Submitted:
24 April 2024
Posted:
24 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Thermal Oxidation of Carbon Paper
2.2. Characterization
2.3. Battery Cell Assembly
2.4. Electrochemical Measurements
2.5. Analysis Methods Post Discharge
3. Results and Discussion
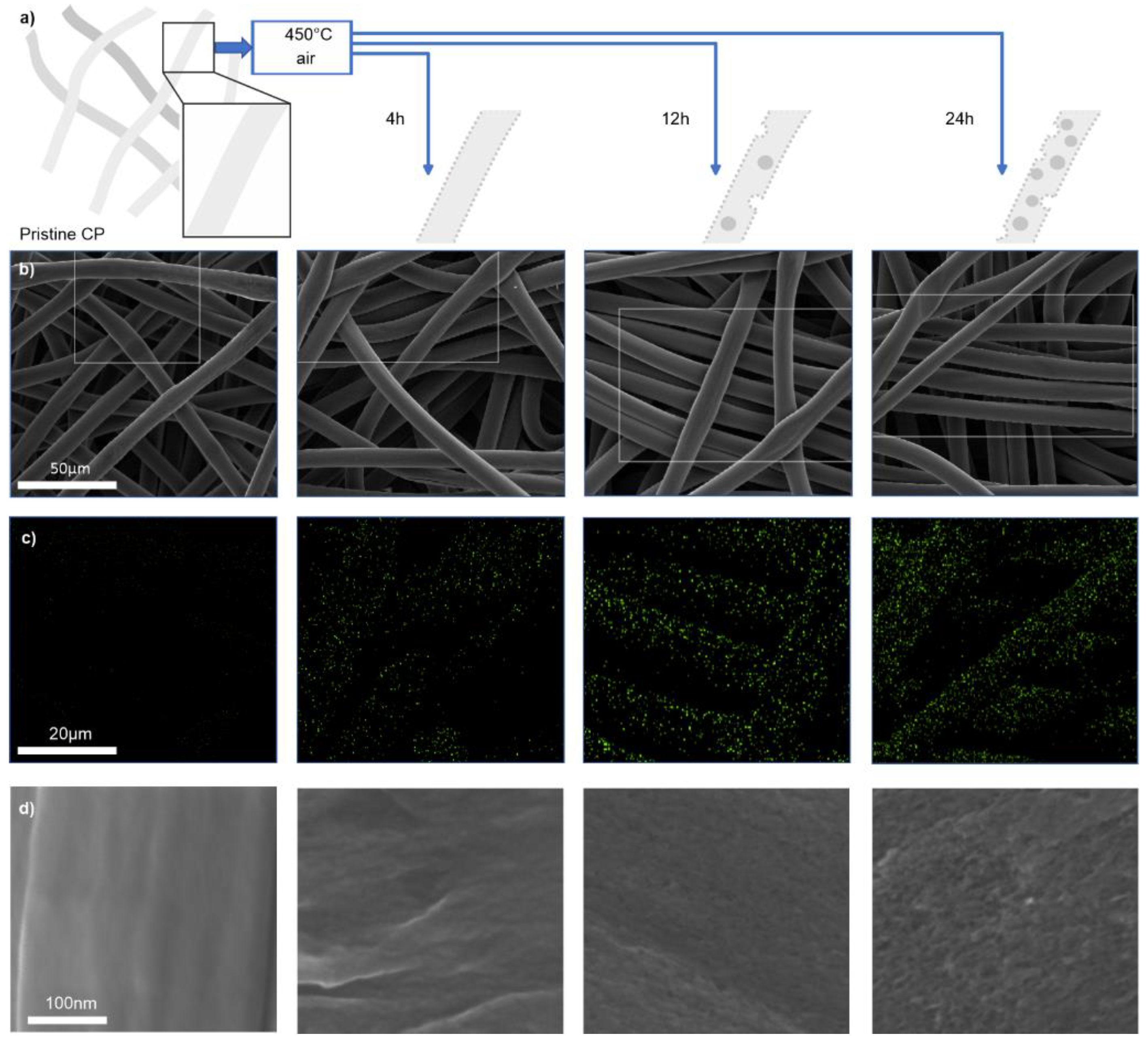
3.1. Thermal Oxidation of Carbon Paper
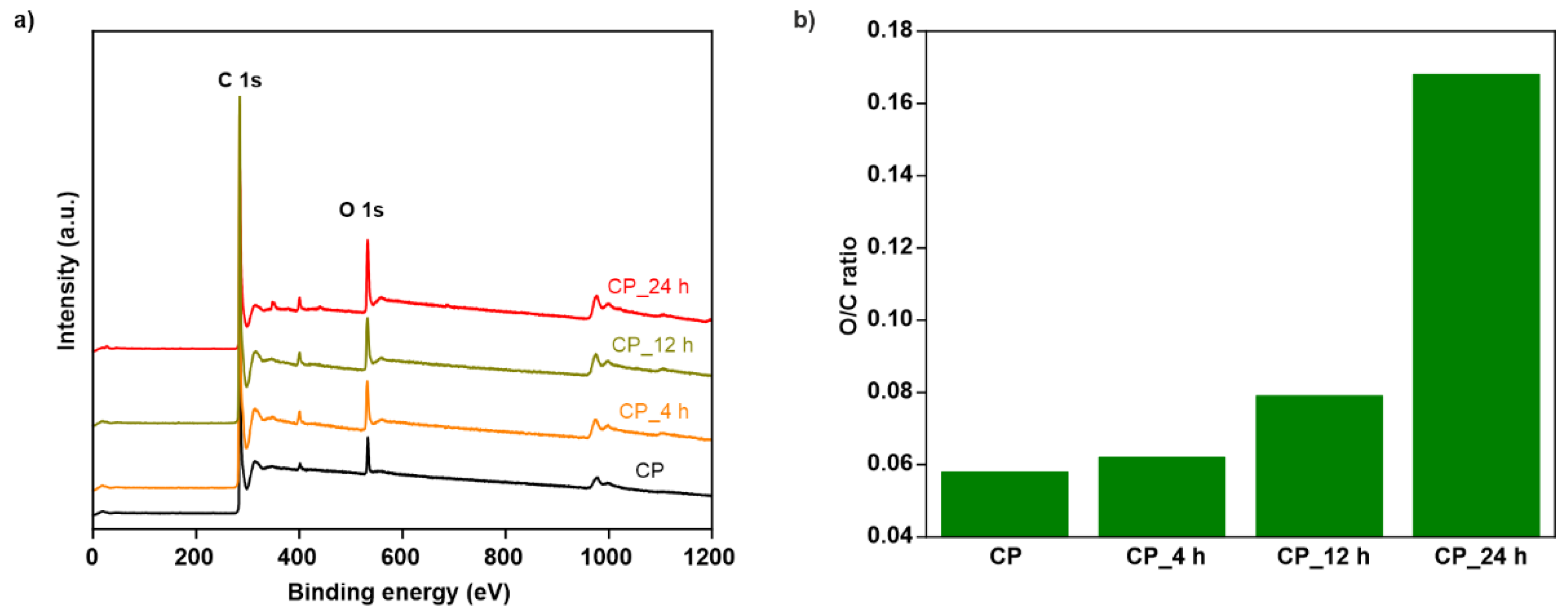
3.1.1. Analysis of Cathode Structure and Surface Properties
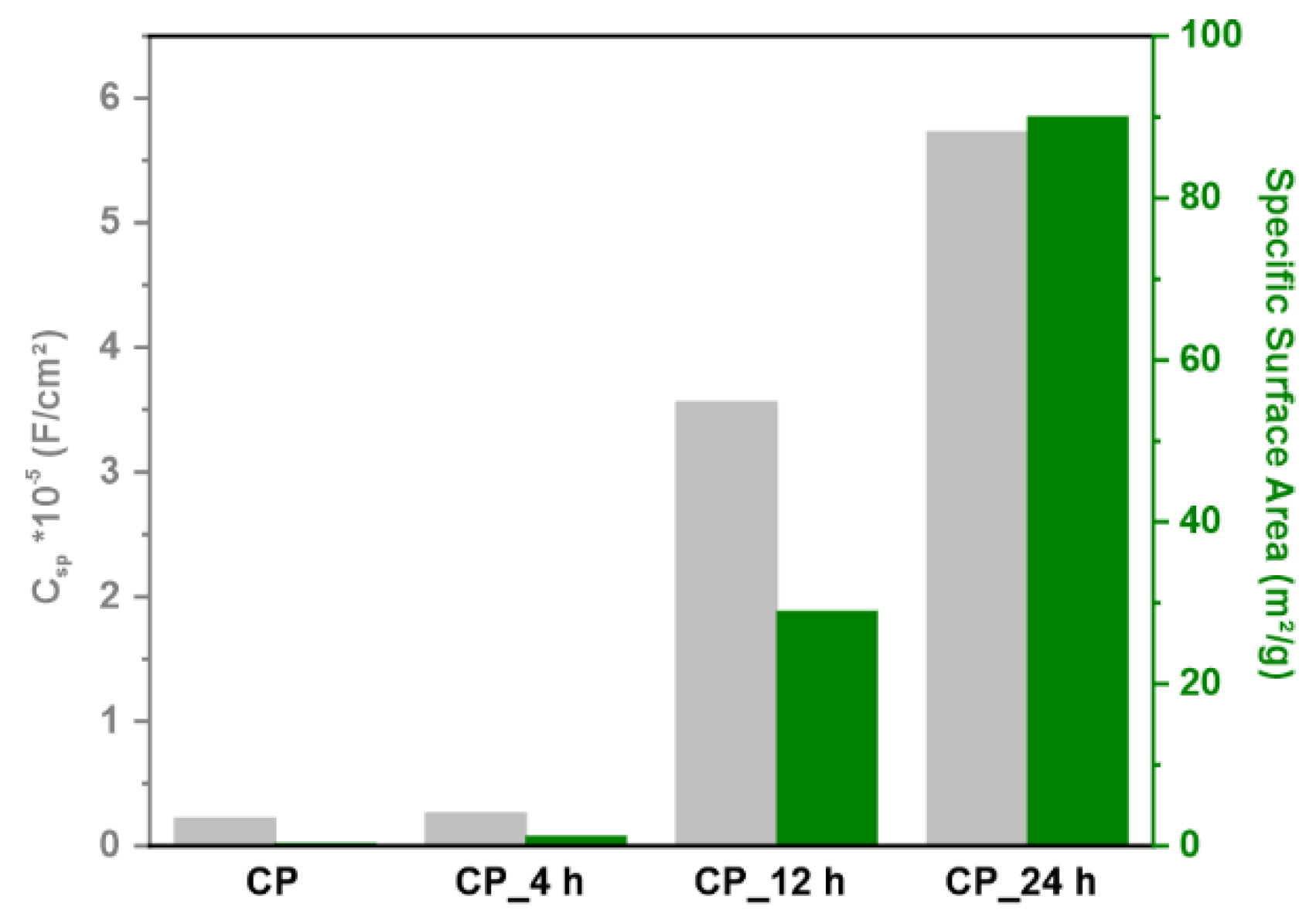
3.1.2. Analysis of Surface Area
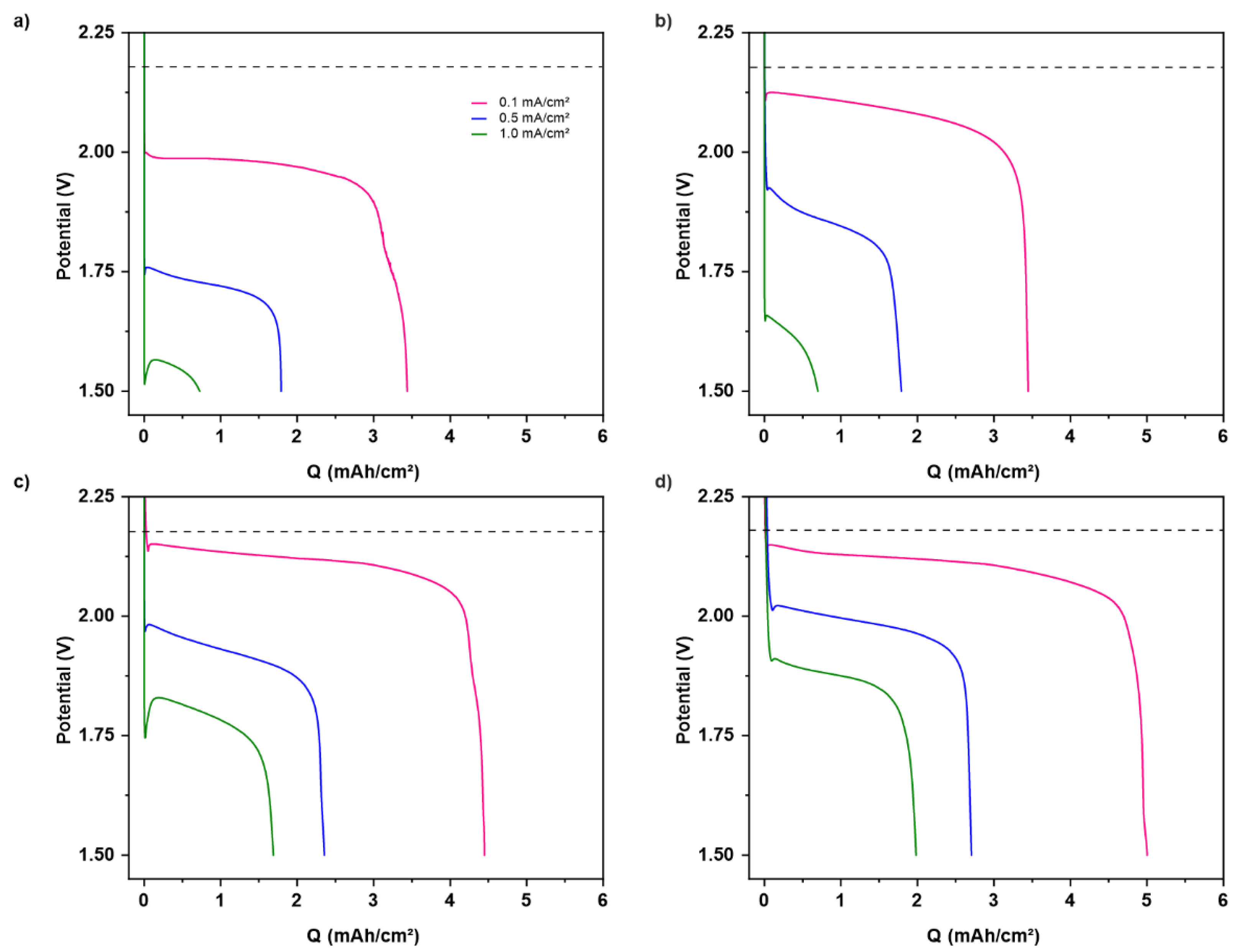
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, N.S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew Chem Int Ed Engl 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium−Air Battery: Promise and Challenges. The Journal of Physical Chemistry Letters 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Ren, X.; Wu, Y. A low-overpotential potassium-oxygen battery based on potassium superoxide. J Am Chem Soc 2013, 135, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nature Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Yao, X.; Dong, Q.; Cheng, Q.; Wang, D. Why Do Lithium–Oxygen Batteries Fail: Parasitic Chemical Reactions and Their Synergistic Effect. Angewandte Chemie International Edition 2016, 55, 11344–11353. [Google Scholar] [CrossRef] [PubMed]
- Mahne, N.; Schafzahl, B.; Leypold, C.; Leypold, M.; Grumm, S.; Leitgeb, A.; Strohmeier, G.A.; Wilkening, M.; Fontaine, O.; Kramer, D.; et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium–oxygen batteries. Nature Energy 2017, 2, 17036. [Google Scholar] [CrossRef]
- Mourad, E.; Petit, Y.K.; Spezia, R.; Samojlov, A.; Summa, F.F.; Prehal, C.; Leypold, C.; Mahne, N.; Slugovc, C.; Fontaine, O.; et al. Singlet oxygen from cation driven superoxide disproportionation and consequences for aprotic metal–O2 batteries. Energy & Environmental Science 2019, 12, 2559–2568. [Google Scholar] [CrossRef]
- Houchins, G.; Pande, V.; Viswanathan, V. Mechanism for Singlet Oxygen Production in Li-Ion and Metal–Air Batteries. ACS Energy Letters 2020, 5, 1893–1899. [Google Scholar] [CrossRef]
- Reinsberg, P.H.; Koellisch, A.; Baltruschat, H. On the importance of ion pair formation and the effect of water in potassium–oxygen batteries. Electrochimica Acta 2019, 313, 223–234. [Google Scholar] [CrossRef]
- Xiao, N.; Rooney, R.T.; Gewirth, A.A.; Wu, Y. The Long-Term Stability of KO2 in K-O2 Batteries. Angewandte Chemie International Edition 2018, 57, 1227–1231. [Google Scholar] [CrossRef]
- Qin, L.; Schkeryantz, L.; Zheng, J.; Xiao, N.; Wu, Y. Superoxide-Based K–O2 Batteries: Highly Reversible Oxygen Redox Solves Challenges in Air Electrodes. Journal of the American Chemical Society 2020, 142, 11629–11640. [Google Scholar] [CrossRef]
- Xiao, N.; Ren, X.; McCulloch, W.D.; Gourdin, G.; Wu, Y. Potassium Superoxide: A Unique Alternative for Metal–Air Batteries. Accounts of Chemical Research 2018, 51, 2335–2343. [Google Scholar] [CrossRef]
- Park, J.; Hwang, J.-Y.; Kwak, W.-J. Potassium–Oxygen Batteries: Significance, Challenges, and Prospects. The Journal of Physical Chemistry Letters 2020, 11, 7849–7856. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, D.-Y.; Huang, G.; Zhang, X.-B. Lithium–Air Batteries: Air-Electrochemistry and Anode Stabilization. Accounts of Chemical Research 2021, 54, 632–641. [Google Scholar] [CrossRef]
- Ren, X.; Lau, K.C.; Yu, M.; Bi, X.; Kreidler, E.; Curtiss, L.A.; Wu, Y. Understanding Side Reactions in K–O2 Batteries for Improved Cycle Life. ACS Applied Materials & Interfaces 2014, 6, 19299–19307. [Google Scholar] [CrossRef]
- Yu, W.; Lau, K.C.; Lei, Y.; Liu, R.; Qin, L.; Yang, W.; Li, B.; Curtiss, L.A.; Zhai, D.; Kang, F. Dendrite-Free Potassium–Oxygen Battery Based on a Liquid Alloy Anode. ACS Applied Materials & Interfaces 2017, 9, 31871–31878. [Google Scholar] [CrossRef]
- McCulloch, W.D.; Ren, X.; Yu, M.; Huang, Z.; Wu, Y. Potassium-Ion Oxygen Battery Based on a High Capacity Antimony Anode. ACS Applied Materials & Interfaces 2015, 7, 26158–26166. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl Mater Interfaces 2017, 9, 4404–4419. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Gourdin, G.; Wu, Y. Simultaneous Stabilization of Potassium Metal and Superoxide in K–O2 Batteries on the Basis of Electrolyte Reactivity. Angewandte Chemie International Edition 2018, 57, 10864–10867. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, P.; Sundaresan, V.B. A Functionally Graded Cathode Architecture for Extending the Cycle-Life of Potassium-Oxygen Batteries. Batteries & Supercaps 2019, 2, 662–662. [Google Scholar]
- Cong, G.; Wang, W.; Lai, N.-C.; Liang, Z.; Lu, Y.-C. A high-rate and long-life organic–oxygen battery. Nature Materials 2019, 18, 390–396. [Google Scholar] [CrossRef]
- Lee, J.T.; Jo, C.; De Volder, M. Bicontinuous phase separation of lithium-ion battery electrodes for ultrahigh areal loading. Proceedings of the National Academy of Sciences 2020, 117, 21155–21161. [Google Scholar] [CrossRef]
- Küpper, J.; Jakobi, S.; Simon, U. PTFE Enhances Discharge Performance of Carbon Cathodes in Potassium-Oxygen Batteries**. Batteries & Supercaps 2021, 4, 1620–1626. [Google Scholar]
- Küpper, J.; Simon, U. The effects of oxygen pressure on the discharge performance of potassium–oxygen batteries. Sustainable Energy & Fuels 2022, 6, 1992–2000. [Google Scholar] [CrossRef]
- Küpper, J.; Li, X.; Simon, U. A Model of the Potassium-Oxygen Battery and its Application in Cathode Design. Journal of The Electrochemical Society 2022, 169, 060539. [Google Scholar] [CrossRef]
- Küpper, J. Enhancing the Discharge Performance of the Potassium-Oxygen Battery. PhD Thesis, RWTH Aachen University, 2022. [Google Scholar]
- Liu, H.; Liu, X.; Li, W.; Guo, X.; Wang, Y.; Wang, G.; Zhao, D. Porous Carbon Composites for Next Generation Rechargeable Lithium Batteries. Advanced Energy Materials 2017, 7, 1700283. [Google Scholar] [CrossRef]
- Liu, Y.S.; Ma, C.; Wang, K.X.; Chen, J.S. Recent advances in porous carbons for electrochemical energy storage. New Carbon Mater. 2023, 38, 1. [Google Scholar] [CrossRef]
- Singh, A.; Yasari, N.; Karan, K.; Roberts, E. Electrocatalytic Activity of Functionalized Carbon Paper Electrodes and Their Correlation to the Fermi Level Derived from Raman Spectra. ACS Applied Energy Materials 2019, 2. [Google Scholar] [CrossRef]
- Greco, K.V.; Bonesteel, J.K.; Chanut, N.; Tai-Chieh Wan, C.; Chiang, Y.-M.; Brushett, F.R. Limited Accessibility to Surface Area Generated by Thermal Pretreatment of Electrodes Reduces Its Impact on Redox Flow Battery Performance. ACS Applied Energy Materials 2021, 4, 13516–13527. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Progress in Materials Science 2023, 135, 101089. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Kwon, S.; Suharto, Y.; Kim, K.J. Facile preparation of an oxygen-functionalized carbon felt electrode to improve VO2+/VO2+ redox chemistry in vanadium redox flow batteries. Journal of Industrial and Engineering Chemistry 2021, 98, 231–236. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Xu, C.; Zhang, H. Activated Carbon Fiber Paper Based Electrodes with High Electrocatalytic Activity for Vanadium Flow Batteries with Improved Power Density. ACS Applied Materials & Interfaces 2017, 9, 4626–4633. [Google Scholar] [CrossRef]
- Yue, Z.R.; Jiang, W.; Wang, L.; Gardner, S.D.; Pittman, C.U. Surface characterization of electrochemically oxidized carbon fibers. Carbon 1999, 37, 1785–1796. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T.M. Recent Advancements in All-Vanadium Redox Flow Batteries. Advanced Materials Interfaces 2016, 3, 1500309. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.; Wei, L. Revealing the Performance Enhancement of Oxygenated Carbonaceous Materials for Vanadium Redox Flow Batteries: Functional Groups or Specific Surface Area? Advanced Sustainable Systems 2018, 2, 1700148. [Google Scholar] [CrossRef]
- Qian, Z.; Sun, B.; Du, L.; Lou, S.; Du, C.; Zuo, P.; Ma, Y.; Cheng, X.; Gao, Y.; Yin, G. Insights into the role of oxygen functional groups and defects in the rechargeable nonaqueous Li–O2 batteries. Electrochimica Acta 2018, 292. [Google Scholar] [CrossRef]
- Yang, S.; Li, L.; Xiao, T.; Zheng, D.; Zhang, Y. Role of surface chemistry in modified ACF (activated carbon fiber)-catalyzed peroxymonosulfate oxidation. Applied Surface Science 2016, 383, 142–150. [Google Scholar] [CrossRef]
- Pacheco, F.G.; Cotta, A.A.C.; Gorgulho, H.F.; Santos, A.P.; Macedo, W.A.A.; Furtado, C.A. Comparative temporal analysis of multiwalled carbon nanotube oxidation reactions: Evaluating chemical modifications on true nanotube surface. Applied Surface Science 2015, 357, 1015–1023. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Nichols, J.E.; Vegge, T.; Luntz, A.C.; McCloskey, B.D.; Hjelm, J. An Electrochemical Impedance Study of the Capacity Limitations in Na–O2 Cells. The Journal of Physical Chemistry C 2016, 120, 10799–10805. [Google Scholar] [CrossRef]
- Wang, F.; Li, X. Effects of the Electrode Wettability on the Deep Discharge Capacity of Li–O2 Batteries. ACS Omega 2018, 3, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Jung, K.-N.; Lee, J.-W. A Mini-Review on Non-Aqueous Lithium-Oxygen Batteries - Electrochemistry and Cathode Materials. J. Electrochem. Sci. Technol 2015, 6, 50–58. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, E.; Ahn, W.-S.; Shim, S.E. Controlling porosity of porous carbon cathode for lithium oxygen batteries: Influence of micro and meso porosity. Journal of Power Sources 2018, 389, 20–27. [Google Scholar] [CrossRef]
- Zhao, T.; Yao, Y.; Yuan, Y.; Wang, M.; Wu, F.; Amine, K.; Lu, J. A universal method to fabricating porous carbon for Li-O2 battery. Nano Energy 2021, 82, 105782. [Google Scholar] [CrossRef]
- Huang, S.; Fan, W.; Guo, X.; Meng, F.; Liu, X. Positive role of surface defects on carbon nanotube cathodes in overpotential and capacity retention of rechargeable lithium-oxygen batteries. ACS Appl Mater Interfaces 2014, 6, 21567–21575. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Mei, D.; Li, X.; Xu, W.; Wang, D.; Graff, G.L.; Bennett, W.D.; Nie, Z.; Saraf, L.V.; Aksay, I.A.; et al. Hierarchically Porous Graphene as a Lithium–Air Battery Electrode. Nano Letters 2011, 11, 5071–5078. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, Y.; Guo, F.; Shen, Y.; Chen, G.; Wei, Y.; Xie, Z.; Zhou, Z. Hierarchical Porous Carbon Nanotube Spheres for High-performance K-O2 Batteries. Chemical Research in Chinese Universities 2021, 37, 254–258. [Google Scholar] [CrossRef]

| Cathode sample | Micropore area (m2/g) | External surface area (m2/g) | Specific surface area (m2/g) |
|---|---|---|---|
| CP | - | - | 0.33 |
| CP_4 h | - | - | 1.2 |
| CP_12 h | 26 | 3 | 29 |
| CP_24 h | 75.7 | 18.2 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





