1. Introduction
Mitochondrial DNA (mtDNA) has their own haploid, circular genome which in humans encodes 37 genes, including 13 proteins of the oxidative phosphorylation (OXPHOS) system [
1]. Because more than 95% mitochondrial proteins are encoded in nuclear DNA (nDNA), proper mitochondrial physiology require the analysis of genetic information encoded by both genomes [
2]. Nucleated cells contain a single diploid genome copy where maternal and paternal information is coded [
3]. By comparison, nucleated cells contain a variable number of mtDNA, from 100 to 10,000 copies depending on cell type, which are exclusively maternally inherited [
4]. mtDNA copy number (mtDNAcn) is typically quantified as the number of mtDNA copies normalized by the number of nDNA copies and has long been used as a metric of mitochondrial health [
5].
Studies show that there is not a simple correlation between mtDNAcn and mitochondrial health; both decreases and increases in mtDNAcn have been associated with heritable and acquired mitochondrial dysfunction [
6]. For example, mtDNAcn depletion is related to lower mitochondrial energy generation [
7]. On the contrary, increased mtDNAcn is related to compensatory upregulation of the OXPHOS system that occurs with compromised mitochondrial function [
8]. The variation of mtDNAcn is related to multiple diseases including, diabetes, cancer, neurological disorders, and cardiovascular diseases [
9,
10,
11].
mtDNAcn varies not only with specific measures of mitochondrial function, but also with environmental factors as well as with races [
12]. White adults with lower mtDNAcn were found to be associated with diabetes [
13]. Furthermore, lower mtDNAcn were found in female Black adults with risk of frailty [
14]. This pattern was also notably in men or Black adults who smoke heavy during their life [
14,
15]. In addition, mtDNAcn was found negatively associated with age and positively associated with adverse experience during early life [
16]. However, the role of mtDNAcn in infants’ neurodevelopmental outcomes related to racial disparity remains unclear.
This study aimed to explore the associations of blood mtDNAcn, health characteristics, and early life experience during NICU hospitalization, with neurodevelopmental outcomes among Black and White preterm infants. To this end, we used a well-validated droplet digital PCR (ddPCR) method for assessing mtDNAcn using genome DNA extracted from blood samples [
5]. These analyses will help to link the early life adverse experience with varied blood mtDNAcn and racial disparity that may contribute to preterm infants’ neurodevelopmental health [
5].
2. Methods
2.1. Experimental Design
A cohort study design was used to investigate the associations of infants’ clinical and medical characteristics, and blood mtDNAcn, with neurodevelopmental outcomes. The study was approved by the Institutional Review Board at two affiliated Level III/IV NICUs in Connecticut.
2.2. Subjects
Medically stable preterm infants were recruited between September 2017 to July 2022 at two NICU sites and who met the inclusion criteria were invited to participate the study. Inclusion criteria were: 1) preterm infants who were at 28 weeks to 32 weeks gestational age (GA) at birth, 2) consent from parents with minimal age requirement (≥ 18 years). Exclusion criteria were infants who have: 1) known congenital or chromosomal abnormalities; 2) severe periventricular/intraventricular hemorrhage (≥ grade III); 3) undergone surgery; and/or 4) illicit substances exposure history during pregnancy.
2.3. Clinical and Medical Characteristics
Preterm infants’ clinical characteristics were collected by research nurses including demographic, delivery method, history of preterm premature rupture of membrane (PPROM), race, birth GA, birth weight, birth length, birth head circumference, and the length of hospital stay.
2.4. Neurobehavioral Measures at NICU
Infants’ neurobehavioral outcomes were measured using the NICU Neonatal Neurobehavioral Scale (NNNS), which included 115 items and was categorized into 13 summary scores [
17]. NNNS were assessed when infants reached their post-menstrual age (PMA) of 36-38 weeks. Suggested by previous findings, we focused on several main summary scores in this study including stress/abstinence (NSTRESS), need for handling (NHANDLE), self-regulation (NREGULATION), and quality of movement (NQMOVE), attention (NATTENTION), arousal (NAROUSAL), reflex (NREFELX), excitability (NEXCITABILITY), and lethargy (NLETHARGY) [
18,
19].
The NSTRESS summary score measures the signs of neonatal abstinence and stress in high-risk preterm infants. The NHANDLE summary score measures to what extent the handling request for the infants to keep infants stay alert. The NREGULATION summary score measures the infants' ability to follow the auditory and visual orientation. The NQMOVE summary score quantifies the ability of infants' maturity and modulation of the movement of limbs. The NATTENTION score measures the ability of infants’ response to visual and auditory stimulation. The NAROUSAL score quantifies the overall level of arousal status during the examination. The NREFELEX score quantifies the presence and the strength of the newborns’ non-optimal responses. The levels of motor, state, and physiologic reactivity of infants were documented as the NEXCITABILITY score and NLETHARGY score, which measure the high and low scores [
19].
Lower scores on NSTRESS, NHANDLE, NREFLEX, NEXCITABILITY, and NLETHARGY subscales, and higher scores on NREGULATION, NQMOVE, NATTENTION subscales indicate better neurobehavioral outcomes. In addition, the moderate scores of NAROUSAL indicate the better response. While the low or high NAROUSAL scores note the infant is either difficult to respond or easily to upset during the examination.
2.5. Neurodevelopmental Outcomes at 1 and 2 Years of Corrected Age
Follow-up neurodevelopment was measured using Bayley Scale of Infant Developmental testing Edition III during the follow-up visits when infants reached 8 to 12 and 18 to 24 months corrected age (CA) [
20]. Bayley scores focused on Cognitive, Language, and Motor composite scores and higher scores reflect better development.
2.6. Blood mtDNAcn Quantification
In this study, an additional 1ml of peripheral venous blood for each infant was collected in EDTA tubes (Fisher Scientific, #02-687-107) as part of the routine blood draw for clinical care in NICU to avoid additional needle sticks to the infant. The blood samples were stored in a freezer at -80 ℃ until genomic DNA extraction. mtDNA and nuclear DNA would be isolated as part of this isolation protocol. Genomic DNA (gDNA) was isolated from 100ul of blood using the DNeasy Blood and Tissue kit from Qiagen (Qiagen, Germantown, MD, USA) following the manufacturer’s protocol. Extracted gDNA samples were checked for purity, concentration, and integrity prior to preparation for the digital droplet PCR copy number assay. Purity ratios were determined for each sample using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Samples were quantified and normalized using the dsDNA High Sensitivity Assay for Qubit 3.0 (Life Technologies, Carlsbad, CA, USA). To further assess DNA quality, gDNA was analyzed on the Agilent TapeStation 4200 (Agilent Technologies, Santa Clara, CA, USA) using the gDNA assay.
To assess mtDNA copy number in these samples, probe assays targeting the mitochondrial genome were obtained from BioRad (BioRad Life Science, Hercules, CA, USA) for use on the BioRad Digital Droplet QX200 system. Probe assay information is as follows: MT-ND1 assay ID: dHsaCPE5029120) with context sequence (CTCTAGCCTAGCCGTTTACTCAATCCTCTGATCAGGGTGAGCATCAAACTCAAACTACGCCC
TGATCGGCGCACTGCGAGCAGTAGCCCAAACAATCTCATATGAAGTCACCCTAGCCATCATTCTACT ATCAACATTACTAATAA) and EIF2C1 ((assay ID: dHsaCP1000002) with context sequence (TGGTTCGGCTTTCACCAGTCTGTGCGCCCTGCCATGTGGAAGATGATGCTCAACATTGATGGTGAGTGGGGAGAGCTATGGAGCCAGGGGCACCCCAAGTCCAGTGACCACACTCCCAGCCTC). gDNA samples were normalized to a standardized concentration of 5ng/μl and prepared for droplet generation following the manufacturer’s protocol for ddPCR Supermix for Probes (No dUTP). Master mix setup followed manufacturer recommendations for 20μl reaction volumes, using 5μl of a 1:10 dilution of normalized gDNA and no restriction enzyme. Emulsions were collected from the microfluidic chip, prepared for thermal cycling and then droplets were read by the QX200 droplet reader. Samples were analyzed by the QuantaLife Software to determine the absolute mitochondrial copy number (copies/ul). The mtDNAcn was then calculated by using the absolute copies of mtDNA molecules from MT-ND1 genes and normalized to EIF2C1 copy number [
5].
2.7. Statistical Analysis
Data analyses were performed using R (version 4.2.0). The general characteristics of the samples were described using descriptive statistics. Quantitative variables were described using means, standard deviations, and qualitative variables were described using frequencies and percentages. The primary outcomes were as the log-transformed mtDNAcn, NNNS subscale scores and the Bayley scores. Linear regression models were performed to investigate the relationship between the primary neurobehavioral outcomes and infant age, gender, race, PPROM, NNNS score, and log-transformed mtDNAcn. Bayesian Information Criterion (BIC) was used as the selection criteria to select a set of candidate models while holding the control variables (infant age, sex, race, PPROM) constant across all models. The race differentiated association between mtDNAcn and NNNS for infants during NICU stay, and the association between mtDNAcn and Bayley scores for infants’ neurodevelopment at one and two years of age were assessed through unadjusted and adjusted linear regression models. Linear models with interaction term between mtDNAcn and race were introduced to assess the race differentiated association between mtDNAcn and NNNS for infants during NICU stay, and the association between mtDNAcn and Bayley scores for infants’ neurodevelopment at age one and two years of age. Comprehensive diagnostics were performed on these candidate models to identify the most parsimonious model that provides the best balance between model fit and complexity. All statistical tests are examined at a 5% level of significance.
3. Results
3.1. Demographic Characteristics of Preterm Infants
The majority of the preterm infants in our study were male (54.55%), white (70.91%), and non-Hispanic (72.73%). The infants were born at 27.97 ± 2.55 weeks of GA with 1055.44 ± 363.74 g birth weight. Most of the study infants had C-section birth (72.73%), had no experience of PPROM (76.36%), and was treated with an antibiotic in the first 3 days after birth (72.73%). The average NICU hospitalization was 74.09 ± 38.16 days (
Table 1).
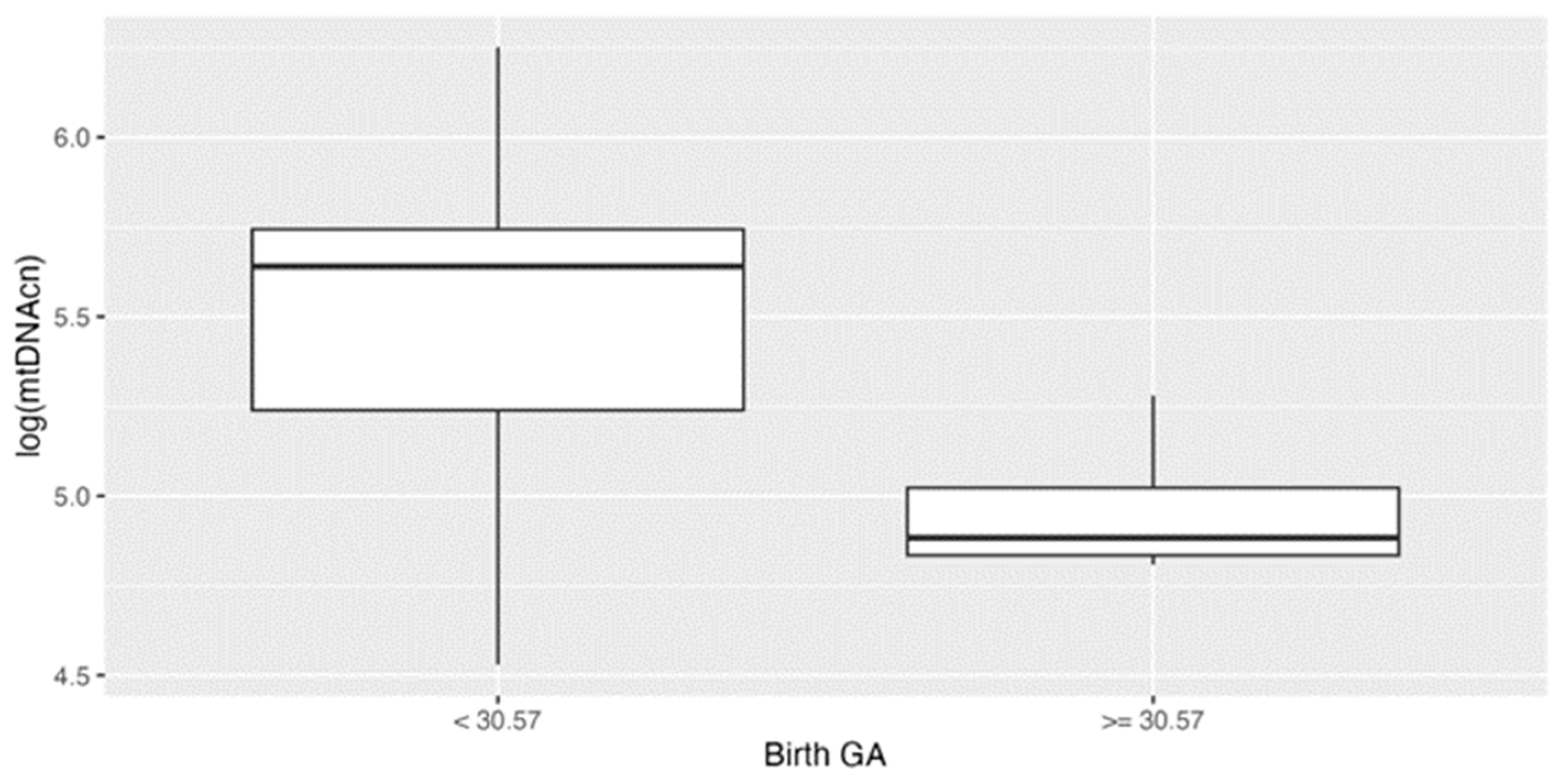
3.2. Mitochondrial DNA Copy Number Varied with Different Age
We previously found the birth GA of 30.57 weeks as a cutoff predicting neurodevelopmental outcomes [
18]. In this study, we found mtDNAcn were significantly higher in younger GA group (< 30.57 weeks) (p<0.001) (
Figure 1A). No significant associations were observed between the level of mtDNAcn and other clinical characteristics, including race.
3.3. Association between mtDNAcn and Neurobehavior at 36 to 38 PMA
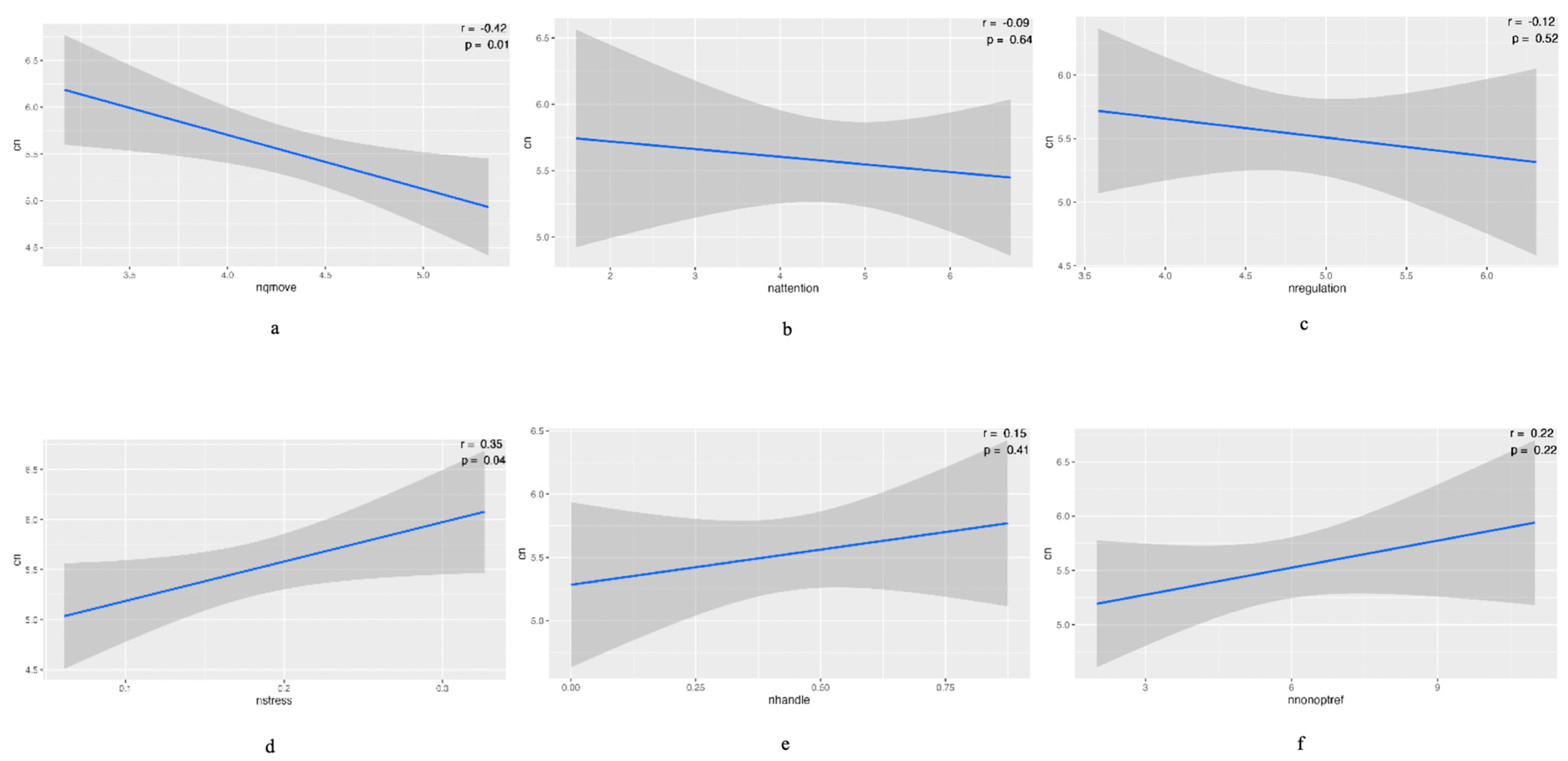
Negative associations were observed between mtDNAcn and NQMOVE (p<0.01), NATTENTION, and NREGULATION. Positive associations were observed between mtDNAcn and NSTRESS (p<0.05), NHANDLE, and NREFELEX. (Figure 2). There was no significant correlation between mtDNAcn and NNNS subscale scores when we adjusted the clinical variables including infants’ race, gender, risk of PPROM, and birth GA. However, we observed the positive association between PPROM and NSTRESS, NEXCITABILITY, and NAROUSAL (p<0.05) (
Table 2). Figure 2. The association between mtDNAcn and neurobehavior at 36 to 38 postmenstrual age (PMA). The negative associations were observed between mtDNAcn and NQMOVE (p<0.01), NATTENTION, and NREGULATION (a to c). The positive associations were observed between mtDNAcn and NSTRESS (p<0.05), NHANDLE, and NREFELEX (d to e).
3.4. Association between mtDNAcn and Neurodevelopment at 1 and 2 Years of Age
When adjusted for the infant demographic and health characteristics including race, gender, risk of PPROM, and birth GA, at follow-up 8 to 12 months CA, infants’ Bayley Cognitive composite scores were lower if they had higher mtDNAcn (p<0.05). Male infants had lower Bayley Cognitive and Motor composite scores (p<0.05) compared to female cohorts (
Table 3). There were no significant associations between Bayley composite scores and mtDNAcn at 18-24 months of CA follow-up.
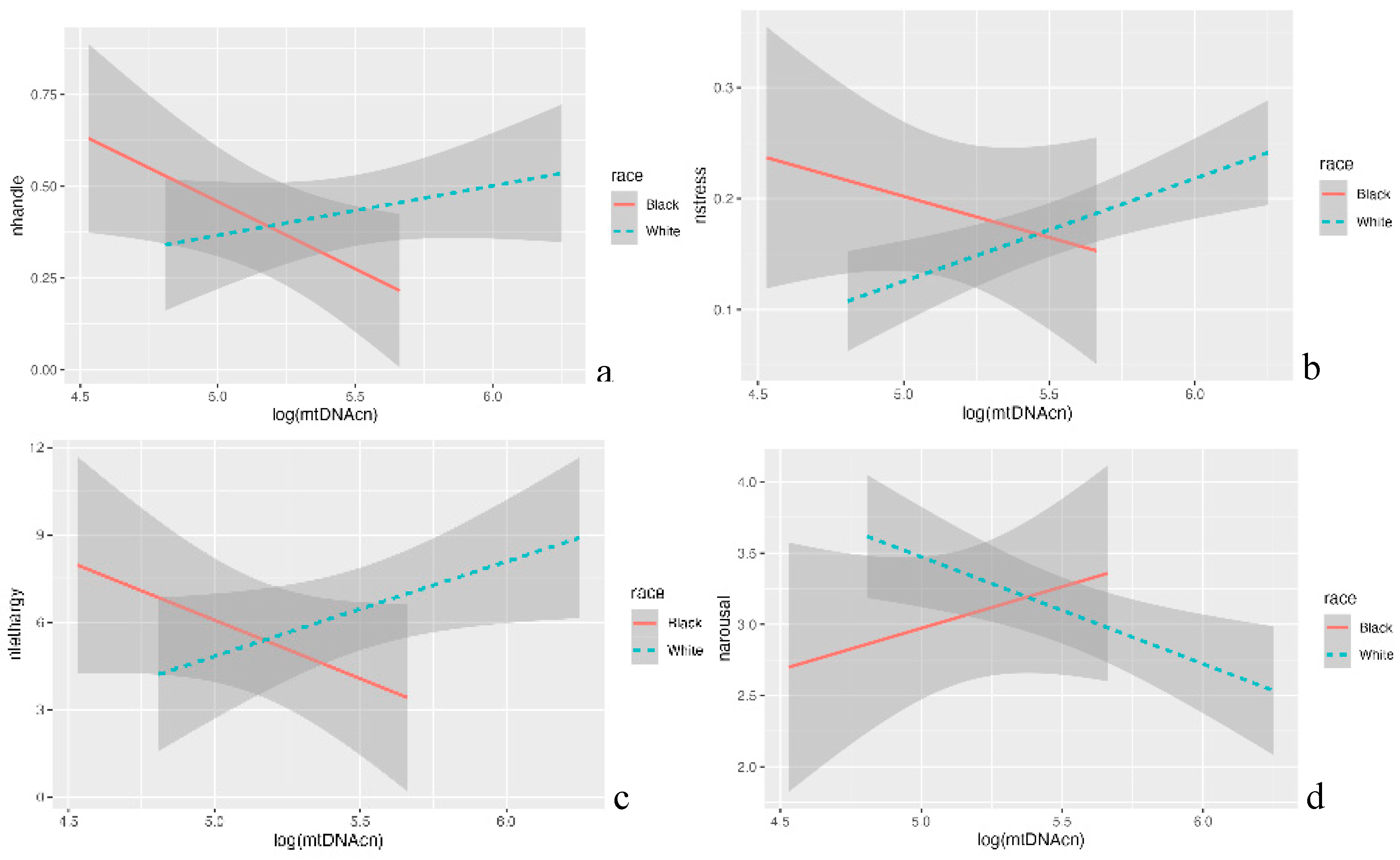
3.5. Racial Differences in the Association between mtDNAcn and Neurodevelopmental Outcomes
Average scores of each NNNS subscale were computed (see supplementary
Table S1). At 36 to 38 weeks of PMA, we observed positive associations between mtDNAcn and NHANDLE (p<0.05), NSTRESS (p<0.05), and NLETHARGY (p<0.05). In addition, we observed the negative association between mtDNAcn and NAROUSAL (p<0.01) in White infants. Interestingly, these correlations were reversed for Black infants (
Figure 3). We also observed the negative association between mtDNAcn and Cognitive and Language composite scores (p<0.05) at 8 to 12 months of CA follow-up white infants. However, the positive association between Black infants’ mtDNAcn and their Cognitive and Language composite scores (p<0.05) at 8 to 12 months of CA follow-up were observed in this study (
Figure 4). There were no significant findings for both White and Black infants at 18 to 24 months of CA follow-up.
4. Discussion
To our knowledge, this study represents the initial investigation into the correlations between blood mtDNAcn and neurobehavior and neurodevelopment of preterm infants, spanning from their NICU stay to 2 years of CA. Consistent with previous studies, we observed a negative association between blood mtDNAcn and birth GA in preterm infants [
21]. Although the underlying mechanisms of age-dependent decrease in mtDNAcn in infants are not fully understood, early life reactive oxygen species (ROS) exposure and compensatory increased level of mtDNAcn could help to preserve mitochondrial function and sustaining energy production [
22]. This adaptation could be particularly crucial to meet the elevated demand of ATP generation during the growth of preterm infants [
23].
Our previous study found that birth before 30.57 weeks of GA could be used as time cutoff to predict the infants’ unfavorable neurobehavioral outcomes [
18]. In the current study, we found that White infants who were born before 30.57 weeks of GA had significant increased mtDNAcn, which indicate the link between increased mtDNAcn and unfavorable neurobehavior, while among Black infants, the opposite was true. Future larger cohort study is warranted to further confirm our findings.
In addition, our study observed the distinct patterns of mtDNAcn variation associated with neurodevelopmental outcomes among preterm infants, highlighting differences based on their racial backgrounds. We observed that White infants with higher mtDNAcn had higher stress and abstinence signs, jittery and startle body movement, and easily aroused to fuss and cry at 36 to 38 weeks of PMA. Conversely, the opposite trend was observed in Black infants. And this association was also reflected in the poor Cognitive status and Language ability in infants during their 1 year of CA follow-up. Although mtDNAcn serves as an indirect measure of mitochondrial function, the link between increased mtDNAcn and decline in language and cognitive abilities in our study suggests an association with over produced ROS, potentially leading to impaired brain cells and compromised mitochondrial function in infants [
24].
The risk of PPROM was identified to be associated with nonoptimal performance in NSTRESS, NEXCITABILITY, and NAROUSAL in preterm infants. Despite not observing a direct association between mtDNAcn and PPROM, existing evidence suggests the maternal inheritance of elevated mtDNAcn as a risk factor for PPROM [
25,
26]. Early study has indicated that the incidence of PPROM could be linked to maternal exposure to smoking and bacterial infection, supporting the hypothesis that an over-produced ROS may induce membrane inflammatory responses [
27,
28].
Although we did not observe significant associations between sex-differentiated mtDNAcn and NNNS or Bayley scores, male infants, identified as an independent risk factors for poor neurodevelopmental [
29], exhibited lower NAROUSAL scores at 36 to 38 PMA and lower cognitive and motor composite scores at 1 year of age compared to female cohorts. The impaired neurodevelopment in male preterm infants may be attributed to factors such as lower energy and fat intake after birth [
30], challenges in adapting to maternal stress [
31], the oxidative status of the placenta [
32], and brain development issues [
33].
4.1. Conclusion and Limitations
In summary, our study provides supporting evidence for the established negative correlation between mtDNAcn and GA. Additionally, it identified race disparity and its correlation between mtDNAcn and neurobehavioral and neurodevelopmental outcomes in infants from birth until 2 years of CA. These findings suggest that elevated mtDNAcn holds potential as a biomarker for mitochondrial stress, perhaps associated with overproduction of ROS in preterm infants. However, due to the limited sample size in this study, a follow-up investigation with a larger participant cohort is needed to strengthen the clinical significance of this data. In addition to an expanded cohort, measuring mtDNAcn across multiple timepoints would be valuable, capturing the variations in mtDNAcn during early and later life in preterm infants.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Infant NNNS subscale scores.
Author Contributions
All authors met the Pediatric Research authorship requirements. Substantial
contributions to conception and design, acquisition of data, or analysis and interpretation of data: all authors.
Drafting and revising the manuscript: T.Z., A.L., R.B., N.A., and X.C. Thoroughly reviewed and critiqued the
manuscript for revision: S.T., A.M., M.C., and Q.C. Final approval of the version to be published: all authors.
Funding Resources
NIH TL1 TR001864 (PI: Tingting Zhao); NIH/NINR F31NR019940 (PI: Tingting Zhao); Eastern Nursing Research Society (ENRS)/Council for the Advancement of Nursing Science Dissertation Award (PI: Tingting Zhao); Sigma Theta Tau International (STTI) Mu Chapter Research Grant (PI: Tingting Zhao). NIH/NINR NR016928 (PI: Xiaomei Cong).
Institutional Review Board Statement
The study was approved by the Institutional Review Board of Connecticut Children’s.Medical Center (protocol code: 16-001 and approval date: December 1, 2023 through December 31, 2026).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
There are no potential conflicts of interest.
References
- Habbane, M. , Montoya, J., Rhouda, T., Sbaoui, Y., Radallah, D., & Emperador, S. Human Mitochondrial DNA: Particularities and Diseases. Biomedicines. 2021. 9(10), 1364. [CrossRef]
- Walker, B. R. , & Moraes, C. T. Nuclear-Mitochondrial Interactions. Biomol. 2022. 12(3), 427. [CrossRef]
- Barlow, D. P., & Bartolomei, M. S. Genomic imprinting in mammals. Cold Spring Harb Perspect Biol. 2014. 6(2), a018382. [CrossRef]
- Wai, T., Ao, A., Zhang, X., Cyr, D., Dufort, D., & Shoubridge, E. A. The role of mitochondrial DNA copy
number in mammalian fertility. Biol Reprod. 2010, 83(1), 52–62. [CrossRef] [PubMed]
- Memon, A. A. , Zöller, B. , Hedelius, A., Wang, X., Stenman, E., Sundquist, J., & Sundquist, K. Quantification of mitochondrial DNA copy number in suspected cancer patients by a well optimized ddPCR method. Biomol Detect Quantif. 2017, 13, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Moraes CT, Shanske S, Tritschler HJ, Aprille JR, Andreetta F, Bonilla E, Schon EA, DiMauro S, mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991, 48, 492–501.
- Zhang, Z., Yang, D., Zhou, B., Luan, Y., Yao, Q., Liu, Y., Yang, S., Jia, J., Xu, Y., Bie, X., Wang, Y., Li, Z., Li,
A., Zheng, H., & He, Y. Decrease of MtDNA copy number affects mitochondrial function and involves in
the pathological consequences of ischaemic stroke. J Cell Mol Med. 2022, 26(15), 4157–4168. [CrossRef]
- Picard, M. Blood mitochondrial DNA copy number: What are we counting?. Mitochondrion. 2021;60:1-11. [CrossRef]
- Memon AA, Vats S, Sundquist J, Li Y, Sundquist K. Mitochondrial DNA Copy Number: Linking Diabetes and Cancer. Antioxid Redox Signal. 2022;37(16-18):1168-1190. [CrossRef]
- Klein HU, Trumpff C, Yang HS, et al. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer's disease brain. Mol Neurodegener. 2021;16(1):75. [CrossRef]
- Quan Y, Xin Y, Tian G, Zhou J, Liu X. Mitochondrial ROS-Modulated mtDNA: A Potential Target for Cardiac Aging. Oxid Med Cell Longev. 2020:9423593. [CrossRef]
- Xiao J, Cohen P, Stern MC, Odedina F, Carpten J, Reams R. Mitochondrial biology and prostate cancer ethnic disparity. Carcinogenesis. 2018;39(11):1311-1319. [CrossRef]
- DeBarmore B, Longchamps RJ, Zhang Y, et al. Mitochondrial DNA copy number and diabetes: the Atherosclerosis Risk in Communities (ARIC) study. BMJ Open Diabetes Res Care. 2020;8(1):e001204. [CrossRef]
- Ashar FN, Moes A, Moore AZ, et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J Mol Med (Berl). 2015;93(2):177-186. [CrossRef]
- Vyas CM, Ogata S, Reynolds CF 3rd, et al. Lifestyle and behavioral factors and mitochondrial DNA copy number in a diverse cohort of mid-life and older adults. PLoS One. 2020;15(8):e0237235. [CrossRef]
- Mendoza-Ortega JA, Reyes-Muñoz E, Nava-Salazar S, et al. Mitochondrial DNA Copy Number Adaptation as a Biological Response Derived from an Earthquake at Intrauterine Stage. Int J Environ Res Public Health. 2021;18(22):11771. [CrossRef]
- Tronick E, Lester BM. Grandchild of the NBAS: the NICU network neurobehavioral scale (NNNS): a review of the research using the NNNS. J Child Adolesc Psychiatr Nurs. 2013;26(3):193-203. [CrossRef]
- Zhao T, Griffith T, Zhang Y, et al. Early-life factors associated with neurobehavioral outcomes in preterm infants during NICU hospitalization. Pediatr Res. 2022;92(6):1695-1704. [CrossRef]
- Montirosso R, Del Prete A, Bellù R, Tronick E, Borgatti R; Neonatal Adequate Care for Quality of Life (NEO-ACQUA) Study Group. Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics. 2012;129(5):e1129-e1137. [CrossRef]
- Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW; Victorian Infant Collaborative Group. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352-356. [CrossRef]
- Xia CY, Liu Y, Yang HR, et al. Reference Intervals of Mitochondrial DNA Copy Number in Peripheral Blood for Chinese Minors and Adults. Chin Med J (Engl). 2017;130(20):2435-2440. [CrossRef]
- Giordano C, Iommarini L, Giordano L, et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy. Brain. 2014;137(Pt 2):335-353. [CrossRef]
- Tan JBC, Boskovic DS, Angeles DM. The Energy Costs of Prematurity and the Neonatal Intensive Care Unit (NICU) Experience. Antioxidants (Basel). 2018;7(3):37. [CrossRef]
- Lee JW, Park KD, Im JA, Kim MY, Lee DC. Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta. 2010;411(7-8):592-596. [CrossRef]
- Kumari R, Suneja A, Mehndiratta M, Guleria K, Malik R. Maternal Serum Vitamin E Levels and its Association with Cord Blood Telomere Length and Mitochondrial DNA Copy Number in Preterm Premature Rupture of Membranes. J Obstet Gynaecol India. 2023;73(1):9-14. [CrossRef]
- Choltus H, Minet-Quinard R, Belville C, et al. Cigarette Smoke Condensate Exposure Induces Receptor for Advanced Glycation End-Products (RAGE)-Dependent Sterile Inflammation in Amniotic Epithelial Cells. Int J Mol Sci. 2021;22(15):8345. [CrossRef]
- Zhang Q, Wang Z, Zhang W, et al. The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels. Nat Cell Biol. 2021;23(8):870-880. [CrossRef]
- Menon R, Richardson LS. Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin Perinatol. 2017;41(7):409-419. [CrossRef]
- Macedo I, Pereira-da-Silva L, Brito L, Cardoso M. Male sex is an independent risk factor for poor neurodevelopmental outcome at 20 months' corrected age, in human milk-fed very preterm infants: a cohort study. Einstein (Sao Paulo). 2019;17(3):eAO4607. [CrossRef]
- Tottman AC, Bloomfield FH, Cormack BE, Harding JE, Taylor J, Alsweiler JM. Sex-specific relationships between early nutrition and neurodevelopment in preterm infants. Pediatr Res. 2020;87(5):872-878. [CrossRef]
- Wainstock T, Shoham-Vardi I, Glasser S, Anteby E, Lerner-Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress. 2015;18(1):49-56. [CrossRef]
- Ruano CSM, Miralles F, Méhats C, Vaiman D. The Impact of Oxidative Stress of Environmental Origin on the Onset of Placental Diseases. Antioxidants (Basel). 2022;11(1):106. [CrossRef]
- Benavides A, Metzger A, Tereshchenko A, et al. Sex-specific alterations in preterm brain. Pediatr Res. 2019;85(1):55-62. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).