Submitted:
28 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Strain Cultivation
2.2. Identification of AnPB
2.3. Morphology and Physiology of Isolates
2.4. 16S rRNA Sequencing and Phylogenetic Study
3. Results and Discussion
3.1. Site Description, Isolation, and Detection of AnPB
3.2. Spectral Analysis
3.3. Phenotypic Features of Strains
3.4. 16S rRNA Gene-Based Phylogenetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Halsey, L.; Vitt, D.; Zoltai, S. Climatic and Physiographic Controls on Wetland Type and Distribution in Manitoba, Canada. Wetlands 1997, 17, 243–262. [Google Scholar] [CrossRef]
- Kennedy, G.; Mayer, T. Natural and Constructed Wetlands in Canada: An Overview. Water Qual. Res. J. Can. 2002, 37, 295–325. [Google Scholar] [CrossRef]
- Wrubleski, D.; Badiou, P.; Goldsborough, G. Coastal Wetlands of Manitoba’s Great Lakes (Canada). In The Wetland Book II: Distribution, Description, and Conservation; Finlayson, C., Milton, G., Prentice, R., Davidson, N., Eds.; Springer: Dordrecht, Netherlands, 2018; Volume 1, pp. 591–604. [Google Scholar]
- Watchorn, K.E.; Goldsborough, L.G.; Wrubleski, D.A.; Mooney, B.G. A Hydrogeomorphic Inventory of Coastal Wetlands of the Manitoba Great Lakes: Lakes Winnipeg, Manitoba, and Winnipegosis. J Great Lakes Res 2012, 38, 115–122. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gale, P.M. Wetland Processes and Water Quality: A Symposium Overview. J. Environ. Qual. 1994, 23, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Brunet, N.N.; Westbrook, C.J. Wetland Drainage in the Canadian Prairies: Nutrient, Salt and Bacteria Characteristics. Agric. Ecosyst. Environ. 2012, 146, 1–12. [Google Scholar] [CrossRef]
- Ducks Unlimited Canada Prairie Pothole Region. Available online: https://www.ducks.ca/places/prairie-pothole-region/ (accessed on 9 February 2024).
- Robarts, R.D.; Waiser, M.J. Effects of Atmospheric Change and Agriculture on the Biogeochemistry and Microbial Ecology of Prairie Wetlands. Great Plains Research 1998, 8, 113–136. [Google Scholar]
- Hamard, S.; Céréghino, R.; Barret, M.; Sytiuk, A.; Lara, E.; Dorrepaal, E.; Kardol, P.; Küttim, M.; Lamentowicz, M.; Leflaive, J.; et al. Contribution of Microbial Photosynthesis to Peatland Carbon Uptake along a Latitudinal Gradient. J. Ecol. 2021, 109, 3424–3441. [Google Scholar] [CrossRef]
- Mellado, M.; Vera, J. Microorganisms That Participate in Biochemical Cycles in Wetlands. Can. J. Microbiol. 2021, 67, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, Z.; Pei, Y. Nutrient Removal and Microbial Community Structure in an Artificial-Natural Coupled Wetland System. Process Saf. Environ. 2021, 147, 1160–1170. [Google Scholar] [CrossRef]
- Gutknecht, J.L.M.; Goodman, R.M.; Balser, T.C. Linking Soil Process and Microbial Ecology in Freshwater Wetland Ecosystems. Plant Soil. 2006, 289, 17–34. [Google Scholar] [CrossRef]
- Yurkov, V. V; Beatty, J.T. Aerobic Anoxygenic Phototrophic Bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 695–724. [Google Scholar] [CrossRef]
- Madigan, M.T.; Jung, D.O. An Overview of Purple Bacteria: Systematics, Physiology, and Habitats. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, Netherlands 2009; Volume 3, pp. 1–15. [Google Scholar]
- Yurkov, V.; Csotonyi, J.T. New Light on Aerobic Anoxygenic Phototrophs. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer: Dordrecht, Netherlands 2009; Volume 3, pp. 31–55. [Google Scholar]
- Szabó-Tugyi, N.; Vörös, L.; Balogh, K.; Botta-Dukát, Z.; Bernát, G.; Schmera, D.; Somogyi, B. Aerobic Anoxygenic Phototrophs Are Highly Abundant in Hypertrophic and Polyhumic Waters. FEMS Microbiol. Ecol. 2019, 95, fiz104. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.; Koblížek, M.; Lew, M.; Medová, H.; Glińska-Lewczuk, K.; Owsianny, P.M. Seasonal Changes of Microbial Communities in Two Shallow Peat Bog Lakes. Folia Microbiol. 2015, 60, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.E.; Gorham, E.; Pratt, D.C. Distribution of Purple Photosynthetic Bacteria in Wetland and Woodland Habitats of Central and Northern Minnesota. J. Bacteriol. 1974, 117, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Head, B.; Maltman, C.; Piercey-Normore, M.; Yurkov, V. Aerobic Anoxygenic Phototrophs in Gold Mine Tailings in Nopiming Provincial Park, Manitoba, Canada. Can. J. Microbiol. 2017, 63, 212–218. [Google Scholar] [CrossRef]
- Kuzyk, S.B.; Ma, X.; Yurkov, V. Seasonal Dynamics of Lake Winnipeg’s Microbial Communities Reveal Aerobic Anoxygenic Phototrophic Populations Coincide with Sunlight Availability. Microorganisms 2022, 10, 1690. [Google Scholar] [CrossRef]
- Rathgeber, C.; Yurkova, N.; Stackebrandt, E.; Schumann, P.; Beatty, J.T.; Yurkov, V. Roseicyclus mahoneyensis gen. nov., sp. nov., an Aerobic Phototrophic Bacterium Isolated from a Meromictic Lake. Int. J. Syst. Evol. Microbiol. 2005, 55, 1597–1603. [Google Scholar] [CrossRef]
- Beveridge, T.J. Use of the Gram Stain in Microbiology. Biotech. Histochem. 2001, 76, 111–118. [Google Scholar] [CrossRef]
- Gregersen, T. Rapid Method for Distinction of Gram-Negative from Gram-Positive Bacteria. Eur. J. Appl. Microbiol. Biotech. 1978, 5, 123–127. [Google Scholar] [CrossRef]
- Bilyj, M.; Lepitzki, D.; Hughes, E.; Swiderski, J.; Stackebrandt, E.; Pacas, C.; Yurkov, V. V. Abundance and Diversity of the Phototrophic Microbial Mat Communities of Sulphur Mountain Banff Springs and Their Significance to the Endangered Snail, Physella Johnsoni. Open J. Ecol. 2014, 4, 488–516. [Google Scholar] [CrossRef]
- Csotonyi, J.T.; Stackebrandt, E.; Swiderski, J.; Schumann, P.; Yurkov, V. An Alphaproteobacterium Capable of Both Aerobic and Anaerobic Anoxygenic Photosynthesis but Incapable of Photoautotrophy: Charonomicrobium ambiphototrophicum, gen. nov., sp. nov. Photosynth. Res. 2011, 107, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, V. V.; Krieger, S.; Stackebrandt, E.; Beatty, J.T. Citromicrobium bathyomarinum, a Novel Aerobic Bacterium Isolated from Deep-Sea Hydrothermal Vent Plume Waters That Contains Photosynthetic Pigment- Protein Complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [CrossRef]
- Kuzyk, S.B.; Jafri, M.; Humphrey, E.; Maltman, C.; Kyndt, J.A.; Yurkov, V. Prosthecate Aerobic Anoxygenic Phototrophs Photocaulis sulfatitolerans gen. nov. sp. nov. and Photocaulis rubescens sp. nov. Isolated from Alpine Meromictic Lakes in British Columbia, Canada. Arch. Microbiol. 2022, 204, 1–15. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [PubMed]
- FortWhyte Alive Our Story. Available online: https://www.fortwhyte.org/about/our-story/ (accessed on 1 February 2024).
- Ayumu, B.B. Water Budget Investigation for FortWhyte Alive; University of Manitoba: Winnipeg, Manitoba, Canada, 2020. [Google Scholar]
- Oak Hammock Marsh History. Available online: https://www.oakhammockmarsh.ca/about/history-of-oak-hammock/ (accessed on 1 February 2024).
- Lew, S.; Lew, M.; Koblížek, M. Influence of Selected Environmental Factors on the Abundance of Aerobic Anoxygenic Phototrophs in Peat-Bog Lakes. Environ. Sci. Pollut. Res. 2016, 23, 13853–13863. [Google Scholar] [CrossRef] [PubMed]
- Gazulla, C.R.; Cabello, A.M.; Sánchez, P.; Gasol, J.M.; Sánchez, O.; Ferrera, I. A Metagenomic and Amplicon Sequencing Combined Approach Reveals the Best Primers to Study Marine Aerobic Anoxygenic Phototrophs. Microb. Ecol. 2023, 86, 2161–2172. [Google Scholar] [CrossRef] [PubMed]
- Mašín, M.; Nedoma, J.; Pechar, L.; Koblížek, M. Distribution of Aerobic Anoxygenic Phototrophs in Temperate Freshwater Systems. Environ. Microbiol. 2008, 10, 1988–1996. [Google Scholar] [CrossRef]
- Pérez, V.; Dorador, C.; Molina, V.; Yáñez, C.; Hengst, M. Rhodobacter sp. Rb3, an Aerobic Anoxygenic Phototroph Which Thrives in the Polyextreme Ecosystem of the Salar de Huasco, in the Chilean Altiplano. Anton. Leeuw. 2018, 111, 1449–1465. [Google Scholar] [CrossRef]
- Kyndt, J.A.; Robertson, S.; Shoffstall, I.B.; Ramaley, R.F.; Meyer, T.E. Genome Sequence and Characterization of a Xanthorhodopsin-Containing, Aerobic Anoxygenic Phototrophic Rhodobacter Species, Isolated from Mesophilic Conditions at Yellowstone National Park. Microorganisms 2022, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Muramatsu, K.; Ueda, Y. Molecular Genetic Analyses of Rhodobacter azotoformans sp. nov. and Related Species of Phototrophic Bacteria. Syst. Appl. Microbiol. 1996, 19, 168–177. [Google Scholar] [CrossRef]
- Takaichi, S.; Shimada, K. Pigment Composition of Two Pigment-Protein Complexes Derived from Anaerobically and Semi-Aerobically Grown Rubrivivax gelatinosus, and Identification of a New Keto-Carotenoid, 2-Ketospirilloxanthin. Plant Cell Physiol. 1999, 40, 613–617. [Google Scholar] [CrossRef]
- Hansen, T.A.; Veldkamp, H. Rhodopseudomonas sulfidophila, nov. spec., a New Species of the Purple Nonsulfur Bacteria. Arch. Mikrobiol. 1973, 92, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Csotonyi, J.T.; Swiderski, J.; Stackebrandt, E.; Yurkov, V. A New Environment for Aerobic Anoxygenic Phototrophic Bacteria: Biological Soil Crusts. Environ. Microbiol. Rep. 2010, 2, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Csotonyi, J.T.; Swiderski, J.; Stackebrandt, E.; Yurkov, V. V. Novel Halophilic Aerobic Anoxygenic Phototrophs from a Canadian Hypersaline Spring System. Extremophiles 2008, 12, 529–539. [Google Scholar] [CrossRef]
- Jia, L.; Feng, X.; Zheng, Z.; Han, L.; Hou, X.; Lu, Z.; Lv, J. Polymorphobacter fuscus sp. nov., Isolated from Permafrost Soil, and Emended Description of the Genus Polymorphobacter. Int. J. Syst. Evol. Microbiol. 2015, 65, 3920–3925. [Google Scholar] [CrossRef]
- Xiao, N.; Jiao, N. Formation of Polyhydroxyalkanoate in Aerobic Anoxygenic Phototrophic Bacteria and Its Relationship to Carbon Source and Light Availability. Appl. Environ. Microbiol. 2011, 77, 7445–7450. [Google Scholar] [CrossRef]
- Liu, X.; Guo, X.; Liu, Y.; Lu, S.; Xi, B.; Zhang, J.; Wang, Z.; Bi, B. A Review on Removing Antibiotics and Antibiotic Resistance Genes from Wastewater by Constructed Wetlands: Performance and Microbial Response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef]
- Ma, J.; Cui, Y.; Li, A.; Zou, X.; Ma, C.; Chen, Z. Antibiotics and Antibiotic Resistance Genes from Wastewater Treated in Constructed Wetlands. Ecol. Eng. 2022, 177, 106548. [Google Scholar] [CrossRef]
- Ohore, O.E.; Qin, Z.; Sanganyado, E.; Wang, Y.; Jiao, X.; Liu, W.; Wang, Z. Ecological Impact of Antibiotics on Bioremediation Performance of Constructed Wetlands: Microbial and Plant Dynamics, and Potential Antibiotic Resistance Genes Hotspots. J. Hazard. Mater. 2022, 424, 127495. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Performance of Full Scale Constructed Wetlands in Removing Antibiotics and Antibiotic Resistance Genes. Sci. Total Environ. 2021, 786, 147368. [Google Scholar] [CrossRef] [PubMed]
- Berezowsky, M. Constructed Wetlands for Remediation of Urban Waste Waters. Geosci. Can. 1995, 22, 129–141. [Google Scholar]
- Yurkov, V.; Hughes, E. Aerobic Anoxygenic Phototrophs: Four Decades of Mystery. In Modern Topics in the Phototrophic Prokaryotes: Environmental and Applied Aspects; Springer International Publishing: Switzerland, 2017; pp. 193–214. [Google Scholar]
- Imhoff, J.F.; Rahn, T.; Künzel, S.; Neulinger, S.C. Photosynthesis is Widely Distributed among Proteobacteria as Demonstrated by the Phylogeny of PufLM Reaction Center Proteins. Front. Microbiol. 2018, 8, 2679. [Google Scholar] [CrossRef]
- Gupta, S.K.; Lal, D.; Lal, R. Novosphingobium panipatense sp. nov. and Novosphingobium mathurense sp. nov., from Oil-Contaminated Soil. Int. J. Syst. Evol. Microbiol. 2009, 59, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.P.; Wang, B.J.; Liu, Y.H.; Liu, S.J. Novosphingobium taihuense sp. nov., a Novel Aromatic-Compound-Degrading Bacterium Isolated from Taihu Lake, China. Int. J. Syst. Evol. Microbiol. 2005, 55, 1229–1232. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From Diversity and Genomics to Functional Role in Environmental Remediation and Plant Growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Chettri, B.; Singh, A.K. Kinetics of Hydrocarbon Degradation by a Newly Isolated Heavy Metal Tolerant Bacterium Novosphingobium panipatense P5:ABC. Bioresour. Technol. 2019, 294, 122190. [Google Scholar] [CrossRef]
- Francis, I.M.; Jochimsen, K.N.; De Vos, P.; van Bruggen, A.H.C. Reclassification of Rhizosphere Bacteria Including Strains Causing Corky Root of Lettuce and Proposal of Rhizorhapis suberifaciens gen. nov., comb. nov., Sphingobium mellinum sp. nov., Sphingobium xanthum sp. nov. and Rhizorhabdus argentea gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1340–1350. [Google Scholar]
- Kim, M.; Bae, J.; Park, W. Rhizorhabdus phycosphaerae sp. nov., Isolated from the Phycosphere of Microcystis aeruginosa. Int. J. Syst. Evol. Microbiol. 2022, 72, 005324. [Google Scholar] [CrossRef]
- Hördt, A.; López, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Göker, M. Analysis of 1,000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar]
- Li, Z.; Li, L.; Sun, H.; Wang, W.; Yang, Y.; Qi, Z.; Liu, X. Ammonia Assimilation: A Double-Edged Sword Influencing Denitrification of Rhodobacter azotoformans and for Nitrogen Removal of Aquaculture Wastewater. Bioresour. Technol. 2022, 345, 126495. [Google Scholar] [CrossRef]
- Costa, S.; Ganzerli, S.; Rugiero, I.; Pellizzari, S.; Pedrini, P.; Tamburini, E. Potential of Rhodobacter capsulatus Grown in Anaerobic-Light or Aerobic-Dark Conditions as Bioremediation Agent for Biological Wastewater Treatments. Water-SUI. 2017, 9, 108. [Google Scholar] [CrossRef]
- Ali, A.; Li, M.; Su, J.; Li, Y.; Wang, Z.; Bai, Y.; Ali, E.F.; Shaheen, S.M. Brevundimonas diminuta Isolated from Mines Polluted Soil Immobilized Cadmium (Cd2+) and Zinc (Zn2+) through Calcium Carbonate Precipitation: Microscopic and Spectroscopic Investigations. Sci. Total Environ. 2022, 813, 152668. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chaturvedi, P.; Chandra, R.; Kumar, S. Identification of Heavy Metals Tolerant Brevundimonas sp. from Rhizospheric Zone of Saccharum Munja L. and Their Efficacy in in-Situ Phytoremediation. Chemosphere 2022, 295, 133823. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Venkateswarlu, K.; Megharaj, M. Anoxygenic Phototrophic Purple Non-Sulfur Bacteria: Tool for Bioremediation of Hazardous Environmental Pollutants. World J. Microbiol. Biotechnol. 2023, 39, 283. [Google Scholar] [CrossRef]

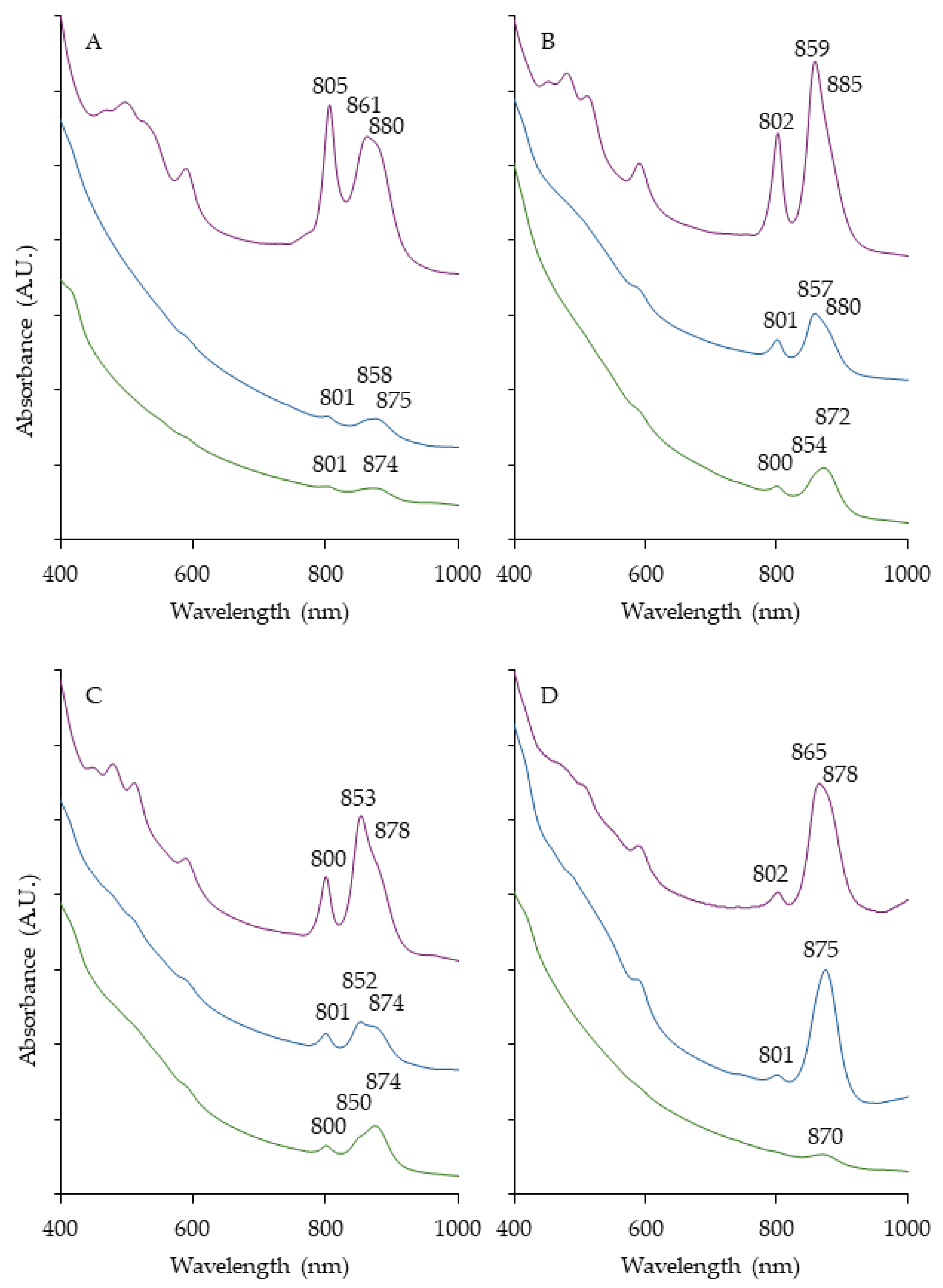
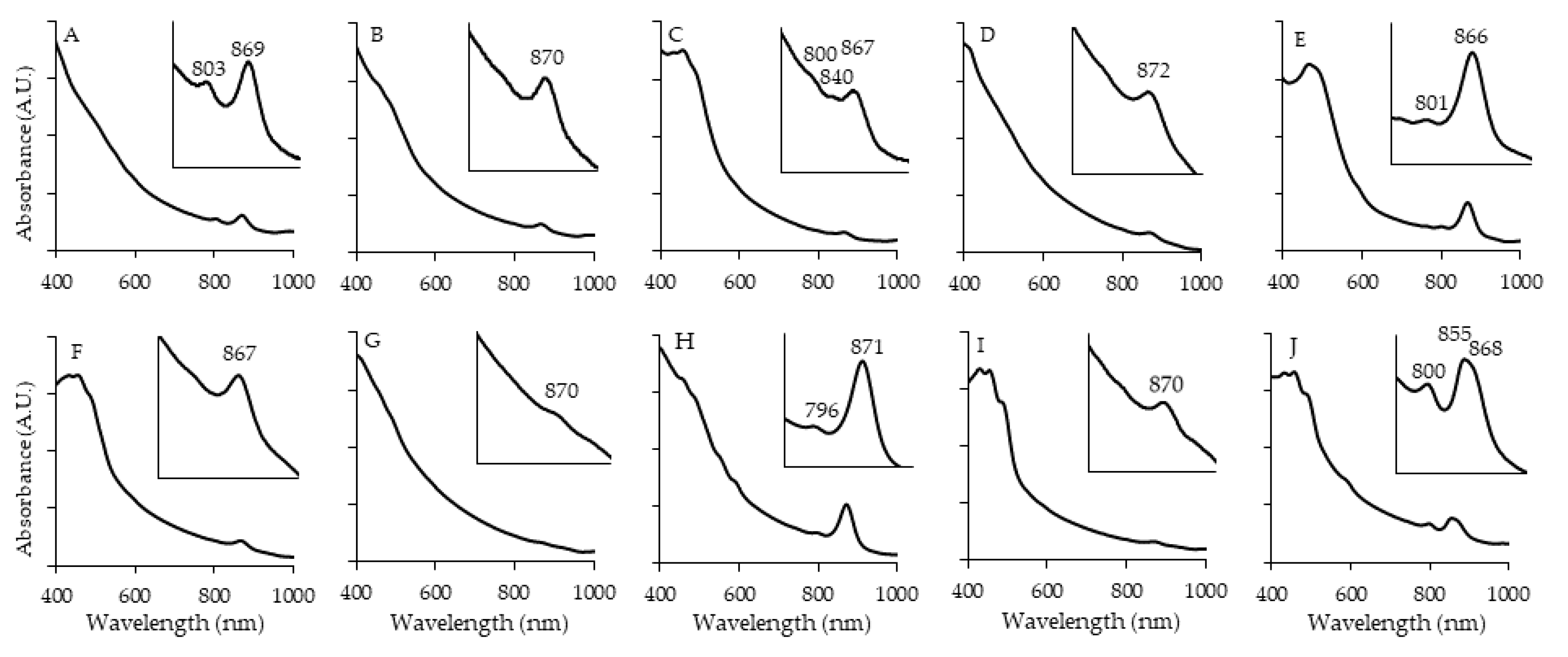
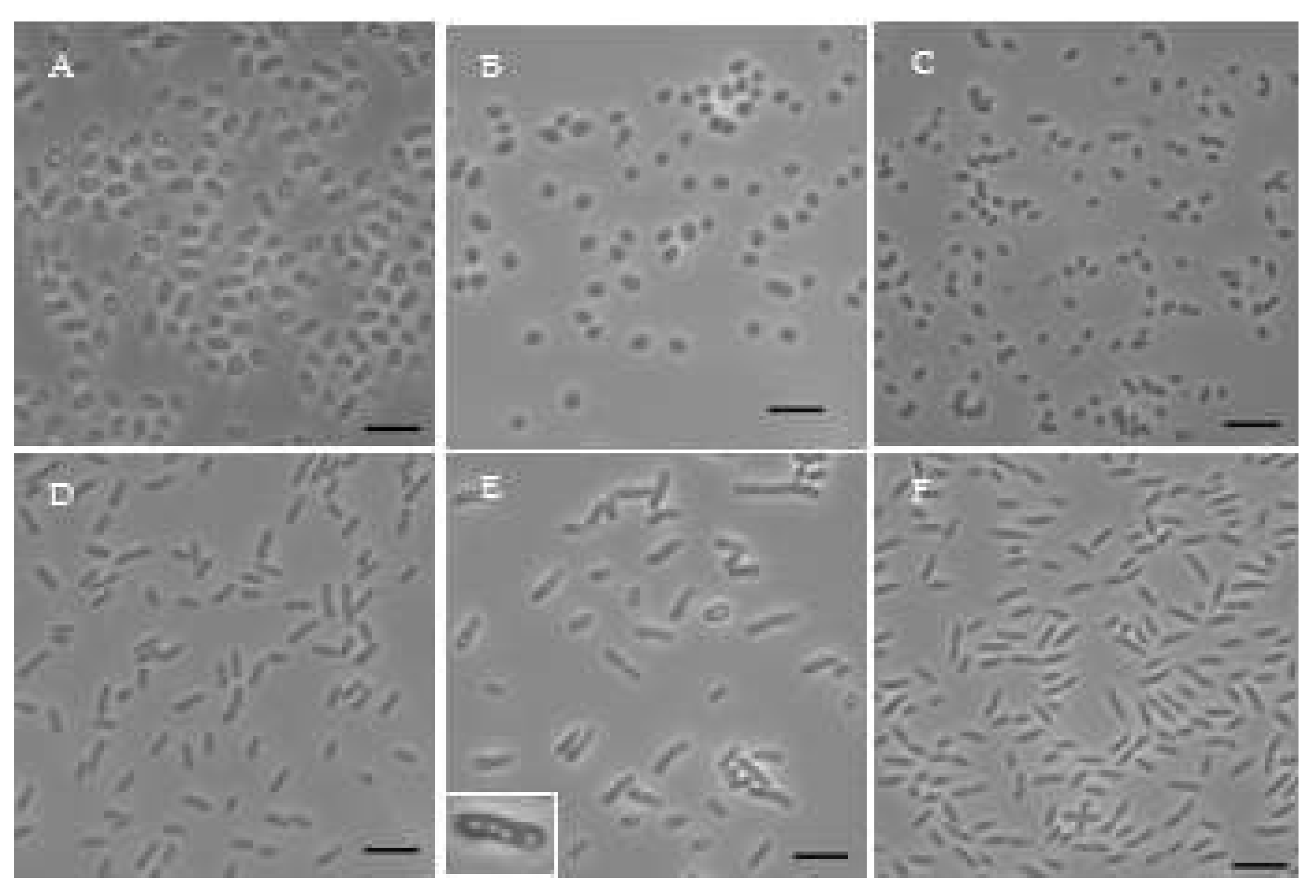
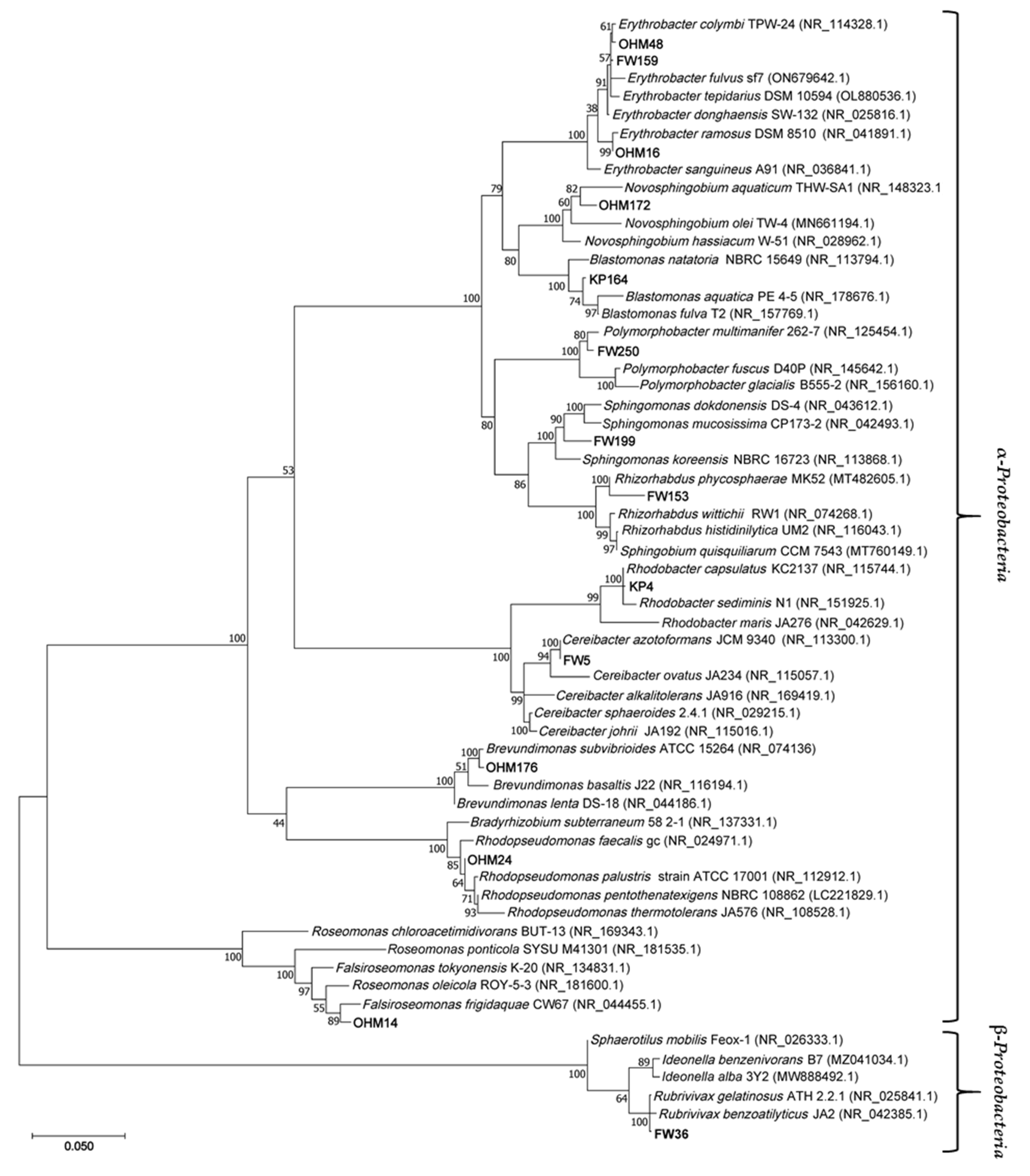
| Site | Colonies | Media | Colony Pigmentation | AnPB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PM | RO | OM | Yellow | Orange | Red | Pink | Brown | |||
| King’s Park | ||||||||||
| 1 | 38 | 15 | 18 | 5 | 8 | 11 | 10 | 3 | 1 | 17 |
| FortWhyte Alive | ||||||||||
| 2 | 47 | 10 | 32 | 5 | 12 | 17 | 8 | 3 | 3 | 27 |
| 3 | 19 | 5 | 8 | 6 | 4 | 5 | 3 | 1 | 1 | 4 |
| 4 | 46 | 2 | 27 | 17 | 8 | 9 | 4 | 11 | 5 | 18 |
| Oak Hammock Marsh | ||||||||||
| 5 | 31 | 5 | 20 | 6 | 6 | 17 | 3 | 1 | 1 | 18 |
| 6 | 41 | 4 | 36 | 1 | 10 | 16 | 4 | 4 | 2 | 14 |
| 7 | 13 | 2 | 5 | 6 | 1 | 1 | 1 | 9 | 0 | 4 |
| Total Colonies | 235 | 43 | 146 | 46 | 49 | 76 | 33 | 32 | 13 | 102 |
| Overall Percentage (%) | - | 18.3 | 62.1 | 19.6 | 20.9 | 32.3 | 14.0 | 13.6 | 5.5 | 43.4 |
| Strain | Sample Site | Dilution | Media | Most related species | 16S rRNA similarity (%) |
|---|---|---|---|---|---|
| FW199 | 3 | 10-3 | PM | Sphingomonas dokdonensis DS-4 | 97.69 |
| OHM172 | 7 | 10-2 | RO | Novosphingobium hassiacum W-51 | 98.65 |
| KP164 | 1 | 10-1 | RO | Blastomonas aquatica PE 4-5 | 99.47 |
| FW250 | 5 | 10-4 | RO | Polymorphobacter multimanifer 262-7 | 99.32 |
| FW153 | 3 | 10-3 | RO | Rhizorhabdus phycosphaerae MK52 | 97.11 |
| OHM176 | 7 | 10-2 | RO | Brevundimonas subvibrioides ATCC 15264 | 99.77 |
| OHM48 | 6 | 10-1 | RO | Erythrobacter colymbi TPW-24 | 99.70 |
| OHM16 | 8 | 10-2 | OM | Erythrobacter ramosus DSM 8510 | 99.77 |
| FW159 | 5 | 10-5 | RO | Erythrobacter donghaensis SW-132 | 99.77 |
| OHM14 | 8 | 100 | OM | Falsiroseomonas frigidaquae CW67 | 98.87 |
| OHM24 | 8 | 100 | PM | Rhodopseudomonas pentothenatexigens NBRC 108862 | 99.54 |
| FW36 | 4 | 10-3 | PM | Rubrivivax gelatinosus ATH 2.2.1 | 99.93 |
| FW5 | 4 | 10-3 | PM | Cereibacter azotoformans JCM 9340 | 100.00 |
| KP4 | 1 | 100 | PM | Rhodobacter capsulatus KC2137 | 99.92 |
| Strain | FW199 | OHM172 | KP164 | FW250 | FW153 | OHM176 | OHM48 | OHM16 | FW159 | OHM14 | OHM24 | FW36 | FW5 | KP4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colony colour | Yellow | Yellow | Yellow | Yellow-Brown | Light Orange | Orange | Orange | Orange | Red | Pink | Pink | Purple-Brown | Red | Red |
| Cell shape | Rod | Rod | Rod | Ovoid | Rod | Rod | Rod | Rod | Rod | Coccoid | Rod | Rod | Coccoid | Ovoid-rod |
| Cell size (µm) | 2.29±0.41 x 0.63±0.02 | 4.16±0.88 x 0.72±0.07 | 3.85±0.36 x 0.81±0.04 | 1.40±0.11 x 0.78±0.03 | 3.08±0.22 x 0.68±0.05 | 3.09±0.44 x 0.60±0.03 | 2.76±0.34 x 0.65±0.03 | 1.66±0.26 x 0.87±0.05 | 2.29±0.32 x 0.58±0.08 | 1.69±0.16 x 1.18±0.08 | 2.48±0.18 x 0.82±0.07 | 3.02±0.30 x 0.72±0.06 | 1.65±0.13 x 1.31±0.08 | 2.46±0.71 x 0.84±0.05 |
| Motility | + | - | - | - | - | + | - | - | - | + | + | + | + | - |
| Temperature range/optimum (°C) | 20-41/32 | 12-37/32 | 12-37/28 | 16-37/32 | 12-41/32 | 12-32/32 | 12-37/37 | 7-41/32 | 16-37/32 | 12-37/32 | 12-41/32 | 12-41/37 | 7-41/37 | 12-41/32 |
| pH range/optimum | 6-10/6 | 6-9/8 | 6-9/6 | 6-9/7 | 6-9/6 | 6-8/6 | 6-9/6 | 6-9/6 | 6-9/7 | 7-9/7 | 6-9/6 | 6-10/6 | 6-11/6 | 6-9/6 |
| Enzyme Activity: | ||||||||||||||
| Catalase | + | + | + | - | - | + | + | + | - | - | - | - | - | - |
| Oxidase | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Nitrate reductase | - | - | - | - | + | - | - | - | - | - | - | + | + | - |
| Hydrolysis of: | ||||||||||||||
| Gelatin | - | - | + | + | - | + | - | + | + | + | - | + | - | + |
| Starch | + | - | + | - | - | - | + | + | + | - | - | + | - | - |
| Tween 20 | + | - | + | - | - | - | - | + | - | - | - | + | - | - |
| Tween 40 | - | + | + | - | - | - | + | + | + | - | - | + | - | - |
| Tween 60 | + | + | + | + | - | + | + | + | + | - | - | + | - | - |
| Tween 80 | + | + | + | - | - | + | + | + | + | - | - | + | - | - |
| Strain | FW199 | OHM172 | KP164 | FW250 | FW153 | OHM176 | OHM48 | OHM16 | FW159 | OHM14 | OHM24 | FW36 | FW5 | KP4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Utilization of: | ||||||||||||||
| Acetate | - | + | + | - | + | - | + | + | - | - | + | + | + | + |
| Butyrate | - | + | + | - | + | - | + | + | + | - | + | + | + | + |
| Citrate | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Ethanol | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Formate | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fructose | + | - | - | - | - | - | - | - | - | - | - | + | + | + |
| Glucose | + | + | + | - | + | + | + | + | - | - | - | + | + | + |
| Glutamate | + | + | - | - | + | + | + | + | + | - | - | + | + | + |
| Lactose | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Malate | - | - | - | - | - | - | - | - | - | - | + | - | - | - |
| Pyruvate | - | + | + | - | + | + | + | + | - | - | + | + | + | + |
| Succinate | - | - | - | - | - | + | + | + | - | - | + | + | + | + |
| Fermentation: | ||||||||||||||
| Fructose | - | - | - | - | - | - | - | - | - | - | - | - | - | + |
| Glucose | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sucrose | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Strain | FW199 | OHM172 | KP164 | FW250 | FW153 | OHM176 | OHM48 | OHM16 | FW159 | OHM14 | OHM24 | FW36 | FW5 | KP4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotics: | ||||||||||||||
| Ampicillin | R | R | S | R | R | R | R | R | R | S | R | S | S | S |
| Chloramphenicol | R | S | S | R | S | S | S | S | S | S | R | S | S | S |
| Erytromycin | S | S | S | R | R | S | S | S | S | S | R | S | S | S |
| Imipenem | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Kanamycin | S | S | S | R | S | S | S | S | S | S | S | S | S | S |
| Naladixic Acid | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Penicilin G | R | R | S | R | R | R | R | R | R | S | R | S | S | S |
| Polymixin B | S | S | S | R | S | S | S | S | S | R | R | S | S | S |
| Streptomycin | R | R | R | S | R | S | R | R | R | S | R | S | S | S |
| Tetracycline | S | S | S | R | S | S | S | S | S | S | R | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





