1. Introduction
The intertwining interaction of two emerging pollutants, namely radionuclides and microplastics, within water systems, seawater, and wastewater poses a significant challenge to the sustainable management of aqueous systems, thus research on their occurrence in the environment as well as how they behave in artificial and natural systems in media including water, seawater, and wastewater should be given the highest priority. Radionuclides, stemming from nuclear activities and events, introduce radioactive isotopes into water bodies, presenting potential hazards to both ecosystems and human health [
1]. Concurrently, microplastics, minute plastic particles prevalent in water due to waste and industrial activities, compound the pollution issue, while their persistence and ability to accumulate in marine life and subsequently enter the human food chain highlight the urgency to mitigate their spread [
2]. The sustainable water management principles advocate for a systemic approach to minimize waste and maximize resource efficiency, emphasizing the importance of managing these pollutants through integrated strategies [
1]. Addressing the coexistence of radionuclides and microplastics, as well as the adsorption and transport of radionuclides by microplastics, demands innovative waste management techniques, improved water treatment technologies, and a transition towards eco-friendly materials to ensure the sustainable management of water resources and the protection of marine ecosystems. Striking a balance in tackling these pollutants aligns with long-term sustainability, aiming for a harmonious relationship between human activities and the environment.
Heavy metals are regarded as environmental contaminants because of their toxic effects, persistence, and biological accumulation in both soil and water systems [
3]. A number of radioactive metals, including uranium (U), and Americium (Am), which belong also in this category, are of particular concern [
4,
5]. Various studies report that microplastics (MPs) are capable of adsorbing heavy metals ions including radionuclides in aqueous systems [
1,
6]. Due to their toxicity and the prolonged half-lives of some actinides, contaminated waters with actinide levels above those advised by the World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA) may result in major health issues [
7,
8]. The accumulation of toxic radioactive metals may endanger the health of living organisms, including humans, thus it is mandatory to measure and monitor the levels of radionuclides in the geosphere, as well as to study their migration into MPs, since the latter can serve as a transfer medium into organisms.
1.1. Uranium and Americium Characteristics and Occurrence
Uranium is a natural element, which exists in the form of three radio-isotopes: uranium-238, uranium-235, and uranium-234 [
1]. Due to their modest radioactivity, there is only a little amount of background radiation in the natural world, with uranium-238 making more than 99% of the naturally existing element, whereas uranium-234 is present in very small quantities (less than 1%) and belongs to the uranium-238 decay series. In aqueous solutions uranium may exist in four distinct oxidation states (e.g., III, IV, V, and VI), with IV and VI oxidation states being the prevalent forms in environmental systems. Uranium can be found in waters, soils, and rocks, with the latter disintegrating into tiny fragments and colloidal particles that can be deposited in soil and carried by air and water into surface waterways such as streams and lakes. Human activities related to the uranium fuel cycle, including mining and milling processes, as well as using chemicals to dissolve uranium from subterranean rocks into groundwater can contribute to the presence of uranium in the biosphere [
9]. On the other hand, americium is a man-made element classified as part of the actinide group, with an atomic number of 95 [
10,
11]. Its chemical behavior is quite similar to that of trivalent lanthanides, and its most stable oxidation state in waters is Am(III). The interaction of dissolved species of americium with colloids, minerals, and rocks has an essential effect on the way that species spreads through the geosphere [
12]. Americium-241 is the most abundant isotope and can be found in the environment through various sources [
11], which include atmospheric nuclear weapons tests, or releases from nuclear power and reprocessing plants (such as Sellafield, La Hague, and Mayak), as well as nuclear-related accidents such as Palomares in 1966, Thule in 1968, Chernobyl in 1986, and Fukushima in 2011 [
11,
13]. Americium-241 is typically found conjugated in minute airborne fragments, and when these fragments are released into the atmosphere, they have the potential to precipitate into soil, water, and sediments. Consequently, americium-241 has entered the environment to different extents and therefore, it is crucial to analyze it in the environment for monitoring purposes and study its migration and impact in the environment, including potential radiation risks [
14,
15].
1.2. Polyurethane Production and Applications
Polyurethanes (PU) are condensation products of polyisocyanates and polyols and have intramolecular urethane bonds (carbonate ester bonds, -NHCOO-) [
16]. It is a versatile polymer that is widely used because of its desired properties such as excellent mechanical strength, resistance to wear and tear, and flexibility [
17]. Polyurethanes are used in a variety of industries, including construction, automotive, furniture, and footwear. They are a key material in products such as sponges, tires, paints, insulation, coats, fibers, foam cushions, and adhesives [
18,
19,
20]. Additionally, it is used in medical devices such as catheters and artificial heart valves, as it is vulnerable to microbial attack [
17]. Fungi and bacteria degrade polyester-polyurethane through enzyme-based hydrolysis of the ester bonds [
20]. In the literature, three types of PU degradation have been identified: fungal and bacterial, and enzymatic degradation [
19,
20]. Polyurethane can be produced in different forms including flexible and rigid foams, coatings, and elastomers. Each form has its unique set of properties and is suitable for different applications [
21].
1.3. Polyurethane Adsorption Studies
PU foam has been extensively used for the accumulation or removal of organic and inorganic species from aqueous solutions, thus it is widely studied in adsorption thermodynamics, particularly in the field of wastewater treatment for the effective removal of pollutants [
22]. Mingtian L. et al (2016) studied the adsorption efficiency of polyurethane foams based on sodium alginate for methylene blue (MB) in a water solution. The adsorption behavior of the PF-SA was significantly influenced by the contact time, dose of the adsorbent, pH of the MB solution, temperature, and initial concentration of the MB. For an MB solution with a concentration lower than 2000 mg L
-1, the elimination efficiency of MB could reach as high as 99% under ideal testing conditions. At higher concentrations, the maximum adsorption capacity for an MB solution could be as high as 1000 mg g
-1 and the pseudo-second-order model provided a good fit to the kinetic data. According to the computed values of ΔG
0, ΔH
0, and ΔS
0 at various temperatures, the adsorption was an endothermic, spontaneous process [
18]. Clares et al. (2015) investigated cadmium removal by Anabaena sp. ATCC 33047, which was immobilized into polyurethane foam. The outcomes indicated that cadmium removal from wastewater was a quick process, fitting better to
Langmuir model, while maximum adsorption capacity reached 162 mg Cd(II) per gram dry biomass [
23].
Bondareva and Fedorova investigated the capacity of a synthetic sorption material attached to a polyurethane foam sheet that is coated with iron(III) oxyhydroxide and manganese(IV) dioxide to purify surface water samples from radionuclides and heavy metals. Several experiments were conducted using water samples contaminated with various toxic metals and radionuclides (e.g., Pb
2+, Hg
2+, Cd
2+, Cu
2+, U-238, Cs-137, Pu-242) to demonstrate the effectiveness of the composite materials for water decontamination from radionuclides and other toxic metals. The simplicity and effectiveness, and preservation of desired qualities were the key benefits of the suggested sorption materials [
24]. Zhu and his team (2018) designed and synthesized a number of compositionally different thermogelling polyurethane copolymers that could be injected into tumor during brachytherapy in order to overcome a potential radiopharmaceutical leakage from the tumor [
25]. Kaur et al. (2016) investigated the potential of radionuclide removal by a hydrogel impregnated polyurethane foam from contaminated blood and other biological fluids and could show high Tc-99 removal ability [
26].
Chen et al. (2022) studied the decontamination of surfaces in the case of nuclear accidents and nuclear decommissioning using an eco-friendly foam detergent [
27]. Youssef et al. (2022) investigated the adsorption of hexavalent uranium onto polyether type polyurethane foam in aquatic solutions. The effect of various parameters such as pH, uranium-ion concentration, temperature, and contact time, on the removal efficiency were examined using batch-type experiments. The outcomes demonstrated that a pseudo-second-order kinetic equation could adequately describe the U(VI) adsorption process (q
e = 26.3), while the
Langmuir isotherm model could accurately describe the adsorption process, and indicated that polyurethane polymer foam is a suitable material for uranium sorption [
28].
1.4. Polylactic Acids Production and Applications
Polylactic acids
(PLAs) are of the most well-known thermoplastic polyester MPs that are environmentally friendly as they are biodegradable, biocompatible, and bio-based polyester and have been shown to be ambitious substitutes for petroleum-derived MPs [
29]. They are formally generated by lactic acid condensation or polymerization by ring-opening of the cyclic dimer lactide. Moreover, their most important advantage is found in their production process, as PLAs can be produced from various biopolymers, such as cellulose, corn, starch or other carbohydrates [
30,
31]. Therefore, PLAs find wide application in everyday life such as food packaging and beverage containers [
32,
33], and the textile industry [
34]. PLAs also find applications in the automotive industry as a substitute for conventional plastics commonly used (e.g., poly(ethylene terephthalate) (PET), acrylonitrile butadiene styrene (ABS), polypropylene (PP) and polycarbonate (PC)) [
35,
36]. In the agricultural sector, PLAs are precursor materials for the manufacture of hydrophobic nets as a replacement for traditional materials applied in outdoor applications [
37]. Additionally, PLAs have application in the biomedical field as a support for one of the scaffolds intended for tissue engineering purposes. Due to their excellent chemical, biomechanical, physical and degradation properties are suitable materials for the restoration of blood vessels, bone, skin, cartilage, nerves, and muscles [
38]. Further, PLAs appear to have several advantages that make them ideal for use in various sectors and aspects of daily life, covering from single-use products to more permanent applications, surpassing existing used polymers, making it the "polymer of the 21st century" [
39]. Their demand is constantly increasing, indicating that the percentage of PLAs released into environmental receivers is expected to increase in near future [
40,
41]. However, the disadvantages of PLAs are mainly found in low flexibility, poor crystallinity, ineffective gas barrier properties and the fact that PLAs are often contain nanoparticles of organic and inorganic origin, including metals [
30,
31]. Recent studies have examined PLA as a potential carrier for various contaminants into the environment, thus posing a threat to freshwater, marine and terrestrial ecosystems [
40,
41].
1.5. Polylactic Acids Adsorption Studies
In a recent study Feng et al. (2023) used Prussian blue nanoparticles-PLA mixtures as adsorbents for the treatment of cesium-containing wastewater and investigated the adsorption thermodynamics, kinetics, and isotherm models [
42]. It was found that at 293 K cesium ion adsorption capacity was 16.5 mg g
-1 and that the metal ion concentration was reduced from 40 mg L
-1 to 4.8 mg L
-1. At the optimum pH (5 < pH < 9), the maximum adsorption capacity was 17.0 mg g
-1, and the equilibrium was attained within 60 min. The adsorption isotherm and kinetic data were well fitted with the
Freundlich model and quasi-second-order fitting model, respectively, and the adsorption process was an endothermic reaction.
Bearing in mind all the above, it can be concluded that studying the interaction between PU or PLA and radionuclides (e.g., U and Am) is deemed necessary and of great interest. The present study deals with various parameters that can affect the ability of MPs to adsorb and capture radionuclides, such as the temperature impact on the actinide ion (uranium-232 and americium-241) adsorption by polyurethane (PU) and polylactic acid (PLA) microplastics in laboratory aqueous solutions (at pH 4, 7 and 9) and natural water (seawater and wastewater) samples. The adsorption efficiency is described in terms of the linear distribution coefficient Kd and the adsorption thermodynamics are described by the standard enthalpy and entropy evaluated from the experimental data obtained from the different systems for the two radionuclides. To the best of our knowledge this is the first study on the adsorption of uranium and americium isotopes with PU and PLA microplastics and also the first study dealing with the impact and the thermodynamics of radionuclide/actinide ion adsorption by microplastics.
2. Results and Discussion
2.1. Temperature Effect on Radionuclide Adsorption by MPs in Lab Solutions
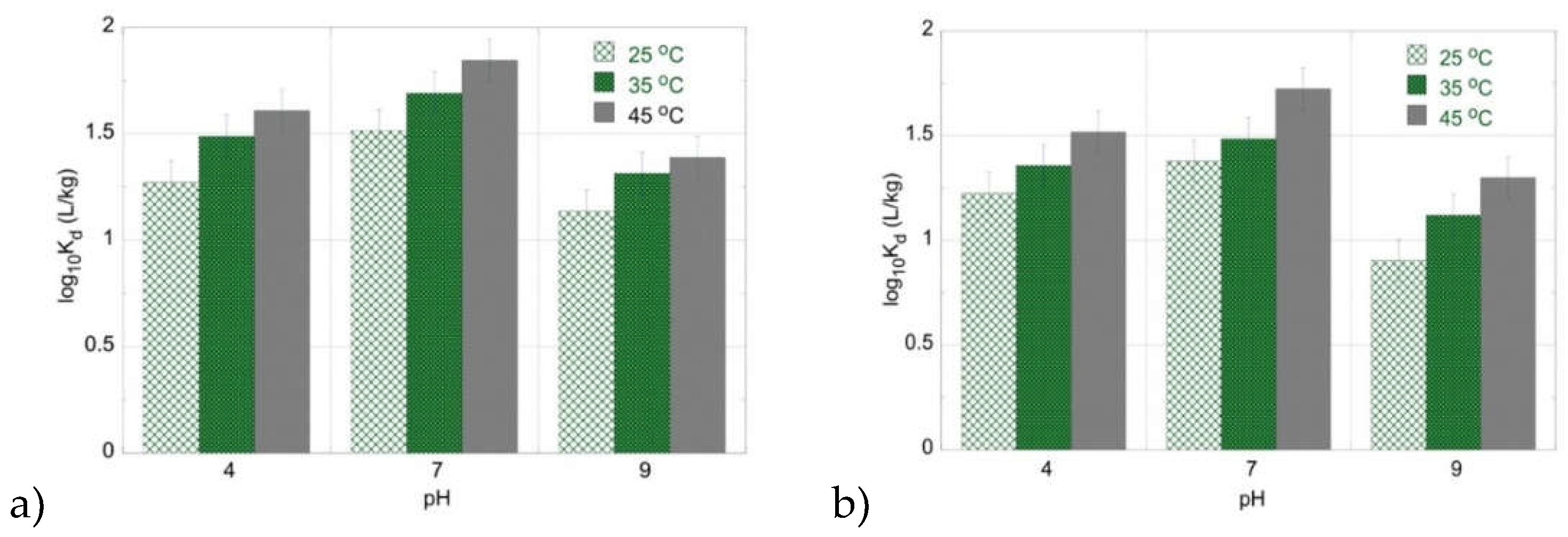
The temperature effect on the adsorption efficiency (K
d) of uranium (uranium-232) by PU and PLA in the acidic, neutral, and alkaline pH region has been determined in de-ionized water solutions and the associated data are visually presented in
Figure 2. The experimental data clearly show that increasing temperature favors the actinide adsorption indicating the endothermic character of the radionuclide adsorption by the microplastics. In addition, it is obvious that the highest adsorption efficiency is observed in the neutral pH region (pH 7).
Figure 1.
logKd values corresponding to the adsorption of U(VI) by a) PU and b) PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 1.
logKd values corresponding to the adsorption of U(VI) by a) PU and b) PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
This can be ascribed to the stabilization of the positively charged UO
22+ cation, which is the predominant U(VI) species, in the acidic solution, as well as to the interaction of the protons with the negatively polarized microplastic surface, which competes cation adsorption (
Figure 3). On the other hand, in the alkaline pH region (pH 9), the predominant formation of the tri-carbonate complex of the hexavalent uranium (UO
2(CO
3)
34-) stabilizes the radionuclide in the aqueous phase and is competitive to adsorption. Moreover, the data summarized in
Figure 2 show that the two different microplastic types (PU and PLA) present almost the same affinity for U(VI) in the acidic pH region (pH 4) and in the neutral and basic pH region (pH 9) PU possesses higher affinity for U(VI).
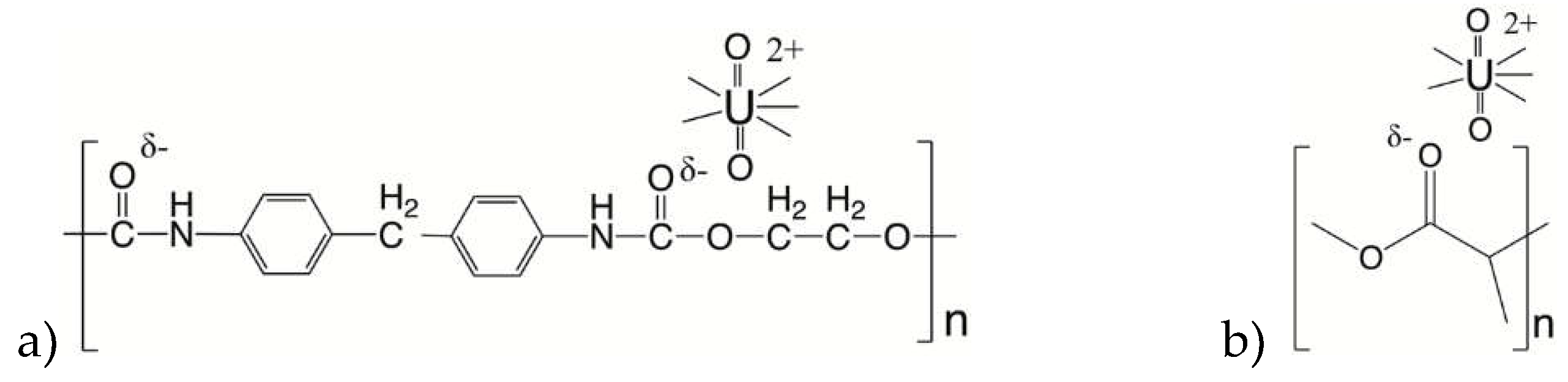
Figure 2.
Schematic interaction of U(VI) with a) PU and b) PLA.
Figure 2.
Schematic interaction of U(VI) with a) PU and b) PLA.
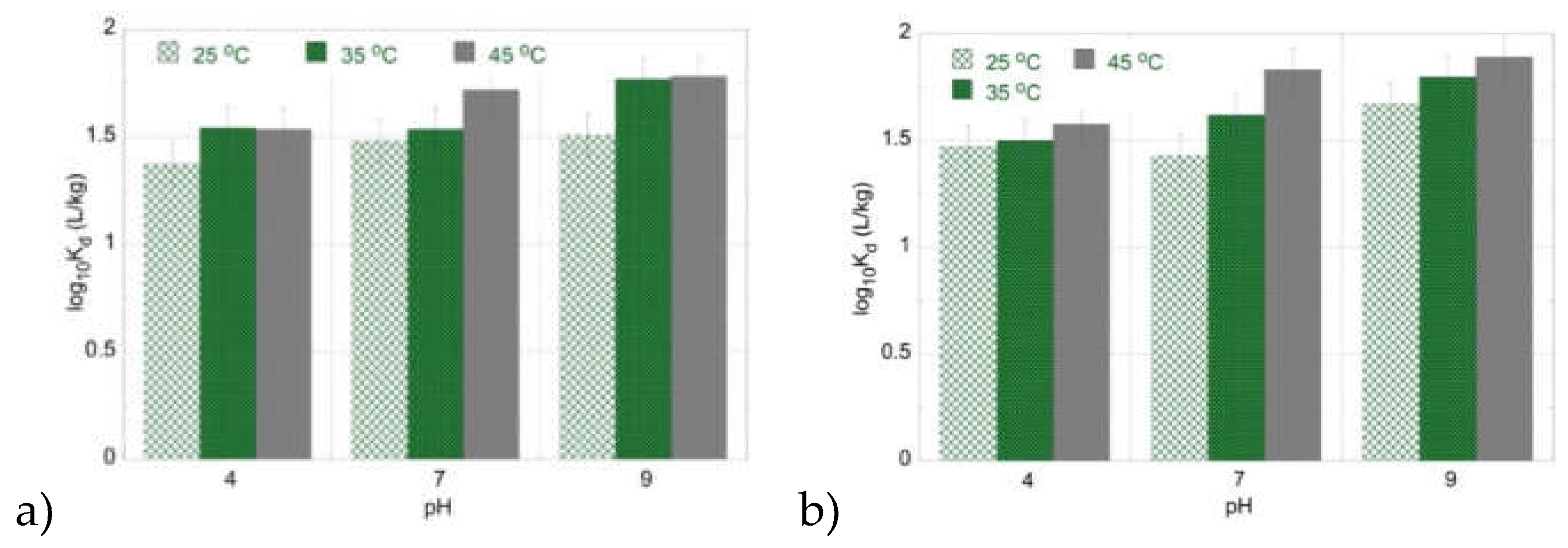
The adsorption of Am(III) by PU and PLA is also an endothermic process, since increasing temperature results generally in higher K
d values (
Figure 4). However, the adsorption efficiency for Am(III) increases with pH and the highest K
d values are obtained in the basic pH region. This could be ascribed to the fact that the Am(III) species formed in the basic pH region (e.g. Am(CO
3)
2- and Am(CO
3)
33-) are compared to UO
2(CO
3)
34- less stable in the aqueous phase, resulting in higher adsorption efficiency of Am(III) in the alkaline pH region compared to U(VI).
Figure 3.
logKd values for the adsorption of Am(III) by a) PU and b) PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 3.
logKd values for the adsorption of Am(III) by a) PU and b) PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Comparison of the present data with data obtained from experiments on the adsorption of Am(III) and U(VI) by oxidized biochar using similar concentration levels suggests that the U(VI) adsorption is similarly an endothermic process, although the K
d values are several orders of magnitude higher (4<logK
d < 5) [
51]. On the contrary the Am(III) adsorption by oxidized biochar is an exothermic reaction and the associated logK
d values lie between 3 and 4 [
52]. The higher K
d values associated with the oxidized biochar can be ascribed to the higher chemical affinity of the carboxylic groups, which are present on the biochar’s surface towards the hard Lewis acids (e.g., Am
3+ and UO
22+). In addition, high K
d values have been determined also for the adsorption of the Am(III) and U(VI) isotopes by X-alginate aerogels [
44]. The K
d values determined in the present study are closer to corresponding K
d values determined for the adsorption of the same radionuclides by PN6 and PE microplastics. The logK
d values evaluated for the U(VI) adsorption by PN6, PVC and PE are 2.2 < logK
d < 3.4, 2.2 < logK
d < 2.4 and 2.1 < logK
d < 2.5, respectively [
46]. On the other hand, the logK
d values evaluated for the Am(III) adsorption by PN6 and PE are 1.2 < logK
d < 2.8 and 0.7 < logK
d < 2.0 [
50].
2.2. Temperature Effect on Radionuclide Adsorption by MPs in Laboratory Solutions Using Radionuclide Mixtures
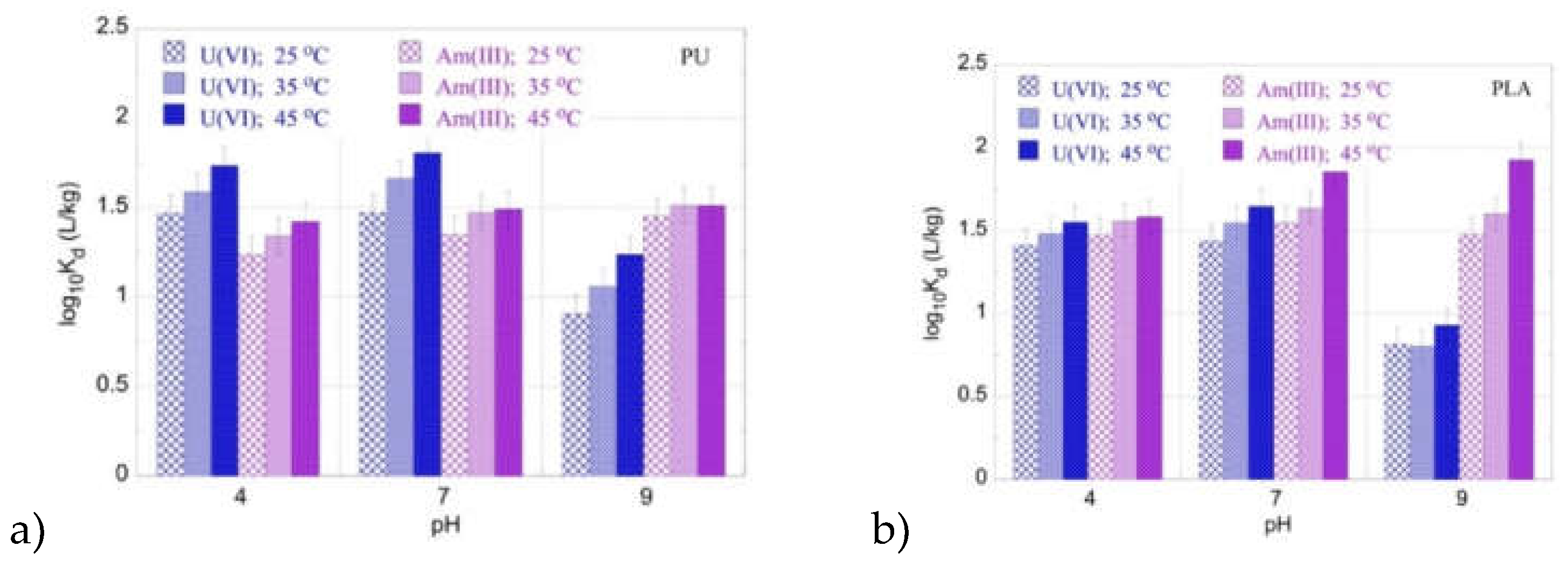
The impact of temperature on of U(VI) and Am(III) adsorption by the PU and PLA microplastics has been also investigated using radionuclide mixtures in order to compare and prove the adsorption behavior observed in the single radionuclide systems. The corresponding data are graphically presented in
Figure 5 and agree with the data obtained in the corresponding single radionuclide systems. This is attributed to the fact that both radionuclides are in ultra-trace levels and their concentration is several orders of magnitude lower than the equivalent concentration of the surface-active sites, which results in a parallel, non-competitive adsorption behavior.
Figure 4.
logKd values for the adsorption of a radionuclide mixture (Am(III) and U(VI)) by a) PU and b) PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the mix solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for each of uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 4.
logKd values for the adsorption of a radionuclide mixture (Am(III) and U(VI)) by a) PU and b) PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the mix solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for each of uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
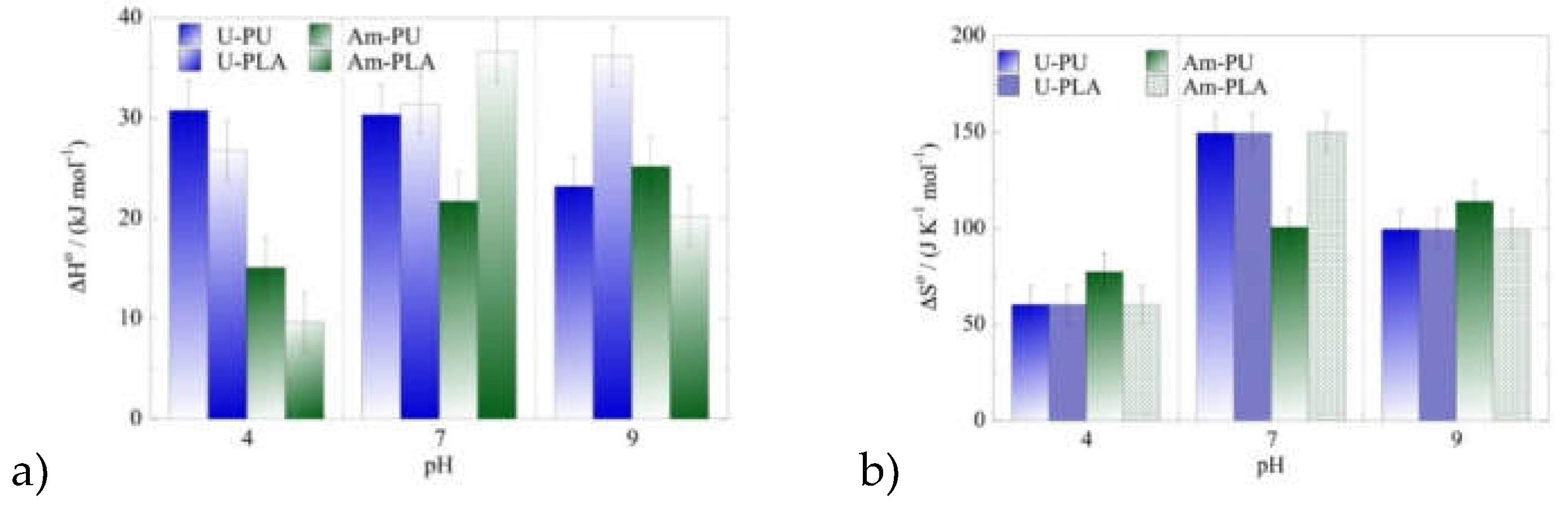
Based on the data obtained from the temperature effect experiments, the thermodynamic parameters ΔH
o (standard enthalpy) and ΔS
o (standard entropy) associated with the adsorption of the two radionuclides by PU and PLA have been evaluated using the linear form of the Van’t Hoff equation (equation 10 and the associated data are graphically shown in
Figure 6.
Figure 5.
a) ΔHo and b) ΔSo values for the adsorption of a radionuclide mixture (Am(III) and U(VI)) by PU and PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the mix solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for each of uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 5.
a) ΔHo and b) ΔSo values for the adsorption of a radionuclide mixture (Am(III) and U(VI)) by PU and PLA as a function of pH at three different temperatures. Experimental conditions: 20 mL of the mix solution in various pH (pH = 4, 7, 9), with 0.5 Bq mL-1 for each of uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
The data presented in
Figure 6 clearly indicate that the adsorption of both radionuclides (e.g., uranium-232 and americium-241) by PU and PLA is an endothermic, entropy-driven process. This assumes that upon adsorption of each U(VI) and Am(III) radionuclide several water molecules are released from the radionuclide coordination sphere resulting in increasing randomness at the solid/solution interface upon adsorption.
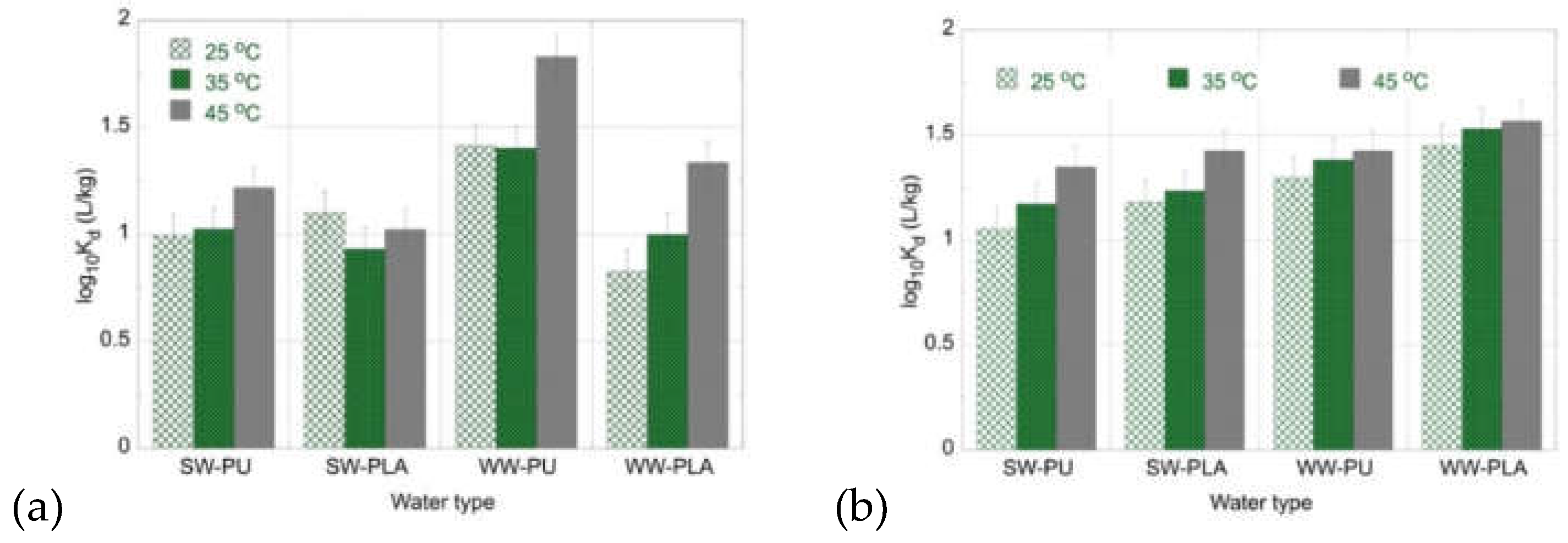
2.3. Temperature Effect on Radionuclide Adsorption by MPs in Environmental Solutions
Furthermore, the temperature effect on the adsorption (logK
d) of U(VI) and Am(III) by PU and PLA has been investigated in seawater and wastewater solutions and the associated data are shown in
Figure 7. Considering that the pH values of the seawater and wastewater solutions is weak alkaline (pH ~8) it appears that the K
d values associated with the environmental waters are significantly lower than the corresponding K
d values evaluated from experiments carried out in de-ionized water solutions. This can be attributed to presence of competitor cations (e.g., Ca
2+ and Fe
3+), which compete for the active sites present on the microplastic surface and thus resulting in lower adsorption efficiencies. A decline of the K
d values in seawaters and wastewaters compared to laboratory solutions is observed also even to a greater extent when applying oxidized biochar [
51,
52], aerogels [
44] and other types of microplastics [
46,
50] to remove Am(III) and U(VI) from natural waters, indicating a higher affinity of PU and PLA for the studied radionuclides. Specifically, in previous studies, we have investigated the uranium-232 adsorption by polyethylene (PE), polyamide naylon (PN6) and polyvinyl chloride (PVC) [
46] and the americium-241 adsorption by PE and PN6 [
50]. In those studies [
46,
50], the parameters that can affect the ability of MPs to adsorb and capture radionuclides included solution pH , contact time, and particle size, but not the temperature effect. The resulting K
d values revealed intriguing insights: approximately 250 ± 15 L kg
-1 for PE, 230 ± 20 L kg
-1 for PN6, and 270 ± 50 L kg
-1 for PVC. Additionally, the adsorption of americium-241 on PE and PN6 resulted in K
d values of 100 and 680 L kg
-1, respectively [
50].
Figure 6.
logKd values evaluated for the adsorption of (a) U(VI) and (b) Am(III) by PU and PLA in seawater (SW) and wastewater (WW) samples at three different temperatures. Experimental conditions: 20 mL solution (SW and WW), 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 6.
logKd values evaluated for the adsorption of (a) U(VI) and (b) Am(III) by PU and PLA in seawater (SW) and wastewater (WW) samples at three different temperatures. Experimental conditions: 20 mL solution (SW and WW), 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
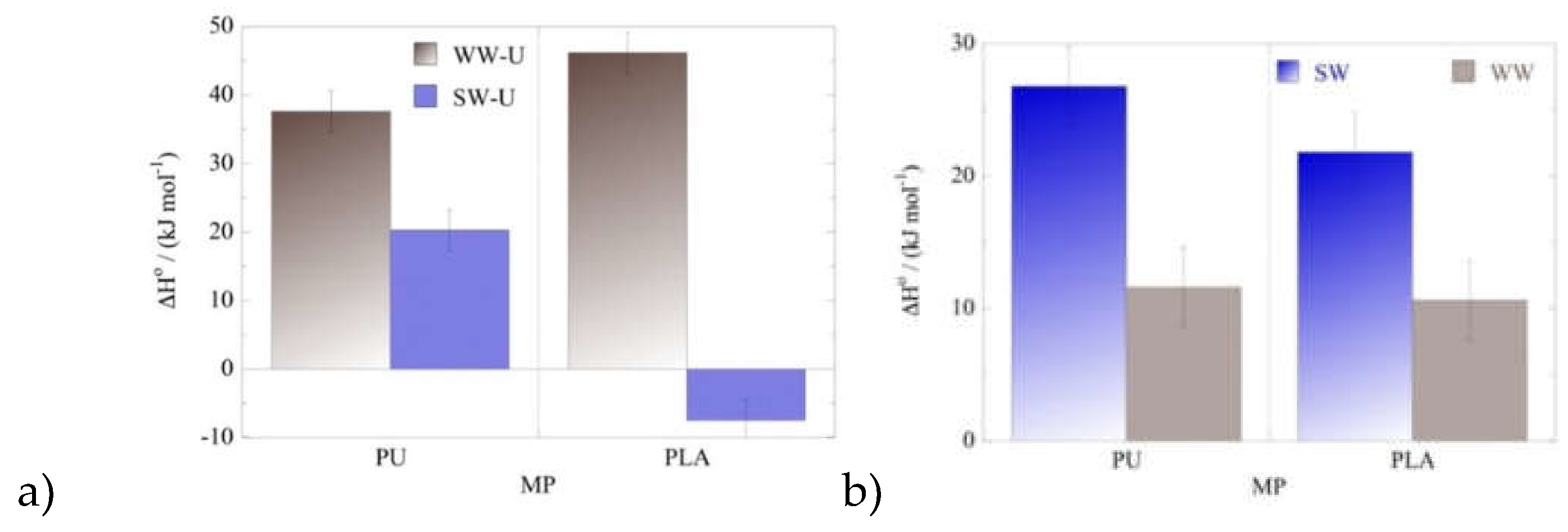
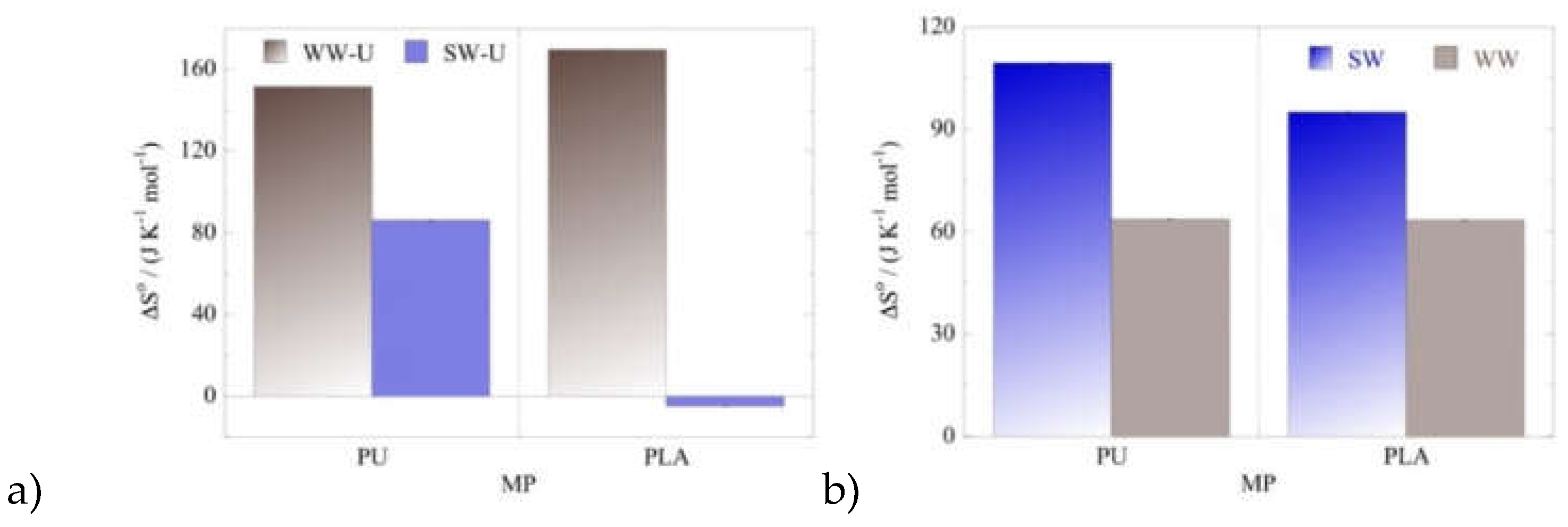
According to the thermodynamic data summarized in
Figure 8 the values of standard enthalpy (ΔH
o) and standard entropy (ΔS
o) are positive except for the U(VI) adsorption by PLA in seawater samples, where the ΔH
o and ΔS
o are both negative assuming degreasing randomness in the system upon adsorption and an enthalpy-driven process. On the contrary, U(VI) adsorption by PU in wastewater and seawater, and U(VI) adsorption by PLA in wastewater in an endothermic, entropy-driven process, indicating that both, surface active moieties present on the microplastic surface as well as aqueous phase composition are key factors governing the mechanism of the uranium adsorption by microplastics.
Figure 7.
ΔHo values evaluated for the adsorption of (a) U(VI) and (b) Am(III) by PU and PLA in seawater (SW) and wastewater (WW) samples at three different temperatures. Experimental conditions: 20 mL solution (SW and WW), 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 7.
ΔHo values evaluated for the adsorption of (a) U(VI) and (b) Am(III) by PU and PLA in seawater (SW) and wastewater (WW) samples at three different temperatures. Experimental conditions: 20 mL solution (SW and WW), 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 8.
ΔSo values evaluated for the adsorption of (a) U(VI) and (b) Am(III) by PU and PLA in seawater (SW) and wastewater (WW) samples at three different temperatures. Experimental conditions: 20 mL solution (SW and WW), 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
Figure 8.
ΔSo values evaluated for the adsorption of (a) U(VI) and (b) Am(III) by PU and PLA in seawater (SW) and wastewater (WW) samples at three different temperatures. Experimental conditions: 20 mL solution (SW and WW), 0.5 Bq mL-1 for both uranium-232 and americium-241 tracers, at different temperatures (25, 35, 45 oC).
3. Materials and Methods
The experiments were carried under ambient conditions in 30 mL polyethylene vials. The standard tracer solutions, which have been used, were uranium-232 (National Physical Laboratory, Teddington, UK) and americium-241 (North America Scientific Inc., Los Angeles, CA, USA) and had a total activity concentration of 4.923 and 12.05 kBq g-1, respectively. These uranium-232 and americium-241 standard solutions were used for the preparation of reference and test solutions, which had generally an initial radioactivity concentration of 0.5 mBq mL-1.
The experiments were performed in laboratory solutions with a total volume of 20 mL. The aqueous solutions used were deionized water (DI) at different pH (4, 7 and 9), seawater (SW, collected from a coastal area of Cyprus) and wastewater (WW, corresponds to secondary treatment effluent, supplied by a local water treatment facility of Cyprus), which were both physico-chemically characterized in previous works [
43,
44]. Analysis of radionuclides uranium-232 and americium-241 was performed by using an alpha spectrometer (Canberra) after electrodepositing a small amount of sample onto stainless steel discs as described elsewhere [
45].
3.1. Batch Experiments
Adsorption experiments were performed similarly to previous studies that have been performed on the sorption of uranium-232 [
46,
47,
48], Np-237 [
49] and americium-241 [
50] on MPs. To the separate and mixtures solutions of uranium-232 and americium-241, were added 0.5 g of the MP material. The final volume of the solution was 20 mL and the activity concentration of each isotope equal to 25 Bq L
-1, corresponding to a molar concentration of [uranium-232] =86 fmol L
-1 and [americium-241] = 0.82 pmol L
-1. The experiments were carried out in PE vials under the various temperatures (25, 35, 45
oC) and pH (4, 7 and 9) for the DI solutions. The solutions were allowed to equilibrate for 10 days, and a small amount (100 µL) taken from the solution was withdrawn and electrodeposited. The concentration of radionuclides was estimated by alpha spectroscopy. The alpha spectrometer used had been previously calibrated by using a standard reference solution and a calibration source (1.02 Bq mL
-1 standard reference solution uranium-232 and 6.6 Bq (total) uranium-238/234, plutonium-239, americium-241 mixed standard on planchet from Eckert & Ziegler).
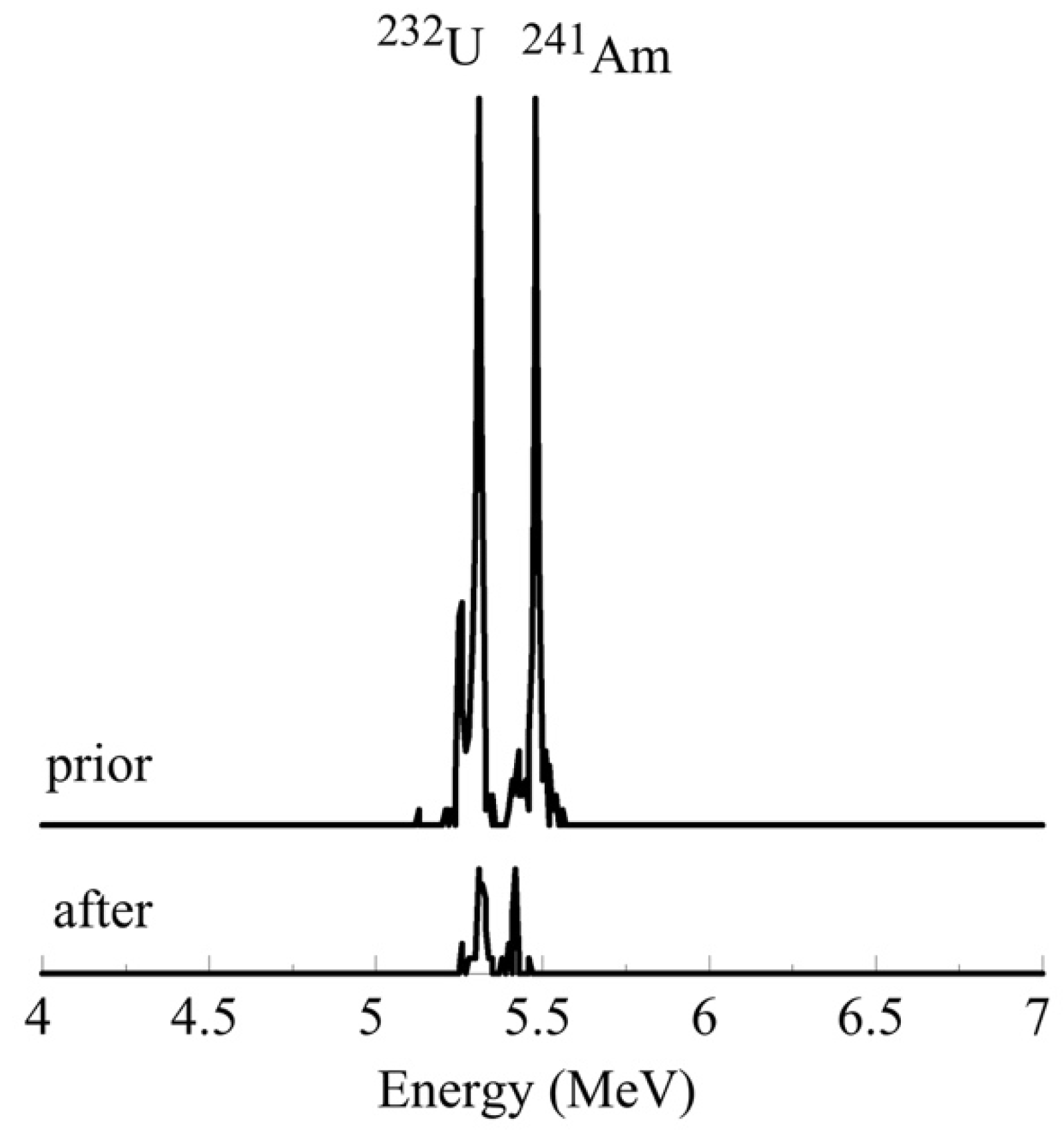
Figure 9.
A characteristic alpha spectrum of the uranium-232 and americium-241 corresponding to a radionuclide mixture solution (a) prior and (b) after radionuclide adsorption by PU microplastics.
Figure 9.
A characteristic alpha spectrum of the uranium-232 and americium-241 corresponding to a radionuclide mixture solution (a) prior and (b) after radionuclide adsorption by PU microplastics.
The distribution coefficient, K
d adequately describes the equilibrium associated with the sorption of uranium-232 and americium-241 as the concentrations of the radionuclides are extremely low and the binding sites (B) of the MP surface are in large excess compared to the initial concentration radionuclides.
where C
ads (Bq g
-1) is the activity of each actinide adsorbed by the MPs, C
aq (Bq L
-1) is the actinide equilibrium concentration in the aqueous solution. The activity of actinides bound on the vial walls must be considered when performing adsorption experiments at ultra-trace levels as it is not negligible. The percent adsorbed is calculated as the percent adsorbed activity of actinide in the aqueous solution relative to the activity of actinide in the reference solution. The adsorption experiments were carried out in duplicate, and the mean values have been used for the calculations and graphical presentations.
The following formula was used to calculate the enthalpy (ΔH
o) and entropy (ΔS
o) of the system:
where K
d is proportional to enthalpy and entropy and inversely proportional to the temperature. The slope is equal to the enthalpy divided to the temperature (T) in K by the gas constants (R) and the intercept is equal to the entropy divided to the gas constants.
4. Conclusions and Future Studies
The adsorption of the uranium-232 and americium-241 radionuclides by polyurethane (PU) and polylactic acid (PLA) microplastics in aqueous laboratory and environmental solutions (e.g., seawater and wastewater) has been investigated as a function of temperature in the acidic, neutral, and alkaline pH region. The temperature increase affects positively the adsorption of uranium-232 and americium-241, which are present under oxic conditions basically in the hexavalent (U(VI)) and trivalent (Am(III)) oxidation state. The highest adsorption efficiency for uranium was observed in the near pH range, whereas for americium, both the neutral and alkaline pH range favor its adsorption by PU and PLA. Regarding the thermodynamics of the adsorption, generally the positive ΔHo and ΔSo values indicate an endothermic and an entropy-driven adsorption mechanism. Only, in the case of the U(VI) adsorption by PLA in seawater samples both ΔHo and ΔSo values become negative suggesting a solid-solution interface enthalpy-driven adsorption process with diminishing randomness.
Future studies could include other radionuclides, in different oxidation states and chemical properties in solution. Furthermore, it would be of particular interest to carry out temperature effect studies at relatively higher concentrations (10-5 M to 10-2 M) of radionuclides and apply various analytical techniques (such as XPS, Raman, LFS, FTIR, SEM-EDX and ζ-potential), as well as to expand the temperature effect investigation also to other MP types (e.g., PN6, PE etc.), that will allow a better understanding of the adsorption mechanism on the surface of the MPs and characterization of the surface species.
Author Contributions
Conceptualization, I.I., D.A.G., and I.P.; methodology, I.I., I.P.; formal analysis, I.I., V.K., I.A., D.A.G. and I.P.; investigation, I.I.; data curation, I.I. and V.K.; writing—original draft preparation, I.I., V.K., I.A., D.A.G. and I.P.; writing—review and editing, , I.I., V.K., I.A., D.A.G. and I.P.; supervision, I.P..; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ioannidis, I.; Kinigopoulou, V.; Giannakoudakis, D.A.; Arkas, M.; Anastopoulos, I.; Triantafyllidis, K.S.; Pashalidis, I. Microplastics and disposable face masks as “Trojan Horse” for radionuclides pollution in water bodies–A review with emphasis on the involved interactions. Sustain. Chem. Environ. 2023, 100005. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; I, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 6730305, 1–14. [Google Scholar] [CrossRef]
- Cantrell, K.J.; Zachara, J.M.; Dresel, P.E.; Krupka, K.M.; Serne, R.J. Geochemical processes data package for the vadose zone in the single-shell tank waste management areas at the hanford site; Pacific Northwest National Lab.(PNNL), Richland, WA (United States). 2007. [Google Scholar]
- Salminen-Paatero, S.S.; Paatero, J. Transfer of Natural Radionuclides in Terrestrial Food Chains—A Review of Investigations in Finland. Int. J. Environ. Res. Public Heal. 2021, 18, 10577. [Google Scholar] [CrossRef] [PubMed]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Chakraborty, A.; Pal, A.; Saha, B.B. A Critical Review of the Removal of Radionuclides from Wastewater Employing Activated Carbon as an Adsorbent. Materials (Basel). 2022, 15, 1–42. [Google Scholar] [CrossRef]
- Nuccetelli, C.; Rusconi, R.; Forte, M. Radioactivity in drinking water: Regulations, monitoring results and radiation protection issues. Ann. Ist. Super. Sanita 2012, 48, 362–373. [Google Scholar] [CrossRef]
- Charalambous, C.; Aletrari, M.; Piera, P.; Nicolaidou-Kanari, P.; Efstathiou, M.; Pashalidis, I. Uranium levels in Cypriot groundwater samples determined by ICP-MS and α-spectroscopy. J. Environ. Radioact. 2013, 116, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Osváth, S.; Vajda, N.; Molnár, Z. Development of a complex method for the determination of actinoides. J. Radioanal. Nucl. Chem. 2009, 281, 461–465. [Google Scholar] [CrossRef]
- Thakur, P.; Mulholland, G.P. Determination of 237Np in environmental and nuclear samples: a review of the analytical method. Appl. Radiat. Isot. 2012, 70, 1747–1778. [Google Scholar] [CrossRef]
- Choppin, G.R. Actinide speciation in the environment. J. Radioanal. Nucl. Chem. 2007, 273, 695–703. [Google Scholar] [CrossRef]
- Radiation, U.N.S.C. on the E. of A. Sources and effects of ionizing radiation. UNSCEAR 1996 report to the General Assembly, with scientific annex. 1996.
- Warwick, P.E.; Croudace, I.W.; Carpenter, R. Review of analytical techniques for the determination of americium-241 in soils and sediments. Appl. Radiat. Isot. 1996, 47, 627–642. [Google Scholar] [CrossRef]
- Hou, X.; Roos, P. Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal. Chim. Acta 2008, 608, 105–139. [Google Scholar] [CrossRef] [PubMed]
- Selvasembian, R.; Gwenzi, W.; Chaukura, N.; Mthembu, S. Recent advances in the polyurethane-based adsorbents for the decontamination of hazardous wastewater pollutants. J. Hazard. Mater. 2021, 417, 125960. [Google Scholar] [CrossRef] [PubMed]
- Nakajima-Kambe, T.; Shigeno-Akutsu, Y.; Nomura, N.; Onuma, F.; Nakahara, T. Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl. Microbiol. Biotechnol. 1999, 51, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.T. Biodegradation of polyurethane: a review. Int. Biodeterior. Biodegradation 2002, 49, 245–252. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications–a review. Rsc Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Hasany, S.M.; Saeed, M.M.; Ahmed, M. Separation of radionuclides by polyurethane foam. J. Radioanal. Nucl. Chem. 2000, 246, 581–587. [Google Scholar] [CrossRef]
- Clares, M.E.; Guerrero, M.G.; García-González, M. Cadmium removal by Anabaena sp. ATCC 33047 immobilized in polyurethane foam. Int. J. Environ. Sci. Technol. 2015, 12, 1793–1798. [Google Scholar] [CrossRef]
- Bondareva, L.; Fedorova, N. New Methodological Approach to Water Purification from Long-Lived Radionuclides and Heavy Metals Under Emergency Conditions. In Proceedings of the Wastewater Technologies and Environmental Treatment: Proceedings of the ICWTET2020; Springer, 2021; pp. 11–22.
- Zhu, J.-L.; Yu, S.W.-K.; Chow, P.K.-H.; Tong, Y.W.; Li, J. Controlling injectability and in vivo stability of thermogelling copolymers for delivery of yttrium-90 through intra-tumoral injection for potential brachytherapy. Biomaterials 2018, 180, 163–172. [Google Scholar] [CrossRef]
- Kaur, A.; Chattopadhyay, S.; Jain, S.; Tyagi, A.; Singh, H. Preparation of hydrogel impregnated antimicrobial polyurethane foam for absorption of radionuclide contaminated blood and biological fluids. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Chen, C.; Xi, H.; Li, Z.; Zhang, H.; Lin, X.; Wang, Y. Removal of uranium by APG/TAS antifreeze foam detergent with high foaming property. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 650, 129589. [Google Scholar] [CrossRef]
- Youssef, W.M.; Hussein, A.E.M.; Taha, M.H.; El-Maadawy, M.M. Uranium (VI) Sorption from Liquid Waste Solution Using Functionalized Polyurethane Polymer: Kinetic and Isotherm Characterizations. Russ. J. Inorg. Chem. 2022, 67, 1058–1068. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Benelfellah, A.; Papadopoulos, L.; Terzopoulou, Z.; Bikiaris, D.N.; Aït Hocine, N. Properties of poly (lactic acid)/montmorillonite/carbon nanotubes nanocomposites: determination of percolation threshold. J. Mater. Sci. 2021, 56, 16887–16901. [Google Scholar] [CrossRef]
- Tarani, E.; Pušnik Črešnar, K.; Zemljič, L.F.; Chrissafis, K.; Papageorgiou, G.Z.; Lambropoulou, D.; Zamboulis, A.; N. Bikiaris, D.; Terzopoulou, Z. Cold crystallization kinetics and thermal degradation of pla composites with metal oxide nanofillers. Appl. Sci. 2021, 11, 3004. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of oligomers from a food contact biopolymer based on polylactic acid (PLA) and polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Mawhorter, C.; Legoski, S. Quantification of microplastics by count, size and morphology in beverage containers using Nile Red and ImageJ. J. Water Health 2021, 19, 79–88. [Google Scholar] [CrossRef]
- Perin, D.; Rigotti, D.; Fredi, G.; Papageorgiou, G.Z.; Bikiaris, D.N.; Dorigato, A. Innovative bio-based poly (lactic acid)/poly (alkylene furanoate) s fiber blends for sustainable textile applications. J. Polym. Environ. 2021, 29, 3948–3963. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly (lactic Acid): A versatile biobased polymer for the future with multifunctional properties—From monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers (Basel). 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, D.; Liu, W.; Zhou, H.; Zhang, Y.; Wang, X. Fabrication of biodegradable poly (lactic acid)/carbon nanotube nanocomposite foams: significant improvement on rheological property and foamability. Int. J. Biol. Macromol. 2020, 163, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Miros-Kudra, P.; Gzyra-Jagieła, K.; Kudra, M. Physicochemical assessment of the biodegradability of agricultural nonwovens made of PLA. Fibres Text. East. Eur. 2021. [Google Scholar] [CrossRef]
- Karava, V.; Siamidi, A.; Vlachou, M.; Christodoulou, E.; Zamboulis, A.; Bikiaris, D.N.; Kyritsis, A.; Klonos, P.A. Block copolymers based on poly (butylene adipate) and poly (l-lactic acid) for biomedical applications: Synthesis, structure and thermodynamical studies. Soft Matter 2021, 17, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Fojt, J.; David, J.; Přikryl, R.; Řezáčová, V.; Kučerík, J. A critical review of the overlooked challenge of determining micro-bioplastics in soil. Sci. Total Environ. 2020, 745, 140975. [Google Scholar] [CrossRef]
- Verdú, I.; González-Pleiter, M.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Microplastics can act as vector of the biocide triclosan exerting damage to freshwater microalgae. Chemosphere 2021, 266, 129193. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ni, J.; Cao, X.; Gao, J.; Yang, L.; Jia, W.; Chen, F.; Feng, S.; Zhang, Y.; Ma, F. Separation and Removal of Radionuclide Cesium from Water by Biodegradable Magnetic Prussian Blue Nanospheres. Processes 2022, 10, 2492. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Appl. Polym. Mater. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Ioannidis, I.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range. Gels 2023, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Kiliari, T.; Pashalidis, I. Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat. Meas. 2010, 45, 966–968. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Microplastics as radionuclide (U-232) carriers. J. Mol. Liq. 2022, 351, 118641. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Single-use surgical face masks as radionuclide (U-232 and Ra-226) carriers. J. Mol. Liq. 2021, 342, 117578. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Uranium desorption from microplastic surfaces. J. Radioanal. Nucl. Chem. 2022. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Neptunium interaction with microplastics in aqueous solutions. J. Mol. Liq. 2022, 356, 119056. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I. Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings 2022, 12, 1452. [Google Scholar] [CrossRef]
- Philippou, M.; Pashalidis, I.; Theocharis, C.R. Uranium Isotope (U-232) Removal from Waters by Biochar Fibers: An Adsorption Study in the Sub-Picomolar Concentration Range. Molecules 2022, 27, 6765. [Google Scholar] [CrossRef]
- Philippou, M.; Pashalidis, I.; Kalderis, D. Removal of 241Am from Aqueous Solutions by Adsorption on Sponge Gourd Biochar. Molecules 2023, 28, 2552. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).