Submitted:
01 May 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Case Reports
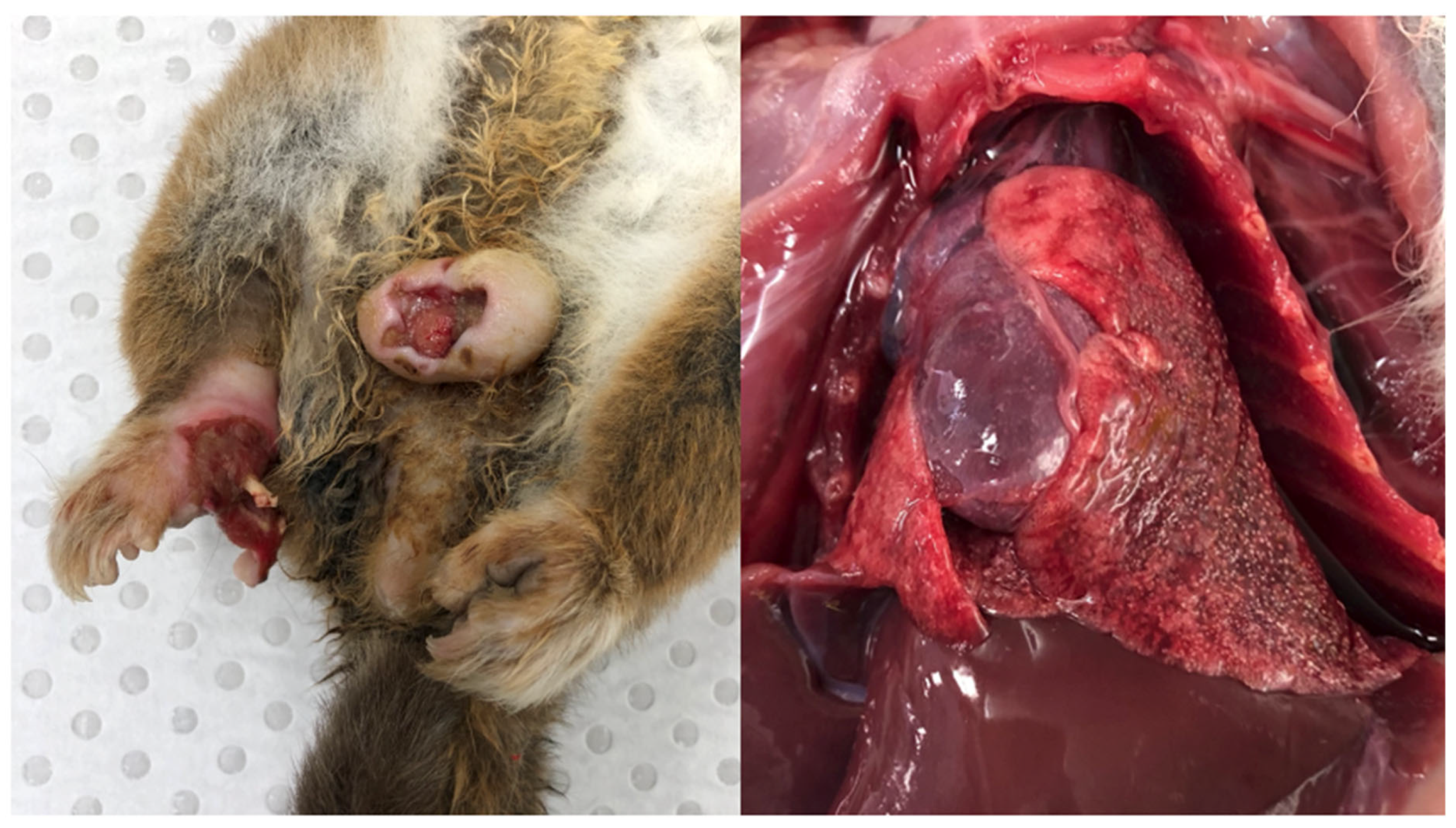
Case 1
History
Post-Mortem Examination
Laboratory Investigations
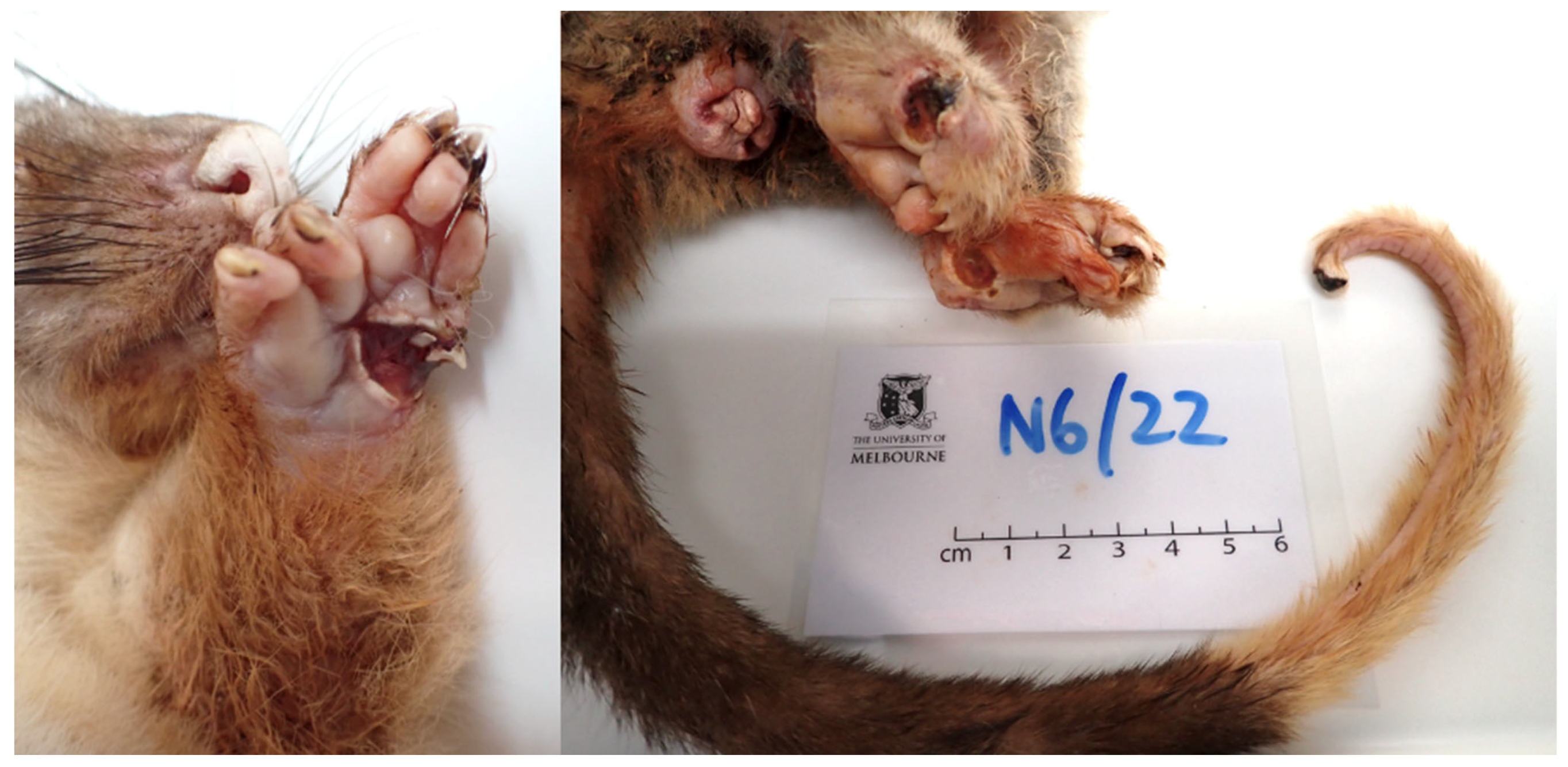
Case 2
History
Post-Mortem Examination
- Single dry erythematous ulcers approximately 1 cm2 in diameter with white margins on the palmar surfaces of each fore paw; two lateral carpal bones of the left paw were exposed;
- Two shallow dry, scabbed lesions approximately 1-1.5 cm2 on the plantar surfaces of each hind paw;
- One shallow erythematous ulcer encircled by white margins surrounding the central cloaca; and
- One approximately 3 mm2 blackened oedematous ulcer with necrotic focus encompassing the distal tail tip.
Laboratory Investigations
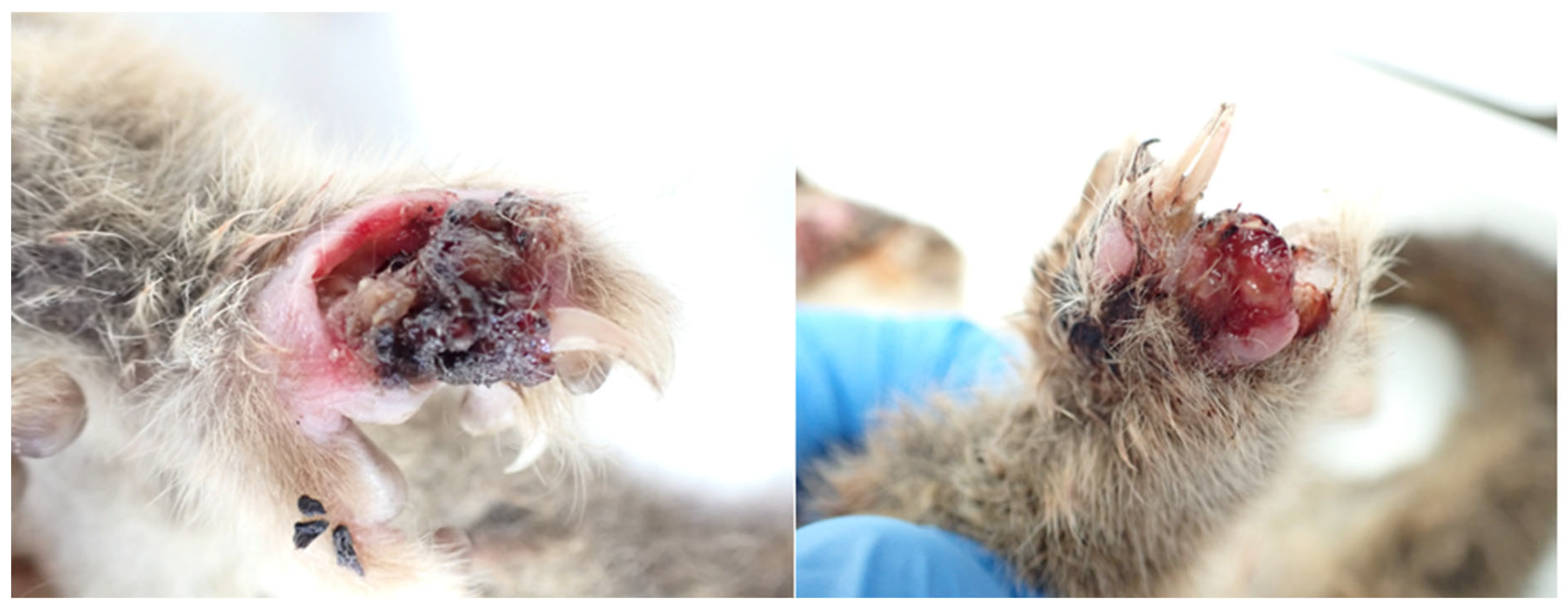
Case 3
History
Post-Mortem Examination
- The right forepaw was markedly oedematous, and bore an approximately 2.5 cm x 1 cm almost circumferential proliferative ulcerative lesion covering most of its palmar aspect and extending partially to the dorsal aspect of digit 1.
- The dorsal surface of the left hind paw was similarly swollen and bore an approximately 2 cm x 1.5 cm ulcer between digits 1 and 2, with deeply undermined caudal wound edges. The ulcer was covered by a plug of necrotic material and extended deep into the underlying muscle and tendon.
- The craniodorsal aspect of digit 4 of the right hind paw was similarly swollen, ulcerated to the level of the underlying subcutis, and contained thick purulent exudate; the nail was absent.
- Two shallow dry circular ulcers were present on the hairless ventral surface of the tail, approximately halfway down the white furred section, and approximately 1.5 cm apart. The proximal ulcer was approximately 3 mm2 and the distal ulcer was approximately 6 mm2.
Laboratory Investigations
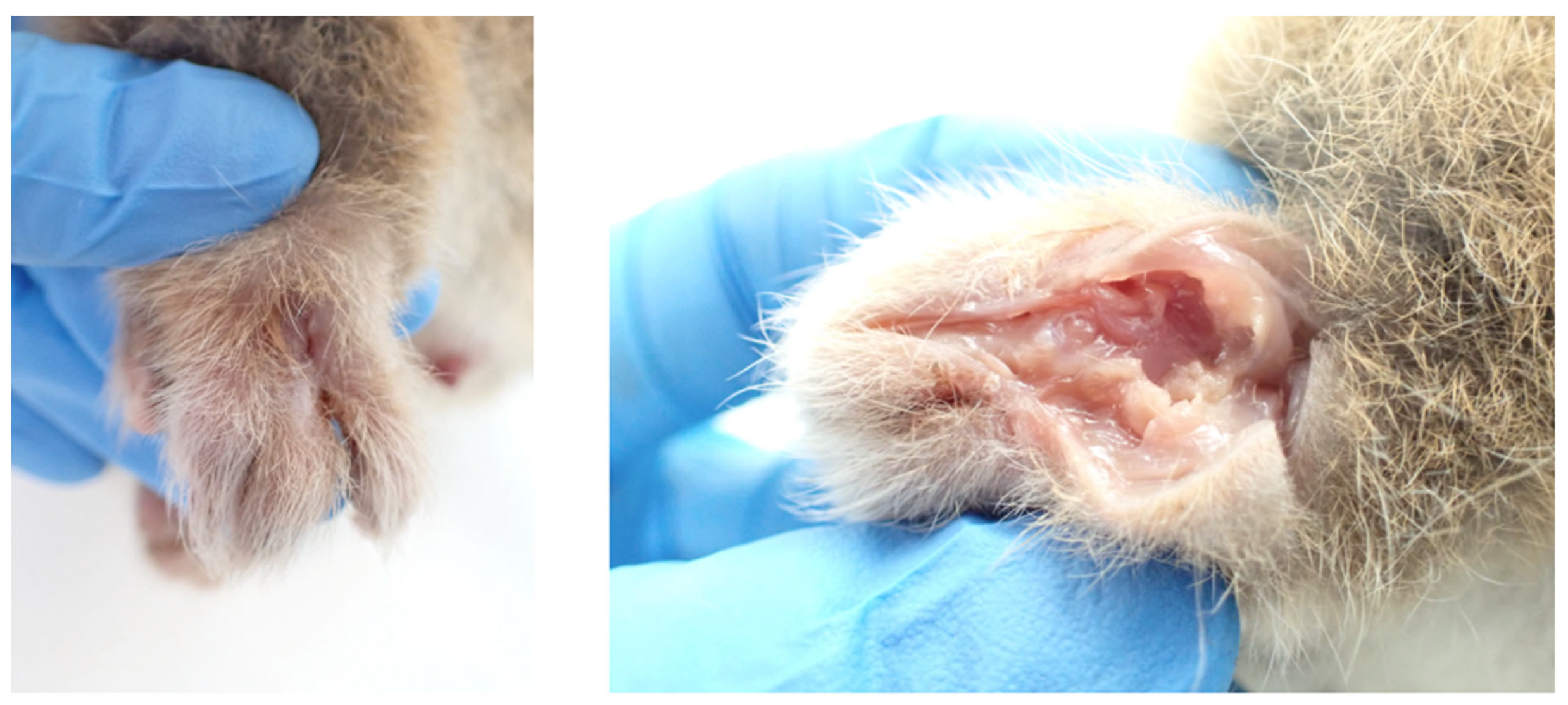
Case 4
History
Post-Mortem Examination
- There was an approximately 4 mm x 2 mm linear epidermal ulcer exuding suppurative material on the dorsal aspect of the markedly oedematous left dorsal hind paw, dissection through which revealed a tunnelling wound that extended through the subcutaneous tissues to a central cavity involving striated muscle, ligaments and tendons (see Figure 4).
- Three discrete, scabbed, dry, 3-5 mm2 ulcerative lesions were present on the ventral surface of the tail: two on the proximal portion, and the third in the proximal portion of the distal white section.
Laboratory Investigations
Discussion
Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mitchell PJ, Jerrett IV, Slee KJ. Skin ulcers caused by Mycobacterium ulcerans in koalas near Bairnsdale, Australia. Pathology 1984;16:256-260. https://pubmed.ncbi.nlm.nih.gov/6514393/.
- 2. Van Zyl A, Daniel J, Wayne J et al. Mycobacterium ulcerans infections in two horses in south-eastern Australia. Aust Vet J 2010;88:101-106. [CrossRef]
- 3. O’Brien CR, McMillan E, Harris O et al. Localised Mycobacterium ulcerans infection in four dogs. Aust Vet J 2011;89:506-510. [CrossRef]
- WHO. WHO Meeting on Buruli ulcer and other skin NTDs- Final report. 25-27 March 2019. World Health Organization (WHO), Geneva, Switzerland, 2019:212. https://apps.who.int/iris/rest/bitstreams/1257101/retrieve.
- DH. Victoria, local public health areas and local government areas surveillance summary report. https://www.health.vic.gov.au/infectious-diseases/local-government-areas-surveillance-report. 2023. Retrieved 12/1/2023.
- Tai AYC, Athan E, Friedman ND et al. Increased Severity and Spread of Mycobacterium ulcerans, Southeastern Australia. Emerg Infect Dis 2018;24:58-64. [CrossRef]
- Fyfe JAM, Lavender CJ, Handasyde KA et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLOS Negl Trop Dis 2010;4:e791. [CrossRef]
- Johnson PDR, Azuolas J, Lavender CJ et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis 2007;13:1653-1660. [CrossRef]
- Mee PT, Buultjens AH, Oliver J et al. A transmission chain linking Mycobacterium ulcerans with Aedes notoscriptus mosquitoes, possums and human Buruli ulcer cases in southeastern Australia. bioRxiv, 2023:39. [CrossRef]
- O’Brien CR, Handasyde KA, Hibble J et al. Clinical, microbiological and pathological findings of Mycobacterium ulcerans infection in three Australian possum species. PLOS Negl Trop Dis 2014;8:e2666. [CrossRef]
- WHO. Treatment of Mycobacterium ulcerans disease (Buruli ulcer)- Guidance for health workers. World Health Organization (WHO), Geneva, Switzerland, 2012:76. https://www.who.int/publications/i/item/9789241503402.
- Ban S, Cox-Whitton K, Grillo T. Mycobacterium ulcerans in a ringtail possum in a new location. Animal Health Surveillance Quarterly 2020;25 (3):13-17. http://www.sciquest.org.nz/elibrary/edition/8052.
- Mellor DJ, Beausoleil NJ, Littlewood KE et al. The 2020 Five Domains Model: including human–animal interactions in assessments of animal welfare. Animals 2020;10:1870. [CrossRef]
- O’Brien DP, Friedman ND, McDonald A et al. Clinical features and risk factors of oedematous Mycobacterium ulcerans lesions in an Australian population: beware cellulitis in an endemic area. PLOS Negl Trop Dis 2014;8:e2612. [CrossRef]
- Yotsu RR, Richardson M, Ishii N. Drugs for treating Buruli ulcer (Mycobacterium ulcerans disease). Cochrane Database Syst Rev 2018. [CrossRef]
- Xu RW, Stinear TP, Johnson PDR, O’Brien DP. Possum bites man: case of Buruli ulcer following possum bite. Med J Aust 2022;216:452-453. https://onlinelibrary.wiley.com/doi/full/10.5694/mja2.51505.
- Wildlife Victoria. Ulcerative lesions in Victorian possums, 2013-2022 [unpublished data]. 2023. www.wildlifevictoria.org.au/.
- DEECA. Victorian Wildlife Rehabilitation Guidelines. https://www.wildlife.vic.gov.au/victorian-wildlife-rehabilitation-guidelines/victorian-wildlife-rehabilitation-guidelines. 2023. Retrieved 10/9/2023.
- Agriculture Victoria. Code of Practice for the Welfare of Wildlife During Rehabilitation. Agriculture Victoria, Canberra, Australia, 2020. https://agriculture.vic.gov.au/livestock-and-animals/animal-welfare-victoria/pocta-act-1986/victorian-codes-of-practice-for-animal-welfare/code-of-practice-for-the-welfare-of-wildlife-during-rehabilitation.
- O’Brien DP, Jenkin G, Buntine J et al. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: guideline update. Med J Aust 2014;200:267-270. [CrossRef]
- Schilling AK, van Hooij A, Lurz PWW et al. Clinical progression of leprosy in Eurasian red squirrels (Sciurus vulgaris) in a naturally infected wild population. J Zoo WIldl Med 2021;52:1159-1166. https://pubmed.ncbi.nlm.nih.gov/34998285/.
- Fitzgerald SD, Kaneene JB. Wildlife reservoirs of bovine tuberculosis worldwide: hosts, pathology, surveillance, and control. Vet Pathol 2013;50:488-499. [CrossRef]
- Miguel E, Grosbois V, Caron A et al. A systemic approach to assess the potential and risks of wildlife culling for infectious disease control. Commun Biol 2020;3. [CrossRef]
- Mysterud A, Rauset GR, Van Moorter B et al. The last moves: The effect of hunting and culling on the risk of disease spread from a population of reindeer. J Appl Ecol 2020;57:2509-2518. [CrossRef]
- Corner LAL, Norton S, Buddle BM, Morris RS. The efficacy of bacille Calmette-Guérin vaccine in wild brushtail possums (Trichosurus vulpecula). Res Vet Sci 2002;73:145-152. [CrossRef]
- Tompkins DM, Ramsey DSL, Cross ML et al. Oral vaccination reduces the incidence of tuberculosis in free-living brushtail possums. Proc Royal Soc B 2009;276:2987-2995. [CrossRef]
- Muhi S, Stinear TP. Systematic review of M. bovis BCG and other candidate vaccines for Buruli ulcer prophylaxis. Vaccine 2021;39:7238-7252. https://www.sciencedirect.com/science/article/pii/S0264410X21007003.
- O’Brien DP, Blasdell K, Muhi S et al. Is BCG vaccination of possums the solution to the Buruli ulcer epidemic in south-eastern Australia? Med J Aust 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




