Submitted:
25 April 2024
Posted:
25 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mice
2.3. Experimental Design
2.4. Whole Transcriptome Analysis of Mouse Small Intestine via RNA Sequencing
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analysis
2.6. Immunoblot Analysis
2.7. Immunofluorescence Analysis
2.8. Metagenomic Analysis of 16S rRNA Genes in Intestinal Microbiota
2.9. Statistical Analyses
3. Results
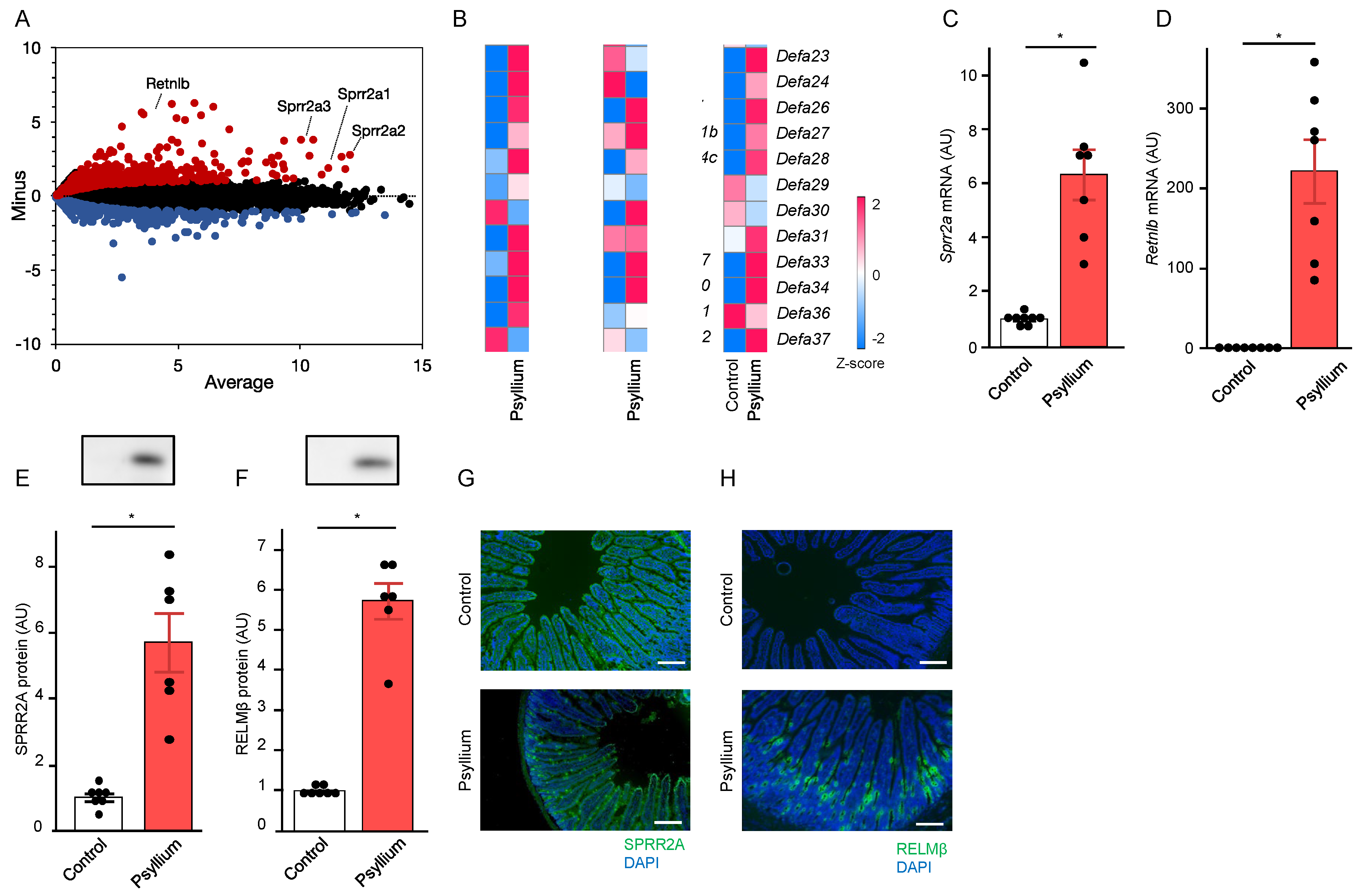
3.1. Psyllium Supplementation Upregulates the Production of Antimicrobial Proteins in the Mouse Small Intestine
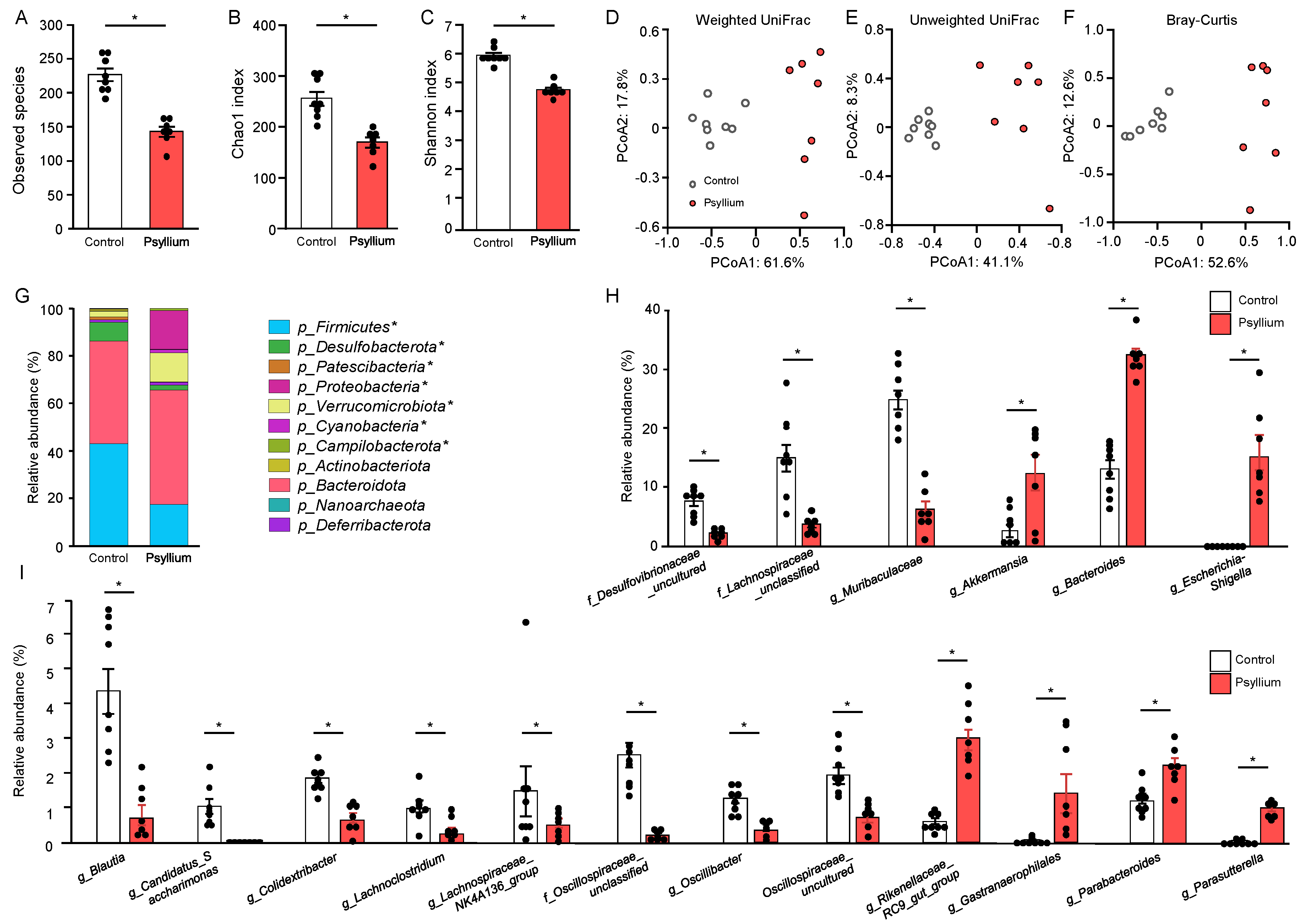
3.2. Psyllium Supplementation Alters Cecal Microbiota Composition
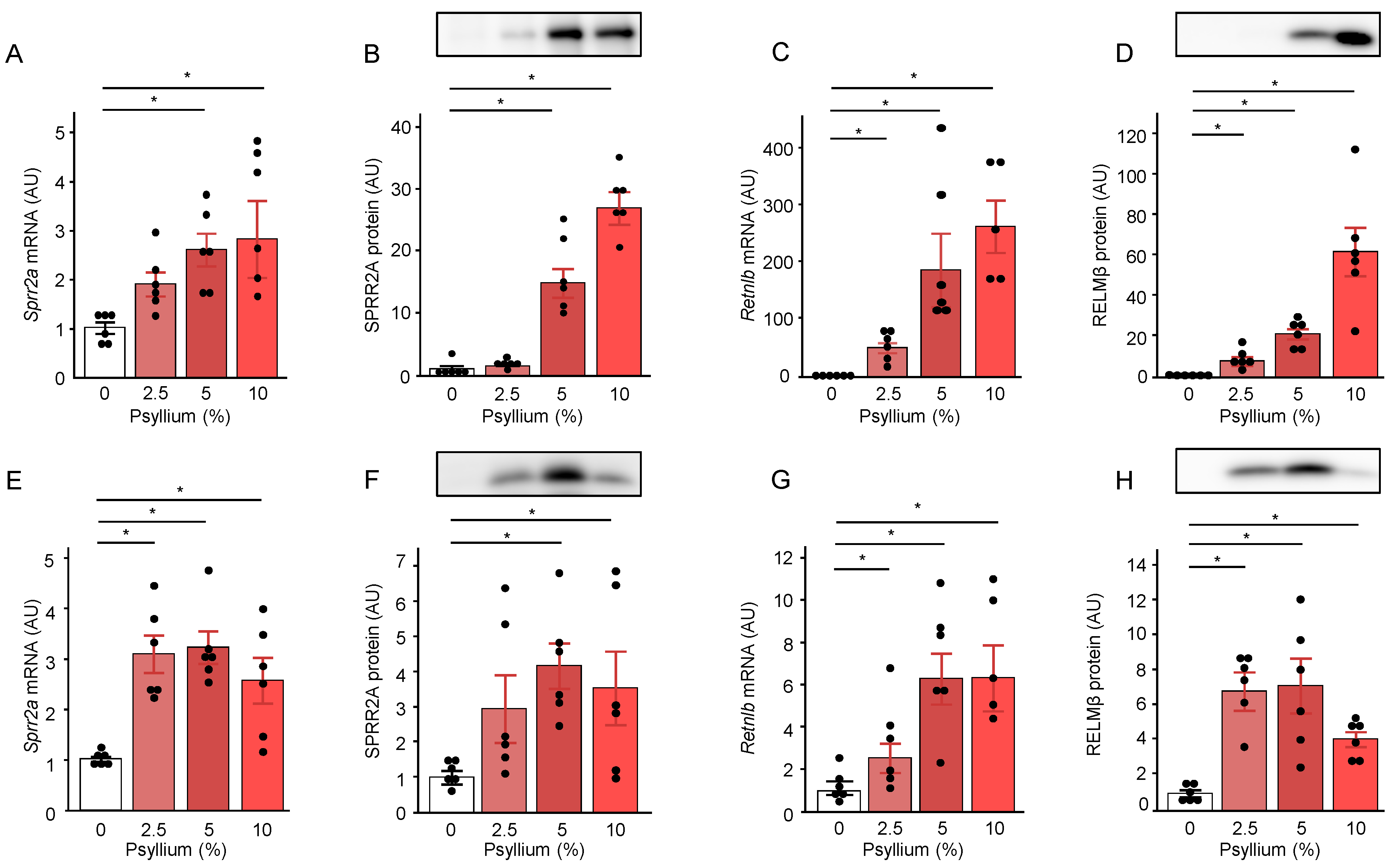
3.3. Psyllium Supplementation Upregulates Antimicrobial Proteins, SPRR2A and RELMβ, in a Dose-Dependent Manner
3.4. Psyllium Fiber Sustains Upregulation of Antimicrobial Proteins for 15 d
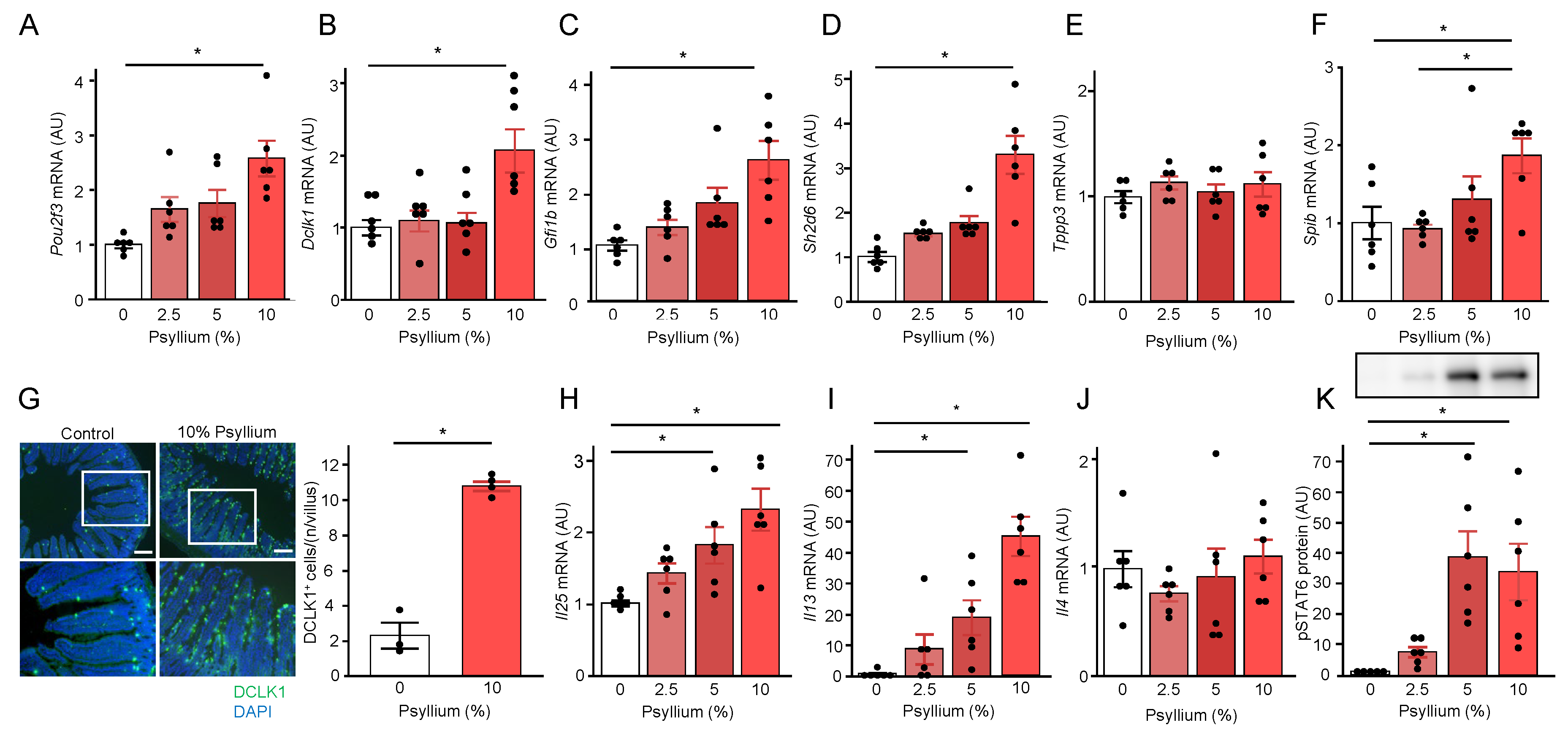
3.5. Psyllium Supplementation Increases Tuft Cell Proliferation via IL-13 Signaling
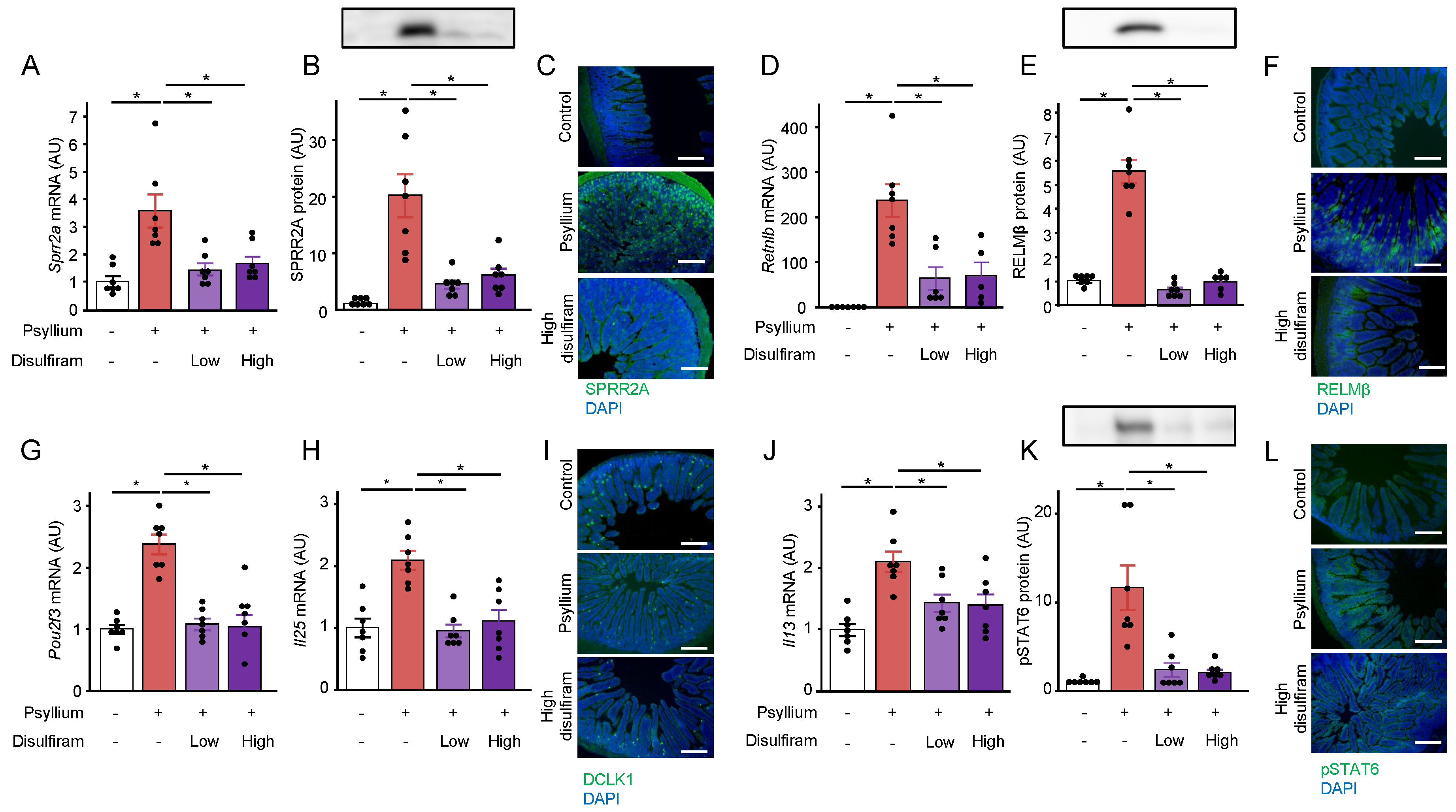
3.6. ILC2 is Involved in the Psyllium-Induced Antimicrobial Protein Production
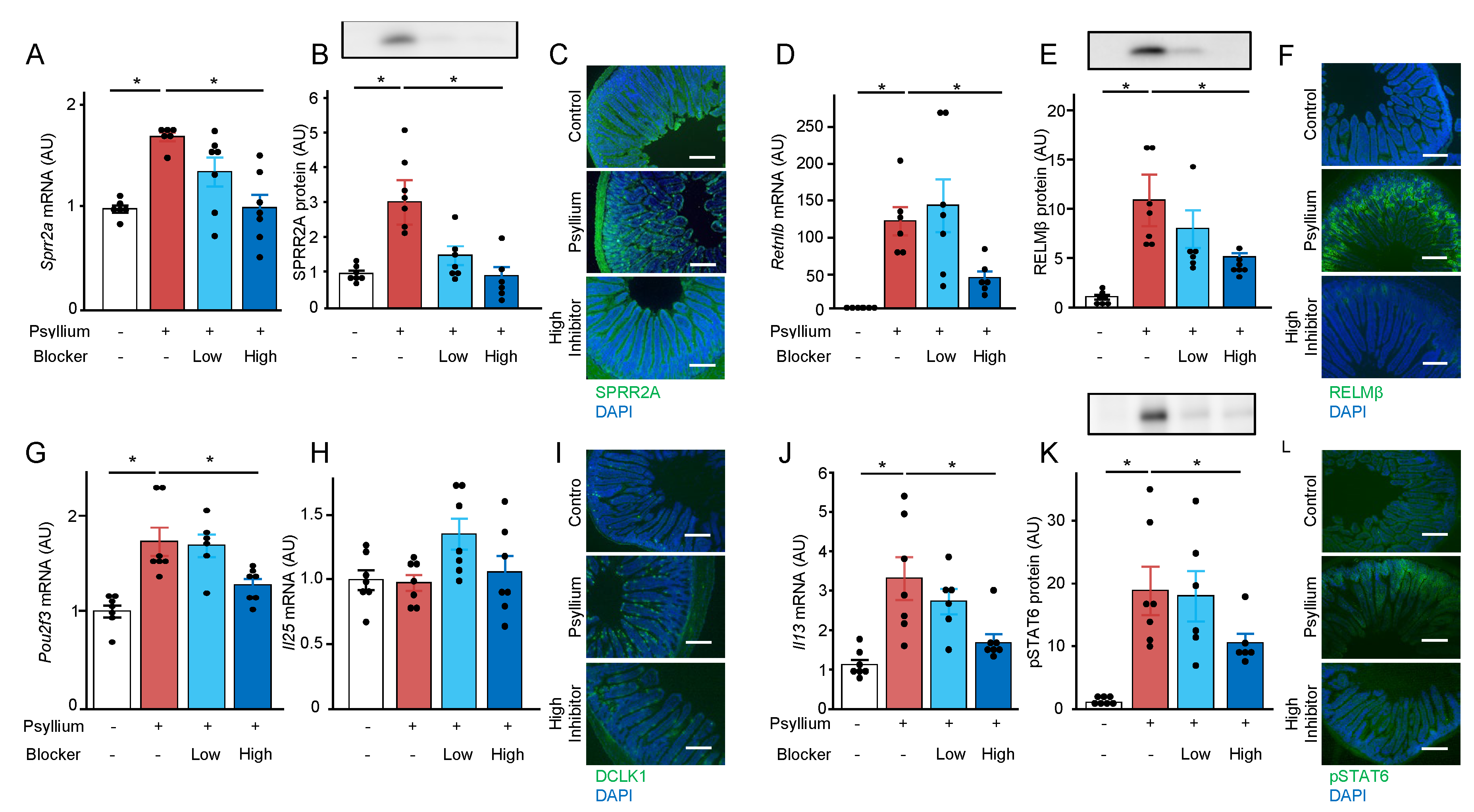
3.7. Bitter Taste Receptors of Tuft Cells are Involved in Psyllium-Induced Antimicrobial Protein Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blyth, G.A.D.; Connors, L.; Fodor, C.; Cobo, E.R. The Network of Colonic Host Defense Peptides as an Innate Immune Defense Against Enteropathogenic Bacteria. Front Immunol 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Abdullah, M.; Määttänen, P.; Pilar, A.V.C.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.L.; Sherman, P.M. Protein Kinase Cσ Signaling Is Required for Dietary Prebiotic-Induced Strengthening of Intestinal Epithelial Barrier Function. Sci Rep 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, T.; Suzuki, T. Guar Gum Fiber Increases Suppressor of Cytokine Signaling-1 Expression via Toll-like Receptor 2 and Dectin-1 Pathways, Regulating Inflammatory Response in Small Intestinal Epithelial Cells. Mol Nutr Food Res 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schoot, A.; Drysdale, C.; Whelan, K.; Dimidi, E. The Effect of Fiber Supplementation on Chronic Constipation in Adults: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. American Journal of Clinical Nutrition 2022, 116, 953–969. [Google Scholar] [CrossRef]
- Ogata, M.; Van Hung, T.; Tari, H.; Arakawa, T.; Suzuki, T. Dietary Psyllium Fiber Increases Intestinal Heat Shock Protein 25 Expression in Mice. Nutrition Research 2017, 39, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bretin, A.; Yeoh, B.S.; Ngo, V.L.; Reddivari, L.; Pellizzon, M.; Vijay-Kumar, M.; Gewirtz, A.T. Psyllium Fiber Protects Mice against Western Diet-Induced Metabolic Syndrome via the Gut Microbiota-Dependent Mechanism. Gut Microbes 2023, 15, 2221095. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Takemura, N.; Sonoyama, K.; Morita, A.; Kawagishi, H.; Aoe, S.; Morita, T. Small Intestinal Goblet Cell Proliferation Induced by Ingestion of Soluble and Insoluble Dietary Fiber Is Characterized by an Increase in Sialylated Mucins in Rats. Journal of Nutrition 2012, 142, 1429–1436. [Google Scholar] [CrossRef]
- Bretin, A.; Zou, J.; San Yeoh, B.; Ngo, V.L.; Winer, S.; Winer, D.A.; Reddivari, L.; Pellizzon, M.; Walters, W.A.; Patterson, A.D.; et al. Psyllium Fiber Protects Against Colitis Via Activation of Bile Acid Sensor Farnesoid X Receptor. CMGH 2023, 15, 1421–1442. [Google Scholar] [CrossRef]
- France, M.M.; Turner, J.R. The Mucosal Barrier at a Glance. J Cell Sci 2017, 130, 307–314. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Pernitzsch, S.R.; Bäumler, A.J. Healthy Hosts Rule within: Ecological Forces Shaping the Gut Microbiota. Mucosal Immunol 2018, 11, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Fraser, L.M.; Barrón, G.M.; Gologorsky, M.B.; Atkinson, S.N.; Gerrick, E.R.; Hayward, M.; Ziegelbauer, J.; Li, J.A.; Nico, K.F.; et al. Tuft Cells Mediate Commensal Remodeling of the Small Intestinal Antimicrobial Landscape. Proc Natl Acad Sci U S A 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, C.; Sifuentes-Dominguez, L.; Zarek, C.M.; Propheter, D.C.; Kuang, Z.; Wang, Y.; Pendse, M.; Ruhn, K.A.; Hassell, B.; et al. Small Proline-Rich Protein 2A Is a Gut Bactericidal Protein Deployed during Helminth Infection. Science (1979) 2021, 374. [Google Scholar] [CrossRef] [PubMed]
- Propheter, D.C.; Chara, A.L.; Harris, T.A.; Ruhn, K.A.; Hooper, L. V. Resistin-like Molecule β Is a Bactericidal Protein That Promotes Spatial Segregation of the Microbiota and the Colonic Epithelium. Proc Natl Acad Sci U S A 2017, 114, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.S.B.; Morampudi, V.; Chan, J.M.; Bhinder, G.; Lau, J.; Yang, H.; Ma, C.; Huang, T.; Ryz, N.; Sham, H.P.; et al. Goblet Cell Derived RELM-β Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. PLoS Pathog 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of Immune Responses by Tuft Cells. Nat Rev Immunol 2019, 19, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Howitt, M.R.; Lavoie, S.; Michaud, M.; Blum, A.M.; Tran, S. V.; Weinstock, J. V.; Gallini, C.A.; Redding, K.; Margolskee, R.F.; Osborne, L.C.; et al. Tuft Cells, Taste-Chemosensory Cells, Orchestrate Parasite Type 2 Immunity in the Gut. Science 2016, 351, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-Cell-Derived IL-25 Regulates an Intestinal ILC2-Epithelial Response Circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Gerbe, F.; Sidot, E.; Smyth, D.J.; Ohmoto, M.; Matsumoto, I.; Dardalhon, V.; Cesses, P.; Garnier, L.; Pouzolles, M.; Brulin, B.; et al. Intestinal Epithelial Tuft Cells Initiate Type 2 Mucosal Immunity to Helminth Parasites. Nature 2016, 529, 226–230. [Google Scholar] [CrossRef]
- McGinty, J.W.; Ting, H.A.; Billipp, T.E.; Nadjsombati, M.S.; Khan, D.M.; Barrett, N.A.; Liang, H.E.; Matsumoto, I.; von Moltke, J. Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-Helminth Immunity in the Small Intestine but Are Dispensable for Anti-Protist Immunity. Immunity 2020, 52, 528–541.e7. [Google Scholar] [CrossRef]
- Luo, X.C.; Chen, Z.H.; Xue, J.B.; Zhao, D.X.; Lu, C.; Li, Y.H.; Li, S.M.; Du, Y.W.; Liu, Q.; Wang, P.; et al. Infection by the Parasitic Helminth Trichinella Spiralis Activates a Tas2r-Mediated Signaling Pathway in Intestinal Tuft Cells. Proc Natl Acad Sci U S A 2019, 116, 5564–5569. [Google Scholar] [CrossRef] [PubMed]
- Mucke, H.A.M. Drug Repurposing Patent Applications January-March 2020. Assay Drug Dev Technol 2020, 18, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hwang, J.T.; Park, J.H.; Kim, M.S.; Wang, S.; Sung, M.J. Allyl Isothiocyanate Ameliorates Angiogenesis and Inflammation in Dextran Sulfate Sodium-Induced Acute Colitis. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, F.; Dey, B.; Yoshida, Y.; Nishimura, S.; Tabata, S. Bitter Taste Receptor Antagonists Inhibit the Bitter Taste of Canola Meal Extract in Chickens. Journal of Poultry Science 2020, 57, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Prandi, S.; Bromke, M.; Hübner, S.; Voigt, A.; Boehm, U.; Meyerhof, W.; Behrens, M. A Subset of Mouse Colonic Goblet Cells Expresses the Bitter Taste Receptor Tas2r131. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.U.; Gouka, R.J.; Gruppen, H.; Driesse, M.; Van Buren, L.; Smit, G.; Vincken, J.P. 6-Methoxyflavanones as Bitter Taste Receptor Blockers for HTAS2R39. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus Murinus in Mice. Cell Rep 2017, 20, 1513–1524. [Google Scholar] [CrossRef]
- Gao, L.; Huang, Y.; Liu, Y.; Mohamed, O.A.A.; Fan, X.; Wang, L.; Li, L.; Ma, J. Bacterial Community Structure and Potential Microbial Coexistence Mechanism Associated with Three Halophytes Adapting to the Extremely Hypersaline Environment. Microorganisms 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; McDonald, D.; Gonzalez, A.; Navas-Molina, J.A.; Jiang, L.; Xu, Z.Z.; Winker, K.; Kado, D.M.; Orwoll, E.; Manary, M.; et al. Phylogenetic Placement of Exact Amplicon Sequences Improves Associations with Clinical Information. mSystems 2018, 3. [Google Scholar] [CrossRef]
- Stockinger, S.; Albers, T.; Duerr, C.U.; Ménard, S.; Pütsep, K.; Andersson, M.; Hornef, M.W. Interleukin-13-Mediated Paneth Cell Degranulation and Antimicrobial Peptide Release. J Innate Immun 2014, 6, 530–541. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhu, X.; Geng, J.; Xu, Y.; Wu, R.; Li, C.; Fan, D.; Qin, X.; Du, Y.; Tian, Y.; et al. Intestinal Tuft-2 Cells Exert Antimicrobial Immunity via Sensing Bacterial Metabolite N-Undecanoylglycine. Immunity 2022, 55, 686–700.e7. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Legraverend, C.; Jay, P. The Intestinal Epithelium Tuft Cells: Specification and Function. Cellular and Molecular Life Sciences 2012, 69, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Moerings, B.G.J.; van Bergenhenegouwen, J.; Furber, M.; Abbring, S.; Schols, H.A.; Witkamp, R.F.; Govers, C.; Mes, J.J. Dectin-1b Activation by Arabinoxylans Induces Trained Immunity in Human Monocyte-Derived Macrophages. Int J Biol Macromol 2022, 209, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Atagozli, T.; Elliott, D.E.; Ince, M.N. Helminth Lessons in Inflammatory Bowel Diseases (IBD). Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat Rev Gastroenterol Hepatol 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hooper, L. V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; O’Leary, C.E.; von Moltke, J.; Liang, H.E.; Ang, Q.Y.; Turnbaugh, P.J.; Radhakrishnan, S.; Pellizzon, M.; Ma, A.; Locksley, R.M. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 2018, 174, 271–284.e14. [Google Scholar] [CrossRef]
- Nadjsombati, M.S.; McGinty, J.W.; Lyons-Cohen, M.R.; Jaffe, J.B.; DiPeso, L.; Schneider, C.; Miller, C.N.; Pollack, J.L.; Nagana Gowda, G.A.; Fontana, M.F.; et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49, 33–41.e7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




