Submitted:
24 April 2024
Posted:
25 April 2024
You are already at the latest version
Abstract
Keywords:
1. Brief Historical Overview and the Presentation of the Problem
2. The Selection of Studies for Review
3. Research Findings
3.1. Cross-Sectional Studies
3.2. Longitudinal Studies
3.3. Treatment with Medical Cannabis
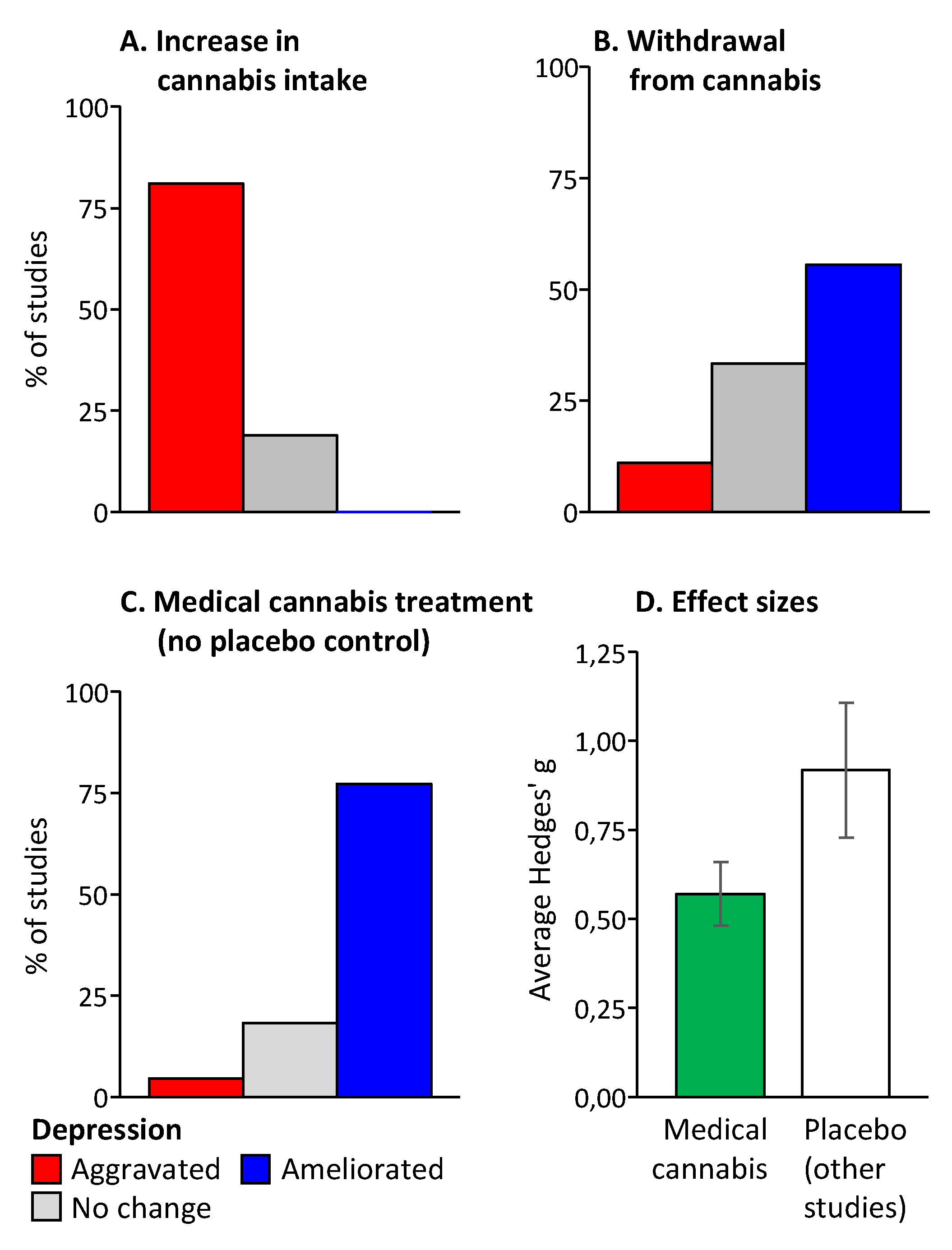
3.4. Overall Assessment
4. Mechanistic Considerations
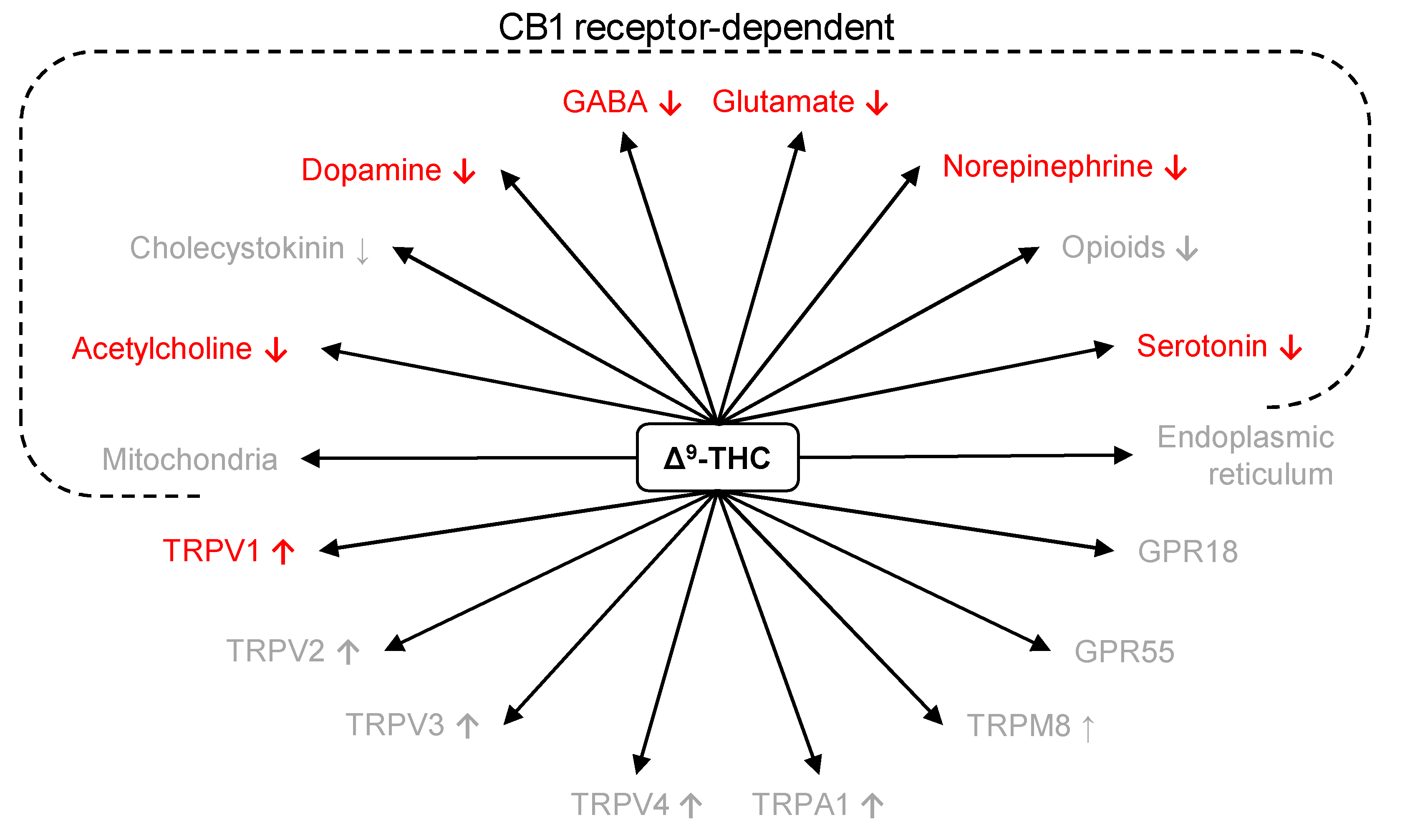
4.1. Neurochemistry
4.2. Pharmacology
5. Concluding Remarks
Funding
Conflicts of Interest
References
- Rudgley, R. Psychoactive plants. In: The Cultural History of Plants; Prance, G., Nesbitt, M., Eds.; Routledge, New York, USA, 2005, pp. 191–205.
- Rudgley, R. The Encyclopedia of Psychoactive Substances. St. Martin’s Griffin, New York, USA, 2000. pp. 191–205.
- Crocq, M. A. History of cannabis and the endocannabinoid system. Dialog Clin Neurosci, 2020, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Musto, D. F. The Marihuana Tax Act of 1937. Arch Gen Psych, 1972, 26, 101–108. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Single Convention on Narcotic Drugs, 1961. Available online: https://www.google.com/search?client=firefox-b-d&q=1961+United+Nations+Single+Convention+on+Narcotic+Drugs#vhid=zephyrhttps://www.unodc.org/pdf/convention_1961_en.pdf&vssid=collectionitem-web-desktophttps://www.unodc.org/pdf/convention_1961_en.pdf (accessed on 12.04.2024).
- Hill, M. N. , Hillard, C. J., Bambico, F. R., Patel, S., Gorzalka, B. B., Gobbi, G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci, 2009, 30, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S. , Sherry, S. , Mahu, I., Stewart, S. Development of brief alcohol and cannabis motives measures: psychometric evaluation using expert feedback and longitudinal methods. Cannabis, 2023, 6, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Cassano, P. , Fava, M. Depression and public health: an overview. J. Psychosom. Res., 2002, 53, 849–857. [Google Scholar] [CrossRef]
- Stachowicz, K. , Sowa-Kućma, M. The treatment of depression - searching for new ideas. Front. Pharmacol., 2022, 13, 988648. [Google Scholar] [CrossRef] [PubMed]
- Denson, T. F. , Earleywine, M. Decreased depression in marijuana users. Addict. Behave., 2006, 31, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Lucido F., H. , Mangini, M. Implementation of the Compassionate Use Act In a Family Medical Practice: Seven years’ clinical experience. O’Shaughnessy’s, 2004, 1, 3–5. [Google Scholar]
- Marijuana Policy Project. Available online. https://www.mpp.org/issues/legalization/effective-arguments-for-regulating-and-taxing-marijuana/ (accessed on 12. 04. 2024.
- Zimmerman, B. , Crumpacker, N., Bayer, R. Is Marijuana the Right Medicine for You: A Factual Guide to Medical Uses of Marijuana. Keats Publishers. Great Barrington, MA, 1998. pp. 1–208.
- McMahon, G. , Lergen C. Prescription Pot: A Leading Advocate’s Heroic Battle to Legalize Medical Marijuana. New Horizon Press, Far Hills, NJ, 2003. pp. 1–200.
- Rosenthal, E. , Mikuriya, T. H., Gieringer, D. Marijuana Medical Handbook. Quick American Archives, Oakland, USA, 1997. pp. 1–270.
- Baral, A. , Hanna, F. , Chimoriya, R., Rana, K. Cannabis use and its impact on mental health in youth in australia and the united states: A scoping review. Epidemiologia, 2024, 5, 106–121. [Google Scholar] [CrossRef]
- Chadwick, B. , Miller, M. L., Hurd, Y. L. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front. Psychiat., 2013, 4, 129. [Google Scholar] [CrossRef]
- Dave, P. A. , Rohit, R. K., Tibrewal, C., Modi, N. S., Bajoria, P. S., Gandhi, S. K., Patel, P. Should marijuana be legalized: a scoping review of associations of marijuana and depression. Cureus, 2023, 15(8), e42835. [CrossRef]
- Lev-Ran, S. , Roerecke, M. , Le Foll, B., George, T. P., McKenzie, K., Rehm, J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol. Med., 2014, 44, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Reece A., S. Chronic toxicology of cannabis. Clin. Toxicol., 2009, 47, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Tondo, L. , Baldessarini, R. J., Hennen, J., Minnai, G. P., Salis, P., Scamonatti, L., Masia, M., Ghiani, C., Mannu, P. Suicide attempts in major affective disorder patients with comorbid substance use disorders. J Clin Psychiat, 1999, 60 Suppl 2, 63–116.
- Bartoli, F. , Crocamo, C. , Carrà, G. Cannabis use disorder and suicide attempts in bipolar disorder: A meta-analysis. Neurosci Biobehav. Rev., 2019, 103, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Leite, R. T. , Nogueira, S.deO., do Nascimento, J. P., de Lima, L. S., da Nóbrega, T. B., Virgínio, M.daS., Moreno, L. M., Sampaio, B. H., de Matos E Souza, F. G. The use of cannabis as a predictor of early onset of bipolar disorder and suicide attempts. Neural Plasticity, 2015, 434127. [CrossRef]
- Shamabadi, A. , Ahmadzade, A. , Pirahesh, K., Hasanzadeh, A., Asadigandomani, H. Suicidality risk after using cannabis and cannabinoids: An umbrella review. Dialog Clin Neurosci., 2023, 25, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Sundram, S. Cannabis and neurodevelopment: implications for psychiatric disorders. Human Psychopharmacol, 2006, 21, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cumbo, N. , Lessner, K. , Marshall, C., Bozorghadad, S., Boehmer, S., Olympia, R. P. Demographics and reported symptoms associated with marijuana use among adolescent and young adult. Cureus, 2023, 15, e47844. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C. , Alvarez, P. , Hammond, C. J., Lilly, F. R. W. Cannabis use associations with adverse psychosocial functioning among north american college students. Subs. Use Misuse, 2023, 58, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, W. , Orozco, G. , Villanueva, G., Merianos, A. L. E-Cigarette and cannabis use patterns, depression, and suicide behaviors among us youth: analysis of 2019 youth risk behavior survey data. Amer J Health Promotion, 2023, 37, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Líbano, J. , Torres-Vallejos, J. , Oyanedel, J. C., González-Campusano, N., Calderón-Herrera, G., Yeomans-Cabrera, M. M. Prevalence and variables associated with depression, anxiety, and stress among Chilean higher education students, post-pandemic. Front. Psychiat., 2023, 14, 1139946. [Google Scholar] [CrossRef]
- Croock, J. , Mpinganjira, M. G., Gathoo, K., Bulmer, R., Lautenberg, S., Dlamini, Q., Londani, P., Solontsi, A., Stevens, C., Francis, J. M. Probable depression and its correlates among undergraduate students in Johannesburg, South Africa. Front. Psychiat., 2023, 14, 1018197. [Google Scholar] [CrossRef]
- Lisano, J. K. , Kisiolek, J. , Flores, V., Smoak, P., Pullen, N. A., Stewart, L. K. Chronic cannabis use is associated with altered monocyte phenotype, immune response, and depression in physically active individuals. Can. J Physiol. Pharmacol., 2023, 101, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Keen, L. , Turner, A. D., Harris, T., George, L., Crump, J. Differences in internalizing symptoms between those with and without Cannabis Use Disorder among HBCU undergraduate students. J. Amer. College Health, 2023, 71, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Rochat, L. , Mobbs, O. , Billieux, J., Khazaal, Y., Zufferey, C. Impulsivity, depressive mood, and cannabis use in a representative sample of french-speaking swiss young men. Psychol. Belgica, 2022, 62, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, W. , Lu, W. , McDonald, A., Yang, J. S. Human capital development factors and black adolescent tobacco and cannabis use. Nicot. Tobacco Res., 2023, 25, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Sumbe, A. , Wilkinson, A. V., Clendennen, S. L., Bataineh, B. S., Sterling, K. L., Chen, B., Harrell, M. B. Association of tobacco and marijuana use with symptoms of depression and anxiety among adolescent and young adult in Texas. Tobacco Prevent. Cessation, 2022, 8, 03. [Google Scholar] [CrossRef] [PubMed]
- Morais, P. R. , Nema Areco, K. C., Fidalgo, T. M., Xavier da Silveira, D. Mental health and quality of life in a population of recreative cannabis users in Brazil. J Psychiat Res., 2022, 146, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Steeger, C. M. , Hitchcock, L. N., Bryan, A. D., Hutchison, K. E., Hill, K. G., Bidwell, L. C. Associations between self-reported cannabis use frequency, potency, and cannabis/health metrics. Int. J. Drug Policy, 2021, 97, 103278. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Artamendi, S. , Martínez-Loredo, V. , López-Núñez, C. Sex differences in comorbidity between substance use and mental health in adolescent: two sides of the same coin. Psicothema, 2021, 33, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hindocha, C. , Brose, L. S., Walsh, H., Cheeseman, H. Cannabis use and co-use in tobacco smokers and non-smokers: prevalence and associations with mental health in a cross-sectional, nationally representative sample of adults in Great Britain, 2020. Addiction, 2021, 116, 2209–2219. [Google Scholar] [CrossRef]
- Weidberg, S. , González-Roz, A. , Castaño, Y., Secades-Villa, R. Emotion dysregulation in relation to cannabis use and mental health among young adults. Addict. Behav., 2023, 144, 107757. [Google Scholar] [CrossRef]
- Rup, J. , Freeman, T. P., Perlman, C., Hammond, D.. Cannabis and mental health: Prevalence of use and modes of cannabis administration by mental health status. Addict. Behav., 2021, 121, 106991. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, J. D. , Mikulich-Gilbertson, S. K., He, J. P., Bhatia, D., Ellingson, J. M., Nguyenkhoa Vu, B., Ries Merikangas, K., Sakai, J. T. Cannabis use is associated with depression severity and suicidality in the national comorbidity survey-adolescent supplement. JAACAP, 2023, 1, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hotham, J. , Cannings-John, R. , Moore, L., Hawkins, J., Bonell, C., Hickman, M., Zammit, S., Hines, L. A., Adara, L., Townson, J., White, J. Association of cannabis, cannabidiol and synthetic cannabinoid use with mental health in UK adolescent. Brit. J. Psychiat., 2023, 223, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Sultan, R. S. , Zhang, A. W., Olfson, M., Kwizera, M. H., Levin, F. R. Nondisordered cannabis use among us adolescent. JAMA, 2023, 6, e2311294. [Google Scholar] [CrossRef] [PubMed]
- Horváth, Z. , Sárosi, P. , Boda, L., Farkas, E., Koós, M., Demetrovics, Z., Urbán, R. The relationship between anxious-depressive symptoms and harmful cannabis use: Multiple mediation models via rumination, negative urgency, protective behavioral strategies and refusal self-efficacy. Comprehens. Psychiat., 2022, 116, 152320. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, K. , Hines, L. , Adams, S., Morgan, C. J., Curran, H. V., Freeman, T. P. High potency cannabis use, mental health symptoms and cannabis dependence: Triangulating the evidence. Addict. Behav, 2023, 144, 107740. [Google Scholar] [CrossRef] [PubMed]
- Freichel, R. , Kroon, E., Kuhns, L., Filbey, F., Veer, I. M., Wiers, R., Cousijn, J. Cannabis use disorder symptoms in weekly cannabis users: a network comparison between daily cigarette users and nondaily cigarette users. Cannab. Cannabinoid Res., 2023, Advance online publication. [CrossRef]
- Lawn, W. , Mokrysz, C. , Lees, R., Trinci, K., Petrilli, K., Skumlien, M., Borissova, A., Ofori, S., Bird, C., Jones, G., Bloomfield, M. A., Das, R. K., Wall, M. B., Freeman, T. P., Curran, H. V. The CannTeen Study: Cannabis use disorder, depression, anxiety, and psychotic-like symptoms in adolescent and adult cannabis users and age-matched controls. J. Psychopharmacol., 2022, 36, 1350–1361. [Google Scholar] [CrossRef]
- Parekh, T. , Fahim, F. Building risk prediction models for daily use of marijuana using machine learning techniques. Drug Alcohol Depend., 2021, 225, 108789. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, V. , Kaur, J. , Singh, R., Jaka, S., Kaur, G., Rawal, E., Mathialagan, K., Amuk Williams, O. C. Predictors of hospitalization for manic episode in alzheimer’s dementia: inputs from an inpatient case-control study. Cureus, 2021, 13, e17333. [Google Scholar] [CrossRef]
- Asper, A. , Binenfeld, E. , Pshitizky, H., Feingold, D. Sociodemographic and clinical correlates of cannabis dependence among Israeli combat veterans. J Subst Abuse Treat., 2022, 139, 108786. [Google Scholar] [CrossRef]
- Fitzke, R. E. , Davis, J. P., Pedersen, E. R. Co-use of tobacco products and cannabis among veterans: a preliminary investigation of prevalence and associations with mental health outcomes. J Psychoact. Drugs, 2022, 54, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Hill, M. L. , Nichter, B. M., Norman, S. B., Loflin, M., Pietrzak, R. H. Burden of cannabis use and disorder in the U.S. veteran population: Psychiatric comorbidity, suicidality, and service utilization. J. Affect. Dis., 2021, 278, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Ashwal-Malka, A. , Tal-Kishner, K. , Feingold, D. Moral injury and cannabis use disorder among Israeli combat veterans: The role of depression and perceived social support. Addict. Behav., 2022, 124, 107114. [Google Scholar] [CrossRef] [PubMed]
- Hill, M. L. , Loflin, M. , Nichter, B., Norman, S. B., Pietrzak, R. H. Prevalence of cannabis use, disorder, and medical card possession in U.S. military veterans: Results from the 2019-2020 National Health and Resilience in Veterans Study. Addict. Behav., 2021, 120, 106963. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A. , Gicas, K. , Stevens, W. D., Sergio, L., Wojtowicz, M. Substance use is associated with worse mental health and altered resting state functional connectivity in female university athletes at baseline: A pilot study. PloS One, 2021, 16, e0253261. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. S. , Cheema, Z. , Singla, A., Cornejo, M., Verma, G. Cannabis use is an independent risk factor for manic episode: A report from 380,265 bipolar inpatients. Subst. Use Misuse, 2022, 57, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. , Salloum, R. G., Jenkins, W., Hales, D. B., Sharma, A. Marijuana use among us adults with cancer: findings from the 2018-2019 Behavioral Risk Factor Surveillance System. J. Cancer Survivor., 2022, 17(4), 1161–1170. [CrossRef]
- Xu, W. , Gilmer, D. O., Starkweather, A., Kim, K. Associations among marijuana use, health-related quality of life, exercise, depression and sleep in cancer survivors. J. Adv. Nursing, 2021, 77, 2386–2397. [Google Scholar] [CrossRef]
- Poghosyan, H. , Noonan, E. J., Badri, P., Braun, I., Young, G. J. Association between daily and non-daily cannabis use and depression among United States adult cancer survivors. Nursing Outlook, 2021, 69, 672–685. [Google Scholar] [CrossRef]
- Jefsen, O. H. , Erlangsen, A. , Nordentoft, M., Hjorthøj, C. Cannabis use disorder and subsequent risk of psychotic and nonpsychotic unipolar depression and bipolar disorder. JAMA Psychiat, 2023, 80, 803–810. [Google Scholar] [CrossRef]
- Livingston, N. A. , Farmer, S. L., Mahoney, C. T., Marx, B. P., Keane, T. M. Longitudinal course of mental health symptoms among veterans with and without cannabis use disorder. Psychol. Addict. Behav, 2022, 36, 131–143. [Google Scholar] [CrossRef]
- Kurtzman, E. T. , Greene, J. Is adversity in childhood linked to marijuana use in adulthood?: findings from the Behavioral Risk Factor Surveillance System. Subst. Use Misuse, 2022, 57, 273–286. [Google Scholar] [CrossRef]
- Baiden, P. , Morgan, M. A., Logan, M. W. sports- and physical activity-related concussions, binge drinking and marijuana use among adolescent: the mediating role of depression and suicidal ideation. Subst. Use Misuse, 2022, 57, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Romano, I. , Patte, K. A., de Groh, M., Jiang, Y., Wade, T. J., Bélanger, R. E., Leatherdale, S. T. Substance-related coping behaviours among youth during the early months of the COVID-19 pandemic. Addict. Behav. Rep., 2021, 14, 100392. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N. , Peyser, N. D., Olgin, J. E., Pletcher, M. J., Beatty, A. L., Modrow, M. F., Carton, T. W., Khatib, R., Djibo, D. A., Ling, P. M., Marcus, G. M. Associations between tobacco and cannabis use and anxiety and depression among adults in the United States: Findings from the COVID-19 citizen science study. PloS One, 2023, 18, e0289058. [Google Scholar] [CrossRef]
- Fitzke, R. E. , Wang, J. , Davis, J. P., Pedersen, E. R. Substance use, depression, and loneliness among American veterans during the COVID-19 pandemic. Amer. J. Addict., 2021, 30, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Bălăeț, M. , Trender, W. , Hellyer, P. J., Hampshire, A. Associations between the use of psychedelics and other recreational drugs with mental health and resilience during the COVID-19 pandemic. Front. Psychiat., 2023, 14, 1184681. [Google Scholar] [CrossRef] [PubMed]
- Obuobi-Donkor, G. , Eboreime, E. , Shalaby, R., Agyapong, B., Agyapong, V. I. O. Prevalence and correlates of cannabis abuse among residents in the community of Fort McMurray, a city in Northern Alberta which had endured multiple natural disasters. Front. Psychiat., 2022, 13, 962169. [Google Scholar] [CrossRef] [PubMed]
- Mezaache, S. , Donadille, C. , Martin, V., Le Brun Gadelius, M., Appel, L., Spire, B., Briand Madrid, L., Bastien, M., Roux, P. Changes in cannabis use and associated correlates during France’s first COVID-19 lockdown in daily cannabis users: results from a large community-based online survey. Harm Reduction J., 2022, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Burke, C. W. , Firmin, E. S., Lanni, S., Ducharme, P., DiSalvo, M., Wilens, T. E. Substance use disorders and psychiatric illness among transitional age youth experiencing homelessness. JAACAP, 2023; 1, 3-11. [CrossRef]
- Ciesluk B, Erridge S, Sodergren MH, Troup LJ. Cannabis use in the UK: a quantitative comparison of individual differences in medical and recreational cannabis users. Front Psychol. 2024, 14, 1279123. [CrossRef]
- Phillips KT, Pedula KL, Simiola V, Satre DD, Choi NG. Psychiatric and substance use disorders among adults over age 50 who use cannabis: A matched cohort study using electronic health record data. Addict Behav., 2024, 150, 107927. [CrossRef]
- Modi, V. , Singh, A. , Shirani, J. Marijuana use and stress cardiomyopathy in the young. Cureus, 2021, 13, e18575. [Google Scholar] [CrossRef] [PubMed]
- Campuzano-Cortina, C. , Feijoó-Fonnegra, L. M., Manzur-Pineda, K., Palacio-Muñoz, M., Rendón-Fonnegra, J., Montoya, L., Berrouet, M. C., Restrepo, D. Comorbidity between depressive symptoms and substance use in-patients hospitalized for non-psychiatric diseases. Rev. Colombiana Psiquiat., 2021, 50, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Oladunjoye, A. F. , Li, E. , Aneni, K., Onigu-Otite, E. Cannabis use disorder, suicide attempts, and self-harm among adolescent: A national inpatient study across the United States. PloS One, 2023, 18, e0292922. [Google Scholar] [CrossRef] [PubMed]
- Miller-Matero LR, Joseph-Mofford G, Abdole L, et al. Alcohol and cannabis use among women with infertility: associations with psychiatric symptoms, attempts to conceive, and engagement in fertility treatment. Arch Womens Ment Health. Published online December 11, 2023. [CrossRef]
- Oseni, E. A. , Blumenthal, M. , Izard, S., Qiu, M., Mone, A., Swaminath, A., Sultan, K. Cannabis use and its association with thirty- and ninety-day hospital readmissions for patients admitted for an inflammatory bowel disease exacerbation. J. Clin. Med. Res., 2023, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Goodhines, P. A. , Wedel, A. V., Dobani, F., Zaso, M. J., Gellis, L. A., Park, A. Cannabis use for sleep aid among high school students: Concurrent and prospective associations with substance use and sleep problems. Addict. Behav., 2022, 134, 107427. [Google Scholar] [CrossRef] [PubMed]
- Brown, Q. L. , Shmulewitz, D. , Sarvet, A. L., Young-Wolff, K. C., Howard, T., Hasin, D. S. Cannabis use, cannabis use disorder and mental health disorders among pregnant and postpartum women in the US: A nationally representative study. Drug. Alcohol Dependen., 2023, 248, 109940. [Google Scholar] [CrossRef] [PubMed]
- Mark, K. , Otieno, L. , Moore, E., Zehra, A., Mitchell, M. Association between continued cannabis use during pregnancy and symptoms of anxiety and depression. Int. Rev. Psychiat., 2021, 33, 528–533. [Google Scholar] [CrossRef]
- Meinhofer, A. , Hinde, J. M., Keyes, K. M., Lugo-Candelas, C. Association of comorbid behavioral and medical conditions with cannabis use disorder in pregnancy. JAMA Psychiat., 2022, 79, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Padwa, H. , Huang, D. , Mooney, L., Grella, C. E., Urada, D., Bell, D. S., Bass, B., Boustead, A. E. Medical conditions of primary care patients with documented cannabis use and cannabis use disorder in electronic health records: a case control study from an academic health system in a medical marijuana state. Subst. Abuse Treatm. Prevent. Policy, 2022, 17, 36. [Google Scholar] [CrossRef]
- Murkar, A. , Kendzerska, T. , Shlik, J., Quilty, L., Saad, M., Robillard, R. Increased cannabis intake during the COVID-19 pandemic is associated with worsening of depression symptoms in people with PTSD. BMC Psychiat., 2022, 22, 554. [Google Scholar] [CrossRef]
- Aas M, Sideli L, Franceschini C, Alameda L, Trotta G, Coco GL, Musetti A, Schimmenti A. The role of interpersonal trauma and substance use in mental health: A large population-based study. Psychiatry Res., 2023, 333:115712. [CrossRef]
- Bryan, J. L. , Hogan, J. , Lindsay, J. A., Ecker, A. H. Cannabis use disorder and post-traumatic stress disorder: The prevalence of comorbidity in veterans of recent conflicts. J. Subst. Abuse Treat., 2021, 122, 108254. [Google Scholar] [CrossRef] [PubMed]
- Dolovich, C. L. , Shaffer, S. R., Graff, L. A, et al. The Association Between Increased Maladaptive Health Behaviours and Elevated Mental Health Symptoms Among Persons with IBD During the COVID-19 Pandemic. J Can Assoc Gastroenterol. 2023, 6:179-185. Published 2023 Sep 13. [CrossRef]
- Guo, Y. , Fleming, C. B., Stevens, A. L., Swaim, R. C., Mason, W. A. Correlates of solitary alcohol and cannabis use among American Indian adolescent. Drug. Alcohol Dependen., 2021, 229(Pt A), 109155. [CrossRef]
- Naguib, Y. M. , Sherif, H. A., Elbalshy, A. T., Edrees, E. A., Sabry, A. E., Sharif, A. F., Aloshari, S. H. A., Kasemy, Z. A. Prevalence and associated risk factors of cannabinoid abuse among Egyptian university students: a cross-sectional study. Environ. Sci. Pollut. Res. Int., 2021, 28, 68706–68716. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J. H. , Oña, G., Alcázar-Córcoles, M. Á., Bouso, J. C. Cannabis and public health: A study assessing regular cannabis users through health indicators. Cannab. Cannabin. Res., 2023, Advance online publication. [CrossRef]
- Mantey, D. S. , Onyinye, O. N., Montgomery, L. Prevalence and correlates of daily blunt use among U.S. African American, Hispanic, and White adults from 2014 to 2018. Psychol. Addict. Behav. 2021, 35, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson ML, Trainor C, Lampe E, Presseller EK, Juarascio A. Cannabis use and binge eating: Examining the relationship between cannabis use and clinical severity among adults with binge eating. Exp Clin Psychopharmacol. 2024 Jan 18. [CrossRef]
- Nugent, S. M. , Latour, E., Lim, J., Shannon, J., Morasco, B. J. Cannabis use is associated with pain severity and interference among cancer survivors. Research Square, 2023, 3126192. [CrossRef]
- Miller-Matero, L. R. , Ross, K. , Arellano, C., Zelenak, L., DePascale, E., Gavrilova, L., Braciszewski, J. M., Hecht, L. M., Haley, E. N., Brescacin, C., Carlin, A. M. Cannabis use following bariatric surgery is associated with anxiety and maladaptive eating. Surg. Obesity Related Dis., 2024, 20, 91–97. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M. C. , Cruz, A. P. M., Teixeira, M. O. Depression, anxiety, and drug usage history indicators among institutionalized juvenile offenders of Brasilia. Psicologia, Reflexao e Critica, 2021, 34, 17. [Google Scholar] [CrossRef] [PubMed]
- Fernando, J. , Stochl, J. , Ersche, K. D. Drug Use in Night Owls May Increase the Risk for Mental Health Problems. Front. Neurosci., 2022, 15, 819566. [Google Scholar] [CrossRef] [PubMed]
- St Cyr K, Nazarov A, Le T, et al. Correlates of cannabis use in a sample of mental health treatment-seeking Canadian armed forces members and veterans. BMC Psychiat.. 2023, 23, 836. [CrossRef] [PubMed]
- Argote, M. , Sescousse, G. , Brunelin, J., Baudin, G., Schaub, M. P., Rabin, R., Schnell, T., Ringen, P. A., Andreassen, O. A., Addington, J. M., Brambilla, P., Delvecchio, G., Bechdolf, A., Wobrock, T., Schneider-Axmann, T., Herzig, D., Mohr, C., Vila-Badia, R., Rodie, J. U., Mallet, J., Rolland, B. Association between cannabis use and symptom dimensions in schizophrenia spectrum disorders: an individual participant data meta-analysis on 3053 individuals. EClinicalMedicine, 2023, 64, 102199. [Google Scholar] [CrossRef] [PubMed]
- Luque, B. , García, V. , Tabernero, C. Depression and cognitive impairment in a spanish sample of psychoactive substance users receiving mental health care. Healthcare, 2022, 10, 887. [Google Scholar] [CrossRef]
- Martin, E. L. , Strickland, J. C., Schlienz, N. J., Munson, J., Jackson, H., Bonn-Miller, M. O., Vandrey, R. Antidepressant and Anxiolytic Effects of Medicinal Cannabis Use in an Observational Trial. Front. Psychiat., 2021, 12, 729800. [Google Scholar] [CrossRef]
- Schlienz, N. J. , Scalsky, R. , Martin, E. L., Jackson, H., Munson, J., Strickland, J. C., Bonn-Miller, M. O., Loflin, M., Vandrey, R. A Cross-Sectional and Prospective Comparison of Medicinal Cannabis Users and Controls on Self-Reported Health. Cannab. Cannabin. Res., 2021, 6, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M. , Matalí, J. L., Sivoli, J., Regina, V. B., Butjosa, A., Dolz, M., Sánchez, B., Barajas, A., Del Cacho, N., Baños, I., Ochoa, S., Usall, J. Early onset psychosis and cannabis use: Prevalence, clinical presentation and influence of daily use. Asian J. Psychiat., 2021, 62, 102714. [Google Scholar] [CrossRef] [PubMed]
- Lydiard, J. B. , Patel, H. , Strugatsky, Y., Thompson, W. K., Pelham, W. E., 3rd, Brown, S. A. Prospective associations between cannabis use and depressive symptoms across adolescence and early adulthood. Psychiat. Res., 2023, 325, 115190. [Google Scholar] [CrossRef] [PubMed]
- London-Nadeau, K. , Rioux, C. , Parent, S., Vitaro, F., Côté, S. M., Boivin, M., Tremblay, R. E., Séguin, J. R., Castellanos-Ryan, N. Longitudinal associations of cannabis, depression, and anxiety in heterosexual and LGB adolescent. J. Abnorm. Psychol., 2021, 130, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Vu, T. T. , Dario, J. P., Mateu-Gelabert, P., Levine, D., Punter, M. A., Borrell, L. N., Ngo, V. K. Substance Use Patterns and Their Association with Depression and Social Factors During COVID-19 Among Harlem Residents in New York City. J. Commun. Health, 2023, 48, 937–944. [Google Scholar] [CrossRef]
- Clendennen, S. L. , Chen, B. , Sumbe, A., Harrell, M. B. Patterns in mental health symptomatology and cigarette, e-cigarette, and marijuana use among Texas youth and young adult amid the coronavirus disease 2019 pandemic. Nicot. Tobacco Res., 2023, 25, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Meanley, S. , Choi, S. K., Thompson, A. B., Meyers, J. L., D’Souza, G., Adimora, A. A., Mimiaga, M. J., Kempf, M. C., Konkle-Parker, D., Cohen, M. H., Teplin, L. A., Murchison, L., Rubin, L. H., Rubtsova, A. A., Weiss, D. J., Aouizerat, B., Friedman, M. R., Plankey, M. W., Wilson, T. E. Short-term binge drinking, marijuana, and recreational drug use trajectories in a prospective cohort of people living with HIV at the start of COVID-19 mitigation efforts in the United States. Drug. Alcohol Dependen., 2022, 231, 109233. [Google Scholar] [CrossRef]
- Choi, N. G. , DiNitto, D. M., Marti, C. N., Choi, B. Y. Cannabis and binge alcohol use among older individuals with major depressive episode. Subst. Abuse, 2022, 43, 657–665. [Google Scholar] [CrossRef]
- Livne O, Malte CA, Olfson M, et al. Trends in prevalence of cannabis use disorder among u.s. veterans with and without psychiatric disorders between 2005 and 2019. Am J Psychiatry., 2024, 181:144-152. [CrossRef]
- Liu, J. , Winickoff, J. P., Hanby, E., Rees, V., Emmons, K. M., Tan, A. S. Prevalence and correlates of past 30-day dual-vaping of nicotine and cannabis among adolescent in five New England states. Drug Alcohol Depend. 2024, 254:111055. [CrossRef]
- Duncan, M. J. , Patte, K. A., Leatherdale, S. T. Hit the chronic… physical activity: are cannabis associated mental health changes in adolescent attenuated by remaining active? Soc Psychiatry Psychiatr Epidemiol, 2021, 56:141-152. [CrossRef]
- Wade, N. E. , Gilbart, E. , Swartz, A. M., Lisdahl, K. M. Assessing aerobic fitness level in relation to affective and behavioral functioning in emerging adult cannabis users. Int. J. Ment. Health Addict, 2021, 19, 546–559. [Google Scholar] [CrossRef]
- Bataineh, B. S. , Wilkinson, A. V., Sumbe, A., Clendennen, S. L., Chen, B., Messiah, S. E., Harrell, M. B. Depressive symptoms and the age of initiation of tobacco and marijuana use among adolescent and young adult. Drug Alcohol Depend, 2023, 252, 110971. [Google Scholar] [CrossRef]
- Bataineh, B. S. , Wilkinson, A. V., Sumbe, A., Clendennen, S. L., Chen, B., Messiah, S. E., Harrell, M. B. The Association Between Tobacco and Cannabis Use and the Age of Onset of Depression and Anxiety Symptoms: Among Adolescent and Young adult. Nicot. Tobacco Res., 2023, 25, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Xu, C. , Wang, S., Su, B. B., Ozuna, K., Mao, C., Dai, Z., Wang, K. (2024). Associations of adolescent substance use and depressive symptoms with adult major depressive disorder in the United States: NSDUH 2016-2019. Journal of affective disorders, 344, 397–406. [CrossRef]
- Nielsen, L. G. , Køster Rimvall, M. , Van Os, J., Verhulst, F., Rask, C. U., Skovgaard, A. M., Olsen, E. M., Jeppesen, P. Precursors of self-reported subclinical hypomania in adolescence: A longitudinal general population study. PloS One, 2021, 16, e0253507. [Google Scholar] [CrossRef] [PubMed]
- Nathan Marti, C. , Arora, S. , Loukas, A. Depressive symptoms predict trajectories of electronic delivery nicotine systems, cigarette, and cannabis use across 4.5 years among college students. Addict. Behav., 2023, 146, 107809. [Google Scholar] [CrossRef]
- Capaldi, D. M. , Tiberio, S. S., Kerr, D. C., Owen, L. D. Associations of cannabis use across adolescence and early adulthood with health and psychosocial adjustment in early adulthood and midadulthood in men. Subst. Abuse Res. Treat., 2022, 16, 11782218221096154. [Google Scholar] [CrossRef]
- Bach, S. L. , Cardoso, T. A., Moreira, F. P., Mondin, T. C., Simjanoski, M., Kapczinski, F. P., Frey, B. N., Souza, L. D. M., da Silva, R. A., Jansen, K. Risk factors for new-onset bipolar disorder in a community cohort: A five-year follow up study. Psychiat. Res., 2021, 303, 114109. [Google Scholar] [CrossRef] [PubMed]
- Marmet, S. , Studer, J. , Wicki, M., Gmel, G. Cannabis use disorder trajectories and their prospective predictors in a large population-based sample of young Swiss men. Addiction, 2021, 116, 560–570. [Google Scholar] [CrossRef]
- Dunbar, M. S. , Davis, J. P., Tucker, J. S., Seelam, R., Rodriguez, A., D’Amico, E. J. Parallel trajectories of vaping and smoking cannabis and their associations with mental and physical well-being among young adult. Drug. Alcohol Dependen., 2023, 251, 110918. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A. C. R. , Montezano, B. B., de Aguiar, K. R., Noronha, L. T., Baldez, D. P., Watts, D., Menezes, A. M. B., Wehrmeister, F. C., Gonçalves, H., Kunz, M., Kapczinski, F., Passos, I. C. Early exposure to cannabis and bipolar disorder incidence: Findings from a 22-year birth cohort study in Brazil. Acta Psychiat. Scand., 2024, Advance online publication. [CrossRef]
- Gripe, I. , Pape, H. , Norström, T. Associations Between Cannabis Use and Mental Distress in Young People: A Longitudinal Study. J Adolesc Health., 2024, 74, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A. , Hielscher, E. , Miettunen, J., Denissoff, A., Alakokkare, A. E., Scott, J. G., Niemelä, S. Adolescent cannabis use, depression and anxiety disorders in the Northern Finland Birth Cohort 1986. BJPsych open, 2021, 7, e137. [Google Scholar] [CrossRef]
- Chan, G. C. K. , Becker, D. , Butterworth, P., Hines, L., Coffey, C., Hall, W., Patton, G. Young-adult compared to adolescent onset of regular cannabis use: A 20-year prospective cohort study of later consequences. Drug Alcohol Rev., 2021, 40, 627–636. [Google Scholar] [CrossRef]
- Bolton, S. , Joyce, D. W., Gordon-Smith, K., Jones, L., Jones, I., Geddes, J., Saunders, K. E. A. Psychosocial markers of age at onset in bipolar disorder: a machine learning approach. BJPsych open, 2022, 8, e133. [Google Scholar] [CrossRef]
- Preuss, U. W. , Hesselbrock, M. N., Hesselbrock, V. M. A prospective comparison of bipolar i and ii subjects with and without comorbid cannabis use disorders from the COGA dataset. Brain Sci., 2023, 13, 1130. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A. N. , Le Foll, B. Survival probabilities and predictors of major depressive episode incidence among individuals with various types of substance use disorders. J. Clin. Psychiat., 2021, 82, 20m13637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. , Duan, Z. , Romm, K. F., Ma, Y., Douglas Evans, W., Bennett, B., Fuss, C., Klinkhammer, K. E., Wysota, C. N., Berg, C. J. Bidirectional associations between depressive symptoms and cigarette, e-cigarette, cannabis, and alcohol use: Cross-lagged panel analyses among young adult before and during COVID-19. Addict. Behav., 2022, 134, 107422. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, H. M. , Spagnolo, J., Bartram, M., Fleury, M. J., Gouin, J. P., Grenier, S., Roberge, P., Shen-Tu, G., Vena, J. E., Lamoureux-Lamarche, C., Wang, J. Factors associated with change in moderate or severe symptoms of anxiety and depression in community-living adults and older adults during the COVID-19 pandemic. Can J Public Health, 2023. [CrossRef]
- McAfee, J. , Boehnke, K. F., Moser, S. M., Brummett, C. M., Waljee, J. F., Bonar, E. E. Perioperative cannabis use: a longitudinal study of associated clinical characteristics and surgical outcomes. Regional Anesth. Pain Med., 2021, 46, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Janes, L. A. , Hammond, J. W., Bonham, A. J., Carlin, A. M., Ghaferi, A. A., Varban, O. A., Ehlers, A. P., Finks, J. F. The effect of marijuana use on short-term outcomes with bariatric surgery. Surg. Obesity Related Dis., 2023, 19, 964–970. [Google Scholar] [CrossRef]
- Albelo, F. D. , Baker, M. , Zhang, T., Schneider, M. B., Jauregui, J. J., Nadarajah, V., Meredith, S. J., Packer, J. D., Henn, R. F., 3rd Impact of pre-operative recreational marijuana use on outcomes two years after orthopaedic surgery. Int. orthopaedics, 2021, 45, 2483–2490. [Google Scholar] [CrossRef]
- Lendel A, Richards R, Benedict J, Lynch C, Schaffir J. Incidence of postpartum depression in low-income cannabis users with and without a history of depression. Arch Womens Ment Health. 2024;27(1):145-151. [CrossRef]
- Leng, Q. L. , Lo, J. O., Rakshe, S., Hildebrand, A. D., Doyle, O. J., Seghete, K. M., Graham, A. (2023). The association between preconception cannabis use and depression and anxiety during pregnancy. General hospital psychiatry, 83, 148–155. [CrossRef]
- Cao, S. , Jones, M., Tooth, L., Mishra, G. D. (2021). Association between preconception cannabis use and risk of postpartum depression: Findings from an Australian longitudinal cohort. Drug. Alcohol Dependen., 226, 108860. [CrossRef]
- Mensah, F. K. , Glover, K., Leane, C., Gartland, D., Nikolof, A., Clark, Y., Gee, G., Brown, S. J. (2024). Understanding cannabis use and mental health difficulties in context with women’s experiences of stressful events and social health issues in pregnancy: The Aboriginal Families Study. Comprehensive psychiatry, 131, 152455. Advance online publication. [CrossRef]
- Hinojosa, C. A. , Liew, A., An, X., Stevens, J. S., Basu, A., van Rooij, S. J. H., House, S. L., Beaudoin, F. L., Zeng, D., Neylan, T. C., Clifford, G. D., Jovanovic, T., Linnstaedt, S. D., Germine, L. T., Rauch, S. L., Haran, J. P., Storrow, A. B., Lewandowski, C., Musey, P. I., Hendry, P. L., … Fani, N. (2024). Associations of alcohol and cannabis use with change in posttraumatic stress disorder and depression symptoms over time in recently trauma-exposed individuals. Psychological medicine, 54(2), 338–349. [CrossRef]
- Kwon, E. , Oshri, A., Zapolski, T. C. B., Zuercher, H., Kogan, S. M. (2023). Substance use trajectories among emerging adult Black men: Risk factors and consequences. Drug and alcohol review, 42(7), 1816–1824. [CrossRef]
- Denissoff, A. , Mustonen, A., Alakokkare, A. E., Scott, J. G., Sami, M. B., Miettunen, J., Niemelä, S. (2022). Is early exposure to cannabis associated with bipolar disorder? Results from a Finnish birth cohort study. Addiction (Abingdon, England), 117(8), 2264–2272. [CrossRef]
- Romm, K. F., Wang, Y., Duan, Z., Bennett, B., Fuss, C., Ma, Y., Blank, M. D., Bray, B. C., Ahluwalia, J. S., Berg, C. J. (2022). Psychosocial predictors of longitudinal changes in tobacco and cannabis use among young adult. Addict. Behav., 129, 107264. [CrossRef]
- Stypulkowski, K. , Thayer, R. E. (2022). Long-Term Recreational Cannabis Use Is Associated With Lower Executive Function and Processing Speed in a Pilot Sample of Older Adults. Journal of geriatric psychiatry and neurology, 35(5), 740–746. [CrossRef]
- Wright, A. C. , Browne, J., Cather, C., Meyer-Kalos, P., Mueser, K. T. (2023). Relationship between patterns of cannabis use and functional and symptomatic trajectories in first-episode psychosis. European archives of psychiatry and clinical neuroscience, 273(4), 765–778. [CrossRef]
- Feinstein, A. , Meza, C., Stefan, C., Staines, W. R. (2021). Discontinuing cannabis improves depression in people with multiple sclerosis: A short report. Multiple sclerosis (Houndmills, Basingstoke, England), 27(4), 636–639. [CrossRef]
- Dabravolskaj, J. , Veugelers, P. J., Amores, A., Leatherdale, S. T., Patte, K. A., Maximova, K. (2023). The impact of 12 modifiable lifestyle behaviours on depressive and anxiety symptoms in middle adolescence: prospective analyses of the Canadian longitudinal COMPASS study. The international journal of behavioral nutrition and physical activity, 20(1), 45. [CrossRef]
- Coughlin, L. N. , Bonar, E. E., Wieringa, J., Zhang, L., Rostker, M. J., Augustiniak, A. N., Goodman, G. J., Lin, L. A. (2023). Pilot trial of a telehealth-delivered behavioral economic intervention promoting cannabis-free activities among adults with cannabis use disorder. Journal of psychiatric research, 163, 202–210. [CrossRef]
- Elison-Davies, S. , Wardell, J. D., Quilty, L. C., Ward, J., Davies, G. (2021). Examining correlates of cannabis users’ engagement with a digital intervention for substance use disorder: An observational study of clients in UK services delivering Breaking Free Online. J. Subst. Abuse Treat., 123, 108261. [CrossRef]
- Sullivan, R. M. , Wallace, A. L., Stinson, E. A., Montoto, K. V., Kaiver, C. M., Wade, N. E., Lisdahl, K. M. (2022). Assessment of Withdrawal, Mood, and Sleep Inventories After Monitored 3-Week Abstinence in Cannabis-Using Adolescent and Young adult. Cannab. Cannabin. Res., 7(5), 690–699. [CrossRef]
- Cooke, M. E. , Gilman, J. M., Lamberth, E., Rychik, N., Tervo-Clemmens, B., Evins, A. E., Schuster, R. M. (2021). Assessing Changes in Symptoms of Depression and Anxiety During Four Weeks of Cannabis Abstinence Among Adolescent. Front. Psychiat., 12, 689957. [CrossRef]
- Sterling, S. , Parthasarathy, S., Jones, A., Weisner, C., Metz, V., Hartman, L., Saba, K., Kline-Simon, A. H. (2022). Young Adult Substance Use and Healthcare Use Associated With Screening, Brief Intervention and Referral to Treatment in Pediatric Primary Care. The Journal of adolescent health: official publication of the Society for Adolescent Medicine, 71(4S), S15–S23. [CrossRef]
- Curry, J. F. , Kaminer, Y., Goldston, D. B., Chan, G., Wells, K. C., Burke, R. H., Inscoe, A. B., Meyer, A. E., Cheek, S. M. (2022). Adaptive Treatment for Youth With Substance Use and Depression: Early Depression Response and Short-term Outcomes. Journal of the American Academy of Child and Adolescent Psychiatry, 61(4), 508–519. [CrossRef]
- Murnion, B. Medicinal cannabis. Aust Prescr. 2015 Dec;38(6):212-5. [CrossRef]
- Mammen, G. , de Freitas L., Rehm J., Rueda S. Cannabinoid concentrations in Canada’s regulated medical cannabis industry. Addict. (Abingdon Engl.) 2017;112:730–732. [CrossRef]
- Yang, Y. , Vyawahare R., Lewis-Bakker M., Clarke H.A., Wong A.H.C., Kotra L.P. Bioactive Chemical Composition of Cannabis Extracts and Cannabinoid Receptors. Molecules. 2020;25:3466. [CrossRef]
- Ebbert JO, Scharf EL, Hurt RT. Medical Cannabis. Mayo Clin Proc. 2018 Dec;93(12):1842-1847. [CrossRef]
- Tait, M. A. , Costa, D. S. J., Campbell, R., Norman, R., Warne, L. N., Schug, S., Rutherford, C. (2023). Health-related quality of life in patients accessing medicinal cannabis in Australia: The QUEST initiative results of a 3-month follow-up observational study. PloS one, 18(9), e0290549. [CrossRef]
- Wang, Y. , Jean Jacques, J., Li, Z., Sibille, K. T., Cook, R. L. (2021). Health Outcomes among Adults Initiating Medical Cannabis for Chronic Pain: A 3-month Prospective Study Incorporating Ecological Momentary Assessment (EMA). Cannabis (Albuquerque, N.M.), 4(2), 69–83. [CrossRef]
- Gambino, A. , Cabras, M., Panagiotakos, E., Calvo, F., Macciotta, A., Cafaro, A., Suria, M., Haddad, G. E., Broccoletti, R., Arduino, P. G. (2021). Evaluating the Suitability and Potential Efficiency of Cannabis sativa Oil for Patients with Primary Burning Mouth Syndrome: A Prospective, Open-Label, Single-Arm Pilot Study. Pain medicine (Malden, Mass.), 22(1), 142–151. [CrossRef]
- Hedges, L. (1981). Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. Journal of Educational Statistics. 6(2), 107-128. [CrossRef]
- Zloczower, O. , Brill, S., Zeitak, Y., Peles, E. (2022). Risk and benefit of cannabis prescription for chronic non-cancer pain. Journal of addictive diseases, 40(2), 157–167. [CrossRef]
- Rapin, L. , Gamaoun, R., El Hage, C., Arboleda, M. F., Prosk, E. (2021). Cannabidiol use and effectiveness: real-world evidence from a Canadian medical cannabis clinic. J. Cannabis Research, 3(1), 19. [CrossRef]
- Tervo-Clemmens, B. , Schmitt, W., Wheeler, G., Cooke, M. E., Schuster, R. M., Hickey, S., Pachas, G. N., Evins, A. E., Gilman, J. M. (2023). Cannabis use and sleep quality in daily life: An electronic daily diary study of adults starting cannabis for health concerns. Drug. Alcohol Dependen., 243, 109760. [CrossRef]
- Gilman, J. M. , Schuster, R. M., Potter, K. W., Schmitt, W., Wheeler, G., Pachas, G. N., Hickey, S., Cooke, M. E., Dechert, A., Plummer, R., Tervo-Clemmens, B., Schoenfeld, D. A., Evins, A. E. (2022). Effect of Medical Marijuana Card Ownership on Pain, Insomnia, and Affective Disorder Symptoms in Adults: A Randomized Clinical Trial. JAMA network open, 5(3), e222106. [CrossRef]
- Meng, H. , Page, M. G., Ajrawat, P., Deshpande, A., Samman, B., Dominicis, M., Ladha, K. S., Fiorellino, J., Huang, A., Kotteeswaran, Y., McClaren-Blades, A., Kotra, L. P., Clarke, H. (2021). Patient-reported outcomes in those consuming medical cannabis: a prospective longitudinal observational study in chronic pain patients. Résultats rapportés par les patients consommant du cannabis médical: une étude observationnelle longitudinale prospective chez des patients souffrant de douleur chronique. Canadian journal of anaesthesia = Journal canadien d’anesthesie, 68(5), 633–644. [CrossRef]
- Specka M, Bonnet U, Schmidberg L, Wichmann J, Keller M, Scholze C, Scherbaum N. Effectiveness of Medical Cannabis for the Treatment of Depression: A Naturalistic Outpatient Study. Pharmacopsychiatry. 2024 Jan 11. [CrossRef]
- Gershoni, T. , Pud, D., Aviram, J., Eisenberg, E. (2023). Wellness of patients with chronic pain is not only about pain intensity. Pain practice: the official journal of World Institute of Pain, 23(2), 145–154. [CrossRef]
- Aviram J, Pud D, Gershoni T, Schiff-Keren B, Ogintz M, Vulfsons S, Yashar T, Adahan HM, Brill S, Amital H, Goor-Aryeh I, Robinson D, Green L, Segal R, Fogelman Y, Tsvieli O, Yellin B, Vysotski Y, Morag O, Tashlykov V, Sheinfeld R, Goor R, Meiri D, Eisenberg E. Medical cannabis treatment for chronic pain: Outcomes and prediction of response. Eur J Pain. 2021 Feb;25(2):359-374. [CrossRef] [PubMed]
- Sagar, K. A. , Dahlgren, M. K., Lambros, A. M., Smith, R. T., El-Abboud, C., Gruber, S. A. (2021). An Observational, Longitudinal Study of Cognition in Medical Cannabis Patients over the Course of 12 Months of Treatment: Preliminary Results. Journal of the International Neuropsychological Society: JINS, 27(6), 648–660. [CrossRef]
- Gruber, S. A. , Smith, R. T., Dahlgren, M. K., Lambros, A. M., Sagar, K. A. (2021). No pain, all gain? Interim analyses from a longitudinal, observational study examining the impact of medical cannabis treatment on chronic pain and related symptoms. Experimental and clinical psychopharmacology, 29(2), 147–156. [CrossRef]
- Abuhasira R, Schwartz L, Novack V. Medical Cannabis Is Not Associated with a Decrease in Activities of Daily Living in Older Adults. Biomedicines. 2023;11(10):2697. Published 2023 Oct 3. [CrossRef]
- Cooke, M. E. , Potter, K. W., Jashinski, J., Pascale, M., Schuster, R. M., Tervo-Clemmens, B., Hoeppner, B. B., Pachas, G. N., Evins, A. E., Gilman, J. M. (2023). Development of cannabis use disorder in medical cannabis users: A 9-month follow-up of a randomized clinical trial testing effects of medical cannabis card ownership. Front. Psychiat., 14, 1083334. [CrossRef]
- Mangoo, S. , Erridge, S., Holvey, C., Coomber, R., Barros, D. A. R., Bhoskar, U., Mwimba, G., Praveen, K., Symeon, C., Sachdeva-Mohan, S., Rucker, J. J., Sodergren, M. H. (2022). Assessment of clinical outcomes of medicinal cannabis therapy for depression: analysis from the UK Medical Cannabis Registry. Expert review of neurotherapeutics, 22(11-12), 995–1008. [CrossRef]
- Sachedina, F. , Chan, C., Damji, R. S., de Sanctis, O. J. (2022). Medical cannabis use in Canada and its impact on anxiety and depression: A retrospective study. Psychiat. Res., 313, 114573. [CrossRef]
- Guarnaccia JB, Khan A, Ayettey R, Treu JA, Comerford B, Njike VY. Patterns of Medical Cannabis Use among Patients Diagnosed with Multiple Sclerosis. Mult Scler Relat Disord. 2021 May;50:102830. [CrossRef] [PubMed]
- Stack, S. K. , Wheate, N. J., Moloney, N. C., Abelev, S. V., Barlow, J. W., Schubert, E. A. (2023). The Effectiveness and Adverse Events of Cannabidiol and Tetrahydrocannabinol Used in the Treatment of Anxiety Disorders in a PTSD Subpopulation: An Interim Analysis of an Observational Study. The Journal of pharmacy technology: jPT: official publication of the Association of Pharmacy Technicians, 39(4), 172–182. [CrossRef]
- Legenbauer, T. , Kirschbaum-Lesch, I., Jörke, C., Kölch, M., Reis, O., Berger, C., Dück, A., Schulte-Markwort, M., Becker-Hebly, I., Bienioschek, S., Schroth, J., Ruckes, C., Deuster, O., Holtmann, M. (2024). Bright Light Therapy as Add-On to Inpatient Treatment in Youth With Moderate to Severe Depression: A Randomized Clinical Trial. JAMA Psychiat., e240103. Advance online publication. [CrossRef]
- Zhang, X. , Chen, S., Zhang, M., Ren, F., Ren, Y., Li, Y., Liu, N., Zhang, Y., Zhang, Q., Wang, R. (2021). Effects of Fermented Milk Containing Lacticaseibacillus paracasei Strain Shirota on Constipation in Patients with Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 13(7), 2238. [CrossRef]
- Komorniak, N. , Kaczmarczyk, M., Łoniewski, I., Martynova-Van Kley, A., Nalian, A., Wroński, M., Kaseja, K., Kowalewski, B., Folwarski, M., Stachowska, E. (2023). Analysis of the Efficacy of Diet and Short-Term Probiotic Intervention on Depressive Symptoms in Patients after Bariatric Surgery: A Randomized Double-Blind Placebo Controlled Pilot Study. Nutrients, 15(23), 4905. [CrossRef]
- Gan, S. L. , Long, Y. Q., Wang, Q. Y., Feng, C. D., Lai, C. X., Liu, C. T., Ding, Y. Y., Liu, H., Peng, K., Ji, F. H. (2023). Effect of esketamine on postoperative depressive symptoms in patients undergoing thoracoscopic lung cancer surgery: A randomized controlled trial. Front. Psychiat., 14, 1128406. [CrossRef]
- Choi, J. I. , Lee, Y. L., Lee, S. Y. (2022). Efficacy and safety of fermented Prunus mume vinegar on fatigue improvement in adults with unexplained fatigue: A randomized controlled trial. Frontiers in nutrition, 9, 990418. [CrossRef]
- Blumenthal, J. A. , Smith, P. J., Jiang, W., Hinderliter, A., Watkins, L. L., Hoffman, B. M., Kraus, W. E., Mabe, S., Liao, L., Davidson, J., Sherwood, A. (2022). Exercise and Escitalopram in the Treatment of Anxiety in Patients with Coronary Heart Disease: One Year Follow-Up of the UNWIND Randomized Clinical Trial. Journal of cardiovascular development and disease, 9(10), 320. [CrossRef]
- Walden, K. E. , Moon, J. M., Hagele, A. M., Allen, L. E., Gaige, C. J., Krieger, J. M., Jäger, R., Mumford, P. W., Pane, M., Kerksick, C. M. (2023). A randomized controlled trial to examine the impact of a multi-strain probiotic on self-reported indicators of depression, anxiety, mood, and associated biomarkers. Frontiers in nutrition, 10, 1219313. [CrossRef]
- Velichkov, M. , Bezur, Z., van Reekum, C. M., Williams, C. M. (2024). A biphasic response to blueberry supplementation on depressive symptoms in emerging adults: a double-blind randomized controlled trial. European journal of nutrition, 10.1007/s00394-023-03311-9. Advance online publication. [CrossRef]
- Ning, H. , Zhou, H., Ren, J., Zhou, G., Yang, N., Wang, Z., Yuan, C., Tian, Z., Chen, J., Shen, L., Zheng, H., Zhao, Y., Wang, H., Liu, W., Liu, Z. (2022). Zishen pingchan granules combined with pramipexole in the improvement of depressive symptoms in Parkinson’s disease: a prospective, multicenter, randomized, double-blind, controlled clinical study. Journal of translational medicine, 20(1), 357. [CrossRef]
- Khera, T. , Helfand, J., Kelly, L., Mueller, A., Shankar, P., Marcantonio, E. R., Subramaniam, B. (2022). Twelve-Month Cognitive and Functional Outcomes Following Cardiac Surgery: The DEXACET Trial of Intravenous Acetaminophen Versus Placebo. Frontiers in pharmacology, 13, 803903. [CrossRef]
- Zaid, A. H. , Thapamagar, S. B., Anholm, J. D., Weaver-Carnahan, L., Duong, L., Specht, L. (2024). Effects of Dronabinol on Dyspnea and Quality of Life in Patients with COPD. Chronic obstructive pulmonary diseases (Miami, Fla.), 10.15326/jcopdf.2023.0401. Advance online publication. [CrossRef]
- Lin, C. H. , Wang, S. H., Lane, H. Y. (2022). Effects of Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, on Perceived Stress and Cognitive Function Among Patients With Late-Life Depression: A Randomized, Double-Blind, Sertraline- and Placebo-Controlled Trial. The international journal of neuropsychopharmacology, 25(7), 545–555. [CrossRef]
- Molassiotis, A. , Suen, L., Lai, C., Chan, B., Wat, K. H. Y., Tang, J., To, K. L., Leung, C. O., Lee, S., Lee, P., Chien, W. T. (2020). The effectiveness of acupressure in the management of depressive symptoms and in improving quality of life in older people living in the community: a randomised sham-controlled trial. Aging mental health, 24(6), 1001–1009. [CrossRef]
- Savard, J. , Moussa, H., Pelletier, J. F., Julien, P., Lacombe, L., Tiguert, R., Caumartin, Y., Dujardin, T., Toren, P., Pouliot, F., Lodde, M., Fradet, Y., Robitaille, K., Fradet, V. (2023). Effects of omega-3 supplementation on psychological symptoms in men with prostate cancer: Secondary analysis of a double-blind placebo-controlled randomized trial. Cancer medicine, 12(19), 20163–20176. [CrossRef]
- Taghvaei, T. , Elyasi, F., Rahbar, Z., Neyestani, F. (2021). Effectiveness of Buspirone in Patients with Functional Dyspepsia: A Randomized, Double-Blind, Placebo-Controlled Study. Middle East journal of digestive diseases, 13(4), 302–313. [CrossRef]
- Camacho-Díaz, B. H. , Arenas-Ocampo, M. L., Osorio-Díaz, P., Jiménez-Aparicio, A. R., Alvarado-Jasso, G. M., Saavedra-Briones, E. V., Valdovinos-Díaz, M. Á., Gómez-Reyes, E. (2023). The Effects of Agave Fructans in a Functional Food Consumed by Patients with Irritable Bowel Syndrome with Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 15(16), 3526. [CrossRef]
- Spierings, E. L. H. , Ning, X., Ramirez Campos, V., Cohen, J. M., Barash, S., Buse, D. C. (2021). Improvements in quality of life and work productivity with up to 6 months of fremanezumab treatment in patients with episodic and chronic migraine and documented inadequate response to 2 to 4 classes of migraine-preventive medications in the phase 3b FOCUS study. Headache, 61(9), 1376–1386. [CrossRef]
- Lewis, G. , Duffy, L., Ades, A., Amos, R., Araya, R., Brabyn, S., Button, K. S., Churchill, R., Derrick, C., Dowrick, C., Gilbody, S., Fawsitt, C., Hollingworth, W., Jones, V., Kendrick, T., Kessler, D., Kounali, D., Khan, N., Lanham, P., Pervin, J., … Lewis, G. (2019). The clinical effectiveness of sertraline in primary care and the role of depression severity and duration (PANDA): a pragmatic, double-blind, placebo-controlled randomised trial. The lancet. Psychiatry, 6(11), 903–914. [CrossRef]
- Zhang, Z. , Zhang, W. H., Lu, Y. X., Lu, B. X., Wang, Y. B., Cui, L. Y., Cheng, H., Yuan, Z. Y., Zhang, J., Gao, D. P., Gong, J. F., Ji, Q. (2023). Intraoperative Low-Dose S-Ketamine Reduces Depressive Symptoms in Patients with Crohn’s Disease Undergoing Bowel Resection: A Randomized Controlled Trial. Journal of clinical medicine, 12(3), 1152. [CrossRef]
- Mannel, M. , Kuhn, U., Schmidt, U., Ploch, M., Murck, H. (2010). St. John’s wort extract LI160 for the treatment of depression with atypical features - a double-blind, randomized, and placebo-controlled trial. Journal of psychiatric research, 44(12), 760–767. [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Rezende, B.; Alencar, A.K.N.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Charac-teristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Haller, J. (2023). Anxiety Modulation by Cannabinoids-The Role of Stress Responses and Coping. Int. J. Mol. Sci., 24(21), 15777. [CrossRef]
- Devane, W.A.; Dysarz, F.A., 3rd; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a canna-binoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Im-munohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Chan, S.C.; Yung, W.H. Presynaptic inhibition of GABAergic inputs to rat substantia nigra pars reticulata neurones by a cannabinoid agonist. Neuroreport 1998, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endocannabinoid signaling in the brain. Science 2002, 296, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002, 68, 247–286. [Google Scholar] [CrossRef]
- Bowers, M.E.; Ressler, K.J. Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expres-sion and extinction retention. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 688–700. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Katona, I.; Sperlágh, B.; Sík, A.; Köfalvi, A.; Vizi, E.S.; Mackie, K.; Freund, T.F. Presynaptically located CB1 can-nabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neuro-Sci. Off. J. Soc. Neurosci. 1999, 19, 4544–4558. [Google Scholar] [CrossRef]
- Schlicker, E.; Kathmann, M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001, 22, 565–572. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Di Marzo, V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and syn-thetic can-nabimimetics: Focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2010, 5, 103–121. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Biringer, R.G. Endocannabinoid signaling pathways: Beyond CB1R and CB2R. J. Cell Commun. Signal. 2021, 15, 335–360. [Google Scholar] [CrossRef] [PubMed]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.Y.; Lu, H.C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that in-creases intra-cellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid signaling in astrocytes. Glia 2023, 71, 44–59. [Google Scholar] [CrossRef]
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 3254–3263. [Google Scholar] [CrossRef]
- Felder, C. C., Joyce, K. E., Briley, E. M., Mansouri, J., Mackie, K., Blond, O., Lai, Y., Ma, A. L., Mitchell, R. L. (1995). Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Molecular pharmacology, 48(3), 443–450.
- McPartland, J. M. , Glass, M., Pertwee, R. G. (2007). Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Brit. J. Pharmacol., 152(5), 583–593. [CrossRef]
- Sharir, H., Abood, M. E. (2010). Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacology therapeutics, 126(3), 301–313. [CrossRef]
- Storozhuk, M. V. , Zholos, A. V. (2018). TRP Channels as Novel Targets for Endogenous Ligands: Focus on Endocannabinoids and Nociceptive Signalling. Current neuropharmacology, 16(2), 137–150. [CrossRef]
- Sanacora, G. , Treccani, G., Popoli, M. (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology, 62(1), 63–77. [CrossRef]
- Luscher, B. , Shen, Q. , Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiat., 2011, 16, 383–406. [Google Scholar] [CrossRef]
- Ressler, K. J. , Nemeroff, C. B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depres. Anxiety, 2000, 12 Suppl 1, 2–19. [CrossRef]
- Brunello, N. , Mendlewicz, J. , Kasper, S., Leonard, B., Montgomery, S., Nelson, J., Paykel, E., Versiani, M., Racagni, G. The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur. Neuropsychopharm., 2002, 12, 461–475. [Google Scholar] [CrossRef]
- Dagytė, G. , Den Boer, J. A., Trentani, A. The cholinergic system and depression. Behavi. Brain Res., 2011, 221, 574–582. [Google Scholar] [CrossRef]
- Dunlop, B. W. , Nemeroff, C. B. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiat., 2007, 64, 327–337. [Google Scholar] [CrossRef]
- Iglesias, L. P. , Aguiar, D. C., Moreira, F. A. TRPV1 blockers as potential new treatments for psychiatric disorders. Behav. Pharmacol., 2022, 33, 2–14. [Google Scholar] [CrossRef]
- Melas, P. A. , Scherma, M. , Fratta, W., Cifani, C., Fadda, P. Cannabidiol as a potential treatment for anxiety and mood disorders: molecular targets and epigenetic insights from preclinical research. Int. J. Mol. Sci., 2021, 22, 1863. [Google Scholar] [CrossRef]
- Vigil, J. M. , Stith, S. S., Brockelman, F., Keeling, K., Hall, B. Systematic combinations of major cannabinoid and terpene contents in Cannabis flower and patient outcomes: a proof-of-concept assessment of the Vigil Index of Cannabis Chemovars. J. Cannabis Res., 2023, 5, 4. [Google Scholar] [CrossRef]
- Martin, M. , Ledent, C. , Parmentier, M., Maldonado, R., Valverde, O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology, 2002, 159, 379–387. [Google Scholar] [CrossRef]
- Piomelli, D. , Tarzia, G. , Duranti, A., Tontini, A., Mor, M., Compton, T. R., Dasse, O., Monaghan, E. P., Parrott, J. A., Putman, D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597). CNS Drug Rev., 2006, 12, 21–38. [Google Scholar] [CrossRef]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 160, pp. 1–24. [Google Scholar]
- Jain, T. , Wager-Miller, J. , Mackie, K., Straiker, A. Diacylglycerol lipaseα (DAGLα) and DAGLβ cooperatively regulate the production of 2-arachidonoyl glycerol in autaptic hippocampal neurons. Mol. Pharmacol., 2013, 84, 296–302. [Google Scholar] [CrossRef]
- Lu, H. C. , Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiat., 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Pertwee R., G. Cannabinoid pharmacology: the first 66 years. Brit. J. Pharmacol., 2006, 147 Suppl 1(Suppl 1), S163–S171. [CrossRef]
- Marsicano, G. , Goodenough, S. , Monory, K., Hermann, H., Eder, M., Cannich, A., Azad, S. C., Cascio, M. G., Gutiérrez, S. O., van der Stelt, M., López-Rodriguez, M. L., Casanova, E., Schütz, G., Zieglgänsberger, W., Di Marzo, V., Behl, C., Lutz, B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science, 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Haller, J. , Bakos, N. , Szirmay, M., Ledent, C., Freund, T. F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci., 2002, 16, 1395–1398. [Google Scholar] [CrossRef]
- Haller, J. , Barna, I. , Barsvari, B., Gyimesi Pelczer, K., Yasar, S., Panlilio, L. V., Goldberg, S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology, 2009, 204, 607–616. [Google Scholar] [CrossRef]
- Degenhardt, L. , Hall, W. , Lynskey, M. Exploring the association between cannabis use and depression. Addiction, 2003, 98, 1493–1504. [Google Scholar] [CrossRef]
| CANNABIS CONSUMPTION WAS ASSOCIATED WITH HIGH DEPRESSION | |||||
|---|---|---|---|---|---|
|
Consumption specifics |
Sample specifics |
Gender at birth |
Age class |
Sample size |
Ref |
| unclear | community sample |
both | Adolescent /young adult |
100+ | [26] |
| unclear | community sample |
both | young adult | 10,000+ | [27] |
| unclear | community sample |
both | adolescent | 2,000+ | [28] |
| unclear | community sample |
both | young adult | 1,000+ | [29] |
| unclear | community sample |
both | adolescent | 1,000+ | [30] |
| unclear | community sample |
both | 18+ | <100 | [31] |
| unclear | community sample |
both | adolescent | 500+ | [32] |
| unclear | community sample |
men | adolescent /young adult |
500+ | [33] |
| unclear | community sample |
both | adolescent | 5,000+ | [34] |
| unclear | community sample |
both | adolescent /young adult |
2,000+ | [35] |
| unclear | community sample |
both | 18+ | 2,000+ | [36] |
| unclear | community sample |
both | young adult /middle-aged |
100+ | [37] |
| unclear | community sample |
both | young adult | 500+ | [38] |
| unclear | community sample |
both | 18+ | 10,000+ | [39] |
| unclear | community sample |
female | adolescent /young adult |
2,000+ | [40] |
| unclear | community sample |
both | 16+ | 10,000+ | [41] |
| lifetime use | community sample |
both | adolescent | 10,000+ | [42] |
| lifetime use | community sample |
both | adolescent | 2,000+ | [43] |
| Non-disord- ered use |
community sample |
both | adolescent | 10,000+ | [44] |
| harmful | community sample |
both | young adult | 500+ | [45] |
| > 1/ month for a year |
community sample |
both | adolescent /young adult |
100+ | [46] |
| >weakly | community sample |
both | young adult | 1,000+ | [47] |
| >weekly | community sample |
both | adolescent /young adult |
100+ | [48] |
| daily for >30 days |
community sample |
both | 18+ | 100,000+ | [49] |
| Cannabis use disorder |
community sample |
both | adolescent | 10,000+ | [44] |
| unclear | Alzheimer disease |
both | elderly | 2,000+ | [50] |
| unclear | Army veterans |
both | young adult | 100+ | [51] |
| unclear | Army veterans |
both | young adult | 1,000+ | [52] |
| unclear medical |
Army veterans |
both | 21+ | 2,000+ | [53] |
| > 3 days /week |
Army veterans |
both | young adult | 100+ | [54] |
| cannabis use disorder |
Army veterans |
both | 21+ | 2,000+ | [55] |
| unclear | Athletes | female | young adult |
<100 | [56] |
| cannabis use disorder |
Bipolar disorder |
both | 18+ | 100,000+ | [57] |
| unclear | Cancer | both | 18+ | 10,000+ | [58] |
| daily | Cancer survivors |
both | middle aged | 1,000+ | [59] |
| daily | Cancer survivors |
both | 18+ | 10,000+ | [60] |
| cannabis use disorder |
Cannabis use disorder |
both | 18+ | 1,000,000+ | [61] |
| cannabis use disorder |
Cannabis use disorder |
both | young adult | 1,000+ | [62] |
| heavy | Childhood adversity |
both | 18+ | 10,000+ | [63] |
| unclear | Concussion | both | adolescent | 10,000+ | [64] |
| unclear | COVID19 | both | adolescent | 2,000+ | [65] |
| unclear | COVID19 | both | 18+ | 10,000+ | [66] |
| unclear | COVID19, army veterans |
both | young adult /middle-aged |
1,000+ | [67] |
| lifetime use | COVID19 | both | 18+ | 2,000+ | [68] |
| abuse | COVID19 | both | adults | 100+ | [69] |
| daily | COVID19 | both | young adult | 2,000+ | [70] |
| cannabis use disorder |
Homelessness | both | adolescent /young adult |
100+ | [71] |
| unclear medical |
Hospitalized (cannabis clinic) |
both | 18+ | 100+ | [72] |
| unclear | Hospitalized (integrated care) |
both | elderly | 500+ | [73] |
| unclear | Hospitalized (stress cardiomyopathy) |
both | 18+ | 10,000+ | [74] |
| problematic use |
Hospitalized (chronic disease) |
both | 18+ | 100+ | [75] |
| Cannabis use disorder* |
Hospitalized | both | adolescent | 100,000+ | [76] |
| unclear | Infertility | female | 18+ | 100+ | [77] |
| unclear | Inflammatory Bowel Disease |
both | 18+ | 1,000+ | [78] |
| unclear | Insomnia | both | adolescent | 100+ | [79] |
| unclear | Pregnancy | female | young adult /middle-aged |
1,000+ | [80] |
| positive drug testing |
Pregnancy | female | young adult /middle-aged |
500+ | [81] |
| cannabis use disorder |
Pregnancy | female | young adult /middle-aged |
1,000,000+ | [82] |
| unclear medical |
Primary care Patients |
both | 18+ | 1,000,000+ | [83] |
| cannabis use disorder |
Primary care patients |
both | 18+ | 1,000,000+ | [83] |
| unclear | PTSD COVID19 |
both | 18+ | 100+ | [84] |
| heavy | PTSD | both | 18+ | 2,000+ | [85] |
| cannabis use disorder |
PTSD | both | 18+ | 10,000+ | [86] |
| unclear | Various (mostly chronic pain) |
both | 18+ | 1,000+ | [87] |
| CANNABIS CONSUMPTION AND DEPRESSION DID NOT ASSOCIATE | |||||
|
Consumption specifics |
Sample specifics |
Gender at birth |
Age class | Sample size | Ref. |
| unclear | community sample |
both | adolescent | 2,000+ | [88] |
| unclear | community sample |
both | young adult | 500+ | [89] |
| regular | community sample |
both | 15+ | 100,000+ | [90] |
| daily | community sample |
both | 18+ | 10,000+ | [91] |
| unclear | Binge eating | both | 18+ | 100+ | [92] |
| unclear | Cancer survivors |
both | elderly | 2,000+ | [93] |
| unclear | Hospitalized (bariatric surg.) |
both | 18+ | 500+ | [94] |
| unclear | Juvenile offenders |
males | adolescent /young adult |
100+ | [95] |
| unclear | Late chronotype | both | young adult | 100+ | [96] |
| daily medical |
PTSD army veterans |
both | middle-age | 100+ | [97] |
| Cannabis use disorder* |
Schizophrenia spectrum dis. |
both | 18+ | 2,000+ | [98] |
| unclear | Substance use disorder |
both | middle-age | 100+ | [99] |
| CANNABIS CONSUMPTION WAS ASSOCIATED WITH LOW DEPRESSION | |||||
|
Consumption specifics |
Sample specifics |
Gender at birth |
Age class |
Sample size |
Ref. |
| unclear | community sample |
both | middle-age | 500+ | [100] |
| occasional | community sample |
both | 18+ | 2,000+ | [36] |
| habitual | community sample |
both | 18+ | 2,000+ | [36] |
| unclear | IBD COVID19 |
both | middle-age | 500+ | [101] |
| unclear | Psychosis first episode |
both | child /adolescent |
100+ | [102] |
| A. CANNABIS USE EMERGES OR INCREASES AFTER DEPRESSION | |||||
|---|---|---|---|---|---|
| Periodcovered |
Consumption change |
Sample specifics |
Age class | Sample size | Ref. |
| 1 year | Self -reported |
community sample |
adolescents /young adults |
500+ | [103] |
| 2 years | Self -reported |
community sample |
adolescents | 1,000+ | [104] |
| Month* | Self -reported |
COVID19 | 18+ | 100+ | [105] |
| 1 year | Self -reported |
COVID19 | adolescents /young adults |
2,000+ | [106] |
| 2 years | Self -reported |
COVID19 | midle-aged /elderly |
2,000+ | [107] |
| 1 year | Self -reported |
Depression, major |
elderly | 10,000+ | [108] |
| 4 years | Diagnostic# | Army veterans |
18+ | 1,000,000+ | [109] |
| B. DEPRESSION EMERGES OR WORSENS AFTER INCREASED CANNABIS USE | |||||
| Period covered |
Consumption change |
Sample specifics |
Age class | Sample size | Ref. |
| 30 days | Self -reported |
community sample |
adolescents | 1,000+ | [110] |
| 1 year | Self -reported |
community sample |
adolescents /young adults |
500+ | [103] |
| 1 year | Self -reported |
community sample |
adolescents | 2,000+ | [111] |
| 1 year | CDDUR | community sample |
adolescents /young adults |
<100 | [112] |
| 1 year | Self -reported |
community sample |
adolescents /young adults |
2,000+ | [113] |
| 2 years | Self -reported |
community sample |
adolescents /young adults |
1,000+ | [114] |
| years-decades | Self -reported |
community sample |
adolescents | 100,000+ | [115] |
| 4 years | ASSIST | ‡community sample |
children | 500+ | [116] |
| 4,5 years | Self -reported |
community sample |
young adults | 2,000+ | [117] |
| ~5 years | Self -reported |
community sample |
adolescents /young adults |
100+ | [118] |
| 5 years | ASSIST | †community sample |
young adults | 1,000+ | [119] |
| 5 years | CUDITr | ♂community sample |
young adults | 5,000+ | [120] |
| 6 years | Self -reported |
community sample |
young adults | 2,000+ | [121] |
| 6 years | Self -reported |
†community sample |
young adults | 2,000+ | [122] |
| 12 years | Self -reported |
♂community sample |
adolescents | 1,000+ | [123] |
| ~17 years | Self -reported |
community sample |
adolescents | 5,000+ | [124] |
| 20 years | Self -reported |
community sample |
adolescents | 1,000+ | [125] |
| < 1 year | Self -reported |
†Bipolar disorder |
midle-aged | 1,000+ | [126] |
| 5 years | Diagnostic# | †Bipolar disorder |
midle-aged | 100+ | [127] |
| 1 year | Diagnostic# | Cannabis use disorder |
18+ | 10,000+ | [128] |
| 1 year | Self -reported |
COVID19 | young adults | 2,000+ | [129] |
| 1 year | Self -reported |
COVID19 | elderly | 10,000+ | [130] |
| 3 & 6 month |
Self -reported |
Hospitalized, surgery |
middle-aged /elderly |
1,000+ | [131] |
| 30 days & 1 year |
Self -reported |
Hospitalized, bariatric surg. |
middle-aged | 5,000+ | [132] |
| 2 years | Self -reported |
Hospitalized, orthopaedic surg. |
18+ | 1,000+ | [133] |
| Month* | Medical records |
♀Pregnant | 18+ | 500+ | [134] |
| Month* | Diagnostic# | ♀Pregnant | young adults /middle-aged |
100+ | [135] |
| ≥1 years | Self -reported |
♀Pregnant | young adults /middle-aged |
500+ | [136] |
| ≥5 years | Self -reported |
♀Pregnant | young adults | 100+ | [137] |
| 12 weeks | PXTSU | PTSD | young adults | 1,000+ | [138] |
| C. NO TEMPORAL RELATIONSHIPS BETWEEN DEPRESSION AND CANNABIS USE | |||||
|
Period covered |
Consumption change |
Sample specifics | Age class | Sample size | Ref. |
| ~5 years | Self -reported |
♂community sample |
young adults | 500+ | [139] |
| <16 years* | Self -reported |
†community sample |
adolescents | 2,000+ | [140] |
| 2 years | Self -reported |
community sample |
young adults | 2,000+ | [141] |
| longterm use | Self -reported |
community sample |
elderly | <100 | [142] |
| 20 years | Self -reported |
community sample |
adolescents | 1,000+ | [125] |
| month* | Self -reported |
COVID19 | 18+ | 100+ | [105] |
| ≤ 24 month* | Self reported |
psychosis, first episode |
young adults | 100+ | [143] |
| CANNABIS WITHDAWAL DECREASED DEPRESSION | ||||||
|---|---|---|---|---|---|---|
|
Withdrawal length |
Withdrawal check |
Sample specifics |
Age class | Sample size |
Comparison group |
Ref. |
| 28 days |
Drug screen |
Multiple sclerosis |
middle-aged | <100 | no withdr. |
[144] |
| few month |
Drug screen |
Pregnancy | young a. /middle-a. |
500+ | no withdr. |
[81] |
| 1 year |
Self report |
Community sample |
adolescents /young a. |
10,000+ | no withdr. |
[145] |
| 1 month |
Self report |
Cannabis use disorder |
18+ | <100 | none | [146] |
| ~5,5 years* |
Self report |
Cannabis use disorder |
14+ | 100+ | none | [147] |
| CANNABIS WITHDAWAL HAD NO EFFECT ON DEPRESSION | ||||||
|
Withdr. length |
Withdr. check |
Sample specifics |
Age class | N |
Comparison group |
Ref. |
| 3 weeks |
Drug screen |
Community sample |
young a. | <100 | non -users |
[148] |
| 4 weeks |
Drug screen |
Community sample |
adolescents /young a. |
100+ | none | [149] |
| 7 years |
Diagno- stic# |
Substance abuse |
adolescents | 1,000+ | none | [150] |
| CANNABIS WITHDAWAL INCREASED DEPRESSION | ||||||
|
Withdr. length |
Withdr. check |
Sample specifics |
Age class | N |
Comparison groups |
Ref. |
| 14 weeks | Drug screen |
Cannabis use disorder |
adolescents /young a. |
<100 | none | [151] |
| A. MEDICAL MARIJUANA AGGRAVATED DEPRESSION | ||||||||
|---|---|---|---|---|---|---|---|---|
| Followup | Instrument | Change | Condition |
Effect size |
Age class |
N |
Comparison group |
Ref. |
| 5-6 years |
Pharm. | diagnostic | Chronic pain |
- | 18+ | 100+ | no disorder |
[160] |
| B. MEDICAL MARIJUANA DID NOT AFFECT DEPRESSION | ||||||||
| Followup | Instrument | Change | Condition |
Effect size |
Age class |
N |
Comparison group |
Ref. |
| 6 month |
ESAS | nil | Various ** |
- | Ma. /Eld |
100+ | none | [161] |
| 12 weeks |
HADS-D | nil | Various *** |
- | 18+ | 100+ | no treatment |
[162] |
| 12 weeks |
HADS-D | nil | Various *** |
- | 18+ | 100+ | no treatment |
[163] |
| vari- able |
PHQ4 | nil | Chronic pain |
- | 25+ | 100+ | none† | [164] |
| C. MEDICAL MARIJUANA ALLEVIATED DEPRESSION | ||||||||
| Followup | Instrument |
In→fin (dif.) |
Condition |
Effect size |
Age class |
N |
Comparison group |
Ref. |
| 18 weeks |
custom | 6.9→3.8 (3.1) |
Major depression |
n.c | Ya /Ma. |
<100 | none | [165] |
| 12 month |
BDI | 3.2→2.2 (1.0) |
Chronic pain |
0,59 | 19+ | 500+ | none | [166] |
| various | BDI | 18.0→11.0 (7.0) |
Chronic pain |
n.c | Ma. | 500+ | none | [167] |
| 1 year |
BDI | 11.3→5.8 (5.5) |
Community sample |
0,79 | Ma. | < 100 | none | [168] |
| 6 month |
BDI | 12.6→5.5 (6.9) |
Chronic pain |
0,87 | 18+ | < 100 | none | [169] |
| 3 month |
DASS-21 | 15.2→10.7 (4.5) |
Various *** |
0,45 | 18+ | 2,000+ | none | [156] |
| 3 month |
ESAS | 3.2→2.3 (0.9) |
Various ** |
n.c | Ma. /Eld |
100+ | none | [161] |
| 6 month |
GDS | 6.4→5.0 (1.4) |
Various** | 0,35 | Eld | 100+ | none | [170] |
| 4 weeks |
GDS | 9.0→3.0 (6.0) |
BMS | n.c | Eld | <100 | none | [158] |
| variable | HADS-D | 11.7→8.6 (3.1) |
Anxiety /depre. |
n.c | Ma. | 500+ | no treatment |
[100] |
| 9 month |
HADS-D | 4.3→3.8 (0.5) |
Various*** | 0,13 | 18+ | 100+ | no treatment |
[171] |
| various | HADS-D | not reported* |
Various ** |
n.c | Adol /Ma. |
100+ | no treatment |
[101] |
| 3 month |
PHQ-8 | 8.5→5.7 (2.8) |
Chronic pain |
0,52 | Ma. /Eld |
<100 | none | [157] |
| 6 month |
PHQ-9 | 12.0→7.0 (5) |
Depres. | n.c | Ya /Ma. |
100+ | none | [172] |
| 2 years |
PHQ-9 | 13.7→7.2 (6.5) |
Various *** |
1,03 | Ma. /Eld |
5,000+ | none | [173] |
| ~3 month |
diagnostic | diagnostic | Multiple Sclerosis |
n.c | Ma. | 100+ | none | [174] |
| various | PROMIS-29 | 61.6→57.5 (4.1) |
Anxiety /PTSD |
0,41 | Ma. | 100+ | none | [175] |
| D. PLACEBO ALLEVIATED DEPRESSION IN CONTROLLED STUDIES | |||||||
|---|---|---|---|---|---|---|---|
| Followup | Instrument |
Initial→final score (dif.) |
Condition |
Effect size |
Age class |
N | Ref. |
| 4 weeks |
BDI | 37.3→29.8 (7.5) |
Major depression |
0,71 | children /adolescents |
227 | [176] |
| 63 days |
BDI | 14.0→7.4 (6.6) |
Constipation depression |
1,00 | middle-aged | 60 | [177] |
| 5 weeks |
BDI | 16.6→8.4 (8.2) |
Bariatric surgery |
1,19 | middle-aged | 38 | [178] |
| 3 month |
BDI | 6.0→2.0 (4.0) |
Lung cancer surgery |
n.c | middle-aged /elderly |
156 | [179] |
| 8 weeks |
BDI | 13.5→5.5 (8.0) |
Unexplained fatigue |
n.c | middle-aged | 80 | [180] |
| 1 year |
BDI | 17.7→8.1 (9.6) |
Coronary heart disease |
n.c | elderly | 128 | [181] |
| 9 weeks |
BDI | 24.5→22.3 (2.2) |
Community sample |
n.c | young adults /middle-aged |
70 | [182] |
| 6 weeks |
BDI | 27.0→14.9 (12.1) |
Depressive symptoms |
n.c | adolescent /young adults |
62 | [183] |
| 12 weeks |
GDS | 7.0→5.8 (1.2) |
Parkinson | 0,37 | middle-aged /elderly |
171 | [184] |
| 1 month |
GDS | 8.0→3.0 (5.0) |
Cardiac surgery |
0,47 | elderly | 83 | [185] |
| 6 weeks |
GDS | 4.4→3.7 (0.7) |
COPD | 0,55 | elderly | 60 | [186] |
| 8 weeks |
GDS | 10.5→8.4 (2.1) |
Major depression |
0,76 | elderly | 117 | [187] |
| 3 month |
GDS | 10.5→8.4 (2.1) |
Community sample |
3,47 | elderly | 80 | [188] |
| 12 month |
HADS-D | 3.2→2.3 (0.9) |
Prostate cancer |
0,32 | middle-aged /elderly |
130 | [189] |
| 8 weeks |
HADS-D | 11.0→7.2 (3.8) |
Functional dyspepsia |
0,79 | not specified |
30 | [190] |
| 30 days |
HADS-D | 14.6→10.5 (4.1) |
Irritable Bowel Syndrome |
1,33 | middle-aged | 42 | [191] |
| 1 year |
HADS-D | 7.0→4.2 (2.8) |
Coronary he- art disease |
n.c | elderly | 128 | [181] |
| 6 month |
PHQ-9 | 3.9→1.9 (2.0) |
Migraine | 0,39 | middle-aged | 807 | [192] |
| 6 weeks |
PHQ-9 | 12.2→8.8 (3.4) |
Depression | 0,59 | 18+ | 653 | [193] |
| 7 days |
PHQ-9 | 15.2→13.0 (2.2) |
Bowel Resection |
0,73 | young adults /middle-aged |
120 | [194] |
| 8 weeks |
PHQ-9 | 11.2→6.3 (4.9) |
Atypical depression |
1,11 | middle-aged | 200 | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





