Submitted:
25 April 2024
Posted:
26 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
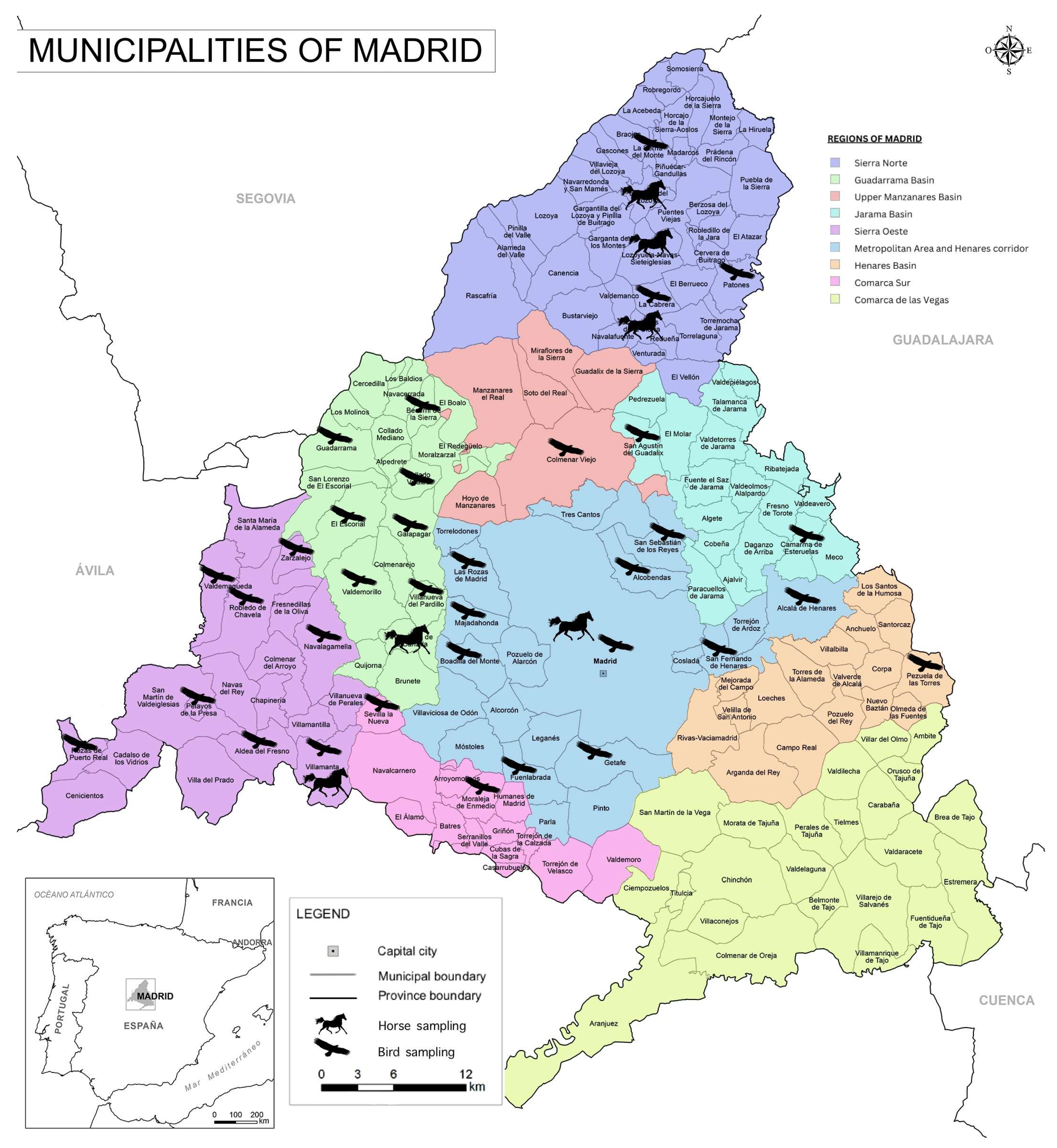
2.1. Samples
2.1.1. Birds
2.1.2. Equines
2.2. Serological Testing
2.3. Statistical Analysis
3. Results
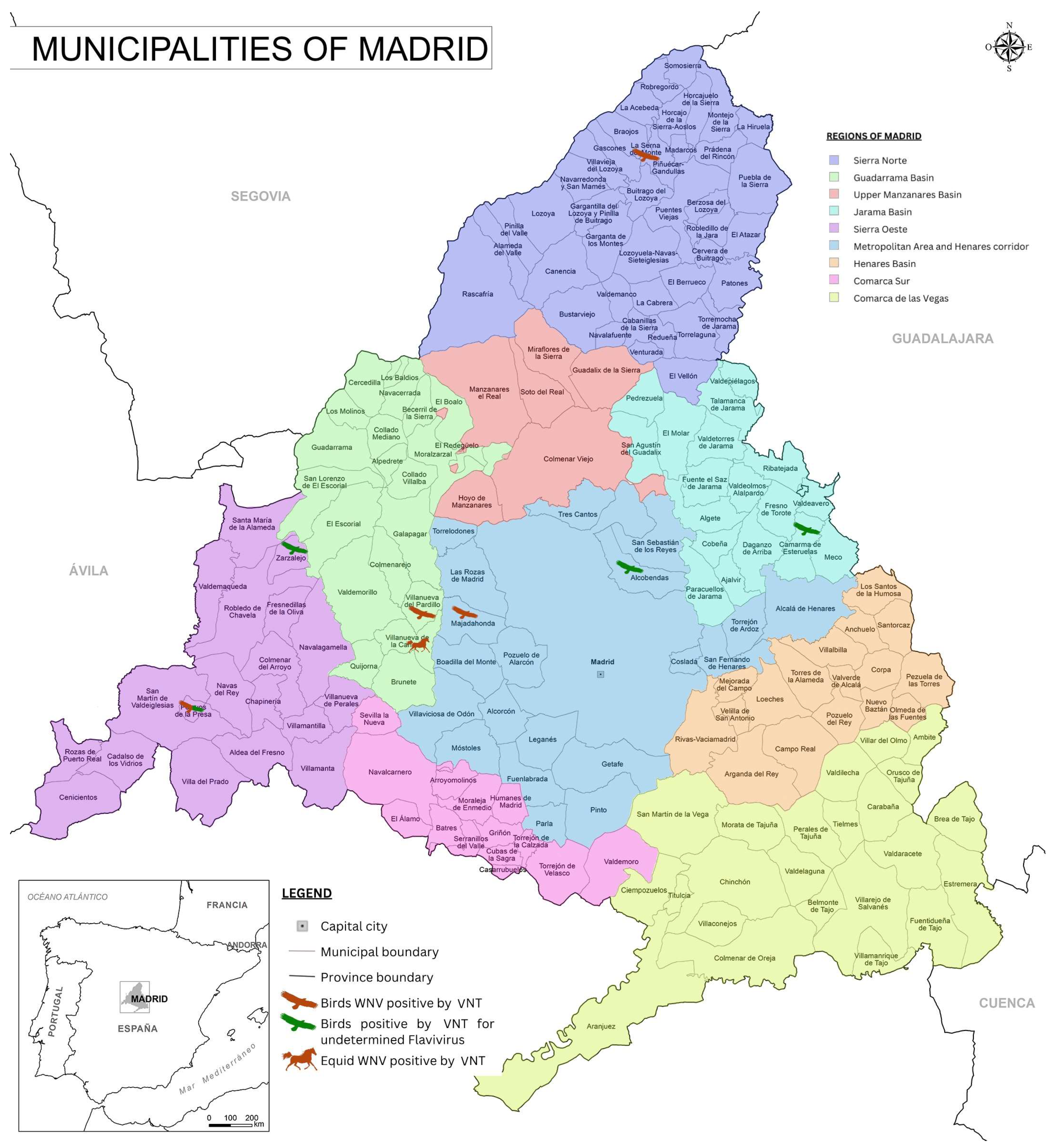
3.1. Birds
3.2. Equids
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Supplementary Materials
References
- Brinton, M.A. Replication Cycle and Molecular Biology of the West Nile Virus. Viruses 2014, 6, 13–53, . [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental Infection of North American Birds with the New York 1999 Strain of West Nile Virus. Emerg. Infect. Dis. 2003, 9, 311–322, . [CrossRef]
- Malkinson, M.; Banet, C.; Weisman, Y.; Pokamunski, S.; King, R.; Drouet, M.T.; Deubel, V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect Dis 2002, 8, 392-397.
- Scherret, J.H.; Poidinger, M.; Mackenzie, J.S.; Broom, A.K.; Deubel, V.; Lipkin, W.I.; Briese, T.; Gould, E.A.; Hall, R.A. The relationships between West Nile and Kunjin viruses. Emerg Infect Dis 2001, 7, 697-705.
- Fall, G.; Di Paola, N.; Faye, M.; Dia, M.; de Melo Freire, C.C.; Loucoubar, C.; de Andrade Zanotto, P.M.; Faye, O.; Sall, A.A. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLOS Negl. Trop. Dis. 2017, 11, e0006078, doi:10.1371/journal.pntd.0006078.
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda 1. Am. J. Trop. Med. Hyg. 1940, s1-20, 471–492, . [CrossRef]
- Joubert, L.; Oudar, J.; Hannoun, C.; Beytout, D.; Corniou, B.; Guillon, J.; Panthier, R. Epidémiologie du virus West Nile: étude d’un foyer en Camargue. IV. La méningo-encéphalomyélite du cheval. Ann Inst Pasteur (Paris) 1970, 118, 239-247.
- Filipe, A.R.; De Andrade, H.R. Arboviruses in the Iberian Peninsula.. 1990, 34, 582–91.
- El Harrack, M.; Le Guenno, B.; Gounon, P. Isolement du virus West Nile au Maroc. Virologie 1997, 1, 248-249.
- Kaptoul, D.; Viladrich, P.F.; Domingo, C.; Niubó, J.; Martínez-Yélamos, S.; De Ory, F.; Tenorio, A. West Nile virus in Spain: Report of the first diagnosed case (in Spain) in a human with aseptic meningitis. Scand. J. Infect. Dis. 2007, 39, 70–71, . [CrossRef]
- Figuerola, J.; Soriguer, R.; Rojo, G.; Tejedor, C.G.; Jimenez-Clavero, M.A. Seroconversion in Wild Birds and Local Circulation of West Nile Virus, Spain. Emerg. Infect. Dis. 2007, 13, 1915–1917, . [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). West Nile virus - infections among humans and outbreaks among equids and/or birds, 13 December 2023. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc (accessed on 22nd January 2024).
- Magallanes, S.; Llorente, F.; Ruiz-López, M.J.; la Puente, J.M.-D.; Soriguer, R.; Calderon, J.; Jímenez-Clavero, M..; Aguilera-Sepúlveda, P.; Figuerola, J. Long-term serological surveillance for West Nile and Usutu virus in horses in south-West Spain. One Heal. 2023, 17, 100578, . [CrossRef]
- Abad-Cobo, A.; Llorente, F.; Barbero, M.d.C.; Cruz-López, F.; Forés, P.; Jiménez-Clavero, M.. Serosurvey Reveals Exposure to West Nile Virus in Asymptomatic Horse Populations in Central Spain Prior to Recent Disease Foci. Transbound. Emerg. Dis. 2017, 64, 1387–1392, . [CrossRef]
- Williams, R.A.J.; Sánchez-Llatas, C.J.; Doménech, A.; Madrid, R.; Fandiño, S.; Cea-Callejo, P.; Gomez-Lucia, E.; Benítez, L. Emerging and Novel Viruses in Passerine Birds. Microorganisms 2023, 11, 2355, . [CrossRef]
- Jiménez-Clavero, M.A.; Sotelo, E.; Fernandez-Pinero, J.; Llorente, F.; Blanco, J.M.; Rodriguez-Ramos, J.; Perez-Ramirez, E.; Höfle, U. West Nile Virus in Golden Eagles, Spain, 2007. Emerg. Infect. Dis. 2008, 14, 1489–1491, . [CrossRef]
- Marzal, A.; Ferraguti, M.; Muriel, J.; Magallanes, S.; Ortiz, J.; García-Longoria, L.; Bravo-Barriga, D.; Guerrero-Carvajal, F.; Aguilera-Sepúlveda, P.; Llorente, F.; et al. Circulation of zoonotic flaviviruses in wild passerine birds in Western Spain. Veter- Microbiol. 2022, 268, 109399, . [CrossRef]
- López, G.; Jiménez-Clavero, M..; Vázquez, A.; Soriguer, R.; Gómez-Tejedor, C.; Tenorio, A.; Figuerola, J. Incidence of West Nile Virus in Birds Arriving in Wildlife Rehabilitation Centers in Southern Spain. Vector-Borne Zoonotic Dis. 2011, 11, 285–290, . [CrossRef]
- Llorente, F.; Pérez-Ramírez, E.; Fernández-Pinero, J.; Soriguer, R.; Figuerola, J.; Jiménez-Clavero, M.. Flaviviruses in Game Birds, Southern Spain, 2011–2012. Emerg. Infect. Dis. 2013, 19, 1023–1025, . [CrossRef]
- Zehender, G.; Veo, C.; Ebranati, E.; Carta, V.; Rovida, F.; Percivalle, E.; Moreno, A.; Lelli, D.; Calzolari, M.; Lavazza, A.; et al. Reconstructing the recent West Nile virus lineage 2 epidemic in Europe and Italy using discrete and continuous phylogeography. PLOS ONE 2017, 12, e0179679–e0179679, . [CrossRef]
- Lu, L.; Zhang, F.; Munnink, B.B.O.; Munger, E.; Sikkema, R.S.; Pappa, S.; Tsioka, K.; Sinigaglia, A.; Molin, E.D.; Shih, B.B.; et al. West Nile virus spread in Europe: Phylogeographic pattern analysis and key drivers. PLOS Pathog. 2024, 20, e1011880, . [CrossRef]
- Sotelo, E.; Fernández-Pinero, J.; Llorente, F.; Vázquez, A.; Moreno, A.; Agüero, M.; Cordioli, P.; Tenorio, A.; Jiménez-Clavero, M.Á. Phylogenetic relationships of Western Mediterranean West Nile virus strains (1996–2010) using full-length genome sequences: single or multiple introductions? J Gen Virol 2011, 92, 2512-2522.
- Rodríguez-Alarcón, L.G.S.M.; Fernández-Martínez, B.; Moros, M.J.S.; Vázquez, A.; Pachés, P.J.; Villacieros, E.G.; Martín, M.B.G.; Borras, J.F.; Lorusso, N.; Aceitero, J.M.R.; et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Eurosurveillance 2021, 26, 2002010, . [CrossRef]
- Ruiz-López, M.J.; Aguilera-Sepúlveda, P.; Cebrián-Camisón, S.; Figuerola, J.; Magallanes, S.; Varona, S.; Cuesta, I.; Cano-Gómez, C.; Sánchez-Mora, P.; Camacho, J.; et al. Re-Emergence of a West Nile Virus (WNV) Variant in South Spain with Rapid Spread Capacity. Viruses 2023, 15, 2372, . [CrossRef]
- Busquets, N.; Laranjo-González, M.; Soler, M.; Nicolás, O.; Rivas, R.; Talavera, S.; Villalba, R.; Miguel, E.S.; Torner, N.; Aranda, C.; et al. Detection of West Nile virus lineage 2 in North-Eastern Spain (Catalonia). Transbound. Emerg. Dis. 2019, 66, 617–621, . [CrossRef]
- Aguilera-Sepúlveda, P.; Napp, S.; Llorente, F.; Solano-Manrique, C.; Molina-López, R.; Obón, E.; Solé, A.; Jiménez-Clavero, M..; Fernández-Pinero, J.; Busquets, N. West Nile Virus Lineage 2 Spreads Westwards in Europe and Overwinters in North-Eastern Spain (2017–2020). Viruses 2022, 14, 569, . [CrossRef]
- Ministerio de Sanidad. Dirección General de Salud Pública. Centro de Coordinación de Alertas y Emergencias Sanitarias. Meningoencefalitis por el virus del Nilo occidental en España 2021. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/docs/20210902_ERR_Nilo_Occidental.pdf (accessed on 5th April 2024).
- European Centre for Disease Prevention and Control (ECDC). Historical data by year - West Nile virus seasonal surveillance. 2023. Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical (accessed on 22nd January 2024).
- Llorente, F.; García-Irazábal, A.; Pérez-Ramírez, E.; Cano-Gómez, C.; Sarasa, M.; Vázquez, A.; Jiménez-Clavero, M.Á. Influence of flavivirus co-circulation in serological diagnostics and surveillance: a model of study using West Nile, Usutu and Bagaza viruses. Transbound Emerg Dis 2019, 66, 2100-2106.
- Jurado-Tarifa, E.; Napp, S.; Lecollinet, S.; Arenas, A.; Beck, C.; Cerdà-Cuéllar, M.; Fernández-Morente, M.; García-Bocanegra, I. Monitoring of West Nile virus, Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 58–64, . [CrossRef]
- Höfle, U.; Gamino, V.; de Mera, I.G.F.; Mangold, A.J.; Ortíz, J.-A.; de la Fuente, J. Usutu Virus in Migratory Song Thrushes, Spain. Emerg. Infect. Dis. 2013, 19, 1173–1175, . [CrossRef]
- Napp, S.; Llorente, F.; Beck, C.; Jose-Cunilleras, E.; Soler, M.; Pailler-García, L.; Amaral, R.; Aguilera-Sepúlveda, P.; Pifarré, M.; Molina-López, R.; et al. Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019. Viruses 2021, 13, 2404, . [CrossRef]
- Agüero, M.; Fernandez-Pinero, J.; Buitrago, D.; Sanchez, A.; Elizalde, M.; Miguel, E.S.; Villalba, R.; Llorente, F.; Jimenez-Clavero, M.A. Bagaza virus in partridges and pheasants, Spain, 2010. Emerg Infect Dis 2011, 17, 1498-1501.
- Arnal, A.; Gómez-Díaz, E.; Cerdà-Cuéllar, M.; Lecollinet, S.; Pearce-Duvet, J.; Busquets, N.; García-Bocanegra, I.; Pagès, N.; Vittecoq, M.; Hammouda, A.; et al. Circulation of a Meaban-Like Virus in Yellow-Legged Gulls and Seabird Ticks in the Western Mediterranean Basin. PLOS ONE 2014, 9, e89601, . [CrossRef]
- Mansfield, K.L.; de la Fuente, J.; Ayllón, N.; de Mera, I.G.F.; Johnson, N.; Gortázar, C.; Alberdi, P.; Marín, J.F.G.; Morales, A.B.; Fooks, A.R.; et al. Identification and characterization of a novel tick-borne flavivirus subtype in goats (Capra hircus) in Spain. J. Gen. Virol. 2015, 96, 1676–1681, . [CrossRef]
- López-Albors, O.; Gil, F.; Vázquez, J.M.; Latorre, R.; Ramíre-Zarzosa, G.; Orenes, M.; Moreno, F. Revisión: nomenclatura e iconografía de las partes de la pluma y sus diferentes tipos. Anal Vet Murcia 1999, 15, 3-16.
- Svensson, L.; Mullarney, K. Guía de Aves de España y Europa; Ediciones Omega, 2023.
- Gómez-Catasús, J.; Carrascal, L.M.; Moraleda, V.; Colsa, J.; Garcés, F.; Schuster, C. Factors Affecting Differential Underestimates of Bird Collision Fatalities at Electric Lines: A Case Study in the Canary Islands. Ardeola 2020, 68, 71–94, . [CrossRef]
- Bravo-Barriga, D.; Aguilera-Sepúlveda, P.; Guerrero-Carvajal, F.; Llorente, F.; Reina, D.; Pérez-Martín, J.E.; Jiménez-Clavero, M..; Frontera, E. West Nile and Usutu virus infections in wild birds admitted to rehabilitation centres in Extremadura, western Spain, 2017–2019. Veter- Microbiol. 2021, 255, 109020, . [CrossRef]
- Pallari, C.T.; Efstathiou, A.; Moysi, M.; Papanikolas, N.; Christodoulou, V.; Mazeris, A.; Koliou, M.; Kirschel, A.N. Evidence of West Nile virus seropositivity in wild birds on the island of Cyprus. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101592, . [CrossRef]
- Chevalier, V.; Reynaud, P.; Lefrançois, T.; Durand, B.; Baillon, F.; Balança, G.; Gaidet, N.; Mondet, B.; Lancelot, R. Predicting West Nile Virus Seroprevalence in Wild Birds in Senegal. Vector-Borne Zoonotic Dis. 2009, 9, 589–596, . [CrossRef]
- Beveroth, T.A.; Ringia, A.M.; Lampman, R.L.; Novak, R.J.; Ward, M.P. CHANGES IN SEROPREVALENCE OF WEST NILE VIRUS ACROSS ILLINOIS IN FREE-RANGING BIRDS FROM 2001 THROUGH 2004. Am. J. Trop. Med. Hyg. 2006, 74, 174–179, . [CrossRef]
- Islam, A.; Islam, S.; Hossain, M.E.; Ferdous, J.; Abedin, J.; Rahman, M.Z.; Rahman, K.; Hoque, A.; Hassan, M.M. Serological Evidence of West Nile Virus in Wild Birds in Bangladesh. Veter- Sci. 2020, 7, 164, . [CrossRef]
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Lecollinet, S.; Pancewicz, S.A.; Kozdruń, W.; Czekaj, H. Occurrence of West Nile Virus Antibodies in Wild Birds, Horses, and Humans in Poland. BioMed Res. Int. 2015, 2015, 1–5, . [CrossRef]
- Amin, M.; Zaim, M.; Edalat, H.; Basseri, H.R.; Yaghoobi-Ershadi, M.R.; Rezaei, F.; Azizi, K.; SalehiVaziri, M.; Ghane, M.; Yousefi, S.; et al. Seroprevalence Study on West Nile Virus (WNV) Infection, a Hidden Viral Disease in Fars Province, Southern Iran. J. Arthropod-Borne Dis. 2020, 14, 173–184, . [CrossRef]
- Paştiu, A.I.; Pap, P.L.; Vágási, C.I.; Niculae, M.; Páll, E.; Domşa, C.; Brudaşcă, F.G.; Spînu, M. Wild Birds in Romania Are More Exposed to West Nile Virus Than to Newcastle Disease Virus. Vector-Borne Zoonotic Dis. 2016, 16, 176–180, . [CrossRef]
- Ferraguti, M.; LA Puente, J.M.-D.; Soriguer, R.; Llorente, F.; Jiménez-Clavero, M..; Figuerola, J. West Nile virus-neutralizing antibodies in wild birds from southern Spain. Epidemiology Infect. 2016, 144, 1907–1911, . [CrossRef]
- Zakhia, R.; Dupuis, A.P.; Khodr, F.; Fadel, M.; Kramer, L.D.; Haddad, N. Evidence of West Nile Virus Circulation in Lebanon. Viruses 2021, 13, 994, . [CrossRef]
- Benjelloun, A.; El Harrak, M.; Calistri, P.; Loutfi, C.; Kabbaj, H.; Conte, A.; Ippoliti, C.; Danzetta, M.L.; Belkadi, B. Seroprevalence of West Nile virus in horses in different Moroccan regions. Vet Med Sci 2017, 3, 198-207.
- Sule, W.F.; Oluwayelu, D.O.; Adedokun, R.A.M.; Rufai, N.; McCracken, F.; Mansfield, K.L.; Johnson, N. High Seroprevelance of West Nile Virus Antibodies Observed in Horses from Southwestern Nigeria. Vector-Borne Zoonotic Dis. 2015, 15, 218–220, . [CrossRef]
- Aquila a-Life. Informe anual de seguimiento de aves liberadas en Madrid 2022. Available online: https://aquila-a-life.org/index.php/es/avances/acciones/monitorizacion/62-acciones-d/476-accion-d1-informe-anual-de-seguimiento-de-aves-liberadas-en-madrid-2022 (accessed on 10 November 2023).
- Instituto Nacional de Estadística. List of place name: Population of the Continuous Municipal Register by Population Unit: Alcobendas. 2024. Available online: https://www.ine.es/en/index.htm (accessed on 25 January 2024).
- Baitchman, E.J.; Tlusty, M.F.; Murphy, H.W. PASSIVE TRANSFER OF MATERNAL ANTIBODIES TO WEST NILE VIRUS IN FLAMINGO CHICKS (PHOENICOPTERUS CHILENSIS AND PHOENICOPTERUS RUBER RUBER). J. Zoo Wildl. Med. 2007, 38, 337–340, . [CrossRef]
- Nemeth, N.M.; Hahn, D.C.; Gould, D.H.; Bowen, R.A. Experimental West Nile Virus Infection in Eastern Screech Owls (Megascops asio). Avian Dis. 2006, 50, 252–258, . [CrossRef]
- Millins, C.; Reid, A.; Curry, P.; Drebot, M.A.; Andonova, M.; Buck, P.; Leighton, F.A. Evaluating the Use of House Sparrow Nestlings as Sentinels for West Nile Virus in Saskatchewan. Vector-Borne Zoonotic Dis. 2011, 11, 53–58, . [CrossRef]
- Nemeth, N.M.; Bowen, R.A. DYNAMICS OF PASSIVE IMMUNITY TO WEST NILE VIRUS IN DOMESTIC CHICKENS (GALLUS GALLUS DOMESTICUS). Am. J. Trop. Med. Hyg. 2007, 76, 310–317, . [CrossRef]
- Xirouchakis, S.M. Breeding biology and reproductive performance of Griffon Vultures Gyps fulvus on the island of Crete (Greece). Bird Study 2010, 57, 213–225, . [CrossRef]
- BirdLife International. IUCN Red List for birds. 2023. Available online: http://datazone.birdlife.org (accessed on 27th October 2023).
- SEO/Birdlife. La Guía de Aves de España. 2023. Available online: https://seo.org/guia-de-aves/ (accessed on 15 November 2023).
- Figuerola, J.; Jiménez-Clavero, M.A.; López, G.; Rubio, C.; Soriguer, R.; Gómez-Tejedor, C.; Tenorio, A. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Veter- Microbiol. 2008, 132, 39–46, . [CrossRef]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M..; Johnson, N. The Role of Birds of Prey in West Nile Virus Epidemiology. Vaccines 2020, 8, 550, . [CrossRef]
- Castillo-Olivares, J.; Mansfield, K.L.; Phipps, L.P.; Johnson, N.; Tearle, J.; Fooks, A.R. Antibody Response in Horses Following Experimental Infection with West Nile Virus Lineages 1 and 2. Transbound. Emerg. Dis. 2011, 58, 206–212, . [CrossRef]
- Jimenez-Clavero, M.A.; Llorente, F.; Sotelo, E.; Soriguer, R.; Gomez-Tejedor, C.; Figuerola, J. West Nile virus serosurveillance in horses in Doñana, Spain, 2005 to 2008. Vet Rec 2010, 167, 379-380.
- European Centre for Disease Prevention and Control. Culex pipiens - Factsheet for experts. 2020. Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/related-public-health-topics/disease-vectors/facts/mosquito-factsheets/culex-pipiens (accessed on 22nd January 2024).
- Höfle, U.; Blanco, J.M.; Crespo, E.; Naranjo, V.; Jiménez-Clavero, M.A.; Sanchez, A.; de la Fuente, J.; Gortazar, C. West Nile virus in the endangered Spanish imperial eagle. Veter- Microbiol. 2008, 129, 171–178, . [CrossRef]
- Aguilera-Sepúlveda, P.; Gómez-Martín, B.; Agüero, M.; Jiménez-Clavero, M..; Fernández-Pinero, J. A new cluster of West Nile virus lineage 1 isolated from a northern goshawk in Spain. Transbound. Emerg. Dis. 2021, 69, 3121–3127, . [CrossRef]
- Alba, A.; Allepuz, A.; Napp, S.; Soler, M.; Selga, I.; Aranda, C.; Casal, J.; Pages, N.; Hayes, E.B.; Busquets, N. Ecological Surveillance for West Nile in Catalonia (Spain), Learning from a Five-Year Period of Follow-up. Zoonoses Public Heal. 2013, 61, 181–191, . [CrossRef]
- Cano-Terriza, D.; Guerra, R.; Lecollinet, S.; Cerdà-Cuéllar, M.; Cabezón, O.; Almería, S.; García-Bocanegra, I. Epidemiological survey of zoonotic pathogens in feral pigeons (Columba livia var. domestica) and sympatric zoo species in Southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2015, 43, 22–27, . [CrossRef]
- Figuerola, J.; Jiménez-Clavero, M.A.; Rojo, G.; Gómez-Tejedor, C.; Soriguer, R. Prevalence of West Nile virus neutralizing antibodies in colonial aquatic birds in southern Spain. Avian Pathol. 2007, 36, 209–212, . [CrossRef]
- Gangoso, L.; Grande, J.M.; Llorente, F.; Jiménez-Clavero, M.Á.; Pérez, J.M.; Figuerola, J. Prevalence of neutralizing antibodies to West Nile virus in Eleonora's Falcons in the Canary Islands. J Wildlife Dis 2010, 46, 1321-1324.
- García-Bocanegra, I.; Busquets, N.; Napp, S.; Alba, A.; Zorrilla, I.; Villalba, R.; Arenas, A. Serosurvey of West Nile Virus and Other Flaviviruses of the Japanese Encephalitis Antigenic Complex in Birds from Andalusia, Southern Spain. Vector-Borne Zoonotic Dis. 2011, 11, 1107–1113, . [CrossRef]
- García-Bocanegra, I.; Franco, J.J.; León, C.I.; Barbero-Moyano, J.; García-Miña, M.V.; Fernández-Molera, V.; Gómez, M.B.; Cano-Terriza, D.; Gonzálvez, M. High exposure of West Nile virus in equid and wild bird populations in Spain following the epidemic outbreak in 2020. Transbound. Emerg. Dis. 2022, 69, 3624–3636, . [CrossRef]
- López, G.; Jiménez-Clavero, M..; Tejedor, C.G.; Soriguer, R.; Figuerola, J. Prevalence of West Nile Virus Neutralizing Antibodies in Spain Is Related to the Behavior of Migratory Birds. Vector-Borne Zoonotic Dis. 2008, 8, 615–622, . [CrossRef]
- la Puente, J.M.-D.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M..; Soriguer, R.; Figuerola, J. Mosquito community influences West Nile virus seroprevalence in wild birds: implications for the risk of spillover into human populations. Sci. Rep. 2018, 8, 1–7, . [CrossRef]
- Napp, S.; Montalvo, T.; Piñol-Baena, C.; Gómez-Martín, M.B.; Nicolás-Francisco, O.; Soler, M.; Busquets, N. Usefulness of Eurasian Magpies (Pica pica) for West Nile virus Surveillance in Non-Endemic and Endemic Situations. Viruses 2019, 11, 716, . [CrossRef]
| cELISA | VNT | ||||||||||
| Order | Family | Species | Common name | N | Pos | Doubt. | Neg | N | Undet. Flavivirus | WNV | Neg |
| BIRDS | |||||||||||
| Accipitriformes | Accipitridae | Aquila adalberti | Spanish imperial eagle | 3 | 3 | ||||||
| Aquila chrysaetos | Golden eagle | 2 | 2 | ||||||||
| Aquila fasciata | Bonelli's eagle | 10 | 4 | 1 | 5 | 5 | 2 | 1 | 2 | ||
| Gyps fulvus | Griffon vulture | 9 | 1 | 5 | 3 | 6 | 1 | 5 | |||
| Circaetus gallicus | Short-toed snake eagle | 1 | 1 | 0 | 1 | 1 | 0 | ||||
| Milvus migrans | Black kite | 5 | 3 | 1 | 1 | 3 | 1 | 2 | |||
| Milvus milvus | Red kite | 17 | 3 | 14 | 3 | 3 | |||||
| Pernis apivorus | European honey buzzard | 1 | 1 | 0 | 1 | 1 | 0 | ||||
| Charadriiformes | Laridae | Larus fuscus | Lesser black-backed gull | 1 | 1 | ||||||
| Ciconiiformes | Ciconiidae | Ciconia ciconia | White stork | 95 | 3 | 3 | 89 | 6 | 1 | 5 | |
| Ciconia nigra | Black stork | 3 | 3 | ||||||||
| Falconiformes | Falconidae | Falco peregrinus | Peregrine falcon | 4 | 4 | ||||||
| Falco tinnunculus | Common kestrel | 1 | 1 | ||||||||
| Otidiformes | Otididae | Otis tarda | Great Bustard | 1 | 1 | ||||||
| Pelecaniformes | Ardeidae | Ardea cinea | Grey heron | 1 | 1 | ||||||
| Strigiformes | Strigidae | Bubo bubo | Eurasian eagle owl | 4 | 1 | 3 | 1 | 1 | |||
| Suliformes | Phalacrocoracidae | Phalacrocorax carbo | Great cormorant | 1 | 1 | 0 | 1 | 1 | |||
| 158 | 13 | 15 | 131 | 27 | 4 | 4 | 19 | ||||
| EQUINES | |||||||||||
| Perissodactyla | Equidae | Equus caballus | Horse | 23 | 1 | 1 | 21 | 2 | 1 | 0 | |
| Equus asinus | Donkey | 2 | 2 | ||||||||
| cELISA | VNT | ||||||
| Common name | Competition % | Result | WNV | USUV | BAGV | Result | |
| BIRDS | |||||||
| Bonelli's eagle | 48.54 | Positive | 1:10 | 1:20 | <1:10 | Undetermined flavivirus | |
| 73.25 | Positive | 1:10 | <1:10 | <1:10 | Undetermined flavivirus | ||
| 87.2 | Positive | 1:20 | 1:5 | 1.5 | WNV | ||
| Griffon vulture | 35.17 | Doubtful | 1:10 | <1:10 | <1:20 | Undetermined flavivirus | |
| European honey buzzard | 90.98 | Positive | 1:40 | 1:10 | <1:10 | WNV | |
| Black kite | 91.27 | Positive | 1:160 | 1:40 | <1:10 | WNV | |
| White stork | 50.54 | Positive | 1:10 | <1:10 | <1:10 | Undetermined flavivirus | |
| Short-toed snake eagle | No data | Positive | 1:80 | 1:10 | <1:10 | WNV | |
| EQUINES | |||||||
| Horse | 94.74 | Positive | 1:320 | 1:20 | <1:10 | WNV | |
| Common name | VNT Result | Age | Migratory status | Sex | Location | Zone |
|---|---|---|---|---|---|---|
| Bonelli's eagle | Undet. flavivirus | Adult | R | Male | Zarzalejo | Sierra Oeste |
| Undet. flavivirus | Adult | R | Male | Pelayos de la Presa | Sierra Oeste | |
| WNV | Adult | R | Female | Pelayos de la Presa | Sierra Oeste | |
| Griffon vulture | Undet. flavivirus | Fledgling | (R) | Indetermined | Camarma de Esteruelas | Jarama Basin |
| European honey buzzard | WNV | Adult | M | Indetermined | La Serna del Monte | Sierra Norte |
| Black kite | WNV | Adult | M | Female | Majadahonda | Metropolitan area |
| White stork | Undet. flavivirus | Nestling | (M) | Indetermined | Alcobendas | Metropolitan area |
| Short-toed snake eagle | WNV | Adult | M | Male | Villanueva del Pardillo | Guadarrama Basin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





