1. Introduction
People with facial defects are a special category of patients whose complex rehabilitation requires complex technological stages of treatment and further psycho-emotional adaptation. Oncologic diseases, congenital defects, gunshot wounds, specific lesions of the maxillofacial region and suicide attempts all lead to the loss of part of the face. Besides, according to World Health Organization statistics, one child with a facial defect is born every 3 minutes.
As a result of surgical interventions in the maxillofacial area, patients often experience disruption of vital body functions such as breathing, digestion, speech formation, etc., which obviously reduces the overall quality of life and aggravates the psychosomatic status.
The main stage of rehabilitation of such patients is maxillofacial prosthetics. Making a facial epithesis helps to replace facial defects and restore vital functions of the body. Improvement of appearance leads to social adaptation of the patient and normalization of his/her quality of life.
The complex technological process of manufacturing facial epithesis includes several surgical, orthopedic and dental stages. In order to achieve a successful and guaranteed result of treatment, it is necessary to coordinate the work of all participants of the process and comprehensive planning of the entire treatment.
Currently, facial epithesis fabrication is performed according to analog algorithms. At the beginning of the prosthetic phase of rehabilitation, silicone casts are taken from the wound surface, which already causes great discomfort for the patient. The subsequent stages of making a plaster model of the defect, creating and customizing the final prosthesis take a couple of weeks.
Traditional methods of epithesis fabrication utilize platinum-based silicones. When the two components are mixed, the silicone polymerizes, which should result in a flexible and durable structure with a Shore A hardness from 10 to 30.
For digital methods of facial epithesis production, the literature describes three ways in which they can be applied. The first method involves the fabrication of a prototype of the future epithesis from standard photopolymer materials by 3D printing, from which, after fitting, a mold is made for casting a permanent structure using conventional technology. The second method consists in manufacturing a special cuvette using additive technologies based on the modeled negative shape of the prototype prosthesis. And the third method, which is under active development by scientists from different countries, is the direct printing of the final prosthesis. However, the optimal material for manufacturing temporary and permanent facial epithetics by additive technology, which would meet the necessary physical, mechanical and aesthetic properties, has not yet been developed.
The use of digital technology for the fabrication of facial prostheses has always been limited due to the lack of 3D defect modeling software and the shortage of material from which to fabricate a prosthesis with the required physical and mechanical properties. The methods known from the literature for additive manufacturing of facial epithesis are not used and are not available due to the complexity of the manufacturing process itself and the economic inaccessibility of the equipment for everyday dental practice.
Thus, in the postoperative period, the patient is forced to live with a disfigured face until the epithesis is fabricated. There have been known cases of suicidal attempts by such patients who could not withstand the psycho-emotional strain. The quick and quality fabrication of immediate facial prostheses while the patient waits for the final construct is an incompletely understood issue.
The solution of all these problems and the development of a comprehensive solution to the issue of rehabilitation of patients with facial defects using digital technologies formed the purpose of our study.
2. Materials and Methods
3D Software
To achieve the goal, the first task was to develop specialized 3D software for modeling facial defects. The functionality of the program should allow virtual modeling of missing parts of the face (ear, eye, nose, orbit). To create a digital platform together with IT specialists it was decided to use the following programming languages (
Table 1):
The software under development was supposed to integrate 3D models of facial parts (ear, nose, eye, orbit) of different shapes and sizes for the subsequent automatic adaptation of virtual epithesis to the wound surface with the possibility of manual correction of the final virtual model. For this purpose, 287 CT scans 15x15 cm were analyzed, from which 50 studies of patients of different ages, male and female, with intact facial parts, of different shapes and sizes were selected. (
Table 2).
The main ideology of the developed technology was the possibility of making facial epithesis without preliminary obtaining silicone impressions from the wound surface. Writing of the program code was to enable automatic conversion of the patient’s head computed tomography from the DICOM to STL format for the subsequent possibility of modeling virtual epitheses.
3D printing material.
Summarizing the data studied in the literature, we can identify the optimal physical and mechanical properties of 3D-printing material from which facial epithesis can be made:
Tensile strength - with constant use and hygiene of the denture, the tensile strength of the material should correspond to values between 53 and 175 N/cm.
Ultimate tensile strength and percent (maximum) elongation - the engineered structural material should have a tensile strength of 2 to 7MPa and high percent elongation.
Dynamic modulus - the material should have a low dynamic modulus and be resistant to deformation.
Hardness is one of the main properties as the facial epithesis should be similar in hardness to the lost part of the face and should correspond to values between 25 and 40 Shore A values.
Rigidity - to avoid changes in the physical properties of the final epithesis under the influence of temperature, the material must have a low glass transition temperature.
Wettability - assessed by measuring the angle of contact between water and the surface of the final structure. Optimal wettability of the material prevents it from spreading and changing its physical and mechanical properties
Water sorption - the level of water adsorbed in the material should be moderate and epithets should not be subjected to deformation during sterilization.
Optimal specific gravity - for comfortable daily use and denture retention, the material developed should not be heavy.
Based on the study of literature sources and detailed analysis of physical and chemical characteristics of known materials for 3D printing, we have identified the main components that have a direct impact on the physical and mechanical properties of the developed material (
Table 3):
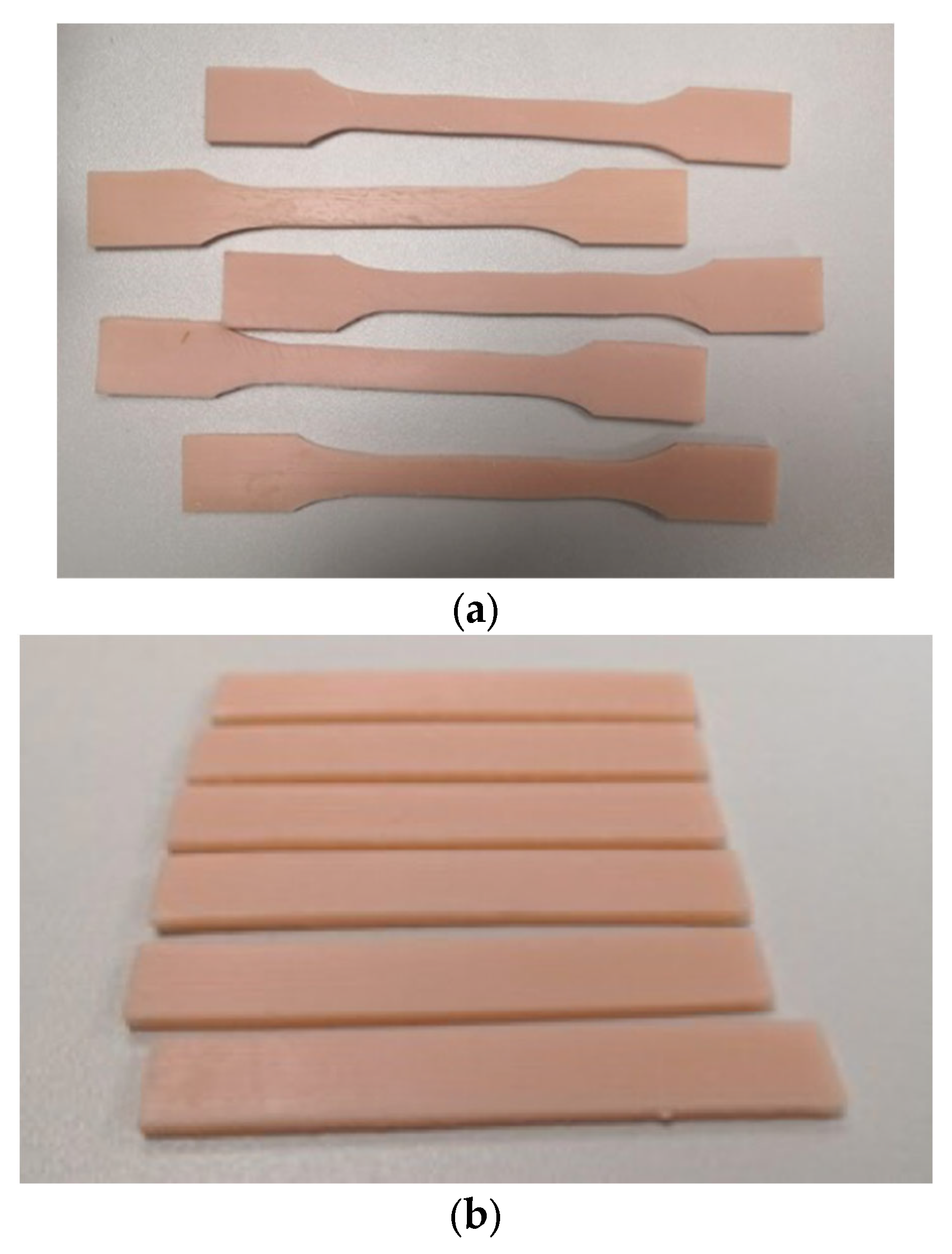
To investigate the physical and mechanical properties of the developed structural material for the manufacture of facial epithesis, two types of specimens were produced by 3D printing - for uniaxial tensile and three-point bending tests. Tensile and three-point bending tests were performed on an Instron 5982 universal testing machine in accordance with the established standards. (
Figure 1)
Modulus of elasticity in bending E, MPa, was calculated by the formula:
where F1 is the load in the area of elastic deformation of the specimen, selected on the rectilinear section of the diagram "load - strain", N;
d - deformation at load F1, mm.
Artificial aging of the samples was carried out in a thermostabilized container Midea 6000 (thermopot). Five samples were made and placed in a thermopot filled with distilled water. The water temperature was maintained at +80 oС. The accuracy of temperature maintenance is – 1 oС.
To assess the biological safety of the developed material, toxicological investigations were carried out using the following methods:
The residual adhesion of microorganisms to the surface of structural material after the photopolymerization process was also evaluated. The adhesion of the following microorganism strains was investigated: : E. coli ATCC 25982; St. aureus ATCC 6538; C. albicans ATCC 10231; St. mutans - 3003.
3. Results
3D software.
Based on the given functional requirements, we have developed three-dimensional software "Phoenix 3D". The uniqueness of our development lies in the complete automation of all processes necessary for the production of facial epithets. The necessity of obtaining silicone impressions from the wound surface of the face is eliminated. To ensure the process of epithesis modeling, it is enough to export the computer tomography of the patient’s head in the format. dicom. Then the process of automatic conversion of the three-dimensional model into the. stl format takes place. (
Figure 2).
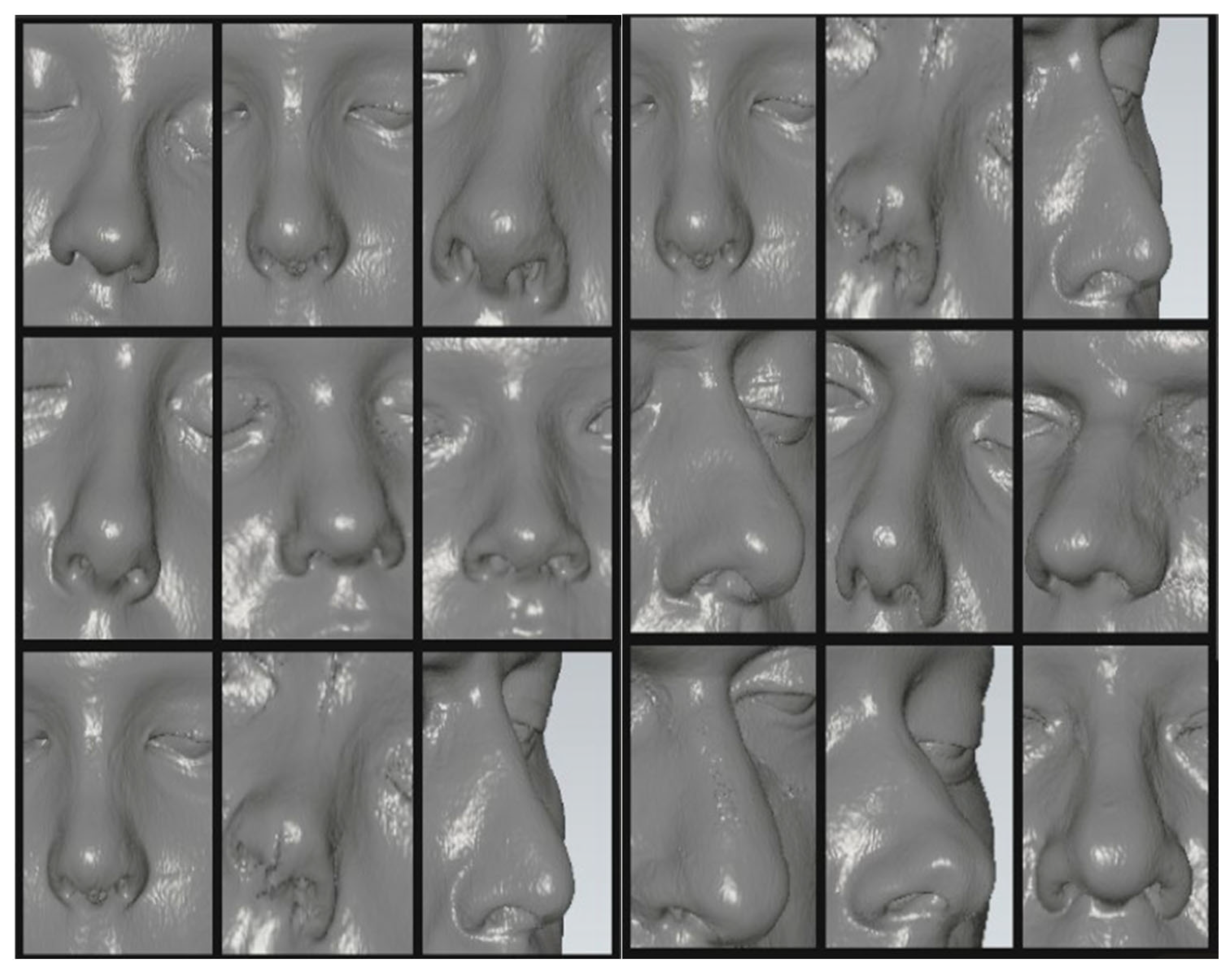
As a result of analyzing 50 CT scans of patients with intact parts of the face, separately were identified the ears, eyes, nose and orbits for creation a virtual digital library and further integration into the Phoenix 3D software. (
Figure 3).
The first step after converting the CT scan into stl format is to mark the defect boundary using the built-in editing tools and select the required facial model from the virtual library. The Phoenix 3D program automatically places the virtual model of the epithesis in the defect area, considering the presence of undercuts and the topography of the prosthetic bed tissues. (
Figure 4).
After positioning the epithesis model in the defect area, specialists have the opportunity to make manual corrections to the design using built-in editing tools. Next, the 3D virtual model of the facial part is exported in stl or .obj format, with subsequent production using additive or subtractive technologies.
Material for 3D printing
Based on the study of literature sources and chemical composition of known polymeric materials, we obtained samples of structural material for the manufacture of facial epithesis. The developed polymer consists of: urethane dimethacrylate, polyethylene glycol dimethacrylate, 2-hydroxyethyl methacrylate and diphenyl (2,4,6-trimethylbenzoyl) phosphinoxide, with the following ratio of components in weight %
urethandimethacrylate - 50,
polyethylene glycol dimethacrylate -41,49,
2-hydroxypropyl methacrylate - 5,
diphenyl (2,4,6-trimethylbenzoyl) phosphinoxide - 3,
titanium dioxide 0.2,
iron oxide pigment brown 0.2,
iron oxide pigment red 0,01,
iron oxide pigment yellow 0.1.
The material was obtained by vigorous mixing on a dissolver of the oligomer and monomer mixture, while heating to at least 60°C with the addition of photoinitiator until homogeneity was achieved. (
Figure 5):
The results of tensile testing of the developed material samples are presented in
Table 4.
h - average thickness of the working (narrow) part of the sample, mm
b - average width of the working (narrow) part of the specimen, mm
A0 - initial cross-section of the sample, mm2
Frr - tensile load at which the specimen failed, N
Frtu - tensile load at reaching the conditional yield point, N
σrr - breaking strength, MPa
σrtu - conditional yield strength, MPa
εrr - relative elongation at break, %
E - tensile modulus of elasticity, MPa
The averaged values of the results of tests on terhpoint bending, are given in
Table 5.
h - thickness of the sample averaged over three measurements, mm
b - specimen width averaged over three measurements, mm
F1 - maximum load at the elastic deformation section of the specimen, N
d - deformation at load F1, mm
E - modulus of elasticity in bending, MPa
The value of the elastic modulus of the material averaged over 6 specimens was calculated - (6.12 ± 1.24) MPa.
As a result of tests of initial samples and samples that underwent artificial aging at 6 and 12 months, it was found that the tensile strength and modulus of elasticity decreases with increasing time of operation and the amount of deformation that the samples withstand decreases.
Measurement of the hardness of the samples by Shore A showed average numerical values within +- 36.
Thus, analyzing the results of the conducted physical and mechanical studies and comparing the obtained numerical values with the known analogues of photopolymer materials, we can conclude that the newly developed material for the fabrication of facial epitheses meets all the necessary strength characteristics.
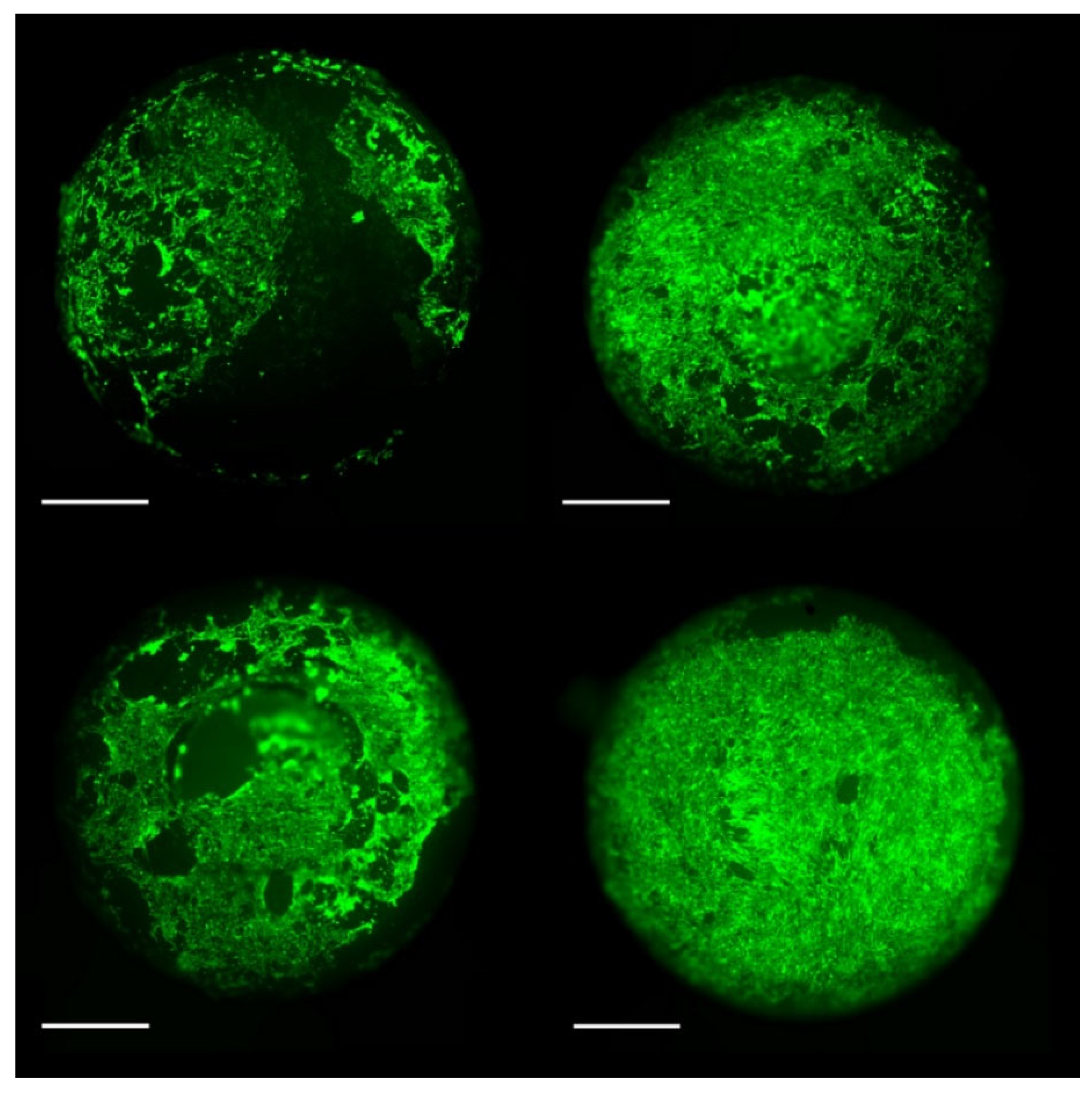
To evaluate the cytological properties of the photopolymer, samples with intact mechanical displacement areas were selected. Then we performed staining with calcein-AM, in which living cells under microscopic magnification have green luminescence and dead cells have red luminescence. No red luminescence of dead cells was detected in the examination of all our samples. (
Figure 6).
The next task was to evaluate the total metabolic activity of the cells using the XTT assay. (
Table 6).
4. Discussion
As a result of all conducted studies of the developed material, the absence of cytotoxic properties was confirmed, which indicates favorable possibilities of photopolymer application in clinical practice.
When conducting microbiological studies and studying the adhesion of strains of microorganisms of conditionally pathogenic group to the presented samples, the following indicators were revealed: (
Table 7).
From the obtained data we can conclude about low adhesion of conditionally pathogenic group of microorganisms to the presented samples.
Сonsidering the physical and mechanical characteristics of the developed material obtained as a result of the conducted complex of researches, the absence of cytotoxic properties, as well as the low level of adhesion of the main opportunistic microorganisms, we can conclude that it is acceptable to use photopolymer material for manufacturing facial immediate prostheses by the method of additive technologies.
Temporary facial prostheses produced using the 3D printing method demonstrate consistent physical and mechanical properties over a period of 12 months, as evidenced by accelerated aging studies. This durability enables their application for up to one year following the initial surgical procedure (
Figure 7).
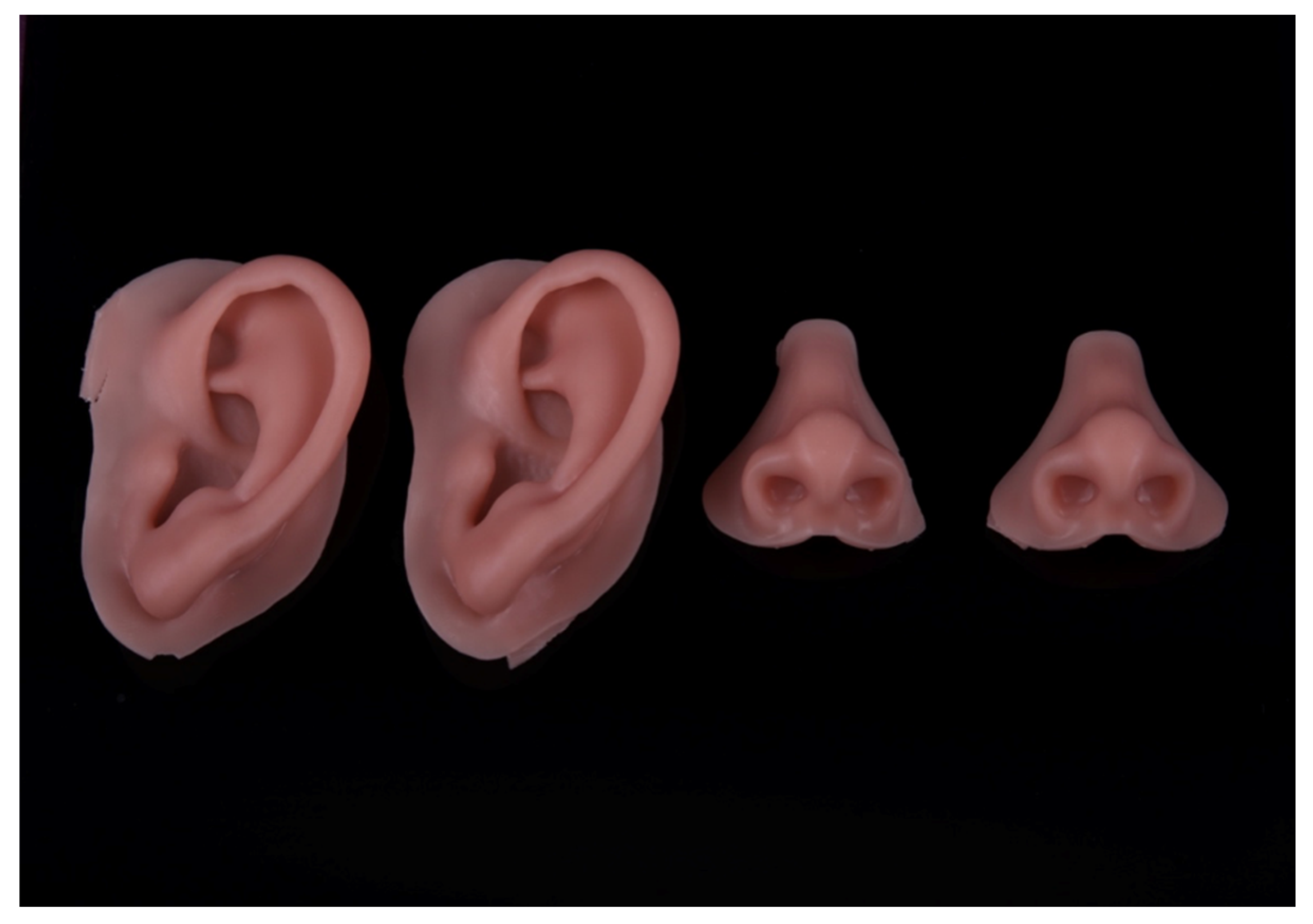
The integration of advanced technology into conventional processes enables the fabrication of intricate facial prostheses, incorporating diverse facial elements. (
Figure 8).
5. Conclusions
The issue of rehabilitation of patients with facial defects is extremely acute. In addition to the annual increase in the number of patients with oncologic diseases of the maxillofacial area, the number of people with shrapnel and gunshot wounds of the face as a result of local wars and conflicts has increased in recent years.
Traditional methods of orthopedic rehabilitation of patients and manufacturing of facial epitheses is a rather complicated and long process. In the postoperative period there is a sharp decrease in the quality of life of this category of patients, violation of the basic functions of the body necessary for vital activity and poor social adaptation.
Direct facial prosthetics in the postoperative period was impossible due to the lack of necessary digital modeling technologies and structural materials for additive or subtractive methods of production. The fabrication of temporary or permanent facial epithesis using digital technologies is an urgent task that can improve the social and functional conditions of patients.
The developed technology of 3D-modeling and additive manufacturing of facial epithesis will allow to produce facial prosthesis directly on the day of surgery. The conducted physical-mechanical, cytotoxic and microbiological studies of the innovative constructive material give grounds to use such a prosthesis for 12 months, until the final epithesis is fabricated, or to fabricate a new one according to the proposed technology.
Author Contributions
Apresyan S.V.- curation of research, development of a program for modeling facial epitheses, preparation and writing of the text of the article; Stepanov A.G.- literature review, collection and analysis of literary sources, development of a program for modeling facial epitheses, preparation and conduct of clinical trials, writing and editing the article; Matelo S.K.- literature review, collection and analysis of literary sources, formation of a database of virtual data, statistical data processing. Kopylov M.K.- lilterature review, collection and analysis of literary sources, formation of a database of virtual data, statistical data processing. Moskovets O.O.- literature review, collection and analysis of literary sources, formation of a database of virtual data, statistical data processing. Sibiryakova A.V.- literature review, collection and analysis of literary sources, formation of a database of virtual data, statistical data processing.
Funding
This research received no external funding.
Data Availability Statement
Data are available on request to the authors.
Acknowledgments
This publication has been supported by the RUDN. University named after Patrice Lumumba, project № 036500-2-000.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antonova, I.N.; Kalakuckij, N.V.; Veselova, K.A.; Kalakuckij, I.N.; Gromova, N.V. Harakteristika proteticheskih materialov dlja protezov lica (obzor). Institut stomatologii. 2019, 1, 94–97, (In Russ.). [Google Scholar]
- Igumnov, A.I.; Apresyan, S.V.; Stepanov, A.G.; Kharazyan, A.E.; Grigoryants, L.S.; Suonio, V.K.; Zrazhevskaya, A.P. Evaluation of clinical effectiveness of hygiene products for the care of facial prostheses. Russ. J. Dent. 2024, 27, 551–560. [Google Scholar] [CrossRef]
- Apresyan, S.V.; Stepanov, A.G.; Retinskaya, M.V.; Suonio, V.K. Development of complex of digital planning of dental treatment and assessment of its clinical effectiveness. Russian Journal of Dentistry. 2020, 24, 135–140. [Google Scholar] [CrossRef]
- Apresyan, S.V.; Stepanov, A.G.; Antonik, M.M.; Degtjarev, N.E.; Kravec, P.L.; Lih- nenko, M.N.; Malazonija, T.T.; Sarkisjan, B.A. Kompleksnoe cifrovoe planirovanie stomatologicheskogo lechenija. M.: Izdatel’stvo Mozartika; 2020. (In Russ.).
- Harazjan AJe, Arutjunov AS, Lebedenko IJu, Arutjunov SD. Jesteticheskoe formirovanie licevogo proteza pri defektah srednej zony lica. Institut sto matologii. 2008;3(40):40‐43. (In Russ.).
- Apresyan, S.V.; Stepanov, A.G.; Suonio, V.K.; Vardanyan, B.A. Manufacture of facial prosthesis by three-dimensional printing. Stomatologiya. 2023, 102, 86–90, (In Russ.). [Google Scholar] [CrossRef]
- D’heygere, V.; Mattheis, S.; Stähr, K.; Bastian, T.; Höing, B.; Lang, S.; Hussain, T. Epithetic nasal reconstruction after total rhinectomy: Oncologic outcomes, immediate and long-term adverse effects, and quality of life. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Livaoğlu, M.; Karacal, N.; Bektaş, D.; Bahadir, O. Reconstruction of full-thickness nasal defect by free anterolateral thigh flap. Acta Otolaryngol. 2009, 129, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Becker, A.M.; Dahlem, K.K.K.; Offergeld, C.; Pfeiffer, J. Aesthetic and functional outcomes in patients with a nasal prosthesis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Chipp, E.; Prinsloo, D.; Rayatt, S. Rhinectomy for the management of nasal malignancies. J. Laryngol. Otol. 2011, 125, 1033–1037. [Google Scholar] [CrossRef]
- Mimica, X.; Yu, Y.; McGill, M.; Barker, C.A.; McBride, S.; Ganly, I.; Cracchiolo, J.R.; Dunn, L.A.; Katabi, N.; Sine, K.; et al. Organ preservation for patients with anterior mucosal squamous cell carcinoma of the nasal cavity: Rhinectomy-free survival in those refusing surgery. Head Neck 2019, 41, 2741–2747. [Google Scholar] [CrossRef]
- Girardi, F.M.; Hauth, L.A.; Abentroth, A.L. Total rhinectomy for nasal carcinomas. Braz. J. Otorhinolaryngol. 2020, 86, 763–766. [Google Scholar] [CrossRef]
- Krakowczyk, Ł.; Szymczyk, C.; Wierzgoń, J.; Oleś, K.; Smyczek, D.; Ulczok, R.; Donocik, K.; Hadasik, G.; Piotrowska, A.; Maciejewski, A. Microvascular nose reconstruction after extended tumor resection. Pol. Przegl. Chir. 2020, 92, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Papaspyrou, G.; Schick, B.; Schneider, M.; Al Kadah, B. Epithetic nasal reconstruction for nasal carcinoma: Retrospective analysis on 22 patients. Eur. Arch. Otorhinolaryngol. 2017, 274, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Shaye, D.A. The history of nasal reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Korfage, A.; Raghoebar, G.M.; Noorda, W.D.; Plaat, B.E.; Vissink, A.; Visser, A. Recommendations for implant-retained nasal prostheses after ablative tumor surgery: Minimal surgical aftercare, high implant survival, and satisfied patients. Head Neck 2016, 38, 619–624. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lünenbürger, H.; Roknic, N.; Klein, M.; Wermker, K. Treatment Outcome of the Transfacial Titanium Epiplating System for Total Nasal Defects. Plast. Reconstr. Surg. 2016, 137, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ribuffo, D.; Serratore, F.; Cigna, E.; Sorvillo, V.; Guerra, M.; Bucher, S.; Scuderi, N. Nasal reconstruction with the two stages vs three stages forehead flap. A three centres experience over ten years. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1866–1872. [Google Scholar] [PubMed]
- Seth, R.; Revenaugh, P.C.; Scharpf, J.; Shipchandler, T.Z.; Fritz, M.A. Free anterolateral thigh fascia lata flap for complex nasal lining defects. JAMA Facial Plast. Surg. 2013, 15, 21–28. [Google Scholar] [CrossRef]
- Agostini, T.; Perello, R.; Russo, G.L.; Spinelli, G. Through-and-through Nasal Reconstruction with the Bi-Pedicled Forehead Flap. Arch. Plast. Surg. 2013, 40, 748–753. [Google Scholar] [CrossRef]
- Paddack, A.C.; Frank, R.W.; Spencer, H.J.; Key, J.M.; Vural, E. Outcomes of paramedian forehead and nasolabial interpolation flaps in nasal reconstruction. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 367–371. [Google Scholar]
- Bolotin, M.V.; Sobolevskiy, V.Y.; Akhundov, A.A.; et al. Microsurgical reconstruction of maxillary defects after limited resections for malignant tumors. Opukholi golovy i shei = Head and Heck Tumors 2021, 11, 18–24. [Google Scholar] [CrossRef]
- Saleh, D.B.; Dearden, A.S.; Smith, J.; Mizen, K.D.; Reid, J.; Eriksen, E.; Fourie, L. Single-stage nasal reconstruction with the islanded forehead flap. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Ethunandan, M.; Downie, I.; Flood, T. Implant-retained nasal prosthesis for reconstruction of large rhinectomy defects: The Salisbury experience. Int. J. Oral Maxillofac. Surg. 2010, 39, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Migliorelli, A.; Sgarzani, R.; Cammaroto, G.; De Vito, A.; Gessaroli, M.; Manuelli, M.; Ciorba, A.; Bianchini, C.; Pelucchi, S.; Meccariello, G. Reconstructive Options after Oncological Rhinectomy: State of the Art. Healthcare (Basel). 2023, 11, 1785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quetz, J.; Ambrosch, P. Total nasal reconstruction: A 6-year experience with the three-stage forehead flap combined with the septal pivot flap. Facial Plast. Surg. 2011, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, T.; Lennon, P.; O’Neill, J.P.; Kinsella, J.; Timon, C. Total rhinectomy, a clinical review of nine cases. Ir. J. Med. Sci. 2016, 185, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Patel, S.; Singh, B.; Wong, R. Jatin Shah’s Head and Neck Surgery and Oncology, 5th ed.; Elsevier Inc.: Philadelphia, PA, USA, 2020. [Google Scholar]
- Becker, C.; Kayser, G.; Pfeiffer, J. Squamous cell cancer of the nasal cavity: New insights and implications for diagnosis and treatment. Head Neck 2016, 38, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Chabrillac, E.; Talawdekar, A.; Garikipati, S.; Varley, I.; Sionis, S.; Beasley, N.; Jackson, R. A single centre’s experience of 23 cases of total rhinectomy for the treatment of squamous cell carcinoma involving the nasal vestibule. Eur. Arch. Otorhinolaryngol. 2022, 279, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C. Treatment of carcinoma of the nasal vestibule by irradiation. Cancer 1976, 38, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Mattheis, S.; Dominas, N.; Hoing, B.; Lang, S.; Stuck, B.A. Regional recurrence in a case series of patients with carcinoma of the nasal cavity—Therapeutic implications. Rhinology 2017, 55, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Faris, C.; Heiser, A.; Quatela, O.; Jackson, M.; Tessler, O.; Jowett, N.; Lee, L.N. Health utility of rhinectomy, surgical nasal reconstruction, and prosthetic rehabilitation. Laryngoscope 2020, 130, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Hosal, S.A.; Aydin, C. Rhinectomy. In Operative Otolaryngology, 3rd ed.; Elsevier Inc.: Philadelphia, PA, USA, 2018; Volume 2, pp. 674–680. [Google Scholar]
- Phillips, T.J. Total nasal reconstruction: A review of the past and present, with a peak into the future. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Cannady, S.B.; Cook, T.A.; Wax, M.K. The total nasal defect and reconstruction. Facial Plast. Surg. Clin. N. Am. 2009, 17, 189–201. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).