1. Introduction
Starch is a reserve polysaccharide of plants. In the native state, starch creates insoluble and semi-crystalline granules with characteristic size and shape according to biological origin. They consist of chains of glucose molecules that can be straight or branched. The branchless form is called amylose in which molecules are linked by α-(1,4) bonds with the ring of oxygen atoms on all the same sides, whereas amylopectin is the name given to the branched form containing branches linked to linear backbone by α-(1,6) linkages. The relative proportion of amylose/amylopectin in starch can be influenced by the choice of plant species or variety, physical separation of one of the components, enzymatically, or by the action of microorganisms [
1]. Amylopectin has stabilizing effects, whereas amylose forms gel. Amylose also possesses a strong tendency to form complexes with lipids and other components. The length and configuration of the chains in amylopectin influence many properties such as crystallinity, pasting and thermal properties, gelatinization temperatures [
2], retrogradation [
3], syneresis, and resistant starch formation and digestibility [
4,
5].
Starch is generally used as a thickener, water binder stabilizer, and gelling agent in food industries [
6]. This processing changes the starch granules and their functional properties. Physicochemical changes during food preparation include gelatinization, the formation of starch-lipids and starch-protein complexes, and the origin of resistant starch. Therefore, they also influence the digestibility.
Gelatinization is a collective term to describe a range of irreversible events occurring when starch is heated in water. The loss of crystalline order during heating is observed. As a result of gelatinization, the in vitro and also in vivo amylolysis rate increases dramatically. It is known that raw corn, wheat, cassava, and smooth pea starches or raw potatoes have considerably lower postprandial responses in blood glucose than the corresponding boiled ingredients [
7].
Starch comprises as much as 70-80% of the total carbohydrates in a normal diet [
8]. Different forms of starch have different rates and ranges of digestion in the human gastrointestinal tract [
9]. Rapid digestion of starch may lead to glucose spikes in blood, which can cause dysregulation of the metabolism and endocrinal systems, leading to chronic diseases such as type 2 diabetes mellitus and obesity [
10]. Overweight and obesity contribute to 3.4 million deaths annually worldwide. In the U.S., data from the National Health and Nutrition Examination Survey (2017-2018) indicates that 42.5% of adults are obese, 9.0% have severe obesity, and 31.1% are overweight [
11]. These figures are rising sharply due to readily available high-calorie food and a sedentary lifestyle [
10]. Obesity is a significant risk factor for many chronic diseases, in addition to diabetes also cardiovascular diseases, nonalcoholic fatty liver disease, dyslipidemia, hypertension, type 2 diabetes mellitus, and certain cancers, making it a significant public health concern [
1].
Starch digestion rate and location in the gastrointestinal tract are critical for human health. All digestive compartments including mouth, stomach, small intestine, and large intestine play critical roles in regulating the overall starch digestion process. A proper investigation of starch digestion patterns should thus be based on the consideration of all these compartments. Main biological factors are summarized as oral mastication and salivation, gastric emptying and motility, small intestinal enzymes and motility, large intestinal resistant starch microbiota interactions and gut-brain feedback control, as well as glucose adsorption and hormonal feedback control [
12].
The α-1,6-glycosidic linkages at the branching sites in amylopectin cannot be broken by α-amylase. The glucose oligosaccharides containing uncleaved α-1,6-glycosidic bonds are the so-called α-limit dextrins. After starch hydrolysis by α-amylase the remaining products, maltose, maltotriose, and the α-limit-dextrins, are hydrolyzed into glucose by the enzyme complexes sucrase-isomaltase and maltase-glucoamylase located in the brush border membrane [
13].
Glucose is absorbed by a sodium-dependent active process. A sodium pump and sodium/potassium ATPase, as well as sodium secreted in the alkaline pancreatic juice, create a sodium gradient across the cell membrane. The sodium ions then enter the cell together with glucose [
14]. Glucose leaves the intestinal cells via the facilitative glucose transporter (GLUT2). Adjacent to the brush border membrane lies the unstirred layer that acts as a diffusion barrier for high-permeability compounds and can affect the rate of transport [
13].
2. Glycemic Index
The glycemic index (GI) is generally defined as the quotient between the area under the blood glucose curve after the consumption of 50 g of carbohydrates from a test food and the area under the curve after the consumption of 50 g of carbohydrate from a reference food (white bread or glucose), multiplied by 100 [
15]. During the past years, the GI of a variety of foods has been determined, and summary tables of the GI of over 750 different food items are available [
16,
17].
Whole wheat bread having a low glycemic index contains resistant starch (see chap. 3), because the glycerides in whole wheat bread bind to starch, which slows the digestion of starch [
18], and also contains dietary fiber components. Whole tissue structures originating from whole kernels or wholemeal flour in bread can decrease enzymatic hydrolysis due to limited accessibility [
19]. Interest in pseudocereals has grown because they contain proteins with better amino acid scores than cereals and a significant proportion of resistant starch, thus having a lower glycemic index; and some other biologically active substances [
20]. Great differences in GI have been observed between different legume products. The botanical origin has an effect and the processing, especially canning and mechanical disruption, produces higher GI values. Also, in the case of legumes, the tissue integrity and softness of the product seem to be important factors [
19].
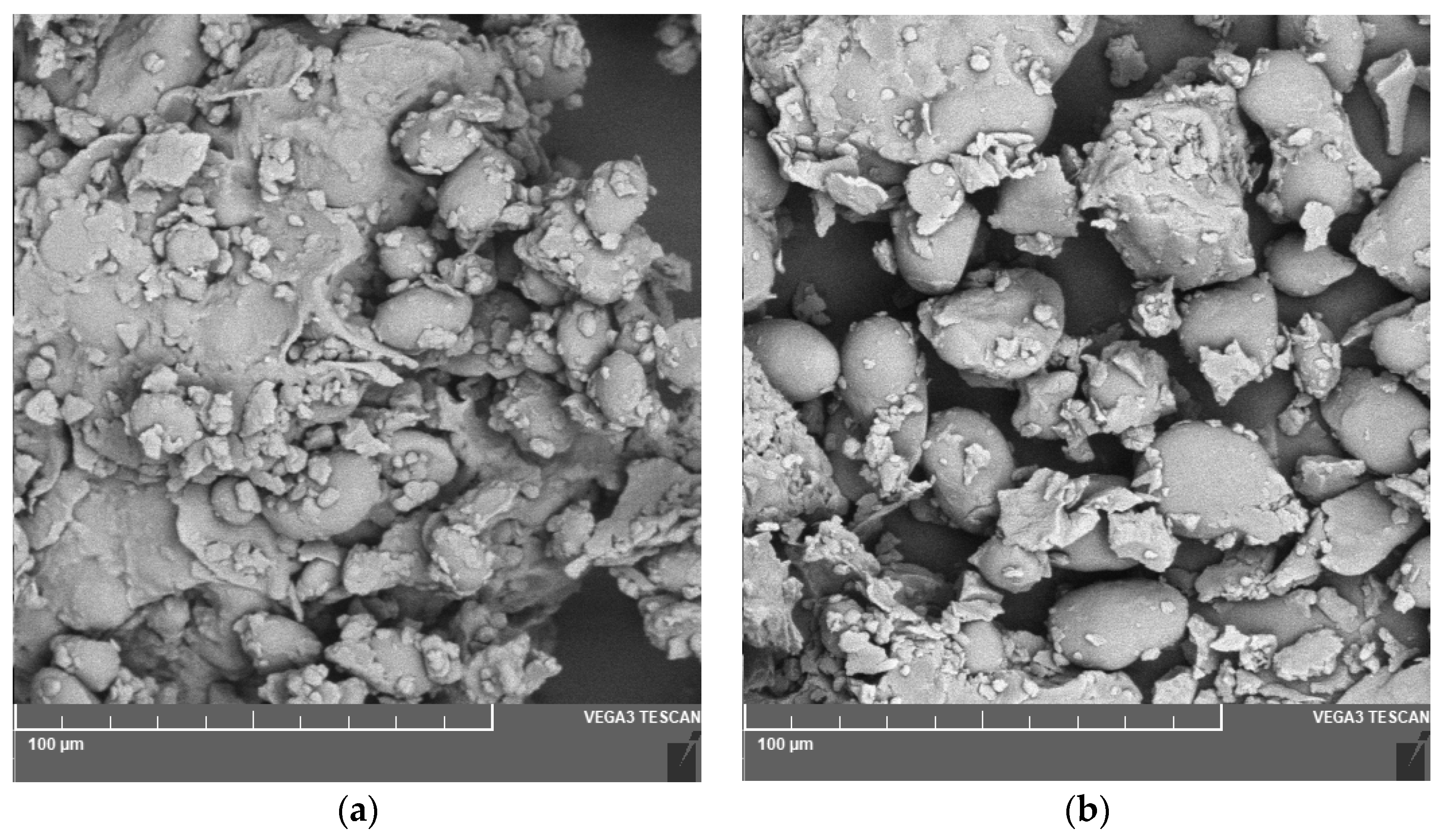
Figure 1.
Microphotography of the flour coming from a) chickpea and b) lentil.
Figure 1.
Microphotography of the flour coming from a) chickpea and b) lentil.
The need to develop low GI products with a high fiber content and low in energy is therefore stressed by the FAO/WHO [
21].
On the other side, some studies have shown that glycemic index only found a weak link between overall disease risk and GI or glycemic load of a diet [
22].
3. Rapidly Digestible Starch, Slowly Digestible Starch and Resistant Starch
Englyst et al. [
23] divided starch into rapidly digestible starch (RDS; starch digested from 0-20 min), slowly digestible starch (SDS; digested from 20-120 min), and resistant starch (RS; remaining starch) based on simulated digestion properties in vitro and bioavailability. This solution has been very simplistic, as it assumes that starch hydrolysis takes place mainly in the intestinal tract, the reaction takes place for the same residence time in the small and large intestine (the same for all people), and the dose of enzymes and their activities are identical. However, the advantage of this solution is that it makes it possible to easily categorize the starch in food in terms of its digestibility.
RDS is rapidly and completely digested in the small intestine. This is associated with elevated plasma glucose and insulin; therefore, it is linked with diabetes, coronary heart disease, and the aging process. Insulin is a peptide hormone produced by beta cells of the pancreatic islets. It regulates the metabolism of carbohydrates by promoting the absorption of glucose from the blood into the liver, fat, skeletal, and muscle cells [
24].
SDS is completely but slowly digested in the small intestine. It is a more desirable form of starch from the nutritional point of view [
9]. SDS fraction generally provides a slow and prolonged release of glucose into the bloodstream [
25]. Diets containing SDS could improve the carbohydrate metabolism of diabetic patients [
26]. Cornstarch in uncooked form (UCCS) is very slowly digestible if ingested. Therefore, it is suitable to give a continuous glucose supply. However, UCCS is not very palatable, which increases the risk of low dietary compliance. In patients with type 1 diabetes on intensive insulin therapy, a conventional bedtime snack is part of the therapy when glucose levels are below an arbitrary level (6.7 or 7 mmol/l). Extending the range of slowly digestible products would increase dietary variety for patients with type 2 diabetes and could improve the dietary treatment of glycogen storage disease type I. One approach to manipulating the rate of starch digestion is to adapt the manufacturing process in such a way that gelatinization of the starch granules is limited. Furthermore, the substitution of finely ground flour for coarse flour as well as the addition of intact grains to bread products is recommended. On the other hand, there are also special finely ground granulated whole grain flours that have a lower proportion of damaged starch (i.e. poorer digestibility) and, conversely, some fine passages of back flours may have a high proportion of damaged starch [
27]. Another strategy is the incorporation of viscous fiber (e.g. β-glucan, pectin) in the food product. Until now this has been proven successful in lowering the glycemic response of bread, oat-bran breakfast cereals, and bars [
14]. The high content of SDS (>35%) was found in the native starch of annatto [
28],
Euryale ferox [
29] and parkia [
8]. A very high SDS content was found in the thermally (80-100 °C) processed starch of gorgon nut [
30].
RS is not digested in the small intestine. RS includes five groups now: the first group is physically encapsulated starch within the plant cells or food/polymer matrix (RS1), the second one is some native starch (RS2), the third one is recrystallized starch (RS3), the fourth one is some chemically modified starch (RS4), and the fifth one is starch-lipid complexes (RS5).
4. Types of Resistant Starch
Type 1 resistant starch (RS1) has recently received tremendous attention from various research areas such as food science, nutrition, microbiology, and pharmaceutical technology due to its ability to resist digestion in the upper gastrointestinal tract. Intact plant tissue shows a lower starch digestibility compared to broken cells and isolated starch, because of the protective effects of cell walls. Other intracellular components inside the cells (i.e., protein/lipid bodies, cytoplasmic matrices, enzyme inhibitors) have been reported to give an extra obstacle to enzyme hydrolysis [
31].
RS2 can be found in raw potatoes, legumes, bananas, or in high amylose starches (HASs). The following HASs are e.g. on the market: corn HASs produced by Ingredion (IL, USA) [
32], by Quanyinxiangyu Biotechnology Co., Ltd. (Beijing, China) [
33] or by National Starch Co., Ltd. (Hamburg, Germany) [
34], having about 70% of amylose content.
Normal rice contains only 14-17% of amylose, therefore Li et al. [
35] labeled Hoshinishiki, as a type of high-amylose rice with 23-25% of amylose. Consumption of high-amylose rice led to significantly lower 24-h mean glucose levels, levels at 2 and 3 h after a meal, and postprandial glucose peak levels within 3 h, as well as significantly higher time in range. A similar trend was observed when the analysis was restricted to patients with type 2 diabetes. These results suggest that high-amylose rice may be a more beneficial staple food for glycemic control than regular rice. Tian et al. [
36] studied gelatinization behavior for eight types of HASs extracted from wheat, barley, corn, and potato. Gelatinization onset temperature, gelatinization peak temperature, and gelatinization conclusion temperature were in the range 53.5-87.8 ⁰C, 68.4-97.0 ⁰C, and 80.6-130.2 ⁰C, respectively. Also, unripe plantain, pumpkin, and winter squash Yinli starch demonstrated the lowest RDS (90%) and the highest RS (>90%) [
37]. Indigenous Himalayan folk rice cultivars are good examples of the dependence RS on amylose content. For an amylose content in the range of 0.7-29.1%, RS was low, i.e., 0.4-2.3% [
38] but for a higher amylose content of 31.8-40.7%, RS rapidly increased to 85.4-92.8% [
39].
Heat moisture treatment and annealing are other treatments that are used in increasing or modifying the resistant starch level in RS2. The modification of resistant starch using these two methods results in structural changes within the amorphous and crystalline regions of starch to different extents [
40].
RS3 is a new crystalline formation of gelatinized starch after retrogradation. The essence of preparing RS3 lies in disrupting the supramolecular structure of native starch, and the non-branched starch molecules recombine into double helixes to form a crystal structure. E.g. Jurkaninová et al. [
41] found that the content of resistant starch in dumplings increased with the time they were stored at low temperatures (about 5 ⁰C). On the other hand, according to Sissons et al. [
42], cold storage of cooked pasta was expected to increase retrograded starch but the increase in resistant starch was minor with no consequent improvement in the extent of starch digestion.
The retrogradation process can also proceed by first enzymatically converting the amylopectin into amylose using debranching enzymes such as pullulanase, isoamylase, or by partial hydrolysis with α-amylase. Starch is subjected to enzymatic or acid hydrolysis to eradicate the amorphous regions and generate starch that is essentially free of amorphous regions and contains at least 90% crystalline material [
40]. Shi et al. [
43] prepared RS3 by treating pea starches with a wrinkled surface (amylose content 79.5%-94.4%) with acid, gelatinization, debranching of amylopectin and recrystallization; then the structure was further modified with heat moisture treatment. RS3 after pressure heating treatment with 30% humidity showed the highest RS content (84.7%).
Han et al. [
33] prepared RS3 from corn HASs by hydrothermal-alkali and hydrothermal-alkali combined ultrasonication. The hydrothermal-alkali alone or in combination with ultrasound disintegrated HASs granules and resulted in a decrease in branching degree and molecular weight. The treated HASs formed RS3 with good thermal stability, high crystallinity, and short-range orderliness during retrogradation. In terms of digestion, the RS content of HASs rose from 47% to 69%, and the resistance of HASs to digestion was significantly improved. The commercial preparative is called Novelose 330 (Ingredion, IL, USA) [
32].
Starches modified with octenyl succinic anhydride are a source of significantly more slowly digestible and resistant starch (RS4) than other modified starches, such as hydroxypropylated starch, acetylated starch, or starches cross-linked by factors used in various combinations. According to Remya et al. [
44] cooked samples of octenyl succinylated potato starch showed SDS and RS in the range of 10.53-34.86% and 3.58-29.1%, respectively, whereas these were 20.46-38.36% and 1.47-27.92%, respectively, for cassava starch.
Resistant starches of the RS4 type can also be obtained using a double modification consisting of initial starch retrogradation (by freezing) and then substitution with acetyl groups and/or cross-linking with adipic acid. Also, starch pyrodextrinization products, resistant to enzymatic digestion, were classified by many authors as RS4 [
45]. Fibersym (RS4) is delivered by MGP Ingredients (KS, USA) [
32].
RS5 is amylose-lipid complexes. Associated helical molecules of amylose form inclusion compounds with lipids. RS5 requires heating the mixture above 90 °C and is heat resistant [
46]. It is more resistant to digestive enzymes than amylose. The susceptibility of amylose-lipid complexes prepared with potato amylose with fatty acids and lysophosphatidylcholine to hydrolysis by glucoamylase from
Rhizopus niveus was reduced through decreasing the ability of starch to adsorb enzymes such as glucoamylase [
40]. A greater resistance to the digestion of amylose-lipid complexes was observed when amylose was complexed with long-chain, saturated monoglycerides compared with complexes with shorter-chain, unsaturated monoglycerides [
47].
Starch digestion is not a simple process. It can run during some time intervals differently. E.g. Wang et al. [
48] dealt with the digestion of waxy corn starch/lauric acid complexes at 37 ⁰C and revealed that the logarithm of slope plots of the complex had a two-stage digestion pattern, with digestion rate of the first stage (
k1 = 0.038 min
−1) being much higher than that of the following stage (
k2 = 0.0116 min
−1) where k represents the first order rate constant.
Dynamic high-pressure microfluidization can be also used for the preparation of starch-lipid complexes. Chen et al. [
49] prepared by this method starch-lipid complexes from lotus seed starch and six saturated fatty acids of different carbon chain lengths and analyzed their semi-crystalline structure and digestibility. Octanoic acid significantly reduced the susceptibility to digestive enzymes, increased slowly digestion starch content (26%), and decreased digestion rate.
5. Effect of Other Parameters on Digestibility
Several factors are known to influence the gastric emptying rate. Meal-related factors are the size, composition, osmolarity, caloric content, and viscosity of a meal [
13]. However, starch digestion is also influenced by physiological factors, such as the degree of chewing, activity of α-amylase in the intestine, and transit time through the stomach and small intestine. Therefore, the rate and extent of digestion vary both within and between individuals [
9].
Food oral processing is the first step of digestion where chewing ability has a strong effect on food digestion. This processing is characterized by a large inter-individual variability that produces boluses with pieces of different particle sizes that may affect the digestion rate of nutrients (i.e. the starch digestion rate and glycemic response). During digestion, food matrix breakdown plays a pivotal role in how nutrients and bioactive compounds are made available for absorption in the human body, therefore regulating their concentration in the blood and utilization in peripheral tissues [
50].
Hoebler et al. [
51] confirmed that the hydrolysis of starch starts in the mouth. By analyzing the composition of spat-out boluses, the authors observed that about 50% of bread starch and 25% of pasta starch were hydrolyzed and transformed into smaller carbohydrate molecules during oral processing. α-amylase has been shown to affect the perception of flavor, mouth-feel, and after-feel attributes of semi-solids through changes in the structural characteristics of starchy products during oral processing [
52].
Yang et al. [
53] found that people with strong chewing ability had a higher rate of saliva secretion, producing more large particles and higher saliva content in the bolus (up to 28%), and a higher degree of oral starch hydrolysis (up to 13.5%). The swallowable bolus in the weak chewing group had larger holes and a looser microstructure with more small rice particles. In addition, an independently developed artificial gastric digestion system (AGDS) was used to explore the starch hydrolysis and gastric emptying of rice. Volunteers were instructed to habitually chew rice samples (104 g) as usual. The steamed rice was of japonica rice, indica rice, or waxy rice. All boluses were weighed and then added to AGDS within 5 min. A total volume of 25 mL of gastric juice (pH 1) was added to the stomach to simulate a fasting state. The system then also continued with the activity of the small intestine. The indica rice particles with a higher degree of fragmentation contacted enzymes more easily and hydrolyzed quicker, thus emptying through the stomach faster (81.76%). However, the oral chewing properties of rice mainly influenced the starch digestion in the stomach and the initial stage of the small intestine (similar to 5 min). This study suggested that the chewing ability and rice variety can influence the bolus properties, which in turn affects gastric emptying and the degree of starch hydrolysis during digestion. The results of gastric retention indicated that the particle size difference produced by oral chewing was the main factor that affected the gastric emptying rate.
Suo et al. [
50] studied factors influencing in vitro starch digestion of pasta. Mastication effort, shape, and their interaction mainly affected the starch digestion rate and the predicted glycemic index. The results suggested that small pasta like risoni, or less mastication effort could be a strategy to have a relatively lower expected glycemic index.
In the stomach, food pieces are subjected to the action of pepsin, acid conditions, and the vigorous grinding action of gastric motility. The rate of gastric emptying is tightly linked to rates of food digestion and nutrient absorption, and is regulated so that the stomach only empties at a rate that mirrors the capacity of the small intestine to digest and absorb the received nutrients. Gastric emptying at half-time correlates with blood glucose and insulin values. This is because the size reduction in the stomach for foods, that leave the mouth as coherent and large particles, will take a longer time and the blood sugar values will be increased gradually. The only exit from the stomach to the small intestine is the polyrus, which allows food pieces less than 2mm in diameter to exit. Liquid phases of a meal are emptied faster from the stomach than solid phases [
19].
The content of the intestine is gradually pushed in a direction from the stomach by peristaltic movements. One of the important influences on the digestibility of starch is the velocity of progress (flow rate) through the intestine. In principle, the flow character can be characterized as a piston flow. The time delay in both the small and large intestine depends on the volumetric flow rate according to the equation:
where τ is the time delay, V - intestinal volume, - volume flow.
Therefore, the contact time between the nutrients and the intestinal mucosa is a factor affecting the rate and extent of intestinal carbohydrate digestion and absorption. The absorption rate of nutrients could increase when intestinal transit is slowed down since a delay in small bowel transit increases the time of contact between the luminal contents and the absorptive epithelium. Only a few studies have investigated the relationship between the transit time and the digestion and absorption of carbohydrates [
13]. In ileostomy patients, Chapman et al. [
54] showed that the absorption of starch is directly related to the small intestinal transit time.
Increasing the flow rate therefore shortens the action time of amylases in the intestine, so part of the slowly digestible starch analyzed in vitro becomes resistant starch. It is known that dietary fiber accelerates this motion. The term dietary fiber (DF) describes indigestible carbohydrates that are linked with improving gut and general health [
55]. It can be a natural part of food or it can be added e.g. by bamboo culm flour [
56].
Soluble fiber strengthens the gluten network and causes a lower release of sugars during in vitro starch digestion. Soluble-viscous DF (psyllium, guar gum, pectin, and β-glucan) decreases the amounts of digestible starch values. This is thought to be due to delayed gastric emptying and delayed absorption of glucose in the small intestine by a reduction in diffusion rate through the intraluminal bulk phase of the small intestine [
13]. Soluble dietary fiber shows a higher water absorption compared to insoluble fiber. The suggested DF ingredients are insoluble DF cellulose and soluble DF guar gum [
57]. A study by Tharakan et al. [
58] with an in vitro GIT model, which included segmentation forces in the small intestine, showed that adding a thickening agent (guar gum) to the simulated intestinal fluids reduced the rate of glucose release after starch hydrolysis.
Fat has the potential to delay gastric emptying. By this mechanism, the reduced glucose response to starch by co-ingestion of fat can be explained [
13]. Also, some foods that our body perceives as unsuitable (surface-ripened cheeses, etc.) can increase the flow through the intestine.
6. How to Increase Digestion of Starch
Faster digestion is suitable for people who need to supply quickly the body with energy. This can be achieved mainly by removing the crystalline lamellae of amylopectin or by avoiding double helices in amylose (retrogradation). As written in the introduction, the simplest procedure to increase the digestibility of starch is its gelatinization (cooking) or hydrolysis to maltodextrins (used, for example, in people with a higher stage of celiac disease).
The physical modification of starch involves breaking the molecular chain, alters the monosaccharide content, and modifies particle size. This modification ultimately leads to changes in the spatial structure of dietary fiber. This approach encompasses methods such as ultrasonic modification, nanotechnology, and microfluidization. These methods not only improve the physical properties of dietary fiber, such as water-holding, swelling, fat-binding, and cation-exchange capacity but also enhance its biological activity thanks to associated bioactive substances such as polyphenols and phenolic acids. Recent studies have indicated that smaller molecular particles result in higher solubility, which improves the digestive and absorptive functions of gut microbiota [
1].
Sissons et al. [
42] suggested subtly manipulating the starch digestion of pasta through some processing procedures. Cooking time had a large impact on pasta starch digestion and reducing cooking from fully cooked to al dente and using pasta of very high protein content (17%), reduced starch digestion extent. The semolina particle size distribution used to prepare pasta impacted pasta quality and starch digestion to a small extent indicating a finer semolina particle size (<180 µm) may promote a more compact structure and help to reduce starch digestion.
Small starch granules have higher enzymatic hydrolysis rates than large granules because of the “outside-in” digestion pattern [
59], reduced amylose content [
60], or increased number of amorphous growth rings in small starch granules [
61]. The digestion rate of starch granules by amylases has been reported to increase as their surface area increases [
22]. Commercial sources of small granules of starch include rice, wheat, and oat [
62]. The suitable size of starch granules can be indicated in non-traditional starches or small particles which can be prepared by some physical technique. The smallest granules (<5 µm) with a high specific area were found in
Agriophyllum squarrosum, amaranth, foxnut, and gorgon nut seeds and the leaves of
Arabidopsis thaliana [
37]. Lindeboom et al. [
62] inform about other potential sources of small granule starch e.g., quinoa (
Chenopodium quinoa Willd.), giant taro (
Alocasia macrorrhiza L.), cow cockle (
Saponaria vaccaria L.), canary grass (Phalaris
canariensis L.), cat tail (
Typha latifolia L.), dasheen (
Colocasia esculenta L.), grain tef (
Eragrostis tef (Zucc.) Trotter) and dropwort (
Filipendula vulgaris Moench), all having granule sizes ranging from 0.5 to 10 µm. On the other hand, Chavez-Salazar et al. [
63] and Puncha-arnon et al. [
64] found 65 μm long particles in unripe plantains and granules of 47 μm in achira rhizomes, respectively. A bimodal size distribution of large and small granules is characteristic of wheat starches as well as those from rye and barley. The two populations are classified as A-granules (>10 µm) and B-granules (<10 µm) and differ somewhat in their physicochemical characteristics and end-use potential [
62].
Digestion patterns of starch granules include surface pitting and erosion, formation of pores, and concentric layered shell structures. The α-amylolysis of waxy wheat starch is characterized by the formation of holes on the granular surface and disruption of the core of the granule. Starch granules from Diamondbird, Batavia, V306, and SM1046 (wheat lines with elevated amylose content) seemed to follow different patterns of α-amylolysis to that observed for waxy wheat starch. Granules from these starches were much less disrupted after 2 h than the waxy starch granules, and the layered structure corresponding to growth rings was not observed in any of the partly hydrolyzed granules. Some fine pitting was noted on the surface and granules had a roughened appearance comparable to the waxy starch granules in the early stages of digestion [
65].
Xie et al. [
66] dealt with the effect of high-pressure microfluidization (HPM) on the microstructure, physicochemical properties, and digestibility of fractionated PS. Results show that the mechanical forces (50-150 MPa) can break the molecules and enhance the digestion rate with increasing pressure. Authors suggested that HPM can be used to modify potato starch with different particle sizes and expand their industrial applications, particularly as an ingredient for infant food, energy food, functional beverages, or special medical food. Similarly, Augustin et al. [
67] dealt with microfluidization of resistant starch. While most resistant starches have physiological functionality, their physical functionality can be problematic because they lack the ability to hold moisture, thicken or form gels. Thus, this physical process offers an alternative route to the traditional chemical methods for modifying the functional properties of food ingredients.
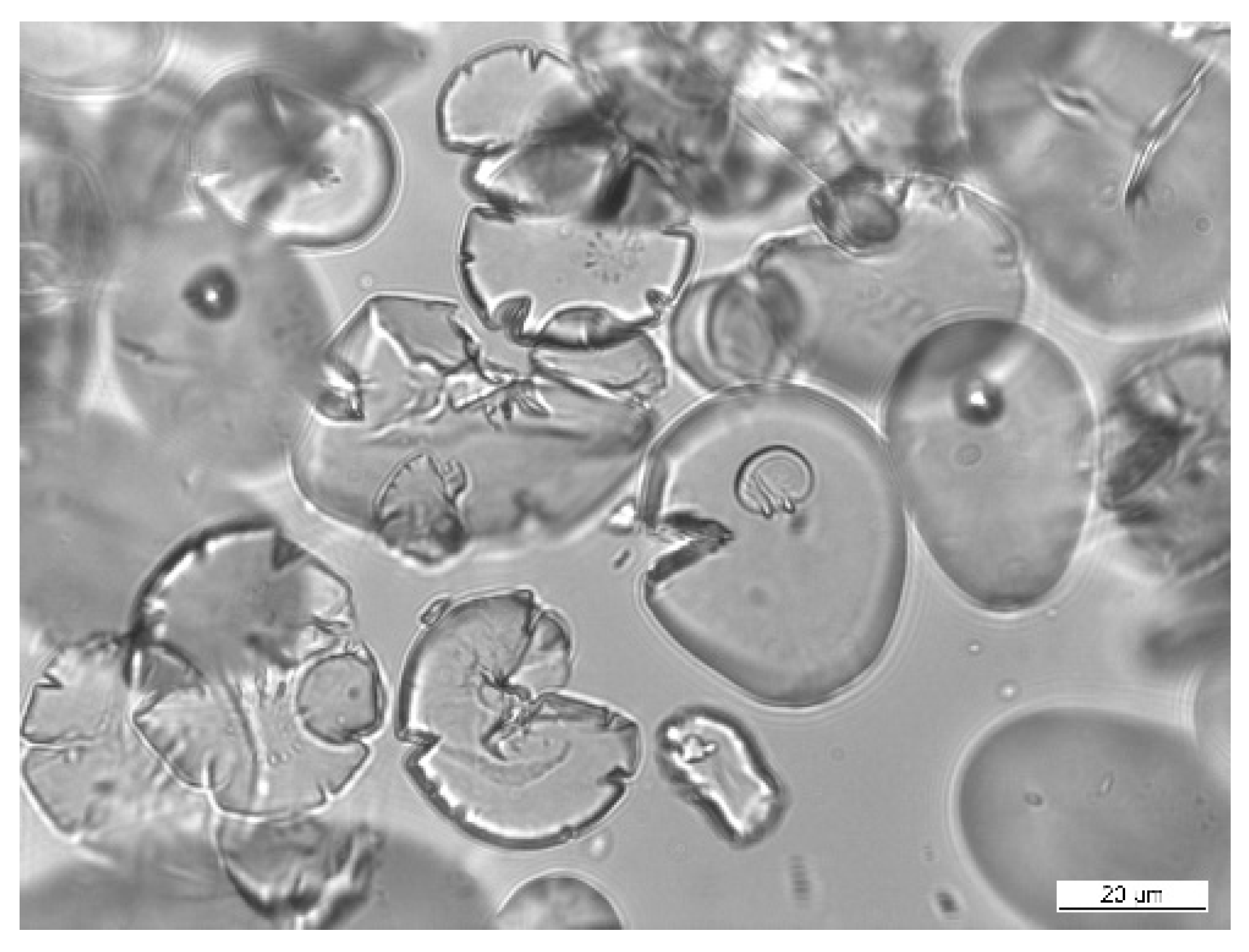
Šárka et al. [
68] used the bead mill DYNO®-MILL type Research Lab (WAB Muttenz, Switzerland) to disintegrate potato starch granules in suspension with isopropanol. The granule shapes were changed to have radial pores on the surface (
Figure 2).
This starch damage is similar to that known for flour grinding. The crystallinity of starch was changed and the ratio of amorphous phase after damage was much higher. Later we also tested the annealing of these particles at 0-4 °C [
69]. The crystallinity improved and was between the native and ultrafine ground starch (
Figure 3). The temperature of gelatinization was lower and gelatinization enthalpy was similar to that of native potato starch.
Many porous foods, such as puffed rice and wheat, corn flakes, and corn chips, produce high glucose and insulin responses [
70]. White wheat bread has a very porous structure, because gluten has the ability to expand during proofing and to retain gases. Saliva easily penetrates through the pores inside the pieces and hydrolysis of starch begins in the mouth. Microstructural studies have shown that after mastification the pieces of white wheat bread are easily broken down by pepsin under conditions mimicking the stomach [
19].
7. Microorganisms in Large Intestine
The gut microbiota is determined by a bacterial ecosystem. It is estimated that each individual houses at least 160 such species from a consortium of 1000 to 1150 prevalent bacterial species. Among these bacteria, 90% of the bacterial phylotypes are members of two phyla—
Bacteroidetes and
Firmicutes, followed by
Actinobacteria and
Proteobacteria. The majority of bacteria in the adult gut are nonsporing anaerobes, the most numerically predominant of which include
Bacteroides spp. and
Bifidobacterium spp.,
Eubacterium spp.,
Clostridium spp.,
Lactobacillus spp.,
Fusobacterium spp., and various Gram-positive cocci. Bacteria that are present in lower numbers include
Enterococcus spp.,
Enterobacteriaceae, methanogens, and dissimilatory sulfate-reducing bacteria. The composition varies according to each individual [
71].
Consuming resistant starches has been shown to enrich specific bacterial groups (
Bifidobacterium adolescentis, Ruminococcus bromii, and
Eubacterium rectale) in some people [
72].
Ruminococcus bromii is a dominant member of the human gut microbiota that plays a key role in releasing energy from dietary starches that escape digestion by host enzymes via its exceptional activity against particulate-resistant starches. Besides, four of the major extracellular starch-degrading enzymes in
R. bromii are attached to the cell surface and/or assembled into complexes via cohesin-dockerin interactions. This provides the first example of the involvement of dockerin and cohesins, best known from their roles in the cellulosomes that are responsible for lignocellulose breakdown, in a starch-degrading enzyme system, and therefore it is referred to as the complexes formed as amylosomes [
73].
In uncooked buckwheat, resistant starch represents approximately one-third of the total starch content. After cooking, the proportion of resistant starch is about 7-10% in heat-treated buckwheat products [
20]. Resistant starch derived from buckwheat has been shown to lower cholesterol concentrations, alleviate obesity, and reduce serum lipopolysaccharide, tumor necrosis factor-α and interleukin 6 concentrations, which are attributed to an increase in the abundance of
Bifidobacterium,
Lactobacillus, and
Enterococcus [
1].
According to Wu et al. [
74] the administration of RS3 for 13 weeks has been shown to improve obesity-induced inflammation, enhance intestinal immunity, improve lipid metabolism disorders, and increase the abundance of
Bifidobacterium and
Roseburia to improve the intestinal environment. However, the results of various studies showed that
Bacteroides, such as
Bifidobacterium and
Roseburia are decreased in type 2 diabetes. Interest in
Roseburia spp. has increased with reports showing that the abundance of these bacteria is also reduced in individuals affected by inflammatory diseases and colorectal cancers. it was found that
R. intestinalis supernatant induced a significant increase in peptide tyrosine tyrosine (PYY) and compared with
B. fragilis,
R. intestinalis produced more butyrate whereas
B. fragilis secreted more propionate and acetate [
75].
According to Martinez et al. [
76] resistant starch from potatoes has markedly increased the relative abundance of
Clostridium chartatabidum and particularly butyrate content, in young adults.
8. Short Chain Fatty Acids
The fermentation of non-digestible substrates like dietary fibers (incl. resistant starch) and endogenous intestinal mucus supports the growth of specialist microbes that produce short chain fatty acids (SCFAs) and gases, causing the lowering of the pH and inhibiting pathogens [
77].
Bacteroides spp., which constitute up to 30% of colonic microbiota, produce a variety of SCFAs [
75]. SCFAs are vital metabolites primarily synthesized by anaerobic bacteria in the colon through the process of fermenting dietary fiber, and the type and concentration of SCFAs in the colon vary depending on the specific food consumed and the composition of the gut microbiome [
1].
SCFAS are acetic, propionic, and butyric acids. Acetate - the most abundant SCFAs and an essential metabolite for the growth of some bacteria - reaches the peripheral tissues where it is used in cholesterol metabolism and lipogenesis [
72]. It acts also as an energy source for the brain, heart, and peripheral tissues and suppresses appetite through central homeostatic mechanisms [
78]. Propionate is a known precursor for hepatic glucose production, which has been reported to suppress feeding behavior in ruminant studies through the stimulation of hepatic vagal afferents [
79]. Higher production of SCFAs correlates with lower diet-induced obesity and with reduced insulin resistance. Gut microbial enzymes contribute to bile acid metabolism, generating unconjugated and secondary bile acids that act as signaling molecules and metabolic regulators to influence important host pathways [
72]. The binding of bile salts to dietary fibers in the small intestine can reduce blood cholesterol levels since they are excreted in the feces, so the body has to utilize endogenous cholesterol to synthesize more bile salts [
22].
Bifidobacteria, Lactobacillus, Clostridium, and
Ruminococcus are conducive to the production of secondary bile acids [
1]. Increased concentrations of propionate in the portal vein would be taken up by the liver and stimulate hepatic gluconeogenesis. An increased hepatic energy status can modulate feeding behavior via the stimulation of hepatic vagal nerve afferents. Increasing acetate and propionate in the peripheral circulation stimulates leptin release from adipocytes via activation of FFAR2. Leptin inhibits some neurons. Acetate can cross the blood-brain barrier and increase POMC and reduce AgRP expression (see chap. 9) [
79].
Butyrate and propionate seem to control gut hormones and reduce appetite and food intake in mice [
72]. Butyric acid is preferred as the energy source by the mucosal cells in the colon, and has been shown to suppress the growth and doubling time of neoplastic cells. Propionates are discussed for their effect on the metabolism of carbohydrates and cholesterol [
9]. In addition, propionate and butyrate can decrease obesity and improve insulin sensitivity [
78]. It further increases fecal bulk, assists glucose tolerance control in diabetes, and causes lower blood lipid levels. Therefore, RS is suitable to add it to food [
37]. According to Du et al. [
80] and Yuan et al. [
81] native beans, winter squash, and pumpkin starches are the RS sources in formulations with desired fiber-like benefits like a lower digestibility. Butyrate has received particular attention for its beneficial effects on both cellular energy metabolism and intestinal homeostasis [
75].
A typical signature of short bowel syndrome patients comprises high saturated aldehydes and medium-chain fatty acids and reduced SCFAs content [
82]. SCFAs concentrations are also decreased in the presence of pathogenic bacteria such as those from
Enterobacteriaceae family including
Salmonella spp., which are often increased in type 2 diabetes [
75].
A high concentration of SCFAs does not in itself mean that it is a quality digestive process in the large intestine. E.g. some findings show that SCFAs can serve as an underlying prognostic marker and treatment target for stroke, which is the second leading contributor to death across the world. On the other side, alterations in intestinal flora after stroke induce major pro-inflammatory T-cell responses, hence exacerbating immune responses and augmenting neuronal apoptosis [
83]. A recent study indicated that patients with cerebral ischemic stroke had more SCFAs producers including
Odoribacter,
Akkermansia,
Ruminococcaceae_UCG_005 and
Victivallis [
84]. Enhanced dysregulated intestinal flora is associated with neurological deficits, neuroinflammation, and more serious stroke consequences. There is a two-way communication network between gut microbiota and the brain, which is comprised of intestinal flora and its metabolites, the autonomic nervous system and enteric nervous system, sympathetic and parasympathetic nerve branches, the neuroimmune system, the neuroendocrine system, and the central nervous system. Through this network, intestinal flora can influence the physiological, behavioral, and cognitive functions of the host and function in basic neurogenic processes like blood-brain barrier formation, myelinogenesis, neurogenesis, and microglia maturity [
83].
The specific products of the gut microbiota have been implicated directly in human health outcomes. Examples include trimethylamine and indolepropionic acid. Indolepropionic acid is highly correlated with dietary fiber intake and has potent radical scavenging activity
in vitro, which seems to reduce the risk of incidence of type 2 diabetes [
72].
Most dietary fiber fermentation initiates in the proximal colon and terminates in the transverse colon, resulting in a limited number of metabolites that reach the distal colon, as evidenced by the decreasing concentrations of SCFAs, lactate, and succinate in the colon from proximal to distal regions [
1].
A gut microbiome-derived metabolite has been shown to have a multitude of properties to prevent ageing-linked physiological decline [
85]. Besides, the gut microbiome significantly influences the development of brain changes and cognitive functions due to its role in regulating the gut-brain axis. Thus, legume-based RS has neuroprotective potential for the aging generation by altering the gut microbiome, offering promising ways for improving brain/neurocognitive health in the aging population. Further study was recommended to investigate in depth the specific neurotransmitters and proteins involved in synaptic plasticity involved in memory regulation and to elucidate the mechanisms underlying RS- or fiber-mediated modulation of neurocognitive health through the diet-microbiome-brain axis [
86].
9. SCFAs as Signaling Molecules
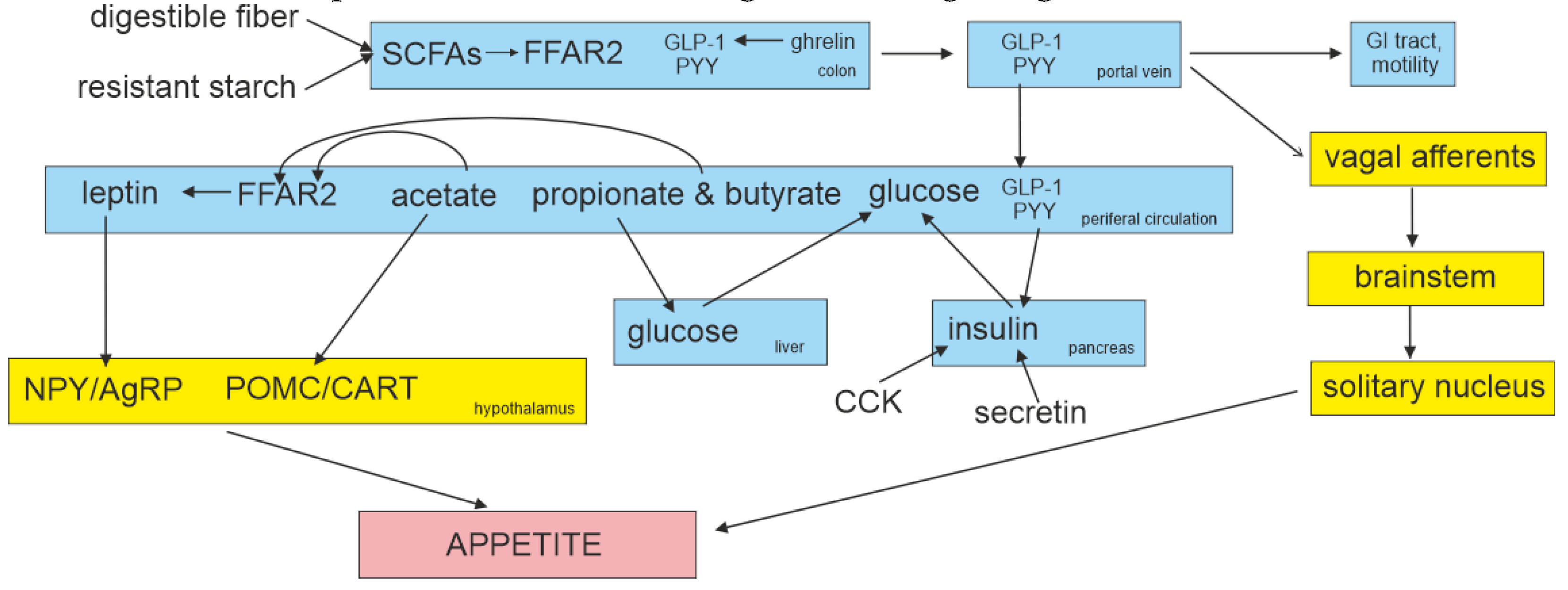
Emerging evidence suggests that gut microbiota can affect host energy homeostasis via various pathways, including the gut-brain axis (e.g., ghrelin, cholecystokinin, neuropeptide Y, agouti-related peptide, pro-opiomelanocortin (POMC), glucagon-like peptide-1 (GLP-1), peptide tyrosine tyrosine (PYY), and leptin), endocrine pathways (e.g., insulin-like growth factor, type 1), and microbial messengers (e.g., bile acids, SCFAs, trimethylamine N-oxide, and indoles) [
1]. Gastrointestinal hormones are peptide hormones secreted by endocrine cells, which are widely distributed throughout the mucosa of the gastrointestinal tract. These hormones regulate intestinal and pancreatic functions, by affecting secretion, motility, absorption, digestion, and cell proliferation [
87].
Secretion and degradation of hormones secreted by the gastrointestinal tract in response to food-derived signals [glucose-dependent insulinotropic polypeptide (GIP) and GLP-1], cholecystokinin (CCK), PYY, motilin, secretin, and gastrin. These hormones control alimentary secretions, which include the release of gastric juice, mucus, and digestive enzymes, and affect intestinal transport (gastric emptying and ileal brake) and also food intake through sensations of hunger, satiety, and appetite [
88]. Within the hypothalamic arcuate nucleus, one discrete group of neurons contains neuropeptide Y (NPY) and agouti-related peptide (AgRP), activation of which enhances food intake. POMC is the precursor of α-melanocyte stimulating hormone, which acts on melanocortin receptors to reduce food intake [
10]. Within the arcuate nucleus, peripheral PYY and GLP-1 increase the activity of the appetite-suppressing POMC/cocaine and amphetamine-regulated transcript (CART) neurons and inhibit appetite-stimulating NPY/AgRP neurons [
79].
Butyrate and other SCFAs are considered pivotal endogenous signaling molecules, acting through their free fatty-acid receptors (FFAR) 2 and 3, which are G-protein coupled receptors linked to heterotrimeric G-proteins attached to the cytoplasmic side of the receptor and stimulation of individual G-proteins trigger different cellular responses through the activation of specific secondary messenger cascades [
79]. Within the gut, the expression of these receptors has been localized to enteroendocrine cells (EEC), in particular, L-cells found at their highest density in the colonic epithelium (L-EEC). Ghrelin was identified to stimulate GLP-1 secretion in murine and human L-cell cultures. SCFAs, by binding to L-EEC FFAR2 and FFAR3, can trigger the secretion of glucagon-like peptide-1 (GLP-1) and peptide PYY [
89].
Both GLP-1 and PYY can stimulate satiation, inhibiting food intake, and attenuating glucose hyperglycemia acting either directly on the hypothalamic centers of appetite control or indirectly via the vagal-brainstem-hypothalamic pathway [
75]. SCFAs can by enhancing PYY and GLP-1 release also alleviate colonic inflammation by augmenting hypoxia-inducible factor-1 to promote the expression of protein interleukin-22 [
89]. PYY and GLP-1 have also been shown to inhibit the motility of the upper gastrointestinal (GI) tract. This slows the gastric emptying of ingested foods and prolongs the stimulation of mechanoreceptors and chemoreceptors in the GI tract that signal centrally via vagal afferents [
79].
Peptide tyrosine tyrosine (PYY) and glucagon-like peptide 1 (GLP-1) are released together following a meal to mediate postprandial satiety. PYY is a 36-amino acid peptide which belongs to the pancreatic polypeptide family [
75]. PYY is produced by the L cells of the gut, with the highest concentrations found in the large bowel and the rectum. Following secretion, the enzyme dipeptidyl peptidase-IV (DPP-IV) cleaves the first two amino acids at the N terminus of PYY
1-36 to generate PYY
3-36, a selective Y2 receptor agonist [
90]. Given that PYY
3-36 is released within 15 min of food intake, this must occur before ingested nutrients reach the distal small intestine and colon. Therefore, the initial post-prandial release of PYY
3-36 seems to be under neural control [
10]. In humans, ad libitum food intake at a buffet meal served 2 hours after the completion of a 90 min intravenous infusion of PYY
3-36 delivered at a dose of 0.8 pmol/kg/min, was reduced by 36% compared with an infusion of saline. The PYY
3-36 infusion generated plasma levels of PYY
3-36 similar to those achieved physiologically after a meal [
91]. Endogenous PYY may also be involved in long-term regulation of body weight by controlling energy expenditure and lipid metabolism [
92].
GLP-1 is an incretin hormone known to potentiate insulin secretion by the pancreas [
75], it is predominantly present in the distal small intestine and colon [
93]. It is released post-prandially into the circulation from the gastrointestinal in both pancreatic cells and colonic epithelium (L-EEC) in proportion to the calories ingested by posttranslational processing of the preproglucagon molecule into GLP-1
7-37 and GLP-1
7-36 amide, which are the biologically active forms of GLP-1 [
94]. As with PYY, a peak occurs before nutrients enter the distal gut and has been shown to be augmented by a high-carbohydrate meal [
10]. In lean humans, infusion of GLP-1
7-36 amide led to a 13.2% reduction in energy intake at the ad libitum buffet meal compared with saline infusion. The reduction in ad libitum energy intake for overweight/obese subjects by GLP-1
7-36 amide was comparable (10.5%) with that of lean subjects [
95]. A summary of the interactions of GLP-1 with the brainstem, hypothalamus and higher brain centers is shown in
Figure 4.
This gut hormone signaling processes can be targeted therapeutically [
93]; GLP-1 receptor agonists -specifically: the active substances exenatide (brand name Bydurenon or Byetta), lixisenatide (Lyxumia), semaglutide (Ozempic, Rybelsus, Wegovy), liraglutide (Saxenda, Victoza), duraglutide (Trulicity) and then insulin in combination with liraglutide (Xulthophy) - are used in the management of type 2 diabetes mellitus and obesity. Most of them are given by injection. They work by increasing insulin release from the pancreas and decreasing excessive glucagon release.
The ability of leptin to directly stimulate central GLP-1 secretion was confirmed [
98]. Leptin communicates to the brain regarding the nutritional status of the body. When leptin is introduced subcutaneously, it is not particularly effective in obese patients unless their serum leptin concentrations reach levels 20- to 30-fold higher than normal. This poor response has been attributed to the inefficient transport of leptin across the blood-brain barrier. To overcome this problem, a scheme for the intranasal delivery of leptin has been devised. The nasal mucosa is highly vascularized, so delivery of a thin layer of medication across its broad surface area can result in rapid absorption of the medication into the bloodstream [
96].
CCK, produced by I cells in the duodenum and the jejunum [
13], stimulates pancreatic enzyme secretion and promotes gallbladder emptying by stimulating efferent vagal neurotransmitter release in the gallbladder ganglia. Secretin, which is produced in a variety of mature enteroendocrine cells (EEC), stimulates pancreatic alkaline electrolyte secretion and has also been implicated in postprandial thermogenesis [
93].
Potato-resistant starch has been found to alleviate liver steatosis, increase the concentrations of peptide tyrosine tyrosine (PYY), hormone glucagon-like peptide-1 (GLP-1), propionic acid, and acetic acid, and increase the expression levels of tight junction proteins by changing the composition of the gut microbiome, such as increasing the relative abundance of
Bifidobacterium,
Ruminococcus,
Bacteroides, and
Coprococcus [
22,
97].
Gastric bypass surgery is one of the most effective treatments for obese patients in which sustained weight loss results from diminished appetite (leading to a sustained weight loss of approximately 30%). Notably, gastric bypass or sleeve gastrectomy surgery patients demonstrate increased levels of PYY and GLP-1, especially post-prandially. Significantly, inhibiting the PYY and GLP-1 responses results in the return of appetite and increases food intake [
98].
10. Role of Dietetic Fiber, Gluten and Phenolics
Diets high in resistant starch or in non-starch polysaccharide fiber (wheat bran) result in the strong and reproducible enrichment of different bacterial species in the human gut [
99]. A sufficient intake of DF decreases mortality and provides several health benefits to humans, including reducing the risk of cardiovascular disease, coronary heart disease, stroke, hypertension, colorectal cancer, and type 2 diabetes [
22,
100]. The researchers reported that the greatest reduction in risk from chronic disease could be achieved by consuming around 25-29 g of dietary fiber a day, which is much higher than the levels consumed by the majority of people, in both developed and developing countries [
22]. Also, RS used as DF, reduces calorie intake (low glycemic index), prevents fat deposition and colon cancer, and enhances mineral absorption under the influence of lower pH [
101]. However, some studies also indicate that major increases in dietary fiber can temporarily reduce the diversity of microbes, as those that digest fiber become specifically enriched, leading to a change in composition and, through competitive interactions, reduced diversity [
72]. On the other side, according to Bergeron et al. [
102] a diet high in resistant starches may increase serum trimethylamine N-oxide, carnitine, betaine, and γ-butyrobetaine concentrations compared to a diet low in resistant starches, suggesting a diet rich in resistant starches does not decrease the markers of cardiovascular disease.
Wheat gluten is an elastic and cohesive protein. It is the major factor affecting the amount of digested starch. It is known that starch encapsulated in a gluten matrix or network could lead to a low digestion rate and extent [
103]. Zeng et al. [
18] found that the addition of wheat gluten (but also of oat or barley protein) and/or β-glucan reduced starch digestion to a certain extent, and their synergistic effect was greater. Similarly, sorghum starch digestion is hindered by a complex protein network called prolamin, known to reduce enzymatic starch breakdown [
104].
However, this action of gluten can be reduced by a contemporary action of some polyphenols, e.g. (-)-epigallocatechin-3-gallate (EGCG). On the other hand, EGCG significantly reduces the digestion extent of wheat starch by 25-30% in the absence of gluten. Both gluten and starch can bind with EGCG in the ternary blends via hydrogen bonding and/or hydrophobic interaction, and decrease the amount of unbound EGCG with competitive inhibitory characteristics in the system [
105].
Animal and in vitro studies indicate that gluten-free bread reduces the microbiota dysbiosis seen in people with gluten sensitivity or coeliac disease. However, a recent large observational study showed, that most people who avoid gluten and have no coeliac disease or proven intolerance, have an increased risk of heart disease, potentially because of the reduced consumption of whole grains [
72]. Bonder et al. [
106] showed that 21 healthy people had substantially different gut microbiota profiles after four weeks on a gluten-free diet. Most people showed a lower abundance of several key beneficial microbe species.
A variety of phenolic compounds have been shown to inhibit the activities of α-amylase and/or α-glucosidase. Acarbose is the most extensively studied and most widely used α-glucosidase inhibitor. The most common adverse side effects of acarbose are gastrointestinal disturbances, like flatulence, diarrhea, and abdominal distension [
13]. The inhibitory phenolic compounds include flavonoids (flavanones, anthocyanins, flavanols, isoflavones, and flavones), phenolic acids, and tannins (proanthocyanidins and ellagitannins) [
107]. Some other in vitro study [
108] has shown that also nanocrystalline cellulose can inhibit the activity of α-amylase and α-glucosidase in model foods containing cooked potato starch and protein.
Inhibition of α-amylase activity to limit the rise in postprandial glucose is one of the most notable effects of dietary plant polyphenols. The rate and extent of inhibition depend on many factors, including the structure of polyphenols and the substrate used [
109]. Phenolic compounds are postulated to bind to active or secondary sites of digestive enzymes [
107] and/or bind to substrate thus reducing starch hydrolysis. They also regulate the composition of gut microbiota and slow down the fermentation of resistant starch
in vitro. On the other hand, a simultaneous supplement of resistant starch and tannic acid could promote the later production of acetate and butyrate and the enrichment of beneficial bacteria. Co-supplement of resistant starch and polyphenols may deliver resistant starch to the distal colon
in vivo, which is beneficial to keep the distal colon healthy and will be investigated [
110]. According to Lachowicz et al. [
111] the flowers and leaves of
Sanguisorba officinalis L. are a good source of polyphenols, including hydrolyzable tannins, phenolic acids, flavonols, and anthocyanins, and exhibit a significant antiradical and reducing potential. In turn, the roots and stalks are a valuable source of flavan-3-ols. The most effective inhibition of α-amylase, α-glucosidase, and pancreatic lipase and antiproliferative activities, reflected in the inhibition of viability of pancreatic ductal adenocarcinoma, colorectal adenocarcinoma, and bladder cancer as well as T-cell leukemia cell, was shown by the flowers and leaves of
S. officinalis L.
To elucidate mechanisms of interactions between a polyphenol and the enzyme, Le et al. [
109] compared the inhibition of porcine pancreatic α-amylase (PPA) by three differently structured polyphenols: p-coumaric acid (p-CA), quercetin (QUER), and cyanidin-3-glucoside (C3G), using solid corn starch as a substrate, which limits the formation of starch-polyphenol complexes due to the compact structure of starch granules. QUER exhibited the strongest inhibitory effect, followed by C3G, and p-CA. The corresponding inhibition modes were determined to be mixed, mixed, and competitive, respectively.
Kan et al. [
112] proved that the presence of gluten had little influence on the inhibitory efficacy of monomeric polyphenols on starch digestibility of bread, but reduced the inhibitory efficacy of polymeric polyphenols on starch digestibility. Co-digestion of bread with both black and green tea polyphenols significantly reduced the kinetic rate and extent of starch digestion. Green tea extract caused a similar reduction in the starch digestibility of both wheat bread and gluten-free bread but black tea extract caused a larger reduction in the starch digestibility of gluten-free bread than in the starch digestibility of wheat bread.
Mung bean husk polyphenols facilitated the synthesis of SCFASs in the colon [
78].
11. Conclusions
The cause of obesity is an imbalance of energy intake and expenditure. The solution is either an increase in energy expenditure, e.g. through sports or other physical movement, and/or a reduction in energy intake. Starch is an important source of energy in food. Its digestibility depends on many factors. The structural arrangement is mainly influenced by heat treatment (gelatinization), by chemical modification, or the formation of amylose-lipid complexes. The texture of food also plays a role. These effects on digestibility can be determined analytically during the simulation of the activity of the digestive tract. In these methods, digestive enzymes are strictly dosed and their effect is limited to a defined time. The methods are very useful for classifying food in terms of digestibility in the intestine into quickly digestible, slowly digestible, and resistant starch. However, they do not cover digestion in other organs such as the oral cavity or stomach. In addition, digestion in the real intestine in many cases does not proceed exactly according to the proposed scheme.
The digestibility of starch is influenced by the rate of transport in the intestine. Some substances slow down the passage through the intestine (e.g. soluble fiber or gluten); the slowing down is caused by an increase in the viscosity of boluses due to macromolecular substances, which, on the other hand, slow down absorption through the wall of the small intestine. Fats also slow down transport in the intestine.
Among the substances that reduce the digestibility of starch is fiber, which accelerates transport in the intestine due to more intense peristaltic movement. Polyphenols bind to active or secondary sites of digestive enzymes and/or bind to substrate thus reducing starch hydrolysis. It is therefore not recommended for healthy individuals to consume regular gluten-free foods in which the proportion of the grain's coating layers is practically zero.
Resistant starch or dietary fiber enriches specific bacterial groups in the large intestine which produce short chain fatty acids (SCFAs). These substances generally have a positive influence on the health of the large intestine, they affect the feeling of satiety and also influence the aging of the human body. SCFAs are considered endogenous signaling molecules, acting through their free fatty-acid receptors 2 and 3 which can trigger the secretion of glucagon-like peptide-1 and peptide PYY, being released together following a meal to mediate postprandial satiety.
For short bowel syndrome patients, or when the action of the gastric enzymes is not sufficient, a larger part of the starch entering the small intestine goes to the large intestine, where it increases the level of SCFAs. But cerebral ischemic stroke can also cause higher levels of SCFAs.
The reduction of overweight consists of increasing the physical activity of a human, reducing the consumption of energy-intensive foods containing quickly digestible starch, and strengthening the fiber in the diet, which also includes resistant starch. In some cases, it is necessary to apply medical treatment, e.g. surgical reduction of the stomach volume, microbiome transplantation, or the application of digestive hormones such as PYY and GLP-1. GLP-1 is also used in the management of type 2 diabetes mellitus. Stomach reduction refers not only to its volume but also to increased levels of PYY and GLP-1. However, always the dietary cooperation of the patient is necessary in all the mentioned procedures.