Submitted:
24 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Acquisition
2.2. Laboratory Analyses
2.3. Laboratory Analyses
2.4. Statistical Analyses
3. Results
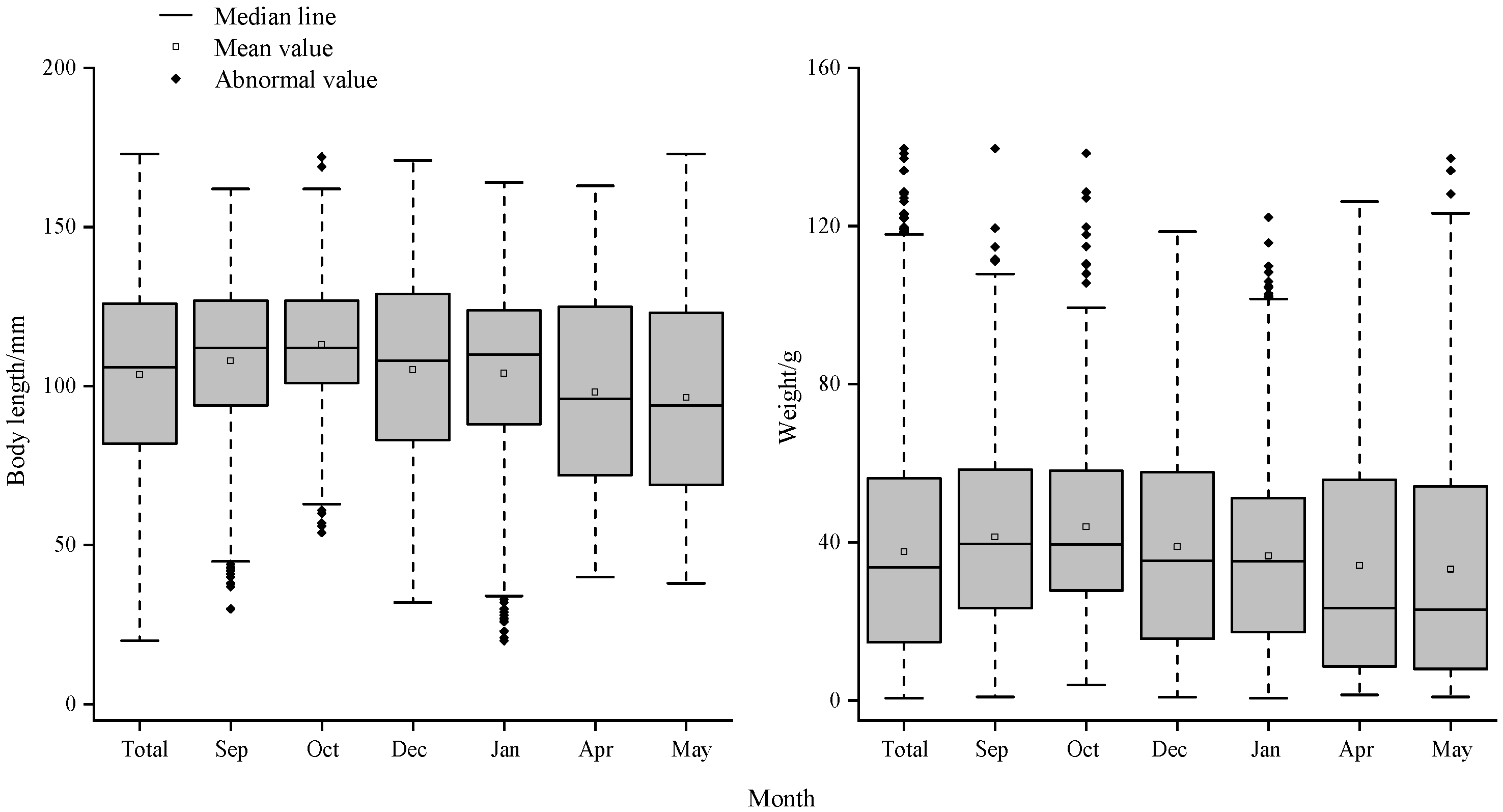
3.1. Seasonal Changes in Body Length and Weight
| Month | Sex | Sample size | Length/cm | Weight/g | |||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | CV | Skewness | Range | Mean | Skewness | |||
| Sep | ♀ | 102 | 8~15.7 | 12.14 | 15.3 | -0.28nd | 15.2~111.2 | 53.76 | 23.35 |
| ♂ | 114 | 7.8~15.6 | 11.3 | 13.7 | 0.23rs | 12.8~106.7 | 42.94 | 20.24 | |
| Oct | ♀ | 427 | 5.6~17.2 | 11.72 | 14.84 | 0.15rs | 5.9~128.6 | 54.85 | 21.41 |
| ♂ | 524 | 6.5~16.9 | 11.36 | 21.14 | 0.11rs | 5.8~138.4 | 46.92 | 17.8 | |
| Dec | ♀ | 390 | 3.4~17.1 | 12.12 | 19.54 | -0.77ls | 1~118.4 | 47.47 | 25.07 |
| ♂ | 393 | 5.6~16 | 10.75 | 21.14 | -0.05ls | 4.5~107.5 | 41.97 | 22.33 | |
| Jan | ♀ | 483 | 6~16.4 | 11.14 | 20.21 | -0.01ls | 5.4~108.4 | 44.57 | 23.99 |
| ♂ | 559 | 6.2~15.8 | 11.4 | 15.28 | -0.21ls | 5.1~94.6 | 42.74 | 19.4 | |
| Apr | ♀ | 287 | 5.2~16.3 | 12.26 | 17.85 | -0.31ls | 3~117.8 | 54.04 | 29.6 |
| ♂ | 349 | 6.1~16 | 11.88 | 16.17 | -0.49ls | 4.8~104.6 | 45.95 | 23.82 | |
| May | ♀ | 430 | 5.2~17.3 | 11.27 | 26.11 | -0.08ls | 3.2~134 | 49.82 | 33.43 |
| ♂ | 405 | 6.2~16.4 | 11.24 | 18.2 | -0.15ls | 5.9~12.31 | 44.41 | 23.04 | |
3.2. Seasonal Changes in Body Length and Weight
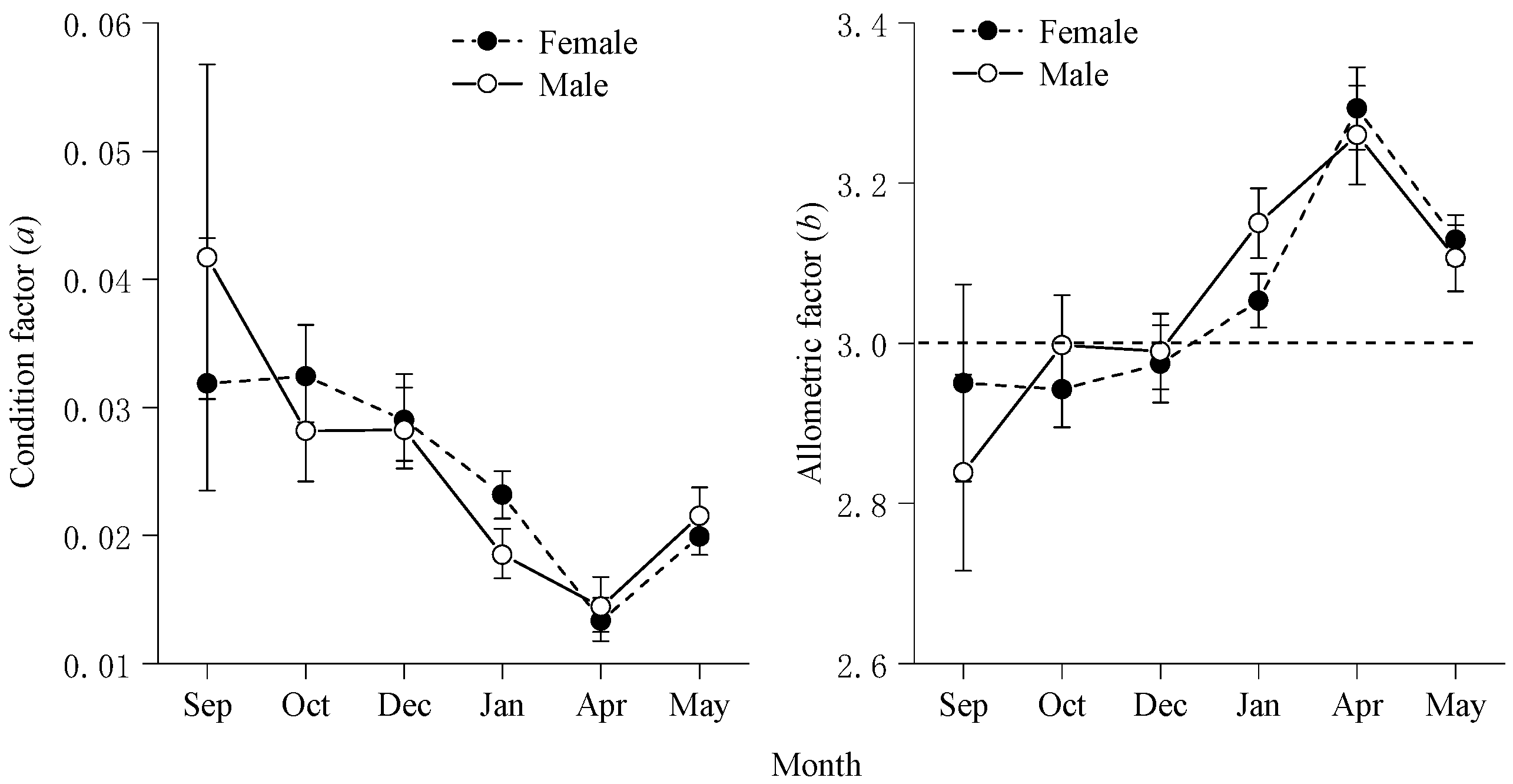
3.3. Changes in the Parameters of the WLR
3.4. Outlier Tests for Parameters of the WLR
3.5. Relevance Analysis
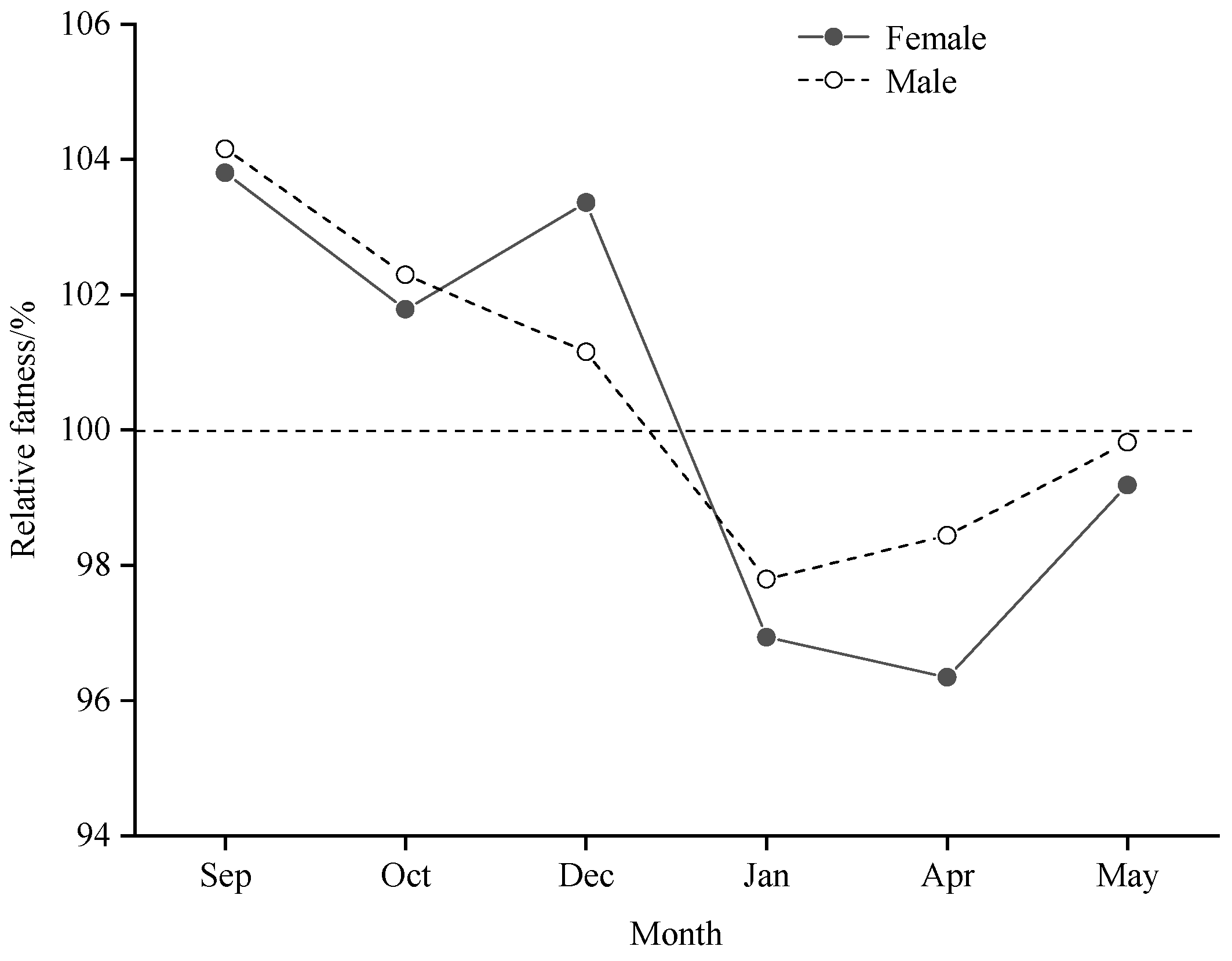
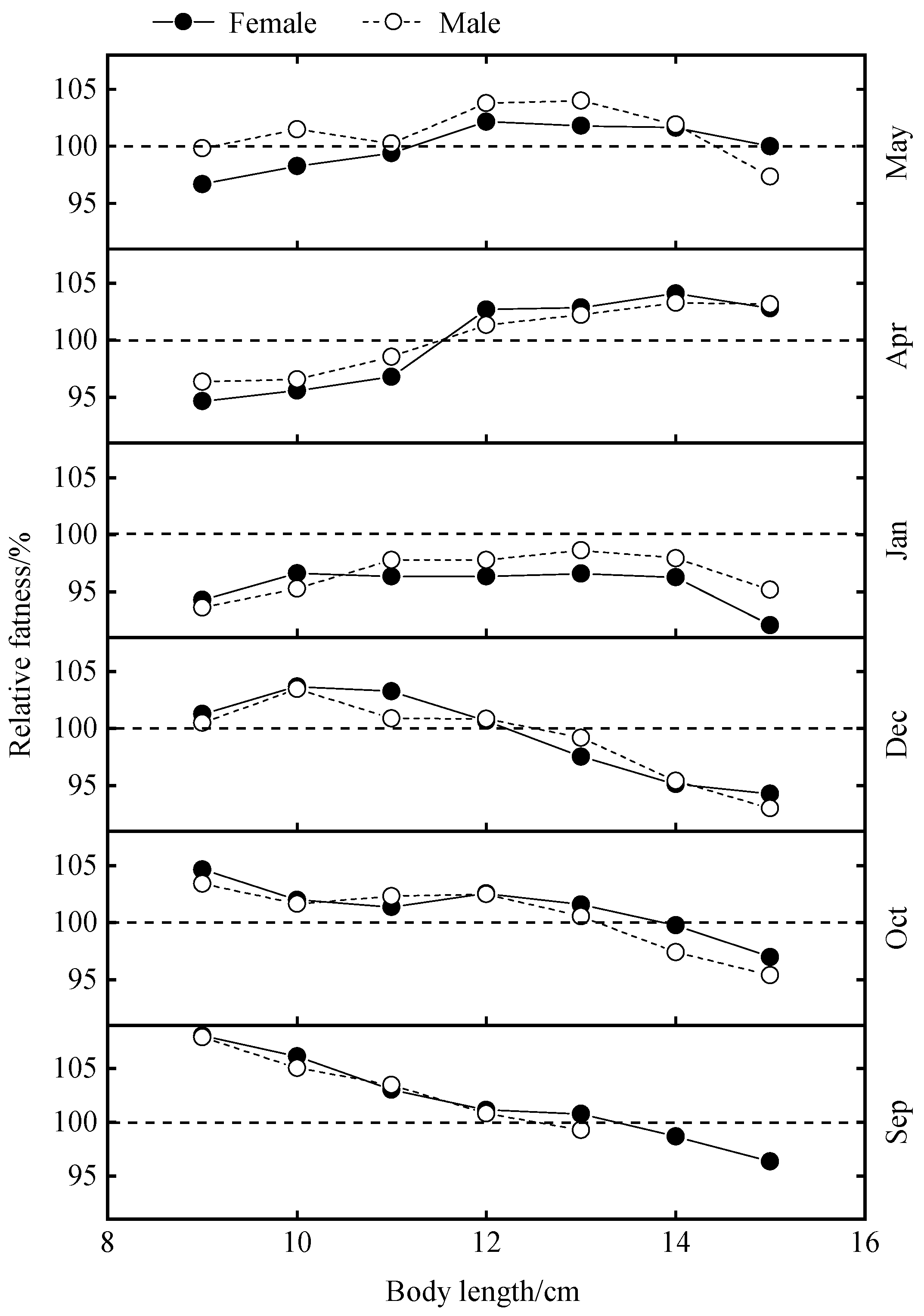
3.6. Seasonal Changes in Relative Fatness
4. Discussion
4.1. Analysis of the WLR
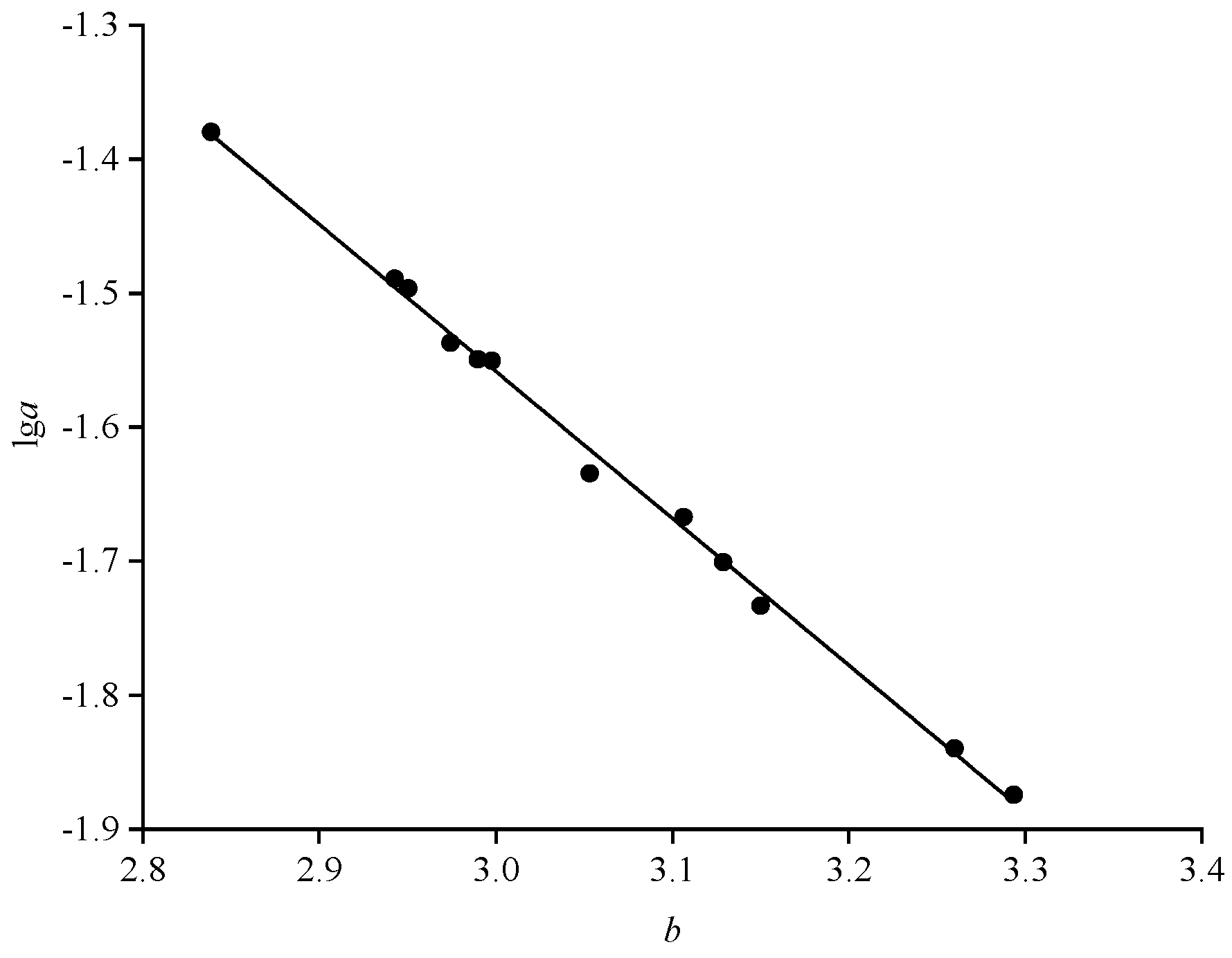
4.2. Relationship between Parameters a and b
4.3. Environmental Factors for Relative Fatness
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggrey-Fynn, J.; Sackey-Mensah, R. Species diversity and relative abundance of fisheries resources found in beach seine along the central coast of ghana. West African J. Appl. Ecol. 2012, 20, 1–9. [Google Scholar]
- Amponsah, S.K.K.; Danson, P.K.O.; Nunoo, F.K. Study of the population parameters of the bigeye grunt, brachydeuterus auritus (valenciennes, 1831) in ghanaian coastal waters and its implications for management. International Journal of Fisheries and Aquatic Studies 2016, 4, 413–419. [Google Scholar]
- Abbey, L.D.; Glover-Amengor, M.; Atikpo, M.O.; Howell, N.K. Proximate and biochemical characterization of burrito (bachydeuterus auritus) and flying gurnard (dactylopterus volitans). Food Sci. Nutr. 2017, 5, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Seto, K.; Belhabib, D.; Mamie, J.; Copeland, D.; Vakily, J.M.; Seilert, H.; Baio, A.; Harper, S.; Zeller, D.; Zylich, K.; Seilert, H. War, fish, and foreign fleets: the marine fisheries catches of sierra leone 1950–2015. Mar. Policy 2017, 83, 153–163. [Google Scholar] [CrossRef]
- Froese, R. Keep it simple: three indicators to deal with overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Zhan, B.Y. Fisheries resource assessment. Beijing, China Agriculture Press, 1995.
- Huang, Z.L.; Chang, J.B. Fractal characteristics of length-weight relationship in fish. Acta hydrobiol. Sin. 1999, 4, 330–336. [Google Scholar]
- Pitcher, T.J.; Hart, P.J.B. Fisheries ecology. London, Croom Helm, 1982.
- Zhang, K.; Chen, Z.Z.; Wang, Y.Z.; Su, D.R.; Qiu, Y.S. Population structure of Priacanthus macracanthus in the Beibu Gulf, and parameters for its growth, mortality and maturity. J. Trop. Oceanograph 2016, 35, 20–28. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Fourtouni, H. Food habits, ontogenetic diet shift and selectivity in zeus faber linnaeus, 1758. J. Fish Biol. 1991, 39, 589–603. [Google Scholar] [CrossRef]
- Li, Z.L.; Jin, X.S.; Shan, X.J.; Dai, F.Q. Inter-annual changes on body weight-length relationship and relative fatness of small yellow croaker (Larimichthys polyactis). J. Fish. Sci. Chin. 2011, 18, 602–610. [Google Scholar] [CrossRef]
- Bavevi, L.; Petrovi, S.; Karamarko, V.; Luzzana, U.; Klanjek, T. Estimating fish energy content and gain from length and wet weight. Eco. Model. 2020, 436, 109280. [Google Scholar] [CrossRef]
- Martínez, M.; Guderley, H.; Dutil, J.D. Condition, prolonged swimming performance and muscle metabolic capacities of cod gadus morhua. J. Exp. Biol. 2003, 206, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, R.W.; Fabrizio, M.C. Condition Indices as Surrogates of Energy Density and Lipid Content in Juveniles of Three Fish Species[J]. T. Am. Fish. Soc. 2017, 146, 1058–1069. [Google Scholar] [CrossRef]
- Jones, R.E.; Petrell, R.J.; Pauly, D. Using modified length–weight relationships to assess the condition of fish. Aquacult. Eng. 1999, 20, 261–276. [Google Scholar] [CrossRef]
- Latour, R.J.; Gartland, J.; Bonzek, C.F. Spatiotemporal trends and drivers of fish condition in chesapeake bay. Mar. Ecol‐prog. Ser. 2017; 579, 1–17. [Google Scholar]
- Haberle, I.; Bavčević, L.; Klanjscek, T. Fish condition as an indicator of stock status: Insights from condition index in a food-limiting environment. Fish Fish. 2023, 24, 567–581. [Google Scholar] [CrossRef]
- Nikolskiy, G.V. The ecology of fishes. London and New York, Academy press, 1963.
- Pauly, D. Some simple methods for the assessment of tropical fish stocks. Rome, FAO Fish Technical Paper, 1983.
- Stergiou, K.I.; Moutopoulos, D.K. A review of length-weight relationships of fishes from Greek marine waters. Naga 2001, 24, 23–39. [Google Scholar]
- Kulbicki, M.; Guillemot, N.; Amand, M. A general approach to length-weight relationships for new caledonian lagoon fishes. Cybium 2005, 29, 235–252. [Google Scholar]
- Carlander, K.D. Handbook of freshwater fishery biology. Ames, The Iowa State University Press, 1997.
- Zhao, G.Q.; Huang, H.L.; Li, L.Z.; Q, T.C.; Fan, R.L.; Feng, C.L.; Li, S.; Yang, J.L. Biological characteristics of bigeye grunt, Brachydeuterus auritus (Valenciennes, 1831) in the Coastal Waters off Sierra Leone. Mar. Fish. 2003, 45, 680–390. [Google Scholar]
- Sylla, S.; Zan-Bi, T.T.; Konan, K.J.; Tia, C.B.; Tidiani, K. Reproductive biology of big-eye grunt Brachydeuterus Auritus in Ivory coast fishery (West Africa). Sci. J. biolo. Sci. 2016, 5, 158–166. [Google Scholar]
- Konoyima, K.J.; Seisay, L.D. Aspects of reproductive biology of Pseudupeneus prayensis collected from the coast off Sierra Leone, West Africa. J. Appl. Biosci. 2021, 158, 16371–16381. [Google Scholar] [CrossRef]
- Le Løeuff, P.; CoselR, R.V. Biodiversity patterns of the marine benthic fauna on the Atlantic coast of tropical Africa in relation to hydroclimatic conditions and paleogeographic events. Acta Oecol. 1998, 19, 309–321. [Google Scholar] [CrossRef]
- Feng, C.L.; Huang, H.L.; Qu, T.C.; Fan, R.L.; Coker, I.C.R.; Seisay, L.D.; Chen, S.; Yang, J.L.; Rao, X.; Li, L.Z. Temporal and spatial patterns of demersal fish assemblages in the coastal water of Sierra Leone[J]. Reg. Stud. Mar. Sci. 2002, 56, 102674. [Google Scholar] [CrossRef]
- Charlotte, K. The use of the scales of the brown trout (Salmo trutta L.) for the back-calculation of growth. ICES J. Mar. Sci. 1962, 27, 304–315. [Google Scholar]
- Safran, P. Theoretical analysis of the weight-length relationship in fish juveniles. Mar. Biol. 1992, 112, 545–551. [Google Scholar] [CrossRef]
- Froese, R. Evaluating length–weight relationships. In: Froese R, Pauly D (Eds), FishBase 2000: concepts, design and data sources. ICLARM, Los Ban˜os, Laguna, Philippines, 2000, 133.
- Cren, E.D.L. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Panfili, J.; Thior, D.; Ecoutin, J.M.; Ndiaye, P.; Albaret, J.J. Influence of salinity on the size at maturity for fish species reproducing in contrasting west african estuaries. J. Fish Biol. 2010, 69, 95–113. [Google Scholar] [CrossRef]
- Longhurst, A.R. Benthic-pelagic coupling and export of organic carbon from a tropical atlantic continental shelf-sierra leone. Estuar. Coast. Shelf Sci. 1983, 17, 261–285. [Google Scholar] [CrossRef]
- Adebiyi, F.A. Growth pattern of the big eye grunt Brachydeuterus auritus (Valenciennes, 1832) off Lagos, Nigeria[J]. Indian J. Fish. 2013, 60, 9–12. [Google Scholar]
- Samb, B. On the biology of Brachydeuterus auritus Senegalese waters. In: Palomares M L D, Samb B, Diouf T, et al. (Eds.), Fish biodiversity: Local studies as basis for global inferences[R]. Brussels: African Caribbean and Pacific Groups of States, European Union, 2003, 1-13.
- Asabere-Ameyaw, A. Observations on the reproductive biology and recruitment of the big eye grunt Brachydeuterus auritus (Pisces: Haemulidae), in Ghana. J. Gh. Sci. Assoc. 2003, 3, 14–21. [Google Scholar]
- Longhurst, A.R. Bionomics of the sciaenidae of tropical west africa. ICES J. Mar. Sci. 1965, 1, 93–114. [Google Scholar] [CrossRef]
- Šantić, M.; Rađa, B.; Pallaoro, A. Diet and feeding strategy of thornback ray Raja clavata[J]. J. Fish Biol. 2012, 81, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Claudino, M.C.; Bastos, R.F. ; Huckembeck; Garcia, M.A. Feeding ecology and prey preferences of a piscivorous fish in the Lagoa do Peixe National Park, a Biosphere Reserve in Southern Brazil[J]. Environ. Biol. Fish. 2012; 93, 1–12. [Google Scholar]
- Gerking, S.D. Feeding Ecology of Fish. San Diego, Academic Press, 1994.
- Ssentongo, G.W.; Ansa-Emmin, M. Marine Fishery Resources of Sierra Leone: A Review of Exploited Fish Stocks[R]. In: CECAF/ECAF Series (FAO), 1986.
- Kantoussan, J.; Ecoutin, J.M.; Simier, M. Effects of salinity on fish assemblage structure: an evaluation based on taxonomic and functional approaches in the casamance estuary (senegal, west africa) - sciencedirect. Estuar. Coast. Shelf Sci. 2012, 113, 152–162. [Google Scholar] [CrossRef]

| Survey indicators | Sep | Oct | Dec | Jan | Apr | May |
|---|---|---|---|---|---|---|
| SST/℃ | 27.76±1.00 | 27.76±0.79 | 29.40±0.48 | 28.64±0.44 | 28.89±0.39 | 29.29±0.47 |
| Sal | 28.13±5.68 | 30.70±2.63 | 31.85±0.79 | 33.90±0.38 | 35.54±0.10 | 35.36±0.47 |
| Dep/m | 30.72±9.40 | 31.40±7.15 | 26.94±8.80 | 25.54±7.59 | 26.66±8.08 | 25.51±8.22 |
| Month | Female | Male | ||||||
| a(×10-2) | b | R2 | t | a(×10-2) | b | R2 | t | |
| Sep | 3.19 | 2.95 | 0.98 | -11.41** | 4.17 | 2.84 | 0.98 | -35.36** |
| Oct | 3.24 | 2.94 | 0.99 | -26.28** | 2.82 | 2.99 | 0.99 | -0.74ns |
| Dec | 2.90 | 2.97 | 0.99 | -7.22** | 2.82 | 2.99 | 0.99 | -3.22* |
| Jan | 2.32 | 3.05 | 0.99 | 27.63** | 1.85 | 3.14 | 0.99 | 64.64** |
| Apr | 1.34 | 3.29 | 0.99 | 125.05** | 1.45 | 3.26 | 0.99 | 88.15** |
| May | 1.99 | 3.13 | 0.99 | 58.55** | 2.15 | 3.11 | 0.99 | 44.41** |
| Parameter | Month | |||||
|---|---|---|---|---|---|---|
| Sep | Oct | Dec | Jan | Apr | May | |
| F | 0.038** | 0.94** | 0.14** | 0.21** | 1.34** | 0.045** |
| Sex | b | Mean body length | Skewness | Proportion of maturity in female | SST | Sal | Dep | |
|---|---|---|---|---|---|---|---|---|
| Female | a | -0.89** | 0.51 | 0.57 | 0.53 | -0.581 | -0.85* | 0.74 |
| b | — | -0.48 | -0.6 | -0.55 | 0.486 | 0.84* | -0.61 | |
| Male | a | -0.9** | 0.07 | -0.86* | — | -0.573 | -0.79* | 0.73 |
| b | — | -0.12 | 0.77 | — | 0.522 | 0.83* | -0.83 | |
| Factor | SST | Sal | Dep | b |
| Correlation Coefficient |
0.63 | 0.88* | -0.97** | 0.88* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




