Submitted:
30 April 2024
Posted:
01 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Variant Screening
Data analysis and variant classification
Literature review
3. Results
Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, J.P.; Patel, P.A.; Shannon, K.M.; Albers, E.L.; Salerno, J.C.; Stein, M.A.; Stephenson, E.A.; Mohan, S.; Shah, M.J.; Asakai, H.; et al. Predictors of myocardial recovery in pediatric tachycardia-induced cardiomyopathy. Hear. Rhythm. 2014, 11, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Medi, C.; Kalman, J.M.; Haqqani, H.; Vohra, J.K.; Morton, J.B.; Sparks, P.B.; Kistler, P.M. Tachycardia-Mediated Cardiomyopathy Secondary to Focal Atrial Tachycardia: Long-Term Outcome After Catheter Ablation. Circ. 2009, 53, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Yang, B.; Li, M.; Zhang, F.; Chen, H.; Gu, K.; Yu, J.; Cao, K.; Chen, M. Tachycardiomyopathy Complicated by Focal Atrial Tachycardia: Incidence, Risk Factors, and Long-Term Outcome. J. Cardiovasc. Electrophysiol. 2014, 25, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.-H.; Kistler, P.M.; Kalman, J.M.; Schilling, R.J.; Hunter, R.J. Comorbidity of atrial fibrillation and heart failure. Nat. Rev. Cardiol. 2016, 13, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Etheridge, S.P.; Marchlinski, F.E.; Spinale, F.G.; Lakkireddy, D.; Olshansky, B. Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. J Am Coll Cardiol. 2015;66:1714-28.
- Walsh, R.; Thomson, K.L.; Ware, J.S.; Funke, B.H.; Woodley, J.; McGuire, K.J.; Mazzarotto, F.; Blair, E.; Seller, A.; Taylor, J.C.; et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. 2017, 19, 192–203. [Google Scholar] [CrossRef]
- Gallagher, J.J. Tachycardia and cardiomyopathy: The chicken-egg dilemma revisited. Circ. 1985, 6, 1172–1173. [Google Scholar] [CrossRef]
- Raymond-Paquin, A.; Nattel, S.; Wakili, R.; Tadros, R. Mechanisms and Clinical Significance of Arrhythmia-Induced Cardiomyopathy. Can. J. Cardiol. 2018, 34, 1449–1460. [Google Scholar] [CrossRef]
- Mares, J.C.; Bar-Cohen, Y. Tachycardia-Induced Cardiomyopathy in a 1-Month-Old Infant. Case Rep. Pediatr. 2012, 2012, 1–4. [Google Scholar] [CrossRef]
- Isojima, T.; Kato, N.; Ito, Y.; Kanzaki, S.; Murata, M. Growth standard charts for Japanese children with mean and standard deviation (SD) values based on the year 2000 national survey. Clin. Pediatr. Endocrinol. 2016, 25, 71–76. [Google Scholar] [CrossRef]
- Van Hare, G.F.; Witherell, C.L.; Lesh, M.D. Follow-up of radiofrequency catheter ablation in children: results in 100 consecutive patients. J Am Coll Cardiol. 1994;23:1651-9.
- Sanchez, C.; Benito, F.; Moreno, F. Reversibility of tachycardia-induced cardiomyopathy after radiofrequency ablation of incessant supraventricular tachycardia in infants. Heart 1995, 74, 332–333. [Google Scholar] [CrossRef]
- Schulze, O.; Kammeraad, J.; Ramanna, H.; Sreeram, N. Catheter ablation for tachyarrhythmia-induced cardiomyopathy in infants. Int. J. Cardiol. 2000, 74, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Felt, J.; Arora, R.; Sethuraman, U. Respiratory Distress in an Infant: An Uncommon Cause for a Common Complaint. J. Emerg. Med. 2016, 50, e57–e60. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, M.; Ruttan, T.K.; Kienstra, A.J.; Wilkinson, M. Making the Quick Diagnosis: A Case of Neonatal Shock. J. Emerg. Med. 2017, 52, e139–e144. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.; Tramma, D.; Thomaidis, K.; Papadopoulou-Legbelou, K. Tachycardia induced cardiomyopathy in an infant with atrial flutter: A challenging but reversible cause of heart failure. Pediatr. Neonatol. 2019, 60, 477–478. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Ferns, S.J. Epicardial Ablation of Persistent Junctional Reciprocating Tachycardia in an Infant With Tachycardia-Induced Cardiomyopathy. JACC: Case Rep. 2021, 3, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Texter, K.M.; Kertesz, N.J.; Friedman, R.A.; Fenrich, A.L. Atrial Flutter in Infants. Circ. 2006, 48, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Tanaka, R.; A Crawford, F.; Zile, M.R. Changes in myocardial blood flow during development of and recovery from tachycardia-induced cardiomyopathy. Circulation 1992, 85, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.W.; Montgomery, C.; Howard, R.J.; A Grima, E.; Armstrong, P.W. Left ventricular myocardial blood flow, metabolism, and effects of treatment with enalapril: further insights into the mechanisms of canine experimental pacing-induced heart failure. . 1993, 121, 294–301. [Google Scholar]
- Horenstein, M.; Saarel, E.; Dick, M.; Karpawich, P. Reversible Symptomatic Dilated Cardiomyopathy in Older Children and Young Adolescents Due to Primary Non-Sinus Supraventricular Tachyarrhythmias. Pediatr. Cardiol. 2003, 24, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, G.; Wijns, W.; Andries, E.; Brugada, P. Tachycardiomyopathy: Mechanisms and Clinical Implications. Pacing Clin. Electrophysiol. 1996, 19, 95–106. [Google Scholar] [CrossRef]
- Huizar, J.F.; Ellenbogen, K.A.; Tan, A.Y.; Kaszala, K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2328-44.
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; de Gasparo, M.; Whitebread, S.; Hebbar, L.; Clair, M.J.; Melton, D.M.; et al. Modulation of the renin-angiotensin pathway through enzyme inhibition specific receptor blockade in pacing-induced heart failure:, I. Effects on left ventricular performance and neurohormonal systems. Circulation. 1997;96:2385-96.
- A Riegger, G.; Elsner, D.; Kromer, E.P.; Daffner, C.; Forssmann, W.G.; Muders, F.; Pascher, E.W.; Kochsiek, K. Atrial natriuretic peptide in congestive heart failure in the dog: plasma levels, cyclic guanosine monophosphate, ultrastructure of atrial myoendocrine cells, and hemodynamic, hormonal, and renal effects. Circulation 1988, 77, 398–406. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, J.V.; Dindar, A.; Griffith, M.J.; A Edgar, R.; Silove, E.D.; Stumper, O.; Wright, J.C. Recovery pattern of left ventricular dysfunction following radiofrequency ablation of incessant supraventricular tachycardia in infants and children. Heart 1998, 79, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Law, Y.M.; Asante-Korang, A.; Austin, E.D.; Dipchand, A.I.; Everitt, M.D.; Hsu, D.T.; Lin, K.Y.; Price, J.F.; Wilkinson, J.D.; et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement From the American Heart Association. Circulation 2019, 140, E9–E68. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Cecconi, A.; Martínez-Vives, P.; Olivera, M.J.; Hernández, S.; López-Melgar, B.; Rojas-González, A.; Díez-Villanueva, P.; Salamanca, J.; Tejelo, J.; et al. Electrocardiogram and CMR to differentiate tachycardia-induced cardiomyopathy from dilated cardiomyopathy in patients admitted for heart failure. Hear. Vessel. 2022, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Spahic, A.; Chen, T.-H.; Geller, J.C.; Saenger, J.; Ohlow, M. Life in the fast lane: clinical and immunohistological characteristics of tachycardia-induced cardiomyopathy—a retrospective study in 684 patients. Herzschrittmachertherapie + Elektrophysiologie 2020, 31, 292–300. [Google Scholar] [CrossRef]
- Mueller, K.A.; Heinzmann, D.; Klingel, K.; Fallier-Becker, P.; Kandolf, R.; Kilias, A.; Walker-Allgaier, B.; Borst, O.; Kumbrink, J.; Kirchner, T.; et al. Histopathological and Immunological Characteristics of Tachycardia-Induced Cardiomyopathy. Circ. 2017, 69, 2160–2172. [Google Scholar] [CrossRef]
- Moore, J.P.; Wang, S.; Albers, E.L.; Salerno, J.C.; Stephenson, E.A.; Shah, M.J.; Pflaumer, A.; Czosek, R.J.; Garnreiter, J.M.; Collins, K.; et al. A Clinical Risk Score to Improve the Diagnosis of Tachycardia-Induced Cardiomyopathy in Childhood. Am. J. Cardiol. 2016, 118, 1074–1080. [Google Scholar] [CrossRef]
- Shinbane, J.S.; A Wood, M.; Jensen, D.; A Ellenbogen, K.; Fitzpatrick, A.P.; Scheinman, M.M. Tachycardia-Induced Cardiomyopathy: A Review of Animal Models and Clinical Studies. Circ. 1997, 29, 709–715. [Google Scholar] [CrossRef]
- O'Brien, P.J.; Moe, G.W.; Nowack, L.M.; Grima, E.A.; Armstrong, P.W. Sarcoplasmic reticulum Ca-release channel and ATP-synthesis activities are early myocardial markers of heart failure produced by rapid ventricular pacing in dogs. Can. J. Physiol. Pharmacol. 1994, 72, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Cruz FE, Cheriex EC, Smeets JL, Atie J, Peres AK, Penn OC, et al. Reversibility of tachycardia-induced cardiomyopathy after cure of incessant supraventricular tachycardia. J Am Coll Cardiol. 1990;16:739-44.
- Lip, G.Y.; Heinzel, F.R.; Gaita, F.; Juanatey, J.R.G.; Le Heuzey, J.Y.; Potpara, T.; Svendsen, J.H.; Vos, M.A.; Anker, S.D.; Coats, A.J.; et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Eur. 2016, 18, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Dohain, A.M.; Lotfy, W.; Abdelmohsen, G.; Sobhy, R.; Abdelaziz, O.; Elsaadany, M.; Abdelsalam, M.H.; Ibrahim, H. Functional recovery of cardiomyopathy induced by atrial tachycardia in children: Insight from cardiac strain imaging. Pacing Clin. Electrophysiol. 2021, 44, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.-H.; Kalman, J.M.; Ellims, A.H.; Iles, L.M.; Medi, C.; Sherratt, C.; Kaye, D.M.; Hare, J.L.; Kistler, P.M.; Taylor, A.J. Diffuse Ventricular Fibrosis Is a Late Outcome of Tachycardia-Mediated Cardiomyopathy After Successful Ablation. Circ. Arrhythmia Electrophysiol. 2013, 6, 697–704. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: a position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Hear. J. 2007, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of Myocardial Fibrosis With Cardiovascular Magnetic Resonance. Circ. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- West, A.M.; Kramer, C.M. Cardiovascular Magnetic Resonance Imaging of Myocardial Infarction, Viability, and Cardiomyopathies. Curr. Probl. Cardiol. 2010, 35, 176–220. [Google Scholar] [CrossRef]
- Hasdemir, C.; Yuksel, A.; Camli, D.; Kartal, Y.; Simsek, E.; Musayev, O.; Isayev, E.; Aydin, M.; Can, L.H. Late Gadolinium Enhancement CMR in Patients with Tachycardia-Induced Cardiomyopathy Caused by Idiopathic Ventricular Arrhythmias. Pacing Clin. Electrophysiol. 2012, 35, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Lashus, A.G.; Case, C.L.; Gillette, P.C. Catheter Ablation Treatment of Supraventricular Tachycardia–Induced Cardiomyopathy. Arch. Pediatr. Adolesc. Med. 1997, 151, 264–6. [Google Scholar] [CrossRef]
- Nerheim, P.; Birger-Botkin, S.; Piracha, L.; Olshansky, B.; B, S.; B, G.; T, N.; F, G.; J, V.; S, C.; et al. Heart Failure and Sudden Death in Patients With Tachycardia-Induced Cardiomyopathy and Recurrent Tachycardia. Circulation 2004, 110, 247–252. [Google Scholar] [CrossRef]
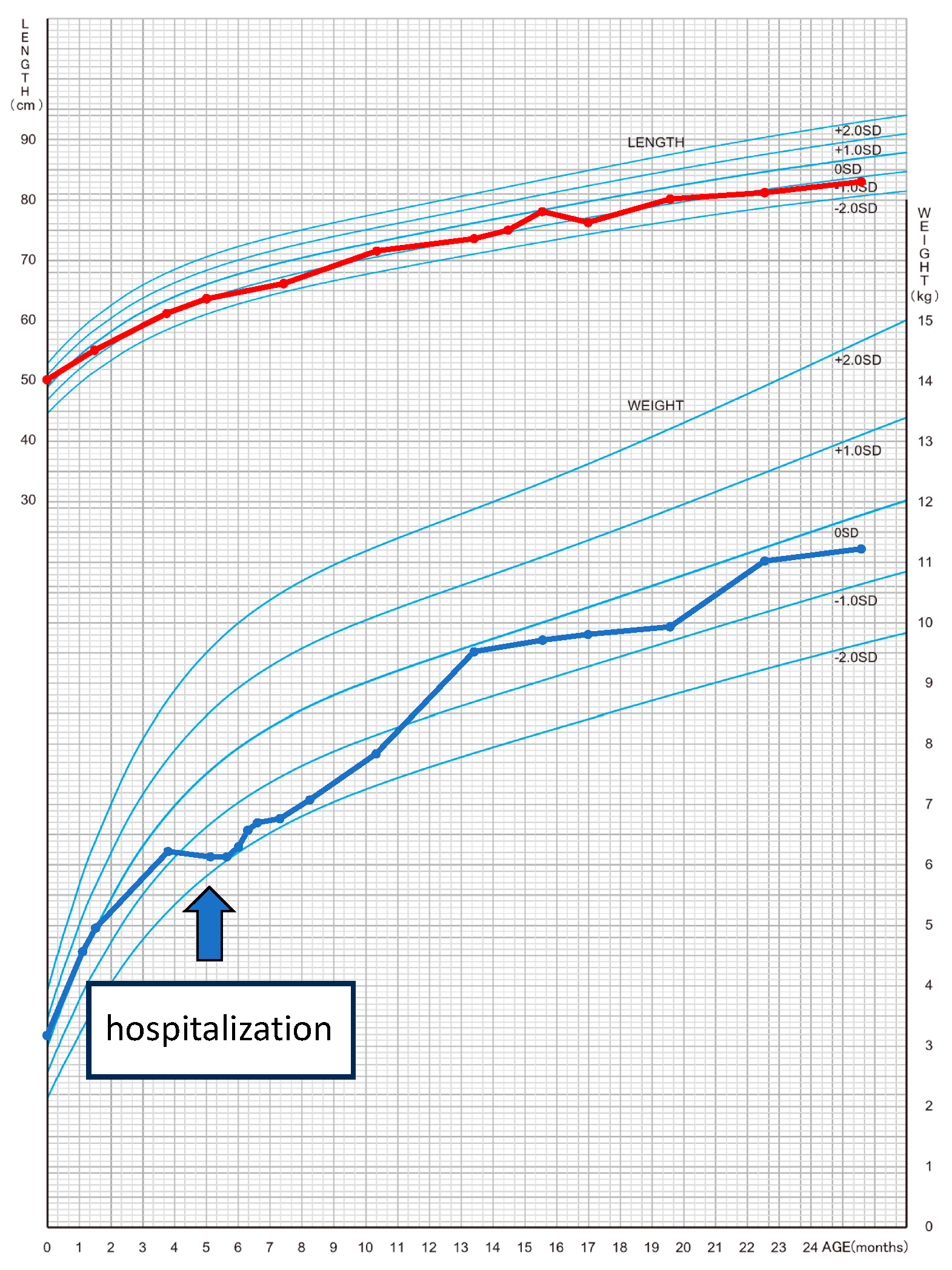
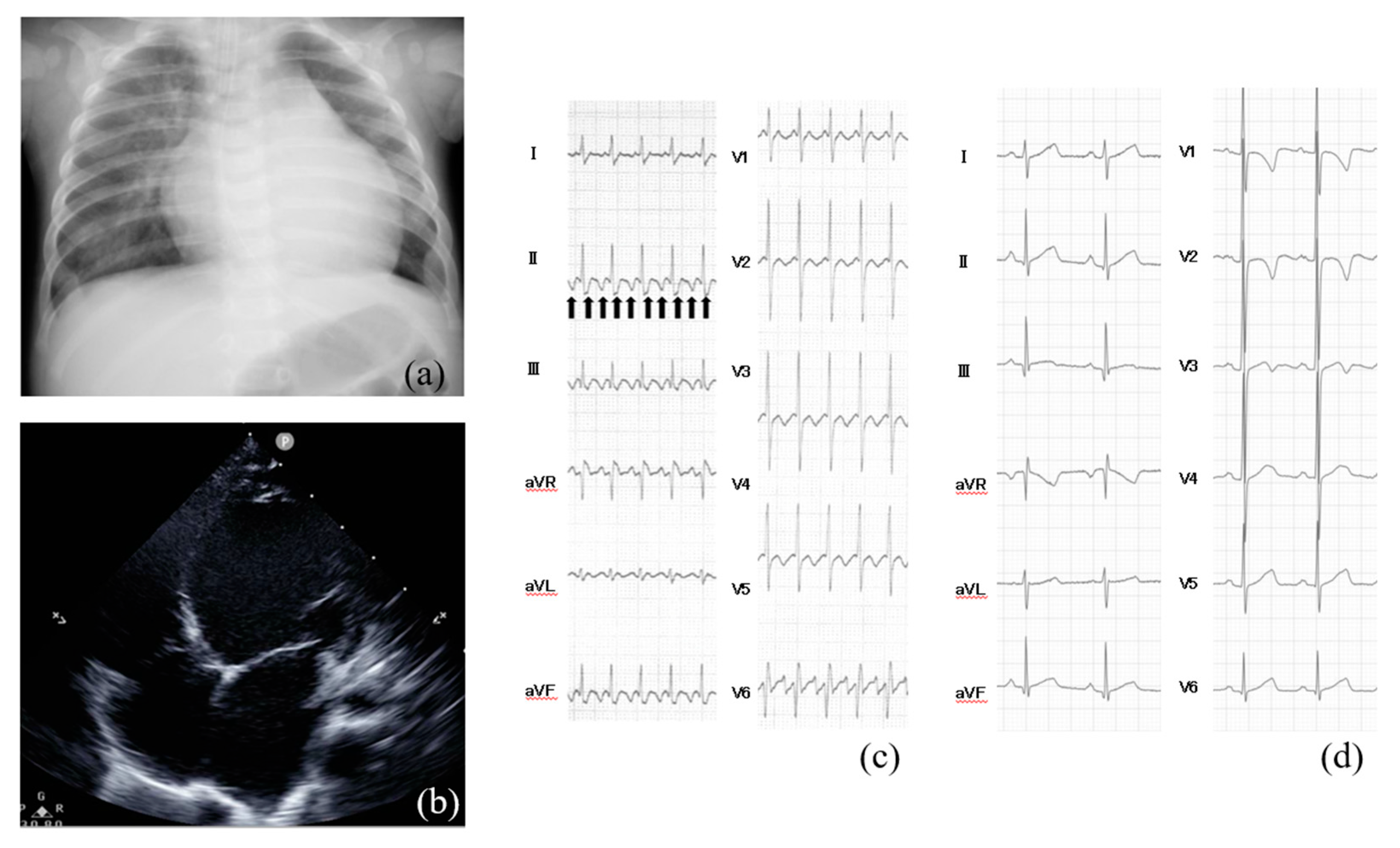
| Author | Age of TIC | Cause of tachycardia | Heart rate (beats/min) | Duration from the onset | LV function on echocardiogram | Medication | Non-pharmacological therapy | LV function on echocardiogram after treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| Van Hare GF, et al.[11] | 5-week-old | PJRT | N/A | N/A | LVFS 5% | flecainide and sotalol | catheter ablation | improved | alive |
| Sanchez C, et al.[12] | 3-month-old | PJRT | 230 | 20 days | LVFS 20% | digoxin and amiodarone | catheter ablation | improved | alive |
| Schulze OC, et al.[13] | 3-week-old | PJRT | 230 | N/A | LVFS 15% | no | catheter ablation | improved | alive |
| Mares JC, et al.[9] | 1-month-old | SVT | 260 | N/A | LVFS 13% | flecainide | no | improved | alive |
| Jon Felt, et al.[14] | 7-week-old | AFL | 195 | N/A | LVFS 17% | amiodarone | electrical cardioversion | improved | alive |
| Gardiner M, et al.[15] | 24-day-old | SVT | 270 | N/A | LVEF 35% | adenosine | no | improved | alive |
| Papadopoulou M, et al.[16] | 1-month-old | AFL | 216 | N/A | LVFS 19-21%, LVEF 42-45% | no | electrical cardioversion | improved | alive |
| McKenzie K, et al.[17] | 3-month-old | SVT | N/A | 1 month | LVEF 22% | digoxin, beta-blocker and amiodarone | catheter ablation | improved | alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





