1. Introduction
Traditionally known for their anti-inflammatory properties through the inhibition of cyclooxygenase enzymes COX-1 and COX-2, non-steroidal anti-inflammatory drugs (NSAIDs) are being investigated for their expanded therapeutic potential in oncological contexts [
1,
2].
Additionally, to modulate inflammation and cellular signalling, these drugs have shown the ability to interfere with tumour progression by inducing effects that may vary from induced cellular apoptosis to angiogenesis and metastasis retardment. These properties are inconsistent with the evidence of COX-2 overexpression in tumours and its association with malignant features, which highlights the importance of exploring the actions of NSAIDs beyond the realm of inflammation [
3,
4,
5].
Taking this reasoning into account, this in silico research addresses the potential interaction between specific NSAIDs - sulindac, celecoxib, diclofenac and nimesulide - and enzymes that are crucial for the cell membranes lipid homeostasis, in particular platelet-activating factor acetyl hydrolase (PAF-AH) and lysophosphatidylcholine acyl transferase 3 (LPCAT3), t enzymes that are part of the Land’s cycle [
6,
7].
The Land's cycle is a process that occurs in response to the frequency of damage to the plasma membrane, promoting membrane remodelling. The regulation of PAF levels is performed by the enzyme platelet-activating factor acetyl hydrolase. Membrane remodelling is facilitated by the action of lysophosphatidylcholine acyltransferase 3, which reintegrates a fatty acid into the sn2 position of the membrane phospholipid [
8].
These enzymes, which are essential for the regulation of phospholipids and oxidised fatty acids, share structural similarities with COX enzymes, both in terms of lipid substrates and the hydrophobic nature of their active sites [
9,
10].
Such structural similarities suggest functional adaptability in the tumour context, where modulation of the cellular microenvironment, including inflammation and angiogenesis, plays an important role. Given the wide range of NSAIDs and their frequent use in clinical and preclinical settings, studying their interaction with PAF-AH and LPCAT3 creates new opportunities for understanding the complex dynamics of tumour progression and potential therapeutic approaches. [
11,
12]
Structurally, LPCAT3 has distinct domains that enhance its interactions with cellular membranes. These domains facilitate efficient access to lipid substrates. The active site of LPCAT3 contains essential amino acids such as serine , histidine and aspartate that form a catalytic triad involved in acyl transfer reactions [
13].
The enzyme's transmembrane domains contain hydrophobic amino acids, including leucine and isoleucine , ensuring stable membrane integration and facilitating interaction with lipid substrates. The active site interface features polar and charged amino acids that are critical for substrate specificity and regulation of enzyme activity. These residues orient the substrate appropriately and stabilize the transition states of the reaction [
14].
This research aims to elucidate, through in silico studies and literature review, how some specific NSAIDs might influence the activity of these key enzymes, potentially contributing to the delineation of more precise and personalised therapeutic strategies in cancer treatment. Furthermore, understanding the interactions between NSAIDs and PAF-AH and LPCAT3 may shed light on the potential mechanisms behind their anti-cancer effects.
The synergistic interaction between PAF-AH and LPCAT3 is vital for lipid homeostasis; while PAF-AH removes potentially harmful oxidized lipids, LPCAT3 ensures that lipoproteins are enriched with polyunsaturated fatty acids (PUFAs), enhancing vascular elasticity and functionality. This balance is crucial for cardiovascular health and other metabolic processes, as we show at the
Figure 1, [
15,
16].
2. Results and Discussion
The following findings are derived from molecular dynamics and molecular docking approaches used to study the lysophosphatidylcholine acyltransferase 3 (LPCAT3) and intracellular platelet activating factor acetyl hydrolase (PAF AH) proteins. These proteins are involved in lipid metabolism, repair, remodelling and/or modulation of inflammation, both key processes in liver and cardiovascular health [
17].
This research delves into the intricate interactions between non-steroidal anti-inflammatory drugs (NSAIDs) and two critical enzymes, PAF-AH and LPCAT3, revealing their potential antitumor properties. This in silico evaluation gathers preliminary evidence that NSAIDs, particularly Acetaminophen (ACE) and Ibuprofen (IBU), can modulate these enzymes, influencing both inflammation and tumour progression. These findings pave the way for oncological therapies based on NSAIDs, exploiting their effects on enzymatic activities that are crucial for cancer development and therapeutic response. [
18,
19].
NSAIDs are a widely used class of compounds in both clinical and dietary contexts. Although they have demonstrated significant benefits in many medical conditions, there are also reports of associated hepatotoxicity [
20].
This hepatotoxicity, also known as drug-induced liver injury (DILI), describes liver damage as acute liver inflammation induced by drugs in general. This is mostly due to long-term enzymatic interactions that are involved in the metabolism of membrane phospholipids, which occurs mainly because of the first-pass effect of drugs metabolised by the liver [
21].
After an initial in silico screening to identify the main NSAIDs indicated as palliative agents in cancer treatment, we evaluated their physical and chemical properties and performed molecular docking to assess their ability to interact in the target enzymes. The results were confronted with clinical findings reported in the currently available literature.
To elucidate the molecular interactions between NSAIDs and these key enzymes involved in inflammatory pathways, we used an integrative approach by combining the DataWarrior analysis software suite [
22], with the sophisticated docking capabilities of GOLD (Genetic Optimization for Ligand Docking) 23].
This methodology facilitated a rigorous evaluation of ligand-protein coupling phenomena, taking advantage of GOLD's piecewise linear potential (PLP) scoring algorithm to quantitatively evaluate the binding affinities of NSAIDs to the active sites of PAF-AH and LPCAT3, which are mainly present in the liver.
With this systematic analysis, the identification of a subset of seven NSAIDs with strong interaction propensities with PAF-AH was possible. Notably, within this subset, paracetamol, and ibuprofen emerged as compounds of interest, demonstrating higher coupling efficiency, as shown in
Table 1.
These results highlight the potential of paracetamol and ibuprofen to modulate the enzymatic activity of PAF-AH, using the PDB code 3D5e, thus contributing to a deeper understanding of the molecular dynamics underlying NSAID-mediated modulation of inflammatory responses.
From the structural standpoint, the efficacy of PAF-AH is linked to the presence of specific residues in its active site that are essential for the correct binding and cleavage of substrates, acting mainly through its catalytic triad composed of residues Ser 273, His 351 and Asp 296. In this in silico analysis the hydrophobic pocket was taken into consideration (
Figure 2 and on the
Figure 3), as it plays an important role in substrate stabilisation and specificity [
20,
21].
The intricate molecular interactions between ACE and the PAF-AH are depicted, highlighting the formation of hydrogen bonds between ACE and a range of amino acid residues located within the enzyme's hydrophobic cavity. Notably, key residues of the catalytic triad, integral to the enzyme's functionality, are indicated with asterisks to underscore their pivotal roles in these interactions.
We can see in
Figure 2B that the ACE is well located in the cavity of the site, where interactions with the secondary site and the catalytic site of the protein will possibly occur. In
Figure 2C we observe close interactions, with less than 2 angstroms, possible hydrogen interactions. We can also observe in
Figure 2 D the close interaction with the amino acid essential to the catalytic site Ser 273 where studies show that if it makes covalent interactions, there is a change in the protein's enzymatic activity [
24].
We observe in
Figure 3B that the compound IBU interacts outside the catalytic triad zone, where it possibly interacts with the protein. Just on the secondary site, as we also observed in
Figure 3C, its interactions occur mainly with Q 352 and Try 160.
In the diagram,
Figure 3D, we see that the interactions are sufficient with the amino acids of the secondary site. Due to the fact, that interaction can physically prevent any substrate from binding to the catalytic triad indirectly, occurring only in the secondary site also known as the substrate stabilizing site [
24]
From the metabolic perspective, there is robust evidence that platelet activation factor (main substrate of PAF-AH) directly affects pathogenic inflammatory response, especially as a cell signalling molecule [
27]. Furthermore, this mechanism had been investigated regarding immunity and many cancer microenvironments.
One study showed that upon activation, PAFRs (Platelet-activating factor receptors) induce tumour macrophage and dendritic cell phenotypes that suppress innate and adaptive immune reactions to tumours [
28]. It has also been suggested that PAFR antagonists, when administered in combination with chemotherapy, may be a promising strategy for cancer treatment [
29].
Another study of metastatic melanoma cell lines showed that the differential expression of PAFR is associated with the regulation of pathways that promote cell migration and metastasis [
30].
Interestingly, in addition to its role in melanoma, PAF-AH has also been associated with BRCA1-mutant breast cancer, suggesting a potential protective effect by negatively regulating key signalling pathways such as the WNT pathway, which is critical for embryonic development [
31,
32].
These findings might be added up to the possibility of a molecular interplay between NSAID and PAF-AH and the role of the enzyme in pathology. Having said that, we emphasize a high complexity of functions that deserves multifactorial analysis for the development of new therapeutic strategies against cancer [
33].
The other protein target for this study was LPCAT3, using the PDB code 7F3X, which is essential in the Lands' pathway, by regulating membrane phospholipid fatty acid composition, directly influencing membrane fluidity and functionality. This regulation is vital for cellular processes such as cell division, signalling, and molecule transport. LPCAT3 synthesizes phospholipids, potential precursors to Platelet-Activating Factor (PAF), while Platelet-Activating Factor Acetyl hydrolase (PAF-AH) breaks down PAF [
34].
In order to assess the binding affinities of NSAIDs to LPCAT3, the results were also obtained through the GOLD software’s PLP scoring algorithm. These metrics, presented in
Table 2, offer insights into how NSAIDs might modulate this enzyme, impacting key biological functions (
Figure 4).
The metabolic implications of LPCAT3, significantly impacts the biology of tumour cells by modifying membrane lipid compositions, thereby affecting crucial cellular processes such as proliferation, migration, and invasion. Modulating LPCAT3 activity or expression can effectively influence membrane dynamics and signalling pathways involved in tumour progression, making LPCAT3 an extremely promising target for cancer therapy [
36].
Pathogenically, LPCAT3 is closely linked to tumours' resistance to ferroptosis, a type of lipid peroxide-mediated cell death. Overexpressing LPCAT3 in tumour cells effectively prevents ferroptosis, thereby promoting survival and growth. Conversely, inhibiting LPCAT3 might significantly inhibit tumour growth by sensitizing cells to this form of cell death [
37].
The interaction of anti-inflammatory agents is stabilized by the tyrosine tunnel so that it binds to the Histidine essential for the enzyme activity of the protein.
This already shows us the possibility of physical inhibition of the protein acting with the PUFA's, meaning that they are unable to stabilize in the site to undergo the enzymatic action of membrane fluid repair carried out by LPCAT3 [
36].
3. Materials and Methods
The study took a multi-faceted approach using two different software tools to analyse and evaluate the binding affinities of compounds to the active sites of liver enzymes. The powerful molecular docking capabilities of GOLD (Genetic Optimisation for Ligand Docking) were used in conjunction with the DataWarrior (DW) software [
22]. This combination enabled molecular docking simulations to be performed that predicted the preferred orientation of the molecules in relation to the active sites of the enzymes under investigation ,[
23].
The chemical structures of the NSAIDs were obtained by data mining for interaction properties with the studied enzymes using the DW software on the ChemBL database. DW was used to obtain parameters for the molecular properties of these compounds, and a parameterised library was generated at a suitable pH for later use by GOLD.
Molecular Docking was carried out by GOLD, where 100 replicates per compound were made from a previously prepared library, the enzymes to obtained LPCAT3 (7F3X) and PAFAH (3D5E) in the PDB (Protein Data Bank) databases. Both proteins already had reference ligands that were used during the tests to guide the results, which also required the use of the normalised score, which verifies the strength of the interaction in relation to the number of atoms in the ligands.
The results were analysed by DW using the piecewise linear potential (PLP) score, a parameter generated by GOLD to objectively quantify the binding affinities of the compounds. This algorithm quantitatively evaluates several factors, such as the structural complementarity of the compound with the target site and the interaction energy. This integrated approach provided valuable information on the interaction between the compounds and the active sites of the PAF AH and LPCAT3 enzymes [
22,
23]. All figures using these studies were generated using Pymol [
38], Biovia Discovery Studio [
39] and LigPlot+ [
40] software.
4. Conclusions
This study links the established and emerging therapeutic roles of NSAIDs, highlighting their potential in cancer therapy while advising against associated cardiovascular risks.
Due to the fact, the negative feedback mechanisms of the regulatory form, which with continuous use medications, much is said about the adaptability of the organism. However, little is said about long-term metabolic dysregulation, silent deregulation and homeostasis. Being seen as expected adverse effects in cancer treatment patients, due to their fragile state of health.
These risks are little associated with enzymes, mainly enzymes that regulate membrane homeostasis, which act on phospholipids, which generate precursors of inflammatory mediators.
Of these enzymes in question, we essentially have PAFAH, in addition to acting directly with PAF, it is also associated with lipid metabolism, as it also has the function of lp-PLA2 (lipoprotein-associated phospholipase), its dysregulation is described as causing atherosclerosis in some cases. Enzyme seen as key to the cardiovascular system, as it is present indirectly, but plays an important role in coagulation and fatty plaques.
This enzyme associated with LPCA3, which has been used as a biomarker for some types of tumours, is also associated with hyperlipidaemia profiles, when deregulated and changes in lipids present in the circulation.
By elucidating the interactions of NSAIDs with key enzymatic pathways, we lay the foundation for future research and therapeutic innovations, aiming to maximize clinical benefits while minimizing risks.
Based on these results, we can relate the effects of compounds on plasma membrane fluidity and acute liver inflammation to long-term interactions and negative feedback mechanisms that lead to dysfunction and lack of repair, accumulation of membrane damage.
Today, the uncontrolled and inappropriate excessive use of NSAIDs is also seen, due to their free sale in some countries.
The medical indication is irregular, for patients to use it when they "feel pain or fever, which can cause more harmful effects than palliative ones, especially in cancer patients, due to the already weakened metabolism of the treatment.
In our study, we demonstrated the potential interaction of several anti-inflammatory drugs with Landis cycle enzymes, which is associated with long-term enzyme dysfunction, leading to diseases such as tumours, lipid dysfunction and the development of atherosclerosis, which can lead to neurological and hepatotoxic effects in long term.
With this computational approach and future research, it will be possible to improve our understanding of NSAIDs, proposing innovative applications in oncology. The ability of these drugs to inhibit LPCAT3 and alter tumour microenvironments suggests new approaches to cancer treatment, awaiting further validation through in vitro, in vivo, and clinical trials.
Author Contributions
Conceptualization, M.H.T. and A.R.; methodology and software, A.R.; validation, A.R; I.N and I.A.; formal analysis, M.A.O and S.S.; investigation, X.X.; A.R.; I.A.; I.N.; C.R.C.C.; G.A.F.; A.B.S.J.; M.R.; M.A.O. resources, A.R; M.H.T and S.S; data curation, A.R.; writing—original draft preparation, A.R; M.H.T; writing—review and editing, A.R.; I.A.; I.N.; C.R.C.C.; G.A.F.; A.B.S.J.; M.R.; M.A.O.; project administration, funding acquisition, visualization and supervision, S.S and M.H.T.; All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from FCT/MCTES (UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020) through national funds. This work received support and help from FCT/MCTES (LA/P/0008/2020 DOI 10.54499/LA/P/0008/2020, UIDP/50006/2020 DOI 10.54499/UIDP/50006/2020 and UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020), through national funds. The author acknowledges FCT by funding 2020.01423.CEECIND/CP1596/CT0003. This work received support and help from FCT PhD Fellowship BD.2023.00566, 17/19942-7, 17/20291-0 and 442831/2023-4, 309271/2022-3, CNPq. This research was funded by grant 2023/01973-4 and 2022/04772-7 São Paulo Research Foundation (FAPESP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
LAQV/REQUIMTE and FCT from PORTUGAL and CNPQ and FAPESP from BRAZIL.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pannunzio, A. , & Coluccia, M. (2018). Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: A review of oncology and medicinal chemistry literature. Pharmaceuticals, 11(4), 101. [CrossRef]
- Wang, P. , Casner, R. G., Nair, M. S., Wang, M., Yu, J., Cerutti, G.,... & Ho, D. D. (2021). Increased resistance of SARS-CoV-2 variant P. 1 to antibody neutralization. Cell host & microbe, 29(5), 747-751.
- Bacchi, S. , Palumbo, P., Sponta, A., & Coppolino, M. F. (2012). Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents), 11(1), 52-64.
- Yu, Y. R. A. , O’Koren, E. G., Hotten, D. F., Kan, M. J., Kopin, D., Nelson, E. R.,... & Gunn, M. D. (2016). A protocol for the comprehensive flow cytometric analysis of immune cells in normal and inflamed murine non-lymphoid tissues. PloS one, 11(3), e0150606. [CrossRef]
- Zappavigna, S. , Cossu, A. M., Grimaldi, A., Bocchetti, M., Ferraro, G. A., Nicoletti, G. F.,... & Caraglia, M. (2020). Anti-inflammatory drugs as anticancer agents. International journal of molecular sciences, 21(7), 2605. [CrossRef]
- Pereira-Leite, C. , Nunes, C., & Reis, S. (2013). Interaction of nonsteroidal anti-inflammatory drugs with membranes: in vitro assessment and relevance for their biological actions. Progress in lipid research, 52(4), 571-584. [CrossRef]
- Peng, H. , Wu, X., Zhao, L., & Feng, Y. (2016). Dynamic analysis of phospholipid metabolism of mouse macrophages treated with common non-steroidal anti-inflammatory drugs. Molecular and cellular biochemistry, 411, 161-171. [CrossRef]
- Murphy, R. C. , & Folco, G. (2019). Lysophospholipid acyltransferases and leukotriene biosynthesis: intersection of the Lands cycle and the arachidonate PI cycle [S]. Journal of Lipid Research, 60(2), 219-226.
- Kita, Y. , Shindou, H., & Shimizu, T. (2019). Cytosolic phospholipase A2 and lysophospholipid acyltransferases. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1864(6), 838-845.
- Korbecki, J. , Rębacz-Maron, E., Kupnicka, P., Chlubek, D., & Baranowska-Bosiacka, I. (2023). Synthesis and significance of arachidonic acid, a substrate for cyclooxygenases, lipoxygenases, and cytochrome P450 pathways in the tumorigenesis of glioblastoma multiforme, including a pan-cancer comparative analysis. Cancers, 15(3), 946. [CrossRef]
- Markworth, J. F. , Vella, L., Lingard, B. S., Tull, D. L., Rupasinghe, T. W., Sinclair, A. J.,... & Cameron-Smith, D. (2013). Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 305(11), R1281-R1296. [CrossRef]
- Mayer, J. M., Testa, B., Vos, M. R. D., Audergon, C., & Etter, J. C. (1995). Interactions between the in vitro metabolism of xenobiotics and fatty acids: The case of ibuprofen and other chiral profens. In Toxicology in Transition: Proceedings of the 1994 EUROTOX Congress Meeting Held in Basel, Switzerland, August 21–24, 1994 (pp. 499-513). Springer Berlin Heidelberg.
- Zhang Q, Yao D, Rao B, Jian L, Chen Y, Hu K, Xia Y, Li S, Shen Y, Qin A, Zhao J, Zhou L, Lei M, Jiang XC, Cao Y. The structural basis for the phospholipid remodeling by lysophosphatidylcholine acyltransferase 3. Nat Commun. 2021 Nov 25;12(1):6869. [CrossRef]
- Rong X, Wang B, Dunham MM, Hedde PN, Wong JS, Gratton E, Young SG, Ford DA, Tontonoz P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife. 2015 Mar 25;4:e06557.
- Chen J, Fang Z, Luo Q, Wang X, Warda M, Das A, Oldoni F, Luo F. Unlocking the mysteries of VLDL: exploring its production, intracellular trafficking, and metabolism as therapeutic targets. Lipids Health Dis. 2024 Jan 12;23(1):14. [CrossRef]
- Rong X, Wang B, Dunham MM, Hedde PN, Wong JS, Gratton E, Young SG, Ford DA, Tontonoz P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife. 2015 Mar 25;4:e06557.
- Moggia, E. , Koti, R., Belgaumkar, A. P., Fazio, F., Pereira, S. P., Davidson, B. R., & Gurusamy, K. S. (2017). Pharmacological interventions for acute pancreatitis. Cochrane Database of Systematic Reviews, (4). [CrossRef]
- Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr Alzheimer Res. 2005 Jul;2(3):355-65. [CrossRef]
- Ghosh R, Alajbegovic A, Gomes AV. NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species. Oxid Med Cell Longev. 2015;2015:536962. [CrossRef]
- Tudor Car, L. , Dhinagaran, D. A., Kyaw, B. M., Kowatsch, T., Joty, S., Theng, Y. L., & Atun, R. (2020). Conversational agents in health care: scoping review and conceptual analysis. Journal of medical Internet research, 22(8), e17158.
- Mirabito Colafella, K. M. , Neuman, R. I., Visser, W., Danser, A. J., & Versmissen, J. (2020). Aspirin for the prevention and treatment of pre-eclampsia: A matter of COX-1 and/or COX-2 inhibition?. Basic & clinical pharmacology & toxicology, 127(2), 132-141.
- J. C. Cole, J. W. M. J. C. Cole, J. W. M. Nissink, R. Taylor in Virtual Screening in Drug Discovery (Eds. B. Shoichet, J. Alvarez), Taylor & Francis CRC Press, Boca Raton, Florida, USA (2005).
- Thomas Sander, Joel Freyss, Modest von Korff, and Christian Rufener. Journal of Chemical Information and Modeling 2015, 55(2), 460–473. [CrossRef]
- Gonzalez, T. D. , & Bahnson, B. J. (2015). Crystal Structure and Atomic Level Analysis of Plasma PAF-AH. In The Enzymes (Vol. 38, pp. 95-116). Academic Press.
- Arai, H. Platelet-activating factor acetylhydrolase. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:83-94.
- Castro Faria Neto HC, Stafforini DM, Prescott SM, Zimmerman GA. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem Inst Oswaldo Cruz. 2005 Mar;100 Suppl 1:83-91.
- Marín-González E, Malmusi D, Camprubí L, Borrell C. The Role of Dissemination as a Fundamental Part of a Research Project. Int J Health Serv. 2017 Apr;47(2):258-276. [CrossRef]
- Jancar S, Chammas R. PAF receptor and tumour growth. Curr Drug Targets. 2014;15(10):982-7.
- da Silva Junior IA, Stone SC, Rossetti RM, Jancar S, Lepique AP. Modulation of Tumor-Associated Macrophages (TAM) Phenotype by Platelet-Activating Factor (PAF) Receptor. J Immunol Res. 2017;2017:5482768. [CrossRef]
- Dalmaso B, Silva-Junior IAD, Jancar S, Del Debbio CB. Platelet-Activating Factor Receptor (PAFR) Regulates Retinal Progenitor/Stem Cells Profile in Ciliary Epithelium Cells. Int J Mol Sci. 2024 Mar 7;25(6):3084. [CrossRef]
- Jung YS, Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. 2020 Feb;52(2):183-191. [CrossRef]
- Travers JB, Rohan JG, Sahu RP. New Insights into the Pathologic Roles of the Platelet-Activating Factor System. Front Endocrinol (Lausanne). 2021 Mar 15;12:624132. [CrossRef]
- Frommeyer TC, Gilbert MM, Brittain GV, Wu T, Nguyen TQ, Rohan CA, Travers JB. UVB-Induced Microvesicle Particle Release and Its Effects on the Cutaneous Microenvironment. Front Immunol. 2022 May 6;13:880850. [CrossRef]
- Wang, B., & Tontonoz, P. (2019). Phospholipid remodeling in physiology and disease. Annual review of physiology, 81, 165-188. [CrossRef]
- Zhang Q, Yao D, Rao B, Jian L, Chen Y, Hu K, Xia Y, Li S, Shen Y, Qin A, Zhao J, Zhou L, Lei M, Jiang XC, Cao Y. The structural basis for the phospholipid remodeling by lysophosphatidylcholine acyltransferase 3. Nat Commun. 2021 Nov 25;12(1):6869. [CrossRef]
- Shao G, Qian Y, Lu L, Liu Y, Wu T, Ji G, Xu H. Research progress in the role and mechanism of LPCAT3 in metabolic related diseases and cancer. J Cancer. 2022 May 1;13(8):2430-2439. [CrossRef]
- Hashidate-Yoshida T, Harayama T, Hishikawa D, Morimoto R, Hamano F, Tokuoka SM, Eto M, Tamura-Nakano M, Yanobu-Takanashi R, Mukumoto Y, Kiyonari H, Okamura T, Kita Y, Shindou H, Shimizu T. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife. 2015 Apr 21;4:e06328. [CrossRef]
- SEELIGER, D.; DE GROOT, B. L. Ligand Dock and binding site analysis with PyMOL and Autodock/Vina. Journal of Computer-Aided Molecular Design, v. 24, n. 5, p. 417-422, 2010.
- BIOVIA FOUNDATION. Discovery Studio – Accelrys. Versão 4.0. San Diego, 2007.
- WALLACE, A. C.; LASKOWSKI, R. A.; THORNTON, J. M. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Engineering, Design and Selection, v. 8, n. 2, p. 127-134, 1995. [CrossRef]
Figure 1.
The Lands cycle is crucial for regulating lipid homeostasis following pro-inflammatory stimuli, involving enzymes such as PAF-AH, cPLA2, COX, and LPCAT3. These enzymes manage the synthesis and degradation of critical lipid mediators like PAF and play essential roles in inflammatory processes: PAF-AH degrades PAF, limiting inflammation by removing bioactive lipids; cPLA2 releases arachidonic acids, precursors to eicosanoids that mediate inflammation, COX converts arachidonic acid into prostaglandins, central to inflammation and pain; LPCAT3 adjusts the lipid composition of membranes, affecting their fluidity and functionality. Plasma lipoproteins transport fatty acids to cells, where they are utilized in membranes or as energy. By balancing lipid synthesis and degradation, the Lands cycle is vital for cellular integrity and the inflammatory response, offering targets for treating inflammatory and cardiovascular diseases. Enzymatic deficiencies in these enzymes can range from temporary relief of inflammation to serious cardiovascular diseases.
Figure 1.
The Lands cycle is crucial for regulating lipid homeostasis following pro-inflammatory stimuli, involving enzymes such as PAF-AH, cPLA2, COX, and LPCAT3. These enzymes manage the synthesis and degradation of critical lipid mediators like PAF and play essential roles in inflammatory processes: PAF-AH degrades PAF, limiting inflammation by removing bioactive lipids; cPLA2 releases arachidonic acids, precursors to eicosanoids that mediate inflammation, COX converts arachidonic acid into prostaglandins, central to inflammation and pain; LPCAT3 adjusts the lipid composition of membranes, affecting their fluidity and functionality. Plasma lipoproteins transport fatty acids to cells, where they are utilized in membranes or as energy. By balancing lipid synthesis and degradation, the Lands cycle is vital for cellular integrity and the inflammatory response, offering targets for treating inflammatory and cardiovascular diseases. Enzymatic deficiencies in these enzymes can range from temporary relief of inflammation to serious cardiovascular diseases.
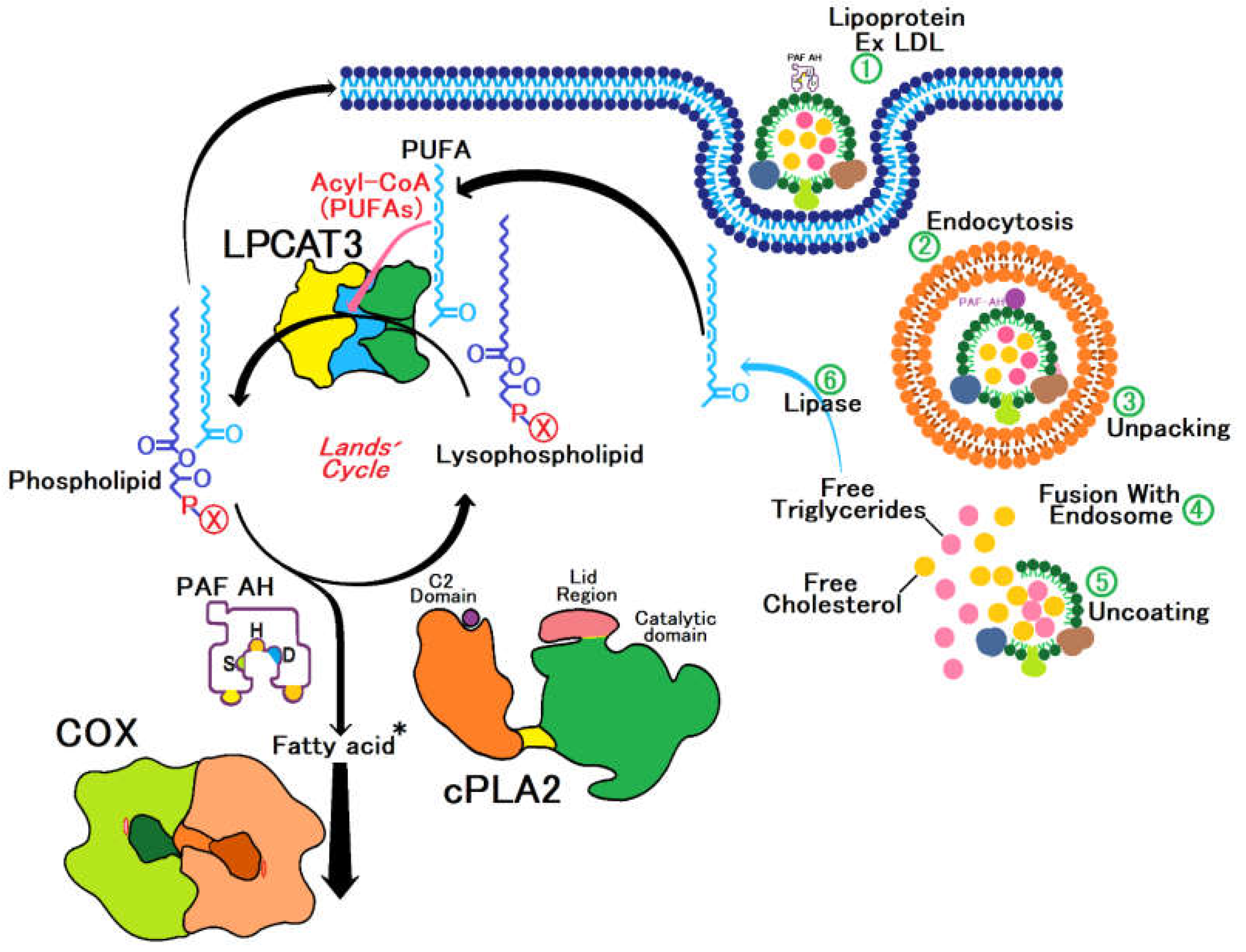
Figure 2.
A showcases the three-dimensional conformation of the PAF-AH enzyme, emphasizing the significant cavity that constitutes the active site. This image was created with Pymol, the surface is coloured according to the electrostatic potential. As the colour legend indicates, the red colour (negative potential) arises from an excess of negative charges near the surface and the blue colour (positive potential) occurs when the surface is positively charged. The white regions correspond to neutral potentials. B, this image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in yellow. C detail how ACE engages with this active site, delineating the spatial alignment and interaction dynamics. This image was created with Pymol, the representation of amino acids on their surface, with the colours depending on the atoms present in them, being red for oxygen, green for carbon and blue for nitrogen, the closest interactions are represented by the colour black. D, this image was created with Biovia Discovery Studio, following colour patterns related to interactions, green for van der Waals, dashed signalling possible hydrogen bond interactions and pink for π-Stacking interactions.
Figure 2.
A showcases the three-dimensional conformation of the PAF-AH enzyme, emphasizing the significant cavity that constitutes the active site. This image was created with Pymol, the surface is coloured according to the electrostatic potential. As the colour legend indicates, the red colour (negative potential) arises from an excess of negative charges near the surface and the blue colour (positive potential) occurs when the surface is positively charged. The white regions correspond to neutral potentials. B, this image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in yellow. C detail how ACE engages with this active site, delineating the spatial alignment and interaction dynamics. This image was created with Pymol, the representation of amino acids on their surface, with the colours depending on the atoms present in them, being red for oxygen, green for carbon and blue for nitrogen, the closest interactions are represented by the colour black. D, this image was created with Biovia Discovery Studio, following colour patterns related to interactions, green for van der Waals, dashed signalling possible hydrogen bond interactions and pink for π-Stacking interactions.
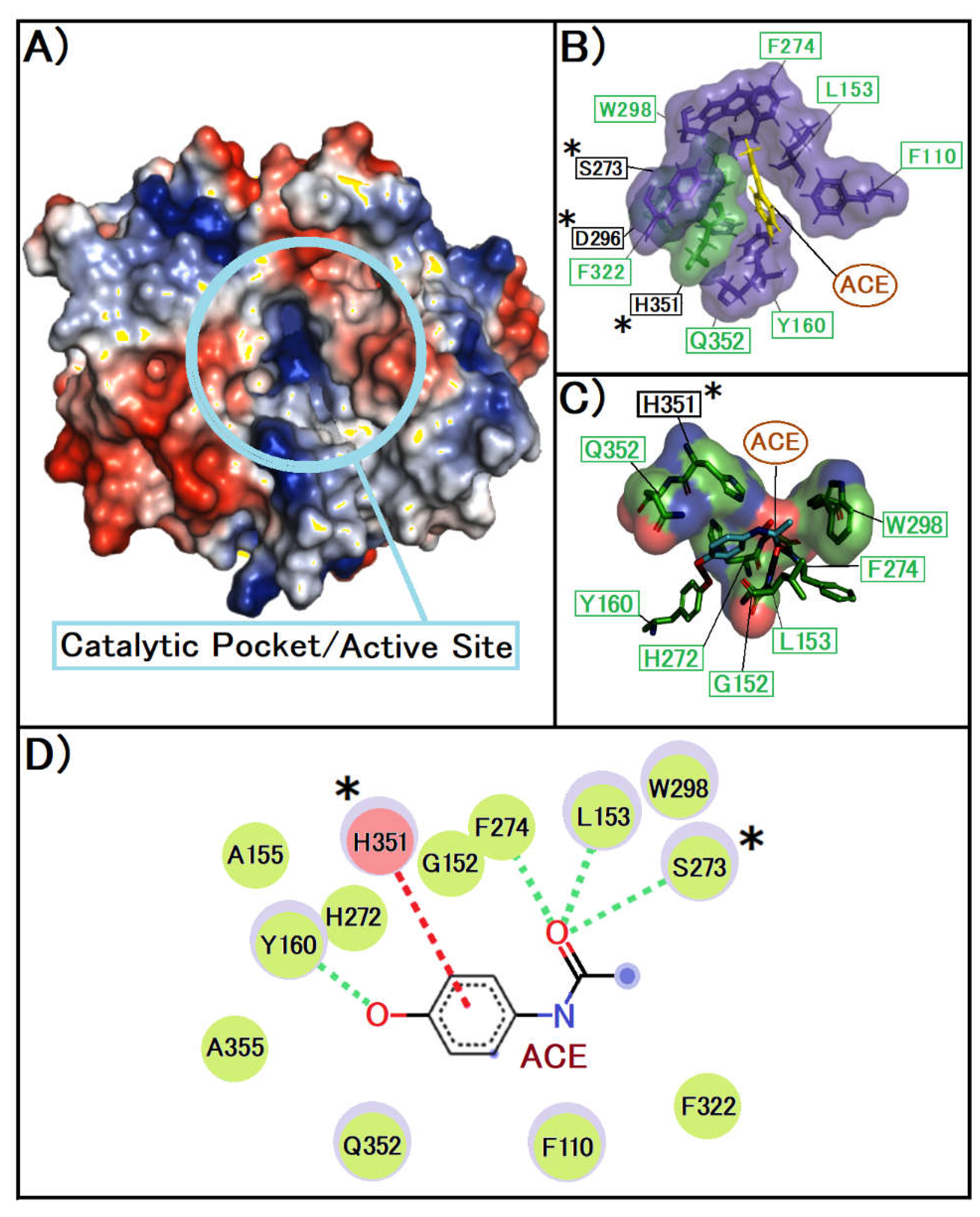
Figure 3.
A presents the three-dimensional model of the PAF-AH enzyme, with a focus on the active site's delineation, this image was created with Pymol, the surface is coloured according to the electrostatic potential. As the colour legend indicates, the red colour (negative potential) arises from an excess of negative charges near the surface and the blue colour (positive potential) occurs when the surface is positively charged. The white regions correspond to neutral potentials. B, This image was created with Pymol, the representation of amino acids on their surface, with the colours depending on the atoms present in them, being red for oxygen, green for carbon and blue for nitrogen, the closest interactions are represented by the colour black C detail the binding mechanism of IBU to the active site, illustrating the precise spatial alignment and interaction dynamics with various residues near the active site and around the catalytic triad, This image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in yellow. D, the intricate molecular interactions between IBU and PAF-AH are depicted, highlighting the formation of hydrogen bonds and hydrophobic interactions between IBU and a range of amino acid residues within the enzyme's hydrophobic cavity. It is important to note that no direct interactions between IBU and the residues of the catalytic triad are observed this image was created with Biovia Discovery Studio, following colours patterns related to interactions, green for van der Waals dashed signalling possible hydrogen bond interactions and pink for π-stacking interactions.
Figure 3.
A presents the three-dimensional model of the PAF-AH enzyme, with a focus on the active site's delineation, this image was created with Pymol, the surface is coloured according to the electrostatic potential. As the colour legend indicates, the red colour (negative potential) arises from an excess of negative charges near the surface and the blue colour (positive potential) occurs when the surface is positively charged. The white regions correspond to neutral potentials. B, This image was created with Pymol, the representation of amino acids on their surface, with the colours depending on the atoms present in them, being red for oxygen, green for carbon and blue for nitrogen, the closest interactions are represented by the colour black C detail the binding mechanism of IBU to the active site, illustrating the precise spatial alignment and interaction dynamics with various residues near the active site and around the catalytic triad, This image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in yellow. D, the intricate molecular interactions between IBU and PAF-AH are depicted, highlighting the formation of hydrogen bonds and hydrophobic interactions between IBU and a range of amino acid residues within the enzyme's hydrophobic cavity. It is important to note that no direct interactions between IBU and the residues of the catalytic triad are observed this image was created with Biovia Discovery Studio, following colours patterns related to interactions, green for van der Waals dashed signalling possible hydrogen bond interactions and pink for π-stacking interactions.
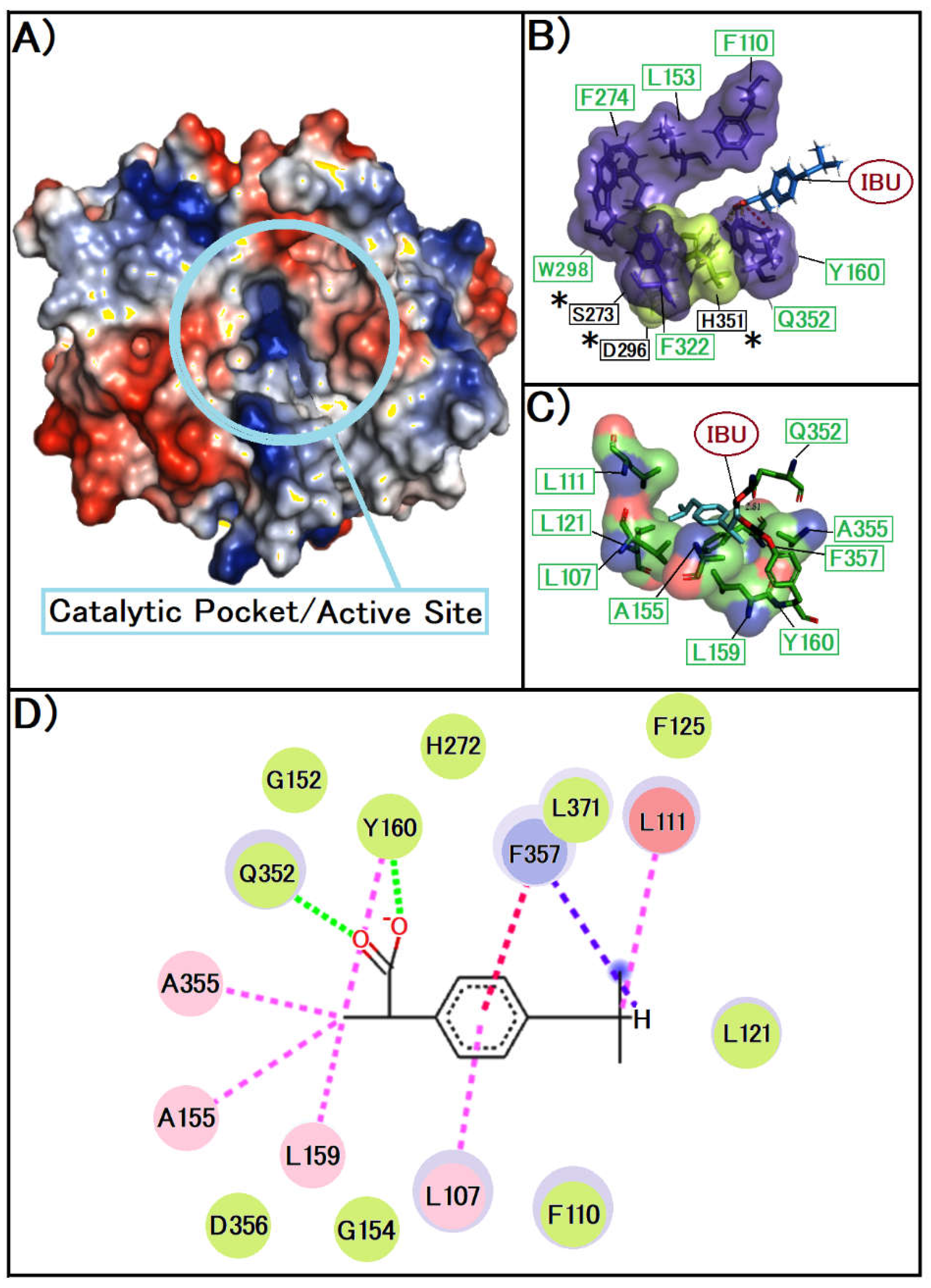
Figure 4.
A, we display the three-dimensional structure of the native LPCAT3 protein, highlighting the active site cavity. This image was created with Pymol, the surface is coloured according to the electrostatic potential. As the colour legend indicates, the red colour (negative potential) arises from an excess of negative charges near the surface and the blue colour (positive potential) occurs when the surface is positively charged. The white regions correspond to neutral potentials. B illustrates the crucial residues involved in the enzymatic activity of LPCAT3. Sections, this image was created with Pymol who show the protein structure in cartoon the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green. C, the image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in Blue. D depict the interaction of Acetaminophen (ACE) with the enzyme's active site. That image was created with Biovia Discovery Studio, following colour patterns related to interactions, green for van der Wall, dashed signalling possible hydrogen bond interactions and pink for staked pi interactions, while E, this image was created with Biovia Discovery Studio, following colour patterns related to interactions, green for van der Wall, dashed signalling possible hydrogen bond interactions, orange the pi alkyl and pink for staked pi interactions and F show the interaction of Ibuprofen (IBU) with the active site of LPCAT3. Asterisks indicate the important residues of the active site, this image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in rose.
Figure 4.
A, we display the three-dimensional structure of the native LPCAT3 protein, highlighting the active site cavity. This image was created with Pymol, the surface is coloured according to the electrostatic potential. As the colour legend indicates, the red colour (negative potential) arises from an excess of negative charges near the surface and the blue colour (positive potential) occurs when the surface is positively charged. The white regions correspond to neutral potentials. B illustrates the crucial residues involved in the enzymatic activity of LPCAT3. Sections, this image was created with Pymol who show the protein structure in cartoon the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green. C, the image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in Blue. D depict the interaction of Acetaminophen (ACE) with the enzyme's active site. That image was created with Biovia Discovery Studio, following colour patterns related to interactions, green for van der Wall, dashed signalling possible hydrogen bond interactions and pink for staked pi interactions, while E, this image was created with Biovia Discovery Studio, following colour patterns related to interactions, green for van der Wall, dashed signalling possible hydrogen bond interactions, orange the pi alkyl and pink for staked pi interactions and F show the interaction of Ibuprofen (IBU) with the active site of LPCAT3. Asterisks indicate the important residues of the active site, this image was created with Pymol, the surface of the amino acids in the secondary site was represented in purple and the amino acids present in the catalytic triad were in green, where in the image the compound is represented in rose.
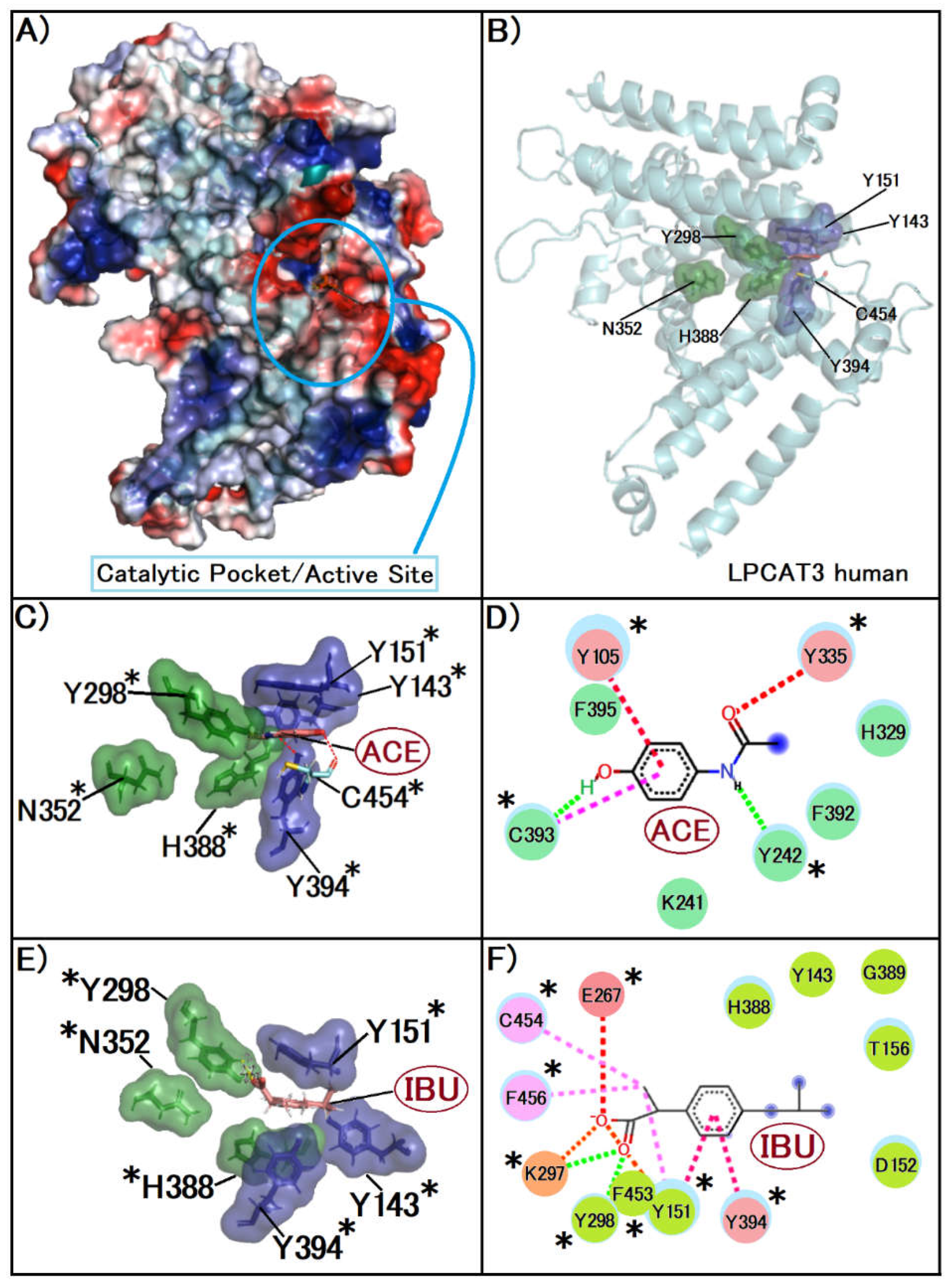
Table 1.
Gold PLP Interaction Analysis of Anti-Inflammatory Drugs with PAF-AH Protein, Highlighting the Optimal Interaction Scores Achieved with Acetaminophen and Ibuprofen.
Table 1.
Gold PLP Interaction Analysis of Anti-Inflammatory Drugs with PAF-AH Protein, Highlighting the Optimal Interaction Scores Achieved with Acetaminophen and Ibuprofen.
| Molecule Name |
Abbreviation |
Origin |
Atoms |
Score Normalized |
PLP Fitness |
|
| Diethyl Phosphate* |
DEP |
Synthetic |
8 |
4.13 |
33.0 |
 |
| Acetaminophen |
ACE |
Synthetic |
11 |
3.50 |
38.5 |
| Ibuprofen |
IBU |
Synthetic |
15 |
3.48 |
52.2 |
| Sulindac |
SUD |
Synthetic |
25 |
2.83 |
70.7 |
| Diclofenac |
DCF |
Synthetic |
19 |
2.77 |
52.6 |
| Nimesulide |
NIM |
Synthetic |
21 |
2.53 |
53.0 |
| Dipyrone |
DIP |
Synthetic |
21 |
2.46 |
51.6 |
| Celecoxib |
CCB |
Synthetic |
26 |
2.38 |
61.9 |
Table 2.
Gold PLP Interaction Analysis of Anti-Inflammatory Drugs with LPCAT3 Protein, Highlighting the Optimal Interaction Scores Achieved with Acetaminophen and Ibuprofen were scored in table.
Table 2.
Gold PLP Interaction Analysis of Anti-Inflammatory Drugs with LPCAT3 Protein, Highlighting the Optimal Interaction Scores Achieved with Acetaminophen and Ibuprofen were scored in table.
| Molecule Name |
Abbreviation |
Origin |
Atoms |
PLP Fitness |
|
| Lysophosphatidylcholine* |
LAP |
Synthetic |
29 |
84.2 |
 |
| Celecoxib |
CCB |
Synthetic |
26 |
72.4 |
| Sulindac |
SUD |
Synthetic |
25 |
66.9 |
| Nimesulide |
NIM |
Synthetic |
21 |
57.2 |
| Diclofenac |
DCF |
Synthetic |
19 |
56.5 |
| Dipyrone |
DIP |
Synthetic |
21 |
54,5 |
| Acetaminophen |
ACE |
Synthetic |
11 |
48,3 |
| Ibuprofen |
IBU |
Synthetic |
15 |
47,3 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).










