Submitted:
30 April 2024
Posted:
30 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Handling of Samples
- -
- plating: acetate buffer+ Bi+ H2O2; deposition potential -0,3 V; duration 60s
- -
- preconcentration: deposition potential -1,4 V; duration 60s; equilibration time 10s
- -
- conditioning: potential 0,3V; duration 30s
3. Results and Discussion
3.1. Physico-Chemical Features of Soil Samples
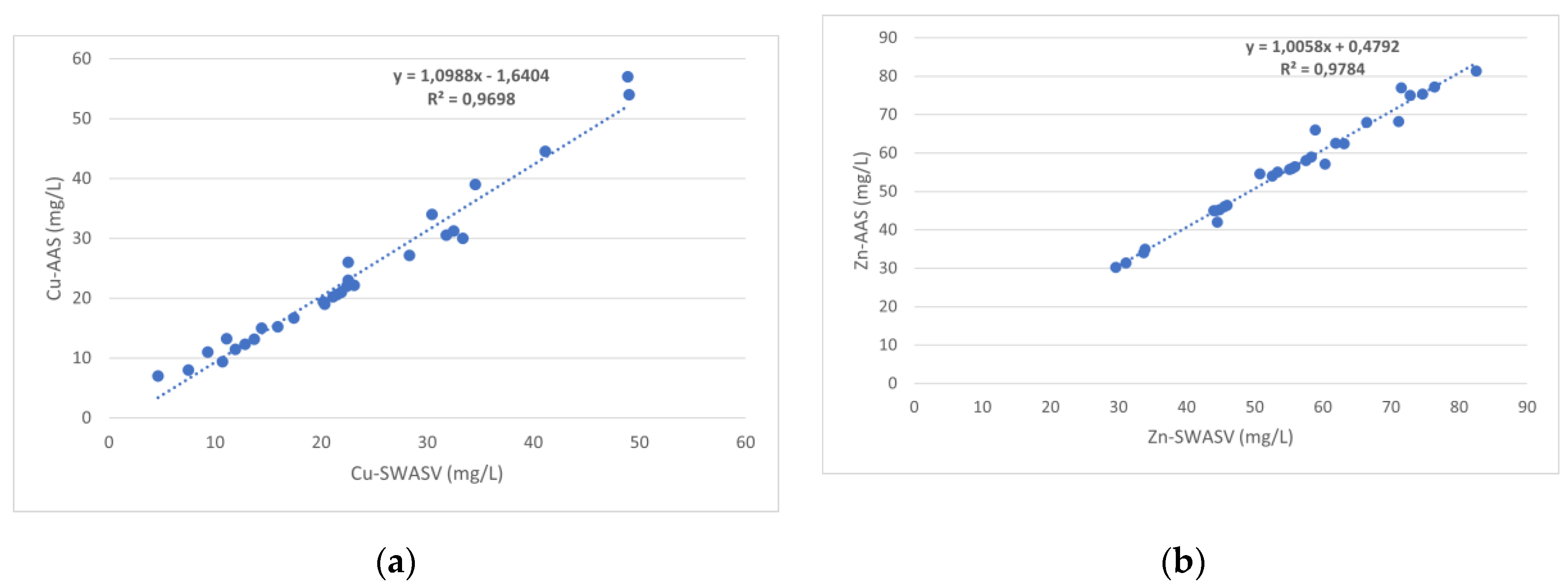
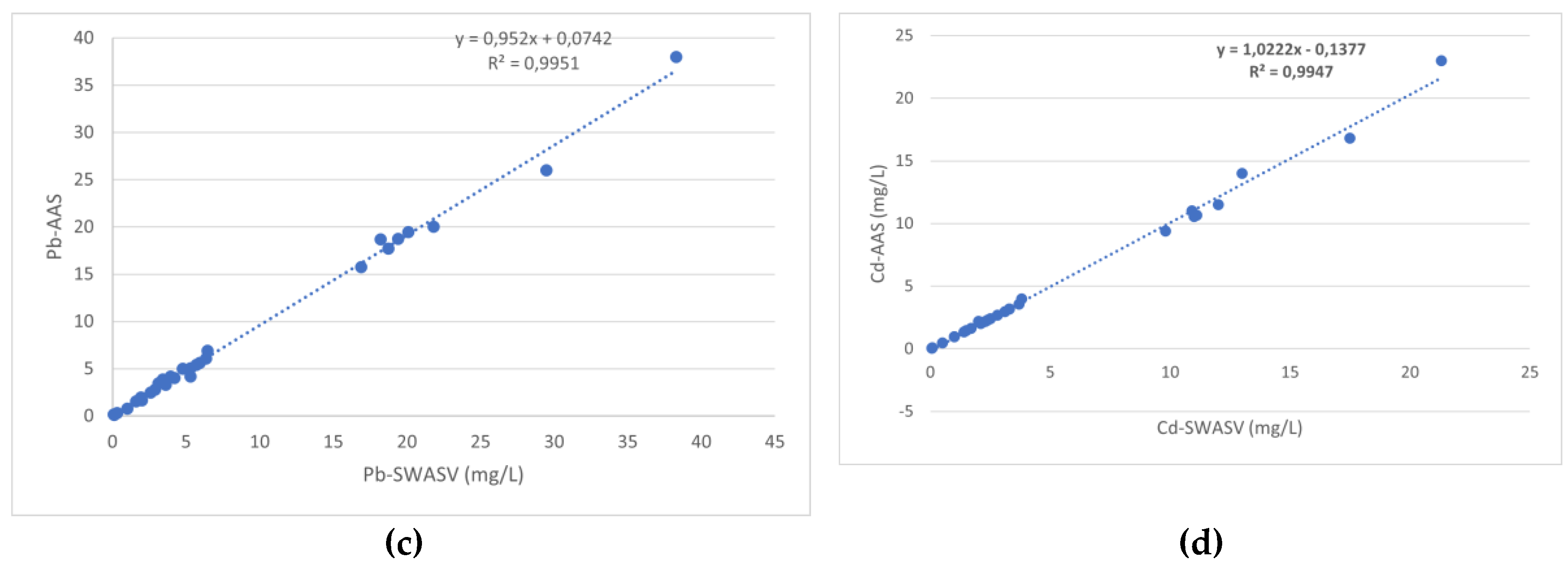
3.2. Voltammetric Determination
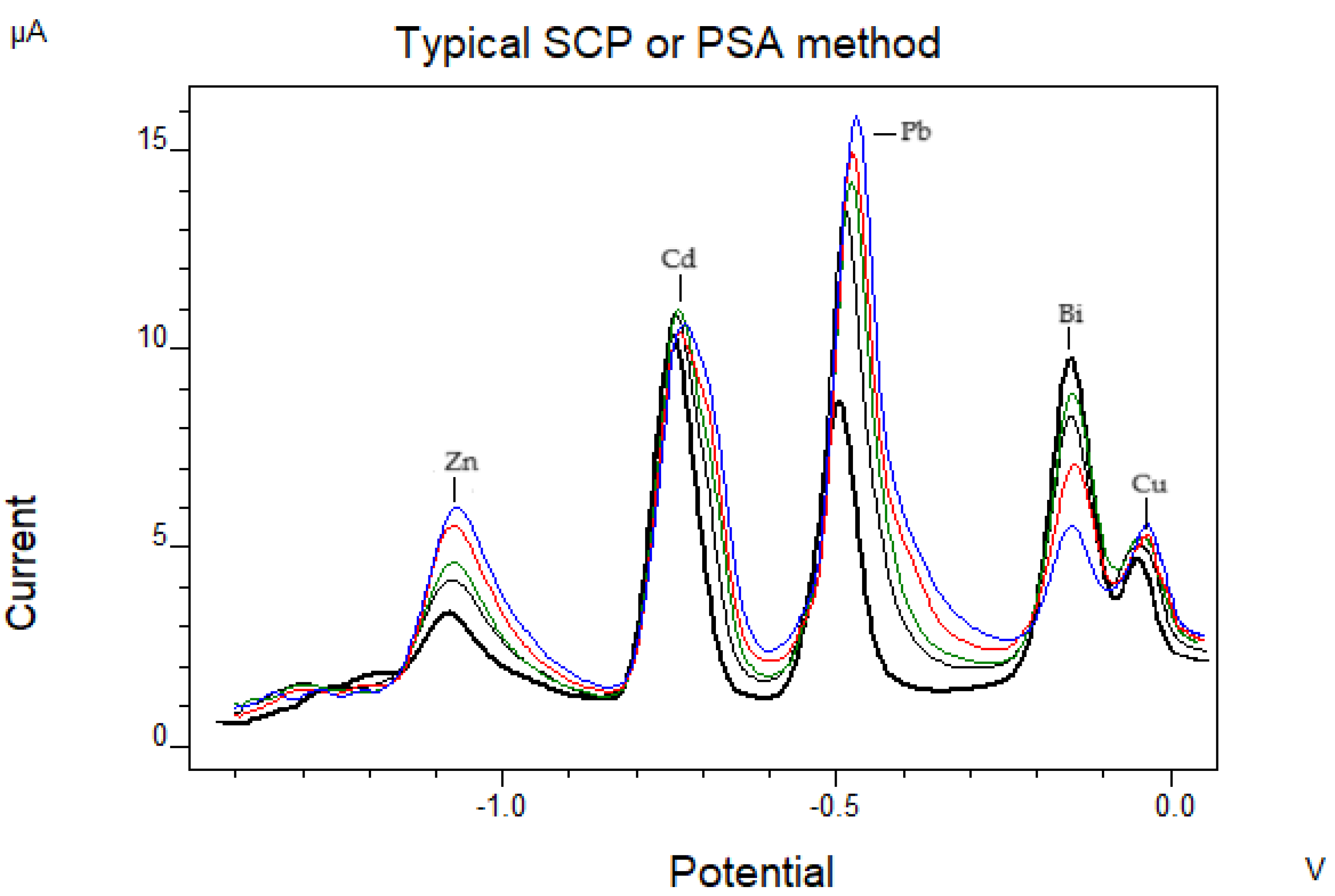
3.2.1. Bismuth Film-Modified Electrode
- -
- Plating of bismuth film in a different supporting electrolyte solution of that of measurement (ex situ)
- -
- Plating of bismuth film in the same supporting electrolyte solution with that of measurement (in situ) [45].
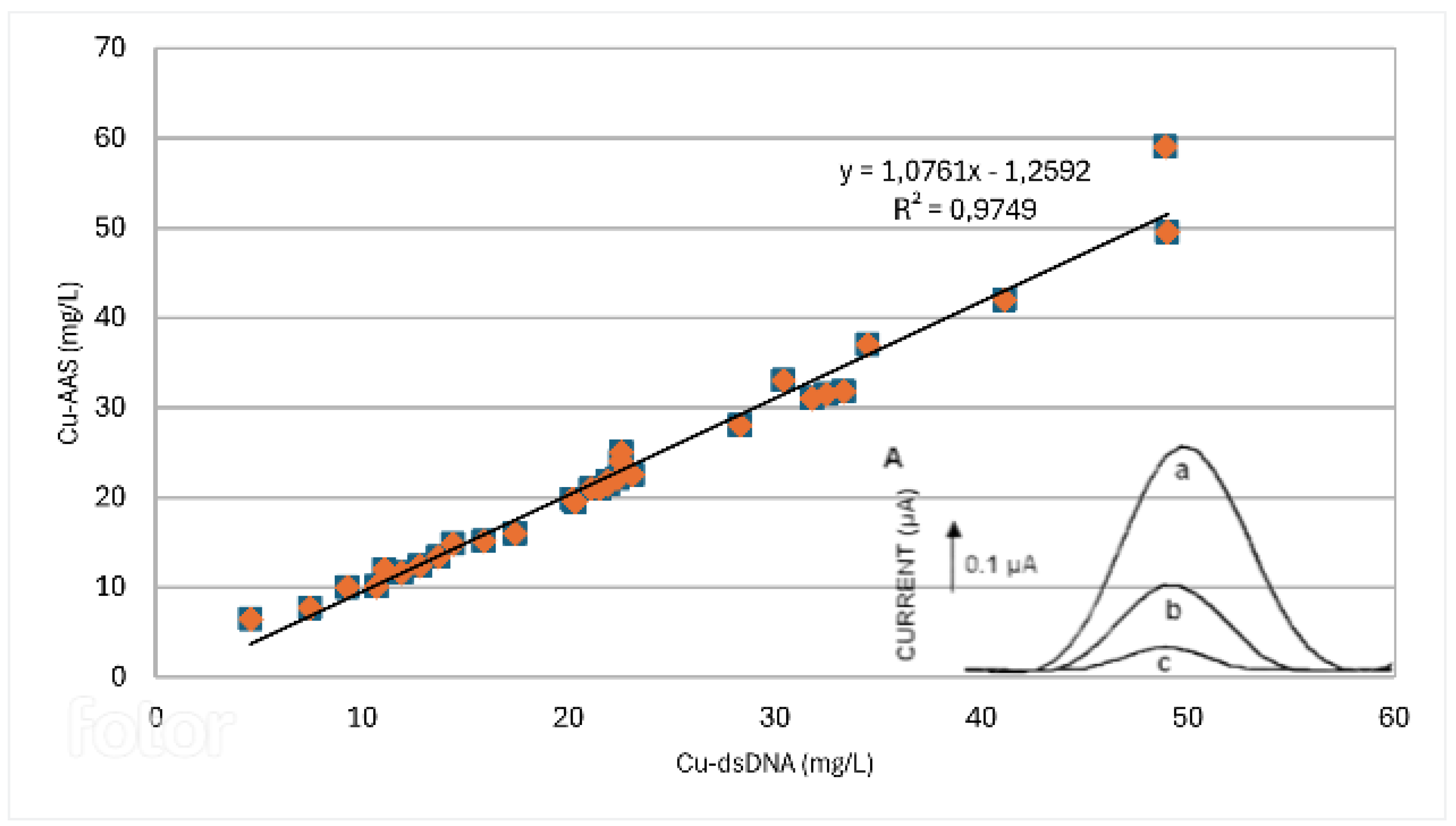
3.2.2. dsDNA Modified Electrode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- B.J. Alloway Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability. In Choice Reviews Online; Alloway, B.J., Ed.; Blackie Academic and Professional: London, England, 2013; Vol. 50 ISBN 9789400744691.
- Kabata-Pendias, A. Trace Elements in Soils and Plants: Fourth Edition; Press, C., Ed.; 4th ed.; Taylor and Francis Group, Ann Arbor, MI, USA, 2010; ISBN 9781420093704.
- Mawari, G.; Kumar, N.; Sarkar, S.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Khan, N.A. Heavy Metal Accumulation in Fruits and Vegetables and Human Health Risk Assessment: Findings From Maharashtra, India. Environ Health Insights 2022, 16, 117863022211191. [CrossRef]
- Jafarzadeh, N.; Heidari, K.; Meshkinian, A.; Kamani, H.; Mohammadi, A.A.; Conti, G.O. Non-Carcinogenic Risk Assessment of Exposure to Heavy Metals in Underground Water Resources in Saraven, Iran: Spatial Distribution, Monte-Carlo Simulation, Sensitive Analysis. Environ Res 2022, 204. [CrossRef]
- Yang, Z.; Zhang, R.; Li, H.; Zhao, X.; Liu, X. Heavy Metal Pollution and Soil Quality Assessment under Different Land Uses in the Red Soil Region, Southern China. Int J Environ Res Public Health 2022, 19. [CrossRef]
- Eid, M.H.; Eissa, M.; Mohamed, E.A.; Ramadan, H.S.; Tamás, M.; Kovács, A.; Szűcs, P. New Approach into Human Health Risk Assessment Associated with Heavy Metals in Surface Water and Groundwater Using Monte Carlo Method. Sci Rep 2024, 14. [CrossRef]
- Golia, E.E.; Dimirkou, A.; Floras, S.A. Spatial Monitoring of Arsenic and Heavy Metals in the Almyros Area, Central Greece. Statistical Approach for Assessing the Sources of Contamination. Environ Monit Assess 2015, 187, 399–412. [CrossRef]
- Mónok, D.; Kardos, L.; Pabar, S.A.; Kotroczó, Z.; Tóth, E.; Végvári, G. Comparison of Soil Properties in Urban and Non-Urban Grasslands in Budapest Area. Soil Use Manag 2021, 37. [CrossRef]
- Scharenbroch, B.C.; Trammell, T.L.; Paltseva, A.; Livesley, S.J.; Edmondson, J. Editorial: Urban Soil Formation, Properties, Classification, Management, and Function. Front Ecol Evol 2022, 10. [CrossRef]
- Meng, Z.; Zheng, Y.; Research, H.X.-A.M.; 2011, undefined Distribution and Ecological Risk Assessment of Heavy Metal Elements in Soil. Trans Tech PublZH Meng, YF Zheng, HF XiaoAdvanced Materials Research, 2011•Trans Tech Publ.
- Liu, R. ping; Xu, Y. ning; Zhang, J. hua; Wang, W. ke; Elwardany, R.M. Effects of Heavy Metal Pollution on Farmland Soils and Crops: A Case Study of the Xiaoqinling Gold Belt, China. China Geology 2020, 3. [CrossRef]
- Ye, W.; Liu, N.; Zhao, G.; Liu, G. Accurate Detection of Cd2+ and Pb2+ Concentrations in Soils by Stripping Voltammetry Peak Areas under the Mutual Interference of Multiple Heavy Metals. Metals (Basel) 2023, 13. [CrossRef]
- Koelmel, J.; pollution, D.A.-E.; 2012, undefined Imaging of Metal Bioaccumulation in Hay-Scented Fern (Dennstaedtia Punctilobula) Rhizomes Growing on Contaminated Soils by Laser Ablation ICP-MS. ElsevierJ Koelmel, D AmarasiriwardenaEnvironmental pollution, 2012•Elsevier.
- Liuzhu, Z.; Sekar, S.; Chen, J.; Lee, S.; Kim, D.Y.; Manikandan, R. A Polyrutin/AgNPs Coated GCE for Simultaneous Anodic Stripping Voltammetric Determination of Pb(II) and Cd(II)Ions in Environmental Samples. Colloids Surf A Physicochem Eng Asp 2022, 648. [CrossRef]
- Massadeh, A.M.; Alomary, A.A.; Mir, S.; Momani, F.A.; Haddad, H.I.; Hadad, Y.A. Analysis of Zn, Cd, As, Cu, Pb, and Fe in Snails as Bioindicators and Soil Samples near Traffic Road by ICP-OES. Environmental Science and Pollution Research 2016, 23. [CrossRef]
- Daşbaşi, T.; Saçmaci, Ş.; Çankaya, N.; Soykan, C. A New Synthesis, Characterization and Application Chelating Resin for Determination of Some Trace Metals in Honey Samples by FAAS. Food Chem 2016, 203. [CrossRef]
- Al-Hamad, A.A.; Ghrefat, H.; Howari, F.; Khawaja, M.A.A.; Zoubi, A. Assessment of Roadside Pollution by Heavy Metals: A Case Study from the District of Bani Kinanah, Irbid, Northern Jordan. Environ Monit Assess 2023, 195. [CrossRef]
- Khan, M.N.; Wasim, A.A.; Sarwar, A.; Rasheed, M.F. Assessment of Heavy Metal Toxicants in the Roadside Soil along the N-5, National Highway, Pakistan. Environ Monit Assess 2011, 182. [CrossRef]
- Siraj, K.; Kitte, S.A. Analysis of Copper, Zinc and Lead Using Atomic Absorption Spectrophotometer in Ground Water of Jimma Town of Southwestern Ethiopia. International Journal of Chemical and Analytical Science 2013, 4. [CrossRef]
- Anemana, T.; Osei, O.; Yeboah, E.; Pekař, M. Simultaneous Determination of Heavy Metals in Competitive Aqueous Solutions and Contaminated Soil Systems. International Journal of Mechanical Engineering 2022, 7, 974–5823.
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A Review of the Identification and Detection of Heavy Metal Ions in the Environment by Voltammetry. Talanta 2018, 178. [CrossRef]
- Nadporozhskaya, M.; Kovsh, N.; Paolesse, R.; Lvova, L. Recent Advances in Chemical Sensors for Soil Analysis: A Review. Chemosensors 2022, 10. [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabó, T.; Trif, L.; Felhősi, I.; Abbi, K.; Labjar, N.; Harmouche, L.; Shaban, A. Biomass Valorization of Walnut Shell into Biochar as a Resource for Electrochemical Simultaneous Detection of Heavy Metal Ions in Water and Soil Samples: Preparation, Characterization, and Applications. Arabian Journal of Chemistry 2022, 15. [CrossRef]
- Salih, F.E.; Ouarzane, A.; El Rhazi, M. Electrochemical Detection of Lead (II) at Bismuth/Poly(1,8-Diaminonaphthalene) Modified Carbon Paste Electrode. Arabian Journal of Chemistry 2017, 10. [CrossRef]
- Silva, J.J.; Paim, L.L.; Stradiotto, N.R. Simultaneous Determination of Iron and Copper in Ethanol Fuel Using Nafion/Carbon Nanotubes Electrode. Electroanalysis 2014, 26. [CrossRef]
- Osteryoung, J.G.; Osteryoung, R.A. Square Wave Voltammetry. Anal Chem 1985, 57, 101A-110A. [CrossRef]
- Thanh, N.M.; Van Hop, N.; Luyen, N.D.; Phong, N.H.; Toan, T.T.T.; Mai, H.D. Simultaneous Determination of Zn(II), Cd(II), Pb(II), and Cu(II) Using Differential Pulse Anodic Stripping Voltammetry at a Bismuth Film-Modified Electrode. Advances in Materials Science and Engineering 2019, 2019. [CrossRef]
- Keramari, V.; Karastogianni, S.; Girousi, S. New Prospects in the Electroanalysis of Heavy Metal Ions (Cd, Pb, Zn, Cu): Development and Application of Novel Electrode Surfaces. Methods Protoc 2023, 6. [CrossRef]
- Golia, E.E.; Papadimou, S.G.; Cavalaris, C.; Tsiropoulos, N.G. Level of Contamination Assessment of Potentially Toxic Elements in the Urban Soils of Volos City (Central Greece). Sustainability 2021, Vol. 13, Page 2029 2021, 13, 2029. [CrossRef]
- Golia, E.E.; Emmanouil, C.; Charizani, A.; Koropouli, A.; Kungolos, A. Assessment of Cu and Zn Contamination and Associated Human Health Risks in Urban Soils from Public Green Spaces in the City of Thessaloniki, Northern Greece. EuroMediterr J Environ Integr 2023, 8. [CrossRef]
- Papadimou, S.G.; Barbayiannis; Golia, E.E. Preliminary Investigation of the Use of Silybum Marianum (L.) Gaertn. as a Cd Accumulator in Contaminated Mediterranean Soils: The Relationships among Cadmium (Cd) Soil Fractions and Plant Cd Content. EuroMediterr J Environ Integr 2024, 9. [CrossRef]
- Dimirkou, A.; Ioannou, Z.; Golia, E.E.; Danalatos, N.; Mitsios, I.K. Sorption of Cadmium and Arsenic by Goethite and Clinoptilolite. Commun Soil Sci Plant Anal 2009, 40. [CrossRef]
- Golia, E.E.; Diakoloukas, V. Soil Parameters Affecting the Levels of Potentially Harmful Metals in Thessaly Area, Greece: A Robust Quadratic Regression Approach of Soil Pollution Prediction. Environmental Science and Pollution Research 2022, 29. [CrossRef]
- ISO/ISO 10390:2005 Soil Quality, Determination of PH. International Standards Organization; Geneve, Switzerland: 2005;
- JAOAC; Chemists, A. of official, Ed.; 14th ed.; Inc. Virginia, USA, 1984; Vol. 6; ISBN 9788578110796.
- ISO/DIS 11466.(1994). Environment Soil Quality. By ISO Standards Compendium, Switzerland; Geneva, Switzerland;
- Lu, H. long; Li, K. wei; Nkoh, J.N.; He, X.; Xu, R. kou; Qian, W.; Shi, R. yong; Hong, Z. neng Effects of PH Variations Caused by Redox Reactions and PH Buffering Capacity on Cd(II) Speciation in Paddy Soils during Submerging/Draining Alternation. Ecotoxicol Environ Saf 2022, 234. [CrossRef]
- Medriano, C.A.; Chan, A.; Sotto, R. De; Bae, S. Different Types of Land Use Influence Soil Physiochemical Properties, the Abundance of Nitrifying Bacteria, and Microbial Interactions in Tropical Urban Soil. SSRN Electronic Journal 2022. [CrossRef]
- Council of the European Communities (1986) The Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture. Council Directive of 12 June 1986. Official Journal of the European Communities No L 181/6.;
- Luo, S.; Chen, R.; Han, J.; Zhang, W.; Petropoulos, E.; Liu, Y.; Feng, Y. Urban Green Space Area Mitigates the Accumulation of Heavy Metals in Urban Soils. Chemosphere 2024, 352. [CrossRef]
- Skorbiłowicz, M.; Skorbiłowicz, E.; Rogowska, W. Heavy Metal Concentrations in Roadside Soils on the Białystok-Budzisko Route in Northeastern Poland. Minerals 2021, 11. [CrossRef]
- Zhang, J.; Liu, K.; He, X.; Li, W.; Zhang, M.; Cai, Q. Evaluation of Heavy Metal Contamination of Soil and the Health Risks in Four Potato-Producing Areas. Front Environ Sci 2023, 11. [CrossRef]
- Tang, S.; Wang, C.; Song, J.; Ihenetu, S.C.; Li, G. Advances in Studies on Heavy Metals in Urban Soil: A Bibliometric Analysis. Sustainability (Switzerland) 2024, 16. [CrossRef]
- Economou, A. Bismuth-Film Electrodes: Recent Developments and Potentialities for Electroanalysis. TrAC - Trends in Analytical Chemistry 2005, 24. [CrossRef]
- Arduini, F.; Calvo, J.Q.; Palleschi, G.; Moscone, D.; Amine, A. Bismuth-Modified Electrodes for Lead Detection. TrAC - Trends in Analytical Chemistry 2010, 29. [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M.; Ogorevc, B. Bismuth-Coated Carbon Electrodes for Anodic Stripping Voltammetry. Anal Chem 2000, 72. [CrossRef]
- Anastasiadou, Z.D.; Jannakoudakis, P.D.; Girousi, S.T. Square Wave Anodic Stripping Voltammetry Determination of Eco-Toxic Metals in Samples of Biological and Environmental Importance. Central European Journal of Chemistry 2010, 8. [CrossRef]
- Anastasiadou, Z.D.; Sipaki, I.; Jannakoudakis, P.D.; Girousi, S.T. Square-Wave Anodic Stripping Voltammetry (Swasv) for the Determination of Ecotoxic Metals, Using a Bismuth-Film Electrode. Anal Lett 2011, 44.
- Stanić, Z.; Girousi, S. Electrochemical Study of the Interaction between DsDNA and Copper(I) Using Carbon Paste and Hanging Mercury Drop Electrode. Talanta 2008, 76. [CrossRef]
- Stanić, Z.; Girousi, S. Electrochemical Study of the Interaction between DsDNA and Copper(II) Using Carbon Paste and Hanging Mercury Drop Electrodes. Microchimica Acta 2009, 164. [CrossRef]
| pH (1:1) |
EC (μS cm-1) |
OM (%) | Clay (%) |
Sand (%) |
CaCO3 (%) |
|
|---|---|---|---|---|---|---|
| Minimum Value | 7.12 | 1224 | 0.55 | 11 | 21 | 8.65 |
| Maximum Value | 8.82 | 4431 | 3.43 | 56 | 67 | 19.34 |
| Mean Value | 7.49 | 2205 | 1.97 | 24 | 44 | 11.33 |
| Relative Standard Deviation |
0.55 | 11.13 | 0.88 | 2.30 | 6.43 | 1.33 |
| Kurtosis Coefficient | 1.339 | -0.385 | -0.543 | -0.008 | -0.082 | -0.760 |
| Skewness Coefficient | 0.690 | 0.643 | -0.213 | 1.0088 | -0.944 | 0.331 |
| N | P | K | Cu | Zn | Pb | Cd | |
|---|---|---|---|---|---|---|---|
| (%) | (mg kg-1) | ||||||
| Minimum Value | 0.1 | 7.81 | 45.12 | 17.44 | 28.13 | 4.54 | 0.17 |
| 10th-perca | 0.12 | 10.41 | 57.61 | 19.45 | 33.14 | 9.15 | 0.24 |
| 50th-percb | 0.15 | 14.66 | 66.87 | 26.78 | 47.31 | 28.55 | 0.76 |
| Average | 0.19 | 16.62 | 71.77 | 29.88 | 49.02 | 29.93 | 0.88 |
| 90th-percc | 0.2 | 17.67 | 88.89 | 32.45 | 52.07 | 39.72 | 0.91 |
| Maximum Value | 0.22 | 22.77 | 97.73 | 34.76 | 69.11 | 43.54 | 1.05 |
| EU Limitsd | 140 | 300 | 300 | 3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





