and tropospheric ozone on physiological and biochemical characteristics of field grown wheat. Biol. Plant. 2003, 47, 625–628. [Google Scholar] [CrossRef]
- Santa-Cruz, D.M. , Pacienza, N.A., Zilli, C.G., Tomaro, M.L., Balestrasse, K.B.; Yannarelli, G.G. Nitric oxide induces specific isoforms of antioxidant enzymes in soybean leaves subjected to enhanced ultraviolet-B radiation. J. Photochem. Photobiol. B: Biology 2014, 141, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.H.; Skinner, D.Z. Differential expression of manganese superoxide dismutase sequence variants in near isogenic lines of wheat during cold acclimation. Plant Cell Rep. 2006, 25, 223–230. [Google Scholar] [CrossRef]
- Tyagi, S.; Sharma, S.; Taneja, M.; Kumar, R.; Sembi, J.K.; Upadhyay, S.K. Superoxide dismutases in bread wheat (Triticum aestivum L.): Comprehensive characterization and expression analysis during development and, biotic and abiotic stresses. Agri Gene 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Chipilski, R.; Moskova, I.; Pencheva, A.; Kocheva, K. Enhancement of maize seed viability after cold storage and induced senescence by priming with synthetic cytokinins. Zemdirbyste Agric. 2023, 110, 33–38. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Borisova, D.; Nikolov, H.; Dimitrov, V.; Bouzekova-Penkova, A. Framework concept of the project “Тhe ancient wheat - growth and physiological characteristics under unfavorable stress factors and possibilities to alleviate the negative effects”. Ninth International Conference on Remote Sensing and Geoinformation of Environment, 2023, 12786, SPIE, 27860A. [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Kramer, G.; Norman, H.; Krizek, D.; Mirecki, R. Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochem. 1991, 30, 2101–2108. [Google Scholar] [CrossRef]
- Swain, T.; Goldstein, L. Methods in Polyphenol Chemistry; Pridham, J.B., Ed.; Pergamon Press: Oxford, UK, 1964; pp. 131–146.
- Edreva, A.; Hadjiiska, E. About the determination of sulfhydril (thiol) group content in plant material. Bulg. J. Plant. Physiol. 1984, 10, 73–82. (in Bulgarian). [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Szeto, Y.T. Total antioxidant capacity of teas by ferric reducing antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Iliev, I.; Azcon-Aguilar, C. Symbiotic association between golden berry (Physalis peruviana) and arbuscular mycorrhizal fungi in heavy metal-contaminated soil. J. Plant Prot. Res. 2017, 57, 173–184. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebigo, M. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Dias, I.; Costa, M. Effect of low salt concentration on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Gronwald, J.W.; Fuerst, E.P.; Eberlein, C.V.; Egli, M.A. Effect of herbicide antidotes on glutathione content and glutathione S-transferase activity of sorghum shoots. Pestic. Biochem. Physiol. 1987, 29, 66–76. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nuc. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Manova, V.; Georgieva, R.; Borisov, B.; Stoilov, L. Efficient removal of cyclobutane pyrimidine dimers in barley: Differential contribution of light-dependent and dark DNA repair pathwys. Physiol. Plant. 2016, 158, 236–253. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 1–11. [Google Scholar] [CrossRef]
- Multiple Primer Analyzer | Thermo Fisher Scientific.
- Karimi, J.; Mohsenzadeh, S.; Niazi, A.; Moghadam, A. Differential expression of mitochondrial manganese superoxide dismutase (SOD) in Triticum aestivum exposed to silver nitrate and silver nanoparticles. Iran. J. Biotechnol. 2017, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pang, D.; Jin, M.; Chen, J.; Kong, X.; Li, W.; Chang, Y.; Li, Y.; Wang, Z. Identification of plant hormones and candidate hub genes regulating flag leaf senescence in wheat response to water deficit stress at the grain-filling stage. Plant Direct. 2019, 3, e00152. [Google Scholar] [CrossRef] [PubMed]
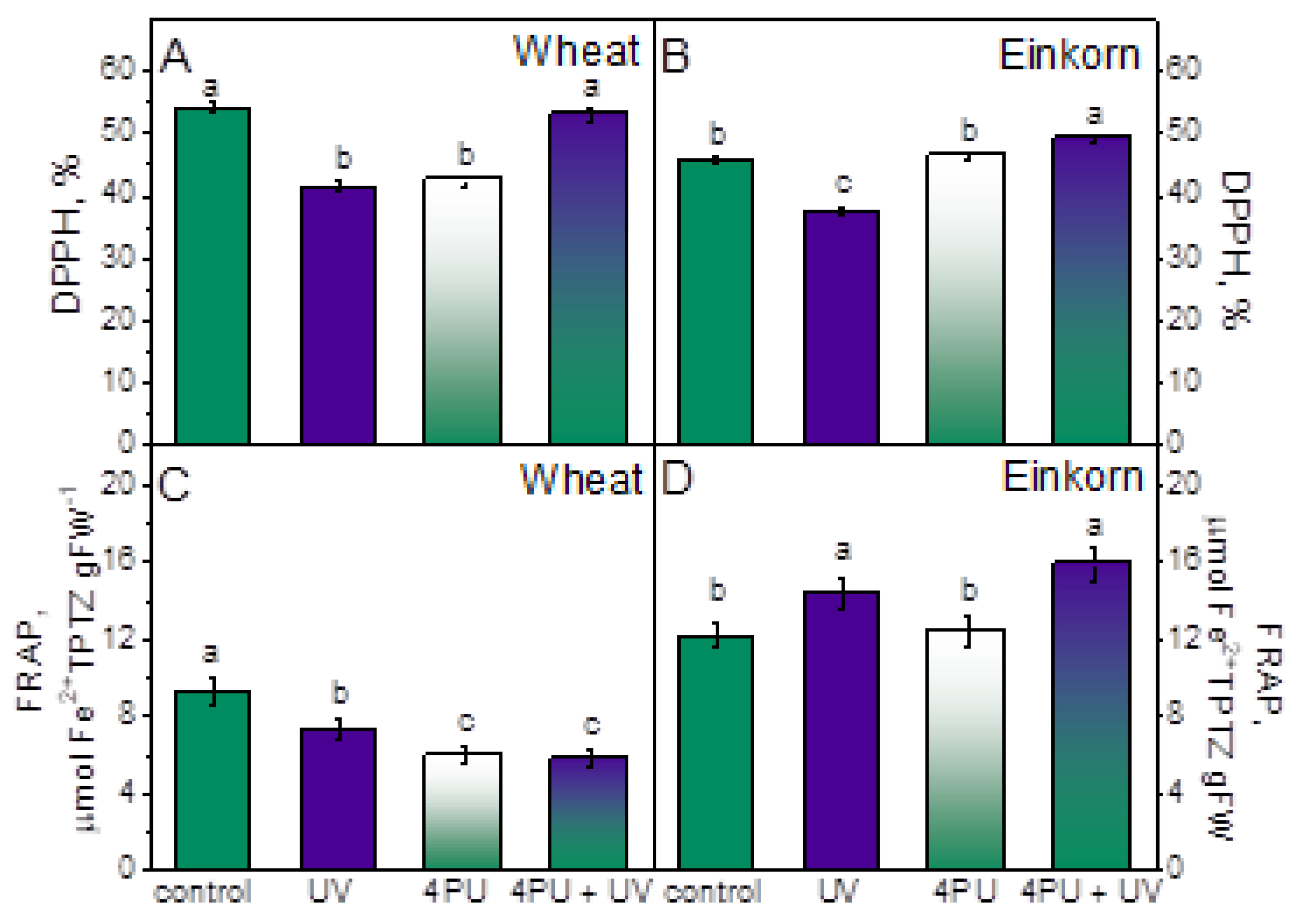
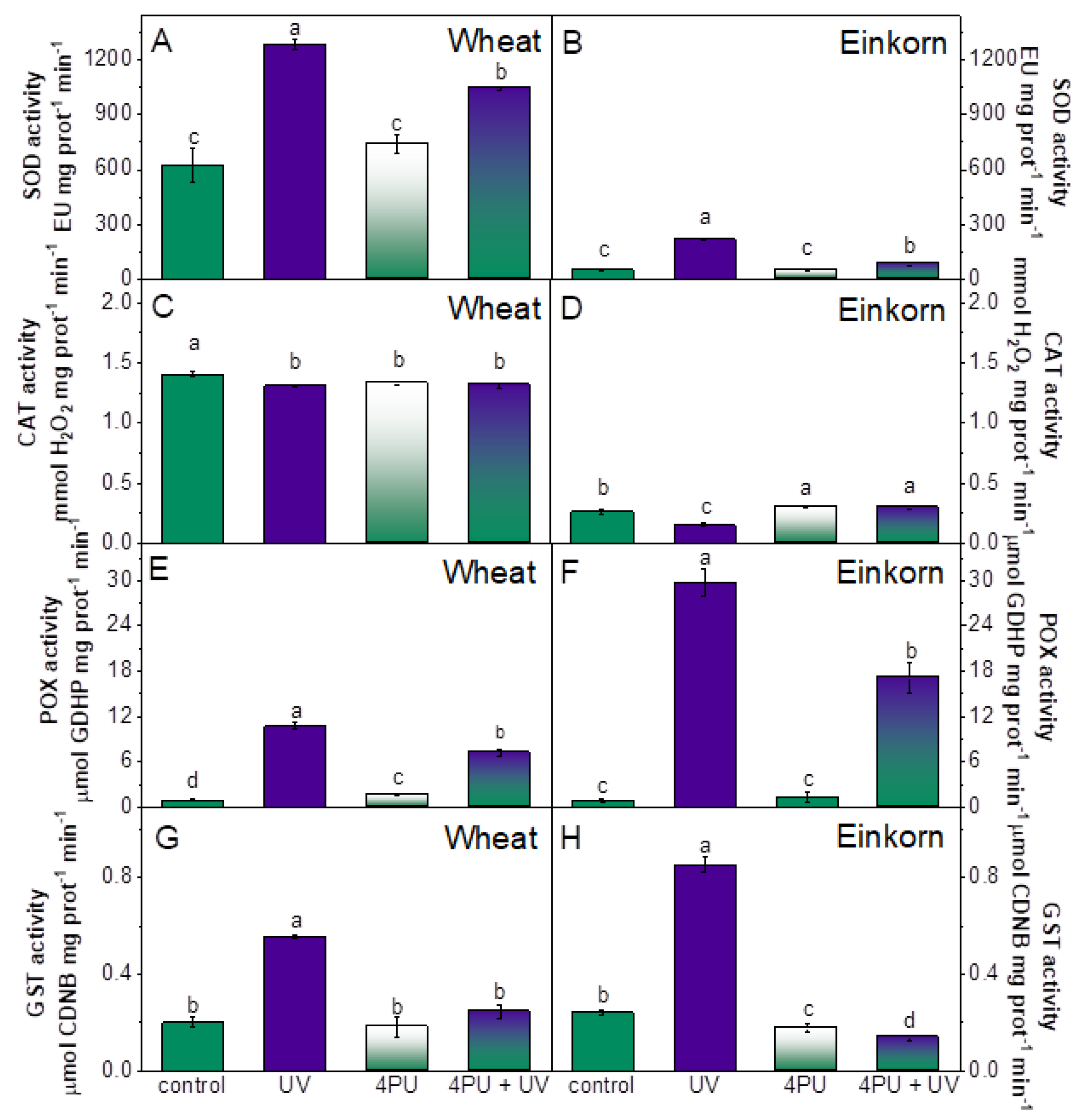
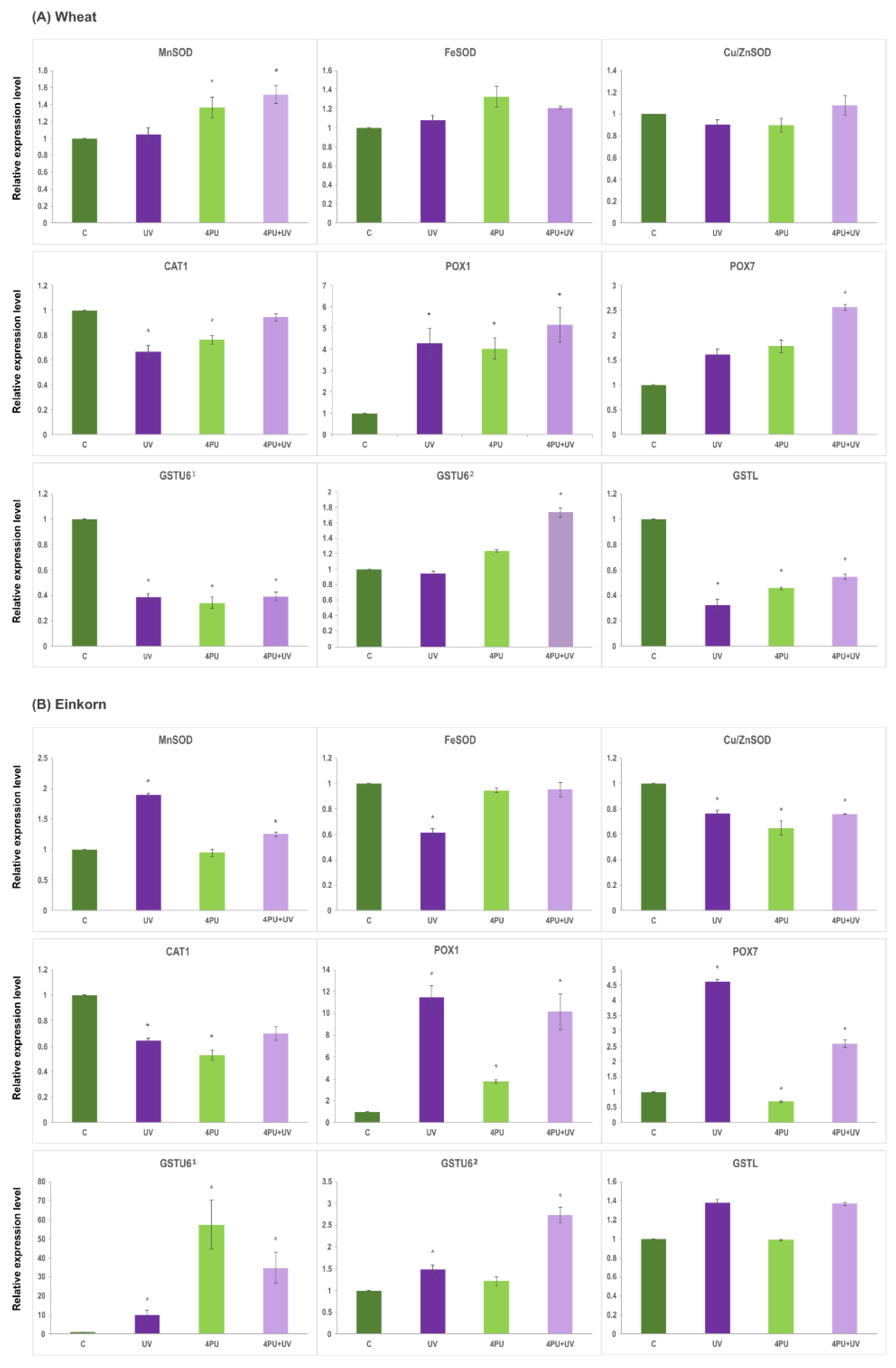

| Variants | Chlorophyll a (mg/g FW) |
Chlorophyll b (mg/g FW) |
Carotenoids (mg/g FW) |
|
|---|---|---|---|---|
| Wheat | ||||
| Control | 1.307 ± 0.036 b | 0.452 ± 0.017 a | 0.799 ± 0.026 a | |
| UV stress | 0.845 ± 0.010 d | 0.270 ± 0.003 c | 0.510 ± 0.006 c | |
| 4PU-30 | 1.419 ± 0.025 a | 0.480 ± 0.011 a | 0.848 ± 0.023 a | |
| 4PU + UV | 1.186 ± 0.015 c | 0.394 ± 0.005 b | 0.670 ± 0.010 b | |
| Einkorn | ||||
| Control | 1.158 ± 0.038 b | 0.392 ± 0.024 b | 0.679 ± 0.024 b | |
| UV stress | 0.756 ± 0.025 c | 0.285 ± 0.011 c | 0.559 ± 0.020 c | |
| 4PU-30 | 1.281 ± 0.036 a | 0.449 ± 0.015 a | 0.750 ± 0.018 a | |
| 4PU + UV | 1.139 ± 0.036 b | 0.395 ± 0.014 b | 0.686 ± 0.023 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





