Submitted:
30 April 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
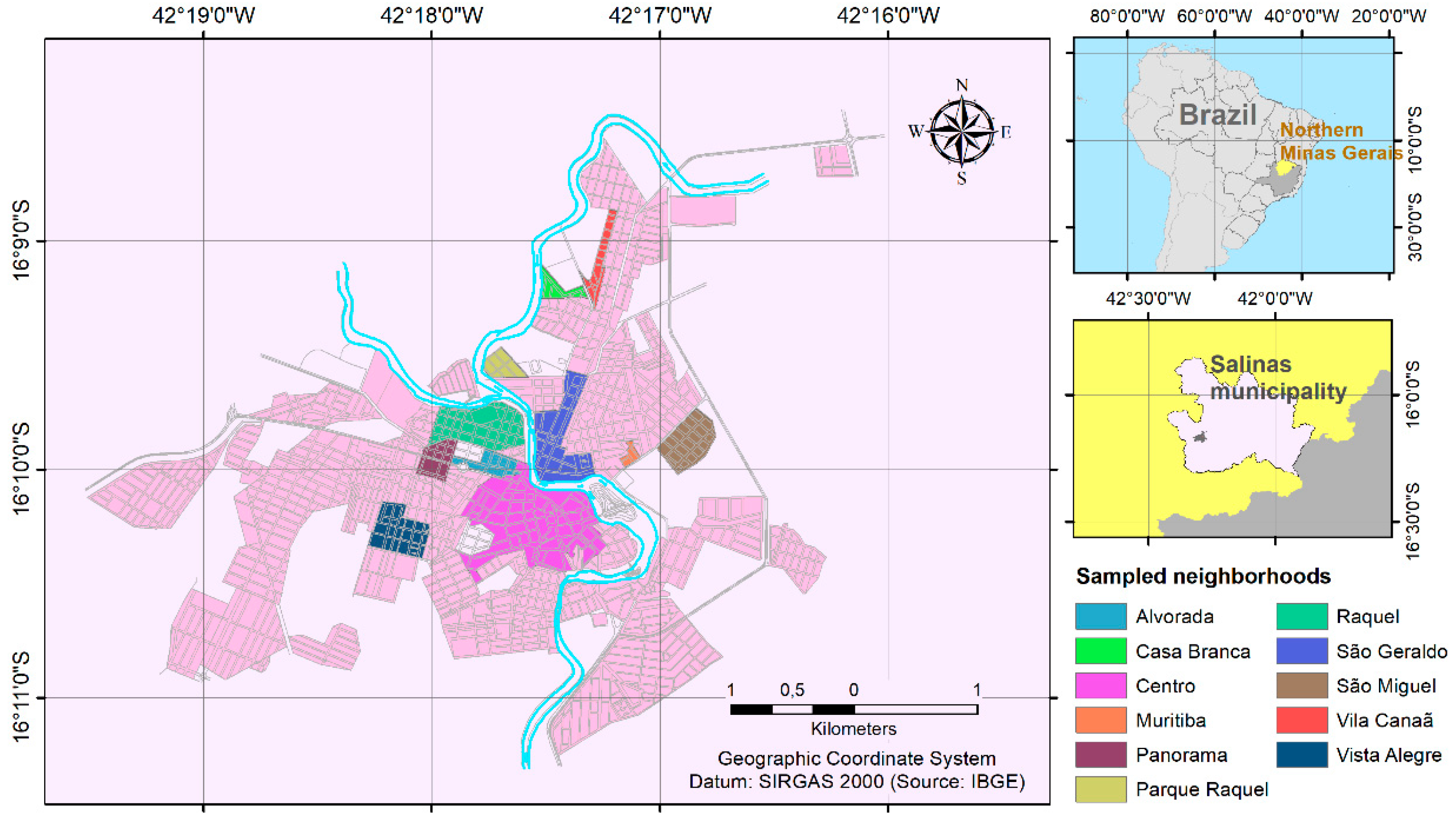
2.1. Study Area
2.2. Mosquito Collection and Rearing Following Instagram Posts
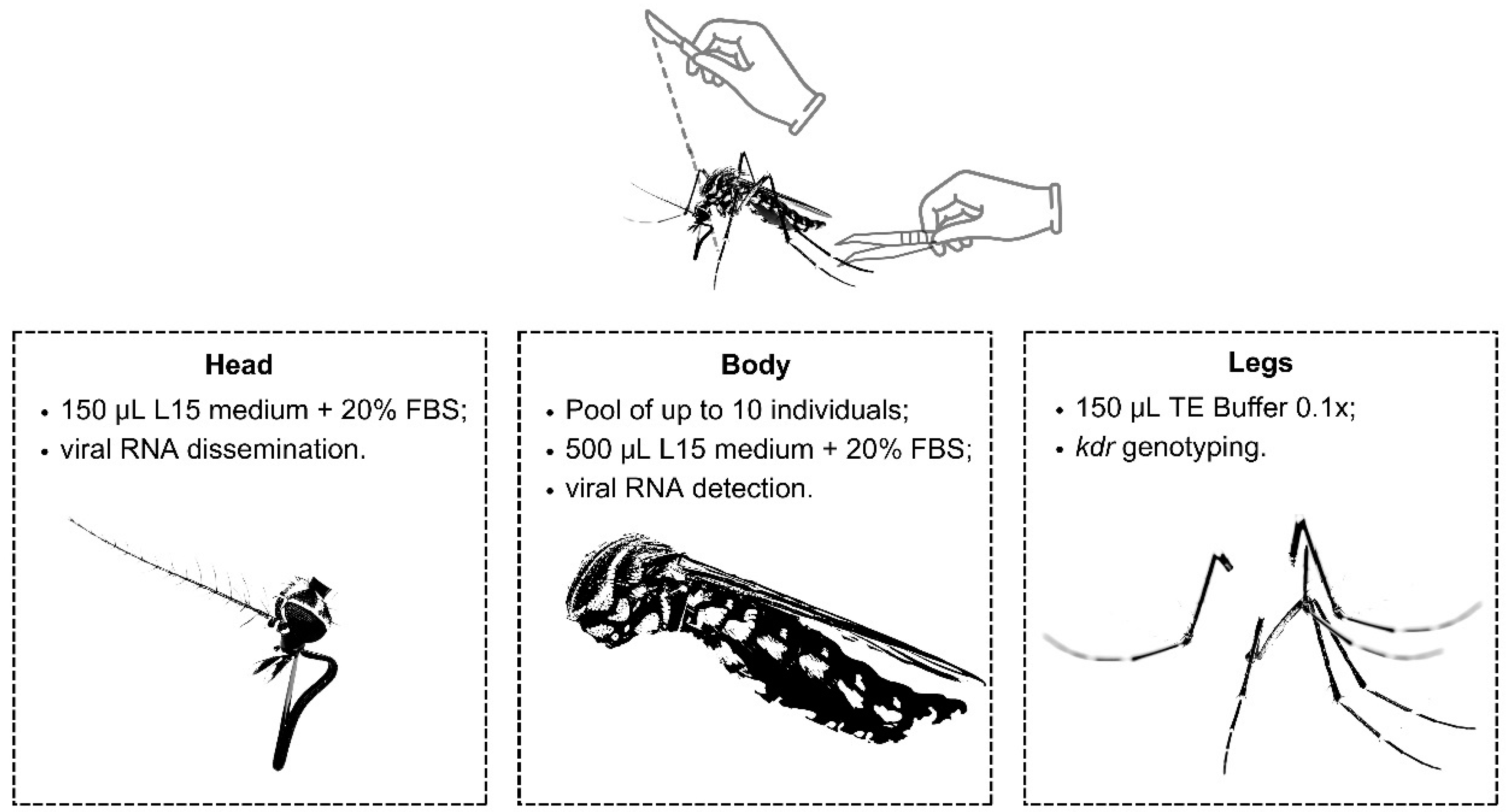
2.3. Taxonomic Identification and CHIKV Molecular Diagnosis in Captured Mosquitoes
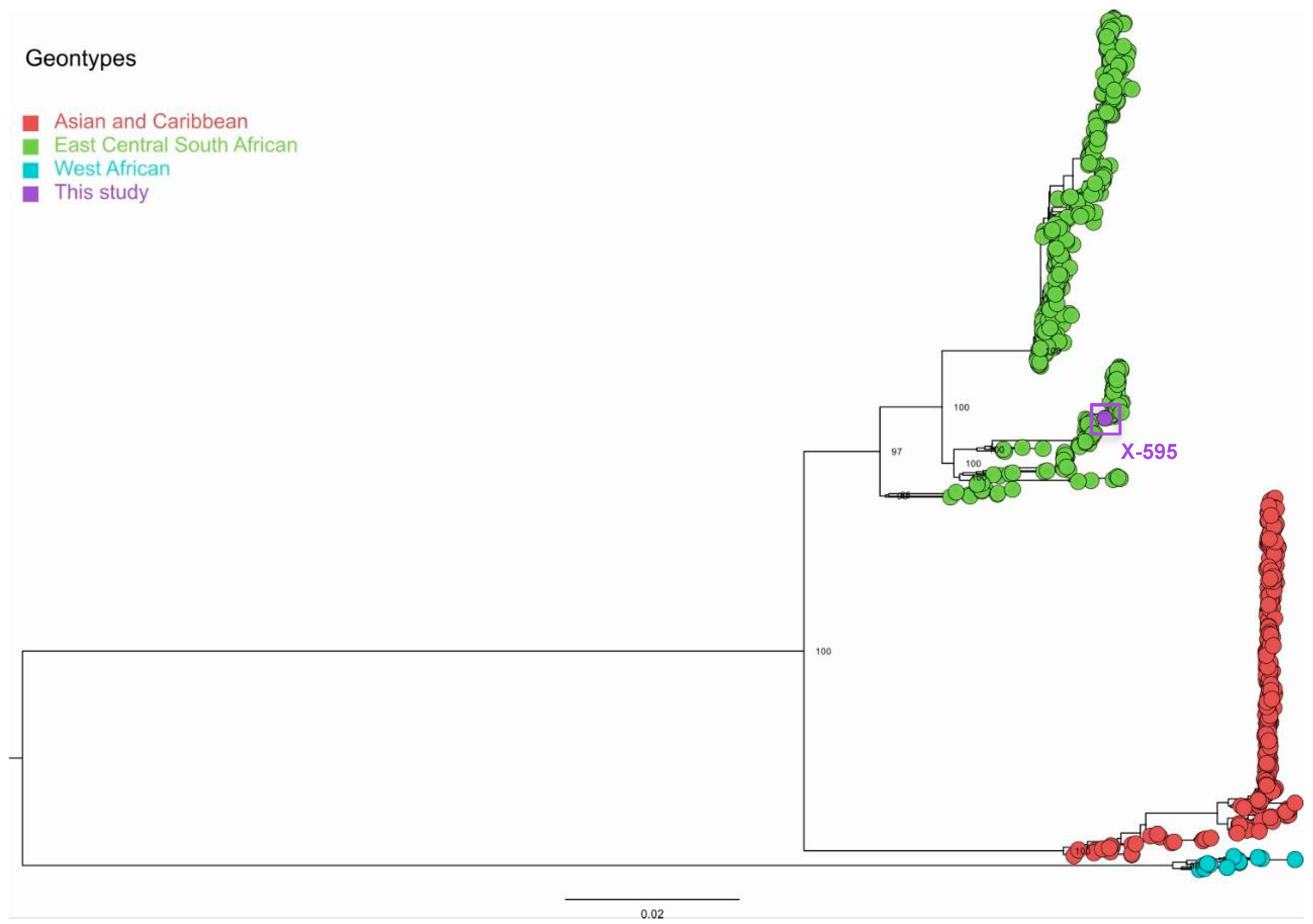
2.4. CHIKV Genome Sequencing and Phylogenomic Analyses
2.5. Analysis of kdr as a Molecular Marker for Pyrethroid Resistance
2.6. Ethical Statements
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, M.C. An Epidemic of Virus Disease in Southern Province, Tanganyika Territory, in 1952–1953. Trans R Soc Trop Med Hyg 1955, 49, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, G.; Gaüzère, B.-A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an Epidemic Arbovirosis. Lancet Infect Dis 2007, 7, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E. Reemergence of Chikungunya Virus. J Virol 2014, 88, 11644–11647. [Google Scholar] [CrossRef] [PubMed]
- Leparc-Goffart, I.; Nougairede, A.; Cassadou, S.; Prat, C.; de Lamballerie, X. Chikungunya in the Americas. Lancet 2014, 383, 514. [Google Scholar] [CrossRef] [PubMed]
- Costa-da-Silva, A.L.; Ioshino, R.S.; Petersen, V.; Lima, A.F.; Cunha, M. dos P.; Wiley, M.R.; Ladner, J.T.; Prieto, K.; Palacios, G.; Costa, D.D.; et al. First Report of Naturally Infected Aedes Aegypti with Chikungunya Virus Genotype ECSA in the Americas. PLoS Negl Trop Dis 2017, 11, e0005630. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Cruz, A.C.; Pinto Nunes Neto, J.; Patroca da Silva, S.; Vieira Pinto da Silva, E.; Juscely Galvão Pereira, G.; Maia Santos, M.; Antônio de Oliveira Monteiro, H.; Barreto dos Santos, F.; José de Paula Souza e Guimarães, R.; Fortes Aragão, C.; et al. Chikungunya Virus Detection in Aedes Aegypti and Culex Quinquefasciatus during an Outbreak in the Amazon Region. Viruses 2020, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.-S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya Isolates from the Outbreak of La Reunion (Indian Ocean) Exhibit Different Patterns of Infection in the Mosquito, Aedes Albopictus. PLoS One 2007, 2, e1168. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rua, A.; Zouache, K.; Girod, R.; Failloux, A.-B.; Lourenco-de-Oliveira, R. High Level of Vector Competence of Aedes Aegypti and Aedes Albopictus from Ten American Countries as a Crucial Factor in the Spread of Chikungunya Virus. J Virol 2014, 88, 6294–6306. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Wiggins, K.; Câmara, D.C.P.; Eastmond, B.; Alto, B.W. Chikungunya Virus Vector Competency of Brazilian and Florida Mosquito Vectors. PLoS Negl Trop Dis 2018, 12, e0006521. [Google Scholar] [CrossRef]
- Carvalho, R.G.; Lourenço-de-Oliveira, R.; Braga, I.A. Updating the Geographical Distribution and Frequency of Aedes Albopictus in Brazil with Remarks Regarding Its Range in the Americas. Mem Inst Oswaldo Cruz 2014, 109, 787–796. [Google Scholar] [CrossRef]
- Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lima-Camara, T.N. The Asian Tiger Mosquito in Brazil: Observations on Biology and Ecological Interactions since Its First Detection in 1986. Acta Trop 2020, 205, 105386. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R. da C.; Cardoso, A. da S.; Souza, J.L. de; Pereira, E. da S.; Amorim, M.F. de; Souza, M.S.M. de; Medeiros, C. de L.; Monteiro, M.F.M.; Meneguetti, D.U. de O.; Paula, M.B. de; et al. First Official Record of Aedes (Stegomyia) Albopictus (Diptera: Culicidae) in the Acre State, Northern Brazil. Rev Inst Med Trop Sao Paulo 2023, 65. [Google Scholar] [CrossRef]
- da Silva Ferreira, R.; de Toni Aquino da Cruz, L.C.; de Souza, V.J.; da Silva Neves, N.A.; de Souza, V.C.; Filho, L.C.F.; da Silva Lemos, P.; de Lima, C.P.S.; Naveca, F.G.; Atanaka, M.; et al. Insect-Specific Viruses and Arboviruses in Adult Male Culicids from Midwestern Brazil. Infection, Genetics and Evolution 2020, 85, 104561. [Google Scholar] [CrossRef] [PubMed]
- da Silva Neves, N.A.; da Silva Ferreira, R.; Morais, D.O.; Pavon, J.A.R.; de Pinho, J.B.; Slhessarenko, R.D. Chikungunya, Zika, Mayaro, and Equine Encephalitis Virus Detection in Adult Culicinae from South Central Mato Grosso, Brazil, during the Rainy Season of 2018. Brazilian Journal of Microbiology 2022, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Mulwa, F.; Mutisya, J.; Koskei, E.; Langat, S.; Nyunja, A.; Koka, H.; Konongoi, S.; Chepkorir, E.; Ofula, V.; et al. Probable Contribution of Culex Quinquefasciatus Mosquitoes to the Circulation of Chikungunya Virus during an Outbreak in Mombasa County, Kenya, 2017–2018. Parasit Vectors 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Smith, G.A.; Mackenzie, J.S. Vector Competence of Australian Mosquitoes for Chikungunya Virus. Vector-Borne and Zoonotic Diseases 2010, 10, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Brasil; Ministério da Saúde; Brasil. Ministério da Saúde. Secretaria de VigDepartamento de Articulação Estratégica de Vigilância em Saúde Guia de Vigilância Em Saúde Available online:. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_5ed_rev_atual.pdf (accessed on 21 April 2024).
- Valle, D.; Bellinato, D.F.; Viana-Medeiros, P.F.; Lima, J.B.P.; Martins Junior, A. de J. Resistance to Temephos and Deltamethrin in Aedes Aegypti from Brazil between 1985 and 2017. Mem Inst Oswaldo Cruz 2019, 114. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.B.; Martins, A.J.; Rodovalho, C. de M.; Bellinato, D.F.; Dias, L. dos S.; Macoris, M. de L. da G.; Andrighetti, M.T.M.; Lima, J.B.P.; Obara, M.T. Assessment of the Susceptibility Status of Aedes Aegypti (Diptera: Culicidae) Populations to Pyriproxyfen and Malathion in a Nation-Wide Monitoring of Insecticide Resistance Performed in Brazil from 2017 to 2018. Parasit Vectors 2020, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Macoris, M. de L.; Martins, A.J.; Andrighetti, M.T.M.; Lima, J.B.P.; Valle, D. Pyrethroid Resistance Persists after Ten Years without Usage against Aedes Aegypti in Governmental Campaigns: Lessons from São Paulo State, Brazil. PLoS Negl Trop Dis 2018, 12, e0006390. [Google Scholar] [CrossRef]
- Moyes, C.L.; Wiebe, A.; Gleave, K.; Trett, A.; Hancock, P.A.; Padonou, G.G.; Chouaïbou, M.S.; Sovi, A.; Abuelmaali, S.A.; Ochomo, E.; et al. Analysis-Ready Datasets for Insecticide Resistance Phenotype and Genotype Frequency in African Malaria Vectors. Sci Data 2019, 6, 121. [Google Scholar] [CrossRef]
- Melo Costa, M.; Campos, K.B.; Brito, L.P.; Roux, E.; Melo Rodovalho, C.; Bellinato, D.F.; Lima, J.B.P.; Martins, A.J. Kdr Genotyping in Aedes Aegypti from Brazil on a Nation-Wide Scale from 2017 to 2018. Sci Rep 2020, 10, 13267. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.S.; Lima, L.F.; Galardo, A.K.R.; Corbel, V.; Lima, J.B.P.; Martins, A.J. Genetic Structure and Kdr Mutations in Aedes Aegypti Populations along a Road Crossing the Amazon Forest in Amapá State, Brazil. Sci Rep 2023, 13, 17167. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.P.; Carrara, L.; Freitas, R.M. de; Lima, J.B.P.; Martins, A.J. Levels of Resistance to Pyrethroid among Distinct Kdr Alleles in Aedes Aegypti Laboratory Lines and Frequency of Kdr Alleles in 27 Natural Populations from Rio de Janeiro, Brazil. Biomed Res Int 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- SES-MG; Secretaria de Estado de Saúde de Minas Gerais Arboviroses Urbanas (Dengue, Chikungunya e Zika). Boletim Epidemiológico No 296 - Semana Epidemiológica 40/2023. Available online: https://www.saude.mg.gov.br/images/1_noticias/06_2023/1-out-nov-dez/09-10-BO_ARBO296.pdf (accessed on 21 April 2024).
- SES-MG; Secretaria de Estado de Saúde de Minas Gerais Painel de Monitoramento de Casos. Vigilância Das Arboviroses Em Minas Gerais: Chikungunya Available online:. Available online: https://www.saude.mg.gov.br/aedes/painel (accessed on 21 April 2024).
- IBGE; Instituto Brasileiro de Geografia e Estatística Áreas Urbanizadas Do Brasil 2019. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101973_informativo.pdf (accessed on 21 April 2024).
- Maia, L.J.; Oliveira, C.H. de; Silva, A.B.; Souza, P.A.A.; Müller, N.F.D.; Cardoso, J. da C.; Ribeiro, B.M.; Abreu, F.V.S. de; Campos, F.S. Arbovirus Surveillance in Mosquitoes: Historical Methods, Emerging Technologies, and Challenges Ahead. Exp Biol Med 2023, 248, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Bonney, R.; Cooper, C.B.; Dickinson, J.; Kelling, S.; Phillips, T.; Rosenberg, K. V.; Shirk, J. Citizen Science: A Developing Tool for Expanding Science Knowledge and Scientific Literacy. Bioscience 2009, 59, 977–984. [Google Scholar] [CrossRef]
- Scolforo, J.R. Mapeamento e Inventário Da Flora Nativa e Dos Reflorestamentos de Minas Gerais. ; Ed. UFLA, Ed. 2006. [Google Scholar]
- Koppen, W. Das Geographische System Der Klimat. Handbuch der Klimatologie 1936, 1–46. [Google Scholar] [CrossRef]
- SES-MG; Secretaria de Estado de Saúde de Minas Gerais Arboviroses Urbanas (Dengue, Chikungunya e Zika). Boletim Epidemiológico No 270 - Semana Epidemiológica 05/2023, 06 de Fevereiro. Available online: https://www.saude.mg.gov.br/images/1_noticias/06_2023/4-jan-fev-marc/BO_ARBO270.pdf (accessed on 21 April 2024).
- Nasci, R.S. A Lightweight Battery-Powered Aspirator for Collecting Resting Mosquitoes in the Field. Mosq News 1981, 41, 808–811. [Google Scholar]
- Dubrulle, M.; Mousson, L.; Moutailler, S.; Vazeille, M.; Failloux, A.-B. Chikungunya Virus and Aedes Mosquitoes: Saliva Is Infectious as Soon as Two Days after Oral Infection. PLoS One 2009, 4, e5895. [Google Scholar] [CrossRef] [PubMed]
- Consoli, R.A.G.B.; Oliveira, R.L. de Principais Mosquitos de Importância Sanitária No Brasil; Editora FIOCRUZ, 1994; ISBN 8575412906.
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia - Volume 2; Edusp, Ed.; 1a.; Edusp: São Paulo, 2002; ISBN 8531406994. [Google Scholar]
- de Oliveira, C.H.; Andrade, M.S.; Campos, F.S.; da C. Cardoso, J. ; Gonçalves-dos-Santos, M.E.; Oliveira, R.S.; Aquino-Teixeira, S.M.; Campos, A.A.; Almeida, M.A.; Simonini-Teixeira, D.; et al. Yellow Fever Virus Maintained by Sabethes Mosquitoes during the Dry Season in Cerrado, a Semiarid Region of Brazil, in 2021. Viruses 2023, 15, 757. [Google Scholar] [CrossRef]
- Pastorino, B.; Bessaud, M.; Grandadam, M.; Murri, S.; Tolou, H.J.; Peyrefitte, C.N. Development of a TaqMan® RT-PCR Assay without RNA Extraction Step for the Detection and Quantification of African Chikungunya Viruses. J Virol Methods 2005, 124, 65–71. [Google Scholar] [CrossRef]
- Quick, J.; Grubaugh, N.D.; Pullan, S.T.; Claro, I.M.; Smith, A.D.; Gangavarapu, K.; Oliveira, G.; Robles-Sikisaka, R.; Rogers, T.F.; Beutler, N.A.; et al. Multiplex PCR Method for MinION and Illumina Sequencing of Zika and Other Virus Genomes Directly from Clinical Samples. Nat Protoc 2017, 12, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.R.R.; Menezes, M.T. de; Salgado-Benvindo, C.; Whittaker, C.; Cox, V.; Chandradeva, N.; Paula, H.H.S. de; Martins, A.F.; Chagas, R.R. das; Brasil, R.D.V.; et al. Epidemiological and Genomic Investigation of Chikungunya Virus in Rio de Janeiro State, Brazil, between 2015 and 2018. PLoS Negl Trop Dis 2023, 17, e0011536. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.; Libin, P.J.K.; Theys, K.; Faria, N.R.; Nunes, M.R.T.; Restovic, M.I.; Freire, M.; Giovanetti, M.; Cuypers, L.; Nowé, A.; et al. A Computational Method for the Identification of Dengue, Zika and Chikungunya Virus Species and Genotypes. PLoS Negl Trop Dis 2019, 13, e0007231. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol Biol Evol 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.G.; Andrade, A.M.S.; Costa, M. da C.N.; Castro, J.S.M.; Oliveira, F.L.S.; Goes, C.S.B.; Maia, M.; Santana, E.B.; Nunes, B.T.D.; Vasconcelos, P.F.C. East/Central/South African Genotype Chikungunya Virus, Brazil, 2014. Emerg Infect Dis 2015, 21, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Souza, U.J.B. de; Santos, R.N. dos; Giovanetti, M.; Alcantara, L.C.J.; Galvão, J.D.; Cardoso, F.D.P.; Brito, F.C.S.; Franco, A.C.; Roehe, P.M.; Ribeiro, B.M.; et al. Genomic Epidemiology Reveals the Circulation of the Chikungunya Virus East/Central/South African Lineage in Tocantins State, North Brazil. Viruses 2022, 14, 2311. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.; Alcantara, L.C.J.; Fonseca, V.; Lima, M.; Castro, E.; Fritsch, H.; Oliveira, C.; Guimarães, N.; Adelino, T.; Evaristo, M.; et al. Increased Interregional Virus Exchange and Nucleotide Diversity Outline the Expansion of Chikungunya Virus in Brazil. Nat Commun 2023, 14, 4413. [Google Scholar] [CrossRef]
- Lessa-Aquino, C.; Trinta, K.S.; Pestana, C.P.; Ribeiro, M.O.; Sucupira, M.V.F.; Boia, M.N.; Baptista, P.A.; Cunha, R. V.; Medeiros, M.A. Detection of East/Central/South African Genotype Chikungunya Virus during an Outbreak in a Southeastern State of Brazil. Epidemiol Infect 2018, 146, 2056–2058. [Google Scholar] [CrossRef]
- Aragão, C.F.; Pinheiro, V.C.S.; Nunes Neto, J.P.; Silva, E.V.P. da; Pereira, G.J.G.; Nascimento, B.L.S. do; Castro, K. da S.; Maia, A.M.; Catete, C.P.; Martins, L.C.; et al. Natural Infection of Aedes Aegypti by Chikungunya and Dengue Type 2 Virus in a Transition Area of North-Northeast Brazil. Viruses 2019, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.F.; de Brito, B.B.; Correia, T.M.L.; Viana, A.I.S.; Carvalho, J.C.; da Silva, F.A.F.; Santos, M.L.C.; da Silveira, E.A.; Neto, H.P.G.; da Silva, N.M.P.; et al. Simultaneous Circulation of Zika, Dengue, and Chikungunya Viruses and Their Vertical Co-Transmission among Aedes Aegypti. Acta Trop 2021, 215, 105819. [Google Scholar] [CrossRef] [PubMed]
- Aragão, C.F.; Cruz, A.C.R.; Nunes Neto, J.P.; Monteiro, H.A. de O.; da Silva, E.V.P.; da Silva, S.P.; Andrade, A.T. dos S.; Tadei, W.P.; Pinheiro, V.C.S. Circulation of Chikungunya Virus in Aedes Aegypti in Maranhão, Northeast Brazil. Acta Trop 2018, 186, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.J.C.; Mourão, F.R.P.; Ribeiro, E.S.D.; Rêgo, M.O. da S.; Frances, P.A. da C.; Souto, R.N.P.; Façanha, M. dos S.; Tahmasebi, R.; Costa, A.C. da Prevalence of Dengue, Zika and Chikungunya Viruses in Aedes (Stegomyia) Aegypti (Diptera: Culicidae) in a Medium-Sized City, Amazon, Brazil. Rev Inst Med Trop Sao Paulo 2020, 62. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Wiggins, K.; Eastmond, B.; Velez, D.; Lounibos, L.P.; Lord, C.C. Transmission Risk of Two Chikungunya Lineages by Invasive Mosquito Vectors from Florida and the Dominican Republic. PLoS Negl Trop Dis 2017, 11, e0005724. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-de-Brito, A.; Ribeiro, I.P.; Miranda, R.M. de; Fernandes, R.S.; Campos, S.S.; Silva, K.A.B. da; Castro, M.G. de; Bonaldo, M.C.; Brasil, P.; Lourenço-de-Oliveira, R. First Detection of Natural Infection of Aedes Aegypti with Zika Virus in Brazil and throughout South America. Mem Inst Oswaldo Cruz 2016, 111, 655–658. [Google Scholar] [CrossRef]
- de Melo Ximenes, M. de F.F.; de Araújo Galvão, J.M.; Inacio, C.L.S.; Macêdo e Silva, V.P.; Pereira, R.L. do N.; Pinheiro, M.P.G.; de Medeiros Silva, M.M.; Gomes, C.E.S. Arbovirus Expansion: New Species of Culicids Infected by the Chikungunya Virus in an Urban Park of Brazil. Acta Trop 2020, 209, 105538. [Google Scholar] [CrossRef]
- Variza, P.F.; Lorenz, C.; Oliveira, J.G. de; Fernandes, M.; Netto, S.A.; Prophiro, J.S. Updated Spatio-Temporal Distribution of Aedes (Stegomyia) Albopictus in Brazil. Acta Trop 2022, 232, 106511. [Google Scholar] [CrossRef]
- Reiter, P.; Fontenille, D.; Paupy, C. Aedes Albopictus as an Epidemic Vector of Chikungunya Virus: Another Emerging Problem? Lancet Infect Dis 2006, 6, 463–464. [Google Scholar] [CrossRef]
- Bonilauri, P.; Bellini, R.; Calzolari, M.; Angelini, R.; Venturi, L.; Fallacara, F.; Cordioli, P.; Angelini, P.; Venturelli, C.; Merialdi, G.; et al. Chikungunya Virus in Aedes Albopictus, Italy. Emerg Infect Dis 2008, 14, 852–854. [Google Scholar] [CrossRef]
- Santos, M.E.G.; Sousa, E.C.; Bitencourt, C.S.; Oliveira, C.H.; Silva, A.J.J.; Ribeiro, J.C.; Franca, A.O.; Mendes, H.H.N.; Abreu, F.V.S. Monitoramento de Mosquitos Aedes Spp. (Diptera: Culicidae) Na Região Urbana Do Município de Salinas-MG, Norte de Minas Gerais.. In Proceedings of the Anais do Congresso da Sociedade Brasileira de Parasitologia - Parasito 2021: A Parasitologia na perspectiva da Saúde Única. ; Sociedade Brasileira de Parasitologia; 2021. [Google Scholar]
- Santos, T.P. Potential of Aedes Albopictus as a Bridge Vector for Enzootic Pathogens at the Urban-Forest Interfacein Brazil - Approved with Minor Corrections. Emerg Microbes Infect 2018. [Google Scholar]
- Corbel, V.; Fonseca, D.M.; Weetman, D.; Pinto, J.; Achee, N.L.; Chandre, F.; Coulibaly, M.B.; Dusfour, I.; Grieco, J.; Juntarajumnong, W.; et al. International Workshop on Insecticide Resistance in Vectors of Arboviruses, December 2016, Rio de Janeiro, Brazil. Parasit Vectors 2017, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Bonney, R.; Shirk, J.L.; Phillips, T.B.; Wiggins, A.; Ballard, H.L.; Miller-Rushing, A.J.; Parrish, J.K. Next Steps for Citizen Science. Science (1979) 2014, 343, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.; Crall, A.; Gray, S.; Phillips, T.; Mellor, D. Citizen Science as a Distinct Field of Inquiry. Bioscience 2015, 65, 208–211. [Google Scholar] [CrossRef]
- Williams, C.R.; Hawthorn-Jackson, D.; Orre-Gordon, S.; O’Sullivan, S. Some Cautions in the Use of Citizen Science: A Case Study of Urban Insect Collection. Transactions of the Royal Society of South Australia 2017, 141, 57–69. [Google Scholar] [CrossRef]
- Liberatore, A.; Bowkett, E.; MacLeod, C.J.; Spurr, E.; Longnecker, N. Social Media as a Platform for a Citizen Science Community of Practice. Citiz Sci 2018, 3, 3. [Google Scholar] [CrossRef]
- Braz Sousa, L.; Fricker, S.R.; Doherty, S.S.; Webb, C.E.; Baldock, K.L.; Williams, C.R. Citizen Science and Smartphone E-Entomology Enables Low-Cost Upscaling of Mosquito Surveillance. Science of The Total Environment 2020, 704, 135349. [Google Scholar] [CrossRef]
- Palmer, J.R.B.; Oltra, A.; Collantes, F.; Delgado, J.A.; Lucientes, J.; Delacour, S.; Bengoa, M.; Eritja, R.; Bartumeus, F. Citizen Science Provides a Reliable and Scalable Tool to Track Disease-Carrying Mosquitoes. Nat Commun 2017, 8, 916. [Google Scholar] [CrossRef]
- Abreu, F.V.S. de; Delatorre, E.; dos Santos, A.A.C.; Ferreira-de-Brito, A.; de Castro, M.G.; Ribeiro, I.P.; Furtado, N.D.; Vargas, W.P.; Ribeiro, M.S.; Meneguete, P.; et al. Combination of Surveillance Tools Reveals That Yellow Fever Virus Can Remain in the Same Atlantic Forest Area at Least for Three Transmission Seasons. Mem Inst Oswaldo Cruz 2019, 114. [Google Scholar] [CrossRef]
- Abreu, F.V.S.; dos Santos, E.; Gomes, M.Q.; Vargas, W.P.; Oliveira Passos, P.H.; Nunes e Silva, C.; Araújo, P.C.; Pires, J.R.; Romano, A.P.M.; Teixeira, D.S.; et al. Capture of Alouatta Guariba Clamitans for the Surveillance of Sylvatic Yellow Fever and Zoonotic Malaria: Which Is the Best Strategy in the Tropical Atlantic Forest? Am J Primatol 2019, 81, e23000. [Google Scholar] [CrossRef]
- Chame, M.; Barbosa, H.J.C.; Gadelha, L.M.R.; Augusto, D.A.; Krempser, E.; Abdalla, L. SISS-Geo: Leveraging Citizen Science to Monitor Wildlife Health Risks in Brazil. J Healthc Inform Res 2019, 3, 414–440. [Google Scholar] [CrossRef] [PubMed]
- Chame, M.; Abdalla, L.; Pinter, A.; Romano, A.P.M.; Krempser, E.; Ramos, D.G.; Passos, P.H. de O.; Silva, P.C.L.; Da Silva, G.M.P.; Gatti, R.R.; et al. Primates in SISS-Geo: Potential Contributions of Mobile Technology, Health Surveillance and Citizen Science to Support Species Conservation in Brazil. Neotropical Primates 2020, 26, 80–89. [Google Scholar] [CrossRef]
- Sousa, L.B.; Craig, A.; Chitkara, U.; Fricker, S.; Webb, C.; Williams, C.; Baldock, K. Methodological Diversity in Citizen Science Mosquito Surveillance: A Scoping Review. Citiz Sci 2022, 7, 8. [Google Scholar] [CrossRef]
- Craig, A.T.; Kama, N.; Fafale, G.; Bugoro, H. Citizen Science as a Tool for Arboviral Vector Surveillance in a Resourced-Constrained Setting: Results of a Pilot Study in Honiara, Solomon Islands, 2019. BMC Public Health 2021, 21, 509. [Google Scholar] [CrossRef]
- Andrade, M.S.; Campos, F.S.; Oliveira, C.H. de; Oliveira, R.S.; Campos, A.A.S.; Almeida, M.A.B. de; Fonseca, V. de S.; Simonini-Teixeira, D.; Sevá, A. da P.; Temponi, A.O.D.; et al. Fast Surveillance Response Reveals the Introduction of a New Yellow Fever Virus Sub-Lineage in 2021, in Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 2022, 117. [Google Scholar] [CrossRef]
| Species | Male | Female | Sum (relative abundance %)# | Pools tested (CHIKV positive) | MIR* |
|---|---|---|---|---|---|
| Ae. aegypti (Linnaeus, 1762) | 70 | 95 | 165 (39.2) | 31 (10) | 60.6 |
| Cx. quinquefasciatus Say, 1823 | 143 | 113 | 256 (60.8) | 26 (2) | 7.8 |
| Total | 213 | 208 | 421 (100) | 57 (12) | 28.5 |
| Cod. Pool | Species | Nº of individuals | Sex | CT* | Heads | Positive heads (CT**) |
|---|---|---|---|---|---|---|
| X-595 | Ae. aegypti | 5 | F | 20.1 | c144, c145, c146, c147, c148 | c144 (21.5); c146 (22.0); c148 (30.1) |
| X-556 | Ae. aegypti | 5 | F | 21.0 | c33, c34, c35, c36, c37 | c34 (21.2) |
| X-585 | Ae. aegypti | 5 | F | 22.9 | c82, c83, c84, c88, 89 | c83 (23.6); c84 (35.8) |
| X-584 | Ae. aegypti | 2 | F | 23.1 | c81, c108 | c81 (21.6) |
| X-579 | Ae. aegypti | 5 | F | 24.1 | c51, c52, c54, c56, c58 | c58 (26.9) |
| X-594 | Ae. aegypti | 5 | F | 24.2 | c139, c140, c141, c142, c143 | c141 (23.0) |
| X-606 | Ae. aegypti | 5 | F | 28.0 | c149, c150, c151, c152, c164 | _ |
| X-555 | Ae. aegypti | 6 | M | 38.0 | c30, c32, c39, c40, c43, c44 | _ |
| X-593 | Ae. aegypti | 7 | M | 38.3 | c126, c128, c134, c135, c136, c137, c138 | _ |
| X-586 | Ae. aegypti | 8 | M | 40.0 | c85, c86, c87, c90, c91, c92, c93, c94 | _ |
| X-623 | Cx. quinquefasciatus | 9 | M | 38.1 | c296, c297, c298, c299, c300, c301, c302, c303, c304 | _ |
| X-619 | Cx. quinquefasciatus | 9 | F | 38.2 | c254, c255, c256, c257, c258, c259, c260, c261, c262 | _ |
| Genotypes | VV+VV+ FF | VV+VV+ FC | VV+VV+ CC | VL+VI+ FC | VL+VI+ CC | LL+II+ CC | VV+Vl+ CC* | VV+VI+ FC | VL+II+ CC |
|---|---|---|---|---|---|---|---|---|---|
| Nº of individuals (Frequency) | 3 (1.8) |
2 (1.2) |
18 (11.0) |
17 (10.4) |
53 (32.3) |
67 (40.9) |
2 (1.2) |
1 (0.6) |
1 (0.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





