1. Introduction
Plasmodium falciparum is the world’s leading and most lethal cause of malaria, making it one of the main challenges to global public health. In 2022, it was responsible for 97% (249 million) of cases and 95% (608,000) of deaths from malaria worldwide [
1].
In the Americas region,
P. falciparum was responsible for 28% of the total 552,000 cases in 2022. Venezuela, Brazil and Colombia accounted for around 73% of malaria cases in this region [
1]. In Brazil, this parasite was responsible for 15.92% of the 142,522 cases reported in the country in 2023
2, according to data from the Malaria Epidemiological Surveillance Information System (Sivep-Malaria) [
2].
Roraima is one of the states that make up the Brazilian Amazon, where 99% of malaria cases occur [
3]. According to Sivep-Malaria, the proportion of
P. falciparum malaria in the state was 29,04%% of the total of 34.555 malaria cases reported in 2023 [
2].
In the global strategy to combat malaria, the World Health Organization (WHO) has presented targets for the period from 2016 to 2030, including a reduction in malaria cases of at least 90% by 2030, with the goal of eliminating malaria in at least 35 countries [
4]. In this context, Brazil launched the
P. falciparum malaria elimination plan in 2015 and in 2022 proposed to eliminate malaria by 2035. One of the intermediate targets of the Brazilian National plan is the elimination of
P. falciparum malaria by 2030 [
5].
A major obstacle to the elimination of malaria is the emergence of
P. falciparum multidrug resistance [
6]. In fact, in 1960, only a decade after starting large-scale use of cloroquine,
P. falciparum resistance to this drug was detected in Colombia, Cambodia, and Thailand and it spread rapidly to other endemic countries, including Brazil [
7]. In the 1970s, the SP combination was introduced to treat
P. falciparum infections in South America, but chemoresistance was also soon reported in Colombia, Brazil, Peru, Venezuela, and Bolivia [
8,
9,
10,
11]. In the 1980s, mefloquine (MQ) was proposed in Brazil as a therapeutic alternative for non-severe malaria and MQ combinated with artemisinin derivatives (ART) for severe malaria cases [
12].
Following reports of treatment failure and the spread of multidrug-resistant
P. falciparum parasites, the WHO recommended ART-based combination therapies (ACTs) for the treatment of uncomplicated
P. falciparum malaria in 2001 [
13]. In addition, the use of ART as monotherapy for malaria treatment was suspended for preventing the emergence of drug resistance [
14]. ACTs combine two active substances with different mechanisms of action: an ART derivative and another antimalarial drug. The first has a short plasma half-life (1 to 2 hours) and aims to rapidly reduce the parasite biomass. The second antimalarial drug has a longer plasma half-life (days to weeks) and is designed to eliminate the remaining parasites [
6,
15].
The Brazilian National Malaria Control Program (PNCM) introduced treatment with ACTs for uncomplicated
P. falciparum malaria in 2005, which led to an increasing malaria decline in the Brazilian Amazon region [
16]. The same epidemiological dynamic can be observed in other endemic countries around the world [
17].
The detection of ART resistance in Southeast Asia, first in Cambodia in 2008 and then in China, Vietnam, Thailand, and Myanmar, has highlighted an obstacle in global malaria elimination effort [
18]. The emergence of this resistance can be attributed to monotherapy with unregulated ART or artesunate (AS) which had been available in this region since the mid-1970s, as well as the availability of these drugs in the private health sector [
19].
In 2010, a mutation associated with ART resistance was identified in Guyana, a South American country. Genomic analyses indicate that the mutation in Guyana is not due to the spread from Southeast Asia but occurred independently [
20].
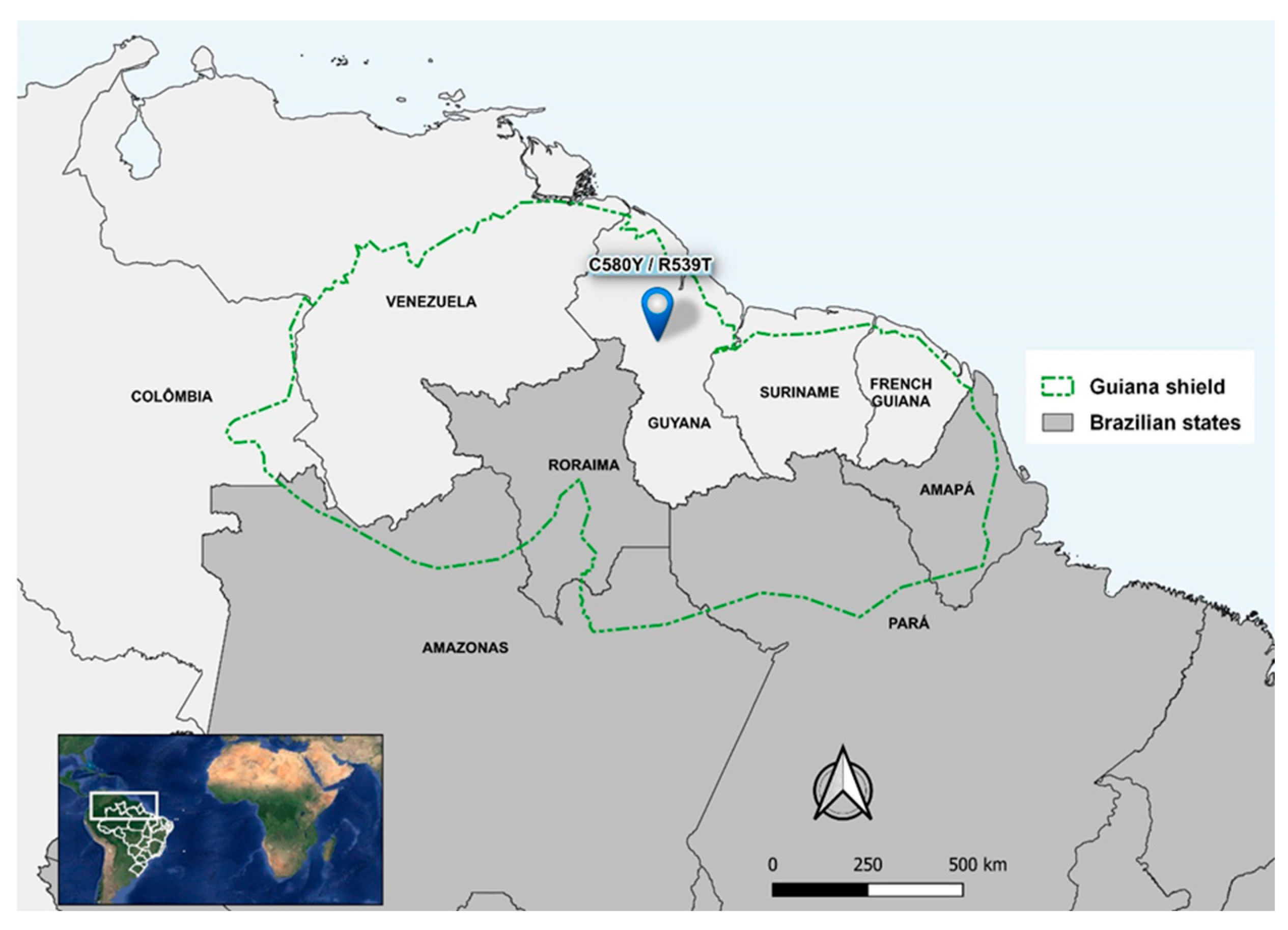
The state of Roraima, together with the western part of Amapá, the northern Amazon and Pará in Brazil and the territories of Guyana, Suriname, French Guiana, Venezuela and Colombia, is part of the Guiana Shield. This region is considered a potential source for the emergence of malaria resistance in South America, as the rich subsoil in gold and other minerals, attracts prospectors to the indigenous forest areas and, therefore, increasing the influx of the human population [
21,
22,
23].
In the last decade, the proportion of
P. falciparum infections in Roraima has increased significantly, probably for delay in diagnosis and treatment in Venezuelans migrants and in gold miners living in illegal mining in the Territory Indigenous Yanomami (TIY) [
24]. Besides, Venezuela’s economic collapse led to a shortage of antimalarial drugs and the search for treatment in neighboring countries, particularly in Brazil, increasing imported malaria cases, including
P. falciparum infections [
25].
In Roraima, there was a 44% rise in malaria cases in 2020 [
24]. In the same year, illegal mining in the TIY increased by 30%. More than half (52%) of the mines in this region are located on the Uraricoera River, but mines have also been found on the banks of the Mucajaí, Couto de Magalhães, Parima and Catrimani rivers [
26].
The mines are located in isolated areas of the forest without access to diagnosis and treatment by the Brazilian Health Unic System (SUS). For this reason, miners with a fever tend to take antimalarials of dubious or even illegal origin to avoid having to stop mining. This scenario may favor the selection of ART-resistant parasites and underline the need for molecular surveillance of antimalarial resistance in this region [
24,
27,
28].
Surveillance of molecular markers of antimalarial resistance is an important strategy for detecting treatment failure and should be implemented to detect resistant parasites early and prevent their spread [
29,
30,
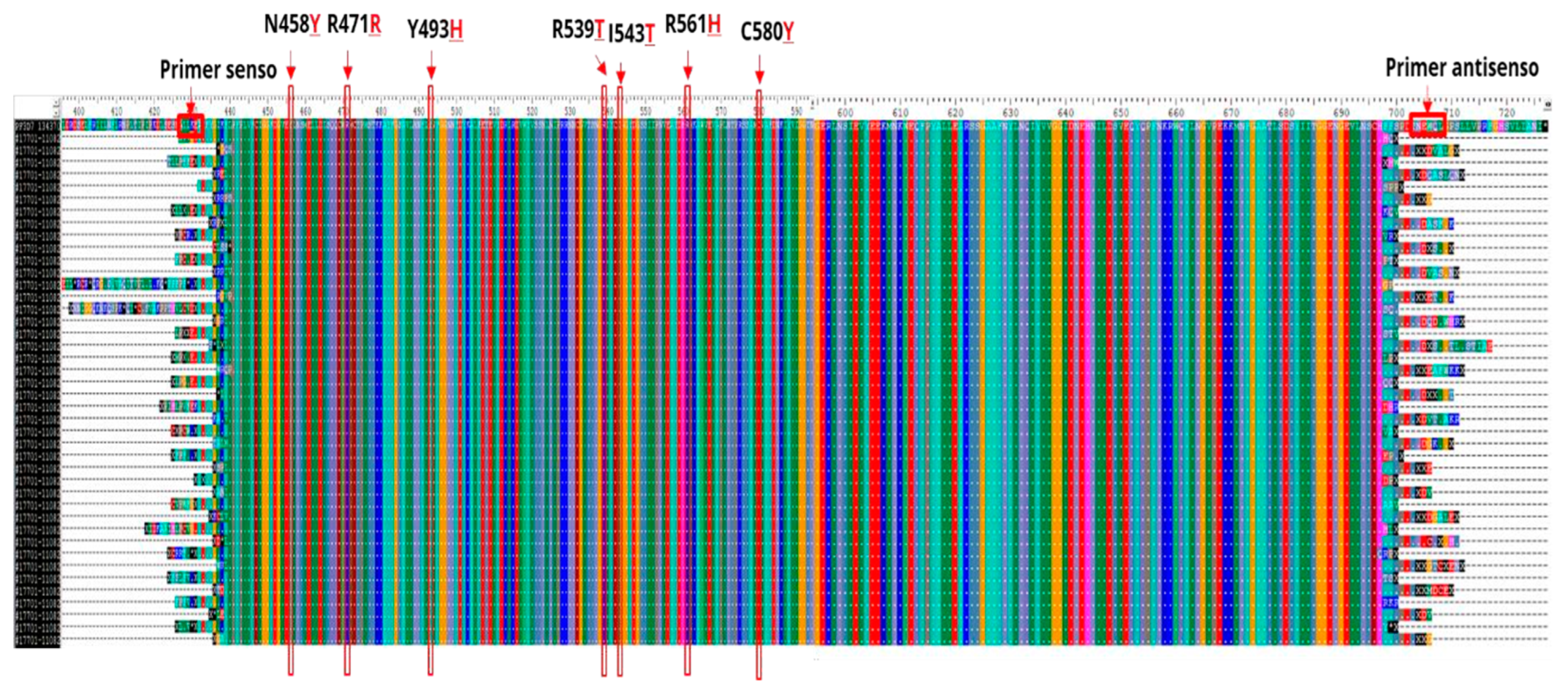
31]. In 2014, mutations in the propeller domain of the Kelch 13 gene on chromosome 13 (
pfk13) were associated with delayed elimination of parasite in vitro and in vivo and this molecular marker have been used for global surveillance of artemisinin resistance (ART-R) [
6,
32]. The following mutations have been validated by in vitro and in vivo studies: C580
Y, R561
H, R539
T, I543
T, P553
L, M476
I, N458
Y, Y493
H, F446
I and P574
L. The mutations P441
L, T449
A, C469
F/Y, A481
V, R515
K, P527
H, N537
I/D, G538
V, V568
G, R622
I and A678
V are considered ART-R molecular markers. In addition, less common
pfk13 variants such as K479
I, G533
A, R575
K, M579
I, D584
V, P667
T, F673
I, and H719
N have also been associated with delayed parasite elimination [
6].
Considering that the future efficacy of ACTs is endangered by the emergence of resistance artemisinin, this study aims to investigate single nucleotide polymorphisms (SNPs) associated with P. falciparum ART-R in the pfk13 gene in the state of Roraima.
2. Materials and Methods
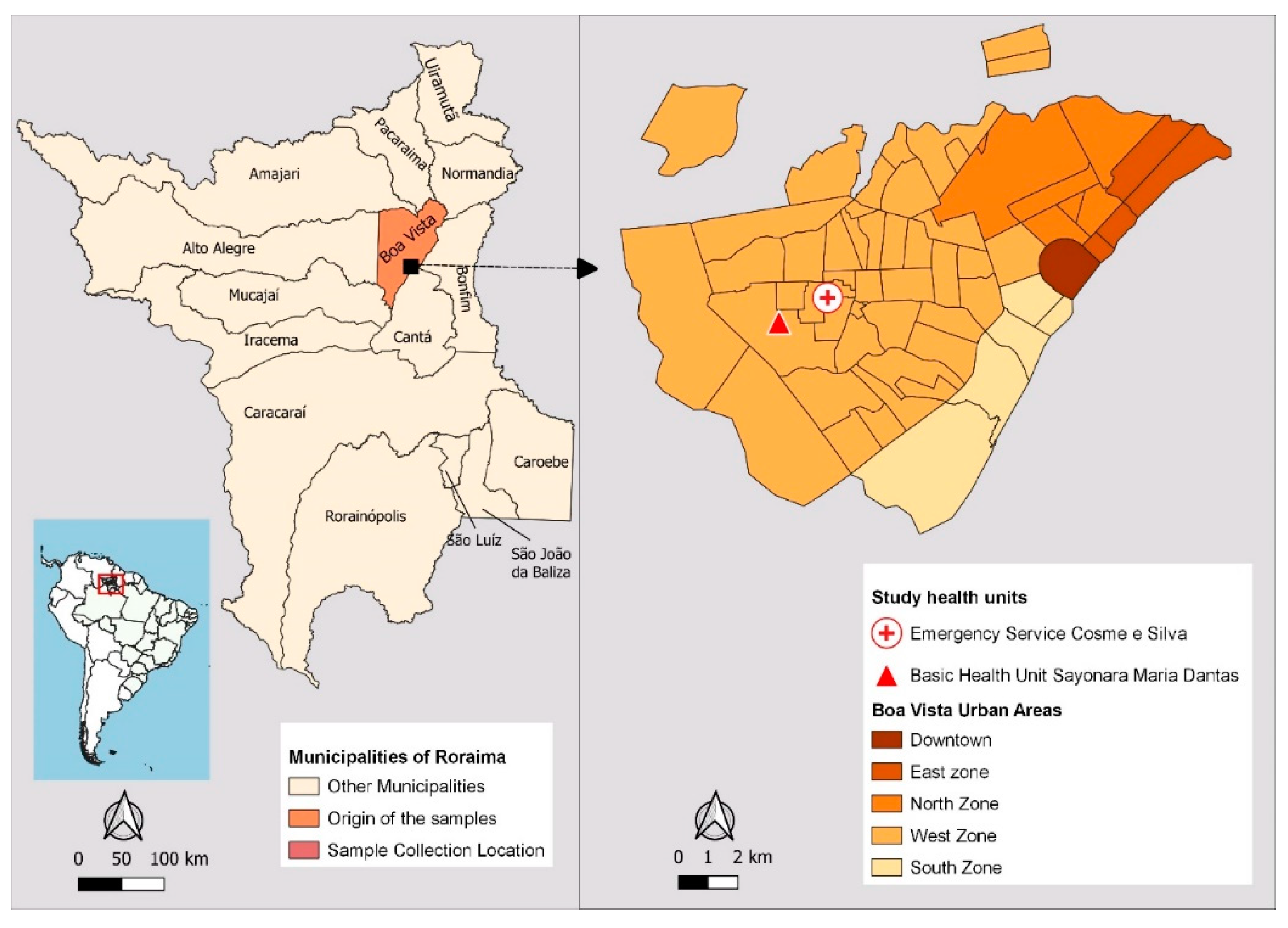
The study site was the municipality of Boa Vista (2°49′10″ N and 60°40′23″ O), the capital of Roraima, which is located in the far north of Brazil and is the only capital above the equator [
33]. Almost the entire Roraima state is part of the Guiana Shield, along with the western part of Amapá and northern of Amazonas and Pará in Brazil. In addition to Brazil, Guyana, Suriname, French Guiana, southern Venezuela and eastern Colombia also comprise the Guiana Shield. There is an intense migratory flow of miners in this Shield and C580
Y and R539
T mutations in the
pfk13 gene have already been identified in Guyana [
20] (
Figure 1).
According to estimates by the Brazilian Institute of Geography and Statistics (IBGE), the city currently has 436,591 inhabitants and is home to 67% of the state’s population. It borders the municipalities of Normandia, Pacaraima and Amajari, to the north; Mucajaí and Alto Alegre, to the south; Bonfim, Cantá and Normandia to the east; and the municipality of Alto Alegre to the west.
Boa Vista is home to the state’s main and most sought-after public health institutions. Two health facilities with the highest number of malaria reports, according to Sivep-Malaria, were selected as sample collection sites: Emergency Service Cosme e Silva and Basic Health Unit Sayonara Maria Dantas, both located in the west zone of the city (
Figure 2). This urban area has the highest employment density, with 70% of the city’s 56 neighborhoods, and the majority of the population has a low monthly income. The West Zone emerged in the 1990s as a result of interregional migration flows, rural exodus and gold prospectors who moved to Boa Vista after mining was banned in the early 1990s [
34].
The samples were collected from December 2021 to June 2022, during the transition period from the rainy season to the dry season, when the mosquito population increases seasonally [
34].
The study was approved by the Research Ethics Committee of the Federal University of Roraima (CEP/UFRR): CAAE 24122619.6.0000.5302, on March 17, 2020. Participants signed the informed consent form (ICF) to take part in the study. Individuals over the age of 18 who had been diagnosed with P. falciparum or mixed malaria (P. vivax + P. falciparum) by a thick blood smear were included. Those excluded from the study were children under 18, indigenous villagers, individuals who were unable to read, or those who refused to sign the informed consent form. Participants in the study were asked questions related to the individual and the disease. Blood was collected by venipuncture of 5 mL of peripheral blood. Part of the blood (around 50 microliters) was transferred directly from the syringe to filter paper (Whatman 903 Protein Saver Cards) and the rest was placed in a vacutainer tube containing EDTA (Becton, Dickinson & Company).
The samples were transported to the Molecular Biology Laboratory of the Biodiversity Research Center of the Universidade Federal de Roraima. The tubes containing blood were centrifuged at 3000× g for 10 minutes to remove the plasma, when the “red blood cell concentrate” (containing leukocytes and platelets) was added to the cryopreservation solution glycerolyte 57 (Baxter) volume by volume (v/v), followed by the aliquoting of each sample. The aliquots with the cryopreservation solution were stored at -20°C until DNA extraction.
Extraction of deoxyribonucleic acid (DNA) was carried out using the column technique (centrifugation method), using the QIAamp DNA blood mini kit (Qiagen), according to the manufacturer’s instructions, from a volume of 500µL of the “red blood cell concentrate”.
The
pfk13 gene fragment was amplified by nested polymerase chain reaction (PCR) [
35]. The following primers were used in the first reaction: K13 F: 5′CGGAGTGACCAAATCTGGGA3′ and K13 R: 5′GGGAATCTGGTGGTAACAGC3′.
A mixture of PCR reagents was prepared with 13.75 µL of ultrapure water, 5 µL Taq 5x hotfirepol®, 0.625 µL of each of the primers (10pmol) and 5 µL of P. falciparum DNA sample was added to each mixture. The PCR conditions in the thermal cycler were as follows: initialization at 95ºC for 15 minutes; 30 cycles of denaturation at 95ºC for 30 seconds, annealing of the primers at 58ºC for 2 minutes and extension at 72ºC for 2 minutes; and final elongation at 72ºC for 10 minutes.
The following primers were used for the second PCR: K13 N_F: 5′GCCTTGTTGAAAGAAGCAGA3′ and K13 N_R: 5′GCCAAGCTGCCATTCATTTG3′. A mixture was prepared with 37.5 µL ultrapure water, 10 µL Taq 5x hotfirepol®, 1.25 µL of each of the primers (10 pmol) and 5 µL of first PCR amplified product was added to each mixture. The PCR conditions were as follows: initialization at 95ºC for 15 minutes; 40 cycles of denaturation at 95ºC for 30 seconds; annealing at 60ºC for 1 minute and extension at 72ºC for 1 minute; and final elongation at 72ºC for 10 minutes.
The final 849 base pairs (bp) amplified product (amplicon) was analyzed after electrophoresis in a 2% agarose gel, stained with Bluegreen (LGC), using a DNrBio-Imagining System/Model: MiniBIS Pro (UV) photodocumentation system. A 100 bp molecular weight marker was used to check the size of the fragments (
Figure 3).
The amplicons were purified using the Wizard® Kit, according to the manufacturer’s instructions. The sequencing reaction was carried out using the Big Dye kit® Terminator Cycle Sequencing Ready Reaction version 3.1 (Applied Biosystems), according to the manufacturer’s instructions. The amplicons were subjected to Sanger-type sequencing using capillary electrophoresis on the ABI PRISM DNA Analyzer 3730 (Applied Biosystems, USA) of the PDTIS/Fiocruz genomic platform and ABI PRISM DNA Analyzer 3500 (Applied Biosystems, USA) of the LabMol/CBio/UFRR.
The entire amplified fragment DNA sequence (codons 427 to 709) was analyzed using the ClustalW multiple sequence aligner in the BioEdit
® software, using the prototype 3D7 (GenBank PF3D7_1343700 available in
https://www.ncbi.nlm.nih.gov/nuccore/), as the reference sequence.
The maps were created using the QGIS program version 3.28.10, mining areas in Roraima were obtained from Mapbiomas [
36]. Geopolitical limits of Brazil and Indigenous Lands were accessed on the IBGE website [
37].
3. Results
A total of 53 samples were collected, 42 from
P. falciparum and 11 from mixed
P. falciparum +
P. vivax malaria infections. Of the total samples collected, 83% (44) were from men and their ages ranged from 18 to 55 years, with a median of 36 years. Regarding the main activity performed in the 15 days before the symptoms, 96% (51) of participants reported mining and only 4% (02) agriculture. Detailed epidemiological information on these patients have recently been published [
38].
Approximately 97% (48) of the samples were amplified by nested PCR for the
pfk13 gene and all amplicons were purified and sequenced. Of the total sequenced samples, 46 (96%) were from patients reporting mining activity, and only 2 (4%) from those reported agricultural activities. The mines were mainly located in the TIY in the municipalities of Alto Alegre (40) and Mucajaí (5) (
Figure 4). In addition, one participant came from gold mines in Itaituba/PA (1). The patients who reported working in agriculture were likely to have been infected in the municipalities of Caracaraí (1) and Alto Alegre (1).
After aligning the nucleotide sample sequences with the 3D7
P. falciparum reference genome strain (PF3D7_1343700), no mutations were found in the
pfk13 gene, neither in the codons associated with ART-R nor in the other codons analyzed. Thus, in the whole amplified fragment, all
P. falciparum sequence samples were identical to the wild type 3D7 reference sequence (
Figure 5).
4. Discussion
It is known that gold miners are considered at risk for malaria and that
P. falciparum is widely distributed in several gold mining regions, especially in the Guiana Shield [
23,
39], where conditions are favorable for the selection of antimalarial-resistant parasites.
The slow elimination of the parasite after treatment with ACT characterizes partial resistance to ART. In the Brazilian endemic areas, the presence of parasites in the blood on day 3 (D3) after the start of therapy (D0) is rare in patients treated with ACT. Therefore, assessment of
P. falciparum clearance on D3 after initiation of treatment is an important parameter for monitoring ART-R [
6]. However, in the present study participants’ parasitemia was not assessed on day D3 after starting treatment with ACT, because it was difficulty to follow the vast majority of participants, who were miners that traveled to Boa Vista only for diagnosis and returned to the mines in TIY just after receiving the medication.
In recent years, illegal mining has increased in Roraima at the TIY, mainly in river areas of Mucajaí, Uraricoera, Catrimani and Parima. More than half (52%) of the total area damaged by mining is concentrated in Uraricoera River [
26]. Access routes to the mining areas are mainly via the rivers and forest areas of the municipalities of Alto Alegre, Amajari, Mucajaí, Caracaraí and Iracema, or by plane via clandestine airstrips hidden in rural areas. The gold miners in this region do not have access to malaria diagnosis and treatment through the SUS which is exclusive for the indigenous peoples living there [
24]. Therefore, for avoid lose working days through travel to distant cities, gold miners buy the drug Artecom
® (dihydroartemisinin-piperaquine), which is not registered in Brazil, that enter illegally through Suriname, Guyana and French Guiana borders. They claimed that a single dose of this drug relieves the symptoms for a few days even though this dose is not able to clear all the parasites. Then, the non-discriminated drug use makes miners a risk group for the selection of
P. falciparum parasites resistant to ART [
38].
Currently, the only treatment for
P. falciparum malaria is the ACTs. Consequently, the emergence of resistance to ART would have a devastating impact on the global goal of malaria elimination and could lead to the development of complete resistance to ART and the selection of resistant parasites against partner drugs as well [
6].
Molecular surveillance of
pfk13 helix domain polymorphism in endemic countries can play an important role in early warning of ART-R parasites [
6,
23,
40,
41]. The C580
Y mutation in the
pfk13 gene has been shown to be relevant for molecular surveillance of ART resistance in Southeast Asia [
42,
43]. This mutation is present in the vast majority of resistant parasites in Cambodia and reaches a prevalence of up to 70% at the border between Thailand and Myanmar [
6]. In 2010, the C580
Y mutation was also identified in 5% (5/98) of parasite samples from Guyana [
20]. The appearance of this mutation in South America raised the question of whether there would a risk of mutant alleles spreading to different hotspots in the Amazon basin through intensive migration between Venezuela, Guyana and Brazil borders, as a result of illegal gold mining. However, between 2016 and 2017, the prevalence of this allele decreased to 1.6% (14/864) [
41]. The in vitro competitive co-cultivation of
pfk13 mutant (C580
Y and R539
T) and non-mutant parasites from Guyana showed that the mutants have a growth deficit compared to the non-mutant wild parasites [
41]. This disadvantage in the fitness of the ART-R mutant parasite could be one of the reasons why these
pfk13 gene mutants have not been fixed and, thus, have not spread from Guyana to neighboring countries in recent years [
41]. However, we must bear in mind that the risk of the spread of such mutants in the Amazon basin, especially those from the Guiana Shield, is proportional to the intensity of mining activities whose main migration patterns include Venezuela, Guyana and the state of Roraima in Brazil [
23].
The World Health Organization drew attention to the presence of
P. falciparum parasites carrying the C580
Y mutation in infected Chinese travelers in Equatorial Guinea and Ghana upon their return to their country and hypothesized that these mutations were likely to have originated in Africa rather than Southwest Asia, but there was no evidence that this mutation had spread to parasite populations in African areas [
6].
In fact, synonymous and non-synonymous mutations in the
pfk13 gene are still rare in Africa. The non-synonymous mutation allele A578
S is the most frequently observed in this continent and also appears to be the most prevalent worldwide, although it occurs in a small proportion in Cambodia, where it does not seem to be associated with the phenotype of clinical or in vitro resistance to ART [
44,
45]. The R539
T and P553
L variants, were identified in a sample from Angola and the M476I in a sample from Equatorial Guinea [
44].
Although 97% of the samples in our study came from individuals infected in mining areas, all sequences analyzed showed non-mutated/wildtype genotypes in the helix-loop-domain of
pfk13. This finding corroborates a study carried out in Roraima which also described the absence of polymorphisms in the
pfk13 gene in parasite samples collected between 2016 and 2017 in the municipalities of Boa Vista, Pacaraima and Rorainópolis [
46].
Samples of
P. falciparum from the Brazilian Amazon basin collected between 1984 and 2011, i.e., in the period before and after the introduction of ART treatment in Brazil, also showed no mutations in the
pfk13 gene [
40], as reported in more recent studies in this region [
47,
48].
In 2013, the mutant
pfk13 A481
V parasite, which is associated with delayed elimination of
P. falciparum in patients in Southeast Asia [
49], was detected in one of the 575 samples of
P. falciparum malaria from Manaus/Brazil, but without clinical signs of ART-R [
23]. The A504
D mutation observed in 2018 in Colombia, also did not lead to ART-R phenotypes [
50]. Thus, further work is needed before these mutations can be considered as markers for quimioresistance to ART in samples from South America.
As the P413
A mutation in the BTB/POZ domain of the
pfk13 gene was recently identified in parasites from an African isolate of
P. falciparum subjected to ART in vitro pressure [
51], we intend to investigate mutations in the BTB/POZ domain, in addition to those here investigated in the
pfk13 gene helix domain.
Finally, considering that the
pfCoronin gene is the main driver of reduced susceptibility to ART in Senegalese parasites developed in vitro [
52], we intend to extend our investigations to this gene, due to the possibility of synergism with
pfk13 in cases of partial resistance to ART [
51].
5. Conclusions
Given the results of this study, treatment with ART derivatives can continue to be used as the first-line treatment for P. falciparum malaria in Brazil. We recommend continuing molecular surveillance to track ART resistance in Roraima, as mutant parasites could be introduced and/or selected due to the influx of miners into the Guiana Shield, which consists of Brazil, French Guiana, Suriname, Guyana, Venezuela, and Colombia.
Author Contributions
Conceptualization: Jacqueline de-Aguiar-Barros, Maria de Fátima Ferreira-da-Cruz, Fabiana Granja. Data curation: Jacqueline de-Aguiar-Barros, Daniel da Silva e Silva, Arthur Camurça Citó. Formal analysis: Jacqueline de-Aguiar-Barros, Maria de Fátima Ferreira-da-Cruz, Fabiana Granja, Rebecca de Abreu-Fernandes, Lucas Tavares de Queiroz, Natália Ketrin Almeida-de-Oliveira Mocelin. Funding acquisition: Maria de Fátima Ferreira-da-Cruz, Fabiana Granja. Investigation: Jacqueline de-Aguiar-Barros, Daniel da Silva e Silva, Maria de Fátima Ferreira-da-Cruz. Methodology: Jacqueline de-Aguiar-Barros, Daniel da Silva e Silva, Rebecca de Abreu-Fernandes, Lucas Tavares de Queiroz, Maria de Fátima Ferreira-da-Cruz, Fabiana Granja. Project administration: Jacqueline de-Aguiar-Barros, Maria de Fátima Ferreira-da-Cruz, Fabiana Granja. Resources: Maria de Fátima Ferreira-da-Cruz, Cláudio Tadeu Daniel-Ribeiro, Fabiana Granja. Software: Jacqueline de-Aguiar-Barros, Arthur Camurça Citó. Supervision: Maria de Fátima Ferreira-da-Cruz, Fabiana Granja. Writing: original draft: Jacqueline de-Aguiar-Barros, Maria de Fátima Ferreira-da-Cruz, Fabiana Granja. Writing—review & editing: Maria de Fátima Ferreira-da-Cruz, Claudio Tadeu Daniel-Ribeiro, Fabiana Granja, Natalia Mocelin, Rebecca de Abreu-Fernandes, Lucas Tavares de Queiroz. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Council for Scientific and Technological Development (CNPq), the Department of Science and Technology in Health / Ministry of Health (DECIT/MS), the Oswaldo Cruz Foundation (Fiocruz), the Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ), the Health Surveillance Secretariat/Ministry of Health (SVS/MS) and the Federal University of Roraima—Nº 60/2022—PRPPG (UFRR). MFFC and CTDR are supported by CNPq, Brazil, through Research Productivity Fellowships, and are “Scientists of Our State” of Faperj.
Institutional Review Board Statement
The study was approved by the Research Ethics Committee of the Federal University of Roraima (CEP/UFRR): CAAE 24122619.6.0000.5302, March 17, 2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Sequences were deposited in Genbank™ with accession numbers PP584057-PP584104.
Acknowledgments
We would like to thank all the patients who volunteered to participate in this study. We thank Hugo Almeida for his support in collecting samples and Jener Franco and José Carlos Nascimento for transporting the team to the field. For their logistical support, we thank the Municipal Malaria Coordination of Boa Vista, the General Health Surveillance Coordination, the Department of Epidemiological Surveillance and the State Malaria Control Centre. To the students and technical staff of LaBMol/CBio/UFRR and the Malaria Research Laboratory/FIOCRUZ for their support in laboratory analysis. And to the microscopists in the malaria rooms at the Sayonara Health Unit and Cosme e Silva Emergency Room.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- World Health Organization: World malaria report 2022. Geneva; 2023. License: CC BY- NC-SA 3.0 IGO.
- Brazil. Ministry of Health. Data for citizens from the Sivep-Malaria, Sinan and E-SUS- VS data sources, for notifications in Brazil from 2007 to 2023. https://public.tableau.com/app/profile/mal.ria.brasil/viz/Dadosparacidado_201925_03_2020/Incio (accessed on 13 Abr 2024).
- Brazil. Ministry of Health. Health Surveillance Secretariat. Epidemiological Bulletin—Malaria 2021. Brasília: Ministry of Health; 2022.
- World Health Organization: Global technical strategy for malaria 2016-2030. Geneva; 2015. License: CC BY-NC-SA 3.0 IGO.
- Brazil. Ministry of Health. Health Surveillance Secretariat. Department of Immunization and Communicable Diseases. Eliminate Malaria Brazil: National Malaria Elimination Plan. Brasília, 2022. 60pp.
- World Health Organization. Artemisinin resistance and artemisinin-based combination therapy efficacy. Geneva; 2018. License: CC BY-NC-SA 3.0 IGO.
- Payne, D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987 Aug;3(8):241-6.
- Cortese JF, Caraballo A, Contreras CE, Plowe CV. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J Infect Dis. 2002 Oct 1;186(7):999-1006. [CrossRef]
- Griffing SM, Mixson-Hayden T, Sridaran S, Alam MT, McCollum AM, Cabezas C. South American Plasmodium falciparum after the malaria eradication era: clonal population expansion and survival of the fittest hybrids. PLoS One. 2011;6(9):e23486. [CrossRef]
- Sridaran S, Rodriguez B, Soto AM, Macedo De Oliveira A, Udhayakumar V. Molecular analysis of chloroquine and sulfadoxine-pyrimethamine resistance-associated alleles in Plasmodium falciparum isolates from Nicaragua. Am J Trop Med Hyg. 2014 May;90(5):840-5. [CrossRef]
- Pelleau S, Moss EL, Dhingra SK, Volney B, Casteras J, Gabryszewski SJ. Adaptive evolution of malaria parasites in French Guiana: Reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc Natl Acad Sci U S A. 2015 Sep 15;112(37):11672-7. [CrossRef]
- Noronha E, Alecrim MG, Romero GA, Macêdo V. Mefloquine resistance in children with falciparum malaria in Manaus, AM, Brazil]. Rev Soc Bras Med Trop. 2000 Mar-Apr;33(2):201-5.
- Tchekounou C, Zida A, Zongo C, Soulama I, Sawadogo PM, Guiguemde KT. Antimalarial drugs resistance genes of Plasmodium falciparum: a review. Ann Parasitol. 2022;68(2):215-225.
- Newton PN, Dondorp A, Green M, Mayxay M, White NJ. Counterfeit artesunate antimalarials in southeast Asia. Lancet. 2003 Jul 12;362(9378):169. [CrossRef]
- Ouji M, Augereau JM, Paloque L, Benoit-Vical F. Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite. 2018;25:24. [CrossRef]
- Lapouble OMM, Santelli ACFS, Muniz-Junqueira MI. Epidemiological situation of malaria in the Brazilian Amazon region, 2003 to 2012. Rev Panam Salud Publica, 2015;38:4.
- Ward KE, Fidock DA, Bridgford JL. Plasmodium falciparum resistance to artemisinin-based combination therapies. Curr Opin Microbiol. 2022 Oct;69:102193. [CrossRef]
- Straimer J, Gnädig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015 Jan 23;347(6220):428-31. [CrossRef]
- Yeung S, Van Damme W, Socheat D, White NJ, Mills A. Access to artemisinin combination therapy for malaria in remote areas of Cambodia. Malar J. 2008 May 29;7:96. [CrossRef]
- Chenet SM, Okoth, SA, Huber, CS, Chandrabose J, Lucchi, NW, et al. Independent Emergence of the Plasmodium falciparum Kelch Propeller Domain Mutant Allele C580Y in Guyana. Journal of Infectious Diseases, 2016, 213 (9), pp.1472-1475. [CrossRef]
- Heemskerk, M, Duijves, C. Small-scale gold mining and conflict in Suriname. In: Small- scale gold mining in the transboundary areas of Brazil, Suriname and French Guiana. Social and Environmental Issues. Report Paramaribo, Suriname: United Nations Development Programme.2011. p. 85-101.
- Douine M, Musset L, Corlin F, Pelleau S, Pasquier J, Mutricy L. Prevalence of Plasmodium spp. in illegal gold miners in French Guiana in 2015: a hidden but critical malaria reservoir. Malar J. 2016 Jun 9;15:315. [CrossRef]
- Mathieu, LC; Singh, P; Monteiro,WM; Magris, M; Cox, H; Lazrek, Y. Kelch13 mutations in Plasmodium falciparum and risk of spreading in Amazon basin countries, Journal of Antimicrobial Chemotherapy, Volume 76, Issue 11, November 2021, p. 2854-2862. [CrossRef]
- De Aguiar Barros J, Granja F, Pequeno P, Marchesini P, Ferreira da Cruz MF. Gold miners augment malaria transmission in indigenous territories of Roraima state, Brazil. Malar J. 2022 Nov 29;21(1):358. [CrossRef]
- Recht J, Siqueira AM, Monteiro WM, Herrera SM, Herrera S, Lacerda MVG. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J. 2017 Jul 4;16(1):273. [CrossRef]
- Yanomami HA, Wnasseduume A. Publishers Hutukara Associação Yanomami & Associação Wanasseduume Ye’kwana. Amazonia. 2021. 52p.
- Nacher M, Guérin PJ, Demar-Pierre M, Djossou F, Nosten F, Carme B. Made in Europe: will artemisinin resistance emerge in French Guiana? Malar J. 2013 May 4;12:152.
- Yan, S.D., Orkis, J., Khan Sohail, S. et al. Digging for care-seeking behaviour among gold miners in the Guyana hinterland: a qualitative doer non-doer analysis of social and behavioural motivations for malaria testing and treatment. Malar J 19, 235 (2020). [CrossRef]
- Mvumbi DM, Kayembe JM, Situakibanza H, Bobanga TL, Nsibu CN, Mvumbi GL. Falciparum malaria molecular drug resistance in the Democratic Republic of Congo: a systematic review. Malar J. 2015 Sep 17;14:354. [CrossRef]
- Costa, G.L., Amaral, L.C., Fontes, C.J.F. et al. Assessment of copy number variation in genes related to drug resistance in Plasmodium vivax and Plasmodium falciparum isolates from the Brazilian Amazon and a systematic review of the literature. Malar J 16, 152 (2017). [CrossRef]
- Pan American Health Organization. Manual de referencia para la vigilancia, el seguimiento y la evaluación de la malaria. Washington, D.C.; 2018. License: CC BYNC-SA 3.0 IGO.
- Pacheco, M.A., Forero-Peña, D.A., Schneider, K.A. et al. Malaria in Venezuela: changes in the complexity of infection reflects the increment in transmission intensity. Malar J 19, 176 (2020). [CrossRef]
- Sales, HJ de; Oliveira, IAD; Galdino, LKA. Production of urban space in Boa Vista, RR: from planning to “disordered” expansion. Terra Livre, 2022; 1:6.
- Barros FSM, Horório, NA. “Man biting rate seasonal variation of malaria vectors in Roraima, Brazil.” Memórias do Instituto Oswaldo Cruz 102 (2007): 299-302.
- Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014 Jan 2;505(7481):50-5. [CrossRef]
- MapBiomas Project—Collection 7.0 of the 2021 Annual Series of Land Use and Coverage Maps of Brazil. Available in: https://brasil.mapbiomas.org/colecoes-mapbiomas/. Accessed on, 2023.
- IBGE—Brazilian Institute of Geography and Statistics. Vectors of geopolitical limits, of Indigenous Lands, Available in: https://www.ibge.gov.br/geociencias/downloads-geociencias.html (accessed on 01 Sept 2023).
- De-Aguiar-Barros, J., Silva, DS., Citó, AC., Peterka, C., Ferreira-da-Cruz, MF. A snapshot of a representative Brazilian state of illegal mining in indigenous areas during the era of malaria elimination. CSP, 2024, In press.
- Murta FLG, Marques LLG, Santos APC, Batista TSB, Mendes MO, Silva ED. Perceptions about malaria among Brazilian gold miners in an Amazonian border area: perspectives for malaria elimination strategies. Malar J. 2021 Jun 26;20(1):286. [CrossRef]
- Inoue J, Jovel I, Morris U, Aydin-Schmidt B, Islam A, Segurado AC. Absence of Plasmodium falciparum K13 Propeller Domain Polymorphisms among Field Isolates Collected from the Brazilian Amazon Basin between 1984 and 2011. Am J Trop Med Hyg. 2018 Dec;99(6):1504-1507. [CrossRef]
- Mathieu LC, Cox H, Early AM, Mok S, Lazrek Y, Paquet JC, Ade MP, Lucchi NW, Grant Q, Udhayakumar V, Alexandre JS, Demar M, Ringwald P, Neafsey DE, Fidock DA, Musset L. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. Elife. 2020 May 12;9:e51015.
- Hassett MR, Roepe PD. Origin and Spread of Evolving Artemisinin-Resistant Plasmodium falciparum Malarial Parasites in Southeast Asia. Am J Trop Med Hyg. 2019 Dec;101(6):1204-1211. [CrossRef]
- Straimer J, Gnädig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015 Jan 23;347(6220):428-31. [CrossRef]
- Huang F, Yan H, Xue JB, Cui YW, Zhou SS, Xia ZG, Abeyasinghe R, Ringwald P, Zhou. XN. Molecular surveillance of pfcrt, pfmdr1 and pfk13-propeller mutations in Plasmodium falciparum isolates imported from Africa to China. Malar J. 2021 Feb 6;20(1):73. [CrossRef]
- Rodrigues ABB, de Abreu-Fernandes R, Neto Z, Jandondo D, Almeida-de-Oliveira NK, de Lavigne Mello AR, Morais J, Daniel-Ribeiro CT, Menard D, Ferreira-da-Cruz MF. Pfkelch13 Plasmodium falciparum Mutations in Huambo, Angola. Pathogens. 2022 May 8;11(5):554.
- Lucchi NW, Abdallah R, Louzada J, Udhayakumar V, Oliveira-Ferreira J. Molecular Surveillance for Polymorphisms Associated with Artemisinin-Based Combination Therapy Resistance in Plasmodium falciparum Isolates Collected in the State of Roraima, Brazil. Am J Trop Med Hyg. 2020 Feb;102(2):310-312. [CrossRef]
- Gomes LR, Lavigne A, Peterka CL, Brasil P, Ménard D, Daniel-Ribeiro CT, Ferreira-da-Cruz MF. Absence of K13 Polymorphism in Plasmodium falciparum from Brazilian Areas Where the Parasite Is Endemic. Antimicrob Agents Chemother. 2018 Sep 24;62(10):e00354-18. [CrossRef]
- Itoh M, Negreiros do Valle S, Farias S, Holanda de Souza TM, Rachid Viana GM, Lucchi N. Efficacy of Artemether-Lumefantrine for Uncomplicated Plasmodium falciparum Malaria in Cruzeiro do Sul, Brazil, 2016. Am J Trop Med Hyg. 2018 Jan;98(1):88-94. [CrossRef]
- WWARN K13 Genotype-Phenotype Study Group. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments-a WWARN individual patient data meta-analysis. BMC Med. 2019 Jan 17;17(1):1. [CrossRef]
- Olivera MJ, Guerra AP, Cortes LJ, et al. Artemether-Lumefantrine Efficacy for the Treatment of Uncomplicated Plasmodium falciparum Malaria in Choco, Colombia after 8 Years as First-Line Treatment. The American Journal of Tropical Medicine and Hygiene. 2020 May;102(5):1056-1063.
- Owoloye A, Olufemi M, Idowu ET, Oyebola KM. Prevalence of potential mediators of artemisinin resistance in African isolates of Plasmodium falciparum. Malar J. 2021 Dec 2;20(1):451. [CrossRef]
- Ullah I, Farringer MA, Burkhard AY, Hathaway E, Willett BC, Khushu M. Artemisinin resistance mutations in Pfcoronin impede hemoglobin uptake. bioRxiv [Preprint]. 2023 Dec 22:2023.12.22.572193.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).