Submitted:
30 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon (AC) from Okara Powder Wastes
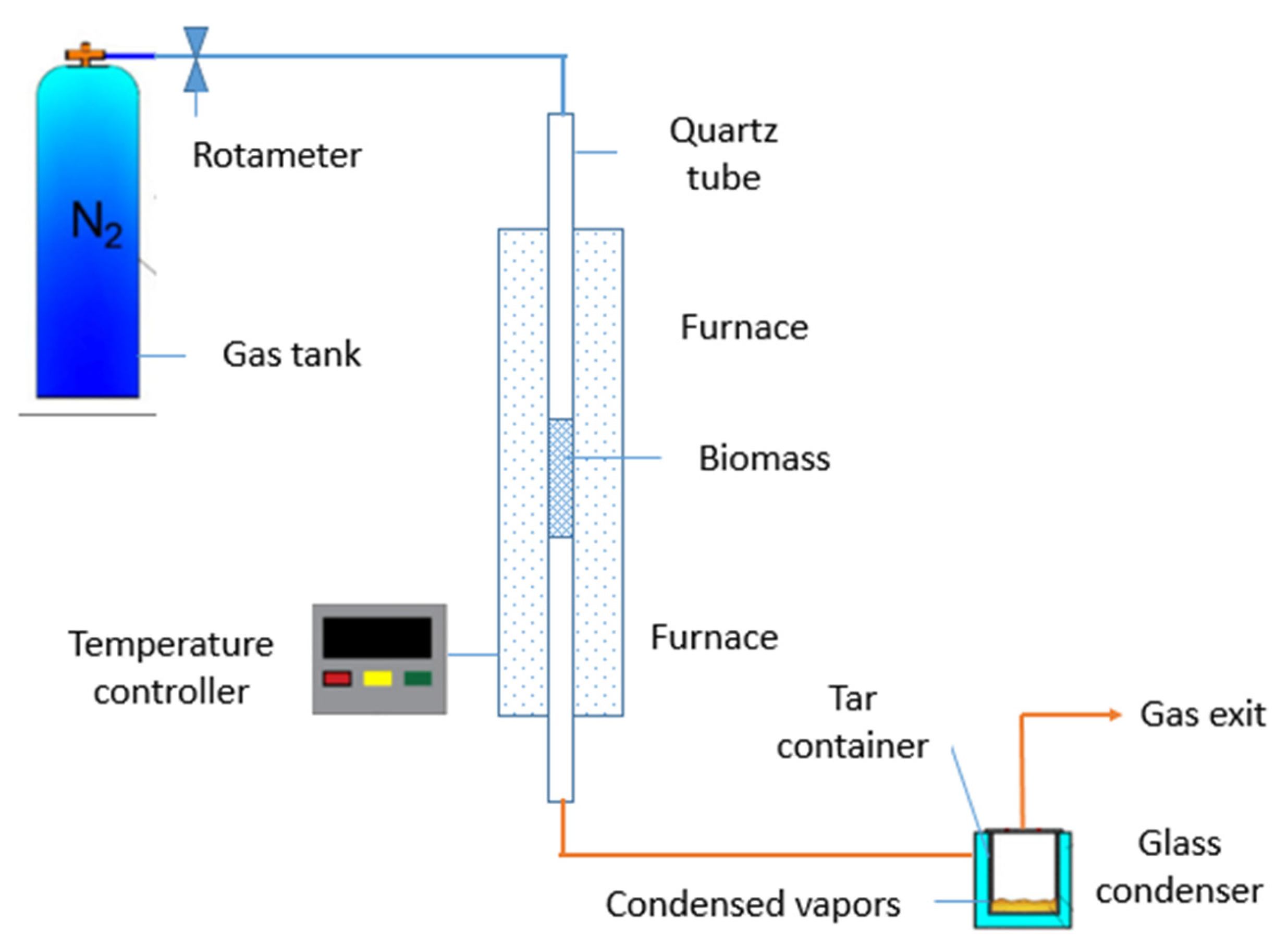
2.1.1. Pyrolysis Experiment Setup
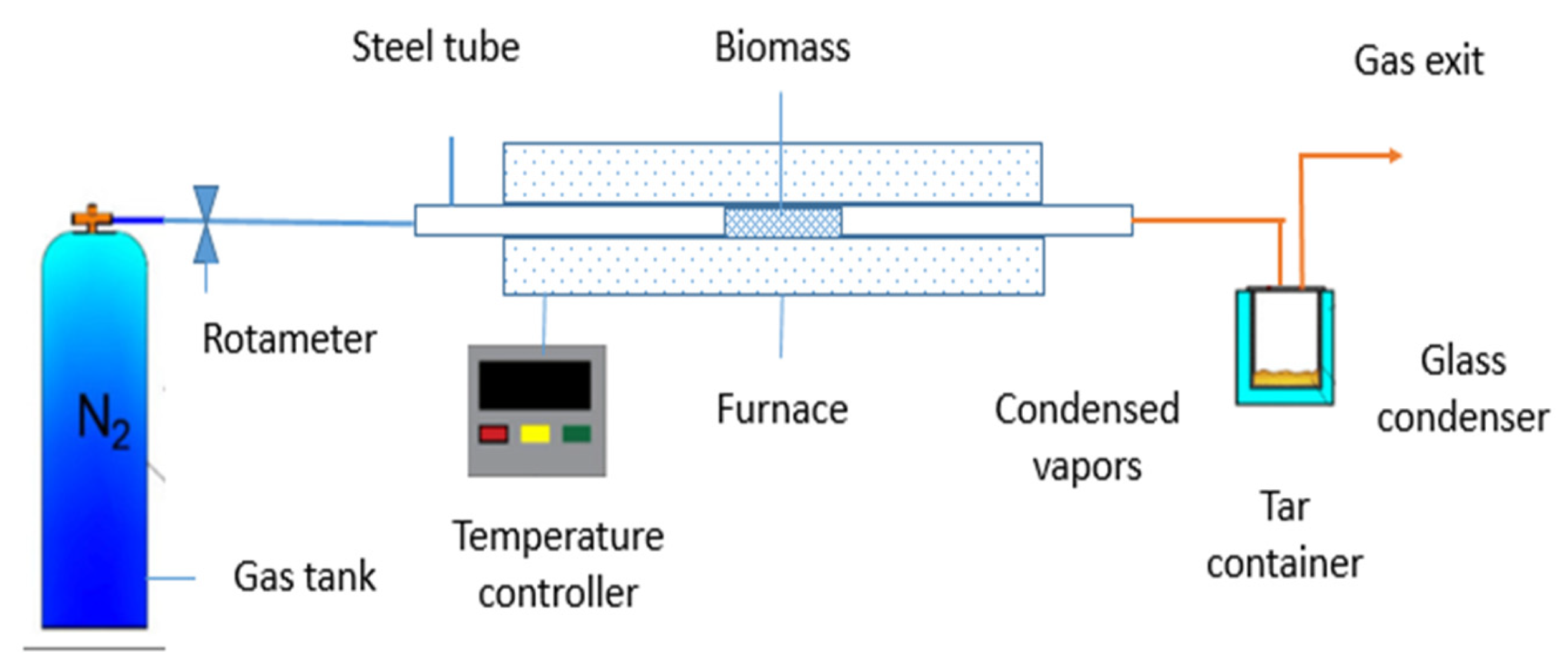
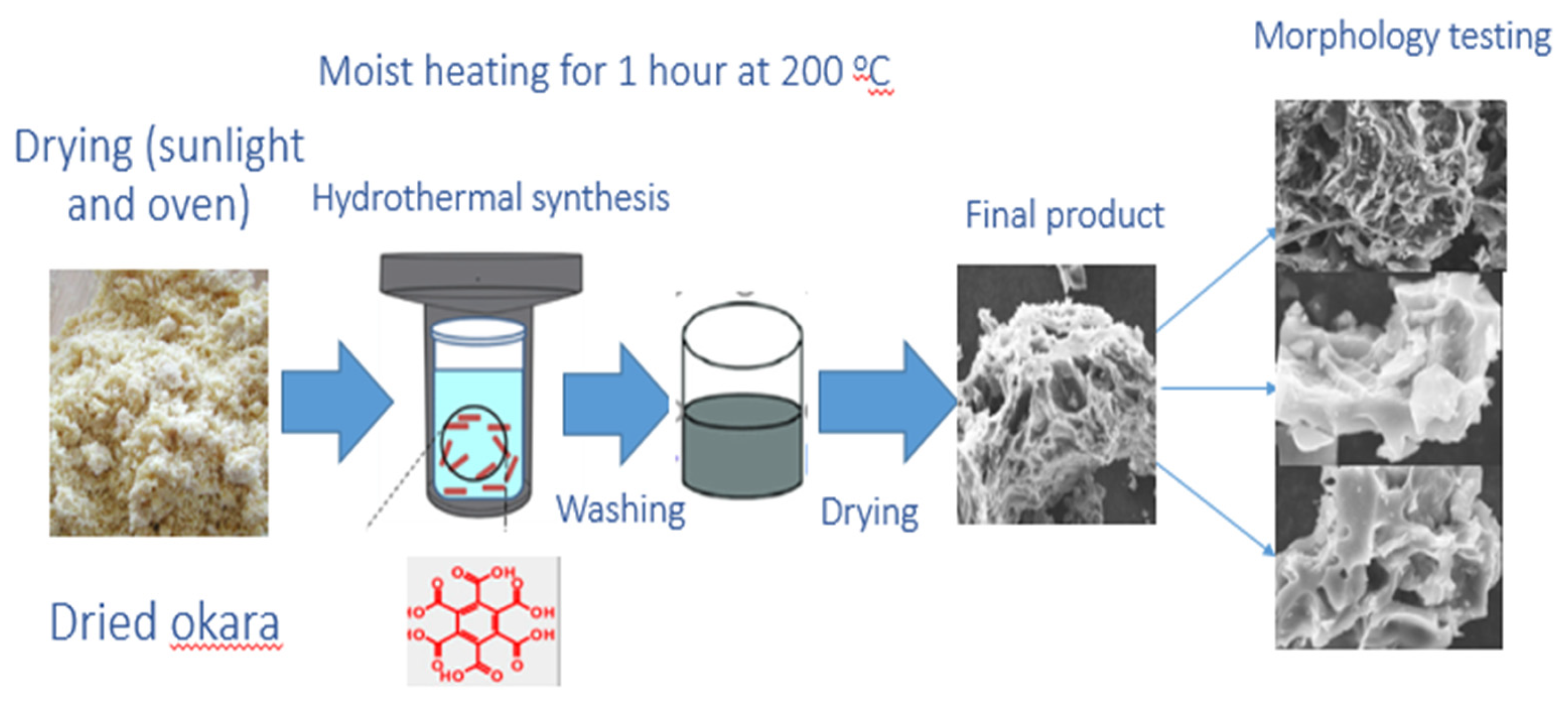
2.1.2. Preparation of Activated Carbon via Hydrothermal Carbonization Method
2.1.3. Activate Biochar/Hydrochar
2.2. Characterization of the Materials
2.3. Calculation of the biomass conversion
3. Results and Discussions
3.1. Characterizations of the Biomass Materials
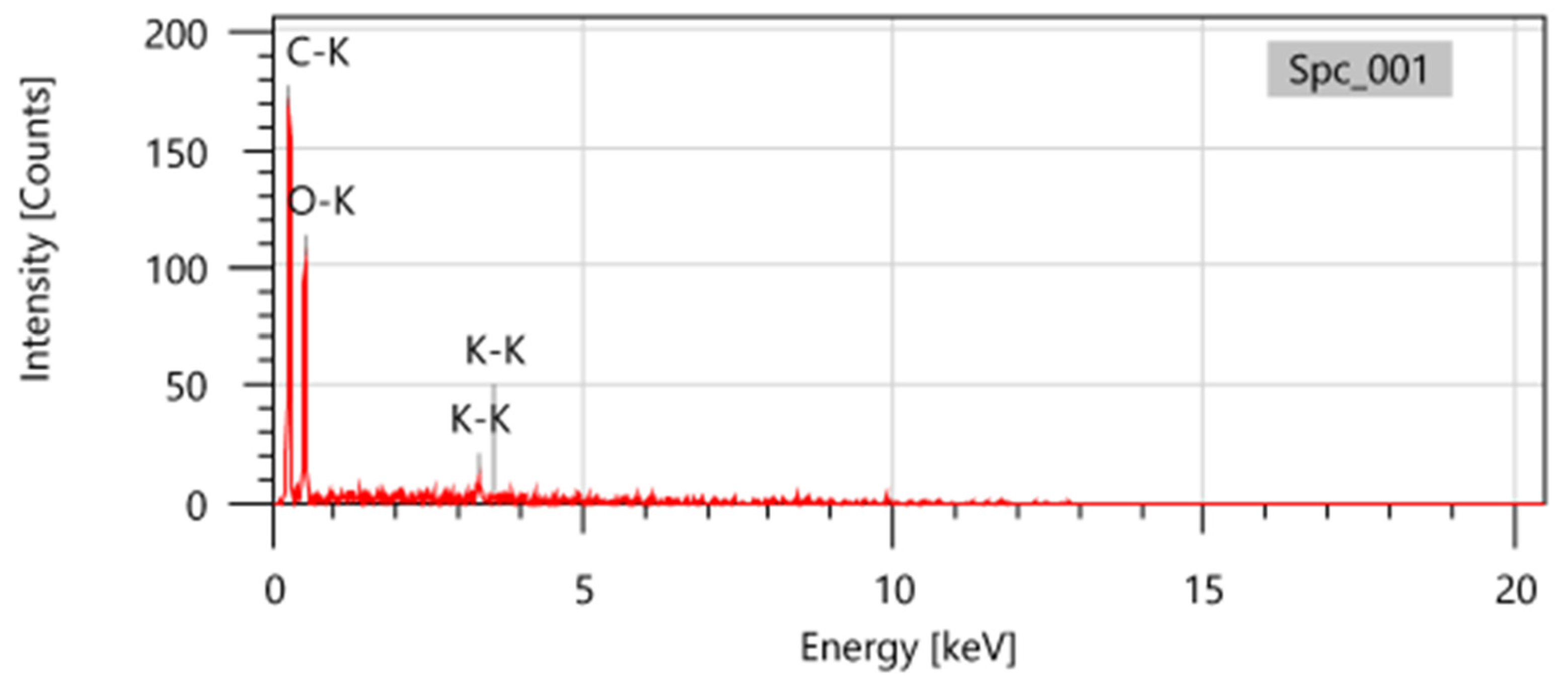
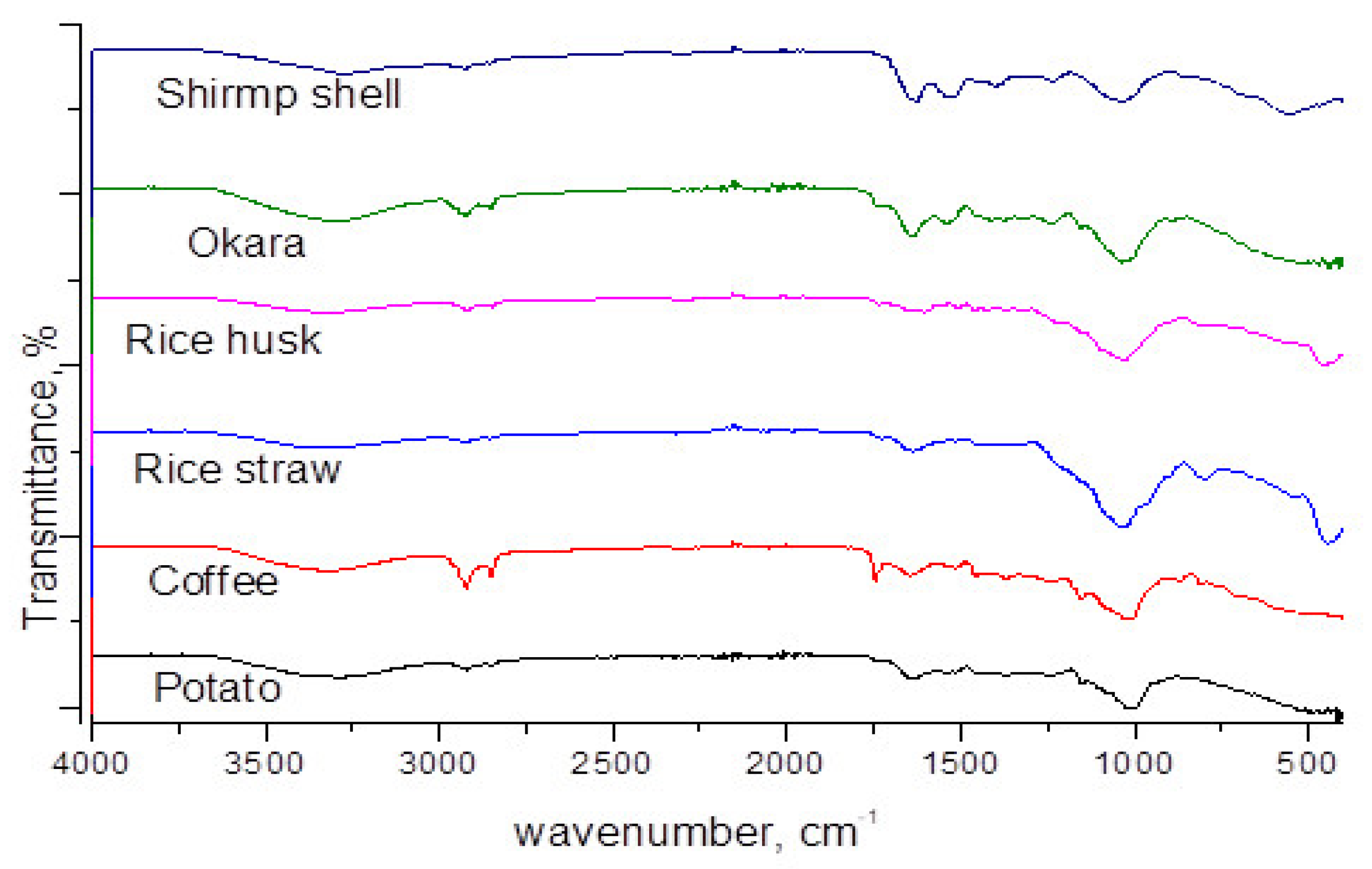
3.1.1. EDX Analysis of Various Biomass
3.1.2. SEM Images of Different Biomass
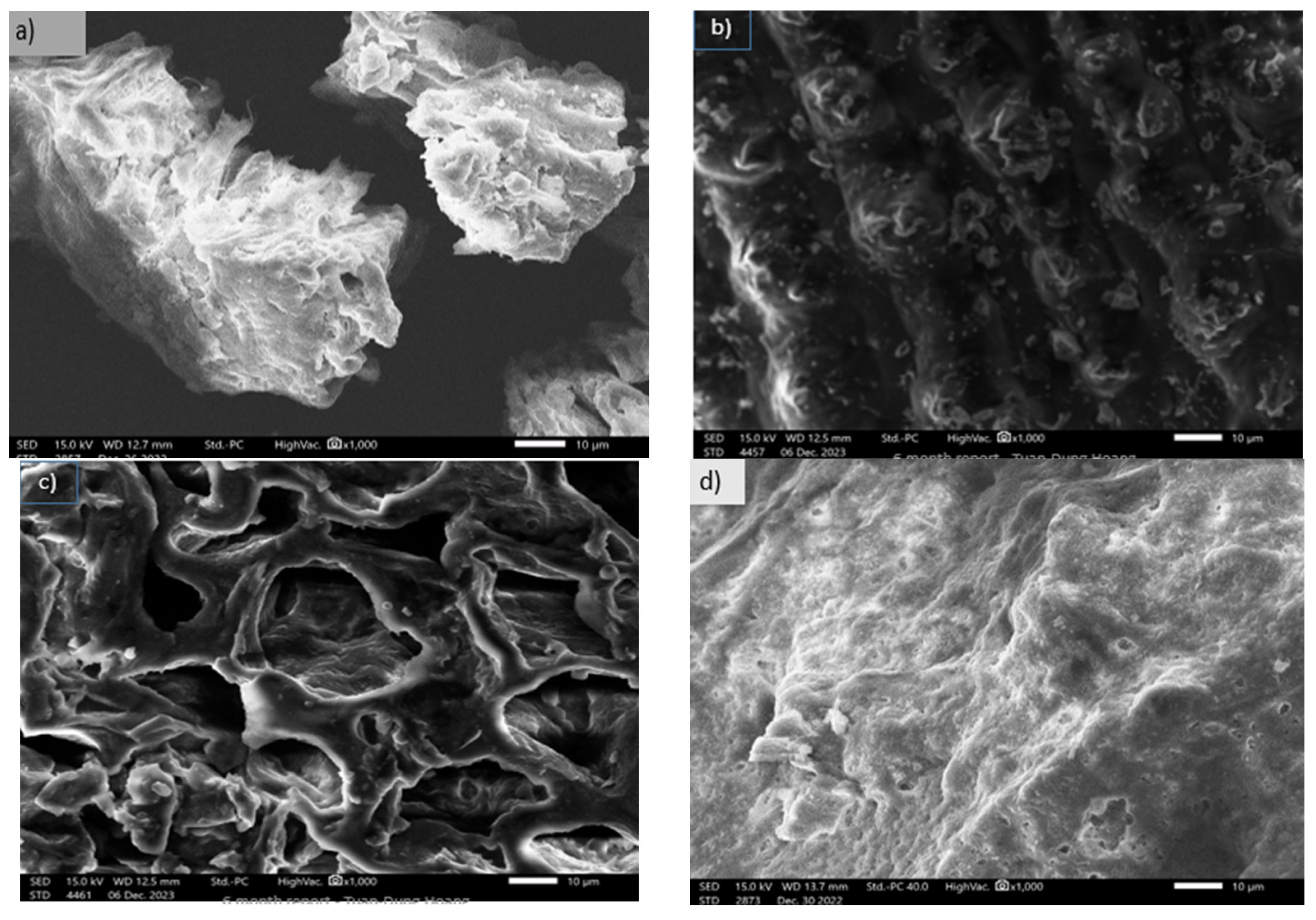
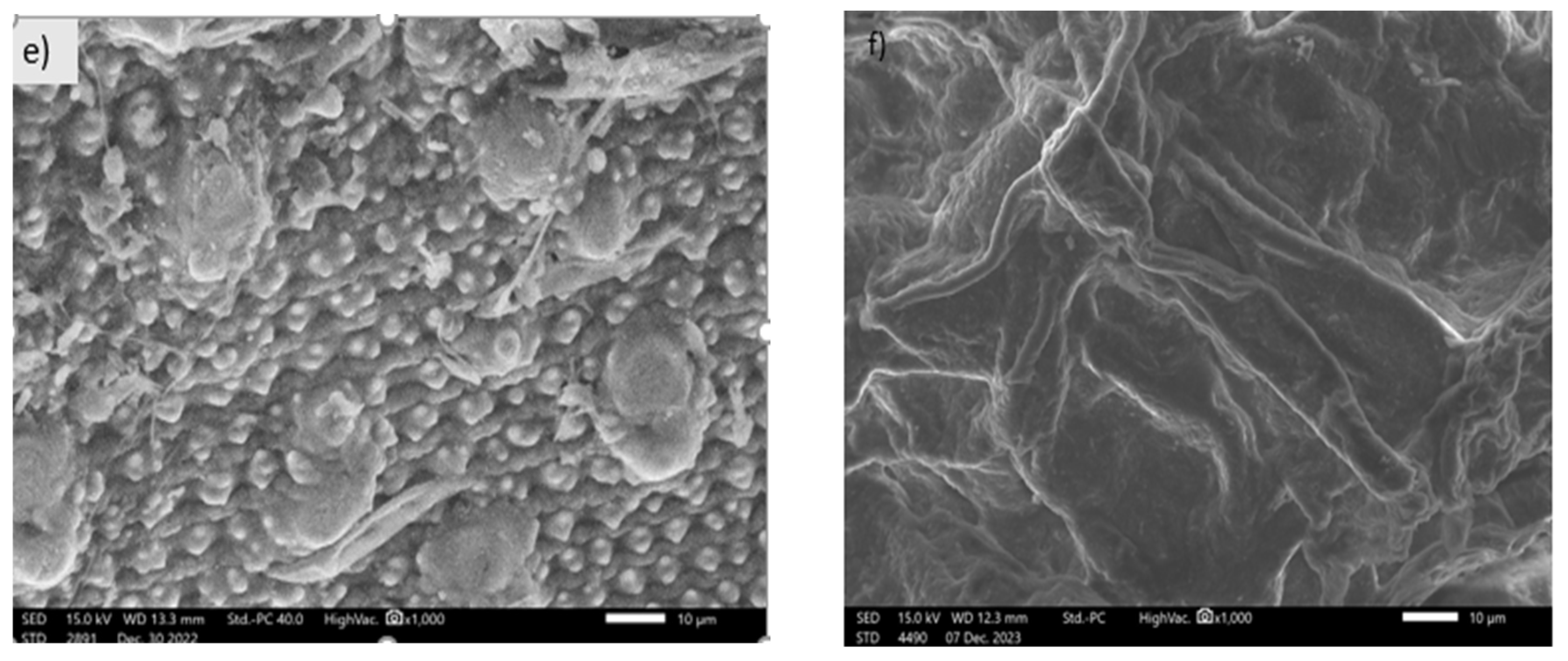
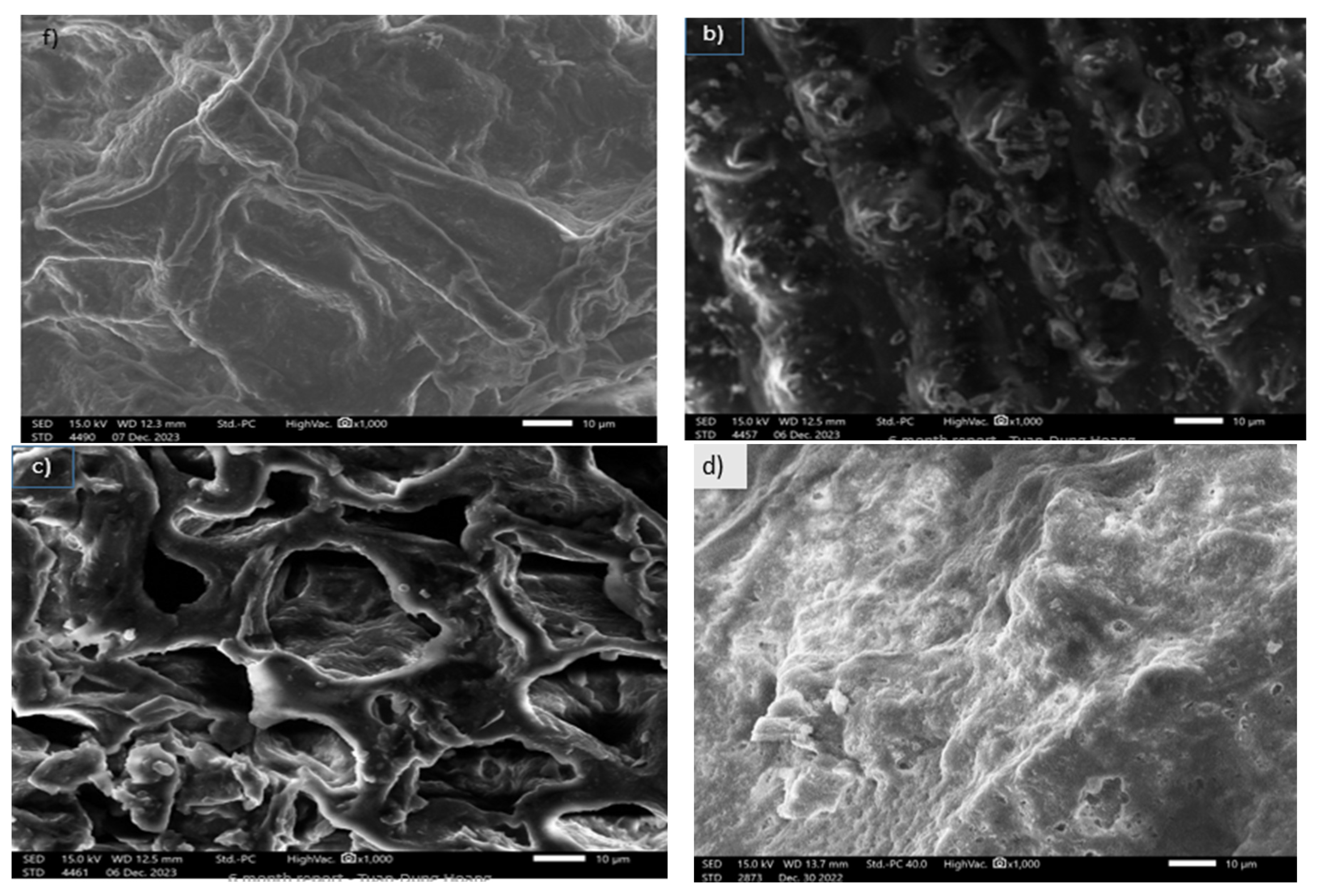
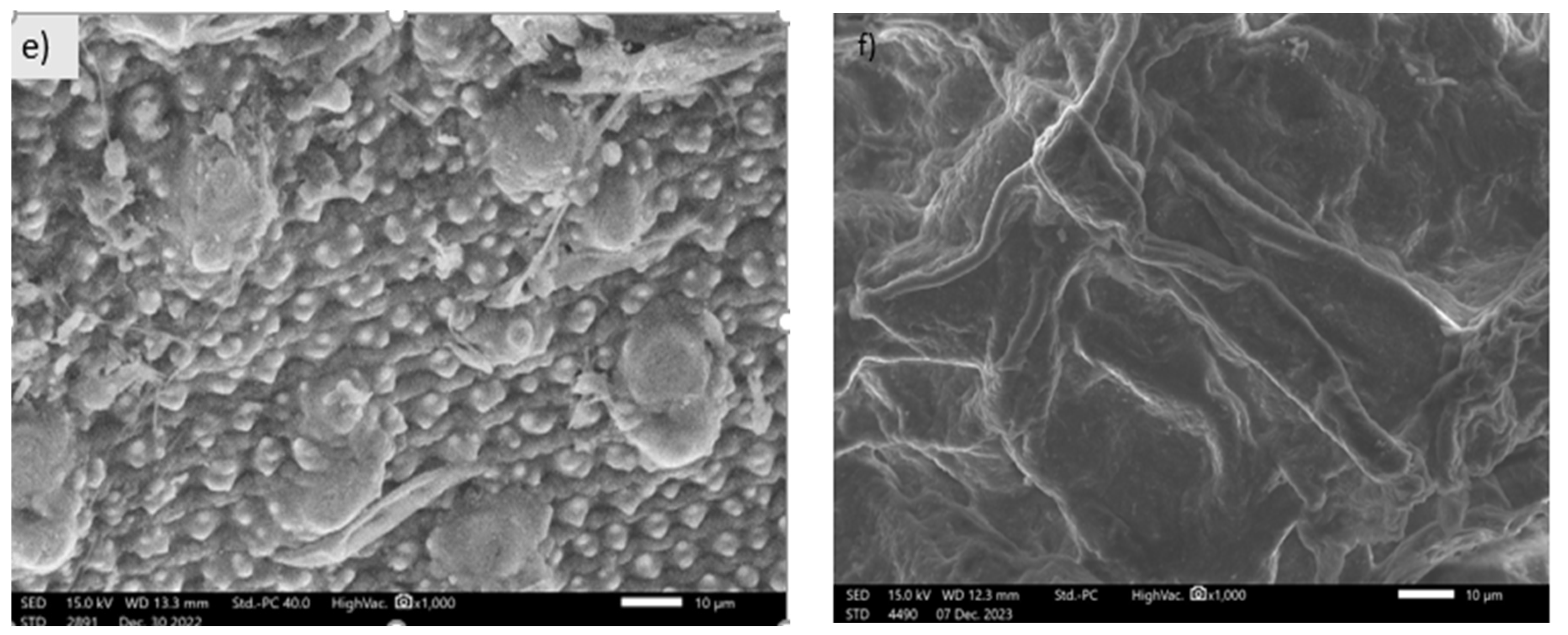
3.1.3. SEM Images of Activated Carbon Synthesized from Okara Powder Waste
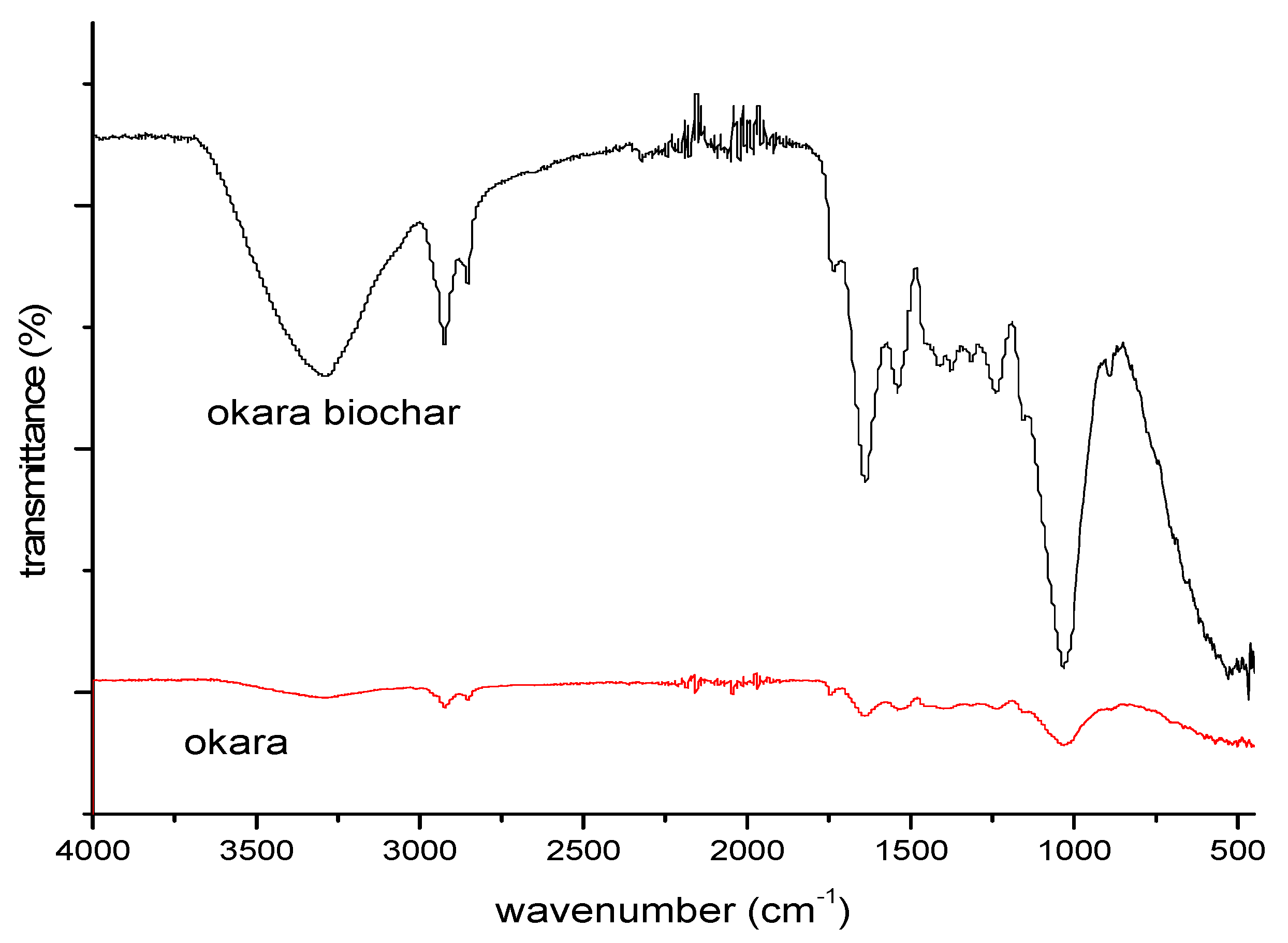
3.1.4. FTIR Analysis
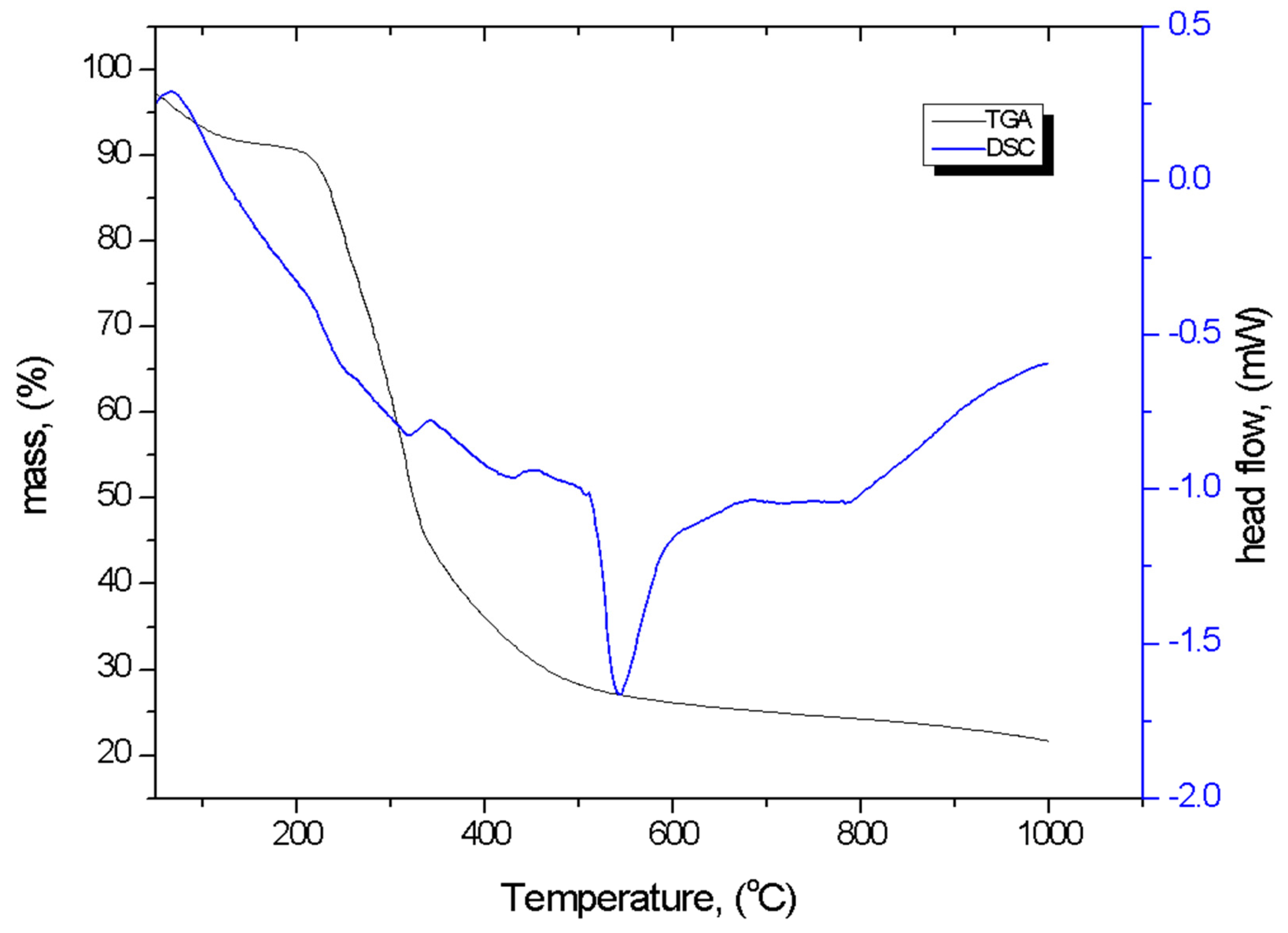
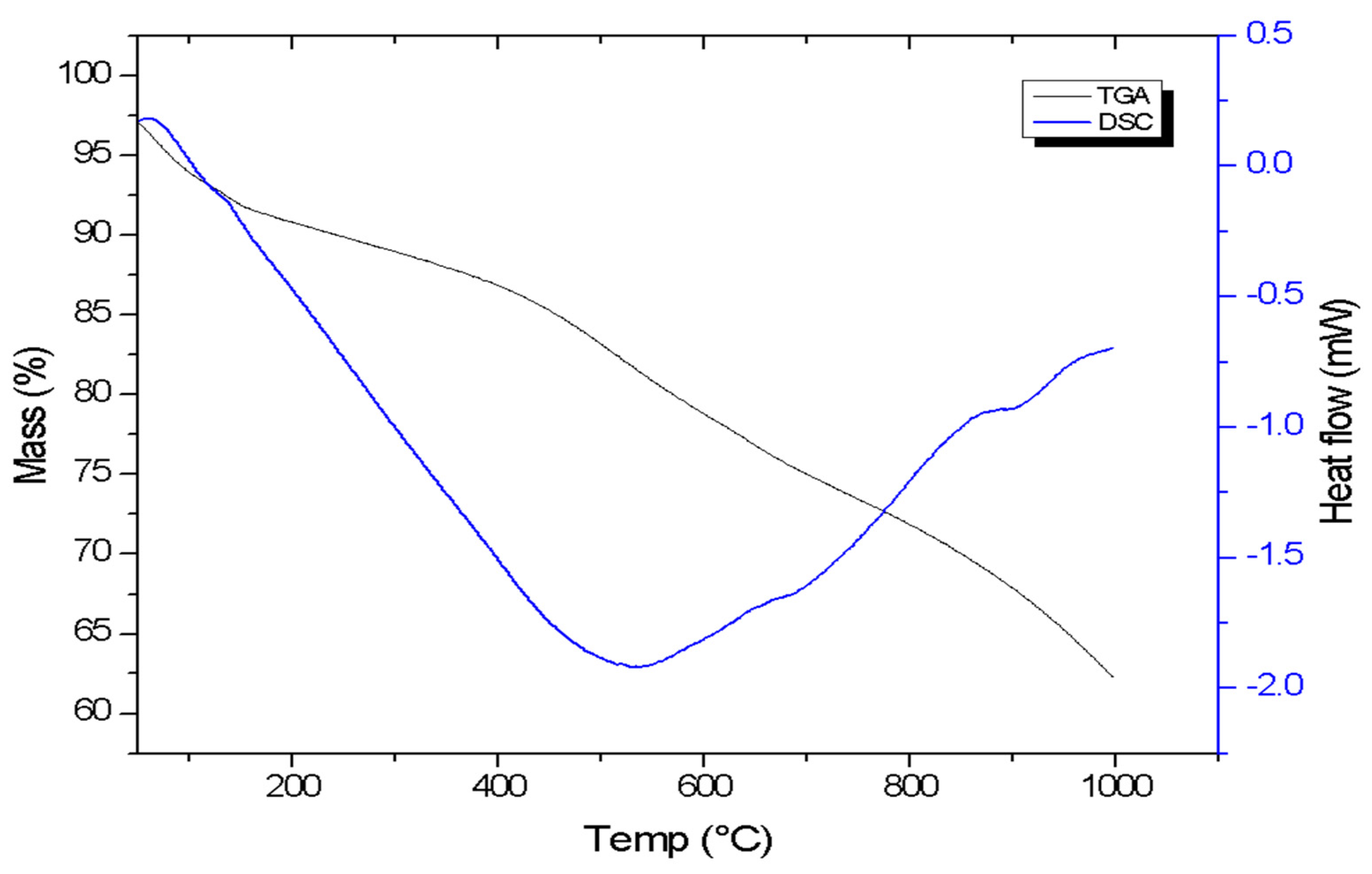
3.1.5. TG-DSC Analysis
3.2. Pyrolysis Products and Their Properties
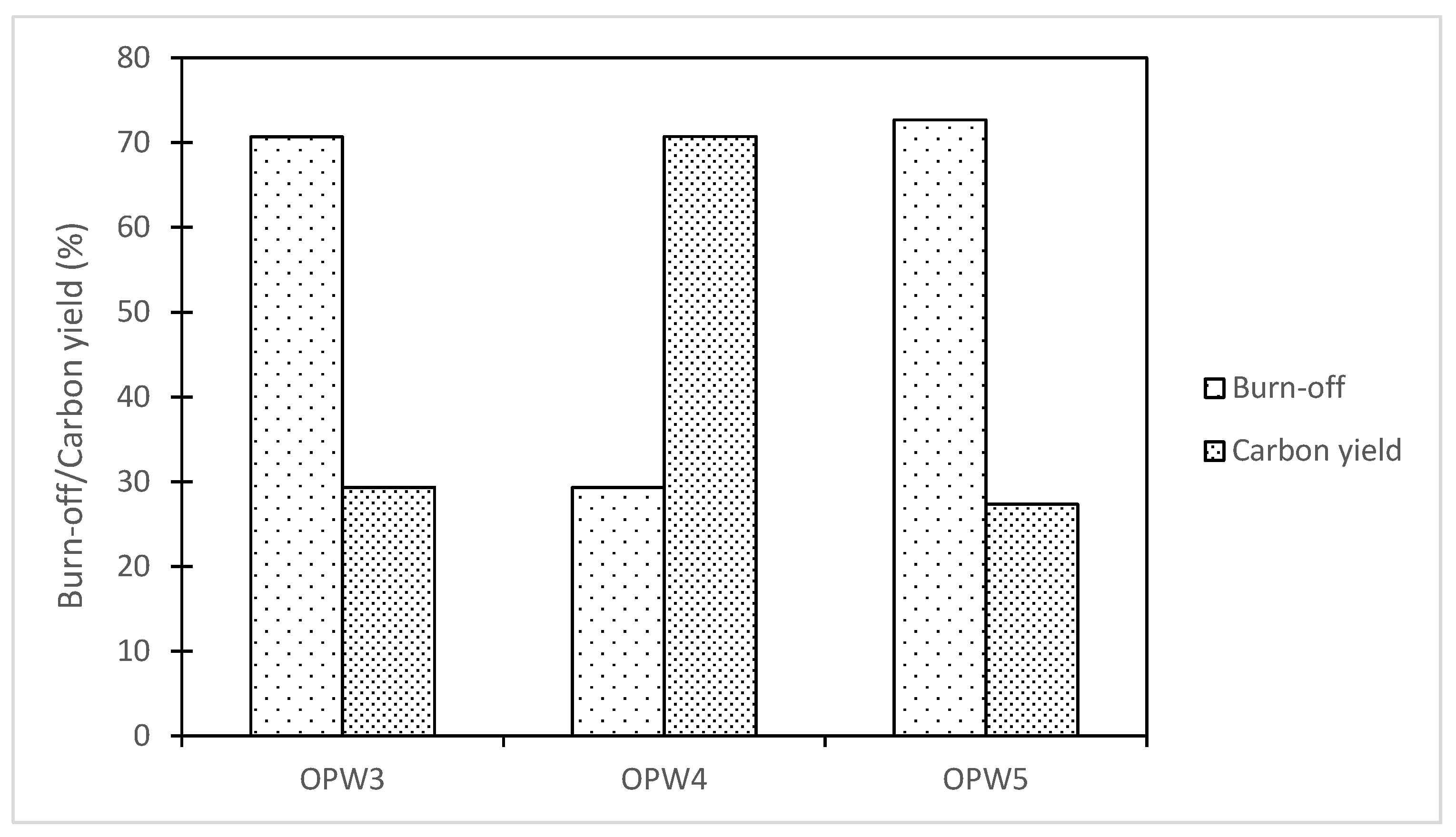
- Biochar and hydrochar yield and burn-off
- Surface areas (S/BET) and pores:
4. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P., et al., Update on CO2 emissions. 2010. 3(12): p. 811-812. [CrossRef]
- Hoang, T.-D., et al., Carbon-Based Synthesized Materials for CO2 Adsorption and Conversion: Its Potential for Carbon Recycling. 2023. 8(4): p. 53. [CrossRef]
- Yoro, K.O. and M.O. Daramola, CO2 emission sources, greenhouse gases, and the global warming effect, in Advances in carbon capture. 2020, Elsevier. p. 3-28.
- Olivier, J.G., K. Schure, and J.J.P.N.E.A.A. Peters, Trends in global CO2 and total greenhouse gas emissions. 2017. 5: p. 1-11.
- Gao, J., S.C.S. Shiong, and Y.J.C.E.J. Liu, Reduction of CO2 to chemicals and Fuels: Thermocatalysis versus electrocatalysis. 2023: p. 145033. [CrossRef]
- Liu, J., et al., Biomass pyrolysis mechanism for carbon-based high-value products. Proceedings of the Combustion Institute, 2023. 39(3): p. 3157-3181. [CrossRef]
- Dziejarski, B., et al., CO2 capture materials: a review of current trends and future challenges. 2023: p. 100483. [CrossRef]
- Furuvik, N.C., et al., Experimental study and SEM-EDS analysis of agglomerates from gasification of biomass in fluidized beds. 2022. 252: p. 124034. [CrossRef]
- Hoang, T.-D. and N.J.F. Nghiem, Recent developments and current status of commercial production of fuel ethanol. 2021. 7(4): p. 314. [CrossRef]
- Sezali, N.A.A., et al., Bio-based nanomaterials for energy application: A review. 2024. [CrossRef]
- Fan, H., et al., Structural Characteristics of Insoluble Dietary Fiber from Okara with Different Particle Sizes and Their Prebiotic Effects in Rats Fed High-Fat Diet. 2022. 11(9): p. 1298. [CrossRef]
- Liu, K., Food use of whole soybeans, in Soybeans. 2008, Elsevier. p. 441-481.
- Xiao, C., Functional soy products, in Functional foods. 2011, Elsevier. p. 534-556.
- Rahman, M.M., et al., A review of okara (soybean curd residue) utilization as animal feed: Nutritive value and animal performance aspects. 2021. 92(1): p. e13594. [CrossRef]
- Li, B., M. Qiao, and F.J.F.R.I. Lu, Composition, nutrition, and utilization of okara (soybean residue). 2012. 28(3): p. 231-252. [CrossRef]
- Abi Bianasari, A., et al., Influence of combined catalysts on the catalytic pyrolysis process of biomass: A systematic literature review. 2024. 309: p. 118437.
- Tran, T.-K., et al., Applications of engineered biochar in remediation of heavy metal (loid) s pollution from wastewater: Current perspectives toward sustainable development goals. 2024: p. 171859. [CrossRef]
- MINH, T.Q., et al., KHẢO SÁT KHẢ NĂNG HẤP PHỤ CIPROFLOXACIN TRONG NƯỚC BẰNG THAN SINH HỌC CÓ NGUỒN GỐC TỪ RONG BIỂN ĐƯỢC ĐIỀU CHẾ THÔNG QUA QUÁ TRÌNH CÁC-BON HÓA THỦY NHIỆT VÀ HOẠT HÓA ZnCl.
- Amalina, F., et al., Biochar production techniques utilizing biomass waste-derived materials and environmental applications–A review. 2022. 7: p. 100134. [CrossRef]
- Toscano Miranda, N., et al., Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renewable and Sustainable Energy Reviews, 2021. 149: p. 111394. [CrossRef]
- Kan, T., V. Strezov, and T.J. Evans, Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renewable and Sustainable Energy Reviews, 2016. 57: p. 1126-1140. [CrossRef]
- Vuppaladadiyam, A.K., et al., A critical review on biomass pyrolysis: Reaction mechanisms, process modeling and potential challenges. Journal of the Energy Institute, 2023. 108: p. 101236. [CrossRef]
- Lee, S.Y., et al., Waste to bioenergy: a review on the recent conversion technologies. BMC Energy, 2019. 1(1): p. 4. [CrossRef]
- Ghodake, G.S., et al., Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. Journal of Cleaner Production, 2021. 297: p. 126645. [CrossRef]
- Sharma, R., et al., A Comprehensive Review on Hydrothermal Carbonization of Biomass and its Applications. Chemistry Africa, 2020. 3(1): p. 1-19. [CrossRef]
- Bach, Q.-V. and Ø. Skreiberg, Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renewable and Sustainable Energy Reviews, 2016. 54: p. 665-677. [CrossRef]
- Yoganandham, S.T., G. Sathyamoorthy, and R.R. Renuka, Emerging extraction techniques: hydrothermal processing, in Sustainable seaweed technologies. 2020, Elsevier. p. 191-205.
- Marzbali, M.H., et al., Wet organic waste treatment via hydrothermal processing: A critical review. Chemosphere, 2021. 279: p. 130557. [CrossRef]
- Hossain, N., et al., Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Scientific Reports, 2020. 10(1): p. 18851. [CrossRef]
- Ullah, H., et al., Physicochemical characteristics and thermal degradation behavior of dry and wet torrefied orange peel obtained by dry/wet torrefaction. Biomass Conversion and Biorefinery, 2023. 13(9): p. 7993-8009. [CrossRef]
- Rodríguez Correa, C., et al., Investigation of the textural and adsorption properties of activated carbon from HTC and pyrolysis carbonizates. Biomass Conversion and Biorefinery, 2018. 8(2): p. 317-328. [CrossRef]
- Mumme, J., et al., Hydrothermal carbonization of digestate in the presence of zeolite: process efficiency and composite properties. 2015. 3(11): p. 2967-2974. [CrossRef]
- Hoang, T.-D., et al., Artificial intelligence in pollution control and management: status and future prospects. 2022: p. 23-43.
- Reza, M.S., et al., Ex Situ Catalytic Pyrolysis of Invasive Pennisetum Purpureum Grass with Activated Carbon for Upgrading Bio-Oil. 2023. 15(9): p. 7628.
- Lee, S.-M., J.-S.J.F. Roh, Nanotubes, and C. Nanostructures, Pore development process according to burn-off of activated carbon black with CO2 gas. 2020. 28(10): p. 808-814. [CrossRef]
- Bedia, J., et al., A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. Journal of Carbon Research, 2018. 4: p. 63. [CrossRef]
- Menya, E., et al., Synthesis and evaluation of activated carbon from rice husks for removal of humic acid from water. Biomass Conversion and Biorefinery, 2022. 12(8): p. 3229-3248. [CrossRef]
- Lazzari, E., et al., Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. 2018. 111: p. 856-864. [CrossRef]
- Anukam, A.I., et al., Characterization of pure and blended pellets made from Norway spruce and pea starch: A comparative study of bonding mechanism relevant to quality. 2019. 12(23): p. 4415. [CrossRef]
- Hoang, A.T., et al., Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. 2021. 223: p. 106997. [CrossRef]
- Xie, L., et al., Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. 2016. 4(5): p. 1637-1646. [CrossRef]
- Nguyen, T.N., et al., Facile green synthesis of carbon quantum dots and biomass-derived activated carbon from banana peels: synthesis and investigation. 2020: p. 1-10. [CrossRef]
- Li, Q., et al., Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. 2021. 103: p. 268-278. [CrossRef]
- Zhang, C., et al., Direct air capture of CO2 by KOH-activated bamboo biochar. 2022. 105: p. 399-405. [CrossRef]
- Malla, F.A., et al., Circular Economy to Decarbonize Electricity, in Renewable Energy in Circular Economy. 2023, Springer. p. 71-87.
- Hoang, T.-D., et al., Biofuel Projects and Current Environmental Policies: Vietnam’s Case and Neighboring Asian Countries, in Biofuels in Circular Economy. 2023, Springer. p. 73-88.
| Biomass precursor | Sample code | Pretreatment methods | Activating agent |
|---|---|---|---|
| Okara powder waste | OPW1 | Carbonization, 800 oC, in an Ar flow over 1h | No activating |
| Okara powder waste | OPW2 | Carbonization at 650 oC in a gas N2 flow for 1 hour | No activating |
| Okara powder waste | OPW3 | Carbonization at 600 oC in a gas N2 flow for 1 hour | No activating |
| Okara powder waste | OPW4 | Hydrothermal carbonization at 200 oC; heating rate is 60 oC/h: temperature of 200 oC is maintained in 4h; weight ratio of OPW: water is 12.8 gram: 40 ml | No activating |
| Okara powder waste | OPW5 | Hydrothermal carbonization at 200 oC; weight ratio is 12.8-gram OPW: 6.4 gr zeolite (4 mm in size): 40 ml water; a temperature of 200 oC is maintained in 4h; the heating rate is 60 oC/h: | No activating |
| Okara powder waste | OPW6 | Hydrothermal carbonization at 200 oC, at a heating rate of 1 60 cC/hour, kept this 200 oC temperature for 4 hours. Then Mix with KOH, and melamine with the mass ratio of hydrochar/KOH/melamine=1:4:2 and then perform the pyrolysis (two-step processes) at 600 oC; in a flow of N2 in 1h; N2 flow rate of 1 l/min |
KOH, melamine (weight ratio of KOH: melamine = 2:1) |
| Okara powder waste | OPW7 | Carbonization 600 oC, N2, 1h to make biochar; after that biochar is mixed with KOH and water; then keep the sample for 48 hours in stirring, the ratio of biochar:KOH: water is 1:4:5 in weight, then filter and dry then calcined at 600 oC in N2 for 1h | KOH |
| Rice husk | RH | Carbonization 600 oC, N2, 1h | No activating |
| Okara powder waste | OPW8 | Carbonization 600 oC, N2, 1h; dry mixing process with KOH(ratio of biochar: KOH = 1.3) then dry at 120oC, then carbonization at 600 oC | KOH |
| Okara powder waste | OPW9 | Carbonization 600 oC, N2, 1h; dry mixing process with KOH (ratio of biochar: KOH = 1.4) then dry at 120 oC, then carbonization at 600 oC | KOH |
| Okara powder waste | OPW10 | Carbonization 600 oC, N2, 1h; then mix biochar:KOH: water ratio of 1:5:10; keep the sample for 5 days, filter, drying, calcine at 600 oC | KOH |
| Elements | Coffee waste | Okara powder waste | Rice straw | Shrimp shell | Rice husk | Potato peels | ||||||
| Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | |
| C | 57.35 | 64.17 | 45.73 | 53.36 | 37.88 | 56.56 | 44.38 | 53.4 | 18.91 | 26.44 | 48.12 | 56.79 |
| O | 42.65 | 35.83 | 52.53 | 46.02 | 9.97 | 11.18 | 48.9 | 44.17 | 55.48 | 58.24 | 46.62 | 41.31 |
| K | 1.74 | 0.62 | 5.77 | 2.65 | 5.26 | 1.91 | ||||||
| Ca | 6.72 | 2.42 | ||||||||||
| Si | 46.37 | 29.61 | 55.61 | 15.31 | ||||||||
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| Biomass precursor | Sample code | Pretreatment methods | Activating agent | SBET (m2/g) | References |
|---|---|---|---|---|---|
| Firewood | - | Carbonization 850 °C | Na2CO3/K2CO3 | 818 | [41] |
| Banana peels | - | Hydrothermal 200 °C, 24 h | No activating agent | 294.6 | [42] |
| Elephant grass, | - | Carbonization at 600 oC, in N2 flow; 1h | KOH | 407 | [34] |
| Lotus leaf | - | Carbonization 500 oC, N2, 1h | Melamine and KOH | 687 | [43] |
| Lotus leaf | - | Carbonization 500 oC, N2, 1h | No activating agent | 3.82 | [43] |
| Lotus leaf | - | Carbonization 500 oC, N2, 1h | Melamine | 4.32 | [43] |
| Okara powder waste | OPW1 | Carbonization, 800 oC, in an Ar flow over 1h | No activating | 1.16 | This work |
| Okara powder waste | OPW2 | Carbonization at 650 oC in a gas N2 flow for 1 hour | No activating | 1.06 | This work |
| Okara powder waste | OPW3 | Carbonization at 600 oC in a gas N2 flow for 1 hour | No activating | 20.73 | This work |
| Okara powder waste | OPW4 | Hydrothermal carbonization at 200 oC; heating rate is 60 oC/h: temperature of 200 oC is maintained in 4h; weight ratio of OPW: water is 12.8 gram: 40 ml |
No activating | 7.01 | This work |
| Okara powder waste | OPW5 | Hydrothermal carbonization at 200 oC; weight ratio is 12.8-gram OPW: 6.4 gr zeolite 4 mm: 40 ml water; temperature of 200 oC is maintained in 4h; heating rate is 60 oC/h: |
Zeolite (4 mm in size) | 14.0 | This work |
| Okara powder waste | OPW6 | Hydrothermal carbonization 200 oC and then pyrolysis (two-step processes) at 600 oC; in a low of N2 in 1h; N2 flow rate of 1 l/m | KOH, melamine | 22.04 | This work |
| Okara powder waste | OPW7 | Carbonization 600 oC, N2, 1h to make biochar; after that biochar is mixed with KOH and water; then keep the sample for 48 hours, then dry at 120oC and calcine at 600oC in N2 flow (1l/min) | KOH (ratio of biochar:KOH: water is 1:4:5 in weight) | 104.32 | This work |
| Rice husk | RH | Carbonization 600 oC, N2, 1h | No activating | 175.48 | This work |
| Okara powder waste | OPW8 | Carbonization 600 oC, N2, 1h; dry mixing process with KOH | KOH (mass ratio of OPW: KOH = 1/3) | 212.60 | This work |
| Okara powder waste | OPW9 | Carbonization 600 oC, N2, 1h; dry mixing process with KOH | KOH (mass ratio of OPW: KOH = 1/4) | 147.85 | This work |
| Okara powder waste | OPW10 | Carbonization 600 oC, N2, 1h; then mix with KOH, biochar:KOH: water mass ratio of 1:5:10; keep the sample for 5 days | KOH (with biochar:KOH: water ratio of 1:5:10; keep the sample for 5 days) | 594.08 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





