1. Introduction
Gliomas are aggressive tumors of the brain which account for about 80% of all primary malignant cerebral tumors[
1,
2]. For their aggressive behavior they require a multimodal management: the current treatment of choice is extensive surgical resection, accompanied by chemotherapy and radiotherapy. When achievable, a gross total (GTR) up to 98% is the first step and it is known to be correlated to a significant survival advantage[
3,
4]. However, the prognosis of high-grade gliomas (HGG) remains poor because of their infiltrative nature and the fast local relapse rate. The literature reports that more than 80% of HGG-recurrences occur within 2 cm of the resection margins. New technologies, including neuronavigation, intraoperative ultrasound, intraoperative neurophysiological monitoring (IONM), exoscopic guidance and fluorescence techniques have been introduced in the last years[
5]. The benefits of fluorescence-guided surgery, particularly with 5- aminolevulinic acid (5-ALA) in resection of HGGs have been documented in several studies [
6]. The U.S. Food and Drug Administration (FDA) approved 5-ALA (Gleolan
®; photonamic GmbH & Co. KG) for use as an intraoperative optical imaging agent in patients with suspected high-grade gliomas in 2017. The approval occurred a decade after European approval and a multicenter, phase III randomized trial which confirmed that surgeons using 5-ALA as a surgical adjunct, could achieve more complete resections of tumors in HGG patients and better patient outcomes than with conventional microsurgery[
1]. 5-ALA is a well-tolerated fluorophore with low rates of adverse events, the most common of them involving liver metabolism, a temporary hypotension and a light sensitivity for the first 24 hours after application. Compared to other fluorophores, as well as the sodium fluorescein (SF), 5-ALA has proven to have more sensitivity and sensibility in detect primary tumor cells thanks to the peculiar accumulation in metabolically active glioma cells in contrast to surrounding healthy brain parenchyma[
7,
8]. After oral administration, 5-ALA passed the blood brain barrier (BBB) and it is converted into the auto fluorescent marker protoporphyrin IX (PpIX), acting like a marker of cancerous cells. Since now, a proper visualization of PPIX fluorescence required the blue filters of the traditional xenon microscopes, a blue-violet light with a wave-length of 375 to 440 nm and an emission filter thus allowing the visualization of pink-red fluorescence, with an emission peak at 635 and 704 nm. The exoscopic guided surgery, thanks to the outer 3D 4k screen and the LED light source, combined with the blue filter could be considered a favorable adjunct compared to the traditional microscopic-guided surgery. In this report, we describe our preliminary experience in the 5-ALA guided HGG surgery adopting the exoscopic guidance, as an alternative or complement to the traditional microscope-guided surgery in order to achieve a better visualization of the surgical field and to obtain the highest degree of tumor resection.
2. Materials and Methods
Ten consecutive patients underwent a surgery for the resection of an intra-axial lesion from February 2022 to February 2023. These procedures were carried out as normal clinical practice at Azienda Ospedaliera Papardo Messina, Sicily. This study did not require an ethical committee approval: all patients were fully informed about the procedure and they gave their written consent. Clinical and personal data were kept confidential.
Statistical Analysis
A descriptive analysis and parametric tests were used (SPSS statistics, Table 5 and 6). The level of statistical significance was set at p < 0.01.
Preoperative Procedures
All the patients, 6 males and 4 females (
Table 1), underwent a preoperative MRI+ gadolinium to assess the tumor volume and the tumor localization. A StealthStation
® Surgical Navigation System (Medtronic, Inc., Minneapolis, MI, USA) was used for pre- operative and intraoperative neuronavigation in all the cases. Neurophysiological intraoperative monitoring by transcranial or cortical stimulation for motor-evoked, visual and somatosensorial potentials was performed in all cases. Antibiotic prophylaxis was performed in all patients through a protocol of 2 g of cephalosporin 2 hours before surgery.
5-ALA Administration
The 5-ALA (Gleolan®; photonamic GmbH & Co. KG) 5 hours before the procedure is supplied to the patient 3-5 hours before the procedure. A dose of 20 mg/kg of Gleolan is administered orally to the patient in 50 ml of drinking water. Then the patient is protected ALA 3 h before surgery and were protected from strong light exposure within the first 24 h after 5-ALA administration to avoid risks related to photosensitivity.
Exoscopic 5-ALA Guided Surgery Method
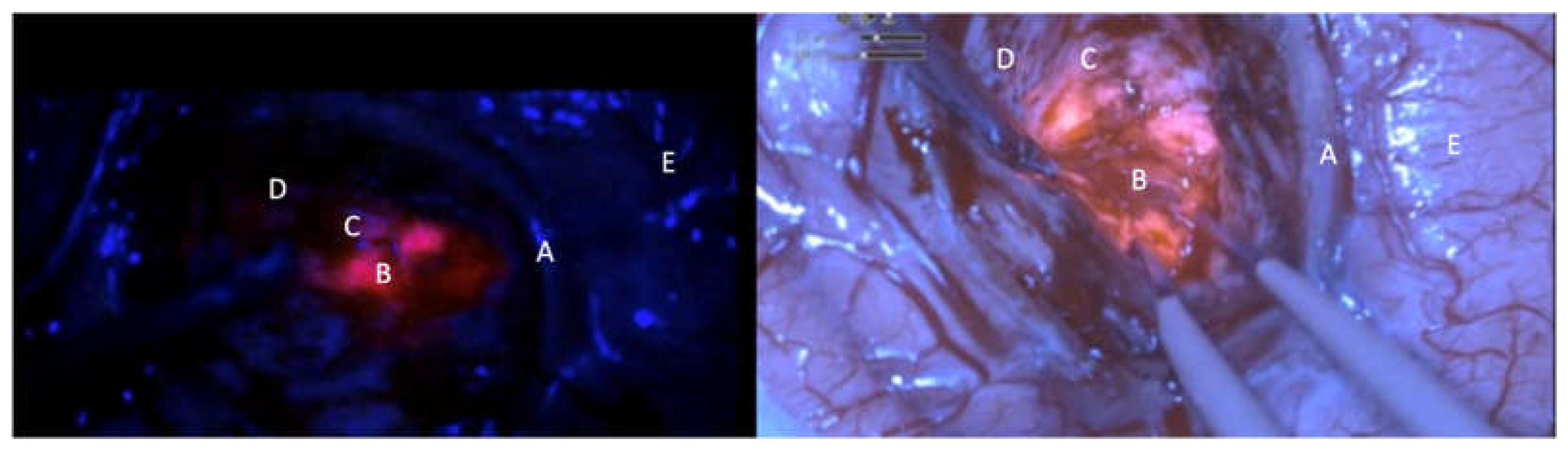
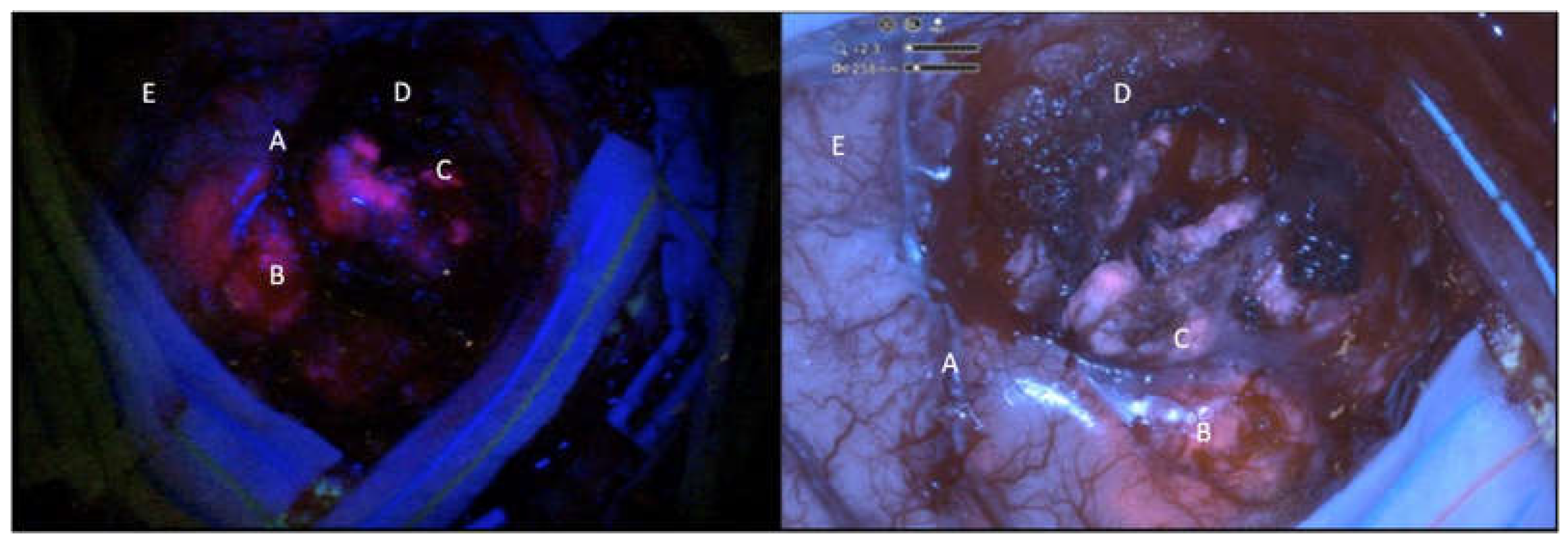
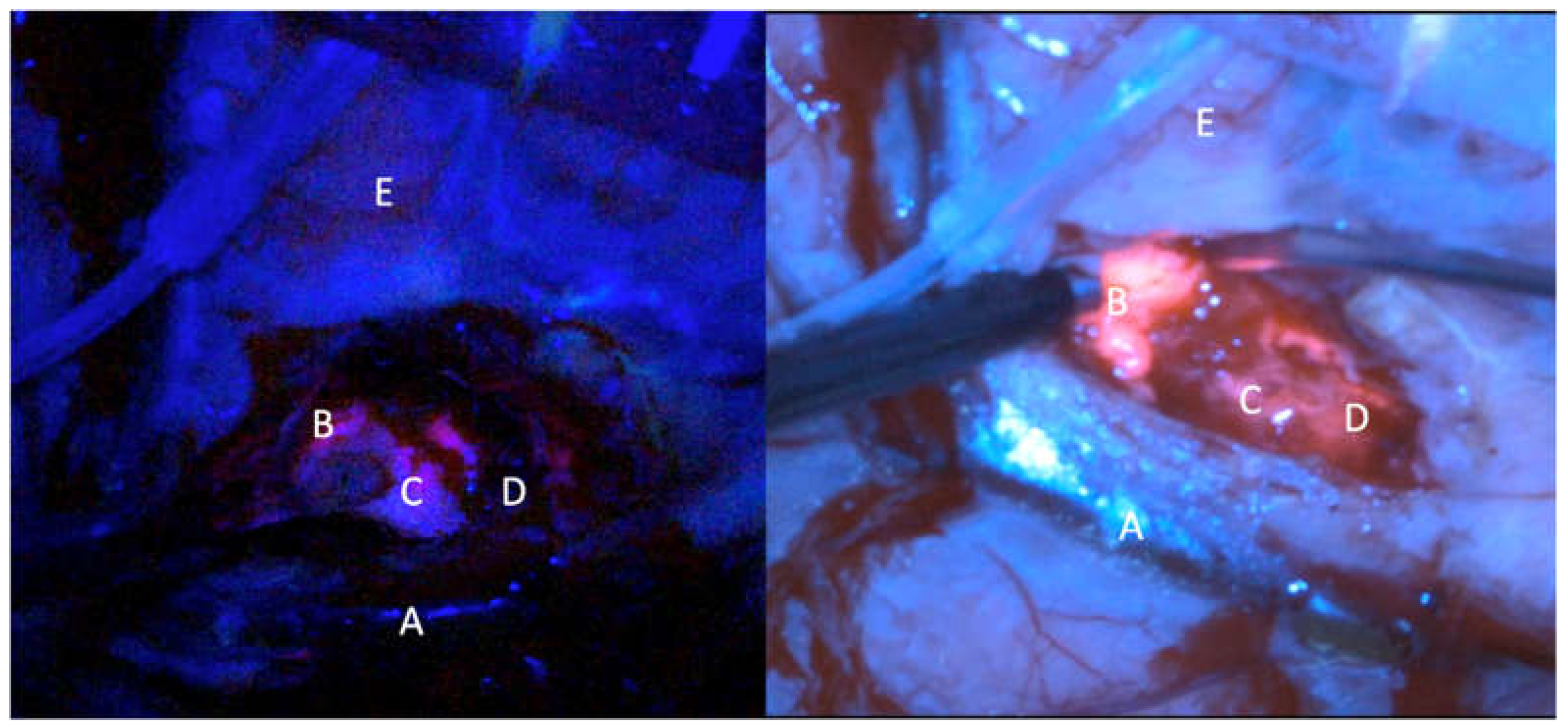
The surgery was performed thanks to the Orbeye exoscope (OE) (Olympus) which is provided by a camera with a LED light source, an ergonomic arm and an outer 3D, 4K 3d 55” screen which allow natural depth of field visualization with high resolution of anatomical details. The blue light excitation (λ=400–410 nm) allows to detect the protoporphyrin IX, the final 5-ALA metabolite accumulated in the tumor cells, strongly fluorescent (peak λ=635 nm). Fluorescence emission was classified as intense (solid or lava like) red fluorescence, corresponding to tumor tissue (vital and solid), faint pink or pinkish fluorescence, corresponding to infiltrating tumor cells. Normal tissue not accumulating protoporphyrin IX reflects the blue-violet light, appearing blue was classified as blue[
9] (
Table 2).
Before the surgery, a traditional operative microscope (OM) (Kinevo i900, ZEISS) provided with the blue filter was draped and used during the procedure just for an illustrative and comparative use. The surgery consisted in a gross total resection in 9 patient and in a subtotal resection in 1 patient. Resection was achieved by alternating white and blue light to complete exeresis. The intensity of the fluorescence was subjectively measured in different picture samples during the surgery in a 1 to 5 (from minimum to maximum) scale. The same frames were analyzed with the microscope and graded in a 1 to 5 scale. The brightness of the surgical field and the detailing of the anatomy were also analyzed comparatively (
Tables 3 and 4).
3. Results
In our series of 10 patients, 4 female and 6 male, underwent a surgery for the exeresis of a high grade glioma; the age range was 39 to 80 years, with a median of 62.1 (interquartile range = 54–71) .No complication or adverse reactions 5-ALA related were observed, except for a case of a slight increase in serum GGT and a case of photosensibilization, both of the cases solved spontaneously. There was no perioperative mortality. The median fluorescence intensity, according to the subjective assessment of the surgeons on a scale of 1–5, was 4.7 in the OE group (IQR = 4.5–5) and 4.0 (IQR 4-4,125) in the OM group; the brightness of the surgical field was evaluated as 4.9(IR 4.9-5) in the OE group and 3.85 in the OM group (IR 3.5-4); the detailing of the anatomy with the blue filter on OE was evaluated with a median of 4.5 (IQR 4-5) in the OE group and 3.2 (IQR 3-3.5) in the OM group. According to the comparative analysis there was a statistical significance between the two groups (p<0.01) (
Tables 5 and 6).
4. Discussion
The introduction of the exoscope in the modern era provided the neurosurgeons with a digital camera system able to deliver intense light and magnification to the deepest areas of the surgical field, allowing the surgeon to see, through a 3D 4k monitor, critical neural and vascular structures as well as tissue differentiation with high magnification. Several studies demonstrated that the exoscope could be considered a safe alternative compared to the OM thanks to a better ergonomic posture of the surgeons during surgical procedures and thanks to the possibility to improve the operational team involvement. Furthermore it is demonstrated that the quality of images and the 3D 4K screen has been increasingly improved in recent years, giving high magnification and enhanced image quality of the surgical field, while also being more ergonomically favorable. Drawback remains about the slight difference of depth perception in exoscopic versus microscopic view[
10,
11]. Several studies have shown that fluorescence-guided tumor resection increases the possibility of achieve the GTR[
12,
13]. The possibility to apply the blue filter to the HGG 5-ALA guided surgery in the exoscopic magnified surgical field is a further advantage. The results of this study demonstrates the ability of the Orbeye exoscope to accurately identify tissue containing tumor cells, compared to a traditional microscope similarly to prior studies using 5-ALA with a standard operative microscope[
6,
8,
14].Recent reviews underlined the feasibility of the 5-ALA guided surgery with the exoscopic guide: Vogelbaum et al. demonstrated that the visualization of 5-ALA induced tumor fluorescence was associated with a positive predictive value as well as other 5-ALA visualization technologies; furthermore the authors reported that this digital system allowed for both an excellent visualization of the fluorescence and the anatomy at the same time, which distinguishes this technology from conventional surgical microscopes(
Figure 1,
Figure 2 and
Figure 3)[
15].
These results are in line with our findings. Papers were published in pediatric neurosurgery applications with good reports in determining the extent of resection[
16]. Ikeda et al., reported a comparative study analyzing technical characteristics of fluorescence-guided surgery with 5-aminolevulinic acid and excitation light source with Olympus Orbeye and a traditional microscope: authors demonstrated that nonfluorescent normal structures without red fluorescence were well recognized under the exoscope; the energy of 405-nm wavelength light in blue light was significantly higher in Orbeye exosco than that in an traditional microscope, especially in the short focal length thus giving the exoscope a good visibility advantage due to blue light energy compared to a traditional microscope. [
17]. In our experience there were no major differences in adapting from a traditional microscope to the exoscope for detecting fluorescence induced by 5-ALA. The major difference was found in the intensity of the fluorescence in the two groups, the brightness of the surgical field and the detailing of the anatomy while using the blue filter. In our opinion a combination of those two technologies may lead to improved levels of extent of resection, control of the surgical field and complication rates.
5. Conclusions
This preliminary experience demonstrates that with the combination of fluorescence induced by 5-aminolevulinic acid and the exoscope system is possible. These two technologies allow the surgeon to perform fully collaborative surgeries, to add ergonomics to the procedure and to have a better visualization of the surgical blue filtered field when compared to the OM.
Funding
This research received no external funding
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Sanai, N. and M.S. Berger, Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol, 2018. 15(2): p. 112-125. [CrossRef]
- Rodriguez-Camacho, A., et al., Glioblastoma Treatment: State-of-the-Art and Future Perspectives. Int J Mol Sci, 2022. 23(13). [CrossRef]
- Lacroix, M., et al., A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg, 2001. 95(2): p. 190-8. [CrossRef]
- Stummer, W., et al., Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery, 2008. 62(3): p. 564-76; discussion 564-76.
- Altieri, R., et al., Glioma Surgery: Technological Advances to Achieve a Maximal Safe Resection. Surg Technol Int, 2015. 27: p. 297-302.
- Stummer, W., et al., Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien), 1998. 140(10): p. 995-1000. [CrossRef]
- Mansouri, A., et al., The role of 5-aminolevulinic acid in enhancing surgery for high-grade glioma, its current boundaries, and future perspectives: A systematic review. Cancer, 2016. 122(16): p. 2469-78.
- Stummer, W., et al., Randomized, Prospective Double-Blinded Study Comparing 3 Different Doses of 5-Aminolevulinic Acid for Fluorescence-Guided Resections of Malignant Gliomas. Neurosurgery, 2017. 81(2): p. 230-239. [CrossRef]
- Widhalm, G., et al., Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev, 2012. 35(3): p. 381-91; discussion 391. [CrossRef]
- 10. Montemurro, N., et al., The Exoscope in Neurosurgery: An Overview of the Current Literature of Intraoperative Use in Brain and Spine Surgery. J Clin Med, 2021. 11(1). [CrossRef]
- Fiani, B., et al., The Role of 3D Exoscope Systems in Neurosurgery: An Optical Innovation. Cureus, 2021. 13(6): p. e15878. [CrossRef]
- Roberts, D.W., et al., Adjuncts for maximizing resection: 5-aminolevuinic acid. Clin Neurosurg, 2012. 59: p. 75-8.
- Roessler, K., et al., Intraoperative tissue fluorescence using 5-aminolevolinic acid (5-ALA) is more sensitive than contrast MRI or amino acid positron emission tomography ((18)F-FET PET) in glioblastoma surgery. Neurol Res, 2012. 34(3): p. 314-7. [CrossRef]
- Orillac, C., W. Stummer, and D.A. Orringer, Fluorescence Guidance and Intraoperative Adjuvants to Maximize Extent of Resection. Neurosurgery, 2021. 89(5): p. 727-736. [CrossRef]
- Vogelbaum, M.A., et al., A Prospective Validation Study of the First 3D Digital Exoscope for Visualization of 5-ALA-Induced Fluorescence in High-Grade Gliomas. World Neurosurg, 2021. 149: p. e498-e503. [CrossRef]
- Maeda, M., et al., 5-ALA fluorescence-guided resection of pediatric low-grade glioma using the ORBEYE 3D digital exoscope: a technical report. Childs Nerv Syst, 2023. 39(4): p. 1061-1064. [CrossRef]
- Ikeda, N., et al., The Characteristic of Light Sources and Fluorescence in the 3-Dimensional Digital Exoscope “ORBEYE” for 5-Aminolevulinic Acid-Induced Fluorescence-Guided Surgery Compared with a Conventional Microscope. World Neurosurg, 2022. 167: p. e1268-e1274. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).