Submitted:
29 April 2024
Posted:
29 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
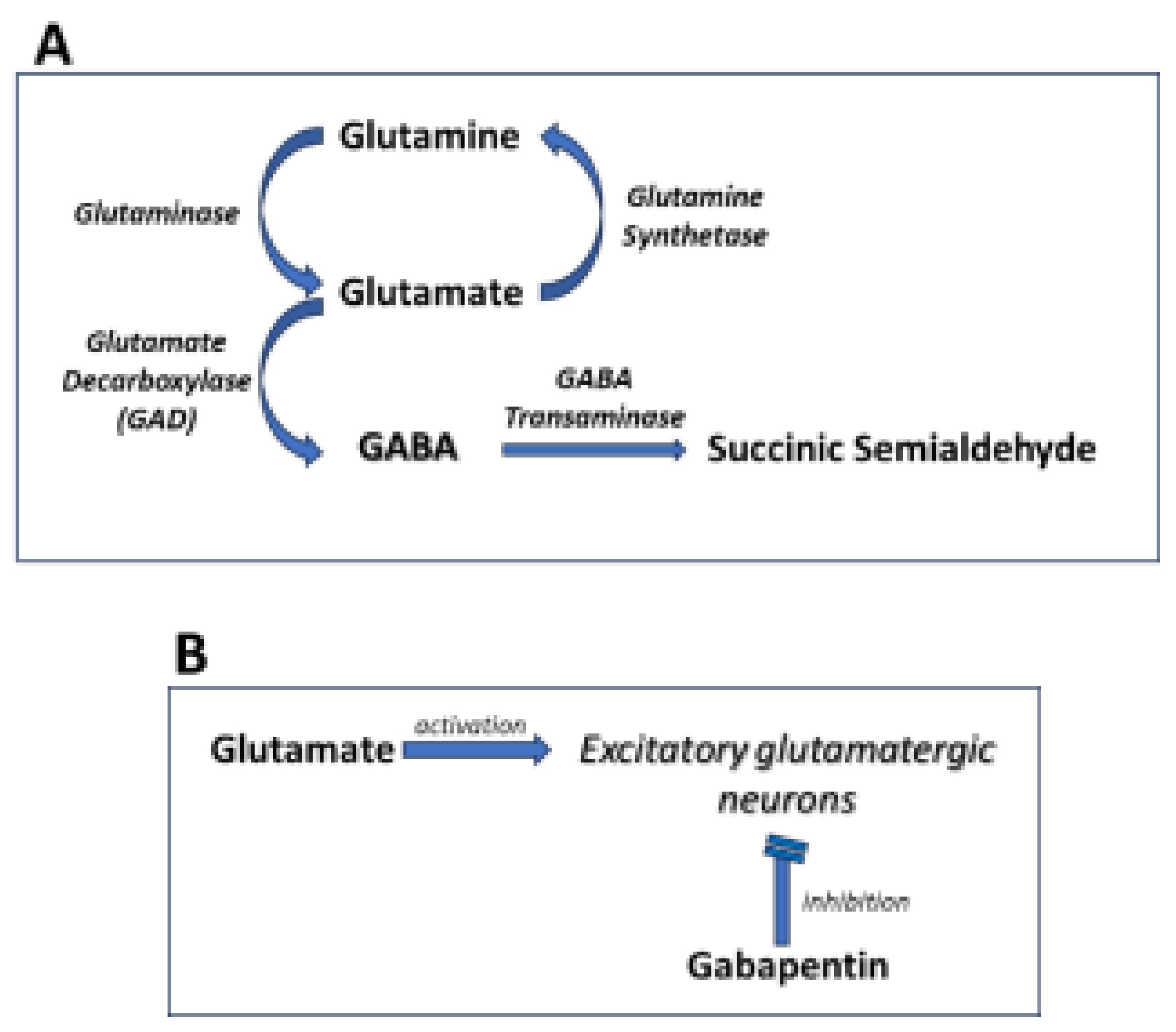
2. Pharmacology of GBP Action
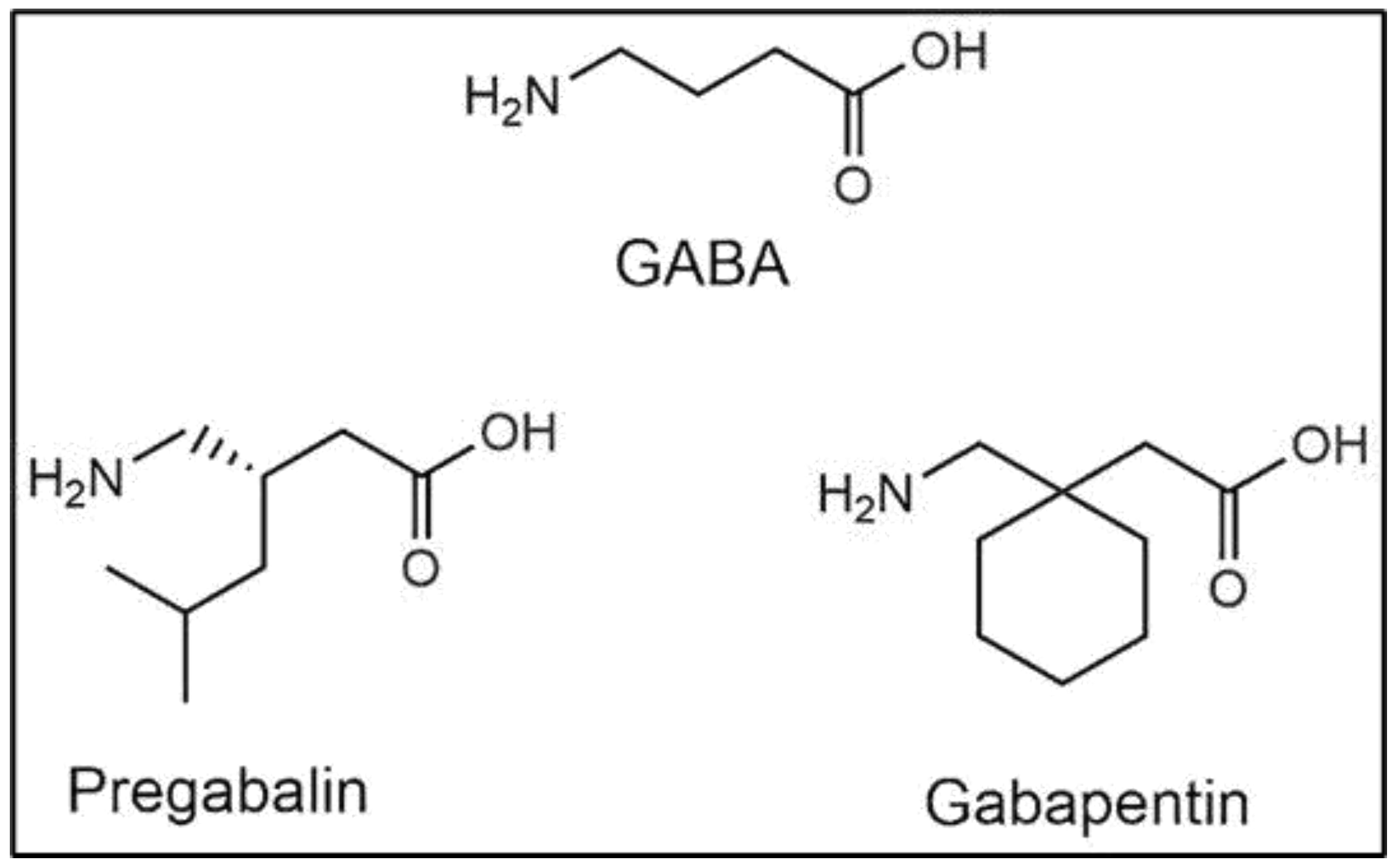
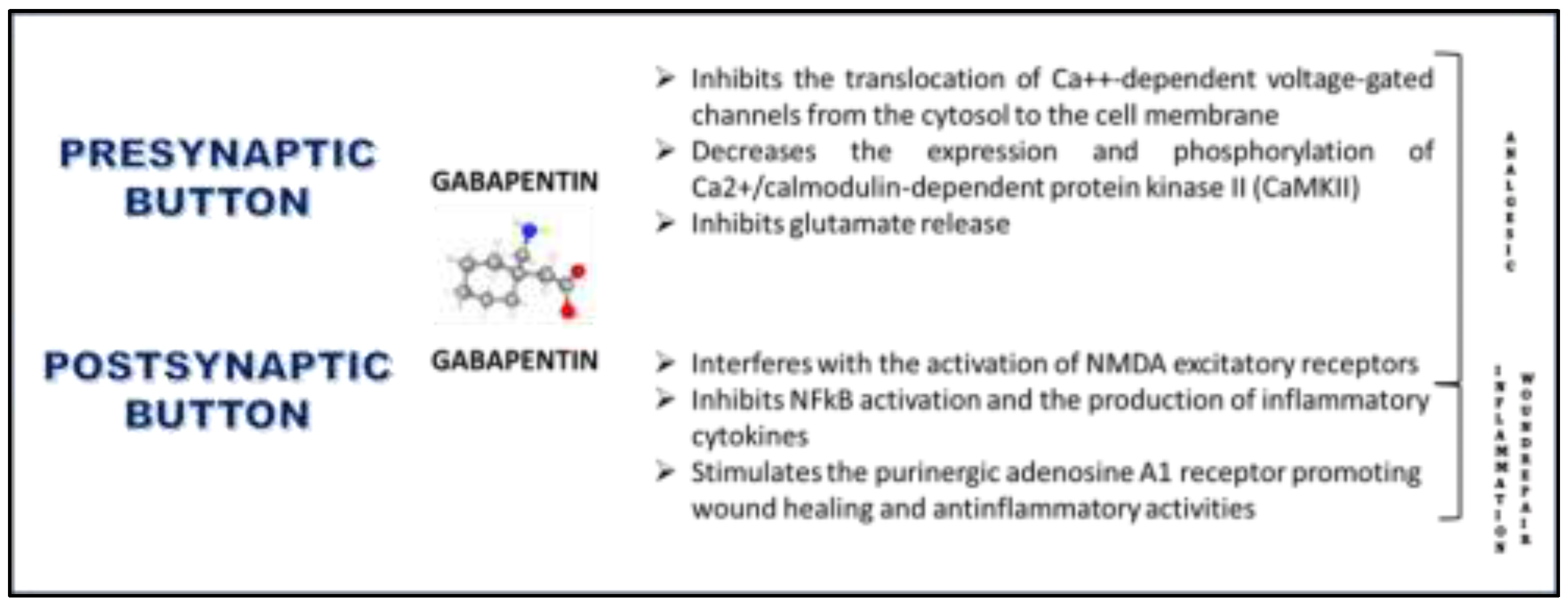
2.1. Effects on Voltage-Gated Calcium Channels
2.2. Effects on CaMKII
2.3. Effects on Glutamate Receptors
2.4. Effects on GABA Synthesis
2.5. Antinflammatory Effects
2.6. Effects on Adenosine A1 Receptors
2.7. Effects on Wound-Healing
3. Current Therapeutic Applications of Systemic GBP
3.1. Psychiatric Disorders
3.2. Anxiety Disorders
3.3. Substance Use Disorders
3.4. Bipolar Disorder
3.5. Insomnia
3.6. Pain Management
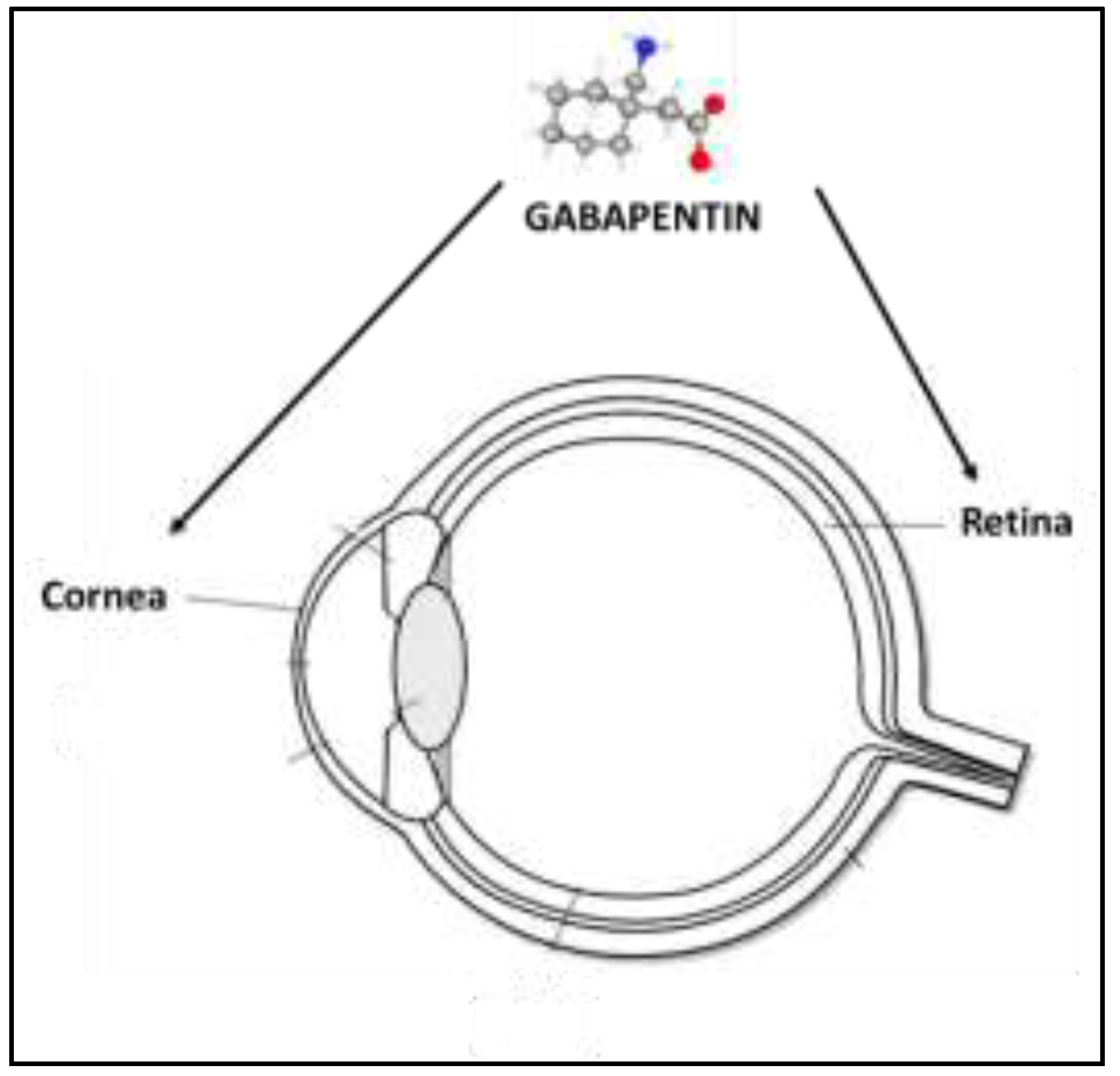
4. Experimental Evidence of Ophthalmic Use of GBP
4.1. Inflammation and Neuropathic Ocular Pain
4.2. Topical Formulation and Treatment with GBP Eye Drops
References
- Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW 4th, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009 Apr;60(1):43-56. [CrossRef]
- Vargas RA. The GABAergic System: An Overview of Physiology, Physiopathology and Therapeutics. Int J Clin Pharmacol Pharmacother. 2018; 3: 142. [CrossRef]
- Osikowicz M, Mika J, Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp Physiol. 2013 Feb;98(2):372-84. [CrossRef]
- Honarmand A, Safavi M, Zare M. Gabapentin: An update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci. 2011 Aug;16(8):1062-9. PMCID: PMC3263084. [PubMed]
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother. 2011 Dec;45(12):1483-90. Epub 2011 Nov 15. [CrossRef] [PubMed]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3628-33. Epub 2008 Feb 25. PMCID: PMC2265195. [CrossRef] [PubMed]
- Tzellos TG, Papazisis G, Amaniti E, Kouvelas D. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. Eur J Clin Pharmacol. 2008 Sep;64(9):851-8. Epub 2008 Jul 8. [CrossRef] [PubMed]
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris). 1997;153 Suppl 1:S39-45. [PubMed]
- Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels (Austin). 2008 Jan-Feb;2(1):4-9. Epub 2008 Apr 4. [CrossRef] [PubMed]
- Ma LL, Liu W, Huang YG, Yang N, Zuo PP. Analgesic effect of gabapentin in a rat model for chronic constrictive injury. Chin Med J (Engl). 2011 Dec;124(24):4304-9. [PubMed]
- Coderre TJ, Kumar N, Lefebvre CD, Yu JS. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2005 Aug;94(4):1131-9. [CrossRef] [PubMed]
- Hara K, Sata T. Inhibitory effect of gabapentin on N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Acta Anaesthesiol Scand. 2007 Jan;51(1):122-8. Epub 2006 Oct 31. [CrossRef] [PubMed]
- Kim YS, Chang HK, Lee JW, Sung YH, Kim SE, Shin MS, Yi JW, Park JH, Kim H, Kim CJ. Protective effect of gabapentin on N-methyl-D-aspartate-induced excitotoxicity in rat hippocampal CA1 neurons. J Pharmacol Sci. 2009 Jan;109(1):144-7. [CrossRef] [PubMed]
- Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, MacLean DM, Zhang Y, Zhou MH, Jayaraman V, Pan HL. The α2δ-1-NMDA Receptor Complex Is Critically Involved in Neuropathic Pain Development and Gabapentin Therapeutic Actions. Cell Rep. 2018 Feb 27;22(9):2307-2321. Erratum in: Cell Rep. 2022 Jan 25;38(4):110308. [CrossRef]
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998 Feb;29(3):233-49. [CrossRef] [PubMed]
- Yu J, Wang DS, Bonin RP, Penna A, Alavian-Ghavanini A, Zurek AA, Rauw G, Baker GB, Orser BA. Gabapentin increases expression of δ subunit-containing GABAA receptors. EBioMedicine. 2019 Apr;42:203-213. Epub 2019 Mar 14. PMCID: PMC6491385. [CrossRef] [PubMed]
- Errante LD, Petroff OA. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure. 2003 Jul;12(5):300-6. [CrossRef] [PubMed]
- Park S, Ahn ES, Han DW, Lee JH, Min KT, Kim H, Hong YW. Pregabalin and gabapentin inhibit substance P-induced NF-kappaB activation in neuroblastoma and glioma cells. J Cell Biochem. 2008 Oct 1;105(2):414-23. [CrossRef] [PubMed]
- Lee BS, Jun IG, Kim SH, Park JY. Intrathecal gabapentin increases interleukin-10 expression and inhibits pro-inflammatory cytokine in a rat model of neuropathic pain. J Korean Med Sci. 2013 Feb;28(2):308-14. Epub 2013 Jan 29. PMCID: PMC3565145. [CrossRef] [PubMed]
- Dias JM, de Brito TV, de Aguiar Magalhães D, da Silva Santos PW, Batista JA, do Nascimento Dias EG, de Barros Fernandes H, Damasceno SR, Silva RO, Aragão KS, Souza MH, Medeiros JV, Barbosa AL. Gabapentin, a synthetic analog of gamma-aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation. 2014 Oct;37(5):1826-36. [CrossRef] [PubMed]
- Li Z, Wang S, Qin Y, Yang B, Wang C, Lu T, Xu J, Zhu L, Yuan C, Han W. Gabapentin attenuates cardiac remodeling after myocardial infarction by inhibiting M1 macrophage polarization through the peroxisome proliferator-activated receptor-γ pathway. Eur J Pharmacol. 2024 Mar 15;967:176398. Epub 2024 Feb 11. [CrossRef] [PubMed]
- Zuchora B, Wielosz M, Urbańska EM. Adenosine A1 receptors and the anticonvulsant potential of drugs effective in the model of 3-nitropropionic acid-induced seizures in mice. Eur Neuropsychopharmacol. 2005 Jan;15(1):85-93. [CrossRef] [PubMed]
- Park JY, Jun IG. The interaction of gabapentin and N6-(2-phenylisopropyl)-adenosine R-(-)isomer (R-PIA) on mechanical allodynia in rats with a spinal nerve ligation. J Korean Med Sci. 2008 Aug;23(4):678-84. PMCID: PMC2526387. [CrossRef] [PubMed]
- Martins DF, Prado MR, Daruge-Neto E, Batisti AP, Emer AA, Mazzardo-Martins L, Santos AR, Piovezan AP. Caffeine prevents antihyperalgesic effect of gabapentin in an animal model of CRPS-I: evidence for the involvement of spinal adenosine A1 receptor. J Peripher Nerv Syst. 2015 Dec;20(4):403-9. [CrossRef] [PubMed]
- Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth. 2018 Jun;120(6):1315-1334. Epub 2018 Apr 12. [CrossRef] [PubMed]
- Sarıtaş TB, Korkmaz M, Sevimli A, Sarıtaş ZK. Comparison of the effects of gabapentin and pregabalin on wound healing in rats. Int Wound J. 2016 Oct;13(5):748-53. Epub 2014 Oct 28. PMCID: PMC7949902. [CrossRef] [PubMed]
- Asfour HZ, Alhakamy NA, Ahmed OAA, Fahmy UA, Md S, El-Moselhy MA, Rizg WY, Alghaith AF, Eid BG, Abdel-Naim AB. Enhanced healing efficacy of an optimized gabapentin-melittin nanoconjugate gel-loaded formulation in excised wounds of diabetic rats. Drug Deliv. 2022 Dec;29(1):1892-1902. PMCID: PMC9246110. [CrossRef] [PubMed]
- Costales B, Goodin AJ. Outpatient Off-Label Gabapentin Use for Psychiatric Indications Among U.S. Adults, 2011-2016. Psychiatr Serv. 2021 Nov 1;72(11):1246-1253. Epub 2021 May 21. [CrossRef] [PubMed]
- Berlin RK, Butler PM, Perloff MD. Gabapentin Therapy in Psychiatric Disorders: A Systematic Review. Prim Care Companion CNS Disord. 2015 Oct 22;17(5):10.4088/PCC.15r01821. PMCID: PMC4732322. [CrossRef] [PubMed]
- Martin JC, Gainer D. Psychiatric Uses of Gabapentin. Innov Clin Neurosci. 2022 Jul-Sep;19(7-9):55-60. PMCID: PMC9507147. [PubMed]
- Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, Iosifescu DV. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front Psychiatry. 2020 Dec 23;11:595584. PMCID: PMC7786299. [CrossRef] [PubMed]
- Gaba A, Shah K, Olson C, Munjal S. Treatment of Acute Intermittent Porphyria-Associated Anxiety Disorder With Gabapentin: Case Report and Review of Literature. J Clin Psychopharmacol. 2024 Jan-Feb 01;44(1):58-60. [CrossRef] [PubMed]
- Felsted JA, Meng A, Ameroso D, Rios M. Sex-specific Effects of α2δ-1 in the Ventromedial Hypothalamus of Female Mice Controlling Glucose and Lipid Balance. Endocrinology. 2020 Jul 1;161(7):bqaa068. PMCID: PMC7286619. [CrossRef] [PubMed]
- İspir GZ, Danışman M, Katar KS. A Hidden Pandemic? Abuse of Gabapentinoids: A Brief Review of Recent Studies. Curr Drug Res Rev. 2023 Nov 28. Epub ahead of print. [CrossRef] [PubMed]
- Modesto-Lowe V, Barron GC, Aronow B, Chaplin M. Gabapentin for alcohol use disorder: A good option, or cause for concern? Cleve Clin J Med. 2019 Dec;86(12):815-823. [CrossRef] [PubMed]
- Buttram ME, Kurtz SP, Ellis MS, Cicero TJ. Gabapentin prescribed during substance abuse treatment: The perspective of treatment providers. J Subst Abuse Treat. 2019 Oct;105:1-4. Epub 2019 Jul 21. PMCID: PMC6709710. [CrossRef] [PubMed]
- Ng QX, Han MX, Teoh SE, Yaow CYL, Lim YL, Chee KT. A Systematic Review of the Clinical Use of Gabapentin and Pregabalin in Bipolar Disorder. Pharmaceuticals (Basel). 2021 Aug 24;14(9):834. PMCID: PMC8469561. [CrossRef] [PubMed]
- Hong JSW, Atkinson LZ, Al-Juffali N, Awad A, Geddes JR, Tunbridge EM, Harrison PJ, Cipriani A. Gabapentin and pregabalin in bipolar disorder, anxiety states, and insomnia: Systematic review, meta-analysis, and rationale. Mol Psychiatry. 2022 Mar;27(3):1339-1349. Epub 2021 Nov 24. PMCID: PMC9095464. [CrossRef] [PubMed]
- Goodman CW, Brett AS. A Clinical Overview of Off-label Use of Gabapentinoid Drugs. JAMA Intern Med. 2019 May 1;179(5):695-701. [CrossRef] [PubMed]
- Pellitteri G, Versace S, Merlino G, Nilo A, Gigli GL, Valente M. A comprehensive update on the ADMET considerations for α2δ calcium channel ligand medications for treating restless legs syndrome. Expert Opin Drug Metab Toxicol. 2024 Mar;20(3):133-142. Epub 2024 Mar 15. [CrossRef] [PubMed]
- Shen Y, Li W, Ma M, Jiang W. Efficacy and Safety of Gabapentin in Improving Sleep Quality of Patients with Sensory Nervous System Diseases: A Meta-Analysis. Altern Ther Health Med. 2023 Jul;29(5):380-385. [PubMed]
- Saul H, Gursul D, Cassidy S, Harrison P. Little evidence supports gabapentinoid use in bipolar disorder or insomnia. BMJ. 2022 Nov 18;379:o2576. [CrossRef] [PubMed]
- Ruan QZ, Robinson CL, Simopoulos TT, Burns JC, Madabhushi SV, Gill JS. Comparative Descriptive Analysis of Physician Versus Patient-Directed Gabapentin Usage In Chronic Pain - A Preliminary Report. Pain Physician. 2023 Oct;26(6):E687-E693. [PubMed]
- Bragg S, Marrison ST, Haley S. Diabetic Peripheral Neuropathy: Prevention and Treatment. Am Fam Physician. 2024 Mar;109(3):226-232. [PubMed]
- Albrecht PJ, Liu Y, Houk G, Ruggiero B, Banov D, Dockum M, Day AJ, Rice FL, Bassani G. Cutaneous targets for topical pain medications in patients with neuropathic pain: individual differential expression of biomarkers supports the need for personalized medicine. Pain Rep. 2024 Feb 16;9(2):e1119. PMCID: PMC10876238. [CrossRef] [PubMed]
- De Stefano G, Di Pietro G, Truini A, Cruccu G, Di Stefano G. Considerations When Using Gabapentinoids to Treat Trigeminal Neuralgia: A Review. Neuropsychiatr Dis Treat. 2023 Sep 19;19:2007-2012. PMCID: PMC10517700. [CrossRef] [PubMed]
- Patel P, Rajput HS, Chavda K, Mistry S, Bhagat S, Hadia R, Saiyed M, Khadela A. Assessing the effectiveness of gabapentin in paclitaxel-induced arthralgia, myalgia, and neuropathic pain: An observational, cohort study. J Oncol Pharm Pract. 2024 Jan 5:10781552231225148. Epub ahead of print. [CrossRef] [PubMed]
- Zerfas I, McGinn R, Smith MA. Pharmacologic Management of Cancer-Related Pain in Pregnant Patients. Drugs. 2023 Aug;83(12):1067-1076. Epub 2023 Jun 22. [CrossRef] [PubMed]
- Drake R, Prael G, Phyo Y, Chang S, Hunt J, Herbert A, Mott C, Hynson J, Phillips M, Cossich M, Mherekumombe M, Kim MS, Chong PH, Abitz M, Bernada M, Avery M, Doogue M, Rowett D, Currow D. Gabapentin for Pain in Pediatric Palliative Care. J Pain Symptom Manage. 2024 Mar;67(3):212-222.e1. Epub 2023 Nov 29. [CrossRef] [PubMed]
- Fleser L, Tibbetts E, Hanson A, Chu EC, Gura K, Tom C, Williams K, Levy P. Evaluating Gabapentin Dosing, Efficacy and Safety in Infants. J Pediatr Pharmacol Ther. 2024 Apr;29(2):159-168. Epub 2024 Apr 8. PMCID: PMC11001217. [CrossRef] [PubMed]
- Anfuso CD, Olivieri M, Fidilio A, Lupo G, Rusciano D, Pezzino S, Gagliano C, Drago F, Bucolo C. Gabapentin Attenuates Ocular Inflammation: In vitro and In vivo Studies. Front Pharmacol. 2017 Apr 4;8:173. PMCID: PMC5378778. [CrossRef] [PubMed]
- Yoon HJ, Kim J, Yoon KC. Treatment Response to Gabapentin in Neuropathic Ocular Pain Associated with Dry Eye. J Clin Med. 2020 Nov 22;9(11):3765. PMCID: PMC7700262. [CrossRef] [PubMed]
- Ongun N, Ongun GT. Is gabapentin effective in dry eye disease and neuropathic ocular pain? Acta Neurol Belg. 2021 Apr;121(2):397-401. Epub 2019 May 27. [CrossRef] [PubMed]
- Nissman SA, Tractenberg RE, Babbar-Goel A, Pasternak JF. Oral gabapentin for the treatment of postoperative pain after photorefractive keratectomy. Am J Ophthalmol. 2008 Apr;145(4):623-629. Epub 2008 Jan 28. [CrossRef] [PubMed]
- Lichtinger A, Purcell TL, Schanzlin DJ, Chayet AS. Gabapentin for postoperative pain after photorefractive keratectomy: a prospective, randomized, double-blind, placebo-controlled trial. J Refract Surg. 2011 Aug;27(8):613-7. Epub 2011 Feb 28. [CrossRef] [PubMed]
- Pakravan M, Roshani M, Yazdani S, Faramazi A, Yaseri M. Pregabalin and gabapentin for post-photorefractive keratectomy pain: a randomized controlled trial. Eur J Ophthalmol. 2012;22 Suppl 7:S106-13. [CrossRef] [PubMed]
- Serna-Ojeda JC, Santana-Cruz O, Quiroz-Casian N, González-Mendoza E, Mercado-Orozco JL, Navas A, Lichtinger A, Graue-Hernandez EO. Pain Management in Corneal Collagen Crosslinking for Keratoconus: A Comparative Case Series. J Ocul Pharmacol Ther. 2019 Jul/Aug;35(6):325-330. Epub 2019 Jun 19. [CrossRef] [PubMed]
- Kavalieratos CS, Dimou T. Gabapentin therapy for painful, blind glaucomatous eye: case report. Pain Med. 2008 Apr;9(3):377-8. [CrossRef] [PubMed]
- Michael R, Jeffers JV, Messenger W, Aref AA. Gabapentin for presumed neuropathic ocular pain. Am J Ophthalmol Case Rep. 2020 Jul 22;19:100836. PMCID: PMC7390772. [CrossRef] [PubMed]
- Jehle T, Lagrèze WA, Blauth E, Knörle R, Schnierle P, Lücking CH, Feuerstein TJ. Gabapentin-lactam (8-aza-spiro [5,4]decan-9-on; GBP-L) inhibits oxygen glucose deprivation-induced [3H]glutmate release and is a neuroprotective agent in amodel of acute retinal ischemia. Naunyn Schmiedebergs Arch Pharmacol. 2000 Jul;362(1):74-81. [CrossRef] [PubMed]
- Lagrèze WA, Müller-Velten R, Feuerstein TJ. The neuroprotective properties of gabapentin-lactam. Graefes Arch Clin Exp Ophthalmol. 2001 Nov;239(11):845-9. [CrossRef] [PubMed]
- Pielen A, Kirsch M, Hofmann HD, Feuerstein TJ, Lagrèze WA. Retinal ganglion cell survival is enhanced by gabapentin-lactam in vitro: evidence for involvement of mitochondrial KATP channels. Graefes Arch Clin Exp Ophthalmol. 2004 Mar;242(3):240-4. Epub 2004 Feb 10. [CrossRef] [PubMed]
- Cammalleri M, Amato R, Olivieri M, Pezzino S, Bagnoli P, Dal Monte M, Rusciano D. Effects of Topical Gabapentin on Ocular Pain and Tear Secretion. Front Pharmacol. 2021 Jun 7;12:671238. PMCID: PMC8216672. [CrossRef] [PubMed]
- Cristaldi M, Olivieri M, Spampinato G, Anfuso CD, Scalia M, Lupo G, Rusciano D. Isolation and Characterization of a New Human Corneal Epithelial Cell Line: HCE-F. Cornea. 2020 Nov;39(11):1419-1425. [CrossRef] [PubMed]
- Yang CJ, Anand A, Huang CC, Lai JY. Unveiling the Power of Gabapentin-Loaded Nanoceria with Multiple Therapeutic Capabilities for the Treatment of Dry Eye Disease. ACS Nano. 2023 Dec 26;17(24):25118-25135Epub 2023 Dec 5. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




