Submitted:
27 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
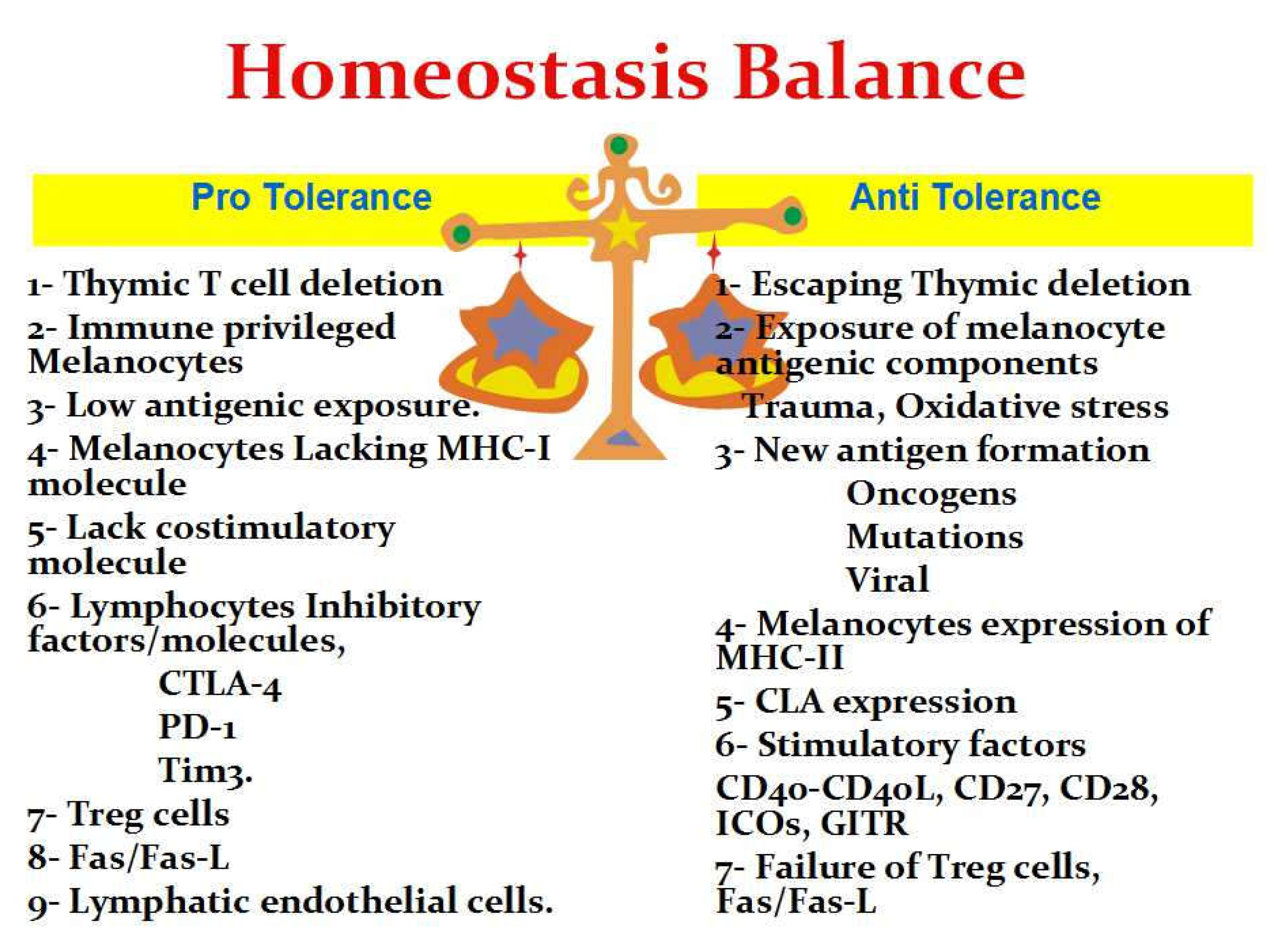
Melanocytes Immune-Privilege
The Central Immune Tolerance
The Peripheral Immune Tolerance
- -
- Cytotoxic T-lymphocyte antigen 4 (CTLA-4), which inhibits CD28-dependent T cell activation, cell cycle progression, and IL-2 production. CTLA-4 inhibition is more pronounced after the initiation of T-cell activation (Alpdogan and van den Brink 2012).
- -
- Programmed cell death-1 (PD-1), also called CD279, is a member of the CD28 family that is expressed on activated T cells and is considered an inhibitory molecule that participates in immune tolerance (Alpdogan and van den Brink 2012).
- -
- T-cell immunoglobulin and mucin-domain containing-3 (Tim-3), which is a co-inhibitory receptor that is expressed on IFN-g-producing T cells and has been shown to suppress their responses upon interaction with their ligand(s) (Rahimi et al. 2019).
- -
- Regulatory T cells (Treg) were first defined as CD4+CD25+ double-positive cells with suppressive functions on immunological response (Alpdogan and van den Brink 2012). Treg cell-mediated suppression of human melanocyte-specific CD8+ T cells causes decreased proliferation, decreased cytokine production, decreased T cell receptor affinity and increased susceptibility to apoptosis. Treg cells can render self-reactive human CD8(+) T cells anergic (Kawakami et al. 2000). Treg may act as cytotoxic T cells that express granzyme A after activation and can kill activated CD4+ and CD8+ T cells by a perforin-dependent mechanism (Alpdogan and van den Brink 2012).
- -
- Fas/Fas-L is another important factor that enhances peripheral tolerance through induction of apoptosis of CTL (Li et al. 2008; Alpdogan and van den Brink 2012).
- -
- Lymphatic endothelial cells (LECs) induce peripheral tolerance by induction of high-level expression of PD-1 with inhibition of the high-affinity IL-2 receptor that is necessary for T(CD8) survival (Lim et al. 2018).
The Breakdown of Tolerance
- -
- loss of proper central deletion due to a thymic failure, which will produce lymphocytes with melanocyte-specific epitopes. Direct evidence has been provided that a large Melan-A/MART-1-specific CD8 T-cell pool is generated during thymic selection (Li et al. 2019). These Melan-A-specific T cells were able to lyse melanoma cells in vitro, indicating their cytotoxic reactivity. While an unusually large repertoire of CD8+ T cells specific for this antigen has been documented, the reasons for its generation have remained mysterious (Romero et al. 2014). The existence of TRP-1 or TRP-2-specific CTL was not proven to induce a relevant breakdown of tolerance that can induce vitiligo (Rausch and Hastings 2012).
- -
- Expression of cutaneous lymphocyte-associated antigen (CLA) on activated T cells and their frequency correlated with the extent of depigmentation and disease activity. Through the expression of CLA, activated T cells can find their way to the skin where they show type 1-cytokine profiles and mediate melanocyte death (Lang et al. 2001).
- -
- Melanocytes expressing MHC-II and melanosomal transmembrane proteins can be transported to the surface of melanocytes and processed as MHC class II antigens via the endocytic pathway (Roberts et al. 2020).
- -
- Other stimulatory factors include:
- -
- Treg cell failure is also possible and decreased Treg cell numbers and function have been reported in patients with several autoimmune diseases including vitiligo (Ben Ahmed et al. 2012).
- -
- PD-1 failure as vitiligo-like lesions developed after anti-PD-1 therapy (Boniface et al. 2017).
- -
- Lymphocytic Fas/Fas-L apoptosis failure can result, and functional polymorphism of the FAS gene was reported with a high vitiligo risk (Li et al. 2008).
Melanocytes Developing New Antigenic Components
Conflict of Interest: None declared.
Funding
References
- Alpdogan O, van den Brink MR. (2012) Immune tolerance and transplantation. Semin Oncol. Dec;39(6):629-42. [CrossRef]
- Andersen MH, Schrama D, Thor Straten P, Becker JC. (2006) Cytotoxic T cells. J Invest Dermatol. Jan;126(1):32-41. [CrossRef]
- Anderson MS, Su MA. (2016) AIRE expands: new roles in immune tolerance and beyond. Nat Rev Immunol. Apr;16(4):247-58. [CrossRef]
- Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB, Ichihashi M. (2012) Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. Apr;132(4):1222-9. [CrossRef]
- Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB. (2008) Evaluation of activatory and inhibitory natural killer cell receptors in non-segmental vitiligo: a flow cytometric study. J Eur Acad Dermatol Venereol. Aug;22(8):970-6. [CrossRef]
- Ben Ahmed M, Zaraa I, Rekik R, Elbeldi-Ferchiou A, Kourda N, Belhadj Hmida N, Abdeladhim M, Karoui O, Ben Osman A, Mokni M, Louzir H. (2012) Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res. Jan;25(1):99-109. [CrossRef]
- Boniface K, Seneschal J, Taïeb A, Merched A. (2017) Vitiligo therapy: restoring immune privilege? Exp Dermatol. Jul;26(7):635-636. [CrossRef]
- Bzioueche H, Simonyté Sjödin K, West CE, Khemis A, Rocchi S, Passeron T, Tulic MK. (2021) Analysis of Matched Skin and Gut Microbiome of Patients with Vitiligo Reveals Deep Skin Dysbiosis: Link with Mitochondrial and Immune Changes. J Invest Dermatol. Sep;141(9):2280-2290. [CrossRef]
- Côté AL, Zhang P, O'Sullivan JA, Jacobs VL, Clemis CR, Sakaguchi S, Guevara-Patiño JA, Turk MJ. (2011) Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T cell responses to tumor-specific antigens. J Immunol. Jan 1;186(1):275-83. [CrossRef]
- Kawakami Y, Suzuki Y, Shofuda T, et al. (2000) T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Res. ;13 Suppl 8:163-169. [CrossRef]
- Khan U, Ghazanfar H.(2018) T Lymphocytes and Autoimmunity. Int Rev Cell Mol Biol. ;341:125-168. [CrossRef]
- Kohil A, Abdalla W, Ibrahim WN, Al-Harbi KM, Al-Haidose A, Al-Asmakh M, Abdallah AM. (2023) The Immunomodulatory Role of Microbiota in Rheumatic Heart Disease: What Do We Know and What Can We Learn from Other Rheumatic Diseases? Medicina (Kaunas). Sep 8;59(9):1629. [CrossRef]
- Lang KS, Caroli CC, Muhm A, Wernet D, Moris A, Schittek B, Knauss-Scherwitz E, Stevanovic S, Rammensee HG, Garbe C. (2001) HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol. Jun;116(6):891-7. [CrossRef]
- Le Poole IC, Luiten RM. (2008) Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun. ;10:227-243. [CrossRef]
- Li M, Sun D, Li C, Zhang Z, Gao L, Li K, Li H, Gao T. (2008) Functional polymorphisms of the FAS gene associated with risk of vitiligo in Chinese populations: a case-control analysis. J Invest Dermatol. Dec;128(12):2820-4. [CrossRef]
- Li Y, Teteloshvili N, Tan S, Rao S, Han A, Yang YG, Creusot RJ. (2019) Humanized Mice Reveal New Insights Into the Thymic Selection of Human Autoreactive CD8+ T Cells. Front Immunol. Feb 4;10:63. [CrossRef]
- Lim WC, Olding M, Healy E, Millar TM. (2018) Human Endothelial Cells Modulate CD4+ T Cell Populations and Enhance Regulatory T Cell Suppressive Capacity. Front Immunol ;9:565. Published Mar 23. [CrossRef]
- Moseley RP, Brown JI, Auld J, et al. (1997) An immunocytochemical study of MHC class I expression on human Langerhans cells and melanocytes. J Pathol. ;181(4):419-425. [CrossRef]
- Rahimi A, Hossein-Nataj H, Hajheydari Z, Aryanian Z, Shayannia A, Ajami A, Asgarian-Omran H. (2019) Expression analysis of PD-1 and Tim-3 immune checkpoint receptors in patients with vitiligo; positive association with disease activity. Exp Dermatol. Jun;28(6):674-681. [CrossRef]
- Rausch MP, Hastings KT. (2012) GILT modulates CD4+ T-cell tolerance to the melanocyte differentiation antigen tyrosinase-related protein 1. J Invest Dermatol. Jan;132(1):154-62. [CrossRef]
- Roberts GHL, Santorico SA, Spritz RA. (2020) The genetic architecture of vitiligo. Pigment Cell Melanoma Res. Jan;33(1):8-15. [CrossRef]
- Romero P, Speiser DE, Rufer N. (2014) Deciphering the unusual HLA-A2/Melan-A/MART-1-specific TCR repertoire in humans. Eur J Immunol. Sep;44(9):2567-70. [CrossRef]
- Shin MK, Im SH, Park HJ, Kim SK, Yim SV, Chung JH, Lee MH. (2011) Association study between polymorphisms of CD28, CTLA4 and ICOS and non-segmental vitiligo in a Korean population. Exp Ther Med. Nov;2(6):1145-1149. [CrossRef]
- Speeckaert R, Lambert J, van Geel N. (2016) Clinical Significance of Serum Soluble CD Molecules to Assess Disease Activity in Vitiligo. JAMA Dermatol. Nov 1;152(11):1194-1200. [CrossRef]
- Wang Y, Li S, Li C. (2019) Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med Sci Monit. Feb 6;25:1017-1023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



