Submitted:
01 May 2024
Posted:
02 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
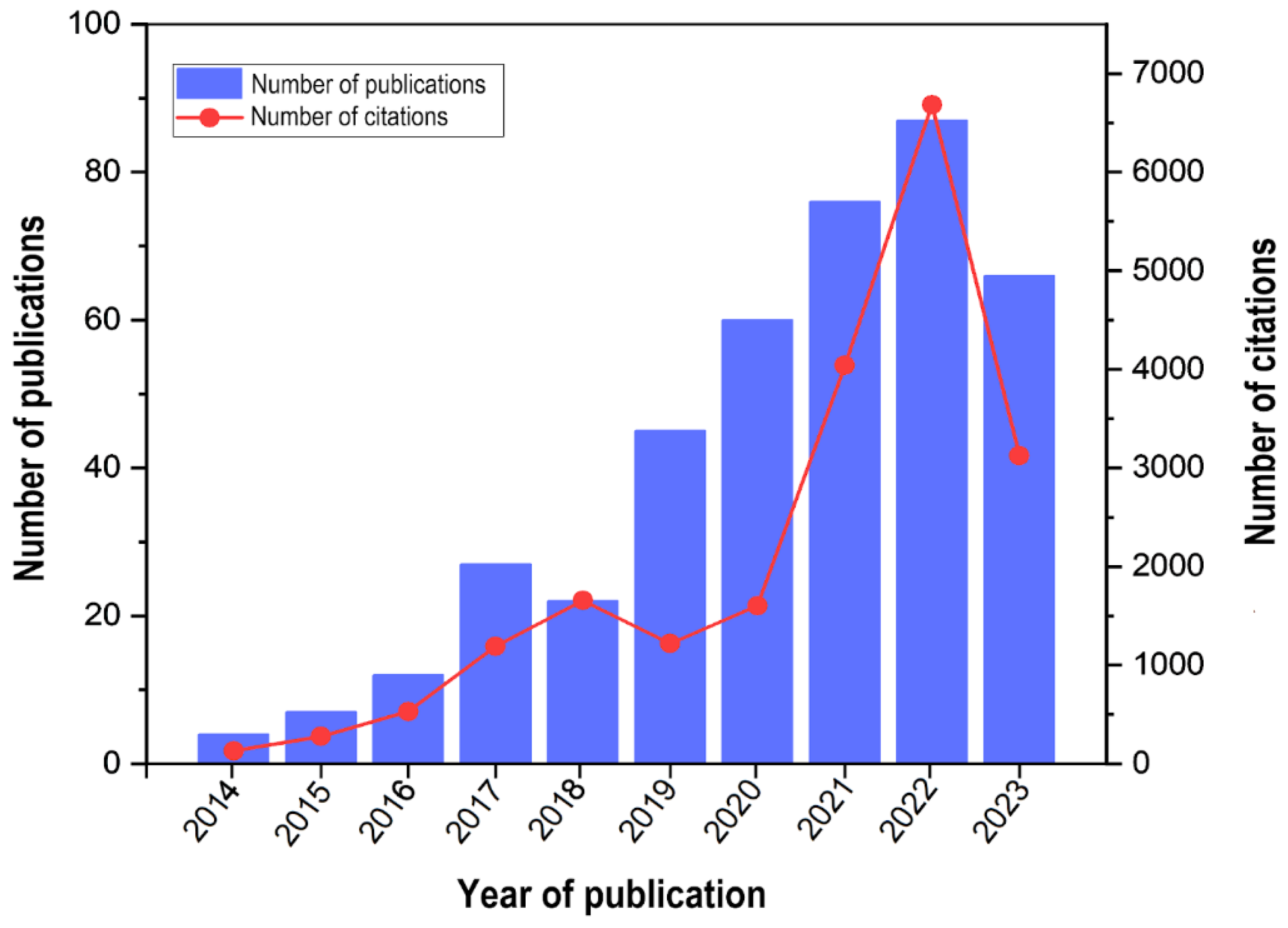
2.1. Dynamics Trends in the Number of Publications
2.2. Keyword Clusters Analysis
2.3. Co-Occurrence Analysis
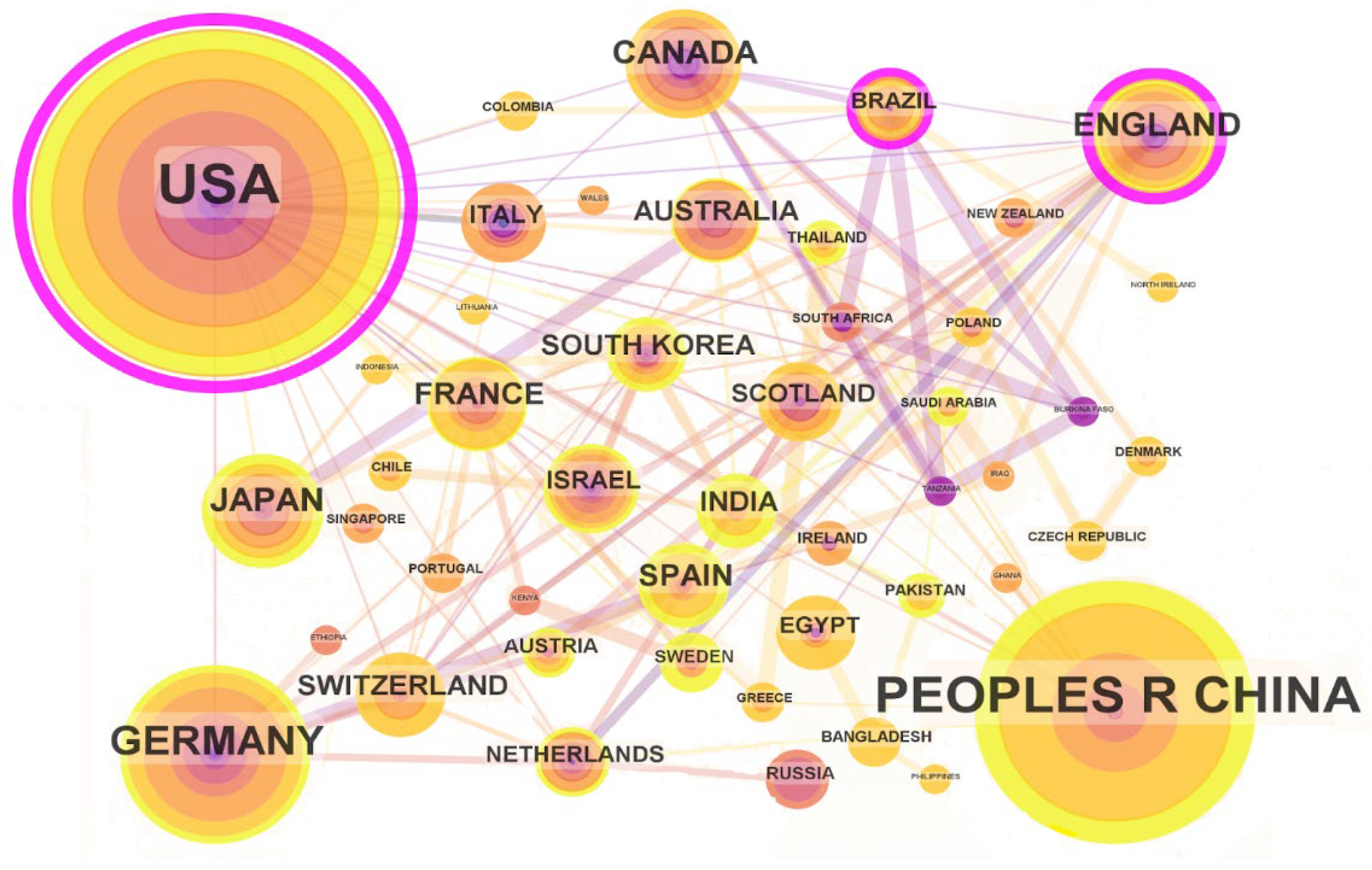
2.3.1. Scientific Production by Country and Institutions
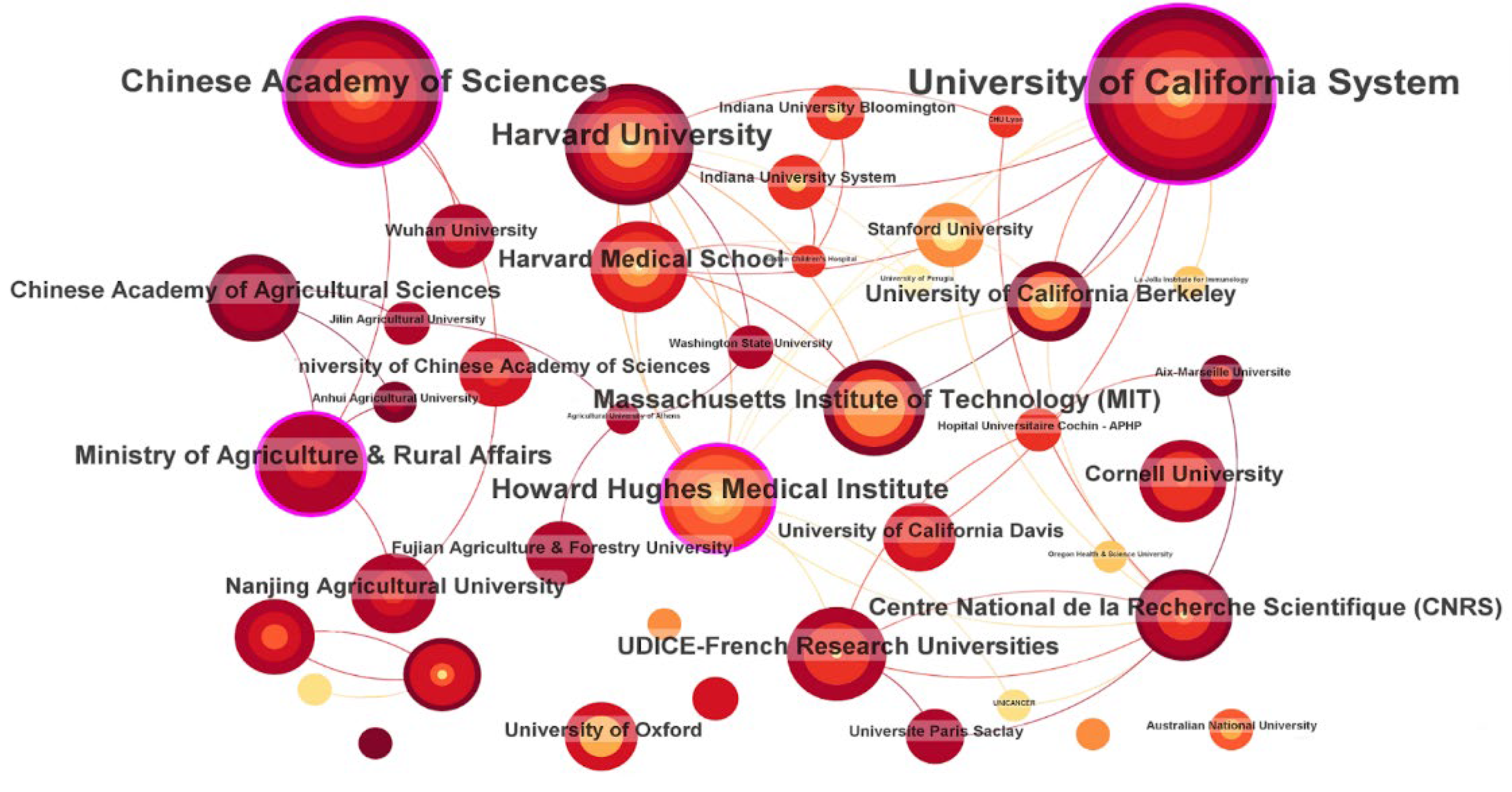
2.3.2. Scientific Production by Institutions
2.4. Co-Citation Analysis
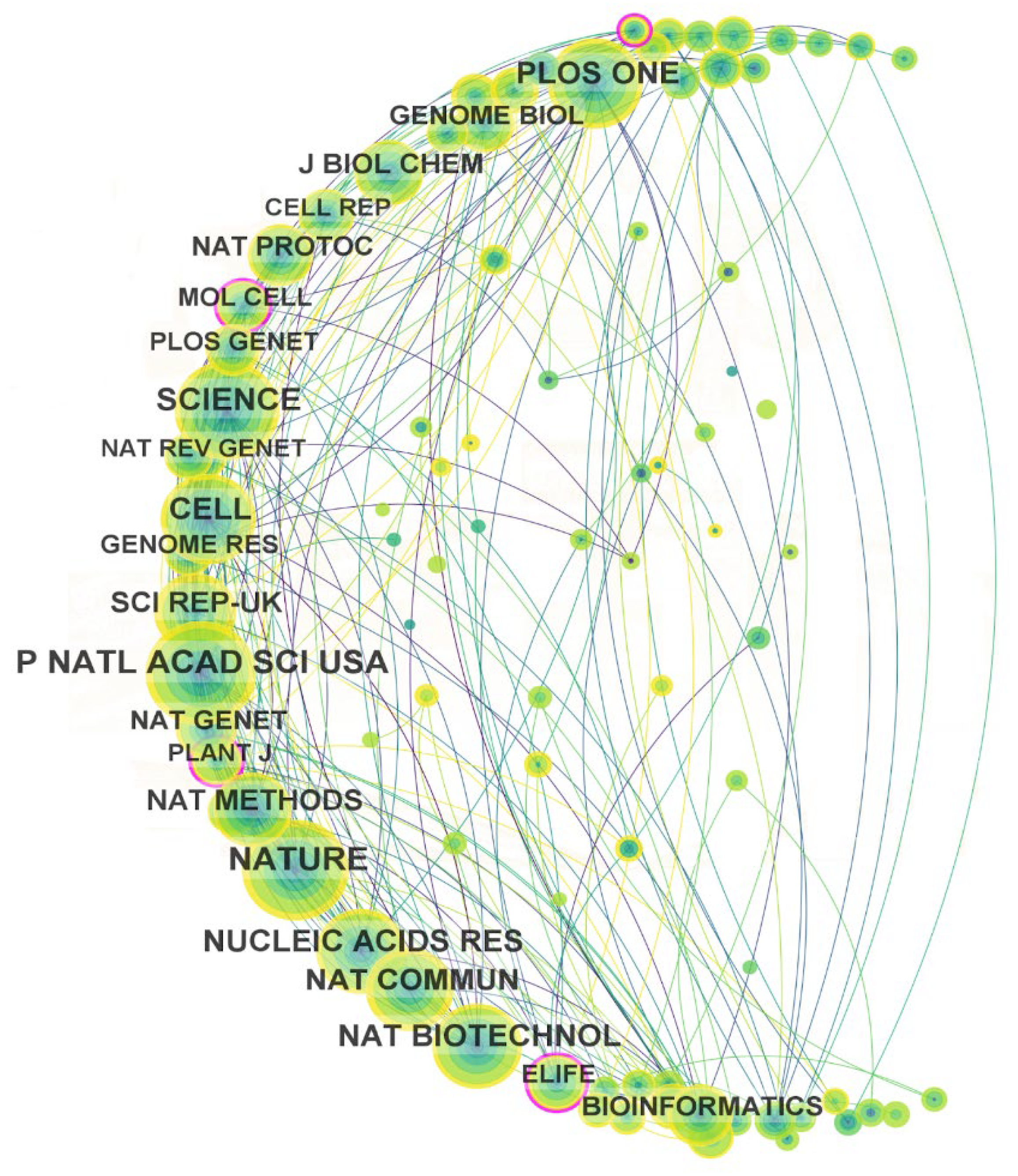
2.4.1. Journal Co-Citation Analysis
2.4.2. Scientific Production by Authors
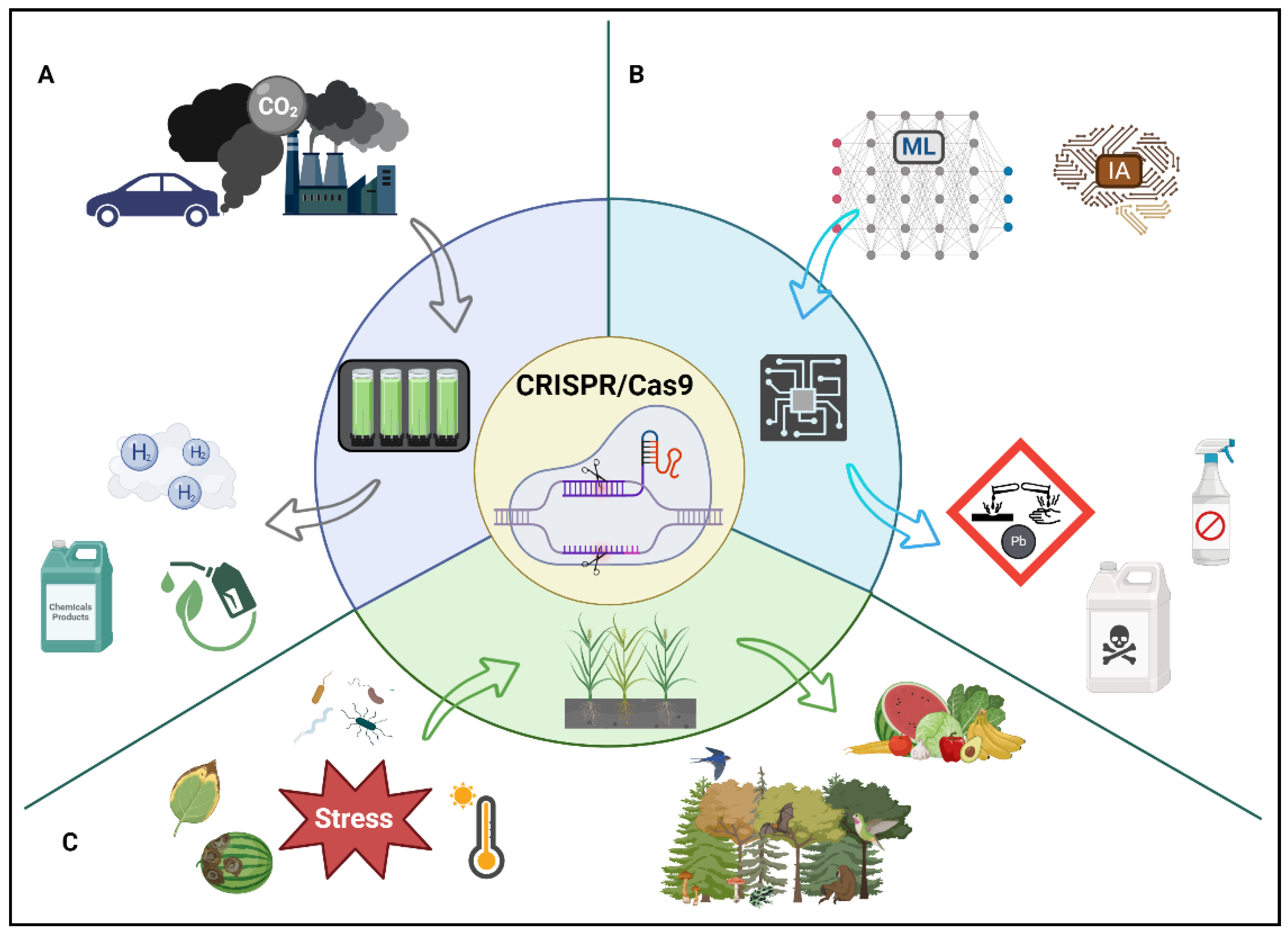
2.5. Applications of CRISPR/Cas9 in Environmental Biotechnology
2.5.1. Fourth-Generation Biofuels
2.5.2. Environmental Monitoring through the Use of Biosensors
2.5.3. CRISPR/Cas9 for Conservation and Sustainability
2.5.4. Enhancing Genetic Resilience
2.5.5. Combating Invasive Species
2.5.6. Sustainable Agriculture
3. Discussion
3.1. Dynamics Trends in the Number of Publications
3.2. Keyword Clusters Analysis
3.3. Co-Occurrence Analysis
3.3.1. Scientific Production by Country and Institutions
3.3.2. Scientific Production by Institutions
3.4. Co-Citation Analysis
3.4.1. Journal Co-Citation Analysis
3.4.2. Scientific Production by Authors
3.5. Applications of CRISPR/Cas9 in Environmental Biotechnology
3.5.1. Fourth-Generation Biofuels
3.5.2. Environmental Monitoring through the Use of Biosensors
3.5.3. CRISPR/Cas9 for Conservation and Sustainability.
3.5.4. Enhancing Genetic Resilience
3.5.5. Combating Invasive Species
3.5.6. Sustainable Agriculture
3.6. Future Perspectives and Final Remarks
- CRISPR/Cas9 technology offers novel ways to reduce biological sources of methane, a potent global warming contributor, by modifying genetically livestock microorganisms [192]. Other efforts that could be used include the combination of fourth-generation biofuels with carbon capture and utilization (CCU) technologies, potentially allowing the development of sustainable energy solutions. By integrating CCU technology, CO2 can be repurposed as a nutrient or substrate more efficiently, accomplishing circular economy goals by reducing the reliance on finite resources [63,64,154,155]. This principle is the basis of new biorefinery processes, which include aerobic and anaerobic microorganisms that currently are sequestrating CO2 [195], and CRISPR/Cas9 could enhance its metabolic pathway. Nevertheless, there are environmental and health concerns that must be addressed and warrant further investigation alongside these technologies [192,196]. These methodologies demonstrate the potential of CRISPR/Cas9 in mitigating GHGs emissions and advancing the carbon neutrality goals of several countries.
- The synergistic utilization of Building Information Modeling (BIM), Artificial Intelligence (AI), and machine learning (ML) with environmental practices has been reported [197,198]. These technologies offer unprecedented opportunities to design, monitor, and optimize genetic interventions for sustainability by CRISPR/Cas9 technology. Further research and development in these areas required a robust database. This information could be obtained from monitoring waste treatment plants or municipal solid waste facilities. Thus, the potential combination of these emerging technologies with biosensors would enable the prediction of harmful substances like pesticides or heavy metals [199]. Additionally, integrating nanotechnology might enhance the sensitivity and selectivity and improve efficiency in environmental monitoring [200]. This novel approach could revolutionize environmental applications. Therefore, integrating BIM, AI, ML, and nanotechnology with environmental practices holds a relevant potential to develop innovative alternatives for environmental monitoring, genetic modifications, and food safety towards to sustainable practices.
- The future of CRISPR/Cas9 technology in environmental and agricultural sciences is promising, representing a relevant shift in managing biodiversity, ecological balance, and food security [201]. Its applications in conserving endangered species, controlling invasive populations, and enhancing crop resilience are promising in addressing some of the most pressing global challenges. The potential to precisely edit genetic sequences allows for targeted interventions, reducing unintended ecological impacts and fostering sustainable practices. As research progresses, the integration of CRISPR/Cas9 into conservation and agricultural strategies promises to revolutionize these fields, balancing ecological integrity with the demands of a growing human population [202]. However, the ethical, regulatory, and ecological considerations surrounding its widespread adoption necessitate careful deliberation and adaptive governance to ensure that the benefits are maximized while minimizing potential risks [203]. The journey of CRISPR/Cas9 from a laboratory tool to a cornerstone of ecological and agricultural innovation underscores its transformative potential, necessitating continued research, dialogue, and responsible implementation to fully realize its benefits for a sustainable future.
| Topic | Application Area | Objective | Outcome | Year | Reference | |
|---|---|---|---|---|---|---|
| 1 | Sustainable Landscape Plants | Sustainability (Agriculture) | Explore CRISPR/Cas9 in sustainable landscape plant development | Discussed potential, no specific outcome detailed | 2020 | [204] |
| 2 | Food System Sustainability | Sustainability (Agriculture) | Assess sustainability of CRISPR food innovations | Methodology advancement, not a direct case study | 2021 | [205] |
| 3 | Gene Editing for Extinction Prevention | Conservation/Law | Governance around using gene editing for conservation | Discussion on regulatory and ethical considerations | 2019 | .[104] |
| 4 | Biodiversity Conservation through Technoscience | Conservation/Bioethics | Discuss the impact of technoscience, including CRISPR, on biodiversity | Philosophical and ethical analysis, no direct outcome | 2018 | [206] |
| 5 | CRISPR/Cas in Fish Aquaculture | Sustainability (Aquaculture) | Discuss the sustainable use of CRISPR/Cas9 in fish aquaculture from a biosafety perspective | Highlighted the need for responsible use, no specific fish case study outcomes | 2021 | [207] |
| 6 | Prospect of CRISPR/Cas9 technology in sustainable landscape plants | Bioethics | Demonstrates CRISPR’s potential in developing sustainable landscape plants, impacting conservation. | The use of CRISPR technology in landscape plants has demonstrated accurate and efficient gene editing | 2020 | [204] |
| 7 | Paths of least resilience: advancing a methodology to assess the sustainability of food system innovations - the case of CRISPR | Sustainability | Evaluates CRISPR’s role in sustainable food system innovations, showcasing its importance in agriculture | A methodology to assess the sustainability of CRISPR technology in the context of food systems innovations considering its potential benefits and risks across various dimensions | 2021 | [205] |
| 8 | Governing Extinction in the Era of Gene Editing | Bioethics | Discusses CRISPR’s impact on preventing extinction and enhancing biodiversity conservation | The paper argues that while current conservation laws may not directly address the specific questions raised by CRISPR, the ESA can provide guidance in governing the use of gene editing | 2019 | [104] |
| 9 | Sustainable use of CRISPR/Cas in fish aquaculture: the biosafety perspective | Sustainability | Highlights CRISPR’s application in sustainable fish aquaculture, emphasizing biosafety | Technical limitations, regulatory and risk assessment challenges of the use of CRISPR/Cas are presented. Strategies for regulatory decisions, risk assessments, and increased public awareness are also provided | 2022 | [207] |
| 10 | Is there a future for genome-editing technologies in conservation? | Animal conservation | Explores the potential and challenges of using CRISPR for conservation efforts | 2016 | [180] | |
| 11 | Can CRISPR gene drive work in pest and beneficial haplodiploid species? | Conservation | Analyzes mathematical models demonstrating that, CRISPR homing gene drive can work in haplodiploids | Altering traits to minimize damage caused by harmful haplodiploids, may be more likely to succeed than control efforts based on introducing traits that reduce pest fitness |
2020 | [186] |
| 12 | Modeling CRISPR gene drives for suppression of invasive rodents using a supervised machine learning framework | Artificial Intelligence, machine learning | Developes a computational model of the release of a suppression gene drive into an island rat population demonstrating it could indeed eradicate rat population within several years | 2021 | [185] |
4. Materials and Methods
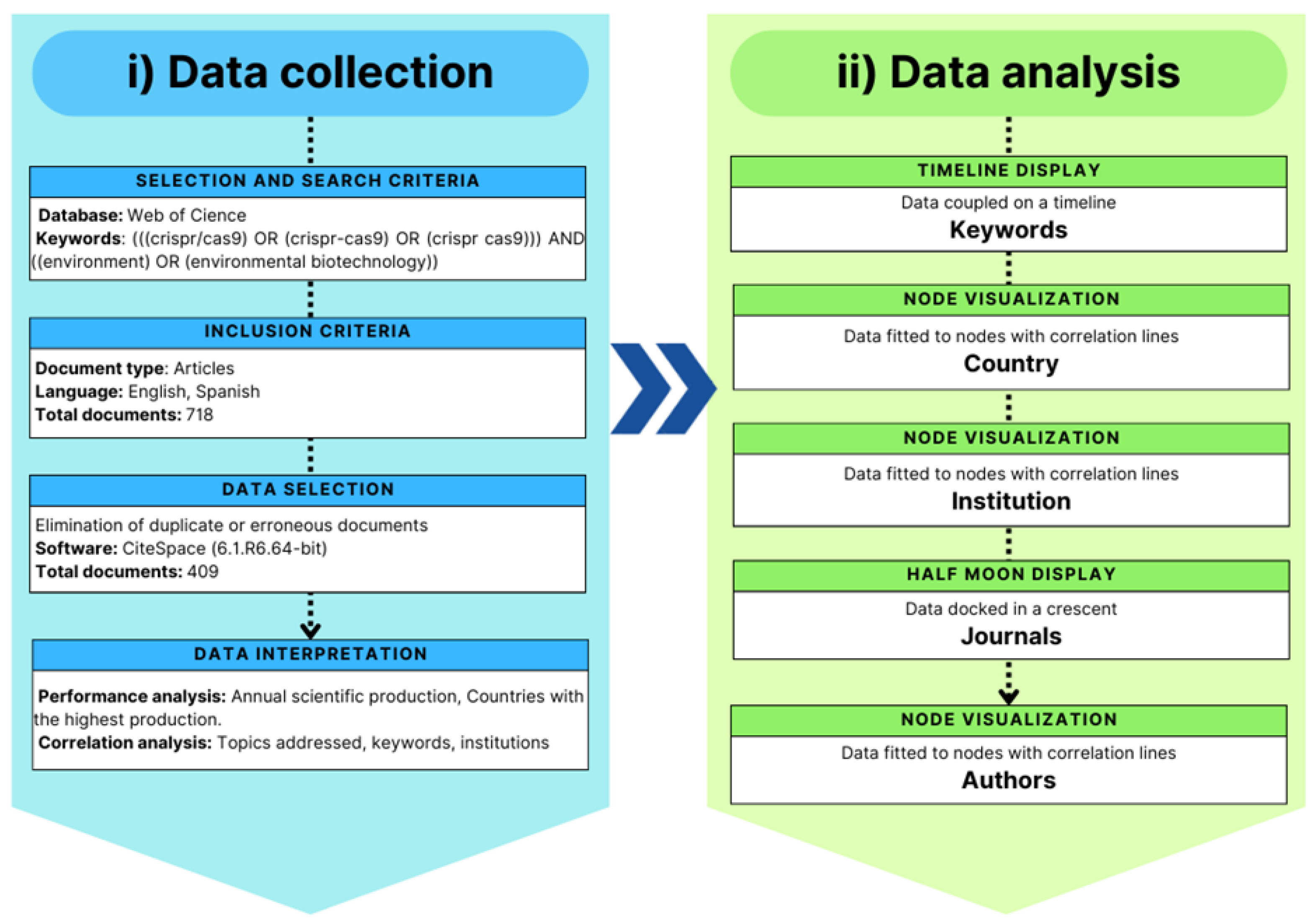
4.1. Exploratory Description Analysis Supported by Databases
4.2. Cutting-Edge Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Al-Ghussain L. Global warming: review on driving forces and mitigation. Environ Prog Sustain Energy 2019;38:13–21. [CrossRef]
- Raharyanti F, Setyaningrum N. Global Warming and its Cause: Natural Science, Social, and Religious Perspective (Literature Study). KnE Soc Sci 2023:25–41. [CrossRef]
- Soares MO, Rabelo EF. Severe ecological impacts caused by one of the worst orphan oil spills worldwide. Mar Environ Res 2023;187:105936. [CrossRef]
- Singh H, Bhardwaj N, Arya SK, Khatri M. Environmental impacts of oil spills and their remediation by magnetic nanomaterials. Environ Nanotechnol Monit Manag 2020;14:100305. [CrossRef]
- Affandi FA, Ishak MY. Impacts of suspended sediment and metal pollution from mining activities on riverine fish population—a review. Environ Sci Pollut Res 2019;26:16939–51. [CrossRef]
- Ahima RS. Global warming threatens human thermoregulation and survival. J Clin Invest 2020;130:559–61. [CrossRef]
- Duchenne-Moutien RA, Neetoo H. Climate Change and Emerging Food Safety Issues: A Review. J Food Prot 2021;84:1884–97. [CrossRef]
- FAO. The state of Food Security and Nutrition in the World 2023 2023. [CrossRef]
- Halkos G, Zisiadou A. Energy Crisis Risk Mitigation through Nuclear Power and RES as Alternative Solutions towards Self-Sufficiency. J Risk Financ Manag 2023;16:45. [CrossRef]
- Hemalatha P, Abda EM, Shah S, Venkatesa Prabhu S, Jayakumar M, Karmegam N, et al. Multi-faceted CRISPR-Cas9 strategy to reduce plant based food loss and waste for sustainable bio-economy – A review. J Environ Manage 2023;332:117382. [CrossRef]
- Chicaiza-Ortiz C, Camacho C, Chicaiza-Ortiz Á, Beihan Z, Jiangyue D, Logroño W, et al. The effectiveness of iron-based additives in enhancing methane and hydrogen production: a systematic review 2023:734–44. [CrossRef]
- Chicaiza Ortiz CD, Navarrete Villa VP, Camacho López CO, Chicaiza Ortiz ÁF. Evaluation of municipal solid waste management system of Quito-Ecuador through life cycle assessment approach 2020.
- Chicaiza C, Bouzerma M, Diéguez-Santana K, Chicaiza Á, Navarrete V, Romero J. Carbon storage technologies applied to rethinking building construction and carbon emissions. IOP Conf. Ser. Earth Environ. Sci., vol. 784, IOP Publishing; 2021, p. 012021.
- Jain M. Environmental Biotechnology. DokumenPub 2014. https://dokumen.pub/environmental-biotechnology-1nbsped-9781783320516-9781842658147.html (accessed November 27, 2023).
- Buddolla V. Environmental Biotechnology: Basic Concepts and Applications 2017. https://www.iberlibro.com/9781783322602/Environmental-Biotechnology-Basic-Concepts-Applications-1783322608/plp (accessed November 27, 2023).
- Zhang D, Zhang Z, Unver T, Zhang B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J Adv Res 2021;29:207–21. [CrossRef]
- Nidhi S, Anand U, Oleksak P, Pooja T, Lal J, Thomas G, et al. Novel CRISPR–Cas Systems: An Updated Review of the Current Achievements, Applications, and Future Research Perspectives 2021. [CrossRef]
- Zaychikova MV, Danilenko VN, Maslov DA. CRISPR-Cas Systems: Prospects for Use in Medicine. Appl Sci 2020;10:9001. [CrossRef]
- Swarts DC, Jinek M. Cas9 versus Cas12a/Cpf1: Structure–function comparisons and implications for genome editing. WIREs RNA 2018;9:e1481. [CrossRef]
- Hatada I, Horii T. CRISPR/Cas9. In: Hatada I, editor. Genome Ed. Anim., vol. 1630, New York, NY: Springer New York; 2017, p. 37–42. [CrossRef]
- Pfeifer K, Ergal İ, Koller M, Basen M, Schuster B, Rittmann SK-MR. Archaea Biotechnology. Biotechnol Adv 2021;47:107668. [CrossRef]
- Ishino Y, Krupovic M, Forterre P. History of CRISPR-Cas from Encounter with a Mysterious Repeated Sequence to Genome Editing Technology. J Bacteriol 2018;200. [CrossRef]
- Liu W, Yang C, Liu Y, Jiang G. CRISPR/Cas9 System and its Research Progress in Gene Therapy. Anticancer Agents Med Chem 2020;19:1912–9. [CrossRef]
- Janik E, Niemcewicz M, Ceremuga M, Krzowski L, Saluk-Bijak J, Bijak M. Various Aspects of a Gene Editing System—CRISPR–Cas9. Int J Mol Sci 2020;21:9604. [CrossRef]
- Ansori ANm, Antonius Y, Susilo RJk, Hayaza S, Kharisma VD, Parikesit AA, et al. Application of CRISPR-Cas9 genome editing technology in various fields: A review. Narra J 2023;3:e184. [CrossRef]
- Wang C, Deng L, Zhang Y, Zhao M, Liang M, Lee L-C, et al. Farmland phytoremediation in bibliometric analysis. J Environ Manage 2024;351:119971. [CrossRef]
- Pande V, Pandey SC, Sati D, Pande V, Samant M. Bioremediation: an emerging effective approach towards environment restoration. Environ Sustain 2020;3:91–103. [CrossRef]
- Shah V, Daverey A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ Technol Innov 2020;18:100774. [CrossRef]
- Basharat Z, Novo L, Yasmin A. Genome Editing Weds CRISPR: What Is in It for Phytoremediation? Plants 2018;7:51. [CrossRef]
- Hashemi Goradel N, Mirzaei H, Sahebkar A, Poursadeghiyan M, Masoudifar A, Malekshahi ZV, et al. Biosensors for the Detection of Environmental and Urban Pollutions. J Cell Biochem 2018;119:207–12. [CrossRef]
- Lander N, Chiurillo MA. State-of-the-art CRISPR /Cas9 Technology for Genome Editing in Trypanosomatids. J Eukaryot Microbiol 2019;66:981–91. [CrossRef]
- Naz M, Benavides-Mendoza A, Tariq M, Zhou J, Wang J, Qi S, et al. CRISPR/Cas9 technology as an innovative approach to enhancing the phytoremediation: Concepts and implications. J Environ Manage 2022;323:116296. [CrossRef]
- Wang J, Chu L, Wojnárovits L, Takács E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci Total Environ 2020;744:140997. [CrossRef]
- Li X, Bao N, Yan Z, Yuan X-Z, Wang S-G, Xia P-F. Degradation of Antibiotic Resistance Genes by VADER with CRISPR-Cas Immunity. Appl Environ Microbiol 2023;89:e00053-23. [CrossRef]
- Tudi M, Daniel Ruan H, Wang L, Lyu J, Sadler R, Connell D, et al. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int J Environ Res Public Health 2021;18:1112. [CrossRef]
- Jose J, Éva C. Plant Biotechnology: Its Importance, Contribution to Agriculture and Environment, and Its Future Prospects. In: Singh J, Bajpai R, Gangwar RK, editors. Biotechnol. Environ. Remediat. 1st ed., Wiley; 2023, p. 9–30. [CrossRef]
- Uthayakumar D, Sharma J, Wensing L, Shapiro RS. CRISPR-Based Genetic Manipulation of Candida Species: Historical Perspectives and Current Approaches. Front Genome Ed 2021;2:606281. [CrossRef]
- Liu Y, Cruz-Morales P, Zargar A, Belcher MS, Pang B, Englund E, et al. Biofuels for a sustainable future. Cell 2021;184:1636–47. [CrossRef]
- Liu L, Supe Tulcan RX, He M, Ouyang W, Zhang Q, Huarez Yarleque CM, et al. Antimony pollution threatens soils and riverine habitats across China: An analysis of antimony concentrations, changes, and risks. Crit Rev Environ Sci Technol 2023:1–20. [CrossRef]
- Wang C, Zhang Y, Deng L, Zhao M, Liang M, Lee L-C, et al. Visualization Network Analysis of Studies on Agricultural Drainage Water Treatment. Processes 2023;11:2952. [CrossRef]
- Zhang Y, Zhao D, Liu H, Huang X, Deng J, Jia R, et al. Research hotspots and frontiers in agricultural multispectral technology: Bibliometrics and scientometrics analysis of the Web of Science. Front Plant Sci 2022;13:955340. [CrossRef]
- Marginson S. Global science and national comparisons: beyond bibliometrics and scientometrics. Comp Educ 2022;58:125–46. [CrossRef]
- Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: An overview and guidelines. J Bus Res 2021;133:285–96. [CrossRef]
- Umeokafor N, Umar T, Evangelinos K. Bibliometric and scientometric analysis-based review of construction safety and health research in developing countries from 1990 to 2021. Saf Sci 2022;156:105897. [CrossRef]
- Chicaiza-Ortiz CD, Rivadeneira-Arias VDC, Herrera-Feijoo RJ, Andrade JC. Biotecnología Ambiental, Aplicaciones y Tendencias. 1st ed. Editorial Grupo AEA; 2023. [CrossRef]
- Xiao J, Wei J, Wu M, Cao X. Bibliometric and Visual Analysis of Crop Water Footprint: A Widely Used Agricultural Water Resources Evaluation Method. Water 2022;14:2866. [CrossRef]
- Dangi AK, Sharma B, Hill RT, Shukla P. Bioremediation through microbes: systems biology and metabolic engineering approach. Crit Rev Biotechnol 2019;39:79–98. [CrossRef]
- Eş I, Gavahian M, Marti-Quijal FJ, Lorenzo JM, Mousavi Khaneghah A, Tsatsanis C, et al. The application of the CRISPR-Cas9 genome editing machinery in food and agricultural science: Current status, future perspectives, and associated challenges. Biotechnol Adv 2019;37:410–21. [CrossRef]
- Wang Q, Coleman JJ. Progress and Challenges: Development and Implementation of CRISPR/Cas9 Technology in Filamentous Fungi. Comput Struct Biotechnol J 2019;17:761–9. [CrossRef]
- Suominen A, Seppänen M, Dedehayir O. A bibliometric review on innovation systems and ecosystems: a research agenda. Eur J Innov Manag 2018;22:335–60. [CrossRef]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012;337:816–21. [CrossRef]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013;339:819–23. [CrossRef]
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015;163:759–71. [CrossRef]
- Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol 2016;34:184–91. [CrossRef]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014;157:1262–78. [CrossRef]
- Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014;507:62–7. [CrossRef]
- Anders C, Niewoehner O, Duerst A, Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 2014;513:569–73. [CrossRef]
- Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014;346:1258096. [CrossRef]
- Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol 2020;38:824–44. [CrossRef]
- Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, et al. An updated evolutionary classification of CRISPR–Cas systems. Nat Rev Microbiol 2015;13:722–36. [CrossRef]
- Gowen CM, Fong SS. Applications of systems biology towards microbial fuel production. Trends Microbiol 2011;19:516–24. [CrossRef]
- Torrentes G. Retrospectiva y prospectiva del Desarrollo de las generaciones de biocombustibles. Cienc Tecnol 2021:53–63. [CrossRef]
- Aro E-M. From first generation biofuels to advanced solar biofuels. Ambio 2015;45:24–31. [CrossRef]
- Escobedo MJ, Calderón AC, Escobedo MJ, Calderón AC. Biomasa microalgal con alto potencial para la producción de biocombustibles. Sci Agropecu 2021;12:265–82. [CrossRef]
- Dalena F, Senatore A, Basile M, Marino D, Basile A. Chapter 1 - From sugars to ethanol—from agricultural wastes to algal sources: An overview. In: Basile A, Dalena F, editors. Second Third Gener. Feedstock, Elsevier; 2019, p. 3–34. [CrossRef]
- Dutta K, Daverey A, Lin J-G. Evolution retrospective for alternative fuels: First to fourth generation. Renew Energy 2014;69:114–22. [CrossRef]
- Fan L, Zhang H, Li J, Wang Y, Leng L, Li J, et al. Algal biorefinery to value-added products by using combined processes based on thermochemical conversion: A review. Algal Res 2020;47:101819. [CrossRef]
- Guo M, Song W, Buhain J. Bioenergy and biofuels: History, status, and perspective. Renew Sustain Energy Rev 2015;42:712–25. [CrossRef]
- Diéguez Santana K, Chicaiza Ortiz CD, Logroño W. Anaerobic digestate: pollutants, ecotoxicology, and legislation, 2022.
- Zhang P, Zhang T, Zhang J, Liu H, Chicaiza-Ortiz C, Lee JTE, et al. A machine learning assisted prediction of potential biochar and its applications in anaerobic digestion for valuable chemicals and energy recovery from organic waste. Carbon Neutrality 2024;3:2. [CrossRef]
- Chicaiza Ortiz CD. Diseño y construcción de un prototipo de fotobiorreactor discontinuo a escala laboratorio para la producción de biomasa algal. B.S. thesis. Escuela Superior Politécnica de Chimborazo, 2017.
- Adeniyi OM, Azimov U, Burluka A. Algae biofuel: Current status and future applications. Renew Sustain Energy Rev 2018;90:316–35. [CrossRef]
- Bibi R, Ahmad Z, Imran M, Hussain S, Ditta A, Mahmood S, et al. Algal bioethanol production technology: A trend towards sustainable development. Renew Sustain Energy Rev 2017;71:976–85. [CrossRef]
- Enamala MK, Enamala S, Chavali M, Donepudi J, Yadavalli R, Kolapalli B, et al. Production of biofuels from microalgae - A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sustain Energy Rev 2018;94:49–68. [CrossRef]
- Javed MR, Noman M, Shahid M, Ahmed T, Khurshid M, Rashid MH, et al. Current situation of biofuel production and its enhancement by CRISPR/Cas9-mediated genome engineering of microbial cells. Microbiol Res 2019;219:1–11. [CrossRef]
- Xiong J, Sun Z, Yu J, Liu H, Wang X. Thermal self-regulatory smart biosensor based on horseradish peroxidase-immobilized phase-change microcapsules for enhancing detection of hazardous substances. Chem Eng J 2022;430:132982. [CrossRef]
- Ullo SL, Sinha GR. Advances in Smart Environment Monitoring Systems Using IoT and Sensors. Sensors 2020;20:3113. [CrossRef]
- Butt MA, Voronkov GS, Grakhova EP, Kutluyarov RV, Kazanskiy NL, Khonina SN. Environmental Monitoring: A Comprehensive Review on Optical Waveguide and Fiber-Based Sensors. Biosensors 2022;12:1038. [CrossRef]
- Zhang yi, Zhu Y, Zhuotong Z, Zeng G, Xiao R, Wang Y, et al. Sensors for the environmental pollutant detection: Are we already there? Coord Chem Rev 2020;431:213681. [CrossRef]
- Gavrilaș S, Ursachi C Ștefan, Perța-Crișan S, Munteanu F-D. Recent Trends in Biosensors for Environmental Quality Monitoring. Sensors 2022;22:1513. [CrossRef]
- Huang C-W, Lin C, Nguyen MK, Hussain A, Bui X-T, Ngo HH. A review of biosensor for environmental monitoring: principle, application, and corresponding achievement of sustainable development goals. Bioengineered 2023;14:58–80. [CrossRef]
- Badihi-Mossberg M, Buchner V, Rishpon J. Electrochemical Biosensors for Pollutants in the Environment. Electroanalysis 2007;19:2015–28. [CrossRef]
- Carpenter AC, Paulsen IT, Williams TC. Blueprints for Biosensors: Design, Limitations, and Applications. Genes 2018;9:375. [CrossRef]
- Otero F, Magner E. Biosensors—Recent Advances and Future Challenges in Electrode Materials. Sensors 2020;20:3561. [CrossRef]
- Kumar T, Naik S, Jujjavarappu SE. A critical review on early-warning electrochemical system on microbial fuel cell-based biosensor for on-site water quality monitoring. Chemosphere 2022;291:133098. [CrossRef]
- Rogers KR. Recent advances in biosensor techniques for environmental monitoring. Anal Chim Acta 2006;568:222–31. [CrossRef]
- Turner APF. Biosensors: sense and sensibility. Chem Soc Rev 2013;42:3184–96. [CrossRef]
- Hashemi Goradel N, Mirzaei H, Sahebkar A, Poursadeghiyan M, Masoudifar A, Malekshahi ZV, et al. Biosensors for the Detection of Environmental and Urban Pollutions. J Cell Biochem 2018;119:207–12. [CrossRef]
- Rodriguez-Mozaz S, Alda MJL de, Marco M-P, Barceló D. Biosensors for environmental monitoring A global perspective. Talanta 2005;65:291–7. [CrossRef]
- Kumar H, Kumari N, Sharma R. Nanocomposites (conducting polymer and nanoparticles) based electrochemical biosensor for the detection of environment pollutant: Its issues and challenges. Environ Impact Assess Rev 2020;85:106438. [CrossRef]
- Nigam VK, Shukla P. Enzyme Based Biosensors for Detection of Environmental Pollutants--A Review. J Microbiol Biotechnol 2015;25:1773–81. [CrossRef]
- Mehrotra P. Biosensors and their applications – A review. J Oral Biol Craniofacial Res 2016;6:153–9. [CrossRef]
- Justino CIL, Duarte AC, Rocha-Santos TAP. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017;17:2918. [CrossRef]
- Wada N, Ueta R, Osakabe Y, Osakabe K. Precision genome editing in plants: state-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol 2020;20:234. [CrossRef]
- Mahmood T, Khalid S, Abdullah M, Ahmed Z, Shah MKN, Ghafoor A, et al. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019;9:105. [CrossRef]
- Khan FS, Goher F, Zhang D, Shi P, Li Z, Htwe YM, et al. Is CRISPR/Cas9 a way forward to fast-track genetic improvement in commercial palms? Prospects and limits. Front Plant Sci 2022;13:1042828. [CrossRef]
- Abdul Aziz M, Brini F, Rouached H, Masmoudi K. Genetically engineered crops for sustainably enhanced food production systems. Front Plant Sci 2022;13:1027828. [CrossRef]
- Ali Q, Yu C, Hussain A, Ali M, Ahmar S, Sohail MA, et al. Genome Engineering Technology for Durable Disease Resistance: Recent Progress and Future Outlooks for Sustainable Agriculture. Front Plant Sci 2022;13:860281. [CrossRef]
- Mestanza-Ramón C, Herrera Feijoo RJ, Chicaiza-Ortiz C, Gaibor ID, Mateo RG. Estimation of Current and Future Suitable Areas for Tapirus pinchaque in Ecuador. Sustainability 2021;13:11486.
- Hassanien A, Saadaoui I, Schipper K, Al-Marri S, Dalgamouni T, Aouida M, et al. Genetic engineering to enhance microalgal-based produced water treatment with emphasis on CRISPR/Cas9: A review. Front Bioeng Biotechnol 2023;10:1104914. [CrossRef]
- Chen W, Yang F, Xu X, Kumar U, He W, You M. Genetic control of Plutella xylostella in omics era. Arch Insect Biochem Physiol 2019;102:e21621. [CrossRef]
- Ferrari L. Farmers’ attitude toward CRISPR/Cas9: The case of blast resistant rice. Agribusiness 2022;38:175–94. [CrossRef]
- Fuchs S, Garrood WT, Beber A, Hammond A, Galizi R, Gribble M, et al. Resistance to a CRISPR-based gene drive at an evolutionarily conserved site is revealed by mimicking genotype fixation. PLOS Genet 2021;17:e1009740. [CrossRef]
- Monast J. Governing Extinction in the Era of Gene Editing 2019.
- Tobin PC. Managing invasive species. F1000Research 2018;7:F1000 Faculty Rev-1686. [CrossRef]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol 2016;34:78–83. [CrossRef]
- Neve P. Gene drive systems: do they have a place in agricultural weed management? Pest Manag Sci 2018;74:2671–9. [CrossRef]
- Asad M, Liu D, Li J, Chen J, Yang G. Development of CRISPR/Cas9-Mediated Gene-Drive Construct Targeting the Phenotypic Gene in Plutella xylostella. Front Physiol 2022;13. [CrossRef]
- Nateghi Rostami M. CRISPR/Cas9 gene drive technology to control transmission of vector-borne parasitic infections. Parasite Immunol 2020;42:e12762. [CrossRef]
- Rybicki EP. CRISPR–Cas9 strikes out in cassava. Nat Biotechnol 2019;37:727–8. [CrossRef]
- Chen W, Page-McCaw PS. CRISPR/Cas9 gene editing 2024. [CrossRef]
- Zhang B. CRISPR/Cas9: A Robust Genome-Editing Tool with Versatile Functions and Endless Application. Int J Mol Sci 2020;21:5111. [CrossRef]
- Burgess DJ. CRISPR screens beyond Cas9. Nat Rev Genet 2020;21:273–273. [CrossRef]
- Kozubek J. Crispr-Cas9 Is Impossible to Stop. JSTOR 2017;18:112–9.
- Rodríguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017;171:470-480.e8. [CrossRef]
- Shahriar SA, Islam MN, Chun CNW, Rahim MdA, Paul NC, Uddain J, et al. Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops. Plants 2021;10:1264. [CrossRef]
- Prabhukarthikeyan SR, Parameswaran C, Keerthana U, Teli B, Jagannadham PTK, Cayalvizhi B, et al. Understanding the Plant-microbe Interactions in CRISPR/Cas9 Era: Indeed a Sprinting Start in Marathon. Curr Genomics 2020;21:429–43. [CrossRef]
- Erpen-Dalla Corte L, M. Mahmoud L, S. Moraes T, Mou Z, W. Grosser J, Dutt M. Development of Improved Fruit, Vegetable, and Ornamental Crops Using the CRISPR/Cas9 Genome Editing Technique. Plants 2019;8:601. [CrossRef]
- Huang W-P, Du Y-J, Yang Y, He J-N, Lei Q, Yang X-Y, et al. Two CRISPR/Cas9 Systems Developed in Thermomyces dupontii and Characterization of Key Gene Functions in Thermolide Biosynthesis and Fungal Adaptation. Appl Environ Microbiol 2020;86:e01486-20. [CrossRef]
- Fan Y, Xie J, Zhang F. Overexpression of miR5505 enhanced drought and salt resistance in rice (Orayza sativa) 2022:2022.01.13.476146. [CrossRef]
- Park J-R, Kim E-G, Jang Y-H, Jan R, Farooq M, Ubaidillah M, et al. Applications of CRISPR/Cas9 as New Strategies for Short Breeding to Drought Gene in Rice. Front Plant Sci 2022;13:850441. [CrossRef]
- Karkute SG, Singh AK, Gupta OP, Singh PM, Singh B. CRISPR/Cas9 Mediated Genome Engineering for Improvement of Horticultural Crops. Front Plant Sci 2017;8. [CrossRef]
- Piatek A, Ali Z, Baazim H, Li L, Abulfaraj A, Al-Shareef S, et al. RNA-guided transcriptional regulation in planta via synthetic dC as9-based transcription factors. Plant Biotechnol J 2015;13:578–89. [CrossRef]
- Ishino Y, Krupovic M, Forterre P. History of CRISPR-Cas from Encounter with a Mysterious Repeated Sequence to Genome Editing Technology. J Bacteriol 2018;200:e00580-17. [CrossRef]
- You L, Tong R, Li M, Liu Y, Xue J, Lu Y. Advancements and Obstacles of CRISPR-Cas9 Technology in Translational Research. Mol Ther Methods Clin Dev 2019;13:359–70. [CrossRef]
- Liu H, Lv Z, Zhang G, Wang X, Wang Y, Wang K. Knowledge mapping and current trends of global research on CRISPR in the field of cancer. Front Cell Dev Biol 2023;11:1178221. [CrossRef]
- Matinvafa MA, Makani S, Parsasharif N, Zahed MA, Movahed E, Ghiasvand S. CRISPR-Cas technology secures sustainability through its applications: a review in green biotechnology. 3 Biotech 2023;13:383. [CrossRef]
- Li Y, Li S, Wang J, Liu G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol 2019;37:730–43. [CrossRef]
- Chennakesavulu K, Singh H, Trivedi PK, Jain M, Yadav SR. State-of-the-Art in CRISPR Technology and Engineering Drought, Salinity, and Thermo-tolerant crop plants. Plant Cell Rep 2022;41:815–31. [CrossRef]
- Gostimskaya I. CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing. Biochem Biokhimiia 2022;87:777–88. [CrossRef]
- Mall AK, Manimekalai R, Misra V, Pandey H, Srivastava S, Sharma A. CRISPR/Cas-Mediated Genome Editing for Sugarcane Improvement. Sugar Tech 2024. [CrossRef]
- Vigentini I, Gebbia M, Belotti A, Foschino R, Roth FP. CRISPR/Cas9 System as a Valuable Genome Editing Tool for Wine Yeasts with Application to Decrease Urea Production. Front Microbiol 2017;8.
- de Mello F da SB, Maneira C, Suarez FUL, Nagamatsu S, Vargas B, Vieira C, et al. Rational engineering of industrial S. cerevisiae: towards xylitol production from sugarcane straw. J Genet Eng Biotechnol 2022;20:80. [CrossRef]
- Vasil IK. A history of plant biotechnology: from the Cell Theory of Schleiden and Schwann to biotech crops. Plant Cell Rep 2008;27:1423–40. [CrossRef]
- Verma N, Singh M. Biosensors for heavy metals. BioMetals 2005;18:121–9. [CrossRef]
- Rai MK. Biotechnological strategies for conservation of rare and endangered medicinal plants. Biodiversitas J Biol Divers 2010;11:157–66. [CrossRef]
- Dey R, Dube SP, Devi S, Raghuwanshi R. Biotechnological approaches in mitigating climate variability and anthropogenic factors. Conserv Halting Reinstating Degraded Nat Resour Dur UN Decade Ecosyst Restor, Nova Science Publishers, Inc.; 2023, p. 105–44.
- Bahmani F, Noroozi M, Kolahchi N, Ghanei M. Bioethics, Emerging Biotechnologies, and Society: Providing an Ethical Framework for Assessing Emerging Biotechnologies. Iran J Med Ethics Hist Med 2023;16.
- Li Y, Wu X, Zhang Y, Zhang Q. CRISPR/Cas genome editing improves abiotic and biotic stress tolerance of crops. Front Genome Ed 2022;4:987817. [CrossRef]
- Vu BN, Vu TV, Yoo JY, Nguyen NT, Ko KS, Kim J-Y, et al. CRISPR-Cas-mediated unfolded protein response control for enhancing plant stress resistance. Front Plant Sci 2023;14:1271368. [CrossRef]
- Liu L, Zhang J, Xu J, Li Y, Guo L, Wang Z, et al. CRISPR/Cas9 targeted mutagenesis of SlLBD40, a lateral organ boundaries domain transcription factor, enhances drought tolerance in tomato. Plant Sci 2020;301:110683. [CrossRef]
- Chen S, Zhang N, Zhou G, Hussain S, Ahmed S, Tian H, et al. Knockout of the entire family of AITR genes in Arabidopsis leads to enhanced drought and salinity tolerance without fitness costs. BMC Plant Biol 2021;21:137. [CrossRef]
- Yu W, Wang L, Zhao R, Sheng J, Zhang S, Li R, et al. Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol 2019;19:354. [CrossRef]
- Baeg G-J, Kim S-H, Choi D-M, Tripathi S, Han Y-J, Kim J-I. CRISPR/Cas9-mediated mutation of 5-oxoprolinase gene confers resistance to sulfonamide compounds in Arabidopsis. Plant Biotechnol Rep 2021;15:753–64. [CrossRef]
- Wang F-Z, Chen M-X, Yu L-J, Xie L-J, Yuan L-B, Qi H, et al. OsARM1, an R2R3 MYB Transcription Factor, Is Involved in Regulation of the Response to Arsenic Stress in Rice. Front Plant Sci 2017;8:1868. [CrossRef]
- Disney A. Social network analysis 101: centrality measures explained. Camb Intell 2020.
- Joint Research Centre (European Commission), Parisi C, Rodríguez-Cerezo E. Current and future market applications of new genomic techniques. LU: Publications Office of the European Union; 2021.
- Pranckutė R. Web of Science (WoS) and Scopus: The Titans of Bibliographic Information in Today’s Academic World. Publications 2021;9:12. [CrossRef]
- Gao C. Precision plant breeding using genome editing technologies. Transgenic Res 2019;28:53–5. [CrossRef]
- Rasetti L. How to Use Impact Metrics. MDPI Blog 2023. https://mdpiblog.wordpress.sciforum.net/2023/06/27/indexing-interview/ (accessed November 22, 2023).
- Kitomi Y, Hanzawa E, Kuya N, Inoue H, Hara N, Kawai S, et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc Natl Acad Sci U S A 2020;117:21242–50. [CrossRef]
- Wu Z-Y, Huang Y-T, Chao W-C, Ho S-P, Cheng J-F, Liu P-Y. Reversal of carbapenem-resistance in Shewanella algae by CRISPR/Cas9 genome editing. J Adv Res 2019;18:61–9. [CrossRef]
- Huang W-P, Du Y-J, Yang Y, He J-N, Lei Q, Yang X-Y, et al. Two CRISPR/Cas9 Systems Developed in Thermomyces dupontii and Characterization of Key Gene Functions in Thermolide Biosynthesis and Fungal Adaptation. Appl Environ Microbiol 2020;86:e01486-20. [CrossRef]
- Patil SA, Gildemyn S, Pant D, Zengler K, Logan BE, Rabaey K. A logical data representation framework for electricity-driven bioproduction processes. Biotechnol Adv 2015;33:736–44. [CrossRef]
- Moravvej Z, Makarem MA, Rahimpour MR. Chapter 20 - The fourth generation of biofuel. In: Basile A, Dalena F, editors. Second Third Gener. Feedstock, Elsevier; 2019, p. 557–97. [CrossRef]
- Fazal T, Mushtaq A, Rehman F, Ullah Khan A, Rashid N, Farooq W, et al. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew Sustain Energy Rev 2018;82:3107–26. [CrossRef]
- Wood DA. 19 - Biodiesel from microalgae. In: Jacob-Lopes E, Zepka LQ, Severo IA, Maroneze MM, editors. 3rd Gener. Biofuels, Woodhead Publishing; 2022, p. 417–38. [CrossRef]
- Sanchez L, Sanz Smachetti ME, Do Nascimento M, Salerno GL, Curatti L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res 2017;22:140–7. [CrossRef]
- Work VH, D’Adamo S, Radakovits R, Jinkerson RE, Posewitz MC. Improving photosynthesis and metabolic networks for the competitive production of phototroph-derived biofuels. Curr Opin Biotechnol 2012;23:290–7. [CrossRef]
- Chen B, Wan C, Mehmood MA, Chang J-S, Bai F, Zhao X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products–A review. Bioresour Technol 2017;244:1198–206. [CrossRef]
- Shin YS, Jeong J, Nguyen THT, Kim JYH, Jin E, Sim SJ. Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour Technol 2019;271:368–74. [CrossRef]
- Nguyen THT, Park S, Jeong J, Shin YS, Sim SJ, Jin E. Enhancing lipid productivity by modulating lipid catabolism using the CRISPR-Cas9 system in Chlamydomonas. J Appl Phycol 2020;32:2829–40. [CrossRef]
- Chang KS, Kim J, Park H, Hong S-J, Lee C-G, Jin E. Enhanced lipid productivity in AGP knockout marine microalga Tetraselmis sp. using a DNA-free CRISPR-Cas9 RNP method. Bioresour Technol 2020;303:122932. [CrossRef]
- Kurita T, Iwai M, Ohta H, Sakuma T, Yamamoto T. Genome editing for biodiesel production in oleaginous microalga, Nannochloropsis species. Gene Genome Ed 2023;6:100027. [CrossRef]
- Tian Y, Liu X, Fan C, Li T, Qin H, Li X, et al. Enhancement of Tobacco (Nicotiana tabacum L.) Seed Lipid Content for Biodiesel Production by CRISPR-Cas9-Mediated Knockout of NtAn1. Front Plant Sci 2021;11.
- Jiang WZ, Henry IM, Lynagh PG, Comai L, Cahoon EB, Weeks DP. Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol J 2017;15:648–57. [CrossRef]
- Do PT, Nguyen CX, Bui HT, Tran LTN, Stacey G, Gillman JD, et al. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2–1A and GmFAD2–1B genes to yield a high oleic, low linoleic and α-linolenic acid phenotype in soybean. BMC Plant Biol 2019;19:311. [CrossRef]
- Lee K-R, Jeon I, Yu H, Kim S-G, Kim H-S, Ahn S-J, et al. Increasing Monounsaturated Fatty Acid Contents in Hexaploid Camelina sativa Seed Oil by FAD2 Gene Knockout Using CRISPR-Cas9. Front Plant Sci 2021;12:702930. [CrossRef]
- Zhang K, Nie L, Cheng Q, Yin Y, Chen K, Qi F, et al. Effective editing for lysophosphatidic acid acyltransferase 2/5 in allotetraploid rapeseed (Brassica napus L.) using CRISPR-Cas9 system. Biotechnol Biofuels 2019;12:225. [CrossRef]
- Kang Y, Su G, Yu Y, Cao J, Wang J, Yan B. CRISPR-Cas12a-Based Aptasensor for On-Site and Highly Sensitive Detection of Microcystin-LR in Freshwater. Environ Sci Technol 2022;56:4101–10. [CrossRef]
- Sheng A, Wang P, Yang J, Tang L, Chen F, Zhang J. MXene Coupled with CRISPR-Cas12a for Analysis of Endotoxin and Bacteria. Anal Chem 2021;93:4676–81. [CrossRef]
- Song J, Song Y, Jang H, Moon J, Kang H, Huh Y-M, et al. Elution-free DNA detection using CRISPR/Cas9-mediated light-up aptamer transcription: Toward all-in-one DNA purification and detection tube. Biosens Bioelectron 2023;225:115085. [CrossRef]
- Zhao Q, Li G, Li X. Aptamer Sensor Based on Hybrid Chain Reaction and CRISPR-Cas9 System for STX Detection. Chemosensors 2023;11:183. [CrossRef]
- Dronina J, Bubniene US, Ramanavicius A. The application of DNA polymerases and Cas9 as representative of DNA-modifying enzymes group in DNA sensor design (review). Biosens Bioelectron 2021;175:112867. [CrossRef]
- Dai Y, Wu Y, Liu G, Gooding JJ. CRISPR Mediated Biosensing Toward Understanding Cellular Biology and Point-of-Care Diagnosis. Angew Chem Int Ed 2020;59:20754–66. [CrossRef]
- Liu CC, Dai Y. Application of CRISPR Cas Systems for Biosensing. Biosensors 2023;13:672. [CrossRef]
- Zhao F, Ding X, Liu Z, Yan X, Chen Y, Jiang Y, et al. Application of CRISPR/Cas9-based genome editing in ecotoxicology. Environ Pollut Barking Essex 1987 2023;336:122458. [CrossRef]
- Abdelmoneim A, Clark CL, Mukai M. Fluorescent Reporter Zebrafish Line for Estrogenic Compound Screening Generated Using a CRISPR/Cas9-Mediated Knock-in System. Toxicol Sci 2020;173:336–46. [CrossRef]
- Li M, Leso M, Buti M, Bellini E, Bertoldi D, Saba A, et al. Phytochelatin synthase de-regulation in Marchantia polymorpha indicates cadmium detoxification as its primary ancestral function in land plants and provides a novel visual bioindicator for detection of this metal. J Hazard Mater 2022;440:129844. [CrossRef]
- Johnson JA, Altwegg R, Evans DM, Ewen JG, Gordon IJ, Pettorelli N, et al. Is there a future for genome-editing technologies in conservation? Anim Conserv 2016;19:97–101. [CrossRef]
- Novak BJ, Maloney T, Phelan R. Advancing a New Toolkit for Conservation: From Science to Policy. CRISPR J 2018;1:11–5. [CrossRef]
- Shashank PR, Parker BM, Rananaware SR, Plotkin D, Couch C, Yang LG, et al. CRISPR-based diagnostics detects invasive insect pests. Mol Ecol Resour 2024;24:e13881. [CrossRef]
- Birand A, Cassey P, Ross JV, Thomas PQ, Prowse TAA. Scalability of genetic biocontrols for eradicating invasive alien mammals. NeoBiota 2022;74:93–103. [CrossRef]
- Faber NR, McFarlane GR, Gaynor RC, Pocrnic I, Whitelaw CBA, Gorjanc G. Novel combination of CRISPR-based gene drives eliminates resistance and localises spread. Sci Rep 2021;11:3719. [CrossRef]
- Champer SE, Oakes N, Sharma R, García-Díaz P, Champer J, Messer PW. Modeling CRISPR gene drives for suppression of invasive rodents using a supervised machine learning framework. PLOS Comput Biol 2021;17:e1009660. [CrossRef]
- Li J, Aidlin Harari O, Doss A, Walling LL, Atkinson PW, Morin S, et al. Can CRISPR gene drive work in pest and beneficial haplodiploid species? Evol Appl 2020;13:2392–403. [CrossRef]
- Tiwari M, Kumar Trivedi P, Pandey A. Emerging tools and paradigm shift of gene editing in cereals, fruits, and horticultural crops for enhancing nutritional value and food security. Food Energy Secur 2021;10:e258. [CrossRef]
- Jaganathan D, Ramasamy K, Sellamuthu G, Jayabalan S, Venkataraman G. CRISPR for Crop Improvement: An Update Review. Front Plant Sci 2018;9. [CrossRef]
- Haque E, Taniguchi H, Hassan MM, Bhowmik P, Karim MR, Śmiech M, et al. Application of CRISPR/Cas9 Genome Editing Technology for the Improvement of Crops Cultivated in Tropical Climates: Recent Progress, Prospects, and Challenges. Front Plant Sci 2018;9. [CrossRef]
- Jaganathan D, Ramasamy K, Sellamuthu G, Jayabalan S, Venkataraman G. CRISPR for Crop Improvement: An Update Review. Front Plant Sci 2018;9:985. [CrossRef]
- El-Mounadi K, Morales-Floriano ML, Garcia-Ruiz H. Principles, Applications, and Biosafety of Plant Genome Editing Using CRISPR-Cas9. Front Plant Sci 2020;11:56. [CrossRef]
- Ahmad M. Plant breeding advancements with “CRISPR-Cas” genome editing technologies will assist future food security. Front Plant Sci 2023;14:1133036. [CrossRef]
- Chen K, Wang Y, Zhang R, Zhang H, Gao C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu Rev Plant Biol 2019;70:667–97. [CrossRef]
- Zaidi SS-A, Mahas A, Vanderschuren H, Mahfouz MM. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol 2020;21:289. [CrossRef]
- Subedi U, Kader K, Jayawardhane KN, Poudel H, Chen G, Acharya S, et al. The Potential of Novel Gene Editing-Based Approaches in Forages and Rumen Archaea for Reducing Livestock Methane Emissions. Agriculture 2022;12:1780. [CrossRef]
- Bajón Fernández Y, Soares A, Vale P, Koch K, Masse AL, Cartmell E. Enhancing the anaerobic digestion process through carbon dioxide enrichment: initial insights into mechanisms of utilization. Environ Technol 2019;40:1744–55. [CrossRef]
- Abdullah B, Syed Muhammad SAF, Shokravi Z, Ismail S, Kassim KA, Mahmood AN, et al. Fourth generation biofuel: A review on risks and mitigation strategies. Renew Sustain Energy Rev 2019;107:37–50. [CrossRef]
- Shivaprakash KN, Swami N, Mysorekar S, Arora R, Gangadharan A, Vohra K, et al. Potential for Artificial Intelligence (AI) and Machine Learning (ML) Applications in Biodiversity Conservation, Managing Forests, and Related Services in India. Sustainability 2022;14:7154. [CrossRef]
- Balakrishnan RM, Uddandarao P, Raval K, Raval R. Chapter 2 - A perspective of advanced biosensors for environmental monitoring. In: Kaur Brar S, Hegde K, Pachapur VL, editors. Tools Tech. Protoc. Monit. Environ. Contam., Elsevier; 2019, p. 19–51. [CrossRef]
- Kumaran A, Jude Serpes N, Gupta T, James A, Sharma A, Kumar D, et al. Advancements in CRISPR-Based Biosensing for Next-Gen Point of Care Diagnostic Application. Biosensors 2023;13:202. [CrossRef]
- Grunewald S. CRISPR’s Creatures: Protecting Wildlife in the Age of Genomic Editing. UCLA J Environ Law Policy 2019;37. [CrossRef]
- Schmidt H, Collier TC, Hanemaaijer MJ, Houston PD, Lee Y, Lanzaro GC. Abundance of conserved CRISPR-Cas9 target sites within the highly polymorphic genomes of Anopheles and Aedes mosquitoes. Nat Commun 2020;11:1425. [CrossRef]
- Kumar V, AlMomin S, Rahman MH, Shajan A. Use of CRISPR in Climate Smart/Resilient Agriculture. In: Bhattacharya A, Parkhi V, Char B, editors. CRISPRCas Genome Ed. Strateg. Potential Crop Improv., Cham: Springer International Publishing; 2020, p. 131–64. [CrossRef]
- Zhang X, Qiu J. Prospect of CRISPR/Cas9 technology in sustainable landscape plants. E3S Web Conf 2020;206:01025. [CrossRef]
- Clément C, Ajena F. Paths of least resilience: advancing a methodology to assess the sustainability of food system innovations - the case of CRISPR. Agroecol Sustain Food Syst 2021;45:637–53. [CrossRef]
- Boëte C. Technoscience and Biodiversity Conservation. Asian Bioeth Rev 2018;10:245–59. [CrossRef]
- Okoli AS, Blix T, Myhr AI, Xu W, Xu X. Sustainable use of CRISPR/Cas in fish aquaculture: the biosafety perspective. Transgenic Res 2022;31:1–21. [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:89. [CrossRef]
- Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J Off Publ Fed Am Soc Exp Biol 2008;22:338–42. [CrossRef]
- Wang Q, Waltman L. Large-scale analysis of the accuracy of the journal classification systems of Web of Science and Scopus. J Informetr 2016;10:347–64. [CrossRef]
- van Eck NJ, Waltman L. VOSviewer Manual 2022.
- Jaeger A, Banks D. Cluster analysis: A modern statistical review -. WIREs Comput Stat - Wiley Online Libr 2023:17. [CrossRef]
- Chen C. Searching for intellectual turning points: Progressive knowledge domain visualization. Proc Natl Acad Sci 2004;101:5303–10. [CrossRef]
- Chen C. CiteSpace: A Practical Guide for Mapping Scientific Literature. 2016.


| Ranking | Count | Centrality | Year | Country |
|---|---|---|---|---|
| 1 | 160 | 0.80 | 2014 | United States |
| 2 | 93 | 0.10 | 2015 | People’s Republic of China |
| 3 | 36 | 0.08 | 2016 | Germany |
| 4 | 20 | 0.28 | 2015 | England |
| 5 | 19 | 0.00 | 2016 | Japan |
| 6 | 16 | 0.06 | 2016 | Canada |
| 7 | 14 | 0.06 | 2015 | France |
| 8 | 13 | 0.04 | 2017 | Spain |
| 9 | 11 | 0.06 | 2017 | Switzerland |
| 10 | 11 | 0.05 | 2017 | Australia |
| Ranking | Organizations | Country | Number of Documents | Year | Centrality |
|---|---|---|---|---|---|
| 1 | University of California System | United States | 31 | 2014 | 0.13 |
| 2 | Chinese Academy of Sciences | People’s Republic of China | 19 | 2017 | 0.18 |
| 3 | Harvard University | United States | 13 | 2014 | 0.10 |
| 4 | Howard Hughes Medical Institute | United States | 12 | 2014 | 0.15 |
| 5 | Ministry of Agriculture & Rural Affairs | People’s Republic of China | 11 | 2018 | 0.08 |
| 6 | University of California Berkeley | United States | 9 | 2014 | 0.00 |
| 7 | Chinese Academy of Agricultural Sciences | People’s Republic of China | 9 | 2021 | 0.07 |
| 8 | Centre National de la Recherche Scientifique (CNRS) | France | 8 | 2015 | 0.10 |
| 9 | UDICE-French Research Universities | France | 8 | 2015 | 0.13 |
| 10 | Massachusetts Institute of Technology (MIT) | United States | 8 | 2014 | 0.01 |
| Ranking | Journal | Country | Number of Documents | SJR 2022 |
Quartile |
|---|---|---|---|---|---|
| 1 | Proceedings of the National Academy of Sciences (PNAS) | United States | 249 | 4.03 | Q1 |
| 2 | Nature | United Kingdom | 245 | 20.96 | Q1 |
| 3 | Science | United States | 244 | 13.33 | Q1 |
| 4 | PloS ONE | United States | 197 | 0.89 | Q1 |
| 5 | Cell | United States | 194 | 26.49 | Q1 |
| 6 | Nucleic Acids Research | United Kingdom | 180 | 8.23 | Q1 |
| 7 | Nature Biotechnology | United Kingdom | 178 | 22.78 | Q1 |
| 8 | Nature Communications | United Kingdom | 161 | 5.12 | Q1 |
| 9 | Scientific Reports | United Kingdom | 147 | 0.97 | Q1 |
| 10 | Nature Methods | United Kingdom | 132 | 14.36 | Q1 |
| Ranking | Authors | Article | Journal | Citations |
|---|---|---|---|---|
| 1 | Jinek M et al. [51] | A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity | Science | 16 |
| 2 | Cong L et al. [52] | Multiplex Genome Engineering Using CRISPR/Cas Systems | Science | 14 |
| 3 | Zetsche B et al. [53] | Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System | Cell | 13 |
| 4 | Doench J et al. [54] | Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9 | Nature Biotechnology | 11 |
| 5 | Hsu P et al. [55] | Development and applications of CRISPR-Cas9 for genome engineering | Cell | 11 |
| 6 | Sternberg S et al. [56] | DNA interrogation by the CRISPR RNA-guided endonuclease Cas9 | Nature | 10 |
| 7 | Anders C et al. [57] | Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease | Nature | 7 |
| 8 | Doudna J et al. [58] | The new frontier of genome engineering with CRISPR-Cas9 | Science | 7 |
| 9 | Anzalone A et al. [59] | Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors | Nature Biotechnology | 7 |
| 10 | Makarova K et al. [60] | An updated evolutionary classification of CRISPR–Cas systems | Nature Reviews Microbiology | 6 |
| Applications | Cluster from bibliometric study | SDG |
|---|---|---|
| Fourth generation biofuels | 1, 2 |  |
| Biosensors | 1, 3 |  |
| Conservation | 1, 4 |  |
| Enhancing genetic resilience | 1, 2 |  |
| Combating invasive species | 1, 4 |  |
| Sustainable agriculture | 1, 3 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





