1. Introduction
Cardiovascular disease (CVD) is the leading cause of death globally. It diminishes the function of vital organs and can lead to severe conditions such as heart attacks or strokes, often remaining undetected until a life-threatening event occurs [
1]. CVD encompasses a spectrum of disorders affecting the heart and blood vessels, including coronary artery disease, cerebrovascular disease, heart failure, and various other conditions [
2,
3,
4]. Among the risk factors contributing to the progression of CVD, dyslipidemia plays a pivotal role [
2,
5,
6]. Dyslipidemia is characterized by elevated levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, and decreased levels of high-density lipoprotein cholesterol (HDL-C) compared to normal values. LDL-C, commonly referred to as “bad” cholesterol, is often associated with atherosclerosis, while HDL-C, known as “good” cholesterol, is typically linked to cardiovascular protection [
5,
6,
7]. Thus, controlling dyslipidemia is a crucial aspect of preventing both primary and recurrent cardiovascular events.
Omega-3 polyunsaturated fatty acids (PUFAs) have garnered considerable interest among researchers for their potential to reduce cardiovascular risk, as evidenced by numerous studies [
8,
9,
10,
11]. Omega-3 PUFAs, containing docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are known to potentially lower the mortality rate from cardiovascular events by enriching phospholipid membranes with DHA and EPA [
12,
13]. Therefore, Omega-3 fatty acids have the potential to enhance the control of arrhythmias, reduce blood pressure, improve arterial and endothelial function, limit platelet aggregation, and regulate abnormal lipid metabolism [
14,
15]. Fish oil and krill oil represent two natural food sources rich in Omega-3 fatty acids [
16,
17,
18]. While some studies suggested that Omega-3 in phospholipid (PL) form in krill oil may be more efficiently absorbed than Omega-3 in triacylglycerol (TAG) form in fish oil, the efficacy of Omega-3 supplementation from these sources remains debated [
18,
19]. Kim et al. (2020) conducted a study comparing the lipid-modifying effects of krill oil and fish oil through network meta-analysis demonstrated equivalent lipid-modifying effects of these oils, contingent on the dosage of Omega-3 [
20]. However, this study solely compared the efficacy of Omega-3 from these oils at different dosages without addressing different formulations, while the formulation is also a crucial factor related to the bioavailability and directly affects their lipid-modifying efficacy [
21,
22]. Hence, our study aims to compare the cardiovascular risk reduction effects of Omega-3 from fish oil and krill oil across various formulations through network meta-analysis, thereby providing an objective and comprehensive understanding of the efficacy of Omega-3 supplementation from these oil sources in the prevention and treatment of CVD.
2. Materials and Methods
2.1. Protocol and Registration
The NMA adhered to the PRISMA-NMA 2015 checklist [
23], followed guidelines from the
“Doing Meta-Analysis with R
” guide [
24] and was registered with PROSPERO under ID: CRD42024502338.
2.2. Eligibility Criteria
Inclusion criteria:
- Randomized controlled trials (RCTs), multi-arm studies.
- Complete presentation of each analyzed variable at the end of each study.
- Study subjects: individuals using FO or KO.
- Studies on subjects who are either people with heart risks or healthy regardless of race or gender.
- Being English articles
Exclusion criteria:
- Unfinished studies
- Without full-text articles
- Studies with withdrawn registrations
- Insufficient information on analyzed variables (TC, TG, HDL-C, LDL-C)
- Interventions with Omega-3 PUFAs but not supplemented in the form of FO or KO.
2.3. PICOS Research Questions
- P: Participants with heart risks or healthy
- I: Interventions consist of FO, KO
- C: Comparators include placebo, soybean oil, sunflower oil, corn oil, olive oil, regular diet, vegetable oil mixture, or no supplement
- O: TC, TG, HDL-C, LDL-C
- S: RCTs and multi-arm studies
2.4. Search Strategy
Identified search terms:
“Fish Oil
”,
“Krill Oil
”,
“Cholesterol
”,
“Triglyceride
”,
“Polyunsaturated Fatty Acid
”,
“CVD
”,
“Health
”. Locate synonyms in MeSH and align with PICOS criteria from January 1993 to December 2023. Search across 5 databases: ClinicalTrials.gov, Cochrane Library, ICTRP-WHO, Embase, and PubMed (Refer to
Supplementary Table S1 for specifics).
2.5. Study Selection (Screening), Data Collection and Data Items
The screening process employs the Convidence semi-automatic screening system, assessing titles, abstracts, and full texts.
The collected data comprised continuous variables: TC, TG, HDL-C and LDL-C.
2.6. Risk of Bias within Individual Studies
Using the PEDro scale with 11 questions corresponding to 10 points: “Poor” with a score of 0-3, “Moderate” with a score of 4-5, “Good” with a score of 6-8 and “Excellent” with a score of 9-10 to evaluate the quality of selected articles.
Processes: Study selection, data collection, and study quality assessment were performed independently by five team members. Differences were resolved through discussion and consensus.
2.7. Summary Measures - Planned Methods of Analysis
The study was conducted on R and R Studio software (version 2023.12.0+369), performing regular network meta-analysis using the netmeta package.
Main summary measures:
Heterogeneity assessment: Total heterogeneity (Q-total) and degrees of freedom (df) were calculated, along with I
2, tau², and tau values to assess the variability in effect estimates that could not be attributed solely to sampling error. Publication bias assessment: Involved calculating the Z-score and P-value from the meta-analysis results, with further testing using the Egger’s test (t) and Standard Error (SE) analysis to identify asymmetries. Crippa and Orsini method [
25] for continuous data. (detail in
Supplementary Table S3).
3. Results
3.1. Study Selection
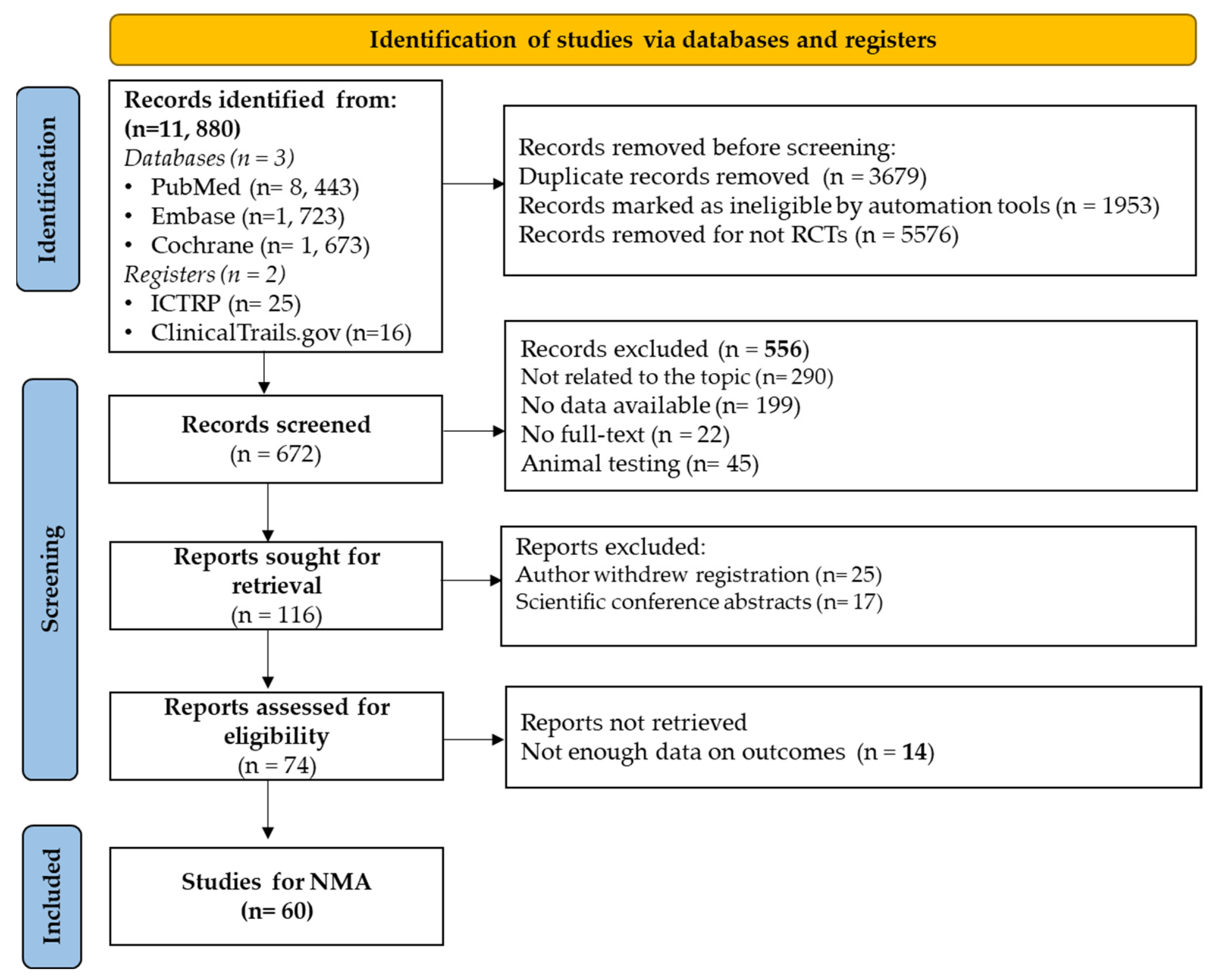
After initial screening, 11880 studies were identified. The Covidence tool helped eliminate duplicates (n = 3679), non-RCT articles (n = 5576), and those not meeting criteria (n = 1953). Among the 672 remaining studies, 294 were excluded due to irrelevance or non-English. Full-text screening of 378 studies resulted in 318 exclusions due to various reasons, including those inaccessible in full text or withdrawn by authors, leaving 60 studies were retained for the network meta-analysis (
Figure 1)
3.2. Study Characteristics
Detailed study characteristics are presented in the
Figure 2 and
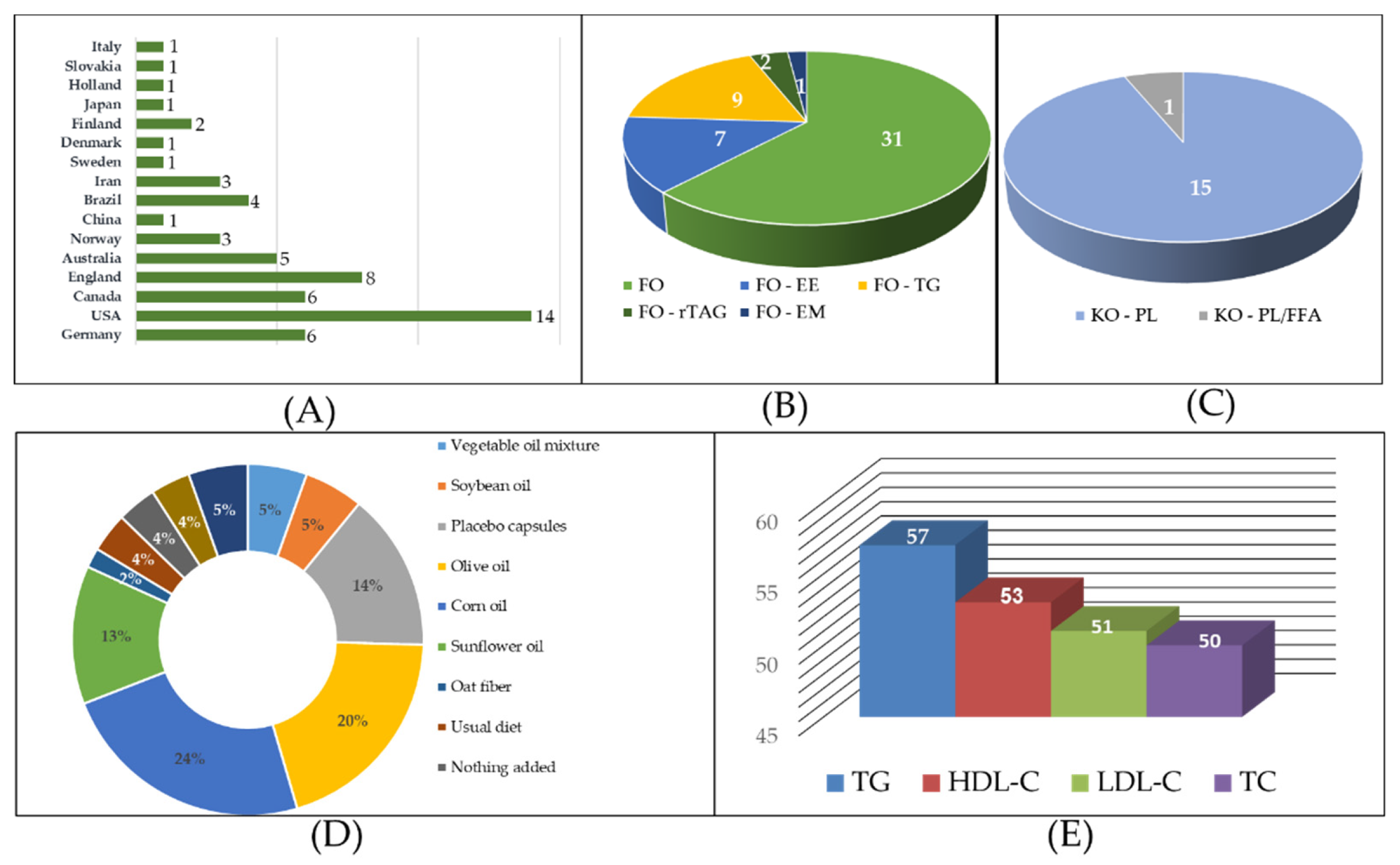
Supplementary Table S4 summarize the basic characteristics of the studies, conducted across 16 countries. The US contributed the highest number of Studies (14), followed by the UK (8), Germany (6), and Canada (6). Participants included individuals at risk of CVD or healthy individuals receiving FO or KO interventions in various forms and dosages. For FO, capsules were the most common form (31 Studies), followed by ethyl ester (EE) (7 Studies), triglyceride (TG) (9 Studies), re-esterified triacylglycerol (rTAG) (2 Studies), and emulsion (EM) (1 study). KO was predominantly in phospholipid (PL) form (15 Studies) and in combination with free fatty acid (PL/FFA) (1 study). Control groups mostly consisted of oils without pharmacological effects, such as corn oil, olive oil, sunflower oil, and placebo capsules. Outcome variables included TC (50 Studies), TG (57 Studies), HDL-C (53 Studies), LDL-C (51 Studies).
3.3. Risk of Bias within Studies
The detailed results are presented in
Figure 3 and
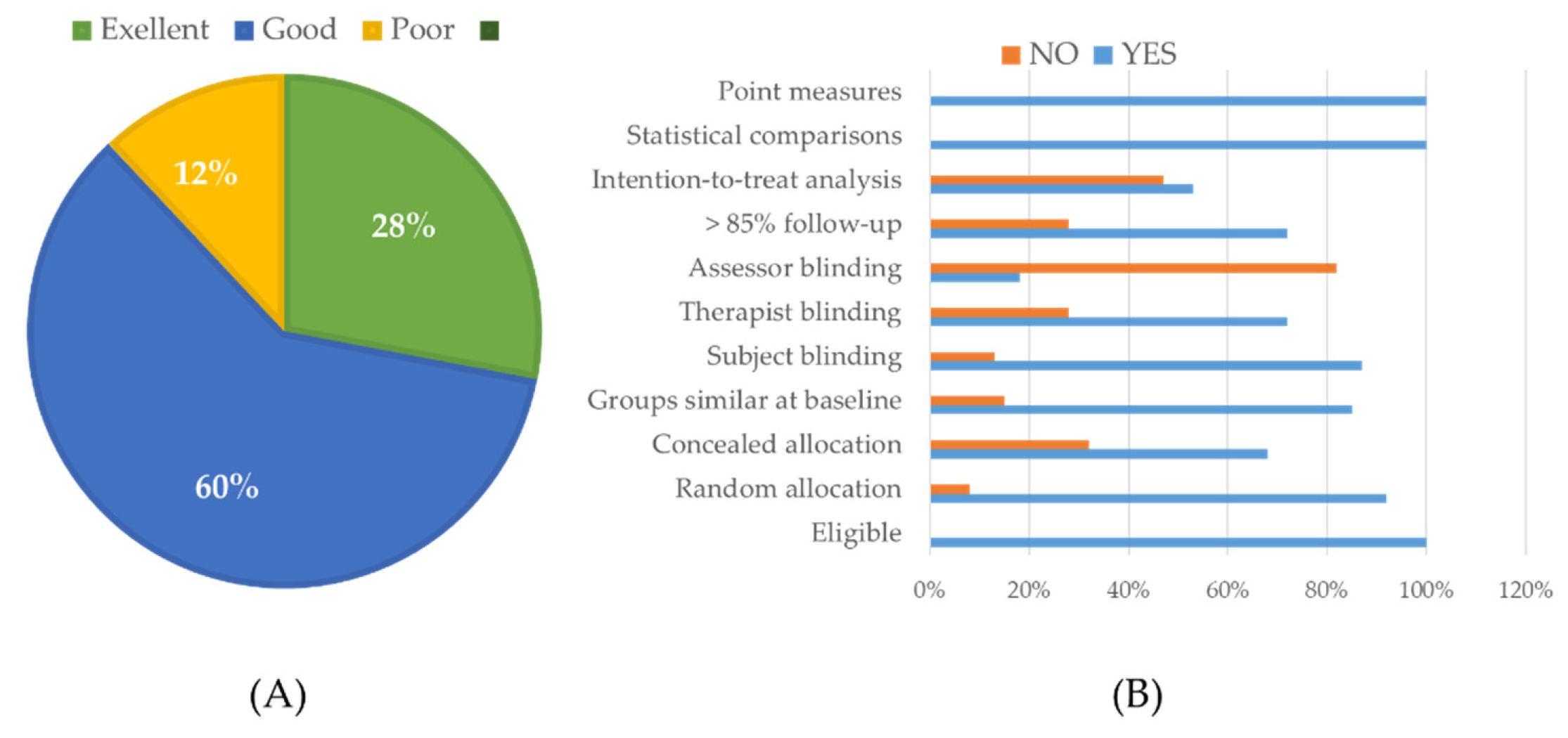
Supplementary Table S5. Out of the total, 17 studies were assessed as having excellent quality (28%), 36 studies as good quality (60%), and the remaining 7 studies as average quality (12%). None of the studies were rated as poor quality. Most of the 60 studies met all three criteria: “Eligible participants”, “Statistical comparison methods between groups”, and “Score measures and variability measures”. Additionally, 7 studies were deemed of moderate quality, with a risk of bias primarily related to lack of information on intention-to-treat (ITT) analysis, allocation concealment, or blinding of subjects (therapists/support staff, outcome assessors/scientists/study authors).
3.4. The Network-Graph
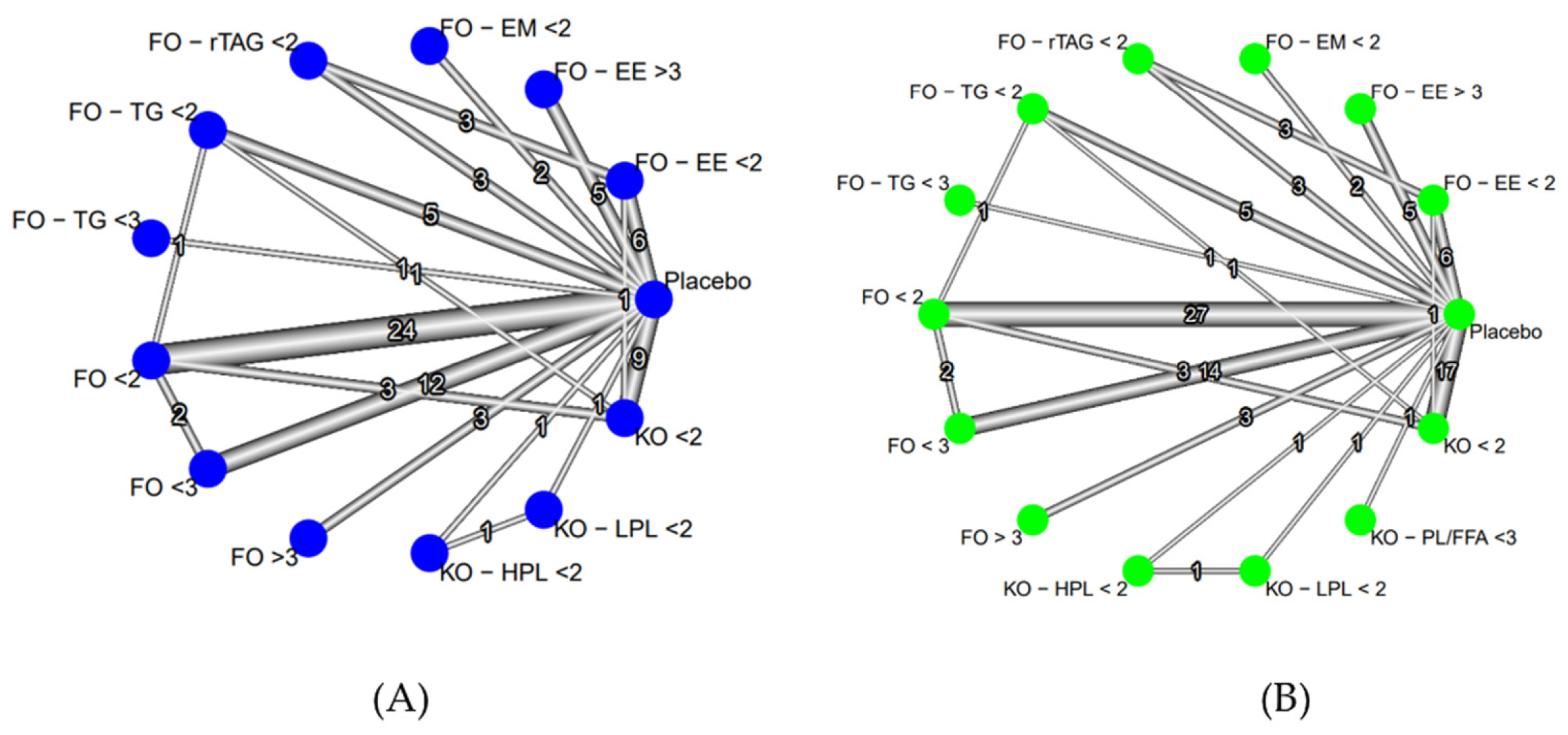
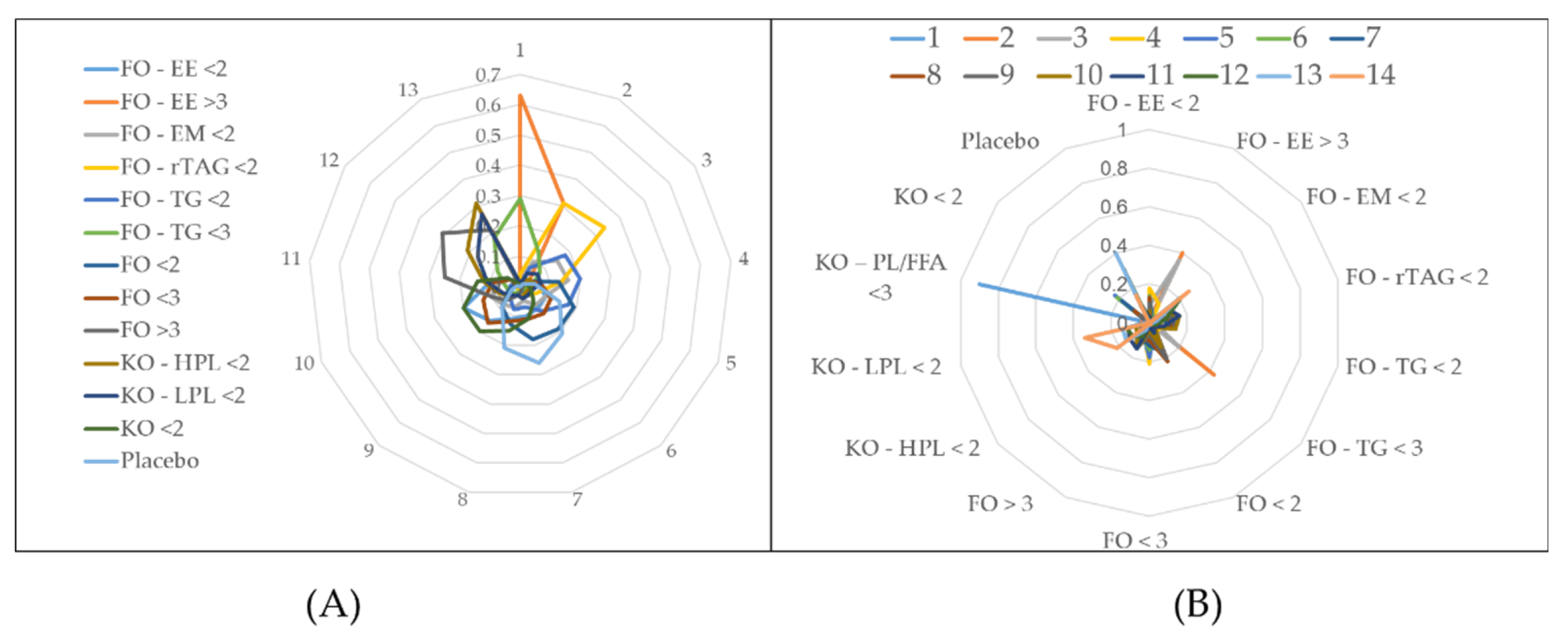
Analyses of lipid indices revealed significant findings across various dosage forms. For Total Cholesterol (TC), 50 studies were scrutinized, yielding 84 comparison pairs across 14 forms. Notably, comparisons centered on FO < 2, FO < 3, KO < 2, and placebo. High heterogeneity was observed (tau2 = 0.0347; tau = 0.1864; I2 = 59.5%), with significant differences in interventions (Q value = 155.43; p < 0.0001) (
Figure 4A,
Supplementary Table S6).
Triglycerides (TG) analysis involved 57 studies, producing 98 comparison pairs. Predominantly, comparisons featured FO < 2, KO < 2, FO < 3, and placebo. High heterogeneity was noted (tau2 = 0.0189; tau = 0.1375; I2 = 84.3%), with significant variability in intervention comparisons (Q total = 489.09; p < 0.0001)
(Figure 4B
, Supplementary Table S7).
High-Density Lipoprotein Cholesterol (HDL-C) analysis comprised 53 studies, revealing 78 comparison pairs. FO < 2, FO < 3, KO < 2, and placebo were frequently compared. Minimal heterogeneity was observed (tau2 = 0.0009; tau = 0.0299; I2 = 17.1%), with no significant differences in interventions (Q total = 67.59; p = 0.138)
(Supplementary Figure S3, Table S12).
In Low-Density Lipoprotein Cholesterol (LDL-C) analysis, 60 studies were reviewed, generating 78 comparison pairs. FO < 2, FO < 3, KO < 2, and placebo were commonly compared. High heterogeneity was present (tau2 = 0.0751; tau = 0.2741; I2 = 87.9%), with substantial differences in intervention comparisons (Q total = 462.14; p < 0.0001)
(Supplementary Figure S7, Table S19).
3.5. Paired Comparison
In the analysis of TC
(Table 1), the intervention FO - EE > 3 demonstrated superior efficacy compared to most interventions, including FO - EE < 2, FO - TG < 2, FO < 2, FO < 3, FO > 3, KO - HPL < 2, KO - LPL < 2, KO < 2, and placebo. Similarly, FO - rTAG < 2 showed higher treatment effectiveness than FO > 3. Only FO - TG < 2, FO - EE > 3, and FO - rTAG < 2 compared with placebo yielded statistically significant results in the TC network meta-analysis (NMA).
Regarding TG (Table 2), significant treatment comparisons include KO - PL/FFA < 3, which is more effective in reducing TG compared to all other intervention oils except FO - EE > 3 and FO - TG < 3. Additionally, FO - EE > 3 showed better treatment effectiveness than FO - EM < 2. Comparisons between placebo and KO < 2, KO - PL/FFA < 3, FO < 3, FO < 2, FO - TG < 3, FO - EE > 3, and FO - EE < 2 yielded statistically significant results in the TG NMA.
For HDL-C (
Supplementary Table S14), significant treatment comparisons include FO - EE < 2, which is more effective in increasing HDL-C compared to FO < 3, FO < 2, and KO < 2. Similarly, FO - EE < 3 is more effective than FO - EE > 3, FO > 3, FO - TG < 2, FO - TG < 3, and FO < 2. Comparisons between FO - EE > 3, FO < 2, FO < 3, FO > 3, and placebo versus FO - EE < 2 with KO < 2 contributed to statistically significant pooled effect sizes for the HDL-C NMA.
In the analysis of LDL-C (
Supplementary Table S21), significant treatment comparisons include FO - EE < 2 and FO - rTAG < 2, which are more effective in reducing LDL-C compared to FO < 3, FO > 3, and KO < 2. Similarly, FO - EE > 3 shows better effectiveness compared to FO - TG < 2, FO < 2, FO < 3, FO > 3, and KO < 2. Comparisons between FO - EE < 2, FO - EE > 3, and placebo versus FO - EE < 2 contributed to statistically significant pooled effect sizes for the LDL-C NMA.
3.6. Treatment Ranking (SUCRA and Rankogram)
For TC, FO - EE > 3 (SUCRA = 96.35%) showed the most effective treatment, while FO - rTAG < 2 (SUCRA = 80.35%), FO – TG < 2 (SUCRA = 63.45%), FO - TG < 3 (SUCRA = 59.96%), and FO - EM < 2 (SUCRA = 56.38%) were effective in reducing TC but had insignificant, with probabilities of 0.63, 0.31, 0.2, 0.29, and 0.16 respectively.
(Figure 5A
, Table 3).
Regarding TG, eight interventions showed the most effective TG reduction with solid evidence: KO - PL/FFA < 3 (SUCRA = 96.82%), FO - EE > 3 (SUCRA = 84.32%), FO - TG < 3 (SUCRA = 77.92%), FO < 3 (SUCRA = 64.02%), KO < 2 (SUCRA = 61.63%), FO - EE < 2 (SUCRA = 59.29%), FO – TG < 2 (SUCRA = 48.68%), and FO < 2 (SUCRA = 45.63%), with corresponding probabilities of 0.90, 0.4, 0.43, 0.21, 0.23, 0.4, 0.14, and 0.22
((Figure 5B
, Table 4).
For HDL-C, five interventions showing the most effectiveness in increasing HDL-C were FO - TG < 3 (SUCRA = 7.72%), FO < 3 (SUCRA = 22.52%), FO < 2 (SUCRA = 28.02%), KO - HPL < 2 (SUCRA = 33.70%), and KO < 2 (SUCRA = 34.78%), with corresponding probabilities of 0.77, 0.27, 0.27, 0.26, and 0.22
(Supplementary Figure S4).
In LDL-C analysis, interventions FO - EE > 3 (SUCRA = 93.80%), FO - EE < 2 (SUCRA = 81.98%) showed the highest effectiveness in reducing LDL-C clearly, while FO - rTAG < 2 (SUCRA = 81.49%), FO - TG < 3 (SUCRA = 63.15%), FO < 2 (SUCRA = 54.15%), and FO - EM < 2 (SUCRA = 50.57%) reducing LDL-C not statistically significant with corresponding probabilities of 0.54, 0.33, 0.26, 0.27, 0.25, and 0.14 respectively.
(Supplementary Figure S9).
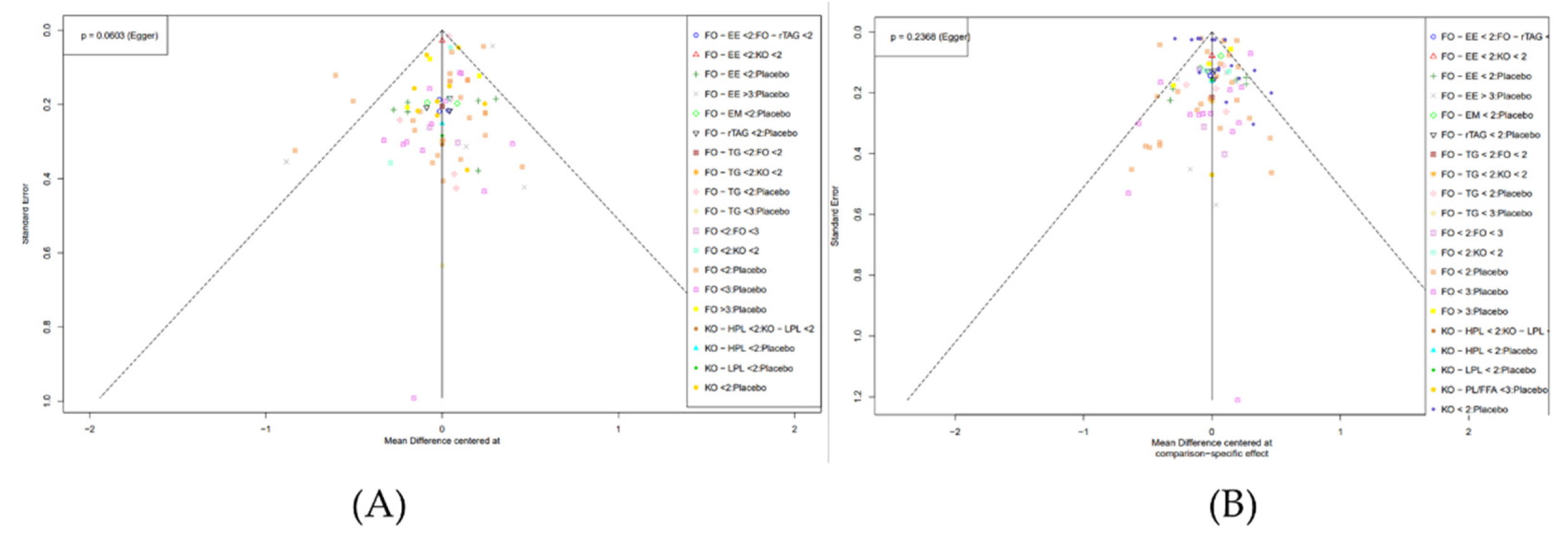
3.7. Publication Bias
Publication bias was assessed using funnel plots and Egger test. For TC variable, no significant asymmetry was observed, and the Egger test showed no publication bias (p = 0.0603 > 0.05) (
Figure 6 (A),
Table 5). Regarding the TG variable, the Egger test indicated no publication bias (p = 0.2368 > 0.05), and most intervention pairs showed accurate results within the confidence interval (
Figure 6 (B),
Table 6). Similarly, for HDL-C level, the Egger test found no publication bias (p = 0.0957 > 0.05), with most intervention pairs demonstrating high accuracy within the confidence interval (
Supplementary Figure S6 & Table S18). However, for LDL-C level, significant asymmetry in the plot suggests potential publication bias, with a deviation observed in the Egger test (p < 0.0001) (
Supplementary Figure S11 & Table S24).
4. Discussion
This research offers a thorough overview of both direct and indirect comparisons involving 14 distinct formulations of FO and KO. The investigation concentrated on four key outcome indicators: TC, TG, HDL-C, and LDL-C. A total of 60 studies were incorporated in the analysis. Bias assessment indicated that 88% of the trials attained either high or excellent quality.
4.1. Total Cholesterol (TC)
According to the World Health Organization (WHO), one-third of ischemic heart disease cases result from high cholesterol, contributing to 2.6 million deaths (4.5% of the global total), emphasizing the crucial role of controlling TC [
26]. Our analysis results indicated that FO - EE > 3 are more effective in reducing TC levels compared to interventions with concentrations ranging from 300 mg to 1900 mg. Conversely, formulations with FO > 3 did not exhibit therapeutic efficacy (SUCRA=18.22%). Our findings suggested that FO supplementation is more effective in reducing TC levels than KO. Additionally, other studies have highlighted the potential of KO to elevate TC levels compared to FO [
27,
28,
29]. Furthermore, we proposed a priority order for FO usage: FO – EE > FO – rTAG > FO – TG, and for KO: KO – HPL > KO – LPL. Research by Cicero A.F.G. [
30] aligns with our findings, showing that both Omega-3 fatty acids and KO can reduce blood TG levels, with esterified Omega-3 demonstrating superior efficacy over KO (p < 0.05). Despite some studies supporting these results, others present conflicting findings. Studies by Nicholls S.J. [
31], A. Kalstad [
32], J. Manson [
33] suggested that adding Omega-3 to conventional therapy does not significantly impact cardiovascular event efficacy. Egger tests conducted to assess publication bias yielded no evidence of bias in NMA TC (p = 0.0603 > 0.05). Linear regression analysis did not provide sufficient statistical evidence of plot asymmetry. However, we acknowledge that the inconsistency arises from comparisons such as FO < 2, FO - EE > 3 vs Placebo, with most of the disparity between direct and indirect evidence arising from FO - TG < 2: FO < 2 and FO < 2: Placebo (
Supplementary Tables S10, S12, S17 and S23).
4.2. Triglyceride (TG)
Omega-3 supplementation has shown promise in improving TG[
34]. Analysis revealed that using KO combined with PL/FFA at EPA + DHA doses ranging from 2000 – 2900 mg (KO - PL/FFA <3, MD = -1.24, 95% CI = [-2.1991; -0.2809], z = -2.53 < -1.96), p = 0.0113) yields the highest efficacy (
Supplementary Figure S1 & Table S6). Other formulations also exhibit considerable treatment effectiveness, with KO - PL/FFA < 3 ranking highest, followed by FO - EE > 3; FO - TG < 3; FO < 3; KO < 2. These findings emphasize KO as a viable alternative to FO for reliably reducing TG levels, even at doses of 2000 - 2900 mg or lower. Even KO capsules with doses below 2000 mg demonstrate superior therapeutic effects compared to FO in EE, TG form or FO capsules at equivalent doses. A meta-analysis further supports this, indicating that even KO containing less than 1000 mg of Omega-3 exhibits similar TG-reducing effects as FO containing 1000 – 2900 Omega-3 [
20]. Research by Wang Y. et al. [
10] confirmed the significant reduction in triglycerides with Omega-3 supplementation (WMD: -18.18 mg/dl; 95% CI: -25.41. - 10.95; p < 0.001), endorsing the efficacy of KO usage. However, a multicenter, randomized, double-blind trial involving 1384 participants found no improvement in lipid indices with 4 g of Omega-3 supplementation per day [
31]. In the NMA TG, while direct evidence studies are limited (only 21.98%), interventions demonstrating high treatment effectiveness all possess direct evidence. Nevertheless, the results post Cochrane
Q’ split (
Supplementary Table S9) revealed 3 placebo comparison pairs with FO < 3, FO < 2, and KO < 2, believed to contribute to NMA TG heterogeneity. Linear regression testing (Egge
r’s test) indicated no evidence of publication bias in NMA TG (p = 0.2368 > 0.05). Most of the effectiveness measures of the intervention pairs were within the confidence interval, with high accuracy, indicating a high level of certainty in the NMA TG results.
4.3. High-Density Lipoprotein Cholesterol (HDL-C)
It can be said that using TG doses of FO (DHA+EPA) from 2000 - 2900 mg per day and FO from 300 - 1900 mg per day both show effectiveness in reducing HDL-C. Regarding krill oil (KO), although studies were conducted at low doses (less than 1000 mg per day), it still exhibited effectiveness in increasing HDL-C levels in both CVD patients and healthy individuals. However, some studies suggested that HDL content remains unchanged with any intervention [
27,
28]. Moreover, in NMA HDL-C, the proportion of studies with direct evidence is low (only 21.98%), while research designs with indirect and direct evidence account for 13.19%, and as high as 91.21% have indirect evidence (totaling 91 comparisons)
(Supplementary Table S16). Nonetheless, there is insufficient statistical evidence to demonstrate graph asymmetry. The majority of intervention pairs’ effectiveness measures fall within the confidence interval, with high precision (with t-values = 1.69, degrees of freedom df = 76, and p-value = 0.0957 < 0.05). The bias estimate is 0.2913 with a standard error (SE) of 0.1727, indicating a level of certainty in this NMA HDL-C result
. (Supplementary Figure S6 & Table S18)
4.4. Low-Density Lipoprotein Cholesterol (LDL-C)
The use of various forms of FO demonstrates significant efficacy in improving LDL-C levels, with priority ranking as follows: FO - EE > 3; FO - EE < 2; FO - rTAG < 2; FO - TG < 3; FO < 2; FO - EM < 2. It can be inferred that utilizing FO-EE doses (DHA + EPA) < 2000 mg and > 3000 mg per day, or lower doses of FO - rTAG below 2000 mg, yields the most pronounced reduction in LDL-C levels. Additionally, research by Xie S.[
35] and Xiao Y.v [
36] corroborated that Omega-3 supplementation has no impact on LDL-C concentrations. Conversely, certain studies suggested that FO usage elevates LDL-C levels and enhances LDL-C oxidation, thus impeding lipid regulation [
31]. Despite our analysis revealing a no
Table disparity between projected and observed data, with a bias estimate of -2.6569 and SE = 0.6215, and the heterogeneity of NMA LDL-C results stemming from comparisons between Placebo and: FO < 2, FO - EE < 2 (p < 0.0001), our study has contributed to a more impartial perspective, offering dependable insights into the dosage, formulation, and efficacy of these oils
. (Supplementary Figure S11 & Table S24)
4.5. Strengths and Limitations
This pilot NMA has several strengths. Firstly, with a substantial inclusion of RCTs (60 RCTs), this NMA offers greater information and stronger evidence compared to RCTs and traditional meta-analyses. Secondly, the study results are highly dependable, with only the LDL-C level exhibiting evidence of publication bias, while the TC, TG, and HDL-C indices show no indication of publication bias. Thirdly, while the previous study by Kim et al. (2020) [
20] concluded that FO and KO had similar effects on lipid modification, current study evaluated the efficacy of Omega-3 from these sources on lipid profiles and cardiovascular risk, offering evidence across various formulations and dosages. As a result, we observed differences in the effects on TC, TG, LDL-C, and HDL-C across various forms and doses of FO and KO. Therefore, our study offers a significant advancement in understanding the cardiovascular risk-reducing effects of Omega-3 from FO and KO, as well as provides important insights for optimizing supplementation strategies and improving patient outcomes. However, this NMA also has a few limitations. Primarily, in all four indicators (TC, TG, HDL-C, LDL-C), the proportion of study designs with indirect evidence is notably high, while the proportion of study designs with direct evidence, as well as those with both direct and indirect evidence, is much lower. Therefore, cautious interpretation of results is warranted in these instances. Furthermore, it’s important to highlight that our study specifically targeted two distinct participant groups: individuals identified as having cardiovascular risks and those classified as healthy. We deliberately excluded subjects with other medical conditions to ensure clarity and maintain focus within our analysis.
5. Conclusions
In summary, this study comprehensively compares interventions involving FO and KO across different dosage forms (capsules, EE, TG, rTAG, PL/FFA, HPL, LPL, EM) and varying dosages (ranging from 300 - 1900 mg, 2000 - 2900 mg, to over 3000 mg) on TC, TG, HDL-C, and LDL-C indices. Most interventions demonstrate effectiveness in reducing TG levels in cardiovascular patients, particularly KO - PL/FFA < 3. Regarding cholesterol levels, positive effects on TC and LDL-C are observed with the use of FO - EE > 3. FO - TG < 3 shows benefits across TC, TG, and HDL-C, while FO - rTAG < 2 is effective in reducing TC and LDL-C.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Table S1. Search terms across databases, Table S2. Selected research, Table S3. Expected analysis methods, Table S4. Research features, Table S5. Research quality, Table S6. Net graph results of TC index, Table S7. Net graph results of TG index, Table S8. Rankogram results of TC index, Figure S1. Forest plot of TG, Figure S2. Forest plot of TC, Figure S3. Net graph estimating the effect of intervention oils on HDL-C, Figure S4. Forest plot of HDL-C, Figure S5. Rankogram and SUCRA results for HDL-C, Figure S6. Net heat chart of HDL-C index, Figure S7. Results of published bias analysis for HDL-C, Figure S8. Net graph estimates the effect of interfering oils on LDL, Figure S9. LDL–C forest chart, Figure S10. Rankogram and SUCRA results for LDL-C, Figure S11. Net heat graph of LDL-C index, Figure S12. Results of published bias analysis for LDL - C index, Table S9 Cochrane Q’ analysis on TG , Table S10 Results of SIDE analysis on TG, Table S11: Decomposition of Q’Cochrane on TC , Table S12: Results of SIDE analysis on TC , Table S13: net graph results of HDL-C, Table S14. Rankogram results of HDL-C , Table S15. Paired treatment comparison league tables on net estimates of HDL-C, Table S16: Cochrane Q’ analysis on HDL-C index, Table S17: SIDE analysis results on HDL-C index, Table S18: Asymmetry test results of funnel chart after linear regression, Table S19: Net graph results of LDL-C, Table S20. Rankogram results of LDL - C index , Table S21. League table comparing treatment in pairs on network estimates of LDL-C , Table S22. Cochrane Q’ analysis on LDL-C index, Table S23: SIDE analysis results on LDL-C index, Table S24: Asymmetry test results of funnel chart after linear regression.
Author Contributions
Conceptualization, T.P.T.P., T.T.D.L. and P.T.N.C.; methodology, T.P.T.P. and T.T.D.L.; software, T.P.T.P., T.T.D.L. and P.T.N.C.; validation, H.A.H., T.V.H., M.T.H.; formal analysis, P.T.N.C., T.T.D.L. and T.P.T.P.; investigation., H.A.H., T.V.H., and M.T.H.; resources, T.P.T.P.; data curation., T.P.T.P., T.T.D.L., T.T.L.P., O.R.M. and P.T.N.C.; writing—original draft preparation, T.P.T.P ,T.T.D.L., T.M.H.V. and T.H.T.L.; writing—review and editing, H.A.H., T.V.H., and M.T.H.; visualization, T.P.T.P., T.T.D.L., P.T.N.C., T.M.H.V., T.H.T.L., V.T.N.H.,.H.T.X.P., O.R.M., T.T.L., M.T.H., H.A.H., and T.V.H.; supervision, H.A.H., T.V.H., and M.T.H.; project administration, T.P.T.P.and T.V.H.; funding acquisition, M.T.H.
Funding
This research was funded by by China Medical University (CMU111-S-23) and China Medical University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used for the analysis are available from the corresponding authors on request.
Acknowledgments
The authors thank Min-Tsang Hsieh, Drug Development Center, China Medical University, Taichung 406040, Taiwan, for his ongoing support in the development of the search strategy.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Berner-Rodoreda, A.; Kanyama, C.; Supady, A.; Bärnighausen, T. Cardiovascular Diseases. In Global Health Essentials; Raviglione, M.C.B., Tediosi, F., Villa, S., Casamitjana, N., Plasència, A., Eds.; Springer International Publishing: Cham, 2023; pp. 157–162. ISBN 978-3-031-33851-9. [Google Scholar]
- Flora, G.D.; Nayak, M.K. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. CPD 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Soulaidopoulos, S.; Madenidou, A.-V.; Daoussis, D.; Melissaropoulos, K.; Mavrogeni, S.; Kitas, G.; Dimitroulas, T. Cardiovascular Disease in the Systemic Vasculitides. CVP 2020, 18, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Ottaviani, G.; Mitchell, R.N. Pathobiology of Cardiovascular Diseases: An Update. Cardiovascular Pathology 2019, 42, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Chae, S.M. Comparison of Efficacy and Safety of Combination Therapy with Statins and Omega-3 Fatty Acids versus Statin Monotherapy in Patients with Dyslipidemia: A Systematic Review and Meta-Analysis. Medicine 2018, 97, e13593. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnia, M.; Asadi, Z.; Zare-Feyzabadi, R.; Yaghooti-Khorasani, M.; Ghazizadeh, H.; Ghaffarian-Zirak, R.; Nosrati-Tirkani, A.; Mohammadi-Bajgiran, M.; Rohban, M.; Sadabadi, F.; et al. Dyslipidemia and Cardiovascular Disease Risk among the MASHAD Study Population. Lipids Health Dis 2020, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Anyaegbunam, U.A.; More, P.; Fontaine, J.-F.; Cate, V.T.; Bauer, K.; Distler, U.; Araldi, E.; Bindila, L.; Wild, P.; Andrade-Navarro, M.A. A Systematic Review of Lipid-Focused Cardiovascular Disease Research: Trends and Opportunities. CIMB 2023, 45, 9904–9916. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Zhou, N.; Shen, Y.; Li, B.; Chen, B.E.; Li, X. Association Between Omega-3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta-Analysis of Randomized Controlled Trials. JAHA 2023, 12, e029512. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hou, H.; Wang, Y.; Li, Y.; Zhang, L.; Zhang, H.; Jin, Q.; Wu, G.; Wang, X. Effects of Omega-3, Omega-6, and Total Dietary Polyunsaturated Fatty Acid Supplementation in Patients with Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Food Funct. 2024, 15, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Shehzad, Q.; Su, Y.; Xu, L.; Yu, L.; Zeng, W.; Fang, Z.; Wu, G.; Wei, W.; et al. Does Omega-3 PUFAs Supplementation Improve Metabolic Syndrome and Related Cardiovascular Diseases? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Critical Reviews in Food Science and Nutrition 2023, 1–28. [Google Scholar] [CrossRef]
- Quispe, R.; Alfaddagh, A.; Kazzi, B.; Zghyer, F.; Marvel, F.A.; Blumenthal, R.S.; Sharma, G.; Martin, S.S. Controversies in the Use of Omega-3 Fatty Acids to Prevent Atherosclerosis. Curr Atheroscler Rep 2022, 24, 571–581. [Google Scholar] [CrossRef]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. ATVB 2020, 40, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Omega-3 Polyunsaturated Fatty Acids Focusing on Eicosapentaenoic Acid and Docosahexaenoic Acid in the Prevention of Cardiovascular Diseases: A Review of the State-of-the-Art. Expert Review of Clinical Pharmacology 2021, 14, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R.V. An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Drenjančević, I.; Pitha, J. Omega-3 Polyunsaturated Fatty Acids—Vascular and Cardiac Effects on the Cellular and Molecular Level (Narrative Review). IJMS 2022, 23, 2104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, L.; Wang, D.; Sun, Y.; Huang, J.; Shahidi, F. Stability and Stabilization of Omega-3 Oils: A Review. Trends in Food Science & Technology 2021, 118, 17–35. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Rong, Y.; Yuan, Y.; Ding, Y.; Shi, W.; Wang, Z. Effects of Different Proteases Enzymatic Extraction on the Lipid Yield and Quality of Antarctic Krill Oil. Food Science & Nutrition 2019, 7, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill ( Euphausia Superba ) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Comp Rev Food Sci Food Safe 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H. (Sabrina); Carne, A.; Bekhit, A.E. Marine Omega-3 (N-3) Phospholipids: A Comprehensive Review of Their Properties, Sources, Bioavailability, and Relation to Brain Health. Comp Rev Food Sci Food Safe 2020, 19, 64–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Yang, I.; Lee, H.S.; Lee, J.-Y.; Kim, K. Lipid-Modifying Effects of Krill Oil vs Fish Oil: A Network Meta-Analysis. Nutrition Reviews 2020, 78, 699–708. [Google Scholar] [CrossRef]
- Aungst, B.J. Optimizing Oral Bioavailability in Drug Discovery: An Overview of Design and Testing Strategies and Formulation Options. Journal of Pharmaceutical Sciences 2017, 106, 921–929. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide; 1st ed.; Chapman and Hall/CRC: Boca Raton, 2021; ISBN 978-1-00-310734-7. [Google Scholar]
- Crippa, A.; Orsini, N. Dose-Response Meta-Analysis of Differences in Means. BMC Medical Research Methodology 2016, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.; Guthold, R.; Cowan, M.; Savin, S.; Bhatti, L.; Armstrong, T.; Bonita, R. The World Health Organization STEPwise Approach to Noncommunicable Disease Risk-Factor Surveillance: Methods, Challenges, and Opportunities. Am J Public Health 2016, 106, 74–78. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced Increase of Omega-3 Index in Healthy Individuals with Response to 4-Week n-3 Fatty Acid Supplementation from Krill Oil versus Fish Oil. Lipids Health Dis. 2013, 12. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic Effects of Krill Oil Are Essentially Similar to Those of Fish Oil but at Lower Dose of EPA and DHA, in Healthy Volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of Krill Oil and Lean and Fatty Fish on Cardiovascular Risk Markers: A Randomised Controlled Trial. J Nutr Sci 2018, 7, e3. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.; Rosticci, M.; Morbini, M.; Cagnati, M.; Grandi, E.; Parini, A.; Borghi, C. Lipid-Lowering and Anti-Inflammatory Effects of Omega 3 Ethyl Esters and Krill Oil: A Randomized, Cross-over, Clinical Trial. 2016, 12, 507–512. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of N-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef]
- Marine N−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer | New England Journal of Medicine. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1811403 (accessed on 25 April 2024).
- Khorshidi, M.; Hazaveh, Z.S.; Alimohammadi-kamalabadi, M.; Jamshidi, S.; Moghaddam, O.M.; Olang, B.; Hatefi, S.; Hosseini, A.; Jamilian, P.; Zarezadeh, M.; et al. Effect of Omega-3 Supplementation on Lipid Profile in Children and Adolescents: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutr J 2023, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; Orsini, N. Dose-Response Meta-Analysis of Differences in Means. BMC Medical Research Methodology 2016, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The Effects of Omega-3 Fatty Acids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Prostaglandins, Leukotrienes and Essential Fatty Acids 2022, 182. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Flow diagram illustrating the study selection process, from identification to inclusion in the meta-analysis, detailing numbers at each stage ((RCT: Randomized Controlled Trial; ICTRP: International Clinical Trials Registry Platform).
Figure 1.
Flow diagram illustrating the study selection process, from identification to inclusion in the meta-analysis, detailing numbers at each stage ((RCT: Randomized Controlled Trial; ICTRP: International Clinical Trials Registry Platform).
Figure 2.
Basic characteristics of the studies: (A) Country of study (B) Basic characteristics of FO group (C) Basic characteristics of KO group (D) Basic characteristics of control group (E) Study outcomes figure. (Legends: FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol).
Figure 2.
Basic characteristics of the studies: (A) Country of study (B) Basic characteristics of FO group (C) Basic characteristics of KO group (D) Basic characteristics of control group (E) Study outcomes figure. (Legends: FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; TC: Total Cholesterol; TG: Triglycerides; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol).
Figure 3.
Assess the risk of bias across studies (A) Quality percentage in studies (B) Proportion of 11 criteria in individual studies (Summarizing the risk of bias within included studies according to the PEDro scale, including individual criteria assessments).
Figure 3.
Assess the risk of bias across studies (A) Quality percentage in studies (B) Proportion of 11 criteria in individual studies (Summarizing the risk of bias within included studies according to the PEDro scale, including individual criteria assessments).
Figure 4.
Net graph estimates the effect of intervention oils on TC level (A) and TG level (B) (random effect model). (TC: Total Cholesterol; TG: Triglycerides; FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; < 2: doses (DHA+EPA) 300 - 1900 mg per day; < 3: doses (DHA+EPA) 2000 - 2900 mg per day; > 3: doses (DHA+EPA) above 3000 mg per day).
Figure 4.
Net graph estimates the effect of intervention oils on TC level (A) and TG level (B) (random effect model). (TC: Total Cholesterol; TG: Triglycerides; FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; < 2: doses (DHA+EPA) 300 - 1900 mg per day; < 3: doses (DHA+EPA) 2000 - 2900 mg per day; > 3: doses (DHA+EPA) above 3000 mg per day).
Figure 5.
Rankograms visualizing the probability rankings of (A) TC level and (B) TG level. (TC: Total Cholesterol); TG: Triglycerides; FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; < 2: doses (DHA+EPA) 300 - 1900 mg per day; < 3: doses (DHA +EPA) 2000 - 2900 mg per day; > 3: doses (DHA+EPA) above 3000 mg per day).
Figure 5.
Rankograms visualizing the probability rankings of (A) TC level and (B) TG level. (TC: Total Cholesterol); TG: Triglycerides; FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; < 2: doses (DHA+EPA) 300 - 1900 mg per day; < 3: doses (DHA +EPA) 2000 - 2900 mg per day; > 3: doses (DHA+EPA) above 3000 mg per day).
Figure 6.
Funnel plot showing the results of the publication bias analysis for the TC level (A) and TG level (B) by Egger’s test.(TC: Total Cholesterol); TG: Triglycerides; FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; < 2: doses (DHA+EPA) 300 - 1900 mg per day; < 3: doses (DHA+EPA) 2000 - 2900 mg per day; > 3: doses (DHA+EPA) above 3000 mg per day).
Figure 6.
Funnel plot showing the results of the publication bias analysis for the TC level (A) and TG level (B) by Egger’s test.(TC: Total Cholesterol); TG: Triglycerides; FO: fish oil; KO: krill oil; FO - EE: fish oil ethyl ester; FO - TG: fish oil triglycerides; FO - rTAG: fish oil re-esterified triacylglycerol; FO - EM: fish oil emulsion; KO - HPL: krill oil high phospholipid; KO - LPL: krill oil low phospholipid; KO - PL/FFA: krill oil phospholipid/free fatty acid; < 2: doses (DHA+EPA) 300 - 1900 mg per day; < 3: doses (DHA+EPA) 2000 - 2900 mg per day; > 3: doses (DHA+EPA) above 3000 mg per day).
Table 1.
The league table compares pairwise treatments on network estimates of the TC level.
Table 1.
The league table compares pairwise treatments on network estimates of the TC level.
| FO - EE <2 |
. |
. |
0.10
[-0.22; 0.41] |
. |
. |
. |
. |
. |
. |
. |
0.24
[-0.13; 0.61] |
-0.05
[-0.28; 0.18] |
0.56
[ 0.23; 0.90]
|
FO - EE >3 |
. |
. |
. |
. |
. |
. |
. |
. |
. |
. |
-0.52
[-0.79; -0.24]
|
0.10
[-0.32; 0.53] |
-0.46
[-0.92; 0.00] |
FO - EM <2 |
. |
. |
. |
. |
. |
. |
. |
. |
. |
-0.06
[-0.43; 0.32] |
0.26
[-0.03; 0.55] |
-0.30
[-0.70; 0.09] |
0.16
[-0.31; 0.63] |
FO - rTAG <2 |
. |
. |
. |
. |
. |
. |
. |
. |
-0.37
[-0.68; -0.06]
|
0.11
[-0.18; 0.40] |
-0.45
[-0.80; -0.11]
|
0.01
[-0.42; 0.44] |
-0.15
[-0.51; 0.21] |
FO - TG <2 |
. |
-0.84
[-1.38; -0.30]
|
. |
. |
. |
. |
0.30
[-0.39; 0.99] |
0.04
[-0.20; 0.29] |
0.21
[-1.10; 1.52] |
-0.36
[-1.68; 0.97] |
0.10
[-1.24; 1.45] |
-0.05
[-1.38; 1.27] |
0.10
[-1.22; 1.41] |
FO - TG <3 |
. |
. |
. |
. |
. |
. |
-0.16
[-1.45; 1.13] |
0.06
[-0.16; 0.28] |
-0.50
[-0.79; -0.21]
|
-0.04
[-0.43; 0.35] |
-0.20
[-0.51; 0.10] |
-0.05
[-0.28; 0.18] |
-0.15
[-1.45; 1.15] |
FO <2 |
0.16
[-0.31; 0.63] |
. |
. |
. |
-0.11
[-0.39; 0.18] |
-0.04
[-0.15; 0.06] |
0.02
[-0.25; 0.28] |
-0.55
[-0.87; -0.23]
|
-0.09
[-0.50; 0.32] |
-0.25
[-0.58; 0.09] |
-0.10
[-0.37; 0.18] |
-0.19
[-1.50; 1.11] |
-0.04
[-0.24; 0.15] |
FO <3 |
. |
. |
. |
. |
0.04
[-0.13; 0.21] |
-0.18
[-0.51; 0.15] |
-0.75
[-1.12; -0.37]
|
-0.28
[-0.74; 0.17] |
-0.44
[-0.83; -0.06]
|
-0.29
[-0.63; 0.04] |
-0.39
[-1.71; 0.93] |
-0.24
[-0.52; 0.04] |
-0.20
[-0.51; 0.11] |
FO >3 |
. |
. |
. |
0.23
[-0.03; 0.49] |
-0.21
[-0.86; 0.43] |
-0.78
[-1.45; -0.11]
|
-0.32
[-1.03; 0.40] |
-0.47
[-1.15; 0.20] |
-0.32
[-0.97; 0.32] |
-0.42
[-1.85; 1.01] |
-0.27
[-0.90; 0.35] |
-0.23
[-0.86; 0.41] |
-0.03
[-0.70; 0.63] |
KO - HPL <2 |
0.05
[-0.66; 0.76] |
. |
0.26
[-0.35; 0.87] |
-0.16
[-0.86; 0.53] |
-0.73
[-1.45; -0.01]
|
-0.27
[-1.03; 0.50] |
-0.42
[-1.15; 0.30] |
-0.27
[-0.97; 0.42] |
-0.37
[-1.83; 1.09] |
-0.22
[-0.90; 0.45] |
-0.18
[-0.87; 0.51] |
0.02
[-0.70; 0.73] |
0.05
[-0.66; 0.76] |
KO - LPL <2 |
. |
0.21
[-0.46; 0.88] |
-0.02
[-0.24; 0.20] |
-0.59
[-0.89; -0.28]
|
-0.13
[-0.53; 0.28] |
-0.28
[-0.60; 0.03] |
-0.14
[-0.38; 0.11] |
-0.23
[-1.53; 1.07] |
-0.08
[-0.25; 0.08] |
-0.04
[-0.26; 0.18] |
0.16
[-0.14; 0.45] |
0.19
[-0.44; 0.82] |
0.14
[-0.54; 0.82] |
KO <2 |
0.16
[-0.01; 0.32] |
0.05
[-0.15; 0.25] |
-0.52
[-0.79; -0.24]
|
-0.06
[-0.43; 0.32] |
-0.21
[-0.50; 0.07] |
-0.06
[-0.28; 0.15] |
-0.16
[-1.45; 1.13] |
-0.01
[-0.12; 0.09] |
0.03
[-0.14; 0.20] |
0.23
[-0.03; 0.49] |
0.26
[-0.35; 0.87] |
0.21
[-0.46; 0.88] |
0.07
[-0.07; 0.22] |
Placebo |
Table 2.
The league table compares pairwise treatments on network estimates of the TG level.
Table 2.
The league table compares pairwise treatments on network estimates of the TG level.
| FO - EE < 2 |
. |
. |
0.06
[-0.16; 0.29] |
. |
. |
. |
. |
. |
. |
. |
. |
-0.11
[-0.42; 0.20] |
-0.21
[-0.39; -0.04]
|
0.18
[-0.09; 0.45] |
FO - EE > 3 |
. |
. |
. |
. |
. |
. |
. |
. |
. |
. |
. |
-0.43
[-0.65; -0.21]
|
-0.23
[-0.51; 0.05] |
-0.41
[-0.73; -0.09]
|
FO - EM < 2 |
. |
. |
. |
. |
. |
. |
. |
. |
. |
. |
-0.02
[-0.25; 0.22] |
-0.08
[-0.29; 0.12] |
-0.26
[-0.56; 0.04] |
0.15
[-0.16; 0.46] |
FO - rTAG < 2 |
. |
. |
. |
. |
. |
. |
. |
. |
. |
-0.04
[-0.26; 0.18] |
-0.05
[-0.27; 0.18] |
-0.23
[-0.50; 0.04] |
0.18
[-0.10; 0.47] |
0.03
[-0.23; 0.29] |
FO - TG < 2 |
. |
-0.33
[-0.83; 0.17] |
. |
. |
. |
. |
. |
0.22
[-0.30; 0.74] |
-0.18
[-0.36; 0.00] |
0.19
[-0.25; 0.63] |
0.01
[-0.46; 0.48] |
0.42
[-0.05; 0.90] |
0.27
[-0.19; 0.73] |
0.24
[-0.21; 0.68] |
FO - TG < 3 |
. |
. |
. |
. |
. |
. |
. |
-0.44
[-0.85; -0.03]
|
-0.05
[-0.23; 0.12] |
-0.23
[-0.47; 0.00] |
0.18
[-0.07; 0.42] |
0.03
[-0.19; 0.24] |
-0.01
[-0.18; 0.17] |
-0.24
[-0.66; 0.18] |
FO < 2 |
0.26
[-0.26; 0.78] |
. |
. |
. |
. |
-0.03
[-0.24; 0.18] |
-0.19
[-0.27; -0.12]
|
0.02
[-0.19; 0.22] |
-0.16
[-0.42; 0.10] |
0.25
[-0.02; 0.52] |
0.10
[-0.14; 0.34] |
0.07
[-0.15; 0.28] |
-0.17
[-0.61; 0.26] |
0.07
[-0.08; 0.22] |
FO < 3 |
. |
. |
. |
. |
. |
-0.26
[-0.40; -0.12]
|
-0.08
[-0.33; 0.17] |
-0.26
[-0.56; 0.03] |
0.15
[-0.16; 0.46] |
0.00
[-0.28; 0.28] |
-0.03
[-0.29; 0.22] |
-0.27
[-0.73; 0.19] |
-0.03
[-0.24; 0.18] |
-0.10
[-0.34; 0.14] |
FO > 3 |
. |
. |
. |
. |
-0.17
[-0.37; 0.03] |
-0.17
[-0.62; 0.27] |
-0.35
[-0.82; 0.12] |
0.06
[-0.42; 0.54] |
-0.09
[-0.55; 0.37] |
-0.12
[-0.57; 0.32] |
-0.36
[-0.95; 0.23] |
-0.12
[-0.54; 0.31] |
-0.19
[-0.63; 0.25] |
-0.09
[-0.55; 0.37] |
KO - HPL < 2 |
-0.07
[-0.49; 0.35] |
. |
. |
-0.08
[-0.50; 0.34] |
-0.24
[-0.68; 0.20] |
-0.42
[-0.88; 0.04] |
-0.01
[-0.48; 0.46] |
-0.16
[-0.61; 0.30] |
-0.19
[-0.63; 0.25] |
-0.43
[-1.01; 0.15] |
-0.19
[-0.60; 0.23] |
-0.26
[-0.69; 0.17] |
-0.16
[-0.61; 0.29] |
-0.07
[-0.49; 0.35] |
KO - LPL < 2 |
. |
. |
-0.01
[-0.42; 0.40] |
0.99
[ 0.02; 1.96]
|
0.81
[-0.17; 1.79] |
1.22
[ 0.23; 2.21]
|
1.07
[ 0.09; 2.05]
|
1.04
[ 0.06; 2.01]
|
0.80
[-0.24; 1.84] |
1.04
[ 0.08; 2.01]
|
0.97
[ 0.00; 1.94]
|
1.07
[ 0.09; 2.05]
|
1.16
[ 0.11; 2.21]
|
1.23
[ 0.19; 2.27]
|
KO - PL/FFA <3 |
. |
-1.24
[-2.20; -0.28]
|
-0.00
[-0.17; 0.16] |
-0.18
[-0.41; 0.05] |
0.23
[-0.01; 0.48] |
0.08
[-0.13; 0.29] |
0.05
[-0.13; 0.22] |
-0.19
[-0.61; 0.23] |
0.05
[-0.05; 0.15] |
-0.02
[-0.17; 0.14] |
0.08
[-0.13; 0.29] |
0.17
[-0.25; 0.59] |
0.24
[-0.17; 0.65] |
-0.99
[-1.95; -0.03]
|
KO < 2 |
-0.25
[-0.33; -0.17]
|
-0.25
[-0.41; -0.10]
|
-0.43
[-0.65; -0.21]
|
-0.02
[-0.25; 0.22] |
-0.17
[-0.37; 0.03] |
-0.20
[-0.36; -0.04]
|
-0.44
[-0.85; -0.03]
|
-0.20
[-0.27; -0.12]
|
-0.27
[-0.40; -0.13]
|
-0.17
[-0.37; 0.03] |
-0.08
[-0.50; 0.34] |
-0.01
[-0.42; 0.40] |
-1.24
[-2.20; -0.28]
|
-0.25
[-0.32; -0.18]
|
Placebo |
Table 3.
SUCRA results of TC level.
Table 3.
SUCRA results of TC level.
| |
SUCRA (%) |
| FO - EE > 3 |
96.35 |
| FO - rTAG < 2 |
80.35 |
| FO - TG < 2 |
63.45 |
| FO - TG < 3 |
59.96 |
| FO - EM < 2 |
56.38 |
| FO < 2 |
54.77 |
| Placebo |
51.42 |
| FO < 3 |
43.97 |
| FO - EE < 2 |
40.45 |
| KO < 2 |
35.56 |
| KO - LPL < 2 |
30.47 |
| KO - HPL < 2 |
23.56 |
| FO > 3 |
15.23 |
Table 4.
SUCRA results of the TG level.
Table 4.
SUCRA results of the TG level.
| |
SUCRA (%) |
| KO – PL/FFA <3 |
96.82 |
| FO - EE > 3 |
84.32 |
| FO - TG < 3 |
77.92 |
| FO < 3 |
64.02 |
| KO < 2 |
61.63 |
| FO - EE < 2 |
59.29 |
| FO - TG < 2 |
48.68 |
| FO < 2 |
45.63 |
| FO > 3 |
42.14 |
| FO - rTAG < 2 |
40.25 |
| KO - HPL < 2 |
30.29 |
| KO - LPL < 2 |
21.46 |
| FO - EM < 2 |
16.66 |
| Placebo |
10.88 |
Table 5.
Asymmetry Egger’s test results of funnel chart after linear regression of TC level. (TC: total-cholesterol; Linear regression test of funnel plot asymmetry: This test assesses whether there is publication bias in the dataset by examining the asymmetry of the funnel plot, which represents the relationship between effect size and study precision. Test result: The t-value, degrees of freedom (df), and p-value indicate the significance of the test. Bias estimate: This represents the degree of bias present in the dataset. A positive value indicates potential publication bias. Details: Additional information about the analysis, including the method used to estimate bias (multiplicative residual heterogeneity variance), the predictor used in the analysis (standard error-SE), the weight assigned to each study (inverse variance), and the reference for the analysis method (Egger et al. (1997) - BMJ))).
Table 5.
Asymmetry Egger’s test results of funnel chart after linear regression of TC level. (TC: total-cholesterol; Linear regression test of funnel plot asymmetry: This test assesses whether there is publication bias in the dataset by examining the asymmetry of the funnel plot, which represents the relationship between effect size and study precision. Test result: The t-value, degrees of freedom (df), and p-value indicate the significance of the test. Bias estimate: This represents the degree of bias present in the dataset. A positive value indicates potential publication bias. Details: Additional information about the analysis, including the method used to estimate bias (multiplicative residual heterogeneity variance), the predictor used in the analysis (standard error-SE), the weight assigned to each study (inverse variance), and the reference for the analysis method (Egger et al. (1997) - BMJ))).
| Linear regression test of funnel plot asymmetry |
| |
t |
df |
p-value |
| Test result |
-1.90 |
82 |
0.0603 |
| Bias estimate |
-0.3558 (SE = 0.1868) |
Details:
- multiplicative residual heterogeneity variance (tau2 = 1.7699)
- predictor: standard error
- weight: inverse variance
- reference: Egger et al. (1997), BMJ |
Table 6.
Asymmetry Egger’s test results of funnel chart after linear regression of TG level. (TG: Triglycerides; Linear regression test of funnel plot asymmetry: This test assesses whether there is publication bias in the dataset by examining the asymmetry of the funnel plot, which represents the relationship between effect size and study precision. Test result: The t-value, degrees of freedom (df), and p-value indicate the significance of the test. Bias estimate: This represents the degree of bias present in the dataset. A positive value indicates potential publication bias. Details: Additional information about the analysis, including the method used to estimate bias (multiplicative residual heterogeneity variance), the predictor used in the analysis (standard error-SE), the weight assigned to each study (inverse variance), and the reference for the analysis method (Egger et al. (1997) - BMJ)).
Table 6.
Asymmetry Egger’s test results of funnel chart after linear regression of TG level. (TG: Triglycerides; Linear regression test of funnel plot asymmetry: This test assesses whether there is publication bias in the dataset by examining the asymmetry of the funnel plot, which represents the relationship between effect size and study precision. Test result: The t-value, degrees of freedom (df), and p-value indicate the significance of the test. Bias estimate: This represents the degree of bias present in the dataset. A positive value indicates potential publication bias. Details: Additional information about the analysis, including the method used to estimate bias (multiplicative residual heterogeneity variance), the predictor used in the analysis (standard error-SE), the weight assigned to each study (inverse variance), and the reference for the analysis method (Egger et al. (1997) - BMJ)).
| Linear regression test of funnel plot asymmetry |
| |
t |
df |
p-value |
| Test result |
1.19 |
96 |
0.2368 |
| Bias estimate |
0.3844 (SE = 0.3229) |
Details:
- multiplicative residual heterogeneity variance (tau2 = 5.7594)
- predictor: standard error
- weight: inverse variance
- reference: Egger et al. (1997), BMJ |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).