Submitted:
30 April 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
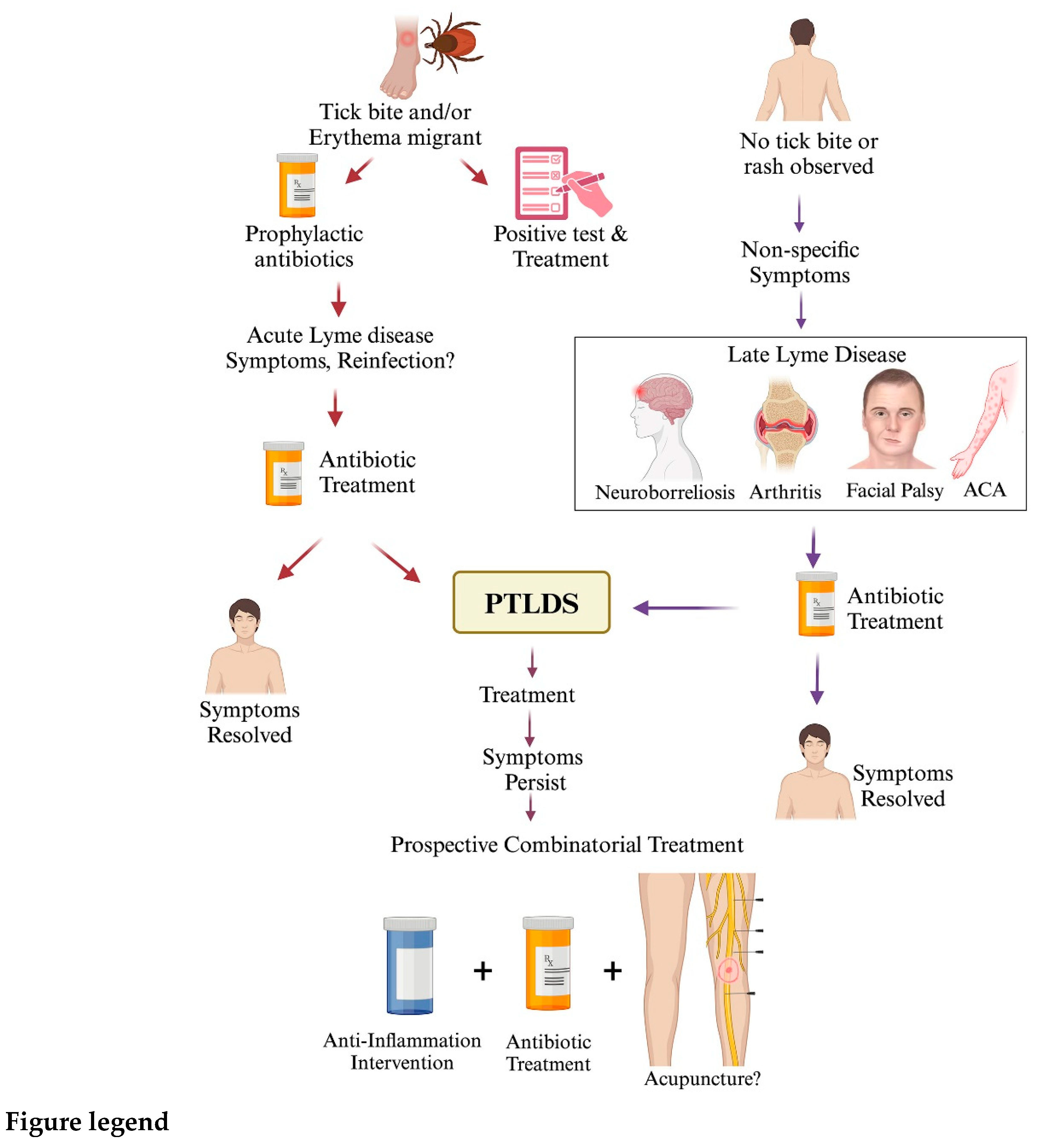
I. Treatment Recommendation for Lyme disease
- A. Prevention, diagnosis and treatment for acute Lyme disease
- 1. Post-exposure prophylaxis following tick bite and erythema migrans detection
- 2. Treatment of other complications in early stages of Lyme disease
- 3. Progression of Lyme disease symptoms post-antibiotic therapy
- B. Late-stage Lyme disease
- 1. Lyme arthritis and neuroborreliosis
- 2. Acrodermatitis chronica atrophicans (ACA)
- 3. Treatment of pregnant women
II. Contribution of host and bacterial factors in Lyme disease
- A. Human genetic factors affecting Lyme disease
- B. Essential components of metabolic pathways in B. burgdorferi
- C. B. burgdorferi persisters and Lyme arthritis
- D. Causes and treatment of PTLDS
- 1. Causes identified for PTLDS
- 2. Approaches to improve quality of life after Lyme disease
III. Vaccines to prevent B. burgdorferi infection and Lyme disease
IV. Future prospective
References
- Schwartz, I.; Margos, G.; Casjens, S.R.; Qiu, W.-G.; Eggers, C.H. Multipartite genome of Lyme disease Borrelia: structure, variation and prophages. Current issues in molecular biology 2021, 42, 409–454. [Google Scholar] [PubMed]
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.; Li, X.; Mead, P.S. Lyme borreliosis. Nature reviews Disease primers 2016, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Adrion, E.R.; Aucott, J.; Lemke, K.W.; Weiner, J.P. Health care costs, utilization and patterns of care following Lyme disease. PloS one 2015, 10, e0116767. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the gut microbiota. The Journal of clinical investigation 2014, 124, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nature Reviews Microbiology 2011, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PloS one 2012, 7, e29914. [Google Scholar] [CrossRef]
- Feng, J.; Shi, W.; Zhang, S.; Zhang, Y. Identification of new compounds with high activity against stationary phase Borrelia burgdorferi from the NCI compound collection. Emerging Microbes & Infections 2015, 4, 1–15. [Google Scholar]
- Hodzic, E.; Imai, D.; Feng, S.; Barthold, S.W. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PloS one 2014, 9, e86907. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Caimano, M.J.; Stevenson, B.; Hu, L.T. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nature reviews microbiology 2012, 10, 87–99. [Google Scholar] [CrossRef]
- Hajdusek, O.; Perner, J. VLA15, a new global Lyme disease vaccine undergoes clinical trials. The Lancet Infectious Diseases 2023, 23, 1105–1106. [Google Scholar] [CrossRef]
- Comstedt, P.; Schüler, W.; Meinke, A.; Lundberg, U. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PloS one 2017, 12, e0184357. [Google Scholar] [CrossRef] [PubMed]
- Lee-Lewandrowski, E.; Chen, Z.; Branda, J.; Baron, J.; Kaufman, H.W. Laboratory Blood-Based Testing for Lyme Disease at a National Reference Laboratory: A 7-Year Experience (2010-2016). American Journal of Clinical Pathology 2019, 152, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S. Epidemiology of Lyme disease. Infectious Disease Clinics 2015, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.; Dattwyler, R.; Shapiro, E.; Halperin, J.; Steere, A.; Klempner, M.; Krause, P.; Bakken, J.; Strle, F.; Stanek, G. Erratum: The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America (Clinical Infectious Diseases (2006) 43,(1089-1134)). Clinical Infectious Diseases 2007, 45. [Google Scholar]
- Knudtzen, F.C.; Andersen, N.S.; Jensen, T.G.; Skarphédinsson, S. Characteristics and clinical outcome of Lyme neuroborreliosis in a high endemic area, 1995–2014: a retrospective cohort study in Denmark. Clinical Infectious Diseases 2017, 65, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Ljøstad, U.; Mygland, Å. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. European Journal of Neurology 2010, 17, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Shadick, N.A.; Phillips, C.B.; Sangha, O.; Logigian, E.L.; Kaplan, R.F.; Wright, E.A.; Fossel, A.H.; Fossel, K.; Berardi, V.; Lew, R.A. Musculoskeletal and neurologic outcomes in patients with previously treated Lyme disease. Annals of Internal Medicine 1999, 131, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Eikeland, R.; Mygland, Å.; Herlofson, K.; Ljøstad, U. Risk factors for a non-favorable outcome after treated E uropean neuroborreliosis. Acta Neurologica Scandinavica 2013, 127, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.W.; Malawista, S.E. Lyme disease: recommendations for diagnosis and treatment. Annals of internal medicine 1991, 114, 472–481. [Google Scholar] [CrossRef]
- Control, C.f.D.; Prevention. Lyme disease data and surveillance. Centers for Disease Control and Prevention, Atlanta, GA: https://www. cdc. gov/lyme/datasurveillance/index. html.[Google Scholar] 2019.
- Mead, P.; Petersen, J.; Hinckley, A. Updated CDC recommendation for serologic diagnosis of Lyme disease. Morbidity and Mortality Weekly Report 2019, 68, 703–703. [Google Scholar] [CrossRef]
- Wormser, G.P.; Schriefer, M.; Aguero-Rosenfeld, M.E.; Levin, A.; Steere, A.C.; Nadelman, R.B.; Nowakowski, J.; Marques, A.; Johnson, B.J.; Dumler, J.S. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagnostic microbiology and infectious disease 2013, 75, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Dressler, F.; Whalen, J.A.; Reinhardt, B.N.; Steere, A.C. Western blotting in the serodiagnosis of Lyme disease. Journal of Infectious Diseases 1993, 167, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Depietropaolo, D.L.; Powers, J.H.; Gill, J.M.; Foy, A.J. Diagnosis of Lyme disease. American family physician 2005, 72, 297–304. [Google Scholar] [PubMed]
- Diseases, C.o.I. Prevention of Lyme disease. Pediatrics 2000, 105, 142–147. [Google Scholar] [CrossRef]
- Eisen, L. Control of ixodid ticks and prevention of tick-borne diseases in the United States: The prospect of a new Lyme disease vaccine and the continuing problem with tick exposure on residential properties. Ticks and tick-borne diseases 2021, 12, 101649. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, R.B.; Nowakowski, J.; Fish, D.; Falco, R.C.; Freeman, K.; McKenna, D.; Welch, P.; Marcus, R.; Agüero-Rosenfeld, M.E.; Dennis, D.T. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. New England Journal of Medicine 2001, 345, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Costello, C.M.; Steere, A.C.; Pinkerton, R.E.; Feder, H.M. A prospective study of tick bites in an endemic area for Lyme disease. The Journal of infectious diseases 1989, 159, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Salzman, M.; Rubin, L.; Sood, S. Prevention of Lyme disease after tick bites. The New England journal of medicine 1993, 328, 137–139. [Google Scholar] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases 2006, 43, 1089–1134. [Google Scholar] [CrossRef]
- Rebman, A.W.; Yang, T.; Yoon, I.; Powell, D.; Geller, S.A.; Aucott, J.N. Initial Presentation and Time to Treatment in Early Lyme Disease. The American journal of tropical medicine and hygiene 2023, tpmd220437–tpmd220437. [Google Scholar] [CrossRef]
- Cornell, K.A.; Knippel, R.J.; Cortright, G.R.; Fonken, M.; Guerrero, C.; Hall, A.R.; Mitchell, K.A.; Thurston, J.H.; Erstad, P.; Tao, A. Characterization of 5′-methylthioadenosine/S-adenosylhomocysteine nucleosidases from Borrelia burgdorferi: Antibiotic targets for Lyme disease. Biochimica et Biophysica Acta (BBA)-General Subjects 2020, 1864, 129455. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Schoen, R.T.; Rahn, D.W.; Sikand, V.K.; Nowakowski, J.; Parenti, D.L.; Holman, M.S.; Persing, D.H.; Steere, A.C. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Annals of internal medicine 2002, 136, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.W.; Paparone, P.; Wormser, G.P.; Nadelman, R.B.; Grunwaldt, E.; Gomez, G.; Wisniewski, M.; Collins, J.J. Comparison of cefuroxime axetil and doxycycline in treatment of patients with early Lyme disease associated with erythema migrans. Antimicrobial agents and chemotherapy 1995, 39, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, K.; Reiso, H.; Berild, D.; Lindbæk, M. Comparison of phenoxymethylpenicillin, amoxicillin, and doxycycline for erythema migrans in general practice. A randomized controlled trial with a 1-year follow-up. Clinical Microbiology and Infection 2018, 24, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.J.; Tata, S.; Berth, W.; Mathiason, M.A.; Agger, W.A. Antibiotic treatment duration and long-term outcomes of patients with early Lyme disease from a Lyme disease–hyperendemic area. Clinical infectious diseases 2010, 50, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, J.; Nadelman, R.B.; Forseter, G.; McKenna, D.; Wormser, G.P. Doxycycline versus tetracycline therapy for Lyme disease associated with erythema migrans. Journal of the American Academy of Dermatology 1995, 32, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Stupica, D.; Lusa, L.; Ružić-Sabljić, E.; Cerar, T.; Strle, F. Treatment of erythema migrans with doxycycline for 10 days versus 15 days. Clinical infectious diseases 2012, 55, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Brady, K.C.; Cho, M.S.; Scavarda, C.A.; McKenna, D. Efficacy of a 14-day course of amoxicillin for patients with erythema migrans. Diagnostic microbiology and infectious disease 2019, 94, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Torbahn, G.; Hofmann, H.; Rücker, G.; Bischoff, K.; Freitag, M.H.; Dersch, R.; Fingerle, V.; Motschall, E.; Meerpohl, J.J.; Schmucker, C. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA dermatology 2018, 154, 1292–1303. [Google Scholar] [CrossRef]
- Chason, M.E.; Monaghan, M.; Wang, J.; Cheng, Y.; DeBiasi, R.L. Symptom resolution in pediatric patients with Lyme disease. Journal of the Pediatric Infectious Diseases Society 2019, 8, 170–173. [Google Scholar] [CrossRef]
- Meissner, H.C.; Steere, A.C. Management of pediatric Lyme disease: updates from 2020 Lyme guidelines. Pediatrics 2022, 149, e2021054980. [Google Scholar] [CrossRef] [PubMed]
- Eppes, S.C.; Childs, J.A. Comparative study of cefuroxime axetil versus amoxicillin in children with early Lyme disease. Pediatrics 2002, 109, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Arnez, M.; Radsel-Medvescek, A.; Pleterski-Rigler, D.; Ruzic-Sabljic, E.; Strle, F. Comparison of cefuroxime axetil and phenoxymethyl penicillin for the treatment of children with solitary erythema migrans. Wiener Klinische Wochenschrift 1999, 111, 916–922. [Google Scholar] [PubMed]
- Cerar, D.; Cerar, T.; Ružić-Sabljić, E.; Wormser, G.P.; Strle, F. Subjective symptoms after treatment of early Lyme disease. The American journal of medicine 2010, 123, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Angelette, A.L.; Rando, L.L.; Wadhwa, R.D.; Barras, A.A.; Delacroix, B.M.; Talbot, N.C.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.M. Tetracycline-, doxycycline-, minocycline-induced pseudotumor cerebri and esophageal perforation. Advances in Therapy 2023, 40, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, R.B.; Luger, S.W.; Frank, E.; Wisniewski, M.; Collins, J.J.; Wormser, G.P. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Annals of internal medicine 1992, 117, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Auwaerter, P.G.; Kobayashi, T.; Wormser, G.P. Guidelines for Lyme disease are updated. The American journal of medicine 2021, 134, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Johnson, L. Long-term outcomes in patients with early lyme disease: more false hope? Clinical Infectious Diseases 2010, 50, 1683–1684. [Google Scholar] [CrossRef]
- Wormser, G.P.; Ramanathan, R.; Nowakowski, J.; McKenna, D.; Holmgren, D.; Visintainer, P.; Dornbush, R.; Singh, B.; Nadelman, R.B. Duration of antibiotic therapy for early Lyme disease: a randomized, double-blind, placebo-controlled trial. Annals of internal medicine 2003, 138, 697–704. [Google Scholar] [CrossRef]
- Eldin, C.; Hansmann, Y. Erythema migrans: Lyme disease does not need prolonged therapy. The Lancet Infectious Diseases 2023, 23, 271–272. [Google Scholar] [CrossRef]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert review of anti-infective therapy 2014, 12, 1103–1135. [Google Scholar] [CrossRef] [PubMed]
- Hercogová, J. Lyme borreliosis. In European Handbook of Dermatological Treatments; Springer, 2023; pp. 599–604. [Google Scholar]
- Hunfeld, K.-P.; Wichelhaus, T.A.; Rödel, R.; Acker, G.; Brade, V.; Kraiczy, P. Comparison of in vitro activities of ketolides, macrolides, and an azalide against the spirochete Borrelia burgdorferi. Antimicrobial agents and chemotherapy 2004, 48, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Labro, M. Anti-inflammatory activity of macrolides: a new therapeutic potential? The Journal of antimicrobial chemotherapy 1998, 41, 37–46. [Google Scholar] [CrossRef]
- Boston, I. Macrolide therapy of chronic Lyme disease. Signature 2003, 9111, P1136–I1142. [Google Scholar]
- Nowakowski, J.; McKenna, D.; Nadelman, R.B.; Cooper, D.; Bittker, S.; Holmgren, D.; Pavia, C.; Johnson, R.C.; Wormser, G.P. Failure of treatment with cephalexin for Lyme disease. Archives of Family Medicine 2000, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Agger, W.; Callister, S.; Jobe, D. In vitro susceptibilities of Borrelia burgdorferi to five oral cephalosporins and ceftriaxone. Antimicrobial agents and chemotherapy 1992, 36, 1788–1790. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Nields, J.A. Lyme disease: a neuropsychiatric illness. American Journal of Psychiatry 1994, 151, 1571–1583. [Google Scholar] [PubMed]
- HANSEN, K.; LEBECH, A.-M. The clinical and epidemiological profile of lyme neuroborreliosis in Denmark 1985–1990: a prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody productionm. Brain 1992, 115, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Oschmann, P.; Dorndorf, W.; Hornig, C.; Schäfer, C.; Wellensiek, H.; Pflughaupt, K. Stages and syndromes of neuroborreliosis. Journal of neurology 1998, 245, 262–272. [Google Scholar] [CrossRef]
- Kaiser, R. Variable CSF findings in early and late Lyme neuroborreliosis: a follow-up study in 47 patients. Journal of neurology 1994, 242, 26–36. [Google Scholar] [CrossRef]
- Berglund, J.; Eitrem, R.; Ornstein, K.; Lindberg, A.; Ringnér, Å.; Elmrud, H.; Carlsson, M.; Runehagen, A.; Svanborg, C.; Norrby, R. An epidemiologic study of Lyme disease in southern Sweden. New England Journal of Medicine 1995, 333, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Fingerle, V.; Pfister, H.-W. Lyme neuroborreliosis—epidemiology, diagnosis and management. Nature Reviews Neurology 2015, 11, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Christen, H.-J. Lyme neuroborreliosis in children. Annals of medicine 1996, 28, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.-W.; Wilske, B. Lyme borreliosis: basic science and clinical aspects. The Lancet 1994, 343, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Nikoskelainen, J.; Hiekkanen, H.; Lauhio, A.; Peltomaa, M.; Pitkäranta, A.; Nyman, D.; Granlund, H.; Carlsson, S.-A.; Seppälä, I. Duration of antibiotic treatment in disseminated Lyme borreliosis: a double-blind, randomized, placebo-controlled, multicenter clinical study. European Journal of Clinical Microbiology & Infectious Diseases 2007, 26, 571–581. [Google Scholar]
- Dersch, R.; Freitag, M.; Schmidt, S.; Sommer, H.; Rauer, S.; Meerpohl, J. Efficacy and safety of pharmacological treatments for acute L yme neuroborreliosis–a systematic review. European journal of neurology 2015, 22, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Mygland, Å.; Ljøstad, U.; Fingerle, V.; Rupprecht, T.; Schmutzhard, E.; Steiner, I. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. European journal of neurology 2010, 17, 8–e4. [Google Scholar] [CrossRef]
- Halperin, J.; Shapiro, E.; Logigian, E.; Belman, A.; Dotevall, L.; Wormser, G.; Krupp, L.; Gronseth, G.; Bever Jr, C. Practice parameter: treatment of nervous system Lyme disease (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2007, 69, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Eikeland, R.; Mygland, Å.; Herlofson, K.; Ljøstad, U. European neuroborreliosis: quality of life 30 months after treatment. Acta Neurologica Scandinavica 2011, 124, 349–354. [Google Scholar] [CrossRef]
- Ljøstad, U.; Skogvoll, E.; Eikeland, R.; Midgard, R.; Skarpaas, T.; Berg, Å.; Mygland, Å. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: a multicentre, non-inferiority, double-blind, randomised trial. The Lancet Neurology 2008, 7, 690–695. [Google Scholar] [CrossRef]
- Karlsson, M.; Hammers-Berggren, S.; Lindquist, L.; Stiernstedt, G.; Svenungsson, B. Comparison of intravenous penicillin G and oral doxycycline for treatment of Lyme neuroborreliosis. Neurology 1994, 44, 1203–1203. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.-W.; Preac-Mursic, V.; Wilske, B.; Schielke, E.; Sörgel, F.; Einhaupl, K.M. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. Journal of Infectious Diseases 1991, 163, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.-W.; Preac-Mursic, V.; Wilske, B.; Einhäupl, K.M. Cefotaxime vs penicillin G for acute neurologic manifestations in Lyme borreliosis: a prospective randomized study. Archives of neurology 1989, 46, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Hassler, D.; Zöller, L.; Haude, M.; Hufnagel, H.; Heinrich, F.; Sonntag, H. Cefotaxime versus penicillin in the late stage of Lyme disease--prospective, randomized therapeutic study. Infection 1990, 18, 16–20. [Google Scholar] [CrossRef] [PubMed]
- STEERE, A.C.; BATSFORD, W.P.; WEINBERG, M.; ALEXANDER, J.; BERGER, H.J.; WOLFSON, S.; MALAWISTA, S.E. Lyme carditis: cardiac abnormalities of Lyme disease. Annals of internal medicine 1980, 93, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Bockenstedt, L.K. Lyme disease and the heart. Circulation 2013, 127, e451–e454. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.D.; Meiman, J.; Mullins, J.; Nelson, R.; Ertel, S.-H.; Cartter, M.; Brown, C.M.; Lijewski, V.; Schiffman, E.; Neitzel, D. Notes from the field: update on Lyme carditis, groups at high risk, and frequency of associated sudden cardiac death--United States. MMWR Morb Mortal Wkly Rep 2014, 63, 982–983. [Google Scholar]
- Kwit, N.A.; Nelson, C.A.; Max, R.; Mead, P.S. Risk factors for clinician-diagnosed Lyme arthritis, facial palsy, carditis, and meningitis in patients from high-incidence states. In Proceedings of the Open Forum Infectious Diseases, 2018; p. ofx254. [Google Scholar]
- RUBIN, D.A.; SORBERA, C.; NIKITIN, P.; MCALLISTER, A.; WORMSER, G.P.; NADELMAN, R.B. Prospective evaluation of heart block complicating early Lyme disease. Pacing and Clinical Electrophysiology 1992, 15, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.J.; Cohn, K.A.; Nigrovic, L.E.; Thompson, A.D.; Hines, E.M.; Lyons, T.W.; Glatz, A.C.; Shah, S.S. Electrocardiograph abnormalities in children with Lyme meningitis. Journal of the Pediatric Infectious Diseases Society 2012, 1, 293–298. [Google Scholar] [CrossRef]
- Oktay, A.A.; Dibs, S.R.; Friedman, H. Sinus pause in association with Lyme carditis. Texas Heart Institute Journal 2015, 42, 248–250. [Google Scholar] [CrossRef]
- Rey, M.-J.; Zimmermann, M.; Adamec, R.; Fleisch, M.; Viquerat, C.; De Freudenreich, J. Intra-hisian 2: 1 atrioventricular block secondary to Lyme disease. European heart journal 1991, 12, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Van der Linde, M.; Crijns, H.; De Koning, J.; Hoogkamp-Korstanje, J.; De Graaf, J.; Piers, D.A.; Van der Galien, A.; Lie, K. Range of atrioventricular conduction disturbances in Lyme borreliosis: a report of four cases and review of other published reports. Heart 1990, 63, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, Y.J.; Brennan, J.J.; Rosenfeld, L.E. Lyme myocarditis presenting as fascicular tachycardia with underlying complete heart block. Journal of cardiovascular electrophysiology 1997, 8, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Petr Kuchynka, M.; Tomas Palecek, M.; Stepan Havranek, M.; Vitkova, I.; Nemecek, E.; Trckova, R.; Krsek, D.; Podzimkova, J.; Fikrle, M.; Danek, B.A. Recent-onset dilated cardiomyopathy associated with Borrelia burgdorferi infection. Herz 2015, 40, 892. [Google Scholar] [CrossRef]
- N’guyen, Y.; Lesaffre, F.; Metz, D.; de Martino, S.; Jaulhac, B.; Andréoletti, L. No serological evidence for Borrelia burgdorferi sensu lato infection in patients with dilated cardiomyopathy in Northern France. Infectious Diseases 2016, 48, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.; Dusleag, J.; Reisinger, E. Reversal by ceftriaxone of dilated cardiomyopathy Borrelia burgdorferi infection. 1992.
- Kubánek, M.; Šramko, M.; Berenová, D.; Hulínská, D.; Hrbáčková, H.; Malušková, J.; Lodererová, A.; Málek, I.; Kautzner, J. Detection of Borrelia burgdorferi sensu lato in endomyocardial biopsy specimens in individuals with recent-onset dilated cardiomyopathy. European journal of heart failure 2012, 14, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Dattwyler, R.J.; Luft, B.J.; Kunkel, M.J.; Finkel, M.F.; Wormser, G.P.; Rush, T.J.; Grunwaldt, E.; Agger, W.A.; Franklin, M.; Oswald, D. Ceftriaxone compared with doxycycline for the treatment of acute disseminated Lyme disease. New England Journal of Medicine 1997, 337, 289–295. [Google Scholar] [CrossRef]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. International journal of systematic and evolutionary microbiology 2016, 66, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Sigal, L.H. Early disseminated Lyme disease: cardiac manifestations. The American journal of medicine 1995, 98, 25S–29S. [Google Scholar] [CrossRef]
- Pinto, D.S. Cardiac manifestations of Lyme disease. Medical Clinics 2002, 86, 285–296. [Google Scholar] [CrossRef]
- Ogrinc, K.; Lusa, L.; Lotrič-Furlan, S.; Bogovič, P.; Stupica, D.; Cerar, T.; Ružić-Sabljić, E.; Strle, F. Course and outcome of early European Lyme neuroborreliosis (Bannwarth syndrome): clinical and laboratory findings. Reviews of Infectious Diseases 2016, 63, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. Verlauf der akuten und chronischen Neuroborreliose nach Behandlung mit Ceftriaxon. Der Nervenarzt 2004, 6, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Stjernberg, L.; Ornstein, K.; Tykesson-Joelsson, K.; Walter, H. 5-y Follow-up study of patients with neuroborreliosis. Scandinavian journal of infectious diseases 2002, 34, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Dersch, R.; Sommer, H.; Rauer, S.; Meerpohl, J. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. Journal of neurology 2016, 263, 17–24. [Google Scholar] [CrossRef] [PubMed]
- STEERE, A.C.; SCHOEN, R.T.; TAYLOR, E. The clinical evolution of Lyme arthritis. Annals of internal medicine 1987, 107, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Caperton, E.M.; Heim-Duthoy, K.L.; Matzke, G.R.; Peterson, P.K.; Johnson, R.C. Ceftriaxone therapy of chronic inflammatory arthritis. Archives of Internal Medicine 1990, 150, 1677–1682. [Google Scholar] [CrossRef]
- Steere, A.C.; Green, J.; Schoen, R.T.; Taylor, E.; Hutchinson, G.J.; Rahn, D.W.; Malawista, S.E. Successful parenteral penicillin therapy of established Lyme arthritis. New England Journal of Medicine 1985, 312, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Dattwyler, R.; Volkman, D.; Halperin, J.; Luft, B. Treatment of late Lyme borreliosis—randomised comparison of ceftriaxone and penicillin. The Lancet 1988, 331, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C. Treatment of Lyme arthritis. 2019, 46, 871–873.
- Steere, A.C.; Levin, R.E.; Molloy, P.J.; Kalish, R.A.; Abraham Iii, J.H.; Liu, N.Y.; Schmid, C.H. Treatment of Lyme arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 1994, 37, 878–888. [Google Scholar]
- Stanek, G.; Wormser, G.P.; Gray, J.; Strle, F. Lyme borreliosis. The Lancet 2012, 379, 461–473. [Google Scholar] [CrossRef]
- Muellegger, R.R.; Glatz, M. Skin manifestations of lyme borreliosis: diagnosis and management. American journal of clinical dermatology 2008, 9, 355–368. [Google Scholar] [CrossRef]
- Lampert, F.; Belohradsky, B.; Förster, C.; Eife, R.; Kollmann, D.; Stochdorph, O.; Gokel, J.; Meister, P.; Lampert, P. Infantile chronic relapsing inflammation of the brain, skin, and joints. The Lancet 1975, 305, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Nadelman, R.B.; Dattwyler, R.J.; Dennis, D.T.; Shapiro, E.D.; Steere, A.C.; Rush, T.J.; Rahn, D.W.; Coyle, P.K.; Persing, D.H. Practice guidelines for the treatment of Lyme disease. Clinical infectious diseases 2000, 31, S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Muanda, F.T.; Sheehy, O.; Bérard, A. Use of antibiotics during pregnancy and risk of spontaneous abortion. Cmaj 2017, 189, E625–E633. [Google Scholar] [CrossRef] [PubMed]
- Muanda, F.T.; Sheehy, O.; Bérard, A. Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. British journal of clinical pharmacology 2017, 83, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Wormser, R.P.; Strle, F.; Myers, R.; Cunha, B.A. How safe is doxycycline for young children or for pregnant or breastfeeding women? Diagnostic microbiology and infectious disease 2019, 93, 238–242. [Google Scholar] [CrossRef]
- Silver, H.M. Lyme disease during pregnancy. Infectious Disease Clinics 1997, 11, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Steere, A.C.; Benach, J.L.; Slade, J.D.; Broome, C.V. Lyme disease during pregnancy. Jama 1986, 255, 3394–3396. [Google Scholar] [CrossRef] [PubMed]
- Strobino, B.A.; Williams, C.L.; Abid, S.; Ghalson, R.; Spierling, P. Lyme disease and pregnancy outcome: A prospective s of two thousand prenatal patients. American journal of obstetrics and gynecology 1993, 169, 367–374. [Google Scholar] [CrossRef]
- Cook, M.J.; Moynan, D.; Avramovic, G.; Lambert, J.S. An In-Depth Review of the Benefits of Antibiotic Use in the Treatment of Borreliosis in Pregnancy. Applied Microbiology 2023, 3, 312–321. [Google Scholar] [CrossRef]
- Lakos, A.; Solymosi, N. Maternal Lyme borreliosis and pregnancy outcome. International Journal of Infectious Diseases 2010, 14, e494–e498. [Google Scholar] [CrossRef]
- Williams, C.; Strobino, B.; Weinstein, A.; Spierling, P.; Medici, F. Maternal Lyme disease and congenital malformations: a cord blood serosurvey in endemic and control areas. Paediatric and Perinatal Epidemiology 1995, 9, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Strobino, B.; Abid, S.; Gewitz, M. Maternal Lyme disease and congenital heart disease: a case-control study in an endemic area. American journal of obstetrics and gynecology 1999, 180, 711–716. [Google Scholar] [CrossRef]
- Shapiro, E.D.; Gerber, M.A.; Holabird, N.B.; Berg, A.T.; Feder Jr, H.M.; Bell, G.L.; Rys, P.N.; Persing, D.H. A controlled trial of antimicrobial prophylaxis for Lyme disease after deer-tick bites. New England Journal of Medicine 1992, 327, 1769–1773. [Google Scholar] [CrossRef]
- Agre, F.; Schwartz, R. The value of early treatment of deer tick bites for the prevention of Lyme disease. American journal of diseases of children 1993, 147, 945–947. [Google Scholar] [CrossRef]
- McGready, R.; Hamilton, K.a.; Simpson, J.a.; Cho, T.; Luxemburger, C.; Edwards, R.; Looareesuwan, S.; White, N.; Nosten, F.; Lindsay, S. Safety of the insect repellent N, N-diethyl-M-toluamide (DEET) in pregnancy. American Journal of Tropical Medicine and Hygiene 2001, 65. [Google Scholar] [CrossRef] [PubMed]
- Koren, G.; Matsui, D.; Bailey, B. DEET-based insect repellents: safety implications for children and pregnant and lactating women. Cmaj 2003, 169, 209–212. [Google Scholar]
- Strausz, S.; Abner, E.; Blacker, G.; Galloway, S.; Hansen, P.; Feng, Q.; Lee, B.T.; Jones, S.E.; Haapaniemi, H.; Raak, S. SCGB1D2 inhibits growth of Borrelia burgdorferi and affects susceptibility to Lyme disease. Nature Communications 2024, 15, 2041. [Google Scholar] [CrossRef]
- Shen, S.; Shin, J.J.; Strle, K.; McHugh, G.; Li, X.; Glickstein, L.J.; Drouin, E.E.; Steere, A.C. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum 2010, 62, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Strle, K.; Sulka, K.B.; Pianta, A.; Crowley, J.T.; Arvikar, S.L.; Anselmo, A.; Sadreyev, R.; Steere, A.C. T-Helper 17 Cell Cytokine Responses in Lyme Disease Correlate With Borrelia burgdorferi Antibodies During Early Infection and With Autoantibodies Late in the Illness in Patients With Antibiotic-Refractory Lyme Arthritis. Clin Infect Dis 2017, 64, 930–938. [Google Scholar] [CrossRef]
- Vudattu, N.K.; Strle, K.; Steere, A.C.; Drouin, E.E. Dysregulation of CD4+CD25(high) T cells in the synovial fluid of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheum 2013, 65, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Shapiro, E.D.; Soffer, G.K. A Review of Post-treatment Lyme Disease Syndrome and Chronic Lyme Disease for the Practicing Immunologist. Clin Rev Allergy Immunol 2021. [Google Scholar] [CrossRef] [PubMed]
- Cerar, T.; Ogrinc, K.; Lotric-Furlan, S.; Kobal, J.; Levicnik-Stezinar, S.; Strle, F.; Ruzic-Sabljic, E. Diagnostic value of cytokines and chemokines in lyme neuroborreliosis. Clin Vaccine Immunol 2013, 20, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Strle, K.; Shin, J.J.; Glickstein, L.J.; Steere, A.C. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 2012, 64, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Hawn, T.R.; Misch, E.A.; Dunstan, S.J.; Thwaites, G.E.; Lan, N.T.; Quy, H.T.; Chau, T.T.; Rodrigues, S.; Nachman, A.; Janer, M.; et al. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol 2007, 37, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, P.J.; Stocks, K.-L.K.; Karozichian, E.S.; Pandit, A.; Hu, L.T. Metabolic modeling predicts unique drug targets in Borrelia burgdorferi. Msystems 2023, 8, e00835–e00823. [Google Scholar] [CrossRef] [PubMed]
- Wagh, D.; Pothineni, V.R.; Inayathullah, M.; Liu, S.; Kim, K.-M.; Rajadas, J. Borreliacidal activity of Borrelia metal transporter A (BmtA) binding small molecules by manganese transport inhibition. Drug design, development and therapy 2015, 805-816.
- Boylan, J.A.; Gherardini, F.C. Determining the cellular targets of reactive oxygen species in Borrelia burgdorferi. Bacterial Pathogenesis: Methods and Protocols 2008, 213-221.
- Boylan, J.A.; Lawrence, K.A.; Downey, J.S.; Gherardini, F.C. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Molecular microbiology 2008, 68, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, T.A.; Lin, Y.-H.; Miller, C.L.; Karna, S.R.; Chambers, J.P.; Seshu, J. Effect of levels of acetate on the mevalonate pathway of Borrelia burgdorferi. PloS one 2012, 7, e38171. [Google Scholar] [CrossRef] [PubMed]
- Stupica, D.; Bajrović, F.F.; Blagus, R.; Cerar Kišek, T.; Collinet-Adler, S.; Ružić-Sabljić, E.; Velušček, M. Association between statin use and clinical course, microbiologic characteristics, and long-term outcome of early Lyme borreliosis. A post hoc analysis of prospective clinical trials of adult patients with erythema migrans. Plos one 2021, 16, e0261194. [Google Scholar] [CrossRef]
- Van Laar, T.A.; Hole, C.; Karna, S.R.; Miller, C.L.; Reddick, R.; Wormley, F.L.; Seshu, J. Statins reduce spirochetal burden and modulate immune responses in the C3H/HeN mouse model of Lyme disease. Microbes and infection 2016, 18, 430–435. [Google Scholar] [CrossRef]
- Parveen, N.; Cornell, K.A.; Bono, J.L.; Chamberland, C.; Rosa, P.; Leong, J.M. Bgp, a secreted glycosaminoglycan-binding protein of Borrelia burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infection and immunity 2006, 74, 3016–3020. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Cornell, K.A. Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Molecular microbiology 2011, 79, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Cornell, K.A.; Primus, S.; Martinez, J.A.; Parveen, N. Assessment of methylthioadenosine/S-adenosylhomocysteine nucleosidases of Borrelia burgdorferi as targets for novel antimicrobials using a novel high-throughput method. Journal of antimicrobial chemotherapy 2009, 63, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, M.; Schlachter, S.; Primus, S.; Wagner, J.; Sweet, B.; Carr, Z.; Cornell, K.A.; Parveen, N. Evaluation of nucleoside analogs as antimicrobials targeting unique enzymes in borrelia burgdorferi. Pathogens 2020, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Brown, A.V.; Matluck, N.E.; Hu, L.T.; Lewis, K. Borrelia burgdorferi, the causative agent of Lyme disease, forms drug-tolerant persister cells. Antimicrobial agents and chemotherapy 2015, 59, 4616–4624. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nature Reviews Microbiology 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerging microbes & infections 2014, 3, 1–8. [Google Scholar]
- Marques, A. Chronic Lyme disease: a review. Infectious disease clinics of North America 2008, 22, 341–360. [Google Scholar] [CrossRef]
- Jutras, B.L.; Lochhead, R.B.; Kloos, Z.A.; Biboy, J.; Strle, K.; Booth, C.J.; Govers, S.K.; Gray, J.; Schumann, P.; Vollmer, W. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proceedings of the National Academy of Sciences 2019, 116, 13498–13507. [Google Scholar] [CrossRef]
- DeHart, T.G.; Kushelman, M.R.; Hildreth, S.B.; Helm, R.F.; Jutras, B.L. The unusual cell wall of the Lyme disease spirochaete Borrelia burgdorferi is shaped by a tick sugar. Nature Microbiology 2021, 6, 1583–1592. [Google Scholar] [CrossRef]
- Melia, M.T.; Auwaerter, P.G. Time for a different approach to Lyme disease and long-term symptoms. N Engl J Med 2016, 374, 1277–1278. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Vrijmoeth, H.D.; van de Schoor, F.; Hovius, J.W. Lyme borreliosis: diagnosis and management. Bmj 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- ED, S. Clinical practice: Lyme disease. N Engl J Med 2014, 371, 683–684. [Google Scholar] [CrossRef]
- Feder Jr, H.M.; Johnson, B.J.; O'Connell, S.; Shapiro, E.D.; Steere, A.C.; Wormser, G.P.; Group, A.H.I.L.D. A critical appraisal of “chronic Lyme disease”. New England Journal of Medicine 2007, 357, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Zubcevik, N.; Mao, C.; Wang, Q.M.; Bose, E.L.; Octavien, R.N.; Crandell, D.; Wood, L.J. Symptom clusters and functional impairment in individuals treated for Lyme borreliosis. Frontiers in Medicine 2020, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M.; Frost, A. Illness perceptions, coping, and health-related quality of life among individuals experiencing chronic Lyme disease. Chronic Illness 2022, 18, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Baker, P.J.; Shapiro, E.D.; Marques, A.; Dattwyler, R.J.; Halperin, J.J.; Wormser, G.P. Treatment trials for post-Lyme disease symptoms revisited. The American journal of medicine 2013, 126, 665–669. [Google Scholar] [CrossRef]
- Chaconas, G.; Castellanos, M.; Verhey, T.B. Changing of the guard: How the Lyme disease spirochete subverts the host immune response. Journal of Biological Chemistry 2020, 295, 301–313. [Google Scholar] [CrossRef]
- Tracy, K.E.; Baumgarth, N. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Frontiers in immunology 2017, 8, 241161. [Google Scholar] [CrossRef]
- Feng, J.; Li, T.; Yee, R.; Yuan, Y.; Bai, C.; Cai, M.; Shi, W.; Embers, M.; Brayton, C.; Saeki, H. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discovery medicine 2019, 27, 125–138. [Google Scholar]
- Caskey, J.R.; Embers, M.E. Persister development by Borrelia burgdorferi populations in vitro. Antimicrobial agents and chemotherapy 2015, 59, 6288–6295. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Zhang, Y. Persister mechanisms in Borrelia burgdorferi: implications for improved intervention. Emerging microbes & infections 2015, 4, 1–3. [Google Scholar]
- Cabello, F.C.; Godfrey, H.P.; Bugrysheva, J.V.; Newman, S.A. Sleeper cells: the stringent response and persistence in the Borreliella (Borrelia) burgdorferi enzootic cycle. Environmental microbiology 2017, 19, 3846–3862. [Google Scholar] [CrossRef]
- Hodzic, E.; Imai, D.M.; Escobar, E. Generality of post-antimicrobial treatment persistence of Borrelia burgdorferi strains N40 and B31 in genetically susceptible and resistant mouse strains. Infection and immunity 2019, 87. [Google Scholar] [CrossRef]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.-F.; Appel, M. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. Journal of clinical microbiology 1997, 35, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Nadelman, R.B.; Nowakowski, J.; Forseter, G.; Bittker, S.; Cooper, D.; Goldberg, N.; McKenna, D.; Wormser, G.P. Failure to isolate Borrelia burgdorferi after antimicrobial therapy in culture-documented Lyme borreliosis associated with erythema migrans: report of a prospective study. The American journal of medicine 1993, 94, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J.; Wormser, G.P. The clinical relevance of studies on Borrelia burgdorferi persisters. The American Journal of Medicine 2017, 130, 1009–1010. [Google Scholar] [CrossRef]
- Hunfeld, K.-P.; Kraiczy, P.; Kekoukh, E.; Schäfer, V.; Brade, V. Standardised in vitro susceptibility testing of Borrelia burgdorferi against well-known and newly developed antimicrobial agents—possible implications for new therapeutic approaches to Lyme disease. International journal of medical microbiology 2002, 291, 125–137. [Google Scholar] [CrossRef]
- Steere, A.C. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. The Journal of clinical investigation 2020, 130, 2148–2151. [Google Scholar] [CrossRef]
- Aucott, J.N.; Soloski, M.J.; Rebman, A.W.; Crowder, L.A.; Lahey, L.J.; Wagner, C.A.; Robinson, W.H.; Bechtold, K.T. CCL19 as a chemokine risk factor for posttreatment Lyme disease syndrome: a prospective clinical cohort study. Clinical and Vaccine Immunology 2016, 23, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Strle, K.; Stupica, D.; Drouin, E.E.; Steere, A.C.; Strle, F. Elevated levels of IL-23 in a subset of patients with post–Lyme disease symptoms following erythema migrans. Clinical infectious diseases 2014, 58, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Li, X.; Wormser, G.P.; Marques, A.; Alaedini, A. Expression of C-reactive protein and serum amyloid A in early to late manifestations of Lyme disease. Clinical infectious diseases 2016, 63, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Jacek, E.; Fallon, B.A.; Chandra, A.; Crow, M.K.; Wormser, G.P.; Alaedini, A. Increased IFNα activity and differential antibody response in patients with a history of Lyme disease and persistent cognitive deficits. Journal of neuroimmunology 2013, 255, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.H.; Johnson, G.M.; Trevino, R.P.; Norton, D.; Levy, L.; Wall, D.; McCall, J. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. New England Journal of Medicine 2001, 345, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. Bmj 2006, 333, 575. [Google Scholar] [CrossRef] [PubMed]
- Batheja, S.; Nields, J.A.; Landa, A.; Fallon, B.A. Post-treatment lyme syndrome and central sensitization. The Journal of neuropsychiatry and clinical neurosciences 2013, 25, 176–186. [Google Scholar] [CrossRef]
- Wills, A.B.; Spaulding, A.B.; Adjemian, J.; Prevots, D.R.; Turk, S.-P.; Williams, C.; Marques, A. Long-term follow-up of patients with Lyme disease: longitudinal analysis of clinical and quality-of-life measures. Clinical Infectious Diseases 2016, 62, 1546–1551. [Google Scholar] [CrossRef]
- Aucott, J.N.; Yang, T.; Yoon, I.; Powell, D.; Geller, S.A.; Rebman, A.W. Risk of post-treatment Lyme disease in patients with ideally-treated early Lyme disease: A prospective cohort study. International Journal of Infectious Diseases 2022, 116, 230–237. [Google Scholar] [CrossRef]
- Hirsch, A.G.; Poulsen, M.N.; Nordberg, C.; Moon, K.A.; Rebman, A.W.; Aucott, J.N.; Heaney, C.D.; Schwartz, B.S. Risk factors and outcomes of treatment delays in Lyme disease: a population-based retrospective cohort study. Frontiers in Medicine 2020, 7, 560018. [Google Scholar] [CrossRef]
- Kaplan, R.; Trevino, R.; Johnson, G.; Levy, L.; Dornbush, R.; Hu, L.; Evans, J.; Weinstein, A.; Schmid, C.; Klempner, M. Cognitive function in post-treatment Lyme disease Do additional antibiotics help? Neurology 2003, 60, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD) A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef]
- Patel, R.; Grogg, K.L.; Edwards, W.D.; Wright, A.J.; Schwenk, N.M. Death from inappropriate therapy for Lyme disease. Clinical infectious diseases 2000, 31, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Berende, A.; ter Hofstede, H.J.; Vos, F.J.; van Middendorp, H.; Vogelaar, M.L.; Tromp, M.; van den Hoogen, F.H.; Donders, A.R.T.; Evers, A.W.; Kullberg, B.J. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. New England Journal of Medicine 2016, 374, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Goodlet, K.J.; Fairman, K.A. Adverse events associated with antibiotics and intravenous therapies for post–Lyme disease syndrome in a commercially insured sample. Clinical Infectious Diseases 2018, 67, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M.; Rumbaugh, J.; Bockenstedt, L.K.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.R.; Belani, K.K.; Bowie, W.R. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme disease. Clinical Infectious Diseases 2021, 72, e1–e48. [Google Scholar] [CrossRef]
- Marzec, N.S. Serious bacterial infections acquired during treatment of patients given a diagnosis of chronic Lyme disease—United States. MMWR. Morbidity and mortality weekly report 2017, 66. [Google Scholar] [CrossRef] [PubMed]
- Stiehm, E.R. Adverse effects of human immunoglobulin therapy. Transfusion medicine reviews 2013, 27, 171–178. [Google Scholar] [CrossRef]
- De Wilde, M.; Speeckaert, M.; Callens, R.; Van Biesen, W. Ceftriaxone-induced immune hemolytic anemia as a life-threatening complication of antibiotic treatment of ‘chronic Lyme disease’. Acta clinica Belgica 2017, 72, 133–137. [Google Scholar] [CrossRef]
- Maksimyan, S.; Syed, M.S.; Soti, V. Post-treatment Lyme disease syndrome: need for diagnosis and treatment. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Bockenstedt, L.K.; Radolf, J.D. Editorial commentary: xenodiagnosis for posttreatment Lyme disease syndrome: resolving the conundrum or adding to it? 2014, 58, 946–948.
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrobial agents and chemotherapy 2008, 52, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Kamp, H.D.; Swanson, K.A.; Wei, R.R.; Dhal, P.K.; Dharanipragada, R.; Kern, A.; Sharma, B.; Sima, R.; Hajdusek, O.; Hu, L.T. Design of a broadly reactive Lyme disease vaccine. npj Vaccines 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Comstedt, P.; Hanner, M.; Schüler, W.; Meinke, A.; Lundberg, U. Design and development of a novel vaccine for protection against Lyme borreliosis. PloS one 2014, 9, e113294. [Google Scholar]
- Comstedt, P.; Hanner, M.; Schüler, W.; Meinke, A.; Schlegl, R.; Lundberg, U. Characterization and optimization of a novel vaccine for protection against Lyme borreliosis. Vaccine 2015, 33, 5982–5988. [Google Scholar] [CrossRef] [PubMed]
- Pine, M.; Arora, G.; Hart, T.M.; Bettini, E.; Gaudette, B.T.; Muramatsu, H.; Tombácz, I.; Kambayashi, T.; Tam, Y.K.; Brisson, D. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease. Molecular Therapy 2023, 31, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Marconi, R.T.; Garcia-Tapia, D.; Hoevers, J.; Honsberger, N.; King, V.L.; Ritter, D.; Schwahn, D.J.; Swearingin, L.; Weber, A.; Winkler, M.T.C. VANGUARD® crLyme: A next generation Lyme disease vaccine that prevents B. burgdorferi infection in dogs. Vaccine: X 2020, 6, 100079. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, A.L.; Lieknina, I.; Kotelovica, S.; Yang, X.; Kraiczy, P.; Pal, U.; Lin, Y.-P.; Tars, K. Eliminating factor H-binding activity of Borrelia burgdorferi CspZ combined with virus-like particle conjugation enhances its efficacy as a Lyme disease vaccine. Frontiers in immunology 2018, 9, 325322. [Google Scholar] [CrossRef]
- Phase 2 Study Of VLA15, A Vaccine Candidate Against Lyme Borreliosis, In A Healthy Pediatric And Adult Study Population. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04801420 (accessed on.
- Dai, J.; Wang, P.; Adusumilli, S.; Booth, C.J.; Narasimhan, S.; Anguita, J.; Fikrig, E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell host & microbe 2009, 6, 482–492. [Google Scholar]
- Bobe, J.R.; Jutras, B.L.; Horn, E.J.; Embers, M.E.; Bailey, A.; Moritz, R.L.; Zhang, Y.; Soloski, M.J.; Ostfeld, R.S.; Marconi, R.T. Recent progress in Lyme disease and remaining challenges. Frontiers in medicine 2021, 8, 666554. [Google Scholar] [CrossRef]
- Levin, S.; Harris, A.A. Principles of combination therapy. Bulletin of the New York Academy of Medicine 1975, 51, 1020. [Google Scholar] [PubMed]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS microbiology letters 2004, 230, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Spoering, A.L.; Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. Journal of bacteriology 2001, 183, 6746–6751. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L.; Quiroz-Gonzalez, S.; Torres-Rosas, R. Nerve stimulation: immunomodulation and control of inflammation. Trends in molecular medicine 2017, 23, 1103–1120. [Google Scholar] [CrossRef] [PubMed]
- Akoolo, L.; Djokic, V.; Rocha, S.C.; Ulloa, L.; Parveen, N. Sciatic–Vagal Nerve Stimulation by Electroacupuncture Alleviates Inflammatory Arthritis in Lyme Disease-Susceptible C3H Mice. Frontiers in immunology 2022, 13, 930287. [Google Scholar] [CrossRef]
- Adams, A.; Hipple, A.; Thompson, S.H. Acupuncture and Chinese Herbs Relieve Long-Term Symptoms of Lyme Disease: A Case Report. Convergent Points: An East-West Case Report Journal 2023, 2. [Google Scholar]
- Bartecchi, C.E. Efficacy of acupuncture vs placebo. In Proceedings of the Mayo Clinic Proceedings; 2006; p. 1263. [Google Scholar]
- Conn, M.; Avery, S.; Bhatia, A.; Alnamos, A.A. Gene Therapy for Post-Treatment Lyme Disease Syndrome.
| Drug | Dosage for adults | Dosage for Children | Duration(Days) | References |
| Doxycycline | 100 mg, twice per day orally | 4.4 mg/kg per day orally, divided into 2 doses | 10–14 | [30,33,35,36,37,38,39,40,41,42] |
| Amoxicillin | 500 mg, three times per day orally | 50 mg/kg per day orally, divided into 3 doses | 14 | [30,33,34,36,40,41,43] |
| Cefuroxime | 500 mg, twice per day orally | 30 mg/kg per day orally, divided into 2 doses | 14 | [30,34,40,43,44,45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





