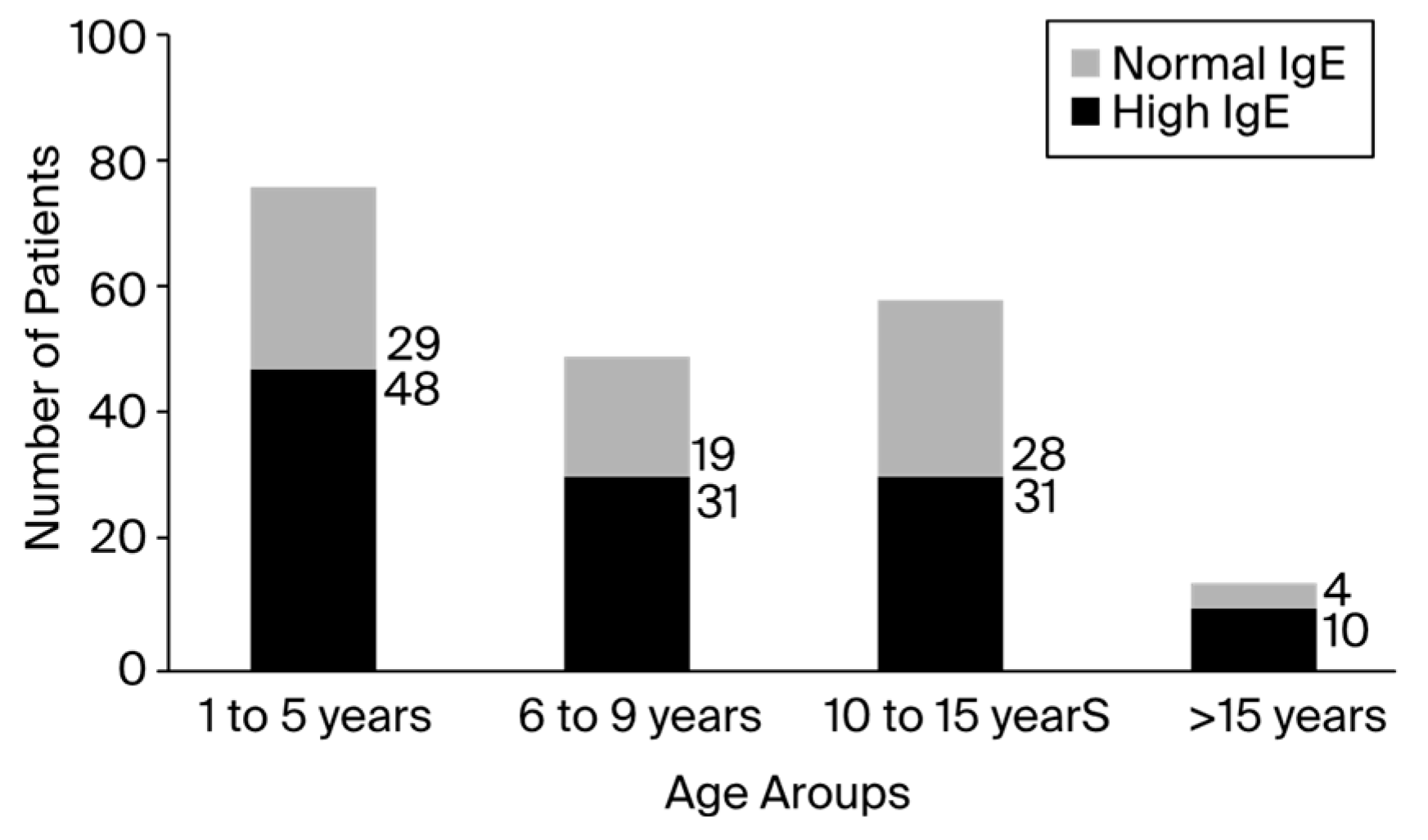1. Introduction
Immunoglobulin E (IgE) antibodies are critical for parasite and toxin defense and can potentially promote antitumor immunity. IgE is also the primary cause of allergic illness symptoms, including life-threatening anaphylaxis [
1,
2,
3].
Many pathological diseases can cause hyper-IgE. The role of IgE is mostly linked to the occurrence of allergy symptoms, which may be followed by an increase in serum levels. Total IgE elevation has also been linked to hyper-IgE disorders, which are rare hereditary immunological deficits [
4]. Other conditions such as infections, tumors, and autoimmune diseases may also increase IgE production [
5]. Nephrotic syndrome, bullous pemphigoids, graft-versus-host disease, bone marrow transplantation, cystic fibrosis, and nephrotic syndrome are additional conditions linked to increased blood IgE levels. Total IgE levels may also increase as a result of using penicillin G or aztreonam or smoking tobacco [
6]. Given the variety of these scenarios, discussing the predictive significance of total IgE would be beneficial for medical professionals.
Hyper-IgE syndrome (HIES), often known as Job’s syndrome, refers to a heterogeneous set of inborn immune system defects, with manifestations such as increased susceptibility to infection and eczema induced by elevated serum IgE [
7]. The pathophysiology of HIES is distinguished by abnormally high IgE serum levels, resulting in a diverse group of patients with rare primary immunodeficiency disorders (PIDDs) [
8,
9]. Patients are prone to developing various disorders including dermatitis, eczema, recurrent skin infections, and pulmonary infections, as well as skeletal and connective tissue abnormalities, depending on the predominant clinical symptoms caused by the different subtypes of HIES [
10,
11]. The prevalence and incidence of HIES are approximately 1:100,000 and 6–10 per year, respectively, with equal preponderance among the sexes [
9,
12,
13]. In 2007, the discovery of dominant-negative signal transducer and activator of transcription gene 3 (STAT3) mutations in HIES patients revealed the genetic foundations of the disease [
7,
12,
14]. HIES has also been linked to a number of unique inborn gene defects. These data from diverse genotypes suggest that IL-6/STAT3 signaling is important in controlling hyper-IgE. Nevertheless, the roles of STAT3 in the synthesis and regulation of IgE remain unknown [
15,
16,
17,
18]. The impaired functioning of Tyk2 and DOCK8 has now been linked to milder types of HIES [
14]. STAT3 functions as a major transcription factor downstream of several cytokine and growth factor receptors, regulating antimicrobial responses and cell survival. Thus, dysfunction of this protein leads to immunodeficiencies and, in some situations, cancer [
14,
19,
20,
21]. However, as the immunological and molecular basis of HIES continues to be elucidated, significant biological and immunological insights into JAK–STAT signaling are emerging. These insights may have consequences for our understanding of the etiology and clinical therapy for HIES [
9,
22].
Atopic diseases are the most common immunological dysregulation syndromes linked to elevated serum IgE levels [
23]. Many monogenic disorders have been identified in the past two decades, each requiring different clinical considerations and treatment approaches. Today, distinct genetic causes characterize numerous diseases, with certain phenotypic similarities likely resulting from mutations within particular pathways [
24]. It is, therefore, critical to understand how and why patients experience increased IgE and infections. There is little known about the factors that contribute to elevated total serum IgE levels in children. Total serum IgE concentrations have been observed to decline with age, with children and adolescents presenting the highest values [
25,
26,
27]. In the pediatric population, the relationship between allergy symptoms and total serum IgE was observed to be dependent on atopic status [
28,
29,
30]. Furthermore, subjects with occupational exposure to dust or gas have higher IgE levels than those who are not exposed, resulting in rhinitis, wheezing, and ongoing asthma [
31]. The purpose of this study is to investigate the biological and clinical range of symptoms in children with increased IgE levels in clinical settings where asthma, atopic dermatitis, or other instances of primary allergic eczema are not usually considered as the final diagnoses. The aim of our study is to identify if the possible causes of high IgE are also dependent on external factors such as environmental factors, age, or associated recurrent pathologies such as infections.
2. Materials and Methods
2.1. Study Design
The clinical research was conducted as a retrospective study, including 200 children (1 to 17 years of age), all of whom were patients admitted to the “Grigore Alexandrescu” Emergency Clinical Hospital for Children in Bucharest. We searched the electronic medical database of the hospital for relevant records from 1 January 2019 to 31 December 2020. All children were previously referred for evaluation due to recurrent respiratory, digestive, or urinary infections. The samples for quantitative measurement of IgE and allergen panels were obtained from venous blood. An anticoagulant-free vacutainer with or without separating gel served as the collecting container. Levels of total serum IgE were measured with an enzyme-linked immunosorbent assay (ELISA). The studied patients were divided into four categories based on age. The first group of patients included children aged 1 to 5, the second group included children aged 6 to 9, the third group included children aged 10 to 15, and the fourth group included children aged above 15 years. The IgE levels were compared with the age-adjusted normal reference ranges, as shown in
Table A1.
The collected patient files were entered into a database. The hospital database was used to collect information such as age, gender, environment, IgE levels, clinical diagnosis, and allergen panel results. The data was statistically evaluated, and the statistical tests applied are detailed in the ‘Descriptive Analysis of the Patients’ Series’ section.
We identified “positive patients” as those who had IgE serum levels over the interval’s superior limit according to the reference values for IgE stratified by age. We defined “negative patients” as those with normal IgE serum levels based on age-specific IgE reference values. The limits for IgE serum levels are presented in
Table A1.
2.2. Descriptive Analysis of the Patients’ Series
The data were analyzed statistically using IBM’s Statistical Analysis Software Package (SPSS) version 29 (2022) and Microsoft Excel 2016 (Redmon, WA, USA). The investigation included descriptive statistics, tests to assess normal distribution (Kolmogorov–Smirnov and Shapiro–Wilks), tests to compare quantitative indicators in different groups (comparison of means), correlation analyses, ROC curves, positive predictive value (PPV), negative predictive value (NPV), and sensitivity and specificity. The chosen significance level was α = 0.05. Thus, if the significance level is not reached for values of p < α, the null hypothesis is rejected.
2.2.1. Inclusion Criteria
The patients included in the study were of both sexes, with an age range between 1 and 17 years, admitted to Pediatric Clinic of “Grigore Alexandrescu” Emergency Clinical Hospital for Children in Bucharest. The children were previously diagnosed with at least two bacterial or viral infections during the last six months and had their IgE levels tested, along with a panel of digestive/respiratory allergens. Only patients who had no prior COVID-19 infection were accepted into the research study due to the complex treatment scheme for the disease.
2.2.2. Exclusion Criteria
The following patients were excluded from the study: patients below 1 year of age, patients not previously diagnosed with at least two bacterial or viral infections during the last six months, and patients who had no IgE level tests or food allergen panels performed. Children who had previously been diagnosed with COVID-19 or had at least one positive COVID-19 test during their stay in the hospital were also excluded from the study.
3. Results
Of the 200 total children, 104 (52%) were boys and 96 (48%) were girls. The boys were slightly younger than the girls, with a mean age of 7.71 ± 4.53 years vs. 8.14 ± 4.80 years, respectively. According to the Kolmogorov–Smirnov and Shapiro–Wilk statistical tests, both genders did not have a normal age distribution (p values were less than 0.01 for both sexes). Overall, the mean age of the group was 7.91 ± 4.65 years. There was a 2.6:1 ratio between the urban and rural patients (urban/rural, 145:55).
Out of the 200 patients, 60% showed significantly elevated total blood IgE levels, especially in the fourth group of patients aged 16 to 17. Although the fourth group of patients had the highest ratio of patients with high IgE levels to patients with normal IgE levels, the first group featured the largest number of patients. According to the registered data, the minimum value of total serum IgE was 0, and the maximum value was 3298, which was reported in an asthmatic patient that presented a clinical diagnosis of anaphylactic shock. The number of patients registered with IgE levels higher than 1000 UI/mL was up to 17 (8.5%). The distribution of patients with high and normal IgE levels among the children varied between group ages, as presented in
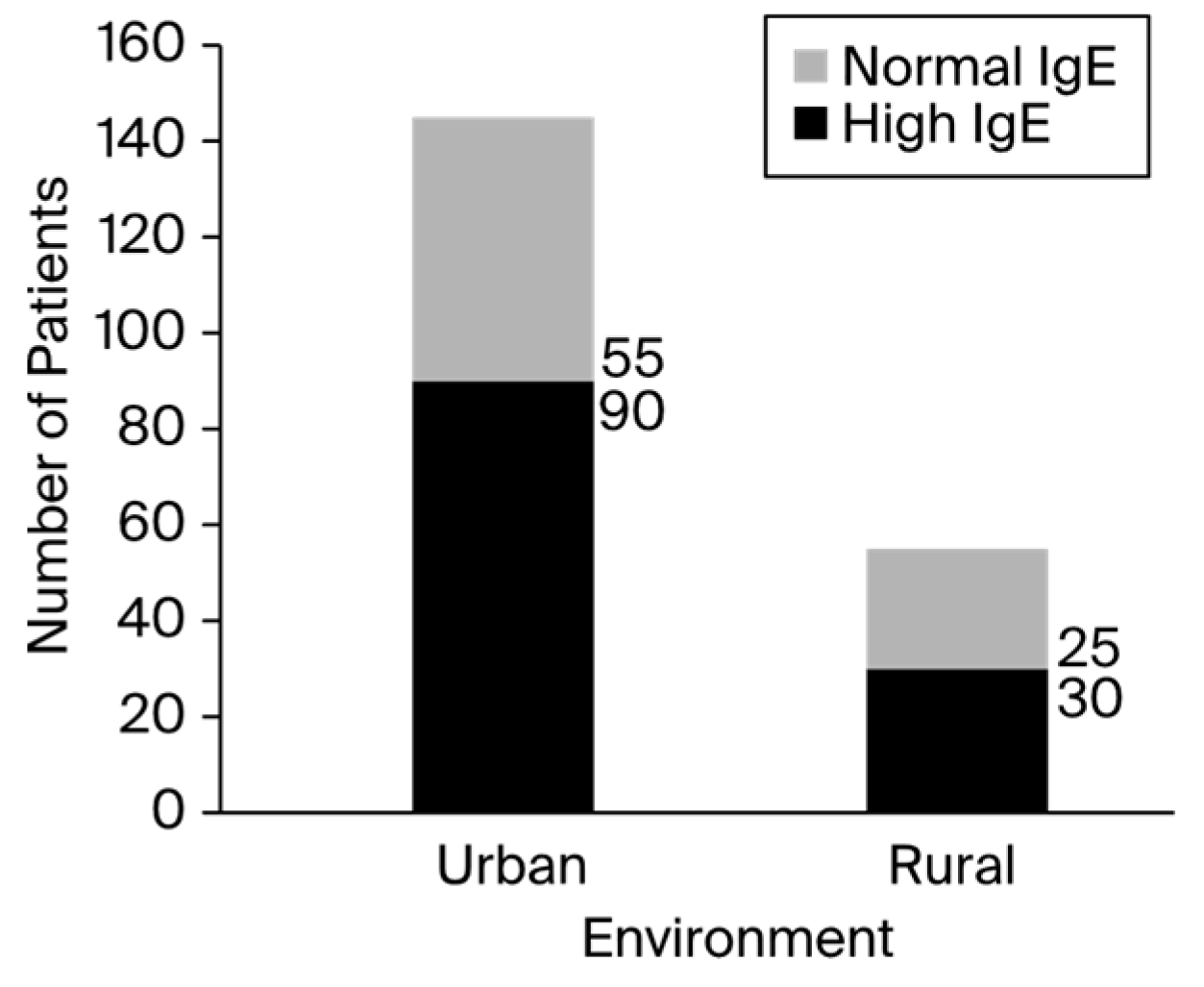
Figure 1. Of the 200 total children, 120 patients presented elevated IgE serum levels, with a male-to-female ratio of 1.06 (male/female, 62:58). The number of positive patients in rural areas was 1.2 times higher than the number of negative patients. In addition, urban environments contained about 1.6 times more positive patients than negative patients, as presented in
Figure 2.
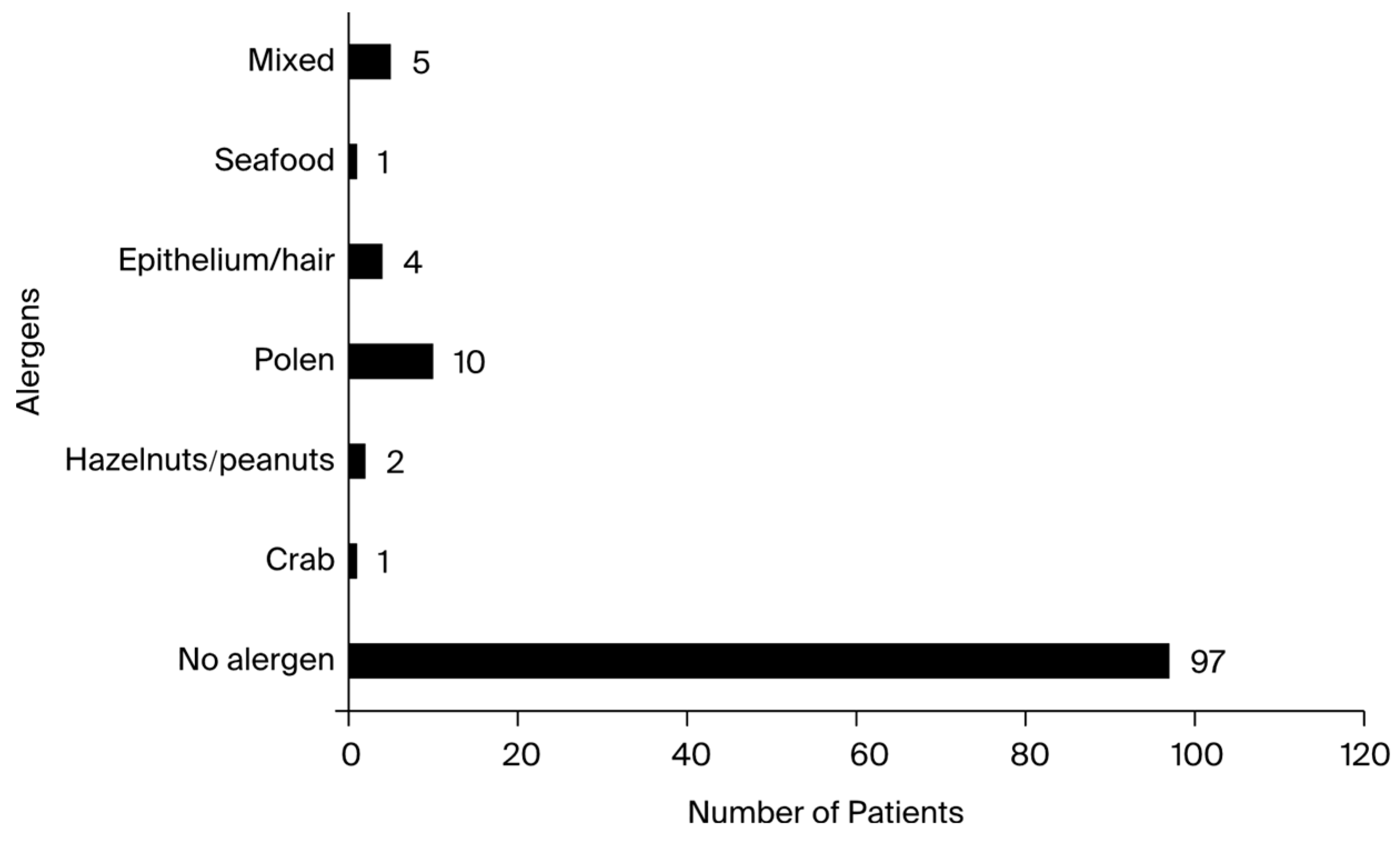
Each of the 200 study participants was assessed for potential allergies. Screening was performed using the Phadiatop test, which is based on a multi-allergen allergosorbent that produces reliable results. For patients with elevated IgE levels, the allergen panels showed a high positive rate. The respiratory panels tested positive for hair, pollen, and epithelial cells without elevated IgE levels in only one patient who was aware of his medical condition.
Table A2 displays the allergen panels. Notably, the patients tested positive for the following allergens: crab, shellfish, hazelnuts, cow’s milk, and peanuts. One patient tested positive for furazolidone before entering the study. Although it was not mentioned in the allergen panel, the patient had a history of anaphylactic shock and was aware of the body’s sensitivity to the substance in question. Patients with a history of atopic dermatitis comprised the majority of those who tested positive for food allergies. The allergen panel results are presented in
Figure 3.
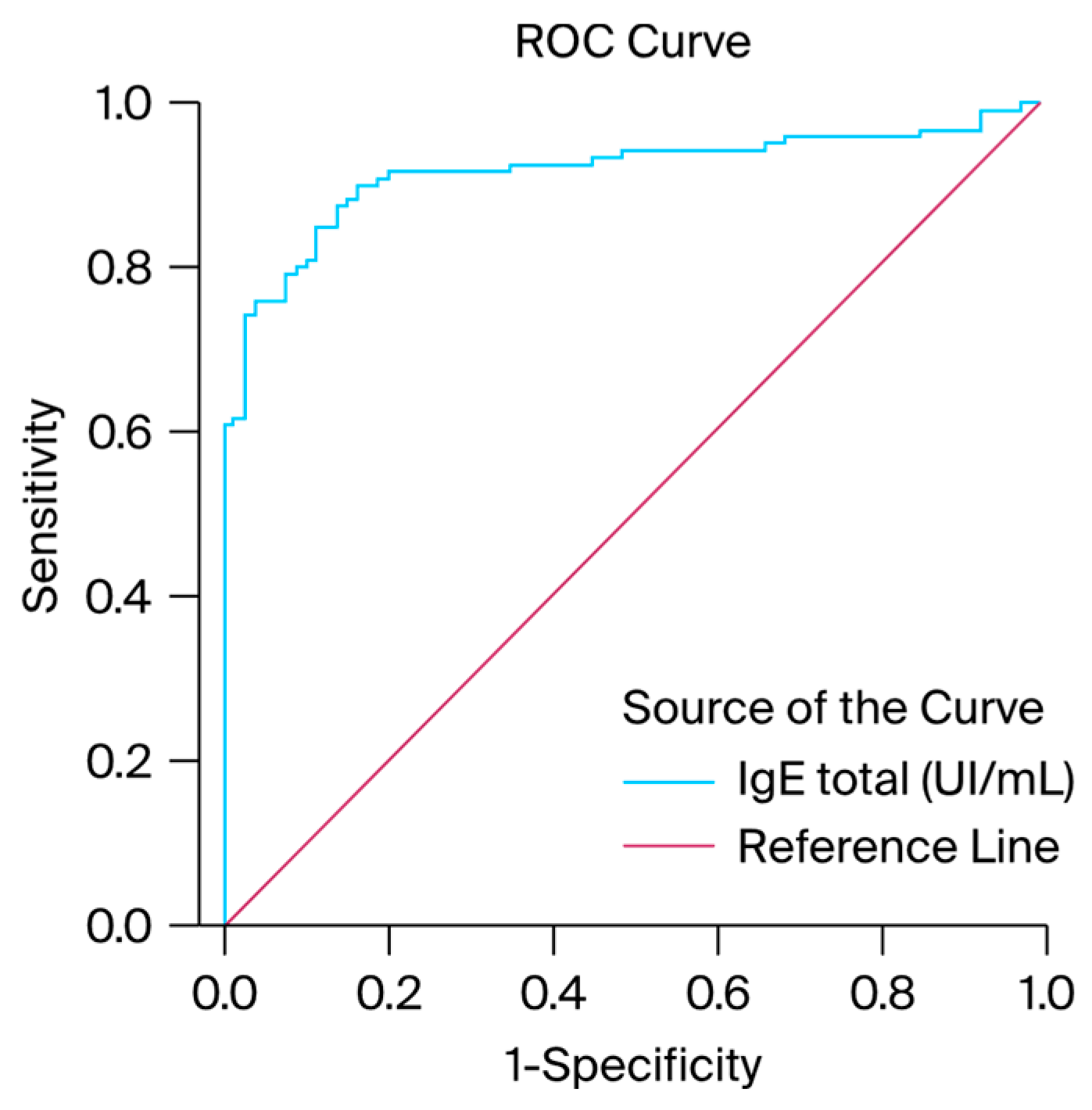
We generated ROC curves for IgE serum levels to assess the possibility of a correlation between a history of infectious diseases among the patients and elevated IgE serum levels. According to the ROC curve results, the IgE values or ROC curves for IgE serum levels achieved statistical significance (AUC = 0.916, with a standard error of 0.021) and exhibited good discriminative capacity for the diagnosis of infectious diseases, as presented in
Table 1. These results indicate that patients may present specific positive IgE levels after recurrent bacterial or viral infections. Total IgE levels exhibited a PPV of 51.9%, NPV of 92.5%, sensitivity of 51.8%, and specificity of 61.5%. Notably, the percentage was determined for the entire study group. Patients with high IgE levels but no allergy manifestation were given a true positive value, while patients with normal IgE levels and allergy manifestation received a negative false value. A graphical representation of the results is presented in
Figure 4. According to the statistical results, the study group had a large dispersion; the Gini index was equal to 83%. The Kolmogorov-Smirnov metric test confirms that the study group is a good fit due to its high value (0.738). The results are presented in
Table 2.
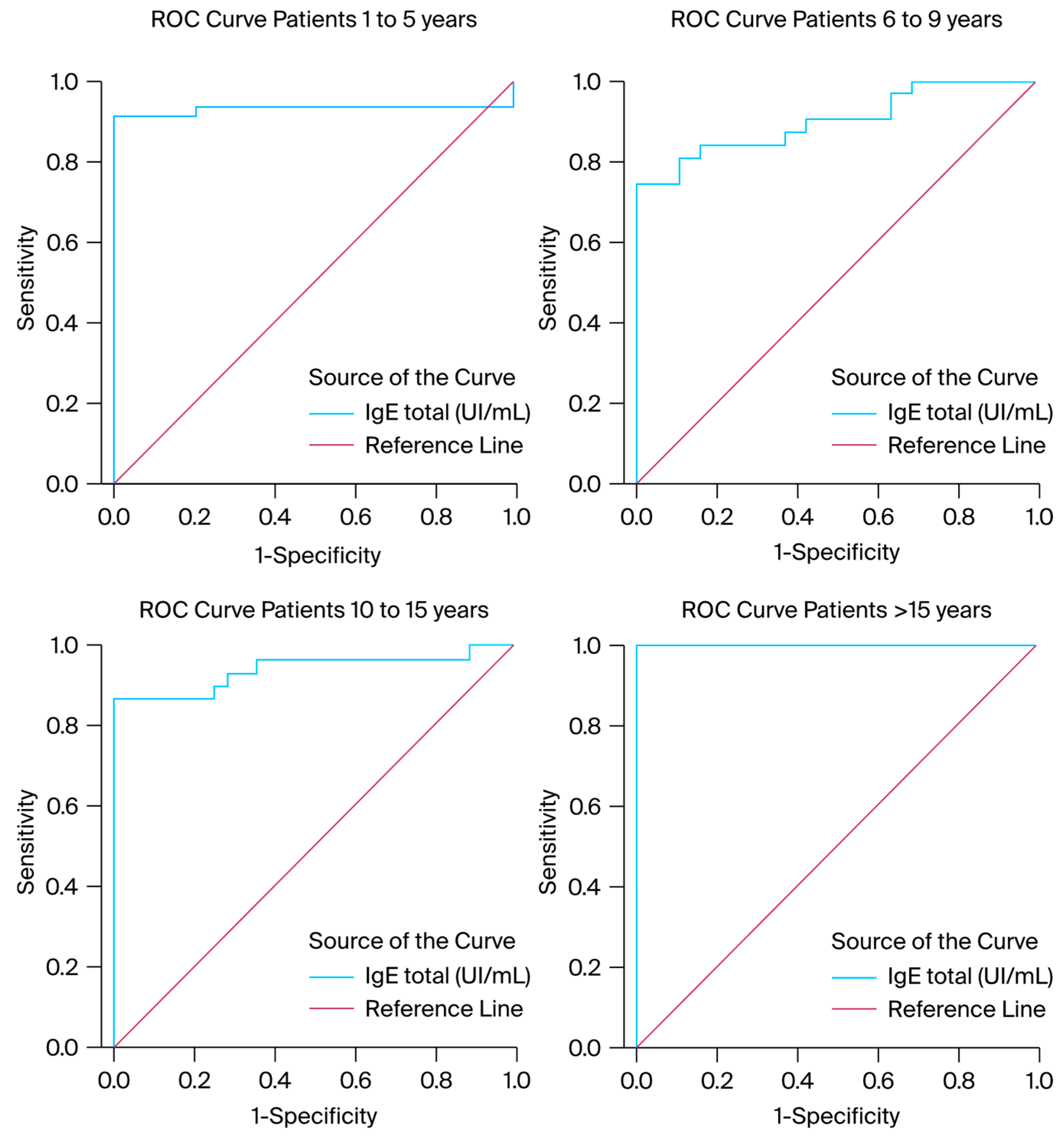
We also produced ROC curves for each group of patients and monitored IgE serum levels to determine the existence of a lower or higher incidence. According to the results, there was a greater possibility of encountering high IgE levels among those older than 15 years (AUC = 1.00) compared to the rest of the pediatric population (AUC 1–5 = 0.933 with a standard error of 0.035, AUC 6–9 = 0.900 with a standard error of 0.043, and AUC 10–15 = 0.942 with a standard error of 0.033). According to the ROC curve results, the IgE values or ROC curves for IgE serum levels in each group of patients reached statistical significance (AUC > 0.900 for each group of patients) and exhibited good discriminative capacity for the diagnosis of infectious diseases. A visual representation of the statistic results is presented in
Figure 5 and
Table 2.
In terms of clinical diagnosis, we discovered that 59 (29.5%) of 200 patients suffered from allergic diseases, 57 (28.5%) from gastrointestinal diseases, and 50 (25%) from respiratory infections. All pathologies with a prevalence of less than 1% were categorized as “others”. The pathologies under this category were as follows: acute non-specific lymphedema, acute non-specific urinary infection, acute pancreatitis, adenoid hypertrophy, allergic rhinitis, anaphylactic shock, celiac disease, erythema infectiosum and nodosum, gastroesophageal reflux disease with esophagitis, hematemesis, Henoch–Schönlein purpura, thrombocytopenic purpura, intestinal occlusion, joint pain with multiple locations, neutropeny, non-specific gastritis with esophagitis, non-specific migraine, non-specific sepsis, non-specific thyroid condition, non-specific urinary infection, palpitations, persistent fever, post-caustic esophageal stenosis, precordial pain, pyelonephritis, syncope and collapse, ulcerative colitis, and uretero-hydronephrosis. The “others” category included up to 34 patients (17%) and was the second most common category of clinical diagnosis after acute non-specific upper airway respiratory infections. The number of reported infections ranged up to 27% (54 patients) and included gastro-intestinal, urinary, and respiratory infections. The clinical characteristics are shown in
Table 3.
4. Discussion
4.1. Infectious Diseases corellated with IgE Mechanisms
The overproduction of IgE antibodies is typically thought to be the primary cause of type I hypersensitivity reactions, also known as anaphylactic reactions [
32]. Anaphylactic hypersensitivity reactions are atypical, excessive reactions solely mediated by antibodies of the IgE type [
1,
11,
22]. This kind of hypersensitivity is characterized primarily by its quick onset, occurring only minutes after contact with the allergen, and its ability to be triggered by any allergen (food, medicine, inhalant, etc.) [
26]. However, according to the present study, the majority of patients with a hyperaccumulation of IgE in their serum displayed a highly varied symptomatology distinct from traditional allergy manifestations. This result suggests that hyper-IgE may have a cause other than allergies, underscoring the need for more research into plausible underlying causes.
According to this study, the most common cause of hospitalization (clinical diagnosis) was acute non-specific ORL infections, various types of non-specific gastrointestinal manifestations, and pathologies based on allergy symptoms. Autosomal-dominant HIES is a primary immunodeficiency condition with multi-organ involvement caused by dominant negative STAT3 mutations. Consequently, the most common symptoms were gastroesophageal reflux disease, dysphagia, and gastrointestinal problems [
33]. Patients with elevated levels of IgE and allergic disease also presented deficiencies in vitamin D, one of the major predictors of asthma and atopic dermatitis [
34,
35]. Thus, patients with hyper-IgE may also benefit from the treatment and care approaches informed by these findings.
4.2. Environment Corellated with Elevated IgE Levels
The development of the mutation that causes the body to produce IgE at an excessive rate might also be influenced by the environment [
28,
36]. Inner-city children differ in their allergen exposure and sensitivity based on geography [
37]. This feature is highlighted by the high percentage of positive instances of patients from an urban setting (90 out of 145 patients), along with the difference between the number of positive and negative cases in urban vs. rural settings [
37]. In contrast to rural areas, where the number of positive patients was 1.2 times higher than the number of negative patients, urban environments corresponded to approximately 1.6 times more positive patients than negative patients. This result suggests that urban areas may have greater rates of infectious transmission [
38]. From an environmental standpoint, metropolitan areas have a higher rate of mutation development than rural ones, which determines the body’s increased production of IgE [
39].
One of the pathologies currently facing pediatricians, dermatologists, and infectious disease specialists is undiagnosed HIES [
7,
40]. To date, there is no known cure for this medical condition [
7], although symptoms can be managed. The only way to improve the clinical panel is by maintaining total serum IgE levels within normal ranges starting from a young age [
30]. This strategy can enhance general quality of life and help avoid severe allergic reactions. An elevated IgE serum level (100 UI/mL ≥ IgE) usually indicates allergies, asthma, eczema, or chronic skin infections, but the majority of patients in this study with elevated IgE serum levels presented only moderate protein-energy malnutrition as the primary diagnosis. This study found that the relationship between asthma (as defined by symptoms and bronchial responsiveness) and total IgE levels is independent of specific IgE levels for common respiratory allergens [
41,
42].
All racial groups (African, Asian, Caucasian, and Hispanic) had HIES. However, White individuals presented a higher frequency of this condition, particularly among the young and extremely young. Among the younger population, the average age at detection of the condition was 11.5 years [
43]. According to the study outcomes, the first age group (1–5 years) contained the most patients, which is understandable given that the majority of infections emerge during the first years of life [
23,
36,
44]. Patients with medication allergies and atopic dermatitis had higher IgE levels, but patients with allergic rhinitis had lower or normal IgE levels. These results are similar to those in a study conducted among the pediatric population [
36]. The second largest age group of patients was 10 to 15 years old. During this time, children are more likely to develop infections due to hormonal changes that indirectly impair the immune system [
45]. This phase makes children’s immune systems more vulnerable and susceptible to several forms of infections [
46]. The last group reported in this study (>15 years) presented a lower incidence of infectious disorders than the previous groups. This result explains why the last group of patients included so few individuals. Although the number of patients in the last group was reduced, the ratio of patients with high IgE serum levels to those with normal IgE levels was 2.5. In other words, the surroundings and the high frequency of recurring infections associated with this particular treatment have the potential to overstimulate the immune system [
11,
12,
22,
23,
28]. Thus, these patients may always develop infections, but the outcome varies from child to child and may not always be predicted solely based on specific IgE levels.
4.3. Elevated IgE Levels Corellated with Negative Allergen Panels
The Phadiatop test disc contains only inhalant allergens, so it cannot be used for screening infants and very young children whose IgE responses, if any, are likely limited to foods [
47]. For patients in this study with elevated IgE levels, the allergen panels showed a high positive rate. Results from examining the food and respiratory allergen panels demonstrated that the individuals who tested positive for the body’s synthesis of hyper-IgE were not allergic to any potential allergens. Despite having higher total serum IgE, 80% of patients tested negative for every allergen in both panels. The epithelium, or pet hair, represented 4.16% of the allergens found, and the pollen of several plant species accounted for 10.41%. Moreover, the majority of individuals who presented sensitivity to the aforementioned allergens also had previous asthma diagnoses. Following this study, the majority of patients were found to be positively allergic to none of the existing allergens in the allergen panels, which contained the most frequently encountered allergens in both the food and respiratory allergen categories. Based on statistical data, each of the other allergens included in this study represented, at most, 1% of the total, along with the diversification of chemically produced foods and current lifestyle. These results suggest a significant increase in the levels of stress experienced by children from a young age, as well as an alarming increase in patients from the pediatric population with allergic and gastric manifestations and the presence of various viral and bacterial infections [
37,
48]. Similar results were also reported by Lin IH et al. in a study of 434 children with atopic allergies, in which more than 60% of the patients had very high total serum IgE levels. Notably, in this study, most of the patients were not found to be positively allergic to any of the existing allergens in the allergen panels, which included the most frequently encountered allergens in both the food and respiratory allergen categories, similar to the study mentioned above [
40]. Kim HY, Choi J, and Ahn K, et al. published a comparable study assessing the total series IgE values in a pediatric population aged 5 to 18 years, in which patients from various Asian nations and backgrounds were monitored and tested [
49].
No patient younger than one year of age exhibited symptoms that necessitated the execution of serological examinations specific to the illness under investigation.
5. Conclusions
The statistical results showed that there is a strong correlation between the frequency of infectious diseases and elevated IgE levels. Furthermore, the environment plays an important role in the sensitization of the immune system. Thus, it is crucial to monitor the immunoglobulins involved in the immune response to infectious microorganisms, as well as their association with IgE levels.
IgE is commonly used as a marker for allergy and parasite infections. However, based on previously published findings and the results of this study, we argue that the treatment strategy should be adjusted and affected children examined for inborn errors of immunity after multiple recurring infections. In cases of exceptionally high IgE levels, genetic testing is required to confirm or exclude HIES and other immunodeficiencies. Furthermore, recurring infections throughout childhood can have a significant impact on one’s immune system. Thus, higher IgE serum levels in adulthood and diseases such as HIES and allergy symptoms without a specific allergen could have their roots in the child’s environment during development.
As a key limitation, IgE levels were evaluated only once after clinical signs occurred. Thus, increased IgE levels in patients presenting various types of allergies may have been impacted by recurring infections. To determine this connection, IgE levels could be evaluated prior to, during, and following infections.
Author Contributions
Conceptualization, S.O. and V.N.; methodology, E.M.; software, S.O.; validation, M.M., D.C. and S.T.; formal analysis, D.B.; investigation, E.I.I.; resources, M.G.; data curation, A.M.D.; writing—original draft preparation, S.O.; writing—review and editing, V.N.; supervision, E.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of ‘Grigore Alexandrescu’ Pediatric Emergency Hospital (the approval code is 28012 from the 10 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study according to the ‘Grigore Alexandrescu’ Pediatric Emergency Hospital protocol.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions such as privacy and ethics.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table A1.
Reference values for IgE stratified by age.
Table A1.
Reference values for IgE stratified by age.
| IgE Levels |
|---|
| Age |
Values (UI/mL) |
| <1 |
<15 |
| 1 to 5 |
<60 |
| 6 to 9 |
<90 |
| 10 to 15 |
<200 |
| >15 |
<100 |
Table A2.
Respiratory and food allergen panels.
Table A2.
Respiratory and food allergen panels.
| Allergen Lists |
|---|
| Respiratory |
Food |
| Pollen Phleum pratense |
Peanuts |
| Pollen graminee mix (gx6) |
Hazelnut |
| Pollen Artemisia vulgaris |
Carrot |
| Pollen Ambrosia elatior |
Potato |
| Parietaria officinalis |
Wheat |
| Pollen Betulla verrucosa |
Gluten |
| Pollen Alnus incana |
Rice |
| Dermatophagoides pteronyssinus |
Soya |
| Dermatophagoides farinae |
Egg white |
| fur and epithelium (cat) |
Yolk |
| fur and epithelium (dog) |
Cow’s milk |
| Blatella germanica |
Alpha-lactalbumin |
Penicillium chrysogenum, Cladosporium herbarum,
Aspergillus fumigatus, Alternaria tenuis |
Beta-lactoglobulin |
| |
Casein |
| |
Crab |
| |
Seafood |
| |
Fish (code) |
| |
Shrimps |
References
- He, J.S.; Narayanan, S.; Subramaniam, S.; Ho, W.Q.; Lafaille, J.J.; Curotto de Lafaille, M.A. Biology of IgE production: IgE cell differentiation and the memory of IgE responses. Curr. Top. Microbiol. Immunol. 2015, 388, 1–19. [Google Scholar] [CrossRef]
- Devereux, G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Belmesk, L.; Muntyanu, A.; Cantin, E.; AlHalees, Z.; Jack, C.S.; Le, M.; Sasseville, D.; Iannattone, L.; Ben-Shoshan, M.; Litvinov, I.V.; et al. Prominent Role of Type 2 Immunity in Skin Diseases: Beyond Atopic Dermatitis. J. Cutan. Med. Surg. 2022, 26, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Devilliers, H.; Turcu, A.; Vernier, N.; Muller, G.; Bielefeld, P.; Bonniaud, P.; Besancenot, J.F. Hyper-IgE in internal medicine. Rev Med. Interne 2018, 39, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.F.; Kleiner, D.E.; Nadiminti, H.; Davis, J.; Quezado, M.; Anderson, V.; Puck, J.M.; Holland, S.M. Causes of death in hyper-IgE syndrome. J. Allergy Clin. Immunol. 2007, 119, 1234–1240. [Google Scholar] [CrossRef]
- Joshi, A.Y.; Iyer, V.N.; Boyce, T.G.; Hagan, J.B.; Park, M.A.; Abraham, R.S. Elevated serum immunoglobulin E (IgE): When to suspect hyper-IgE syndrome-A 10-year pediatric tertiary care center experience. Allergy Asthma Proc. 2009, 30, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Tsilifis, C.; Freeman, A.F.; Gennery, A.R. STAT3 Hyper-IgE Syndrome-an Update and Unanswered Questions. J. Clin. Immunol. 2021, 41, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, L.; Chen, T.X. Clinical Manifestation of Hyper IgE Syndrome Including Otitis Media. Curr. Allergy Asthma Rep. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Mohebbi, M.; Mehravaran, S.; Mazloumi, M.; Jahanbani-Ardakani, H.; Abtahi, S.H. Hyperimmunoglobulin E syndrome: Genetics, immunopathogenesis, clinical findings, and treatment modalities. J. Res. Med. Sci. 2017, 22, 53. [Google Scholar] [CrossRef]
- Minegishi, Y. Hyper-IgE syndrome, 2021 update. Allergol. Int. 2021, 70, 407–414. [Google Scholar] [CrossRef]
- Bergerson, J.R.E.; Freeman, A.F. An Update on Syndromes with a Hyper-IgE Phenotype. Immunol. Allergy Clin. N. Am. 2019, 39, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. STAT3 and the Hyper-IgE syndrome: Clinical presentation, genetic origin, pathogenesis, novel findings and remaining uncertainties. Jakstat 2013, 2, e23435. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.Y.; Iyer, V.N.; Hagan, J.B.; St Sauver, J.L.; Boyce, T.G. Incidence and temporal trends of primary immunodeficiency: A population-based cohort study. Mayo Clin. Proc. 2009, 84, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Camargo, C.A., Jr.; Bohlke, K.; Jick, H.; Miller, R.L.; Sheikh, A.; Simons, F.E. Epidemiology of anaphylaxis: Findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann. Allergy Asthma Immunol. 2006, 97, 596–602. [Google Scholar] [CrossRef] [PubMed]
- The Diagnosis and Management of Anaphylaxis. Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 1998, 101, S465–S528. [Google Scholar]
- Aleksandrova, K.; Egea Rodrigues, C.; Floegel, A.; Ahrens, W. Omics Biomarkers in Obesity: Novel Etiological Insights and Targets for Precision Prevention. Curr. Obes. Rep. 2020, 9, 219–230. [Google Scholar] [CrossRef]
- Chandesris, M.O.; Melki, I.; Natividad, A.; Puel, A.; Fieschi, C.; Yun, L.; Thumerelle, C.; Oksenhendler, E.; Boutboul, D.; Thomas, C.; et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: Molecular, cellular, and clinical features from a French national survey. Medicine 2012, 91, e1–e19. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Holland, S.M.; Staudt, L.M. JAKs and STATs in immunity, immunodeficiency, and cancer. N. Engl. J. Med. 2013, 368, 161–170. [Google Scholar] [CrossRef]
- Minegishi, Y.; Saito, M.; Tsuchiya, S.; Tsuge, I.; Takada, H.; Hara, T.; Kawamura, N.; Ariga, T.; Pasic, S.; Stojkovic, O.; et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 2007, 448, 1058–1062. [Google Scholar] [CrossRef]
- Goussetis, E.; Peristeri, I.; Kitra, V.; Traeger-Synodinos, J.; Theodosaki, M.; Psarra, K.; Kanariou, M.; Tzortzatou-Stathopoulou, F.; Petrakou, E.; Fylaktou, I.; et al. Successful long-term immunologic reconstitution by allogeneic hematopoietic stem cell transplantation cures patients with autosomal dominant hyper-IgE syndrome. J. Allergy Clin. Immunol. 2010, 126, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.M.; DeLeo, F.R.; Elloumi, H.Z.; Hsu, A.P.; Uzel, G.; Brodsky, N.; Freeman, A.F.; Demidowich, A.; Davis, J.; Turner, M.L.; et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 2007, 357, 1608–1619. [Google Scholar] [CrossRef]
- Freeman, A.F.; Milner, J.D. The Child with Elevated IgE and Infection Susceptibility. Curr. Allergy Asthma Rep. 2020, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Duréault, A.; Tcherakian, C.; Poiree, S.; Catherinot, E.; Danion, F.; Jouvion, G.; Bougnoux, M.E.; Mahlaoui, N.; Givel, C.; Castelle, M.; et al. Spectrum of Pulmonary Aspergillosis in Hyper-IgE Syndrome with Autosomal-Dominant STAT3 Deficiency. J. Allergy Clin. Immunol. Pract. 2019, 7, 1986–1995.e3. [Google Scholar] [CrossRef] [PubMed]
- Court, C.S.; Cook, D.G.; Strachan, D.P. The descriptive epidemiology of house dust mite-specific and total immunoglobin E in England using a nationally representative sample. Clin. Exp. Allergy 2002, 32, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Barbee, R.A.; Halonen, M.; Kaltenborn, W.; Lebowitz, M.; Burrows, B. A longitudinal study of serum IgE in a community cohort: Correlations with age, sex, smoking, and atopic status. J. Allergy Clin. Immunol. 1987, 79, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Barbee, R.A.; Halonen, M.; Lebowitz, M.; Burrows, B. Distribution of IgE in a community population sample: Correlations with age, sex, and allergen skin test reactivity. J. Allergy Clin. Immunol. 1981, 68, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, D.L.; Halonen, M.; Burrows, B. Relationships between total serum IgE, atopy, and smoking: A twenty-year follow-up analysis. J. Allergy Clin. Immunol. 1994, 94, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Zetterström, O.; Osterman, K.; Machado, L.; Johansson, S.G. Another smoking hazard: Raised serum IgE concentration and increased risk of occupational allergy. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 1215–1217. [Google Scholar] [CrossRef]
- Burrows, B.; Halonen, M.; Lebowitz, M.D.; Knudson, R.J.; Barbee, R.A. The relationship of serum immunoglobulin E, allergy skin tests, and smoking to respiratory disorders. J. Allergy Clin. Immunol. 1982, 70, 199–204. [Google Scholar] [CrossRef]
- Omenaas, E.; Bakke, P.; Elsayed, S.; Hanoa, R.; Gulsvik, A. Total and specific serum IgE levels in adults: Relationship to sex, age and environmental factors. Clin. Exp. Allergy 1994, 24, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Eckl-Dorna, J.; Villazala-Merino, S.; Campion, N.J.; Byazrova, M.; Filatov, A.; Kudlay, D.; Karsonova, A.; Riabova, K.; Khaitov, M.; Karaulov, A.; et al. Tracing IgE-Producing Cells in Allergic Patients. Cells 2019, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Bagi, P.; Strongin, A.; Heimall, J.; Zhao, X.; Lawrence, M.G.; Trivedi, A.; Henderson, C.; Hsu, A.; Quezado, M.; et al. Gastrointestinal Manifestations of STAT3-Deficient Hyper-IgE Syndrome. J. Clin. Immunol. 2017, 37, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Ehlayel, M.S.; Tulic, M.K.; Hamid, Q. Vitamin D deficiency as a strong predictor of asthma in children. Int. Arch. Allergy Immunol. 2012, 157, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, U.; Filimoniuk, A.; Kowalczuk-Krystoń, M.; Alifier, M.; Karpińska, J.; Kaczmarski, M.G.; Lebensztejn, D.M. Association of antioxidants and vitamin D level with inflammation in children with atopic dermatitis. Int. J. Dermatol. 2019, 58, 1056–1061. [Google Scholar] [CrossRef]
- Kostova, P.; Papochieva, V.; Miteva, D.; Georgieva, B.; Mileva, S.; Shahid, M.; Lukanov, T.; Petrova, G. Elevated IgE Levels-An Allergy or an Underlying Inborn Error of Immunity in Children with Recurrent Infections? Antibodies 2023, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Gruchalla, R.S.; Pongracic, J.; Plaut, M.; Evans, R., 3rd; Visness, C.M.; Walter, M.; Crain, E.F.; Kattan, M.; Morgan, W.J.; Steinbach, S.; et al. Inner City Asthma Study: Relationships among sensitivity, allergen exposure, and asthma morbidity. J. Allergy Clin. Immunol. 2005, 115, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Tesema, G.A.; Worku, M.G.; Alamneh, T.S.; Teshale, A.B.; Yeshaw, Y.; Alem, A.Z.; Ayalew, H.G.; Liyew, A.M.; Tessema, Z.T. Understanding the rural-urban disparity in acute respiratory infection symptoms among under-five children in Sub-Saharan Africa: A multivariate decomposition analysis. BMC Public Health 2022, 22, 2013. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.L.; Krawiec, M.; Koplin, J.J.; Santos, A.F. Update on food allergy. Pediatr. Allergy Immunol. 2021, 32, 647–657. [Google Scholar] [CrossRef]
- Lin, I.H.; Tsai, M.-C.; Chen, J.-P.; Fu, L.-S. Allergic children with extremely high total IgE but no allergen identified in the initial screening panel. J. Microbiol. Immunol. Infect. 2021, 54, 474–481. [Google Scholar] [CrossRef]
- Sunyer, J.; Antó, J.M.; Castellsagué, J.; Soriano, J.B.; Roca, J. Total serum IgE is associated with asthma independently of specific IgE levels. The Spanish Group of the European Study of Asthma. Eur. Respir. J. 1996, 9, 1880–1884. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.R.; Burrows, B.; Flannery, E.M.; Herbison, G.P.; Hewitt, C.J.; Holdaway, M.D. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N. Engl. J. Med. 1991, 325, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.J.; Davies, A.M.; Bax, H.J.; Karagiannis, S.N. IgE Antibodies: From Structure to Function and Clinical Translation. Antibodies 2019, 8, 19. [Google Scholar] [CrossRef]
- Sanders, S.L.; Agwan, S.; Hassan, M.; Bont, L.J.; Venekamp, R.P. Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection. Cochrane Database Syst. Rev. 2023, 10, Cd009417. [Google Scholar] [CrossRef]
- Moise-Silverman, J.; Silverman, L.A. A review of the genetics and epigenetics of central precocious puberty. Front. Endocrinol. 2022, 13, 1029137. [Google Scholar] [CrossRef]
- Monto, A.S. Epidemiology of viral respiratory infections. Am. J. Med. 2002, 112 (Suppl. 6A), 4s–12s. [Google Scholar] [CrossRef]
- Merrett, J.; Merrett, T.G. Phadiatop—A novel IgE antibody screening test. Clin. Exp. Allergy 1987, 17, 409–416. [Google Scholar] [CrossRef]
- Dahdah, L.; Leone, G.; Artesani, M.; Riccardi, C.; Mazzina, O. Apheresis in food allergies. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 227–231. [Google Scholar] [CrossRef]
- Kim, H.Y.; Choi, J.; Ahn, K.; Hahm, M.I.; Lee, S.Y.; Kim, W.K.; Chae, Y.; Park, Y.M.; Han, M.Y.; Lee, K.J.; et al. Reference Values and Utility of Serum Total Immunoglobulin E for Predicting Atopy and Allergic Diseases in Korean Schoolchildren. J. Korean Med. Sci. 2017, 32, 803–809. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).