1. Introduction
PD is the second most common degenerative disease which is characterized with motor and non-motor symptoms [
1]. Hereditary and environmental factors are both implicated in pathogenesis. A-synuclein misfolding and aggregation, mitochondrial dysfunction, reduced protein clearance, neuroinflammation and oxidative stress are putative pathophysiological processes. It is proposed that concerning gut-brain axis, vagus nerve acts like “a highway” to carry aggregated α-synuclein from gastrointestinal system to lower brainstem [
2]. Even the molecular basis of inflammation is yet to be determined there is enhancing evidence which support the role of inflammation in evolution of Parkinson’s disease. Both innate and adaptive immune abnormalities such as increased levels of proinflammatory cytokines and altered immune cell population (such as monocytes and progenitors) have been shown in PD [
3,
4]. Bradykinesia, resting tremor, rigidity, postural instability are the major motor symptoms. Non-motor symptoms such as cognitive dysfunction, depression, psychosis, apathy, impulse control disorder, autonomic dysfunction can be debilitating as much as motor symptoms. Drooling, orthostatic hypotension, supine hypertension, urinary retention/incontinence, erectile dysfunction, gastrointestinal motility disorders, hyperhidrosis are frequently encountered autonomic disorders [
5]. Unified Parkinson’s Disease Rating Scale (UPDRS) is used to measure and follow the symptom severity [
6]. Symptomatic treatment of PH relieves some of the symptoms however with some undesired consequences such as blood pressure and heart rate changes.
The autonomic nervous system controls homeostasis by regulating unconscious, involuntary and vital bodily functions like heartbeat, blood pressure, respiratory functions and gastrointestinal system motility, urinary and secretory functions [
7,
8]. However, the autonomic involvement in PD has been known for a long-time epidemiological information is limited due to inadequate attention to symptoms, lack of clear definitions of symptoms and scales to measure them objectively [
9]. Dysautonomia symptoms may appear as cardiovascular, urinary, and sexual dysfunctions or thermoregulatory and pupillomotor abnormalities. Orthostatic hypotension (OH) which is the most encountered cardiovascular symptom (%30) in PD patients from the beginning, affects the daily activities in a negative way [
10]. Supine hypertension (SH) is observed as much as 34 % in PD patients which is thought to be a sign of autonomic cardiovascular dysfunction which accompany OH. It is suggested that SH is related to myocardial infarction, cognitive dysfunction and stroke in the long term [
11,
12]. Studies concerning the nighttime variation of blood pressure in PD patients revealed different results. Reverse dipping phenomenon shows nighttime blood pressure abnormality which is also a biomarker of cardiovascular autonomic dysfunction.
Valsalva maneuver, hyperventilation, isometric contraction test, cold pressure test, orthostatic tests, head tilt test, ambulatory blood pressure monitoring and Holter monitoring are the tests to detect autonomic cardiovascular involvement [
10]. Blood pressure (BP) has a circadian rhythm which is characterized by a decrease during the nighttime sleep and increase during the awakening. In the morning our activation of sympathetic system, release of renin and cortisol hormones occur so that blood pressure and heart rate increase. This increase in blood pressure is called morning surge (MS). Blood pressure MS is a physiological phenomenon however when it is exaggerated it becomes pathological which is thought to be related myocardial infarction, stroke and death [
13]. Old age, abnormal glucose metabolism, smoking, endothelial dysfunction, atherosclerosis may cause exaggerated MBPS [
14]. This abnormal activity can be documented with ambulatory blood pressure monitoring (ABPM) for 24 hours.
Fibrinogen has a regulatory function for inflammation and atherosclerosis. High fibrinogen levels are related with increased inflammation and affects blood viscosity, platelet aggregation and endothelial cell function. In contrast albumin has anti-inflammatory and antioxidant properties so that low serum levels reflect high systemic inflammatory state. Low albumin level is an independent predictor of high BP [
15]. Fibrinogen/albumin ratio is implicated in physio pathologic process of cerebrovascular and cardiovascular diseases [
16].
Based on these data we wanted to search if there is a relationship between the morning surge and inflammatory biomarkers such as fibrinogen to albumin ratio in PD patients.
2. Materials and Methods
This retrospective study was carried out in Neurology and Cardiology Departments of Balikesir University School of Medicine Hospital. The ethical approval of the study was given by Ethical Committee of Balikesir University Faculty of Medicine. The patient group included 50 PD patients over 18 years of age who were followed up with 24-hour ABPM recordings (Schiller MT-300 BP, Baar, Switzerland) for blood pressure changes in the movement disorders outpatient clinic of the Neurology Department.
Serum fibrinogen, albumin, hsCRP values were obtained from the digital archive of the hospital. FAR was obtained by dividing the fibrinogen value by the albumin value. Fifty age- and gender-matched patients without any systemic disease and with normal 24-hour ABPM records were taken from the Cardiology Department archive and constituted the control group.
In PD, Hoehn-Yahr and UPDRS motor scores calculated by a physician specialized in movement disorders just before the 24-hour ambulatory blood pressure measurement were obtained from the follow-up files.
Ambulatory Blood Pressure Measurement
ABPM was measured with a Schiller MT-300 BP, Baar, Switzerland. Ambulatory blood pressure measurement was performed automatically every 15 minutes during the day and every 30 minutes at night for 24 hours with a cuff attached to the left arm. The sleeping and waking times of all patients were recorded. The volunteers' sleeping and waking times were automatically assessed 24 hours a day and night. Automatically. Mean systolic blood pressure in the first 2 hours after waking up was taken as MBPS.
MBPS, the difference between the mean systolic BP (SBP) over 2 hours following the awakening and the average of three BP values centered on the lowest nocturnal BP [
17].
When the publications on MBPS were scanned; since a threshold value could not be determined exactly, low-value MS (<37 mmHg) and high-value MS (≥37 mmHg) were taken as low-value MBPS (<37 mmHg) and high-value MBPS (≥37 mmHg) determined based on a meta-analysis including eight different populations by Le et al. [
18]. Patients who had kidney, liver disease or inflammatory disorders were excluded.
3. Statistical Analysis
Data were analyzed with IBM SPSS V23 and R software. Compliance with normal distribution was analyzed by Kolmogorov-Smirnov test. Independent two-sample t test was used to compare normally distributed data and Mann-Whitney U test was used to compare non-normally distributed data. Yates correction was used to compare categorical variables according to groups. To examine the effect of independent variables on MBPS values, MBPS values were analyzed by Robust regression analysis using MASS package since MBPS values did not show normal distribution. ROC analysis was used to determine the cut-off value of the Morning Surge parameter. Analysis results were presented as mean±s. deviation and median (minimum - maximum) for quantitative data and frequency (percentage) for categorical data. Significance level was taken as p<0.050.
4. Results
Fifty idiopathic PD patients and 50 control cases were included. PD and control group were not differed according to age and sex (
Table 1 and
Table 2.) Eleven of the PD patients (22%) had additional illness (5: diabetes, 3: diabetes and hypertension, 1: patient diabetes, coronary artery disease, hyperlipidemia, 2: depressive disorder). Demographic data of the participants are given on
Table 1.
Thirty-nine (78%) of PD patients had accompanying illness. Twenty-two (44%) of the group had Hoehn-Yahr stage 1 disease. Patients used oral levodopa, dopamine agonists and jejuna dopa infusion (
Table 1)
UPDRS motor scale median value 10 ,00; creatinine median value was 0.92 in PD; 0,70 in control group. Even both values were in normal limits the difference between the groups was statistically significant (p<0,001). (PD 21,63 control 18,63) The difference of median values of MBPS between the groups was significant (p<0,005). There was no significant difference between the groups according to age BMI, FAR, diastolic BP, night systolic BP, dipper and night diastolic BP, average BP (p>0,050). There is also no difference between the groups concerning other parameters. (p>0,050).
In PD group the effects of independent variables over morning surge are analyzed with Robust regression analysis and statistically significant regression model is created (F=1,970; p=0,047). When BMI value increased one-unit MBPS values increased 1,62 units (p=0,012). When FAR value increases one-unit MBPS value decreases 0,264 units (p=0,009). Other variables had no significant effect on MBPS (p>0,050).
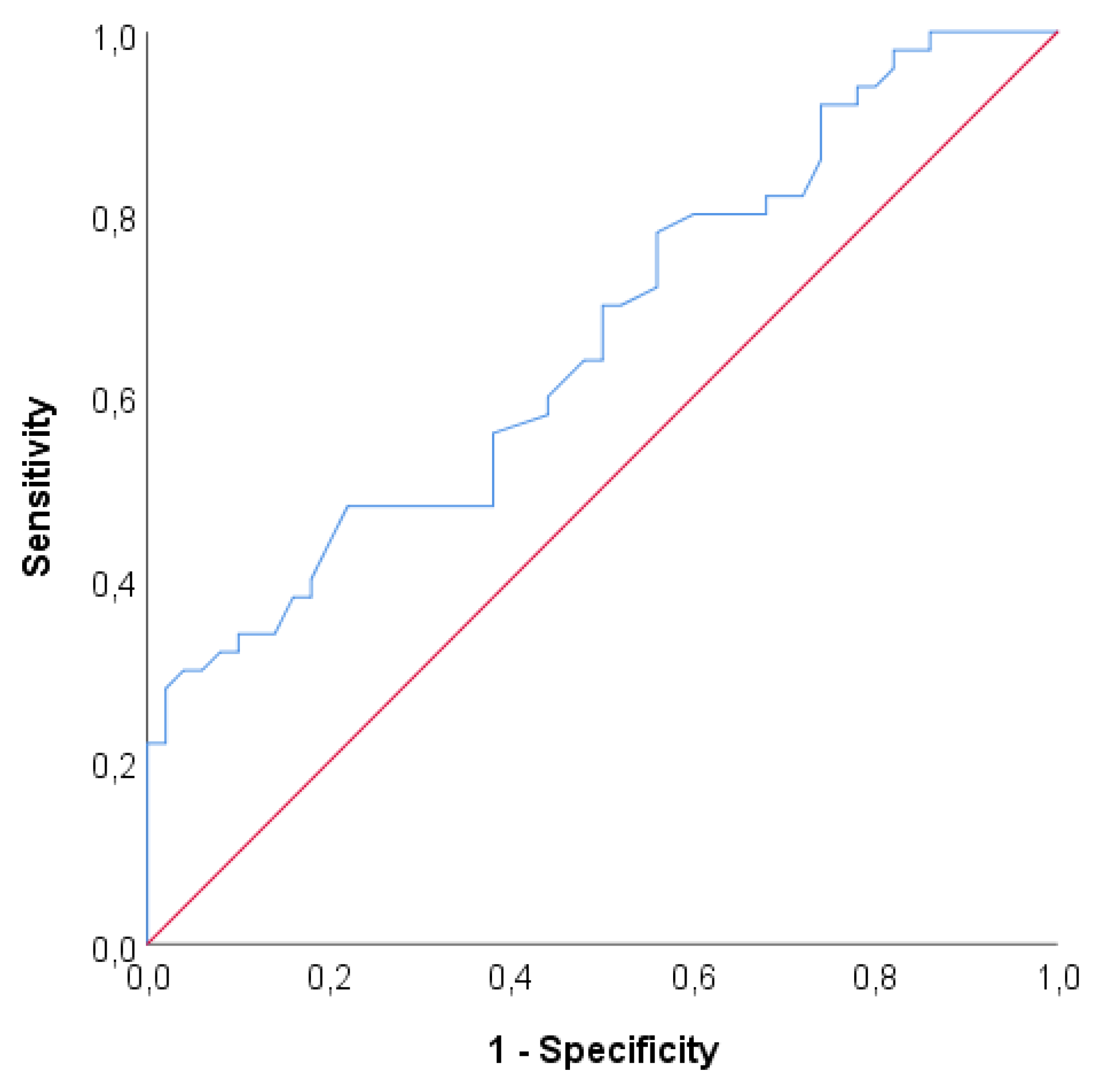
Table 4.
Results of ROC analysis PPV: Positive predictive value, NPV: Negative predictive value.
Table 4.
Results of ROC analysis PPV: Positive predictive value, NPV: Negative predictive value.
| |
AUC (%95 CI) |
p |
Cutoff |
Sensitivity |
Specificity |
PPV |
NPV |
| Morning Surge |
0,665 (0,559 - 0,77) |
0,005 |
25 |
48% |
78% |
68,57% |
60% |
With ROC analysis area under the curve value is found to be 0,665 which is statistically significant to discriminate Parkinson’s disease (p=0,005). If the cut off value is set to 25 Sensitivity= 48%, Specificity =78%, PPV = 68,57 % and NPV = 60%.
5. Discussion
Survey of PD patients has prolonged due to improvement of PD and systemic disease treatments. Additionally, world population grows older and old age is a risk factor for PD. It means more numbers of PD patients will be expecting to be treated and cared. The social and economic aspects of this increase create an important concern [
19,
20]. Even PD has been known for a long time there many questions yet to be answered. Although it has been reported that patients with PD have more risk for myocardial infarction, congestive heart failure, stroke and other cardiovascular causes of death than people without PD, blood pressure change in PD is a less investigated problem [
21,
22]. Another fact is high blood pressure increases the risk for PD [
23]. Hypotension is more expected symptom in patients with autonomic disorders however studies showed fluctuation of blood pressure and nighttime hypertension is possible in PD patients [
24]. Fluctuation of BP may be due to reduced baroreceptor sensitivity caused by Lewy body invasion of autonomic centers and dorsal motor nucleus of glossopharyngeal and vagal nerves, sympathetic postganglionic nerves as well as age related degeneration of these structures. BP abnormalities may be present before the motor symptoms emerge [
25]. Drugs which are used to treat parkinsonism and other cardiovascular disorders may aggravate BP related symptoms which may cause reduced quality of life and organ damages [
26]. When blood pressure measured only once in a day the information about the blood pressure is very limited so that 24-hour ambulatory blood pressure monitoring is very important to show circadian BP changes. [
27]. Tulba et al reported in a metanalysis of 40 studies which evaluated 24 hours ABPM that anormal blood pressure profiles were commonly encountered: high blood pressure in 38.13% of patients (938/2460), orthostatic hypotension in 38.68% (941/2433), supine hypertension in 27.76% (445/1603) and nocturnal hypertension in 38.91% (737/1894). Dipping status was also altered often, 40.46% of patients (477/1179) being reverse dippers and 35.67% (310/869) reduced dippers [
28]. In our study we did not observe any significant difference concerning above mentioned blood pressure parameters. The only significantly different BP parameter was morning surge. BP changes in PD patients especially nighttime changes are usually overlooked. Complexity of pathophysiological processes and drug effects make it more difficult to analyze the nature of these changes. The mechanism of nighttime hypertension and non-dipping is not well understood yet. O'Brien et al defined the dippers and in 1988 [
29]. The dippers have been divided into normal dippers (≥10% to <20% fall) and extreme dippers (≥20% fall). Aging, sleep disorders, diabetes, heart failure, obesity and chronic renal insufficiency may affect nigh time BP. High salt intake, high natriuresis, decrease of parasympathetic reactivity during the night might be responsible for abnormal changes of BP. In addition, when dippers compared non-dippers urine catecholamine excretion decreases during the night in non-dippers. Because of that increased sensitivity of α1 receptors cause higher level of vasoconstriction [
30]. Other factors which are suggested to be related with non-dipping are short sleep duration, fractionated sleep, and less sleep depth [
31]. Both nighttime hypertension and non-dipping is thought to be predictors of cardiovascular events. However, some authors reported low reproducibility of nocturnal dipping patterns. So that before reaching conclusion and planning therapeutic interventions more solid evidence should be gathered [
32].
Our study did not show any significant difference between two groups concerning dipping. However, PH dipping median values (4,96 (-20,75 - 22,59)) slightly lower than control group (6,87 (-12,40 - 23,08)) Majority of our patients had symptoms which are staged as Hoehn-Yahr I. It is possible that change of dipping patterns occurs later phases of the disease as the sleep disorder sand autonomic dysfunction appear. Another possibility is affecting the drugs on sleep and dipping patterns. Early morning changes of blood pressure is related with cardiovascular diseases. Myocardial infarction, sudden death and strokes are frequently seen in the morning and the occurrence of neurohormonal changes in the early hours of the day. so that blood pressure increase in the morning draws more attention [
17]. During the awakening activation of sympathetic nervous system and depression of vagal tonus result in increased blood pressure, heart rate, vasomotor tone, blood viscosity.
In a study analyzing 5464 people from eight countries is reported that accounting for covariables and the night: day ratio of systolic pressure, the hazard ratio of all-cause mortality was 1.32 (95% CI: 1.09 to 1.59;0.004) in the top decile of the systolic sleep-through MBPS (37.0 mm Hg). Another result of this study is MBPS in systolic blood pressure below 20 mm Hg is probably not associated with increased cardiovascular. However, if this value is above the 28 mmHg constitutes a risk for cardiovascular disease [
18,
33].
In our study the MBPS median value of PD patients (21,63) was higher than control group (18,63). The effect analyzed independent variables showed that there was appositive correlation between the BMI and MBPS. When BMI increased one-unit MBPS increased 1,62 unit. When cut off point of MBPS set on 25 sensitivity and specificity were 48% and 78% respectively. The role of neuroinflammation in PD pathophysiology is not clearly understood yet. However, dopamine deficiency may cause inflammation by triggering innate immunity. Gut- brain axis is another supposed inflammatory pathway. It can be said that neuroinflammation effect both peripheral and central nervous system. Higher levels of serum and CSF IL1β, IL2, IL6, IFN γ, TNF α and higher count of CD4 + lymphocyte is detected in PD (3). High variability of blood pressure also induces vascular inflammation. Inflammatory markers such as fibrinogen, TNF-a, IL-6 and high sensitivity CRP are related with blood pressure changes (34). Fibrinogen/albumin ratio (FAR) has been shown to be an effective index in cardiovascular and cancer diseases (35,36). High FAR is found to be an independent risk factor for the exaggerated morning surge in patients with hypertension. Because of that it is suggested that inflammation might be a part of MBPS pathogenesis (14). However, our study did not show any positive relation between the FAR and MBPS. A larger study may clear this conflicting results.
The increased MBPS can be considered as a sign of autonomic dysfunction. Significant difference of creatinine levels may be an early sign of organ damage however the levels are in the normal limits. Patients should be followed to learn if this difference has any effect on prognosis in relation with mortality and morbidity.
PH is a neurodegenerative disease that has complex pathophysiology and clinical findings. BP changes due to the autonomic involvement deserves to be searched thoroughly to prevent possible cardiovascular and cerebral events. We found exaggerated MBPS levels in PD patients than control group which point out possible autonomic involvement. There is no similar study in literature as far as our knowledge goes. We suggest that larger studies searching the relation of MBPS and clinical findings in PD patients.
The limitations our study the number of the patients, absence of echocardiographic evaluation. Some of the patients were under treatment of antihypertensive drugs we did not evaluate their effects on blood pressure. The orthostatic symptoms which are also reflections of the autonomic dysfunction were not evaluated either.
Author Contributions
Ummu S Sari, Seda E Yıldırım, Gulseren Buyukserbetci and Figen Esmeli have given substantial contributions to the conception or the design of the manuscript, Mesut Sackes to acquisition, analysis and interpretation of the data. All authors have participated to drafting the manuscript, Ummu S Sari revised it critically. All authors read and approved the final version of the manuscript. All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Balikesir University (2022/92).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jankovic, J.; Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008,79(4):368-76.
- Breen, D.P.; Halliday, G.M.; Lang, A.E. Gut-brain axis and the spread of α-synuclein pathology: Vagal highway or dead end? Mov Disord 2019, 34(3):307-316.
- Pajares, M.; I Rojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson's Disease: Mechanisms and Therapeutic Implications. Cells 2020, 14;9(7):1687. [CrossRef]
- Tan, E, K.; Chao, Y.X.; West, A.; Chan, L.L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat Rev Neurol 2020, 16(6):303-318.
- Seppi, K.; Ray, C. K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez, L.S.; Weintraub, D.; Sampaio, C.; the collaborators of the Parkinson's Disease Update on Non-Motor Symptoms Study Group on behalf of the Movement Disorders Society Evidence-Based Medicine Committee. Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov Disord 2019,34(2):180-198. [CrossRef]
- Marek, K.; Chowdhury, S.; Siderowf, A.; Lasch, S.; Coffey, C.S.; Caspell-Garcia, C. et al; Parkinson's Progression Markers Initiative. The Parkinson's progression markers initiative (PPMI) - establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018,5(12):1460-1477. 1. [CrossRef]
- Vernino, S. Autoimmune Autonomic Disorders. Continuum (Minneap Minn) 2020 Feb,26(1):44-57.
- Cheshire, W.P Jr.; Goldstein, D.S. The physical examination as a window into autonomic disorders. Clin Auton Res 2018,28(1):23-33.
- Schapira, A.H.V; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017,18(7):435-450. [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson's disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol Dis 2020; 134:104700.
- Goldstein, D.S.; Pechnik, S.; Holmes, C.; Eldadah, B.; Sharabi, Y. Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 2003,42(2):136-42.
- Fanciulli, A.; Göbel, G.; Ndayisaba, J.P.; Granata. R.; Duerr, S.; Strano, S.et al. Supine hypertension in parkinson's disease and multiple system atrophy. Clinical Autonomic Research 2016, 26(2), 97-105.
- Tanindi, A.; Ugurlu, M.; Tore, H.F. Blood pressure morning surge, exercise blood pressure response and autonomic nervous system. Scand Cardiovasc J 2015,49(4):220-7.
- Özdemir, M.; Yurtdaş, M.; Asoğlu, R.; Yildirim, T.; Aladağ, N.; Asoğlu. E. Fibrinogen to albumin ratio as a powerful predictor of the exaggerated morning blood pressure surge in newly diagnosed treatment-naive hypertensive patients. Clin Exp Hypertens 2020;16,42(8):692-699. [CrossRef]
- Ahbap, E.; Sakaci, T.; Kara, E.; Sahutoglu, T.; Koc, Y.; Basturk, T. et al. The relationship between serum albumin levels and 24-h ambulatory blood pressure monitoring recordings in non-diabetic essential hypertensive patients. Clinics (Sao Paulo) 2016,71(5):257-63.
- Rong, H.; Qing, D.; Lei, C.; Ziyan, W.; Jianzhou, C.; Rong, G.; et al. The association between fibrinogen-to-albumin ratio (FAR) and adverse prognosis in patients with acute decompensated heart failure at different glucose metabolic states. Cardiovasc Diabetol 2022, 21, 241. [Google Scholar]
- Bilo, G.; Grillo, A.; Guida, V.; Parati, G. Morning blood pressure surge: pathophysiology, clinical relevance and therapeutic aspects. Integr Blood Press Control 2018; 24,11:47-56.
- Li, Y.; Thijs, L.; Hansen, T.W.; Kikuya, M.; Boggia, J.; Richart, T. et al. International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes Investigators. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension 2010,55 (4)1040–48.
- Gonera, E.G.; van't Hof, M.; Berger, H.J.; van Weel, C.; Horstink, M.W. Symptoms and duration of the prodromal phase in Parkinson's disease. Mov Disord 1997,12(6):871-6.
- Dorsey, E.R.; Bloem, B.R. The Parkinson Pandemic-A Call to Action. JAMA Neurol 2018,75(1):9-10.
- Park, J.H.; Kim, D.H.; Park, Y.G.; Kwon, D.Y.; Choi, M.; Jung, J.H, et al. Association of Parkinson Disease with Risk of Cardiovascular Disease and All-Cause Mortality: A Nationwide, Population-Based Cohort Study. Circulation 2020,141(14):1205-1207.
- Juraschek, S.P.; Daya, N.; Appel, L.J.; Miller, E.R 3rd.; McEvoy, J.W.; Matsushita, K. et al. Orthostatic Hypotension and Risk of Clinical and Subclinical Cardiovascular Disease in Middle-Aged Adults. J Am Heart Assoc 2018,7(10):e008884.
- Chen, J.; Zhang, C.; Wu, Y.; Zhang, D. Association between Hypertension and the Risk of Parkinson's Disease: A Meta-Analysis of Analytical Studies. Neuroepidemiology 2019,52(3-4):181-192.
- Ellis, T.; Boudreau, J.K.; DeAngelis, T.R.; Brown, L.E.; Cavanaugh, J.T.; Earhart, G.M. et al. Barriers to exercise in people with Parkinson disease. Phys Ther 2013,93(5):628-36.
- Stuebner, E.; Vichayanrat, E, Low, D.A.; Mathias, C.J.; Isenmann, S.; Haensch, C.A.; Twenty-four-hour non-invasive ambulatory blood pressure and heart rate monitoring in Parkinson's disease. Front Neurol 2013,15; 4:49.
- Katsi, V.; Papakonstantinou, I.; Solomou, E.; Antonopoulos, A.S.; Vlachopoulos, C.; Tsioufis, K. Management of Hypertension and Blood Pressure Dysregulation in Patients with Parkinson’s Disease a Systematic Review. Article in Current Hypertension Reports 2021.
- Ziemssen, T.; Reichmann, H. Cardiovascular autonomic dysfunction in Parkinson's disease. J Neurol Sci 2010,289(1-2):74-80.
- Tulbă, D.; Cozma, L; Bălănescu, P.; Buzea, A.; Băicuș, C.; Popescu, B.O. Blood Pressure Patterns in Patients with Parkinson's Disease: A Systematic Review. J Pers Med 2021,11(2):129.
- O'Brien, E.; Sheridan, J.; O'Malley, K. Dippers and non-dippers. Lancet 1988,2(8607):397.
- Tang, A.; Yang, E.; Ebinger, J.E. Non-Dipping Blood Pressure or Nocturnal Hypertension: Does One Matter More? Curr Hypertens Rep 2023. [CrossRef] [PubMed]
- Forshaw, PE.; Correia, A.T.L.; Roden, L.C.; Lambert, E.V.; Rae, D.E. Sleep characteristics associated with nocturnal blood pressure nondipping in healthy individuals: a systematic review. Blood Press Monit 2022,27(6):357-370.
- Burgos-Alonso, N.; Ruiz Arzalluz, M.V.; Garcia-Alvarez, A.; Fernandez-Fernandez de Quincoces, D.; Grandes, G. Reproducibility study of nocturnal blood pressure dipping in patients with high cardiovascular risk. J Clin Hypertens (Greenwich) 2021,23(5):1041-1050.
- Sogunuru, G.P.; Kario, K.; Shin, J.; Chen, C.H.; Buranakitjaroen, P.; Chia, Y.C. et al. HOPE Asia Network. Morning surge in blood pressure and blood pressure variability in Asia: Evidence and statement from the HOPE Asia Network. J Clin Hypertens (Greenwich) 2019,21(2):324-334.
- Kim, K.I.; Lee, J.H.; Chang, H.J.; Cho, Y.S.; Youn, T.J.; Chung, W.Y. et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J 2008,72(2):293-8.
- Kayapinar, O.; Ozde, C.; Kaya, A. Relationship between the reciprocal change in inflammation-related biomarkers (Fibrinogen-toAlbumin and hsCRP-to-Albumin ratios) and the presence and severity of coronary slow flow. Clin Appl Thromb Hemost 2019,25:1076029619835383.
- Xu, W.Y.; Zhang, H.H.; Xiong, J.P.; Yang, X.B.; Bai, Y.; Lin, J.Z. et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol 2018,24(29):3281-3292.
Table 1.
Distribution of categorical variables by groups.
Table 1.
Distribution of categorical variables by groups.
| |
Control s |
Patients |
Total |
Test statistics |
P |
| Sex |
|
|
|
|
|
| Female |
20 (40) |
24 (48) |
44 (44) |
0,365 |
0,546* |
| Male |
30 (60) |
26 (52) |
56 (56) |
| Accompanying illness |
|
|
|
|
|
| No |
50 (100) |
39 (78) |
89 (89) |
--- |
--- |
| Yes |
0 (0) |
11 (22) |
11 (11) |
| Oral L-dopa |
|
|
|
|
|
| No |
50 (100) |
12 (24) |
62 (62) |
--- |
--- |
| Yes |
0 (0) |
38 (76) |
38 (38) |
| Dopa Agonist |
|
|
|
|
|
| No |
50 (100) |
13 (26) |
63 (63) |
--- |
--- |
| Yes |
0 (0) |
37 (74) |
37 (37) |
| Jejunal dopa |
|
|
|
|
|
| No |
50 (100) |
46 (92) |
96 (96) |
--- |
--- |
| Yes |
0 (0) |
4 (8) |
4 (4) |
| Hoen Yahr |
|
|
|
|
|
| 0 |
50 (100) |
0 (0) |
50 (50) |
--- |
--- |
| 1 |
0 (0) |
22 (44) |
22 (22) |
| 1,5 |
0 (0) |
3 (6) |
3 (3) |
| 2 |
0 (0) |
10 (20) |
10 (10) |
| 2,5 |
0 (0) |
2 (4) |
2 (2) |
| 3 |
0 (0) |
8 (16) |
8 (8) |
| 4 |
0 (0) |
5 (10) |
5 (5) |
Table 2.
Comparison of quantitative data by groups.
Table 2.
Comparison of quantitative data by groups.
| |
Controls |
Patients |
Total |
Test statistic |
p |
| Mean ±SD |
Median (min. - max.) |
Mean±s. deviation |
Median (min. - max.) |
Mean±s. deviation |
Median (min. - max.) |
| Age |
62,34±10,52 |
63,50 (40,00 - 80,00) |
66,26±9,51 |
67,00 (39,00 - 81,00) |
64,30±10,17 |
65,00 (39,00 - 81,00) |
t=-1,954 |
0,054 |
| ParkinsonDuration |
--- |
--- |
4,32±3,18 |
3,00 (1,00 - 12,00) |
2,16±3,12 |
0,50 (0,00 - 12,00) |
--- |
--- |
| BMI |
25,58±2,22 |
25,72 (21,48 - 29,98) |
27,20±2,81 |
27,30 (19,80 - 34,00) |
26,39±2,65 |
26,19 (19,80 - 34,00) |
t=-1,954 |
0,054 |
| UPDRS-M |
0,00±0,00 |
0,00 (0,00 - 0,00) |
16,62±12,88 |
10,00 (4,00 - 44,00) |
8,31±12,32 |
2,00 (0,00 - 44,00) |
U=0 |
<0,001 |
| hsCRP |
3,15±0,00 |
3,15 (3,14 - 3,15) |
3,00±1,04 |
3,15 (0,14 - 4,60) |
3,07±0,74 |
3,15 (0,14 - 4,60) |
U=1067 |
0,142 |
| FAR |
75,41±7,97 |
74,59 (59,76 - 95,29) |
77,65±17,52 |
76,35 (35,60 - 132,00) |
76,53±13,59 |
74,86 (35,60 - 132,00) |
t=-0,826 |
0,412 |
| Creatinine |
0,69±0,24 |
0,70 (0,30 - 1,20) |
0,92±0,23 |
0,92 (0,40 - 1,45) |
0,80±0,26 |
0,80 (0,30 - 1,45) |
U=644 |
<0,001 |
| SisBP 24 h |
124,09±9,52 |
127,50 (103,00 - 138,00) |
123,96±15,38 |
121,45 (94,40 - 166,00) |
124,03±12,73 |
124,70 (94,40 - 166,00) |
U=1177,5 |
0,617 |
| DiaBP 24 h |
72,16±6,46 |
72,00 (56,00 - 85,00) |
70,97±9,23 |
71,15 (49,90 - 92,60) |
71,56±7,95 |
71,65 (49,90 - 92,60) |
t=-1,954 |
0,054 |
| DayDiaBP |
72,62±10,59 |
73,00 (16,50 - 87,00) |
71,27±8,38 |
71,40 (50,80 - 89,10) |
71,95±9,53 |
72,30 (16,50 - 89,10) |
U=1053 |
0,174 |
| DaySisBP |
125,40±9,46 |
129,00 (103,00 - 140,00) |
124,63±15,45 |
122,30 (96,80 - 167,60) |
125,01±12,75 |
125,00 (96,80 - 167,60) |
U=1128 |
0,400 |
| NightSisBP |
116,68±12,96 |
117,00 (90,00 - 138,00) |
117,54±17,66 |
115,65 (83,90 - 159,30) |
117,11±15,41 |
116,25 (83,90 - 159,30) |
t=-0,28 |
0,780 |
| Dipper |
6,93±7,86 |
6,87 (-12,40 - 23,08) |
5,60±9,07 |
4,96 (-20,75 - 22,59) |
6,26±8,47 |
5,61 (-20,75 - 23,08) |
t=-1,954 |
0,054 |
| MBPS |
18,31±7,26 |
18,63 (3,00 - 31,25) |
24,55±10,44 |
21,63 (9,00 - 55,50) |
21,43±9,48 |
21,00 (3,00 - 55,50) |
U=838 |
0,005 |
| NightDiaBP |
66,01±7,12 |
65,75 (53,00 - 83,00) |
64,67±11,45 |
64,60 (42,70 - 97,10) |
65,34±9,51 |
65,20 (42,70 - 97,10) |
t=-1,954 |
0,054 |
Table 3.
The effects of independent variables over morning surge are analyzed with Robust regression analysis.
Table 3.
The effects of independent variables over morning surge are analyzed with Robust regression analysis.
| Independent variables |
β1 (%95 CI) |
S. error |
β2 |
t |
P |
r |
VIF |
| Fixed |
24,659 (-32,292 - 81,61) |
27,886 |
0,000 |
0,884 |
0,384 |
--- |
--- |
| BMI |
1,62 (0,389 - 2,85) |
0,602 |
0,476 |
2,689 |
0,012 |
0,401 |
2,112 |
| hsCRP |
0,41 (-2,258 - 3,077) |
1,306 |
0,045 |
0,314 |
0,756 |
-0,009 |
1,381 |
| FAR |
-0,264 (-0,457 - -0,071) |
0,095 |
-0,501 |
-2,788 |
0,009 |
0,023 |
2,176 |
| Creatinine |
5,073 (-7,755 - 17,9) |
6,281 |
0,128 |
0,808 |
0,426 |
-0,090 |
1,686 |
| Dipper |
0,115 (-0,3 - 0,529) |
0,203 |
0,115 |
0,565 |
0,577 |
0,137 |
2,798 |
| Parkinson Duration |
-0,632 (-1,75 - 0,487) |
0,548 |
-0,213 |
-1,153 |
0,258 |
-0,066 |
2,294 |
| SistBP 24 h |
-0,063 (-0,325 - 0,199) |
0,128 |
-0,104 |
-0,491 |
0,627 |
-0,183 |
3,024 |
| DiaBP 24 h |
-0,328 (-0,762 - 0,107) |
0,213 |
-0,329 |
-1,538 |
0,134 |
-0,307 |
3,091 |
| NightDiaBP |
-0,025 (-0,397 - 0,346) |
0,182 |
-0,032 |
-0,139 |
0,890 |
-0,264 |
3,533 |
| Gender (Reference: Male) |
5,667 (-2,217 - 13,551) |
3,860 |
0,305 |
1,468 |
0,153 |
0,123 |
2,911 |
| Comorbidity |
3,89 (-2,877 - 10,656) |
3,313 |
0,168 |
1,174 |
0,250 |
0,264 |
1,383 |
| Dopa Oral |
2,307 (-5,837 - 10,451) |
3,988 |
0,110 |
0,578 |
0,567 |
0,210 |
2,418 |
| Dopa Agonist |
-1,81 (-10,717 - 7,097) |
4,361 |
-0,088 |
-0,415 |
0,681 |
-0,061 |
3,028 |
| Dopa Infusion |
-11,291 (-24,839 - 2,257) |
6,634 |
-0,345 |
-1,702 |
0,099 |
-0,102 |
2,766 |
| Hoen Yahr |
|
|
|
|
|
|
|
| 1,5 |
-1,887 (-14,168 - 10,394) |
6,013 |
-0,051 |
-0,314 |
0,756 |
-0,050 |
1,746 |
| 2 |
1,895 (-6,6 - 10,389) |
4,159 |
0,076 |
0,456 |
0,652 |
0,076 |
1,875 |
| 2,5 |
-5,869 (-20,786 - 9,049) |
7,304 |
-0,130 |
-0,803 |
0,428 |
-0,026 |
1,758 |
| 3 |
5,605 (-3,362 - 14,573) |
4,391 |
0,224 |
1,277 |
0,212 |
0,256 |
2,075 |
| 4 |
11,3 (-1,045 - 23,646) |
6,045 |
0,381 |
1,869 |
0,071 |
0,041 |
2,801 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).