1. Introduction
Migraine is a primary headache disorder which lasts up to 72 hours and is usually characterized by moderate or severe recurrent episodes of pulsating pain with most often unilateral appearance and a range of accompanying symptoms. Its first appearance in patients is more often in puberty, with the peak between 35 and 45 years and is more present in the female population. As a global burden, it is presented in approximately 15% of the population [
1,
2,
3]. In 2018 it is classified into three main groups: migraine without aura, migraine with aura and chronic migraine, according to the International Headache classification, 3rd edition [
4]. Aura represents transient focal neurological symptoms that usually precede, but sometimes accompany the headache phase of migraine attack and is present in one third of individuals who have migraine. Migraine is one of the most prevalent neurological diseases and although its pathogenesis is not fully understood, an increasing amount of evidence supports the involvement of the neurovascular system in the development of the disease. Previous knowledge, based on Wolff’s and Graham’s vascular theory of the pathophysiology of migraine in which the aura and headache are due to vasoconstriction and vasodilation of blood vessels of the brain, are not accepted in their entirety today, but they are not abandoned completely. According to the neurogenic (or neurovascular) theory, the migraine patients in the condition without a headache, have hyper excitability of the neurons of the cerebral cortex, especially the occipital cortex. Observing the pathophysiology of migraine with aura, the theory of cortical spreading depression explains the symptoms of the aura, followed by the activation of the trigeminal vascular system and the development of the next phase of migraine attack - pain. The release of neurotransmitters and vasoactive intestinal peptides is increased with activation and sensitization of the trigeminal vascular system (TGVS). This activation of injurious receptors results in the transmission of pain signals to the centre via the trigeminal nociceptive afferent fibres, hence causing pain. [
5,
6,
7,
8]. Visual aura is known as the most common aura symptom during migraine attacks. Transient vasospasm during visual aura or even migraine without aura can cause decreased blood flow and perfusion deficit in the ocular vasculature, similar to that of the cranium. Cerebral and ocular vascular changes and subsequent transitory constriction of the retinal and ciliary arteries lead to possible ischemic damage of the optic nerve, retina or choroid causing retinal layers thickness changes [
9]. Optical coherence tomography (OCT) represents a simple and noninvasive procedure to assess such morphological retinal and choroidal changes in individuals with neurodegenerative diseases but also in migraine patients, utilizing infrared wavelengths with sensitivity of 8-10 micrometers [
10,
11,
12]. Optical coherence tomography (OCT) can be used to quantitatively and qualitatively detect and measure thickness of retinal layers such as retinal nerve fiber layer (RNFL), ganglion cell layer (GCL) and inner plexiform layer (IPL). Several recent clinical studies have reported alteration in the retinal perfusion, microvascular alterations and consequent changes in the thickness of specific retinal layers in migraine individuals but different and even contradictory results have been obtained. We performed extensive clinical study and investigated the impact of migraine with or without aura on possible chances of thickness of seven retinal layers but in this paper we will focus primarily on the inner plexiform layer (IPL).
2. Methods
This case-control study was conducted in 175 participants, 88 individuals had a diagnosis of migraine and 87 were healthy/control participants. The study protocol adhered to the tenets of the Declaration of Helsinki and was previously approved by the Hospital Ethical Board. Each participant was informed about the study and signed an informed consent form before participation in the study. 88 participants who had a diagnosis of migraine with or without aura were treated by neurologist in the Department of Neurology, University Hospital Center Osijek, Croatia. Key (general) criteria for participants with migraine: age older than 18 years, diagnosis of migraine with or without aura confirmed by neurologist, no pathological findings on native magnetic resonance imaging (MRI) of the brain and brain blood vessels including white matter lesions, no pathological findings in neurological examination. Exclusion criteria for the group of cases, i.e., participants with migraine: inability to cooperate with to the patient due to the presence of cognitive or psychiatric disorders, suspected or confirmed neurodegenerative or demyelinating disease of the central nervous system, head trauma, nystagmus verified by neurological examination, myopia or hyperopia > 2 diopters, intracranial operations, eye diseases, ocular hypertension (>20 mm Hg), history of glaucoma, diabetic maculopathy, diabetic retinopathy, epiretinal membrane, vitreomacular traction, any other pathology of the posterior pole of the eye, post-traumatic eye events, eye infections, optic nerve conduction disorders verified by visual evoked potentials, systemic diseases affecting the brain, optic nerve and eye (diabetes mellitus, hypertension or low blood pressure, dyslipidemia, coagulation disorders, cardiomyopathy) or if they were taking any medication that could affect retinal thickness. Subjects who met all the inclusion criteria and none of the exclusion criteria were invited to participate in optical coherence study (OCT) after providing written informed consent. 87 participants were healthy individuals who also had to be older than 18 years and be able to sign informed consent form. Same exclusion criteria were established for groups of healthy controls and they were matched by gender and age.
Procedure: The study was conducted using a high-resolution spectral-domain scan Heidelberg Spectralis® OCT device. It provides a capture rate of 40000 A-scans per second with image magnification of 10 micrometers per pixel. Measurements of the inner plexiform layer in all participants were obtained in the same room, under the same conditions during the morning hours for all participants. For all subjects with migraines, the OCT scan was performed for a minimum of 48 hours after the last migraine acute attack and the application of acute therapy for a migraine attack. All the scans were performed by the same ophthalmologic technician. The system was self-centered on fovea and papilla; all scans were fully automatically aligned and focused using real-time eye tracking. All captured scans were automatically saved and analyzed by the OCT software.
Statistical analysis: Data processing was done in the statistical program PSPP v.3 Statistical methods which were used to answer the hypotheses of the work were Student’s t-test and Pearson’s coefficient correlations. The defined level of significance was p <0.05. The normality of the distribution of optical results coherence tomography (OCT) was checked with the Kolmogorov Smirnov test. The used measure of dispersion of the results is the arithmetic mean and standard deviation.
3. Results
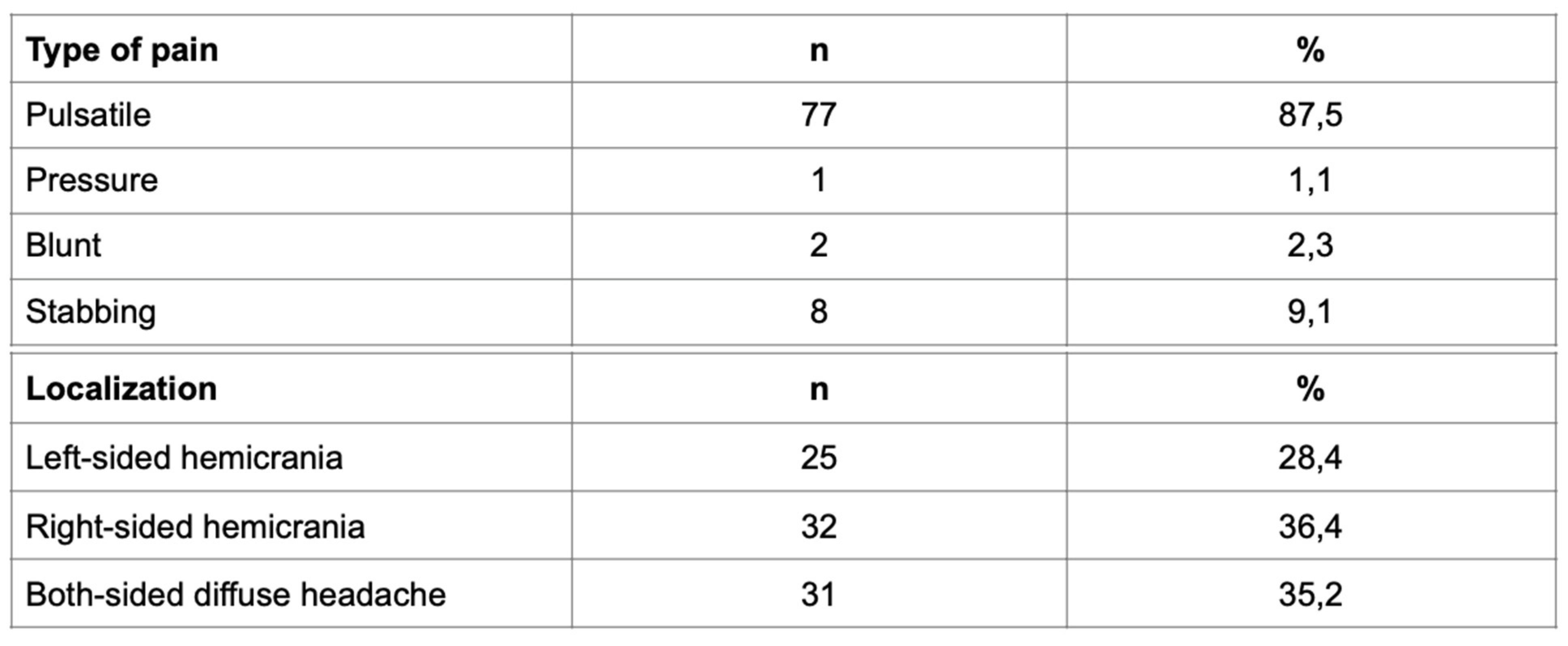
Overall, 175 respondents participated in the research, 146 (84%) of them were female with an average age of 38±11 and 29 (16%) were male patients with an average age of 36±9. 88 (50.3%) respondents had a diagnosis of migraine and 87 (49.7%) were healthy/control subjects. In patients with migraine, 49 (56%) of them reported migraine without aura, whereas 39 (44%) patients had migraine with aura. Furthermore, a positive family history of migraine was documented in 31 (35%) patients; in 26 (84%) respondents migraine was found in parents, and in 5 patients (16%), it was documented in siblings. In the same group, detailed analysis revealed the incidence of migraine with aura in 11 (28%) patients, and without aura in 20 (41%) patients. All participants with migraine with aura reported visual aura; among them 51% of participants have had fortification spectra and 39% of them have had partial vision loss (scotoma). Character of the pain and the localization of migraine can be seen in
Table 1.
Table 1.
Descriptive data presenting the type of migraine pain and its localization.
Table 1.
Descriptive data presenting the type of migraine pain and its localization.
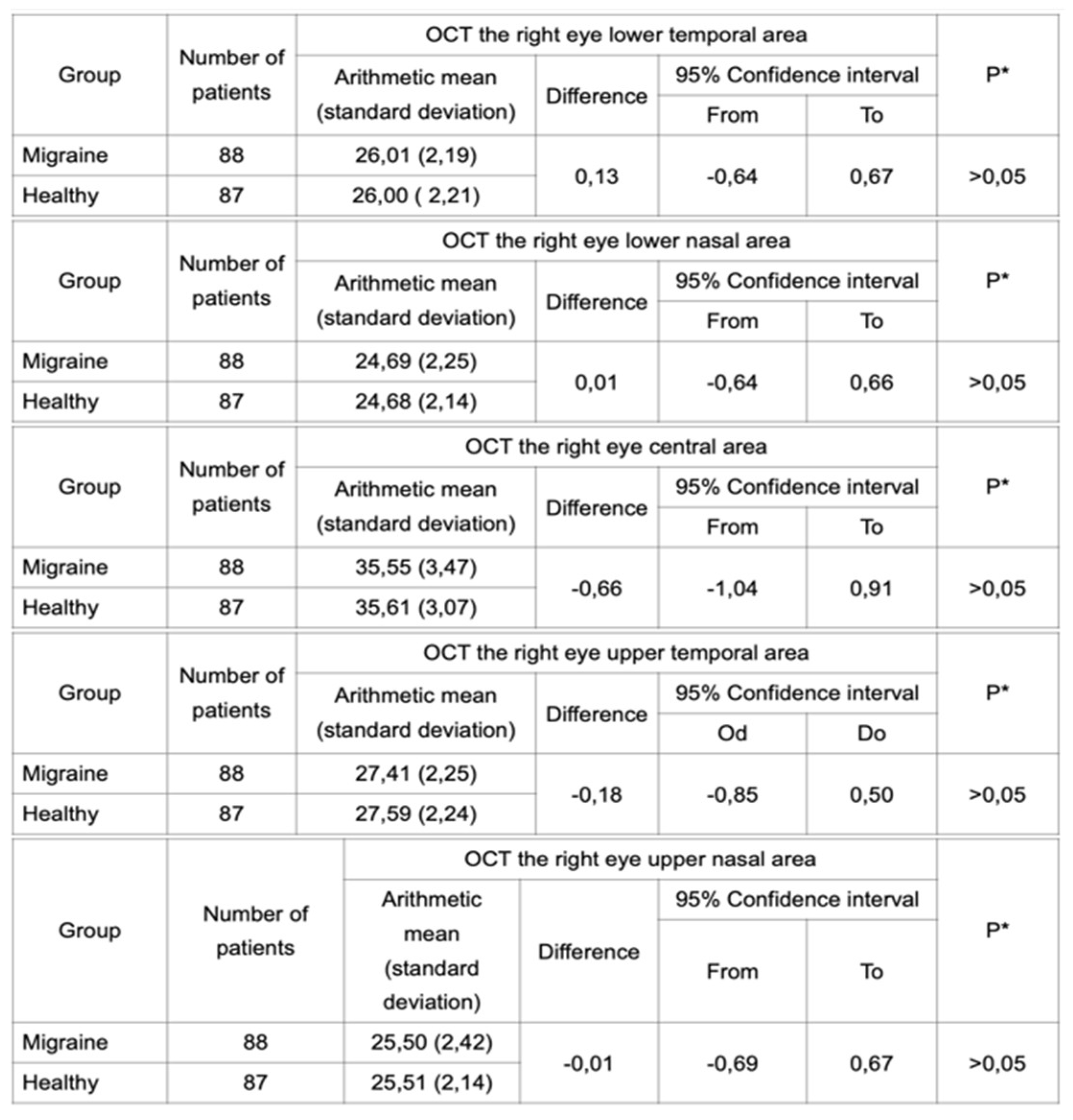
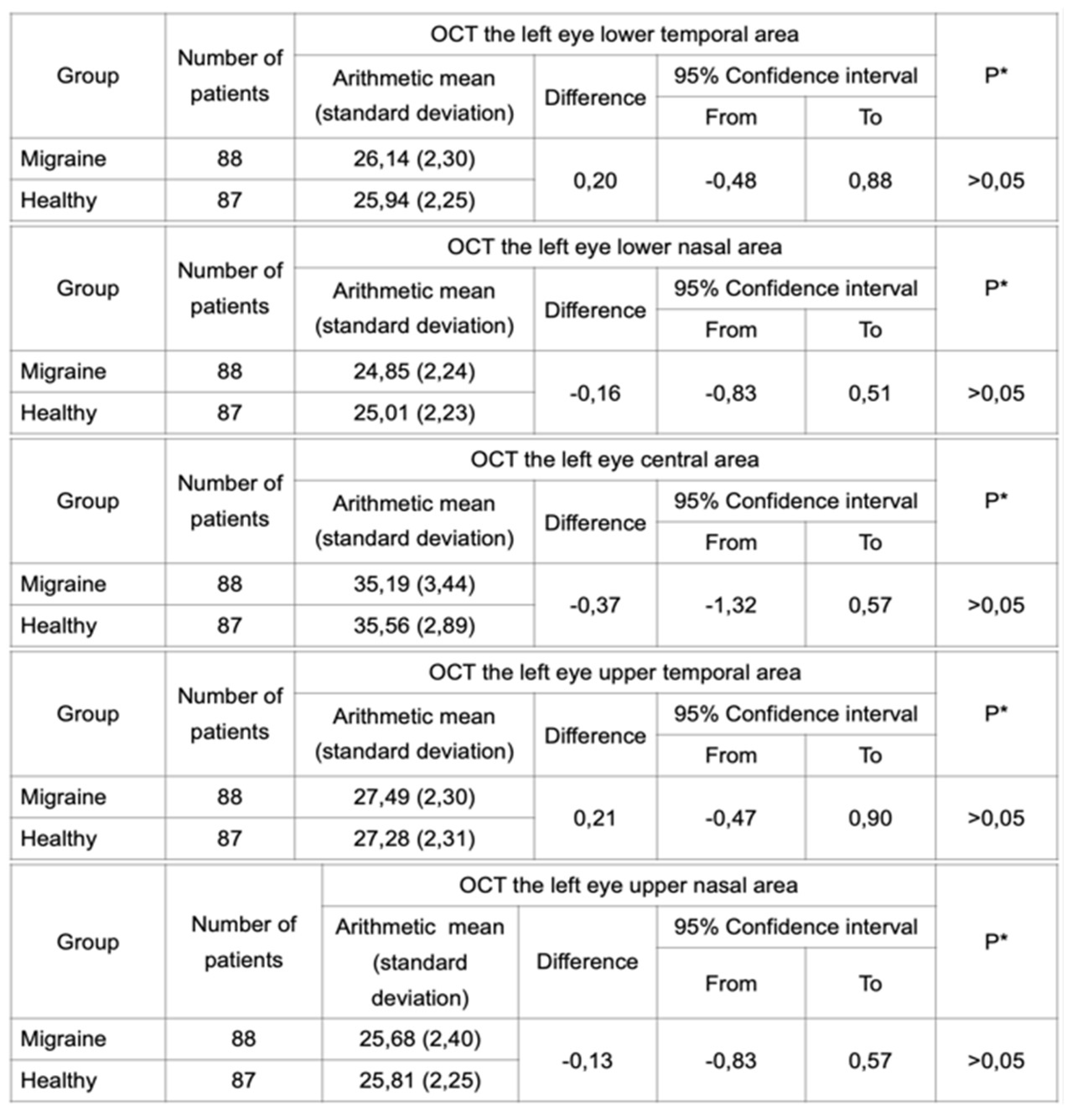
Student’s t-test was performed in independent samples for each eye separately to analyze a difference in thickness of the IPL. Two groups of subjects were analyzed: patients with migraine and healthy patients. OCT was performed in both groups and the results did not reveal any statistically significant difference between these groups (
Table 2 and
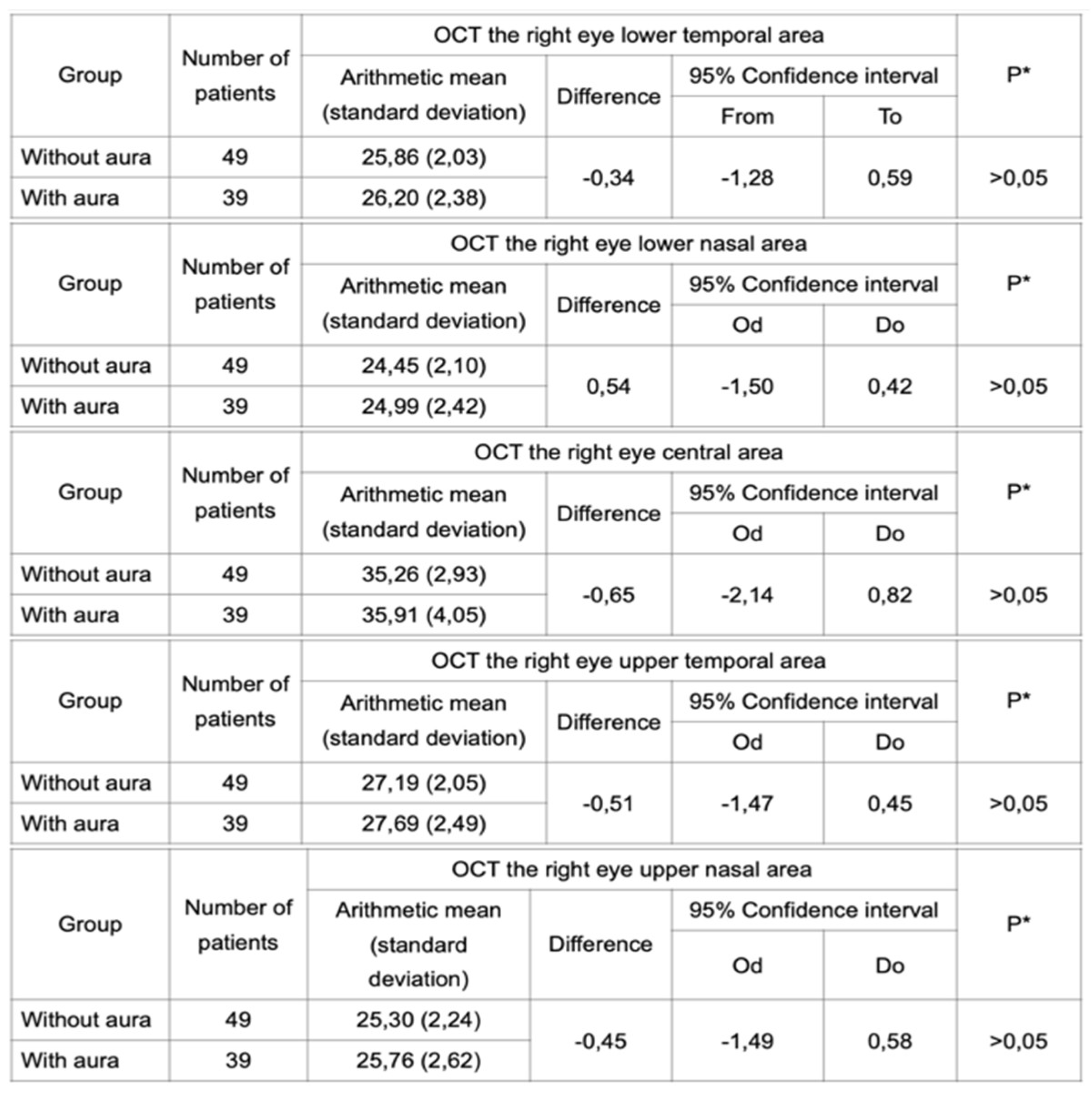
Table 3). Furthermore, the same test was performed in groups of subjects with migraine with aura and migraine without aura. Statistical analysis did not reveal any difference in IPL thickness between these two groups (
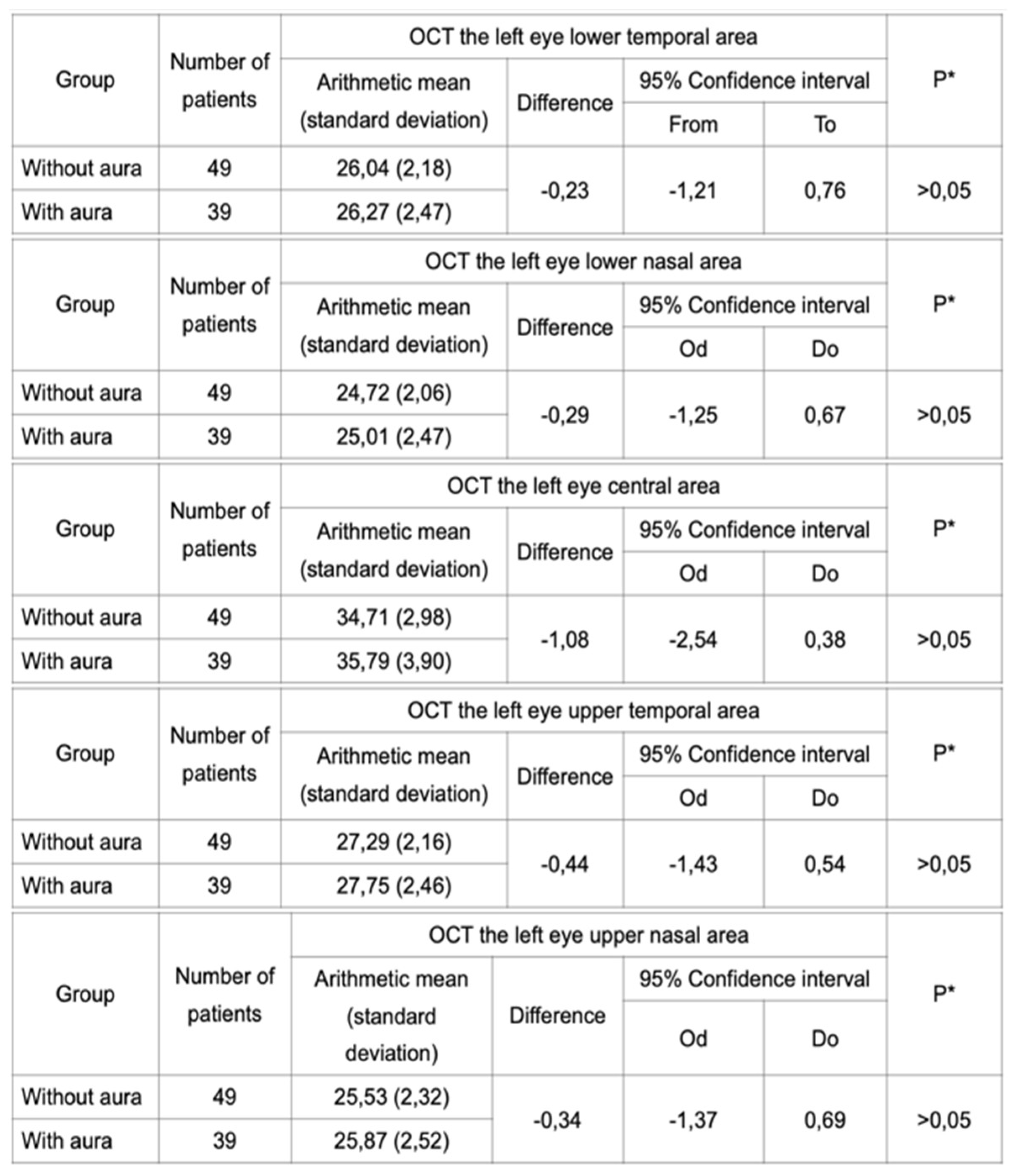
Table 4 and
Table 5).
Table 2.
OCT performed for the right eye in a group of migraineurs and healthy subjects did not reveal a statistically significant difference in the IPL thickness.
Table 2.
OCT performed for the right eye in a group of migraineurs and healthy subjects did not reveal a statistically significant difference in the IPL thickness.
Table 3.
OCT performed for the left eye in a group of migraineurs and healthy subjects did not reveal a statistically significant difference in the IPL thickness.
Table 3.
OCT performed for the left eye in a group of migraineurs and healthy subjects did not reveal a statistically significant difference in the IPL thickness.
Table 4.
OCT performed for the right eye in a group of migraineurs with and without aura did not reveal a statistically significant difference in the IPL thickness.
Table 4.
OCT performed for the right eye in a group of migraineurs with and without aura did not reveal a statistically significant difference in the IPL thickness.
Table 5.
OCT performed for the left eye in a group of migraneurs with and without aura did not reveal a statistically significant difference in the IPL thickness.
Table 5.
OCT performed for the left eye in a group of migraneurs with and without aura did not reveal a statistically significant difference in the IPL thickness.
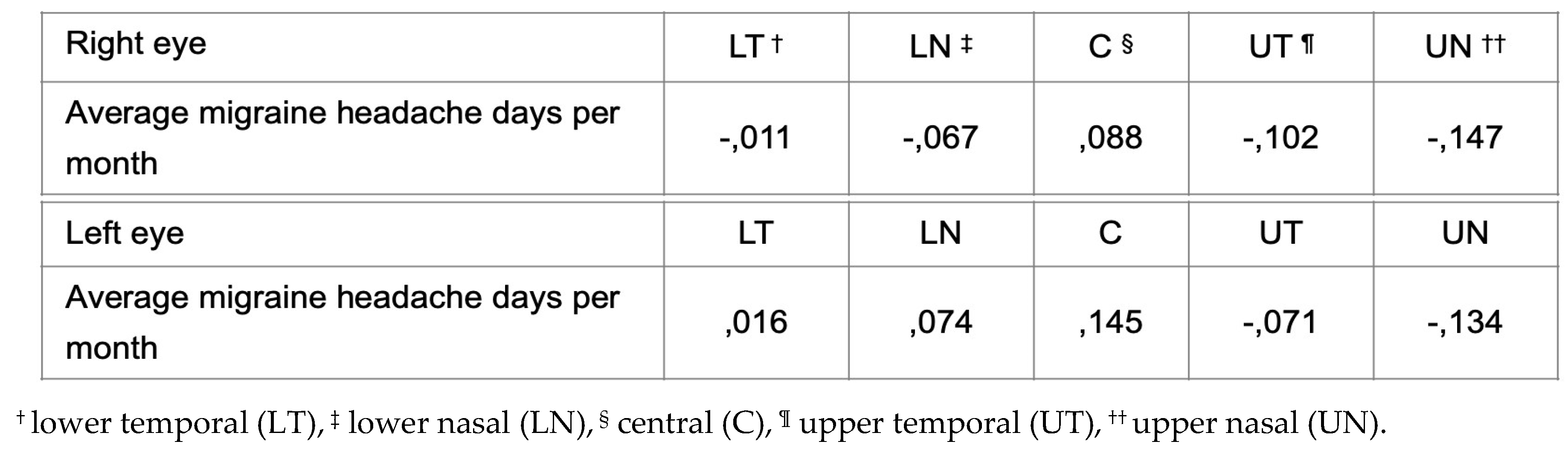
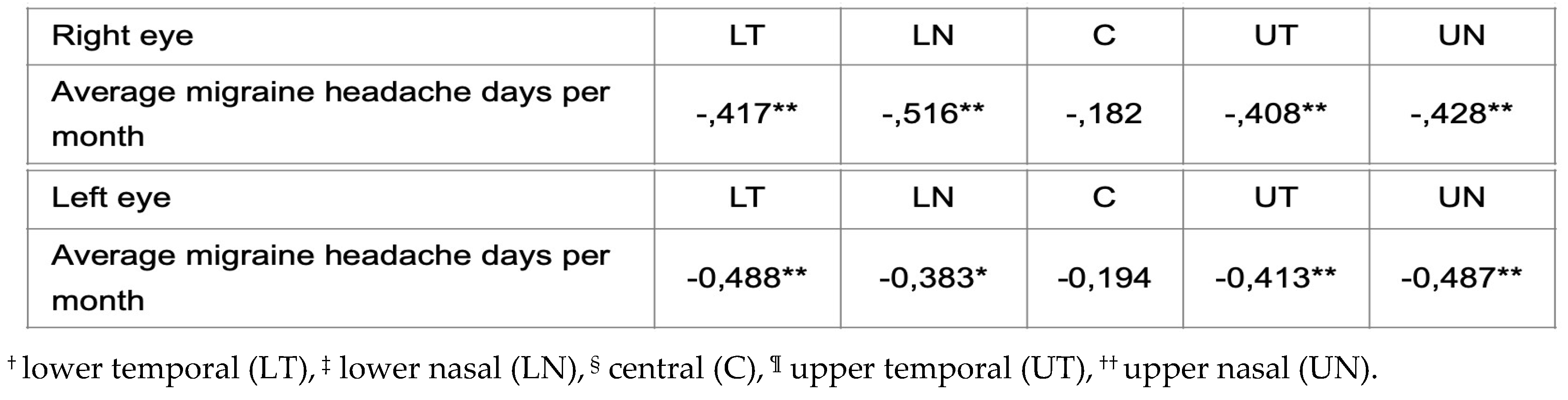
Authors performed two separate Pearson’s tests in order to evaluate the association between the average number of migraine headache days per month and changes in the thickness of the IPL using optical coherence tomography (OCT) in subjects with migraine. A test was performed for the group of patients with migraine without aura and the second test was made for the group of patients with migraine with aura. The results in the first group did not reveal a statistically significant difference. However, statistically significant moderate negative correlation in all quadrants of both eyes except the central area was found in the group of patients with migraine with aura. The result indicates the proportion of average migraine headache days per month and changes in the thickness of the IPL, which is presented in
Table 6 and
Table 7.
Table 6.
Pearson’s test for the patients with migraine without aura revealed no significant differences in the IPL thickness in all quadrants.
Table 6.
Pearson’s test for the patients with migraine without aura revealed no significant differences in the IPL thickness in all quadrants.
Table 7.
Pearson’s test for the patients with migraine without aura revealed moderate differences in the IPL thickness in all quadrants except the central area.
Table 7.
Pearson’s test for the patients with migraine without aura revealed moderate differences in the IPL thickness in all quadrants except the central area.
4. Discussion
Our study was conducted in order to find potential retinal layer changes in a specific layer of the IPL which consists of synaptic connections between the axons of bipolar cells and dendrites of ganglion cells. The IPL contains the synapse between the second-order and third-order neuron in the visual pathway and potential gaps in examination of migraine patients in this layer emerged. Migraines mostly affect the female population [
13], as was also seen in the sample of our subjects included in the study; the proportion of women in the sample was 84%. The beginning of migraine can occur at any age, but the first notable crisis usually occurs during adolescence with the peak between 30 and 50 years of age. Migraine usually becomes significantly less intense and frequent during the following decades. Our study confirmed these claims as the mean age of our patients was 38±11and the presence of the male gender was negligible. Migraine is one of the most prevalent neurological conditions worldwide, with an estimated more than one billion cases occurring yearly [
14]. As a cyclic disorder, it has different phases: premonitory phase, transient neurological symptoms, intense headache attack and postdrome phase [
15,
16]. Underlying theory of the development and the etiology of migraines remain to be elucidated despite recent advancements in diagnosis, even though neurogenic and vasogenic theories have been proposed. The vasogenic theory in the development of migraine prevailed during the 20th century, which proposed vasodilation as the cause of migraine pain. Nevertheless, the neurogenic theory has gained more momentum lately and has been described as an incorrect cerebral interpretation of normal sensory input in the trigeminal sensory system as the source of migraine discomfort [
17]. Regardless of theory, studies of decreased retinal thickness in migraine patients were recently conducted. The use of OCT as the main tool in retinal pathology provides pertinent information regarding diagnosis and diversity of conditions that lead to retinal tissue degeneration and alterations. This technique allows an accurate measurement of the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL) or macular volume. As a non-invasive technique based on the principle of low-coherence light interferometry, it became a generally accepted method increasingly used in analysis of the anterior eye segment. Technically, OCT is using infrared light reflection in order to acquire axial cross-sectional images with resolution of less than 10 microns [
18]. The importance of OCT becomes more obvious as the retina is an extension of the central nervous system and provides the corridor into abnormal processes of the brain. Lately, OCT has been introduced as a diagnostic tool that has provided concrete proof of RNFL thinning and GCL abnormalities in a variety of neurological conditions involving disorders of the nervous system. Findings of these studies suggest a relationship between specific retinal layers thickness, brain atrophy, and clinically manifested visual dysfunction, making the eye a useful model for researching multiple sclerosis and neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease [
12,
19,
20,
21]. Structural changes of the optic nerve in migraine are caused by compromised choroidal blood flow which leads to a focal ischemic damage of the optic disk. In order to confirm the connection between migraine and retinal layers changes, several studies have been conducted so far in the last decade, with inconsistent results. As expected, in our study we found female predominance among our subjects; migraine without aura (MwoA) was found in 56% and with aura (MwA) in 44% of patients, respectively. Familiar inheritance in MwA was found in 85% of subjects and chronic migraine was found in only 6% of our subjects. We examined 175 subjects, i.e., 350 eyes and results of the OCT scanning of the IPL of the left and the right eye did not reveal significant differences among previously defined groups of subjects. Also, comparing groups of patients with and without aura, OCT analysis did not reveal any significant differences between these two groups. The association between frequency of migraine and thickness of IPL among groups of subjects with MwoA and MwA according to Pearson’s test did not reveal any statistically significant differences in the group of subjects with MwoA. Nevertheless, statistically significant moderate negative correlation in all quadrants of both eyes except the central area was found in subjects with MwA.
Regardless of our results, only recently in 2023 Liinamaa et al. published the largest cohort study on retinal neural tissue and vascular changes [
22]. Among 375 migraine patients they did not find any statistically significant differences between healthy controls and migraineurs in average RNFL. They also emphasized that migraine did not have any impact on other retinal layers subfields; this study should be taken seriously according to its total number of migraine subjects and healthy controls. Our results are consistent with the aforementioned study, although notably smaller groups of migraineurs and healthy controls were included in our study. Other studies reported lesser RNFL thickness in migraineurs when compared to healthy controls and some of them observed thinner RNFL in specific quadrants. Raga-Martinez et al. in 2023 reported lesser thickness of the superior quadrant of the peripapillary RNFL, also the same study reported reduced mean thickness of GCL-IPL in chronic migraine patients [
12]. Similarly to our results, Tak el al. in 2018 detected no significant differences of IPL among investigated groups [
23]. Jie et al. published in 2023 meta-analysis of sixteen identified studies in which they found decreasing of RNFL in subjects with MwA and MwoA; also, in subjects with MwA retinal microvascular impairments were found [
5]. We can conclude that our results displayed a similar outcome with aforementioned meta-analysis; we can also emphasize that the number of the eyes examined in our study was notably greater in comparison to any single study included in the previously mentioned meta-analysis. To summarize, we did not find significant changes of IPL in migraine patients. Moreover, the advantage of our study was a completely homogenous group of patients and healthy controls with a relatively large number of subjects. Also, as the first study conducted in the Republic of Croatia it is of our great interest to compare our population with previously conducted studies. According to presented results, authors can conclude that there was no influence of migraine on the changes of examined IPL; although we have to emphasize the need for further investigation of the relationship between average migraine headache days per month and the thickness of the IPL.
References
- Ashina, M. Migraine. N. Engl. J. Med. 383, 1866–1876 (2020).
- Eigenbrodt, A.K., Ashina, H., Khan, S. et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17, 501–514 (2021). [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018 Jan;38(1):1-211. [CrossRef] [PubMed]
- Liu Z, Jie C, Wang J, Hou X, Zhang W, Wang J, Deng Y and Li Y (2023) Retina and microvascular alterations in migraine: a systemic review and meta-analysis. Front. Neurol. 14:1241778. [CrossRef]
- Reggio E, Chisari CG, Ferrigno G, Patti F, Donzuso G, Sciacca G, et al. Migraine causes retinal and choroidal structural changes: evaluation with ocular coherence tomography. J Neurol. 2017;264(3):494–502.
- Shayestagul NA, Christensen CE, Amin FM, Ashina S, Ashina M. Measurement of blood fow velocity in the middle cerebral artery during spontaneous migraine attacks: a systematic review. Headache. 2017;57(6):852–61.
- Zhang X., Levy D., Kainz V., Noseda R., Jakubowski M., Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2010;69:855–865. [CrossRef]
- Abdelghaffar, M., Hussein, M., Thabet, N.H. et al. The potential impact of migraine headache on retinal nerve fiber layer thickness. Egypt J Neurol Psychiatry Neurosurg 58, 141 (2022). [CrossRef]
- Goadsby, P.; Charbit, A.; Andreou, A.; Akerman, S.; Holland, P. Neurobiology of migraine. Neuroscience 2009, 161, 327–341.
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Schuman, J.S. Optical coherence tomography: History, current status, and laboratory work. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2425–2436.
- Raga-Martínez, I.; Povedano-Montero, F.J.; Hernández-Gallego, J.; López-Muñoz, F. Decrease Retinal Thickness in Patients with Chronic Migraine Evaluated by Optical Coherence Tomography. Diagnostics 2023, 13, 5. [CrossRef]
- Allais G, Chiarle G, Sinigaglia S, Airola G, Schiapparelli P, Benedetto C. Gender-related differences in migraine. Neurol Sci. 2020 Dec;41(Suppl 2):429-436. [CrossRef] [PubMed] [PubMed Central]
- Safiri S, Pourfathi H, Eagan A, Mansournia MA, Khodayari MT, Sullman MJM, et al.. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. PAIN. (2022) 163:e293–309. [CrossRef]
- Sacco S, Braschinsky M, Ducros A, Lampl C, Little P, van den Brink AM, et al.. European headache federation consensus on the definition of resistant and refractory migraine. J Headache Pain. (2020) 21:76. [CrossRef]
- Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. (2019) 20:117. [CrossRef]
- Shibata Y. Migraine Pathophysiology Revisited: Proposal of a New Molecular Theory of Migraine Pathophysiology and Headache Diagnostic Criteria. Int J Mol Sci. 2022 Oct 27;23(21):13002. [CrossRef] [PubMed] [PubMed Central]
- Razaghi, G., Hedayati, E., Hejazi, M. et al. Measurement of retinal nerve fiber layer thickness with a deep learning algorithm in ischemic optic neuropathy and optic neuritis. Sci Rep 12, 17109 (2022). [CrossRef]
- Maldonado R.S., Mettu P., El-Dairi M., Bhatti M.T. The application of optical coherence tomography in neurologic diseases. Neurol. Clin. Pract. 2015;5:460–469. [CrossRef]
- Saidha S., Al-Louzi O., Ratchford J.N., Bhargava P., Oh J., Newsome S.D., Prince J.L., Pham D., Roy S., van Zijl P., et al. Optical coherence tomography reflects brain at-rophy in multiple sclerosis: A four-year study. Ann. Neurol. 2015;78:801–813. [CrossRef]
- Ascaso F.J., Cruz N., Modrego P.J., Lopez-Anton R., Santabárbara J., Pascual L.F., Lobo A., Cristóbal J.A. Retinal alterations in mild cognitive im-pairment and Alzheimer’s disease: An optical coherence tomography study. J. Neurol. 2014;261:1522–1530. [CrossRef]
- Ristioja, S., Leiviskä, I.L., Saarela, V.O. & Liinamaa, M.J. (2023) Retinal neural tissue and vascular calibres in migraine: the Northern Finland Birth Cohort Eye Study. Acta Ophthalmologica, 00, 1–10. [CrossRef]
- Tak, A.Z.A., Sengul, Y. & Bilak, Ş. (2018) Evaluation of white matter hyperintensities and retinal fiber layer, ganglion cell layer, inner-plexiform layer, and choroidal layer in migraine patients. Neurological Sciences, 39, 489–496.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).