1. Assessing Judgment Bias in Ambassador Animals: Two Case Studies
There is general agreement among researchers that nonhuman animals experience at least some emotional states (Scarantino et al., 2021). Yet, the research regarding animal emotional states is rife with inconsistencies regarding definitions of emotions and relevant terminology (Nematipour et al., 2022; see Vonk et al., 2024 for a review). What is certain is that presuming emotional states allows researchers to predict animal behavior (Mendl et al., 2009); thus, researchers in various fields, such as neuroscience, psychopharmacology, and animal welfare, have worked to develop valid assessments of these states, in addition to confirmation that animals experience these states (Kremer et al., 2020). Much of the past research on animal emotional states has focused on negative states (Proctor et al., 2013), which makes detection of positive emotional states—a necessary focus for the field of animal welfare (Mellor, 2016)—difficult.
A recent welfare assessment method, cognitive bias testing, allows for the assessment of emotional biases that affect how information is processed (Harding et al., 2004). The judgment bias task, one of several variations of the cognitive bias test, assesses optimism and pessimism through an organism’s response to ambiguous stimuli (Bethell, 2015), thereby providing indicators of positive as well as negative emotional states. An individual’s affective state can influence the perception of ambiguity through multiple means. That is, feeling optimistic or pessimistic can influence what an individual pays attention to, how they judge stimuli, what they remember, and more (e.g., Coles & Heimberg, 2002; Dunn et al., 2009; Eysenck et al., 1991; Novak et al., 2015). In addition, an individual’s affect may bias their tendency towards risk aversion and prediction of expected utility (Mendl et al., 2009). An individual that is optimistic would anticipate receiving a reward when interacting with ambiguous stimuli, whereas an individual that is pessimistic would not anticipate a reward or might expect something aversive from interacting with ambiguous stimuli. Optimism reflects a positive emotion state, whereas pessimism reflects a negative emotion state. Therefore, assessing an animal’s judgment bias might shed light on their affective state in various contexts.
A multitude of animals from insects to primates have demonstrated judgment biases in response to manipulations presumed to impact affective states (Lagisz et al., 2020). However, of the non-pharmacological studies utilizing judgment bias tests, the majority assessed mammals (Lagisz et al., 2020). Fewer studies have examined the judgment biases of birds (canaries, Serinus canaria: Lalot et al., 2017; chickens, Gallus domesticus: Anderson et al., 2021; Deakin et al., 2016; Lourenço-Silva et al., 2023; Seehuus et al., 2013; Wichman et al., 2012; Zidar et al., 2018; crows: McCoy et al., 2019; European starlings, Sturnus vulgaris: Bateson & Matheson, 2007; Bateson et al., 2015; Brilot et al., 2010; Gott et al., 2019; Matheson et al., 2008; Japanese quails, Coturnix japonica: Horváth et al., 2016; ravens, Corvus corax: Adriaense et al., 2019; red junglefowl, Gallus gallus: Munari, 2021), although the judgment bias test is becoming a popular welfare assessment method in livestock poultry (Košťál et al., 2020). Even fewer studies have examined judgment biases of insects and fish (bumblebees, Bombus terrestris: Solvi et al., 2016; convict cichlid, Amatitlania siquia: Laubu et al., 2019; fruit flies, Drosophila melanogaster: Deakin et al., 2018; honeybees, Apis mellifera: Bateson et al., 2011; Schlüns et al., 2017), and no published studies have examined judgment biases of reptiles (Lagisz et al., 2020). The studies on poultry have primarily manipulated the environment and its complexity to understand environmental impacts on affective states but no studies have assessed the impact of humans directly.
With increasing use of judgment bias tests in agricultural settings, it is surprising that zoos have yet to fully adopt this methodology (Clegg, 2018). Perhaps this underutilization is due to the sometimes extensive training required. Only a small number of zoo animals have been assessed; Bottlenose dolphins (Tursiops truncatus; Clegg & Delfour, 2018; Clegg et al., 2017) Western lowland gorillas (Gorilla gorilla gorilla, McGuire & Vonk, 2018; McGuire, Vonk, Fuller, & Allard, 2017), and an American black bear (Ursus Americanus, McGuire, Vonk, & Johnson-Ulrich, 2017; Vonk et al., 2021). Other methods involving less training, such as evaluating response slowing to threatening stimuli, have been used to assess environmental effects on zoo animals. For example, Cronin et al. (2018) found Japanese macaques (Macaca fuscata) exhibited response slowing towards conspecific images compared to control images during more extreme anthropogenic noise conditions, but no change was found for chimpanzees and gorillas. McGuire and Vonk (2020) found that gorillas responded more slowly to direct versus averted gorilla faces, but this pattern did not depend upon housing condition. None of these studies assessed the impacts of interactions with humans.
A recent and more directly relevant study found that one of two grizzly bears (Ursus arctos horribilis) appeared pessimistic during the absence of a training session with food reinforcement, suggesting that the presence of food was more impactful on affect than the presence of visitors (Bernstein-Kurtycz et al., 2024). This methodology could be implemented with ambassador animals that typically have more training experience and can be separated from conspecifics, making them ideal subjects for a methodology requiring training. These animals participate in education presentations meant to teach visitors about animals and conservation, yet not much is known about the impact of participation on the ambassador animals (Spooner et al., 2021). They are often exposed to handling by visitors, which is not a typical experience for most zoo animals and could have significant impacts on their welfare. The AZA has created an Ambassador Animal Evaluation Tool to assess an animal’s suitability for education programs (AZA, n.d.), but there are minimal guidelines on judging welfare in an ambassador animal (Spooner et al., 2021). A study examining ambassador animal welfare at 17 AZA-accredited zoos for several armadillo species, African hedgehogs (Atelerix albiventris), and red-tailed hawks (Buteo jamaicensis) showed no effect of education programs specifically, but fecal glucocorticoid metabolites and undesirable behaviors increased with greater handling durations, and rest behavior decreased (Baird et al., 2016). Another study across multiple zoos demonstrated that giraffes (Giraffa camelopardalis) spent less time ruminating (i.e., masticating, regurgitating, and swallowing cud) and more time idle when utilized in all-day visitor feeding programs, but not in part-day programs, and there was no association with stereotypic behaviors (Orban et al., 2016). In dromedary camels (Camelus dromedarius), salivary cortisol levels were lower when camels were being utilized for visitor rides as compared to seasons when they were not, suggesting the either the visitor rides or the food reinforcement received during such rides were enriching (Majchrzak et al., 2015). These studies demonstrate mixed findings in regard to welfare impacts on ambassador animals. Thus, implementing the judgment bias methodology could contribute to a better understanding of how education presentations might affect the welfare of ambassador animals.
To address this paucity of research and demonstrate the utility of the judgment bias test for assessing zoo animal welfare, we assessed the effects of human interaction on judgment biases in two ambassador animals at the Alexandria Zoo: a domestic chicken (Gallus domesticus) and a red tegu (Salvator rufescens). Chickens are a common subject for judgment bias research with farm animals, but this is the first study on the judgment bias of a zoo-housed chicken used in ambassador programs, and the first study of a judgment bias of a lizard. The red tegu is an omnivorous terrestrial lizard native to South America which grows to a relatively large body size (Fitzgerald et al., 1993; Jarnevich et al., 2018) and is commonly kept as a pet. Although other studies have assessed the welfare of ambassador animals, they have largely utilized behavioral and/or physiological measures (Spooner et al., 2021), which do not provide a clear indication of affect.
We modified a procedure developed by Harding et al. (2004) and adapted recently for use with crows (McCoy et al., 2019). The ambassador animals were required to learn that one location was associated with a large reward, whereas another location was associated with no reward. Then, one novel location was introduced by the researchers located equidistant between the previously known locations, and the latency to approach this novel ambiguous location was compared to the latencies for the known locations. A latency to approach the ambiguous location similar to the no reward location indicated pessimism, and a latency to approach the ambiguous location similar to the latency to approach the large reward location indicated optimism. We assessed the impact of human experiences that were thought to be potentially aversive to each animal, as indicated by their primary care staff, which included two caretakers. Both the tegu and the chicken took part in visitor interactions. We assessed the impact of keeper handling (perch or hold) for the chicken and the impact of visitor touch (touch or no touch) for the tegu. Based on the care staff’s assessment of the animals’ preferences, we expected that the chicken would demonstrate pessimism in the hold condition (i.e., staff keeping their arms around the chicken preventing wing movement) as compared to the perch condition, and the tegu would demonstrate pessimism in the touch condition as compared to the no touch condition. Previous research has also indicated that visitor touch may be aversive for prolonged periods, albeit in different species (Baird et al., 2016). Whether the impact of these programs is positive or negative, or potentially neutral, this study provided meaningful data for zoos and demonstrated the utility of the judgment bias protocol in assessing welfare.
2. Study 1
Both studies were reviewed and approved by Oakland University’s IACUC (Protocol # 2022-1190).
2.1. Method
2.1.1. Subject
The subject was an adult female chicken (Gallus domesticus) that served as an education animal at the Alexandria Zoo in Alexandria, Louisiana. This chicken was housed with a crested screamer (Chauna torquata) in an outdoor habitat near the zoo’s education buildings, which was on a path not often frequented by zoo visitors. At the time of the study, she was a recent addition to the zoo. Thus, this animal had minimal training experience from participating in previous educational programs and was naive to the judgment bias test. On days when testing occurred, daily feeding occurred after testing.
2.1.2. Materials
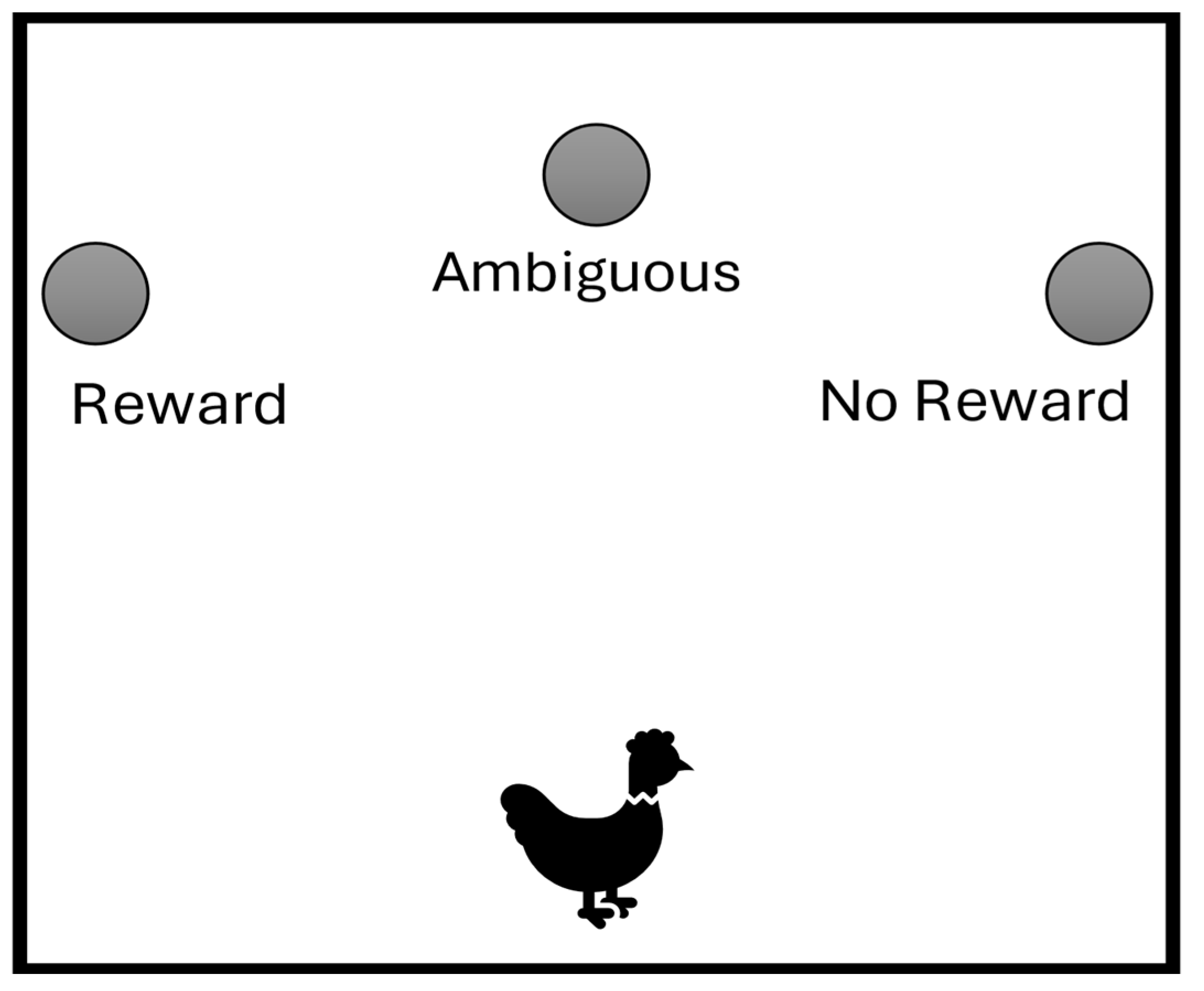
All testing occurred within an indoor education building attached to the chicken’s home habitat. This area was secluded from visitors, but the chicken was exposed to random keeper entrances and noises from their two-way radios, as well as noises from the reptiles and birds housed in this building. Trials were carried out within a caged square arena made out of moveable metal fencing. This fencing was 2.4 m by 2.2 m with sides approximately .7–.9 m tall. (see
Figure 1). The height was .9 m in the back half of the arena, whereas the height was .7 m in the front half of the arena due to differences in the two portions of moveable fencing utilized. One metal bowl was utilized as a food container throughout the study. The bowl was opaque so that the chicken could not see the reward within the container until she approached the container. This bowl was placed in four different locations depending on what phase and what trial was occurring. In habituation trials, the container was placed in the middle center of the testing arena. During training trials, the container would be placed on either the right (no reward) or left (reward) side of the testing arena. The middle (ambiguous) location was equidistant from both of these locations in addition to being the same distance from the animal’s starting point. The container had food placed within it before trials started so that scent could not be used as a cue. Rewards consisted of high value food (mealworms) as rated by the bird’s keepers and approved to be used across multiple trials by the veterinarian. A GoPro HERO10 was used to film all trials.
2.2. Procedure
Sessions occurred at approximately 11:00 am on Mondays, Sundays, and Thursdays. The researcher tested both the chicken and the tegu each day, and the order of sessions were randomly decided. The indoor trial arena, the containers, and the camera were set up prior to retrieving the chicken for sessions. The container was placed in particular locations depending on the trial (reward, no reward, and ambiguous). The reward and no reward locations were 2.3 m apart from each other, and both locations were 2.1 m from the starting point of the arena. On ambiguous trials, the same container was placed equidistant (1.2 m) from the high reward and no reward locations, and this location was also 2.1m from the starting point of the arena. Once the trial was set up, the keeper retrieved the subject from its outdoor habitat and brought the bird to the entrance of the arena. Between trials, the bird was picked up by the keeper and positioned so that the setup of the arena was not visible to the bird. The keeper was blind as to which container location contained the reward on the first trial, although they inevitably saw the reward locations as they viewed the bird across later trials. All trials were recorded for later coding by naïve coders. The researcher recorded the bird’s latency to move from the starting position to the container (i.e., touching the container or positioning their head directly over top of the container) live during testing. This latency was used as the criterion for advancing to the next phase.
Habituation. One session (5 trials each) of habituation occurred per day. The chicken was required to receive a minimum of one session of habituation, but habituation continued until she could retrieve the food in under 1 minute in 4 out of 5 trials within a session. At the start of a trial, the keeper placed the chicken at the middle front of the arena (see
Figure 1) facing a baited container directly in front of her, so that this location was neutral compared to the testing locations. The experimenter (JT) and the keeper stood quietly behind the arena in the opposite direction of the baited container so that they were not visible to the chicken when she was moving toward the container. The trial was scored as successful if the chicken retrieved the food in under one minute. All habituation trials continued until the reward had been retrieved. If the chicken had not retrieved the reward after three minutes and was not moving toward the food, the keeper attempted to direct the animal toward the baited container. This phase ensured the chicken was familiar with the container, knew that the container contained food, and retrieved the food quickly.
Training. One training session (six trials each) occurred per day until criterion was met. Of these six trials, three trials were high reward trials and three trials were no reward trials, presented in a pseudorandomized order. The last of the six trials was always a reward trial. Before each training session, the chicken was given a single habituation trial to maintain motivation. At the start of a training trial, the chicken was placed at the middle front of the arena (see
Figure 1). The experimenter and the keeper then stood quietly behind the arena so that they were not visible to the chicken when she was moving toward the container. All trials ended after three minutes to ensure the chicken had time to eat the reward. Trial length was kept constant in training and testing regardless of whether the reward was retrieved to ensure that the chicken did not approach more quickly on ambiguous trials in testing to hasten the next rewarded trial (not because she was optimistic). As seen in
Figure 1, the locations of the high reward container and no reward container are indicated by the left and right circles. Only one container was present on each trial, so that high reward trials consisted of only the high reward container, and no reward trials consisted of only the no reward container. The experimenter set up the high and no reward containers with the same procedure so as not to cue the chicken to their difference. To move to the testing phase, the chicken was required to reach a specific time difference in their approach to the no reward and high reward conditions. Specifically, the final three no reward trials were paired with the final three high reward trials and the chicken was required to demonstrate a faster time on each of the three pairs of trials across two consecutive sessions to move to testing.
Testing. The chicken received eight 5-trial sessions of testing after completing training. Two sessions occurred per day. Testing sessions occurred on a separate day from the last training session. The testing sessions consisted of 2 high reward trials, 2 no reward trials, and 1 ambiguous trial. The locations of the high reward and no reward containers remained the same as in previous phases, and the ambiguous container was equidistant from both of these containers and the same distance from the starting point. The ambiguous trials were randomly presented among the 5 trials within a session, with the constraint that the first trial was never an ambiguous trial. On ambiguous trials, the container was baited 50% of the time but was scented with food on every trial. To further differentiate this ambiguous container, it was filled with newspaper shreds to cover the potential food so that the bird would not know if it contained food upon approach.
Once a trial started, the experimenter and the keeper stood quietly behind the arena so that they were not visible to the bird when it was moving toward the container. All trials ended once three minutes passed. Limiting testing to eight sessions helped to ensure that the ambiguous location remained ambiguous; however, some learning may have occurred as sessions progressed. Four sessions were conducted after the chicken experienced a football hold (i.e., keeper’s arms wrapped around the wings) while interacting with zoo visitors and four sessions were conducted after the chicken experienced a perched hold (i.e., feet on top of the keeper’s arm with the other arm in front of the chicken’s chest) while interacting with zoo visitors. Each day of testing, the chicken received a football hold condition and a perched hold condition in randomized order. In both conditions, the chicken was carried by a familiar keeper around outside areas in the zoo and experienced physical contact from at least three humans. This interaction lasted around 10 minutes each time, and then the chicken was immediately returned to the testing arena. Once habituation, training, and testing was complete, coders naïve to the manipulation recorded latencies to approach the food locations and foraging behavior from the video recordings.
2.3. Data Analysis
All analyses were conducted via IBM SPSS version 29.0.1.1. To assess the impact of visitors on the subjects, we ran generalized linear mixed models with trial type (reward, no reward, ambiguous) and condition (perched or football hold) and their interaction as fixed factors, session as a random factor, and latencies to reach the container as the outcome variable. Session was included as a random factor to help control for changes in the subject’s behaviors across sessions due to factors such as weather, care staff present, etc. We compared latencies of ambiguous trials to both reward and no reward trials. The gamma distribution was used as the data were not normally distributed and it led to a better fitting model than the gaussian distribution, as indicated by a lower corrected AIC. We applied a Satterthwaite approximation, as this is appropriate for small sample sizes. To probe interactions, separate GLMMs were conducted for the two conditions.
3. Results
3.1. Reliability
A primary coder coded all videos and a secondary coder coded 20% of the same videos. Reliability between coders for latency to approach was assessed via ICC, α = .999.
Training
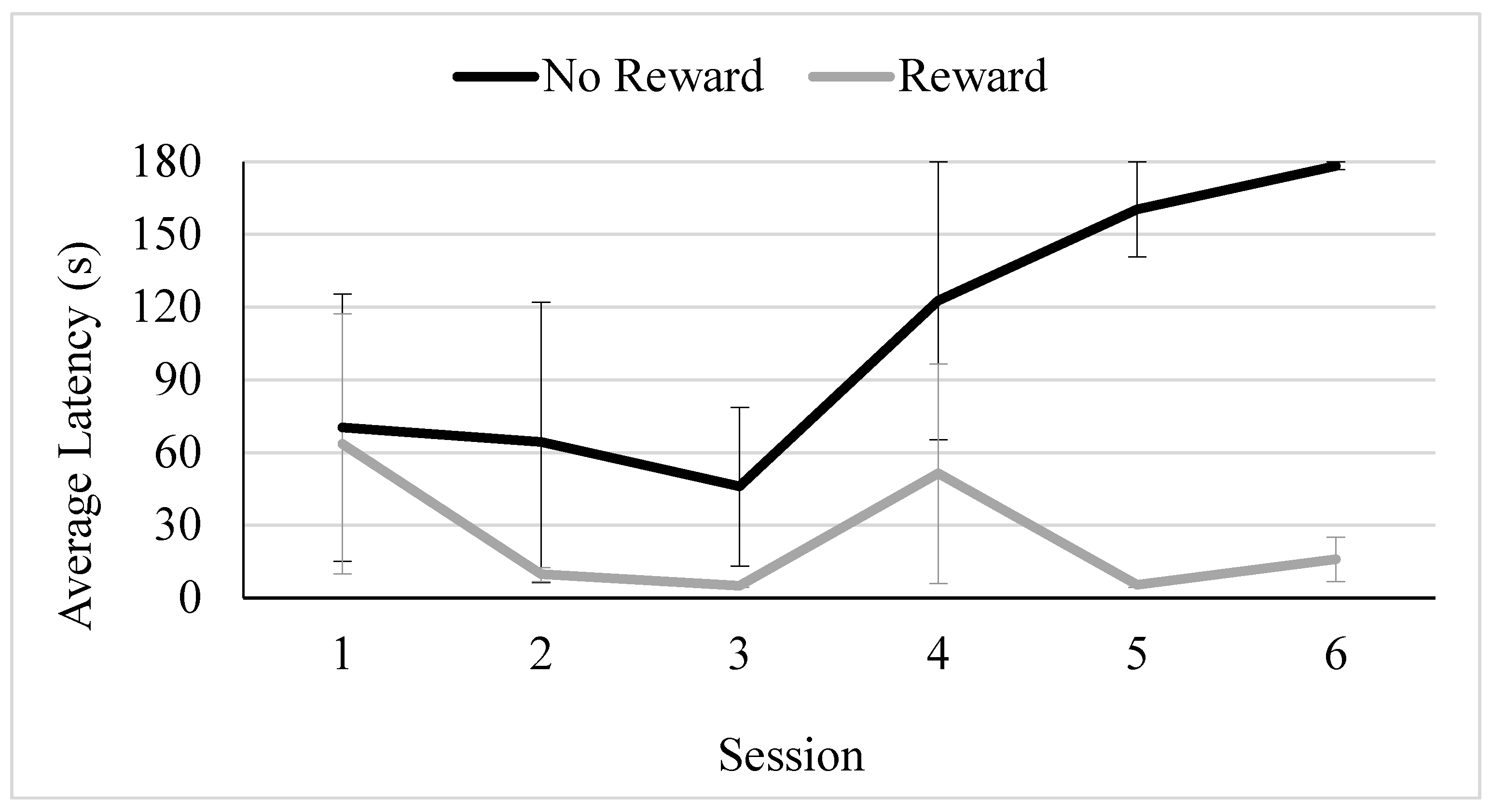
The chicken passed criterion in the habituation phase after five sessions and then moved on to training.
Figure 2 shows the chicken’s average approach time on training sessions by trial type (reward and no reward). The chicken passed the training criterion in session 6, advancing to testing sessions when the discrimination was learned sufficiently.
3.2. Testing
The model fit with a corrected AIC of 262.30. There was no main effect of condition (
F(1,34) = .044,
p = .835; held:
M = 129.399 s,
SE = 116.142 s, perched:
M = 45.298 s,
SE = 8.964 s). There was a significant main effect of trial type (
F(2,5) = 14.678,
p < .001) but this was qualified by a significant interaction between condition and trial type (
F(2,5) = 10.390,
p <.001) . Separate GLMMs for each condition revealed that the main effect of trial type was significant for both conditions (Held,
F(2,2) = 46.286,
p = .004, Perch,
F(2,2) = 26.504,
p <.001). Models fit well with corrected AICs of 110.697 and 119.307. In the Held condition, the chicken did not approach the ambiguous location significantly more slowly than she approached the reward condition (
t (2) = -.426,
p = .705, 95%CI: -32.438, 25.655), or more quickly than she approached the non-reward location (
t (2) = 3.867,
p = .066, 95%CI: -24.668, 310.156). In the Perch condition, the chicken did not approach the ambiguous location significantly more slowly than she approached the reward condition (
t (2) = -1.839,
p = .085, 95%CI: -246.725, 17.511), or significantly more quickly than she approached the non-reward location (
t (2) = -1.807,
p = .090, 95%CI: -238.539, 19.020). Although
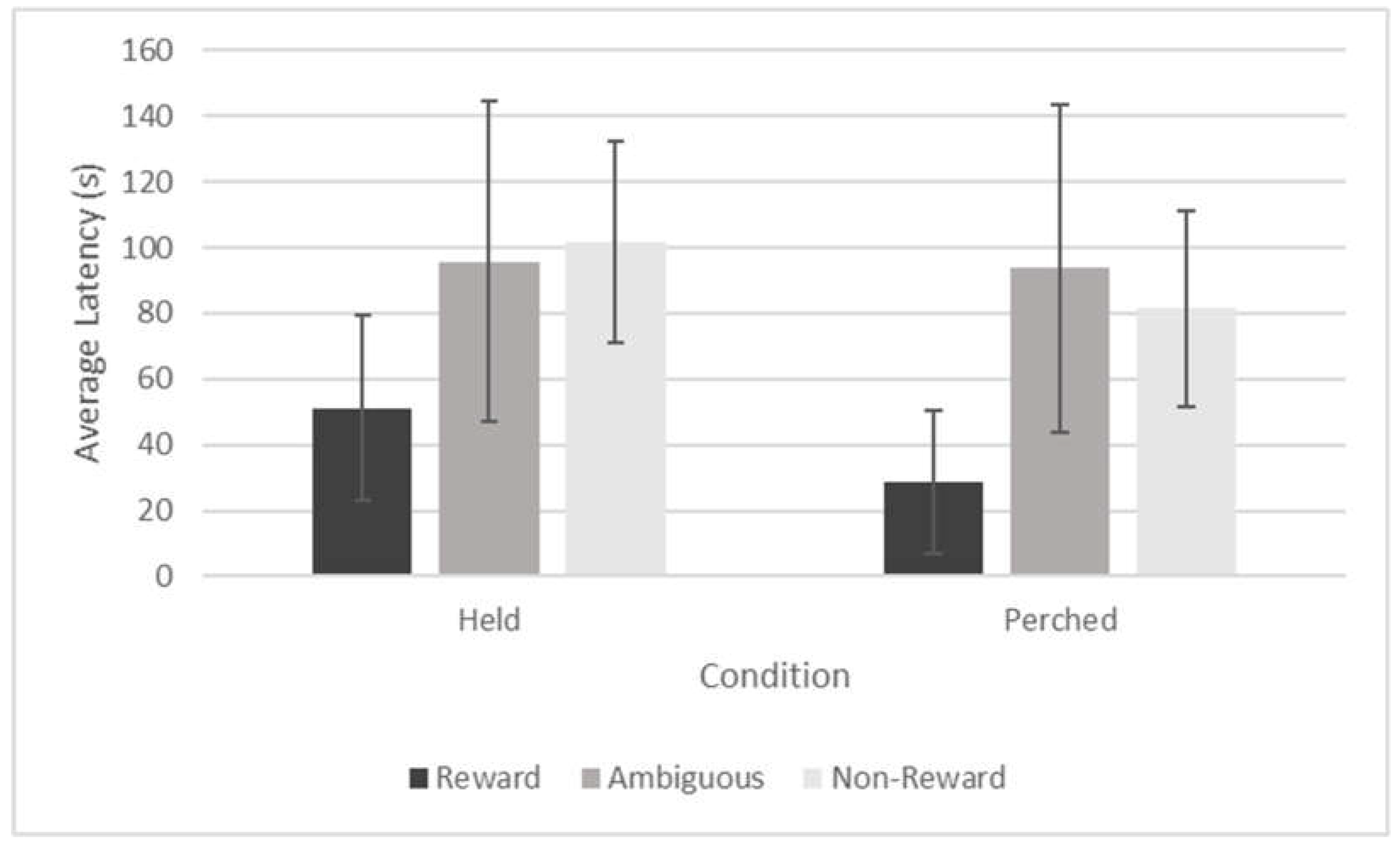
Figure 3 shows that the pattern of approach differed somewhat according to condition, in both conditions, the chicken’s approach latency was more similar to her approach time to the non-reward than to reward location.
3.3. Discussion
There was a significant difference between the chicken’s average approach time in the ambiguous trials compared to the reward trials with the average approach latency greater in the ambiguous trials compared to the reward trials. This, coupled with the finding that the average latency to approach in ambiguous trials did not significantly differ from the average latency in no reward trials suggests pessimism. Although the main effect of trial type interacted with condition, the overall pattern was one in which latencies to approach on ambiguous trials were numerically more similar to latencies on non-reward compared to reward trials (
Figure 3), suggesting that the chicken may experience a negative affective state during those interactions, which is not differentially impacted by the style of hold. However, future studies would need to include a baseline condition without human interaction to further assess whether the chicken was pessimistic due to visitor interactions, or to both hold types, or just in general. This condition was not included here because it is critical to minimize exposure to the ambiguous location to maintain the condition of ambiguity, and therefore, we limited the number of test sessions.
4. Study 2
Method
Subject
A single male red tegu (Salvator rufescens) that served as an education animal at the Alexandria Zoo in Alexandria, Louisiana was tested. The tegu was housed in an indoor habitat within the same educational building that testing occurred, although testing occurred in a different room. This building was infrequently open to the public, and the tegu was housed in a back room, so the tegu’s interactions with visitors were primarily limited to educational and other on-site programs. These educational programs provided the subject with minimal training experience, but he was naïve to the judgment bias test. On days when testing occurred, daily feeding occurred after testing.
Materials
All testing occurred within an indoor education building that housed the tegu’s home habitat. This area was secluded from visitors, but the tegu was exposed to the same noises in this building as the chicken, although he may have been more habituated to these noises as this was his daily habitat. Trials were carried out within an approximately square cardboard arena. This arena was 1.6 m by 1.7 m with sides .9 m tall (set up similarly to
Figure 1), smaller than the chicken’s arena to account for the tegu’s slower speed. One opaque blue plastic lidded soap container was utilized as a food container throughout the study. This container was placed in the same locations as the setup for the chicken for habituation, training, and testing trials. The reward and no reward locations were 1.5 m apart from each other, and both locations were 1.6 m from the entrance to the arena. On ambiguous trials, the container was placed equidistant (.8 m) from the high reward and no reward locations, and this location was also 1.6 m from the starting point of the arena. The container had food placed within it before trials started so that scent could not be used as a cue. Rewards consisted of high value food (meat cubes) as rated by the tegu’s keepers and approved to be used across multiple trials by the veterinarian. A GoPro was used to film all trials.
Procedure
The details of the procedure for the tegu were similar to those of the chicken. However, the tegu received a different manipulation. During testing, the manipulation for the tegu centered around physical interaction with visitors. Four sessions were conducted after the tegu experienced physical touch of various visitors, and four sessions were conducted after the tegu experienced nonphysical interaction with various visitors. Each day of testing, the tegu received a visitor touch condition and a no visitor touch condition in randomized order. In both conditions, the tegu was carried by a familiar keeper around outside areas in the zoo and experienced interaction with at least three humans (aside from the keeper). Similar to the chicken, this interaction lasted around 10 minutes each time, and then the tegu was immediately returned to the testing arena to begin sessions. The analytic approach was the same as above.
5. Results
5.1. Training
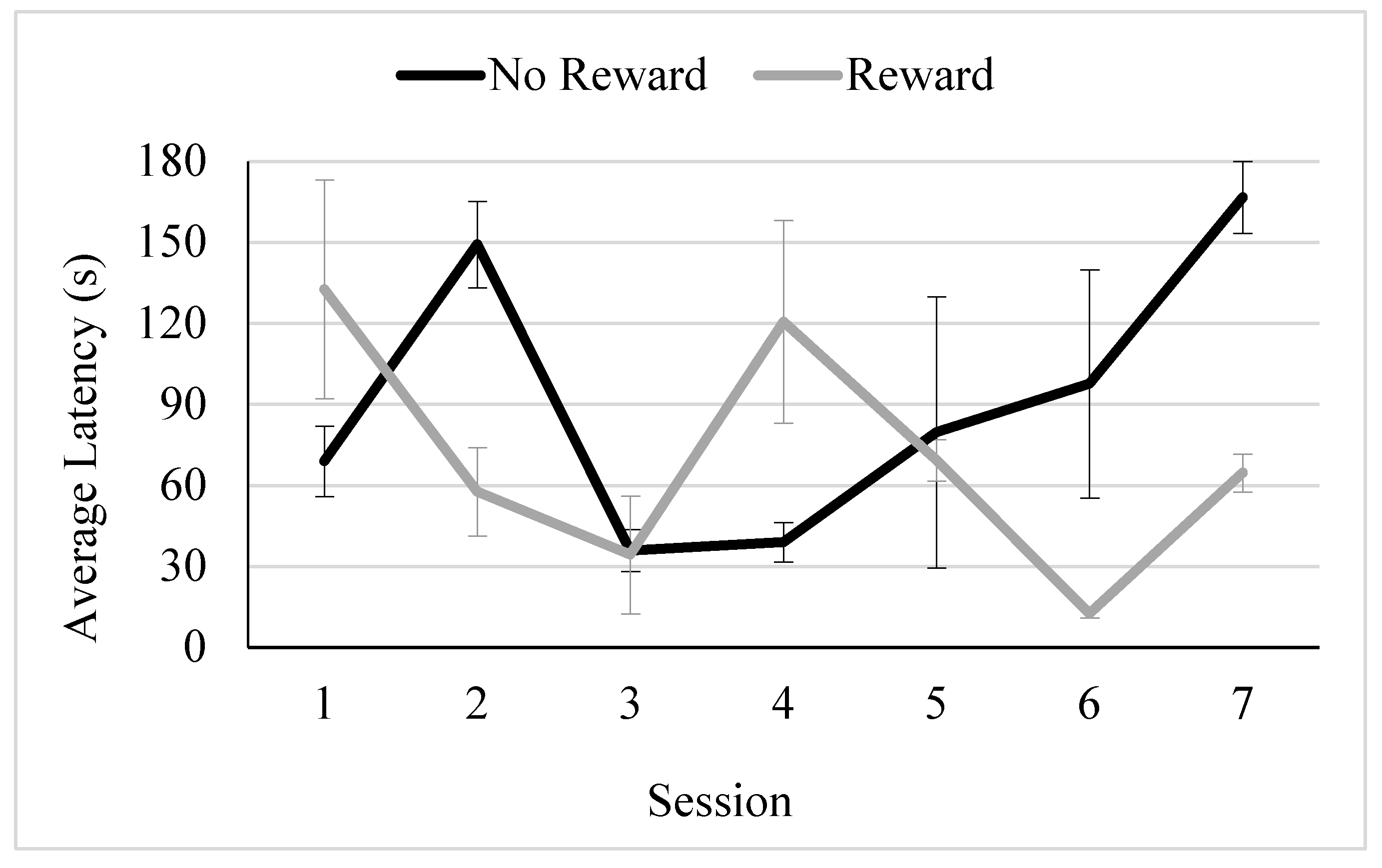
The tegu passed criterion in the habituation phase after two sessions, and then advanced to training. His average latencies to approach in the training phase on both trial types (reward, no reward) are depicted in
Figure 4. The tegu passed criterion on session 7, which allowed him to advance to testing sessions after demonstrating discrimination between trial types.
5.2. Testing
Reliability between coders for latency to approach was excellent; ICC, α = 1.00.
The model fit with a corrected AIC of 181.460. Results of the GLMM demonstrated no main effect of condition (
F(1,9) = 1.068,
p = .329; visitor touch:
M = 56.100 s,
SE = 5.590 s, no visitor touch:
M = 49.580 s,
SE = 3.275 s), but there was a main effect of trial type (
F(2,6) = 66.471,
p < .001). The tegu approached more slowly on ambiguous trials (
M = 56.778 s,
SE = 5.212 s;) compared to reward trials (
M = 16.652 s,
SE = 1.785 s,
t = -3.192,
p = .043,
SE = .277, CI [-1.716, -.051]) and more quickly compared to no reward trials (
M = 123.428 s,
SE = 12.209 s,
t = 7.579,
p = .027,
SE = .184, CI [.431, 2.352]). However, these effects were qualified by a significant interaction between condition and trial type (
F(2,6) = 10.344,
p = .012). The tegu’s approach times between the ambiguous trial type and the no reward trial (
t = -4.556,
p = .006,
SE = .270, CI [-1.926, -.533]), but not the reward trial type (
t = -2.433,
p = .079,
SE = .282, CI [-1.506, .133]), significantly differed across conditions (See
Figure 5).
Separate GLMMs were conducted to probe the interaction further. Models fit well with corrected AICs of 194.437 and 204.031. In the no touch condition, there was a significant effect of position (
F (2,17) = 8.831,
p = .002). Latency to approach on reward trials (
t = -2.994,
p = .008, 95% CI[-63.122, -10.936]) but not no reward trials (
t = 1.131,
p = .274, 95% CI[-16.957, 56.152]) significantly differed from latency to approach on ambiguous trials. In the touch condition, there was also a significant effect of position (
F (2,7) = 231.318,
p < .001). Latency to approach on reward trials (
t = -2.672,
p = .026, CI[-43.711, -3.543]) and no reward trials (
t = 14.569,
p <.001, 95% CI[-108.724, 148.892]) significantly differed from latency to approach on ambiguous trials. Thus, the tegu was somewhat pessimistic in the no touch but was neither optimistic nor pessimistic in the touch condition. However, as can be seen in
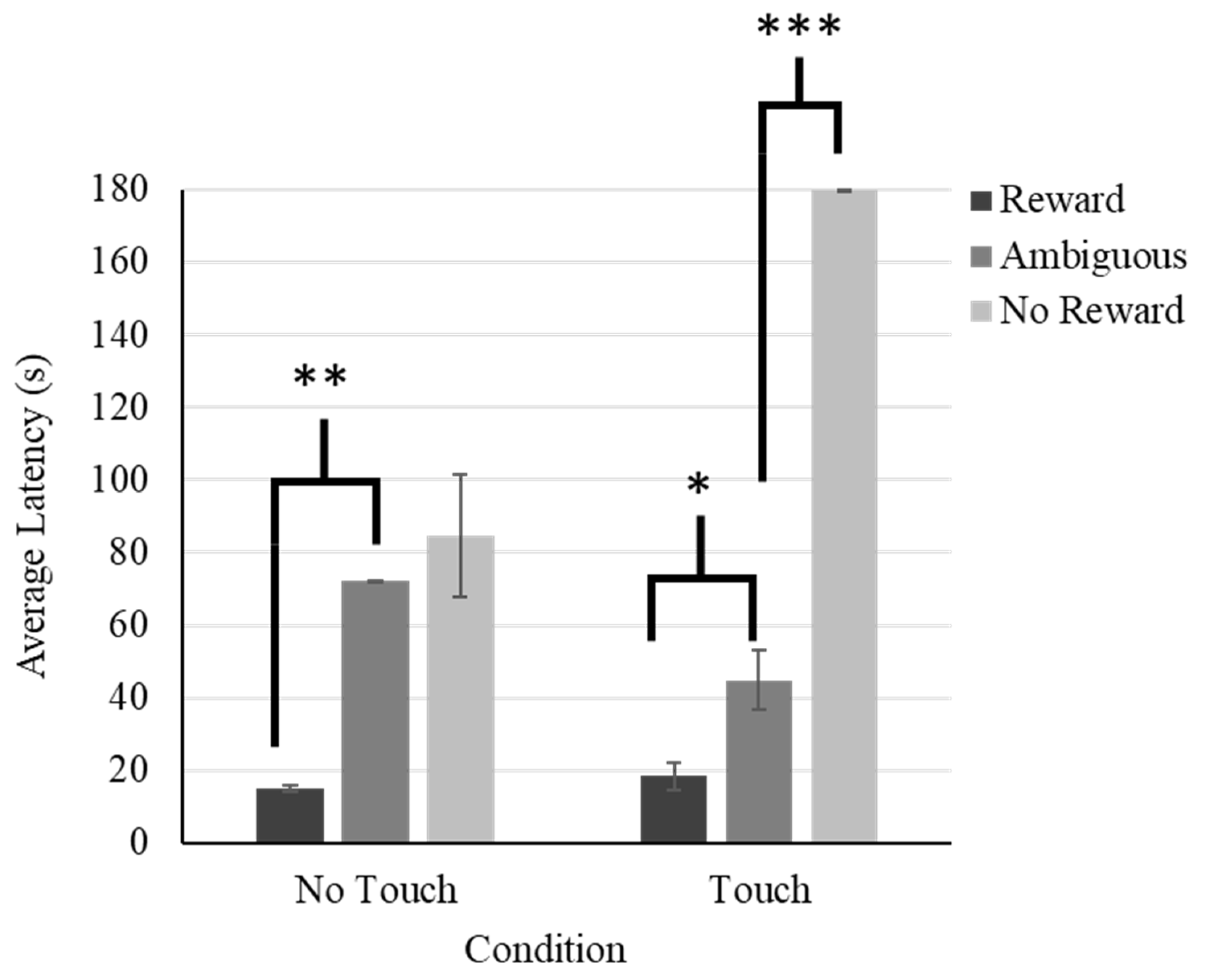
Figure 5, his latency to approach on ambiguous trials was more similar to the reward, compared to the non-reward trials in the touch condition.
5.3. Discussion
The designation of optimism and pessimism depends upon the comparison of the subject’s approach latency to the ambiguous stimulus to both reward and no reward conditions. That the tegu responded significantly more slowly to the ambiguous stimulus compared to the reward stimulus, but also significantly more quickly than to the no reward stimulus suggests neither optimism or pessimism overall. The tegu’s intermediate approach latency on ambiguous trials may suggest that he perceived the intermediate spatial position of the ambiguous stimulus and was suitably uncertain as to what to expect on these trials. Interestingly, the difference in his latency to approach the location on the ambiguous and no reward trials depended upon condition. Whereas the data are suggestive of pessimism in the no touch condition, the data in the touch condition are not consistent with pessimism, with the tegu approaching the ambiguous stimulus much more quickly than the no reward stimulus. Somewhat surprisingly, this suggests that the tegu may have been experiencing less negative affect in the condition with touch compared to the condition without.
6. General Discussion
These results indicate distinct differences between the chicken and the tegu in their affect following visitor interactions. The tegu did not appear to be negatively affected by the touch of visitors, specifically. The lack of evidence for negative affect following the visitor touch condition indicates little cost to his psychological well-being from participating in the program. However, his response to the ambiguous stimulus in the no touch condition was indicative of pessimism, suggesting that his visitor interactions should include a touch component. On the other hand, the chicken demonstrated pessimism in general following visitor interactions regardless of the manner in which she was held by keepers. This interpretation is consistent with previous research on the negative effects of prolonged human handling in hawks, hedgehogs, and armadillos, which showed increased fecal glucocorticoid levels and undesirable behaviors in such conditions (Baird et al., 2016). Thus, the chicken’s interactions with visitors may need to be reconsidered. It is difficult to integrate these results with other findings as there is minimal research on changes in affect following human interactions in zoo animals. Of the few previous studies on cognitive biases in zoo animals, only one examined the influence of humans on three primate species, and this was an indirect effect of anthropogenic noise (i.e., air show noise). Only the macaques, compared to the gorillas and chimpanzees, showed response slowing to conspecific images compared to control images when anthropogenic noise occurred (Cronin et al., 2018). Response slowing on simple tasks in these studies is thought to indicate anxiety, similar to results in humans (Bar-Haim et al., 2007), or that subjects’ attention is focused on vigilance behaviors (Cronin et al., 2018; Ebitz et al., 2013), and is expected to be more extreme with threatening content. However, the macaques did not show response slowing to forward facing faces, which were thought to be threatening, compared to averted faces, which could have been due to the researchers’ use of both female and male faces or lack of statistical power. Interestingly, the macaques were a more recent addition to the zoo (i.e., data were taken during their second year of exposure to the noise) compared to the apes that had 13 years of exposure to the air show. The chicken in the current study was also a recent addition to the zoo. This pattern reinforces the idea that habituation to human influences is an important factor to determining whether human influences will be aversive. Both the gorillas and chimpanzees in Cronin et al. (2018), as well as the tegu here, had been housed at their respective zoos for longer periods of time and displayed neutral affects after exposure to more intense human influences (i.e., touch for the tegu and loud jet noises for the apes). These comparisons must be tempered by the fact that noises from jets in an air show substantially differ from direct human touch.
The manipulation utilized in Bernstein-Kurtycz et al.’s (2024) study of grizzly bears was more comparable to the manipulation in the current study. Grizzly bears were tested after four experimental conditions varying by the occurrence of two factors: a visitor tour behind the scenes and a keeper training session. One bear responded more slowly to the ambiguous stimulus compared to the reward, but not the no reward stimuli, suggesting pessimism only in conditions where food reinforcement was withheld but did not seem affected by the presence or absence of zoo visitors during a tour. The bears displayed a lower proportion of frustration behaviors when presented with food reinforcement compared to when there was no reinforcement regardless of whether visitors were present, validating the results from the judgment bias test. It is important to note, however, that proportions of frustration behaviors were extremely low overall. Unfortunately, we did not assess behavioral impacts in the chicken or the tegu. These subjects also did not receive food reinforcement during the visitor interactions independent of the test rewards. However, the tegu and chicken both have a history of food reinforcement when involved in training and education sessions with keepers, which could influence their perceptions of these sessions and visitor interactions. It is possible that visitor presence did not have a large impact on the bears, as compared to our chicken, because the bears were not touched by visitors. In addition, the bears were able to move around somewhat freely, potentially giving them more of an escape opportunity and preventing negative effects (Sherwen & Hemsworth, 2019), whereas the chicken and tegu were being held by care staff. Even so, our tegu showed less pessimism in the no touch, compared to the touch condition. Habituation may account for these results as well, as the bears had been trained for visitor tours previously. Therefore, the bears and tegu may have become more used to visitor presence, and associated visitors with the provision of rewards, in their time at the zoos compared to the chicken.
However, these results must be interpreted cautiously due to the short-term interactions assessed here, as the animals were exposed to visitors for an approximately ten-minute span before being tested. These animals have been frequently exposed to visitors in their past given their ambassador animal status; thus, a ten-minute exposure period may not have been long enough to cause any of the effects found here. Indeed, the judgment bias task has been considered less effective for assessment of short-term effects as animals may show a positive judgment bias given the supposed stressor has come to an end (Bethell, 2015). Therefore, the results for the tegu in the touch condition could indicate relief at the removal of the stimulus (see also Doyle et al., 2009; Freymond et al., 2014). On the other hand, the pessimism across conditions demonstrated by the chicken could have been a general affective bias caused by long-term factors. Future research could benefit from assessing judgment biases following longer, more intensive, and more consistent visitor interactions.
Despite limitations, these results demonstrate the promise of the judgment bias task for ambassador animal assessment. The chicken and the tegu both learned the discrimination between the reward and no reward location relatively quickly and comparably—the tegu in seven sessions and the chicken in six sessions. Previous studies have demonstrated the usefulness of the task in chickens (Košťál et al., 2020), although its promise for reptiles had only been theorized before this project (Benn et al., 2019). The current data show that tegus may learn spatial discriminations relatively quickly, which will be useful for future explorations of their cognition. We utilized a location-based discrimination with three distinct locations; however, other discriminations could be utilized based on the needs or abilities of various animals, such as colors, sizes, sounds, etc. (see Bethell, 2015, for a review).
Although this study aimed to fill gaps within the literature, there were several limitations. First, assessing the impact of human interactions meant that many different variables, such as the individual keeper handling the animal, differences in visitor behavior, and testing environment could be affecting the judgment bias of the animals. Previous research has indicated that these variables can impact animal behavior over and above visitor effects (Rose et al., 2020). Testing took place in Louisiana in August, during an abnormal drought, and in a building without air conditioning, meaning temperature and weather could have affected the animals more than any human interaction. We attempted to control some variation by including session in the models as a random factor. The models were also lacking power due to the minimal number of subjects and trials, which were kept to a minimum to keep the ambiguous location ambiguous over successive exposures. It could also be that further keeper interaction during the testing procedure overshadowed any effects of the change in hold during visitor interactions for the chicken. The chicken was held in a manner to prevent her flying into the arena when she was placed inside for testing; thus, the results could indicate the chicken’s general dislike of handling. It would have been ideal to include baseline conditions in which the chicken was not handled at all and/or visitors were not present. Lastly, this study involved an extremely small sample from one zoo, so it is important to recognize that the results may not generalize to other ambassador animals.
Even with these limitations, judgment biases are just beginning to be better studied in birds (e.g., Košťál et al., 2020; McCoy et al., 2019), and the current research extends this mainly agriculturally-based literature to zoo animal welfare. In addition, this is the first lizard, to the authors’ knowledge, to be assessed with a judgment bias task, although the promise of judgment bias tests as a welfare assessment tool for reptiles has been acknowledged previously (Benn et al., 2019; Bethell, 2015). Given that affect can alter cognition (Mendl et al., 2009), it is worth investigating the judgment biases of lesser studied animals and the circumstances that may impact these biases. Ambassador animals often experience close human interaction for prolonged periods, yet not enough research has investigated their impact on the welfare of the animals (Spooner et al., 2021). The current research suggests that various differences in human interaction could affect the judgment biases of these ambassador animals differently depending on the individual, but further research is needed to understand if this practice should be reconsidered or encouraged for ambassador animals as a whole.
Author Contributions
J.T.: conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing, visualization. J.V.: methodology, conceptualization, formal analysis, writing—review and editing. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were provided by the Oakland University IACUC.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon request from the corresponding author.
Acknowledgments
We are thankful to the animal caretakers and administrators at the Alexandria Zoo, who facilitated and participated in this study. We are also grateful to the students that assisted in coding the animals’ responses, Sophia Vecchi and Faith Andrzejewski.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adriaense, J. E. , Martin, J. S., Schiestl, M., Lamm, C., & Bugnyar, T. Negative emotional contagion and cognitive bias in common ravens (Corvus corax). Proceedings of the National Academy of Sciences 2019, 116, 11547–11552. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M. G. , Campbell, A. M., Crump, A., Arnott, G., & Jacobs, L. Environmental complexity positively impacts affective states of broiler chickens. Scientific Reports 2021, 11, 16966. [Google Scholar] [CrossRef] [PubMed]
- AZA. (n.d.). Animal Ambassador Policy. Retrieved February 23, 2024, from https://assets.speakcdn.com/assets/2332/ambassador_animal_evaluation_tool.pdf.
- Baird, B. A. , Kuhar, C. W., Lukas, K. E., Amendolagine, L. A., Fuller, G. A., Nemet, J.,... & Schook, M. W. Program animal welfare: Using behavioral and physiological measures to assess the well-being of animals used for education programs in zoos. Applied Animal Behaviour Science 2016, 176, 150–162. [Google Scholar] [CrossRef]
- Bar-Haim, Y. , Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & Van Ijzendoorn, M. H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin 2007, 133, 1. [Google Scholar] [CrossRef]
- Bateson, M. , Desire, S., Gartside, S. E., & Wright, G. A. Agitated honeybees exhibit pessimistic cognitive biases. Current Biology 2011, 21, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M. , Emmerson, M., Ergün, G., Monaghan, P., & Nettle, D. Opposite effects of early-life competition and developmental telomere attrition on cognitive biases in juvenile European starlings. Plos One 2015, 10, e0132602. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M. , & Matheson, S. M. Performance on a categorisation task suggests that removal of environmental enrichment induces ‘pessimism’ in captive European starlings (Sturnus vulgaris). Animal Welfare 2007, 16, 33–36. [Google Scholar]
- Benn, A. L. , McLelland, D. J., & Whittaker, A. L. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality®® protocol to the pygmy blue-tongue skink, Tiliqua adelaidensis, using animal-based measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Bernstein-Kurtycz, L.M. , Vonk, J., Zinter, M., Carroscia, J.M., Koester, D.C., Snyder, R.J., Willis, M.A., & Lukas, K.E. Lack of food reinforcement is hard to “bear”: Validating a judgment bias task to assess underlying affect in grizzly bears. Journal of Applied Animal Welfare Science, 2024. [Google Scholar] [CrossRef]
- Bethell, E. J. A “how-to” guide for designing judgment bias studies to assess captive animal welfare. Journal of Applied Animal Welfare Science 2015, 18, S18–S42. [Google Scholar] [CrossRef]
- Brilot, B. O. , Asher, L., & Bateson, M. Stereotyping starlings are more ‘pessimistic’. Animal Cognition 2010, 13, 721–731. [Google Scholar] [CrossRef]
- Clegg, I. L. Cognitive bias in zoo animals: an optimistic outlook for welfare assessment. Animals 2018, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Clegg, I. L. , & Delfour, F. Cognitive judgement bias is associated with frequency of anticipatory behavior in bottlenose dolphins. Zoo Biology 2018, 37, 67–73. [Google Scholar] [CrossRef]
- Clegg, I. L. , Rödel, H. G., & Delfour, F. Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behavioural Brain Research 2017, 322, 115–122. [Google Scholar] [PubMed]
- Coles, M. E. , & Heimberg, R. G. Memory biases in the anxiety disorders: Current status. Clinical Psychology Review 2002, 22, 587–627. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K. A. , Bethell, E. J., Jacobson, S. L., Egelkamp, C., Hopper, L. M., & Ross, S. R. Evaluating mood changes in response to anthropogenic noise with a response-slowing task in three species of zoo-housed primates. Animal Behavior & Cognition 2018, 5, 209–221. [Google Scholar]
- Deakin, A. , Browne, W. J., Hodge, J. J., Paul, E. S., & Mendl, M. A screen-peck task for investigating cognitive bias in laying hens. PLoS One 2016, 11, e0158222. [Google Scholar] [CrossRef] [PubMed]
- Deakin, A. , Mendl, M., Browne, W. J., Paul, E. S., & Hodge, J. J. State-dependent judgement bias in Drosophila: evidence for evolutionarily primitive affective processes. Biology Letters 2018, 14, 20170779. [Google Scholar]
- Doyle, R. E. , Fisher, A. D., Hinch, G. N., Boissy, A., & Lee, C. Release from restraint generates a positive judgement bias in sheep. Applied Animal Behaviour Science 2010, 122, 28–34. [Google Scholar] [CrossRef]
- Dunn, B. D. , Stefanovitch, I., Buchan, K., Lawrence, A. D., & Dalgleish, T. A reduction in positive self-judgment bias is uniquely related to the anhedonic symptoms of depression. Behaviour Research and Therapy 2009, 47, 374–381. [Google Scholar] [CrossRef]
- Ebitz, R. B. , Watson, K. K., & Platt, M. L. Oxytocin blunts social vigilance in the rhesus macaque. Proceedings of the National Academy of Sciences 2013, 110, 11630–11635. [Google Scholar]
- Eysenck, M. W. , Mogg, K., May, J., Richards, A., & Mathews, A. Bias in interpretation of ambiguous sentences related to threat in anxiety. Journal of Abnormal Psychology 1991, 100, 144. [Google Scholar] [PubMed]
- Fitzgerald, L. A. , Cruz, F. B., & Perotti, G. The reproductive cycle and the size at maturity of Tupinambis rufescens (Sauria: Teiidae) in the dry Chaco of Argentina. Journal of Herpetology.
- Freymond, S. B. , Briefer, E. F., Zollinger, A., Gindrat-von Allmen, Y., Wyss, C., & Bachmann, I. Behaviour of horses in a judgment bias test associated with positive or negative reinforcement. Applied Animal Behaviour Science 2014, 158, 34–45. [Google Scholar]
- Gott, A. , Andrews, C., Bedford, T., Nettle, D., & Bateson, M. Developmental history and stress responsiveness are related to response inhibition, but not judgement bias, in a cohort of European starlings (Sturnus vulgaris). Animal Cognition 2019, 22, 99–111. [Google Scholar] [PubMed]
- Harding, E. J. , Paul, E. S., & Mendl, M. Cognitive bias and affective state. Nature 2004, 427, 312–312. [Google Scholar] [PubMed]
- Horváth, M. , Pichová, K., & Košťál, Ľ. The effects of housing conditions on judgement bias in Japanese quail. Applied Animal Behaviour Science 2016, 185, 121–130. [Google Scholar]
- Jarnevich, C. S. , Hayes, M. A., Fitzgerald, L. A., Yackel Adams, A. A., Falk, B. G., Collier, M. A.,... & Reed, R. N. Modeling the distributions of tegu lizards in native and potential invasive ranges. Scientific Reports 2018, 8, 10193. [Google Scholar] [PubMed]
- Košťál, Ľ. , Skalná, Z., & Pichová, K. Use of cognitive bias as a welfare tool in poultry. Journal of Animal Science 2020, 98, S63–S79. [Google Scholar] [PubMed]
- Kremer, L. , Holkenborg, S. K., Reimert, I., Bolhuis, J. E., & Webb, L. E. The nuts and bolts of animal emotion. Neuroscience & Biobehavioral Reviews 2020, 113, 273–286. [Google Scholar]
- Lagisz, M. , Zidar, J., Nakagawa, S., Neville, V., Sorato, E., Paul, E. S.,... & Løvlie, H. Optimism, pessimism and judgement bias in animals: a systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 2020, 118, 3–17. [Google Scholar]
- Lalot, M. , Ung, D., Péron, F., d’Ettorre, P., & Bovet, D. You know what? I’m happy. Cognitive bias is not related to personality but is induced by pair-housing in canaries (Serinus canaria). Behavioural Processes 2017, 134, 70–77. [Google Scholar]
- Laubu, C. , Louâpre, P., & Dechaume-Moncharmont, F. X. Pair-bonding influences affective state in a monogamous fish species. Proceedings of the Royal Society B 2019, 286, 20190760. [Google Scholar] [PubMed]
- Lourenço-Silva, M. I. , Ulans, A., Campbell, A. M., Almeida Paz, I. C. L., & Jacobs, L. Social-pair judgment bias testing in slow-growing broiler chickens raised in low-or high-complexity environments. Scientific Reports 2023, 13, 9393. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, Y. N. , Mastromonaco, G. F., Korver, W., & Burness, G. Use of salivary cortisol to evaluate the influence of rides in dromedary camels. General and Comparative Endocrinology 2015, 211, 123–130. [Google Scholar] [PubMed]
- Matheson, S. M. , Asher, L., & Bateson, M. Larger, enriched cages are associated with ‘optimistic’ response biases in captive European starlings (Sturnus vulgaris). Applied Animal Behaviour Science 2008, 109, 374–383. [Google Scholar] [CrossRef]
- McCoy, D. E. , Schiestl, M., Neilands, P., Hassall, R., Gray, R. D., & Taylor, A. H. New caledonian crows behave optimistically after using tools. Current Biology 2019, 29, 2737–2742. [Google Scholar] [PubMed]
- McGuire, M. C. , Vonk, J., Fuller, G., & Allard, S. Using an ambiguous cue paradigm to assess cognitive bias in gorillas (Gorilla gorilla gorilla) during a forage manipulation. Animal Behavior and Cognition 2017, 4, 91–104. [Google Scholar]
- McGuire, M. C. , Vonk, J., & Johnson-Ulrich, Z. Ambiguous results when using the ambiguous-cue paradigm to assess learning and cognitive bias in gorillas and a black bear. Behavioral Sciences 2017, 7, 51. [Google Scholar] [CrossRef]
- McGuire, M. C. , & Vonk, J. Gorillas (Gorilla gorilla gorilla) fail to learn abstract cues of differential outcomes in a novel cognitive bias test. Animal Behavior and Cognition 2018, 5, 103–117. [Google Scholar]
- Mellor, D. J. Updating animal welfare thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Mendl, M. , Burman, O. H., Parker, R. M., & Paul, E. S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Applied Animal Behaviour Science 2009, 118, 161–181. [Google Scholar]
- Munari, A. (2021). Exploring a simplified affective state test in the red junglefowl [Master’s Thesis, Linköping]. DiVA.
- Nematipour, B. , Bračić, M., & Krohs, U. Cognitive bias in animal behavior science: A philosophical perspective. Animal Cognition 2022, 25, 975–990. [Google Scholar] [PubMed]
- Novak, J. , Bailoo, J. D., Melotti, L., Rommen, J., & Würbel, H. An exploration based cognitive bias test for mice: Effects of handling method and stereotypic behaviour. PLoS One 2015, 10, e0130718. [Google Scholar]
- Orban, D. A. , Siegford, J. M., & Snider, R. J. Effects of guest feeding programs on captive giraffe behavior. Zoo Biology 2016, 35, 157–166. [Google Scholar] [PubMed]
- Proctor, H. S. , Carder, G., & Cornish, A. R. Searching for animal sentience: A systematic review of the scientific literature. Animals 2013, 3, 882–906. [Google Scholar]
- Rose, P. E. , Scales, J. S., & Brereton, J. E. Why the “visitor effect” is complicated. Unraveling individual animal 2020, visitor number, and climatic influences on behavior, space use and interactions with keepers—A case study on captive hornbills. Frontiers in Veterinary Science, 7, 236.
- Scarantino, A. & de Sousa, R. (2021) Emotion. In: Edward N. Zalta (ed) The Stanford encyclopedia of philosophy, (2021 ed). Metaphysics Research Lab, Stanford University.
- Schlüns, H. , Welling, H., Federici, J. R., & Lewejohann, L. The glass is not yet half empty: agitation but not Varroa treatment causes cognitive bias in honey bees. Animal Cognition 2017, 20, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, B. , Mendl, M., Keeling, L. J., & Blokhuis, H. Disrupting motivational sequences in chicks: Are there affective consequences? Applied Animal Behaviour Science 2013, 148, 85–92. [Google Scholar] [CrossRef]
- Sherwen, S. L. , & Hemsworth, P. H. The visitor effect on zoo animals: Implications and opportunities for zoo animal welfare. Animals 2019, 9, 366. [Google Scholar] [PubMed]
- Solvi, C. , Baciadonna, L., & Chittka, L. Unexpected rewards induce dopamine-dependent positive emotion–like state changes in bumblebees. Science 2016, 353, 1529–1531. [Google Scholar] [CrossRef] [PubMed]
- Spooner, S. L. , Farnworth, M. J., Ward, S. J., & Whitehouse-Tedd, K. M. Conservation education: Are zoo animals effective ambassadors and is there any cost to their welfare? Journal of Zoological and Botanical Gardens 2021, 2, 41–65. [Google Scholar] [CrossRef]
- McGuire, M. , & Vonk, J. M. In or out: Response slowing across housing conditions as a measure of affect in three Western lowland gorillas (Gorilla gorilla gorilla). PeerJ 2020, 8, e9525. [Google Scholar] [CrossRef]
- Vonk, J. , McGuire, M. C., & Johnson-Ulrich, Z. Bearing fruit: Piloting a novel judgment bias task in an American black bear. Zoo Biology 2021, 40, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J. , Torgerson, L., Edge, J. & Benton, B. (2024). More than a feeling: The comparative psychology of emotion. In L. Al-Shawaf & T.K. Shackelford (Eds). The Oxford handbook of evolution and the emotions. [pp. 769-796]. Oxford.
- Wichman, A. , Keeling, L. J., & Forkman, B. Cognitive bias and anticipatory behaviour of laying hens housed in basic and enriched pens. Applied Animal Behaviour Science 2012, 140, 62–69. [Google Scholar] [CrossRef]
- Zidar, J. , Campderrich, I., Jansson, E., Wichman, A., Winberg, S., Keeling, L., & Løvlie, H. Environmental complexity buffers against stress-induced negative judgement bias in female chickens. Scientific Reports 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).