Submitted:
02 May 2024
Posted:
03 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
Location of the Experiment and Preparation of the Honey Bee Colonies
Bee pollen Collection from Colonies with Reduced Feeding
Exposure of Forager Bees to Isolated Fungicides or in Combination
Gene expression Assessment
RNA Extraction and cDNA Library Construction
Molecular Determination of Gene Expression
Statistical Analyses
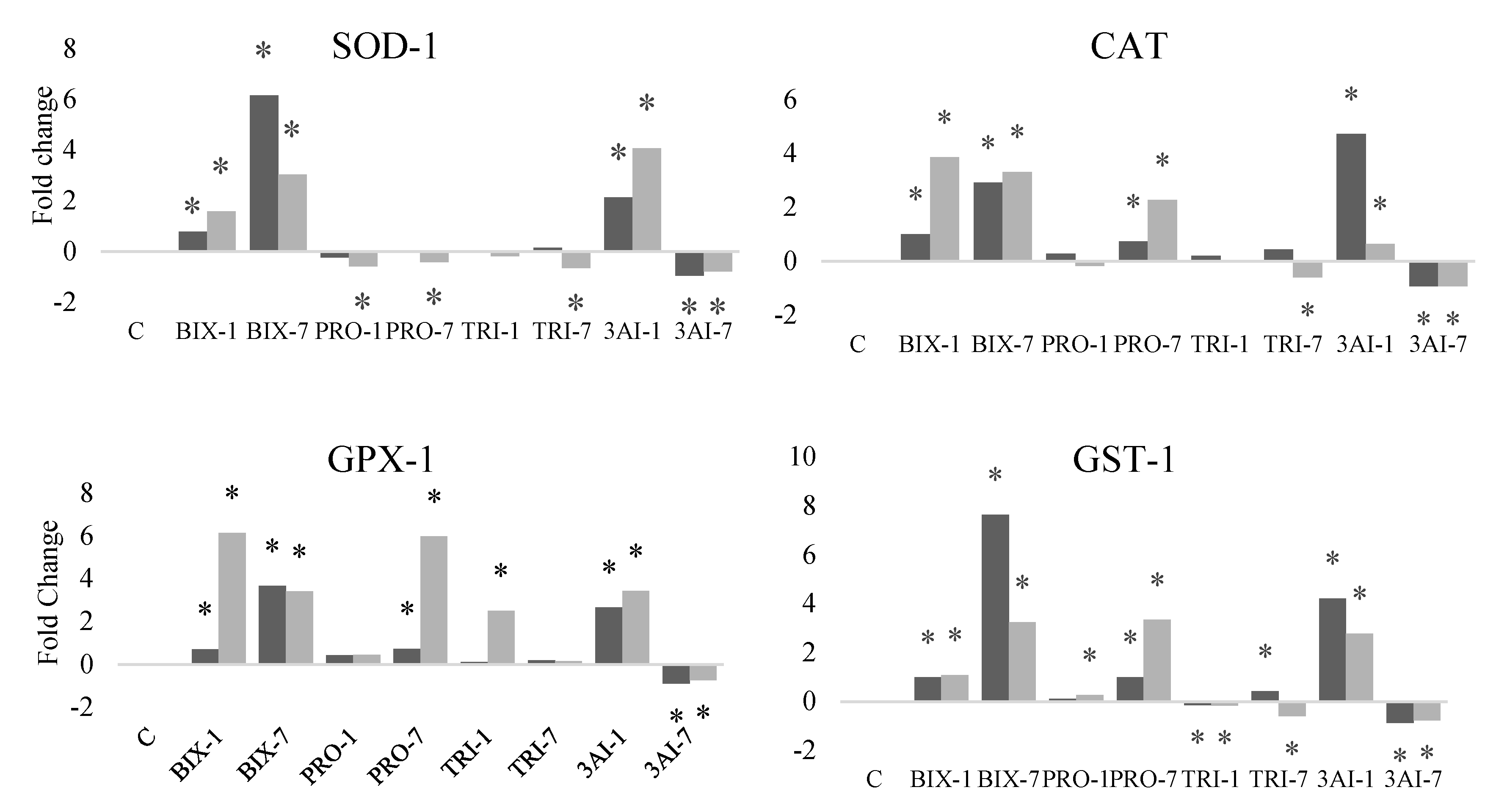
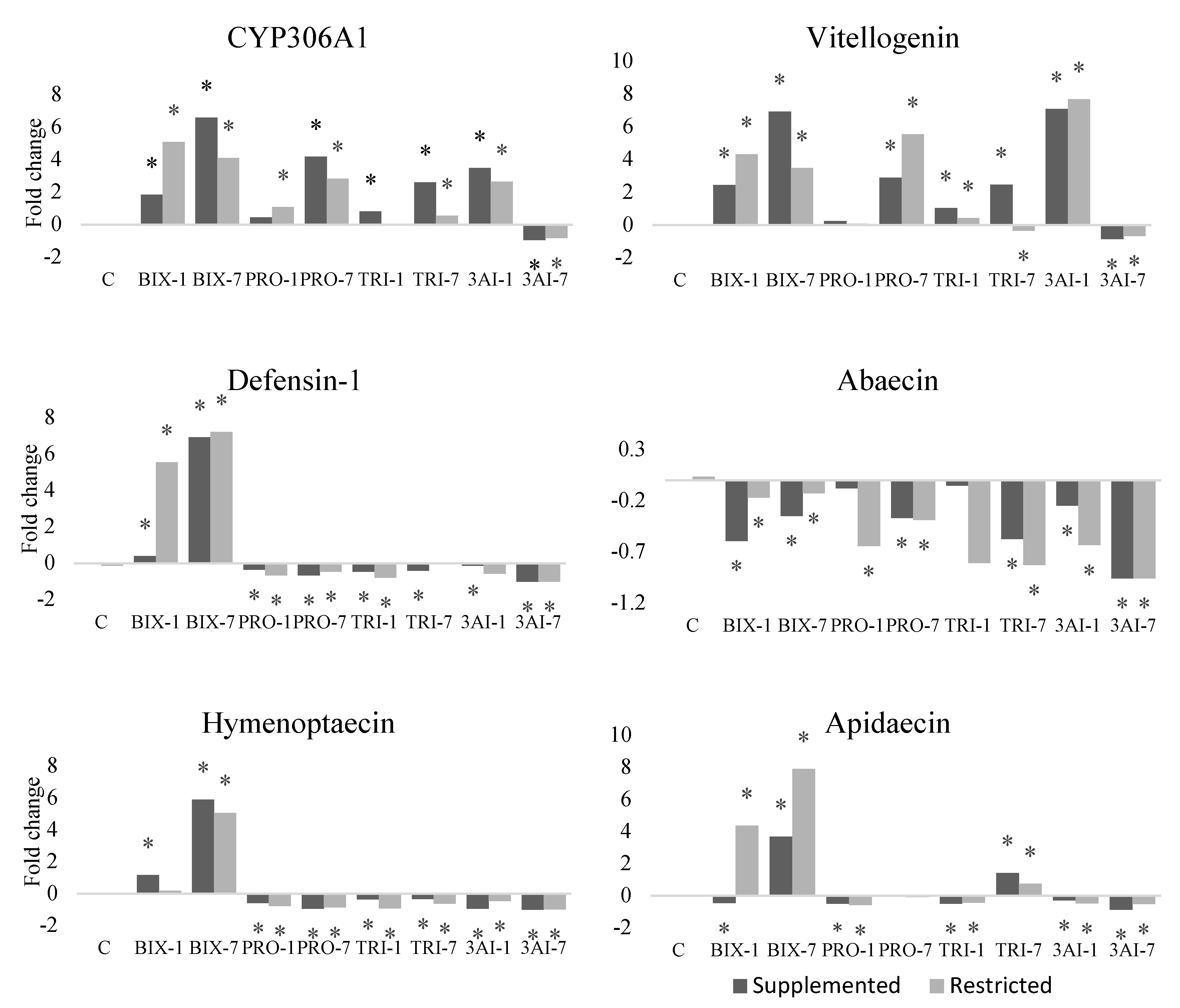
3. Results
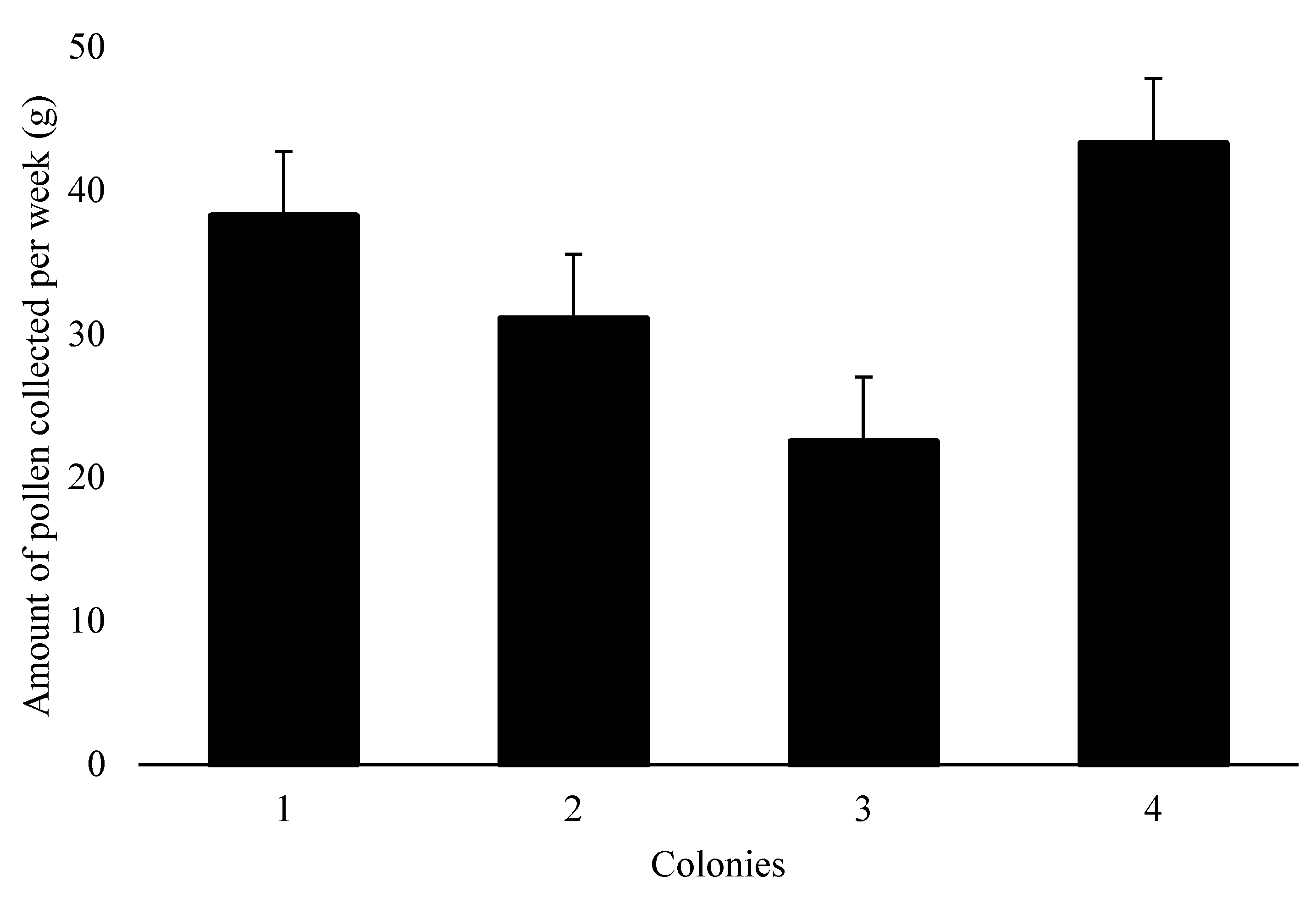
Feeding Management of Colonies
4. Discussion
Colony Feeding Management
Gene Expression Changes in Bees from Colonies with Supplemented or Reduced Feeding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, C.; Bosch, J.; Bortolotti, L.; Medrzycki, P.; Teper, D.; Molowny-Horas, R.; Sgolastra, F. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci. Rep. 2021, 11, 6821. [Google Scholar] [CrossRef] [PubMed]
- Balieira, K.V.B.; Mazzo, M.; Bizerra, P.F.V.; Guimarães, A.R.J.S.; Nicodemo, D.; Mingatto, F.E. Imidacloprid-induced oxidative stress in honey bees and the antioxidant action of caffeine. Apidol. 2018, 49, 562–572. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Bayer. Fox® Xpro: fungicida, 2019. São Paulo: Bayer S. A. Available online: https://www.agro.bayer.com.br/d/fungicida-bcs-fox-xpro-br (accessed on 15 April 2024).
- Bayer. Ficha de Informações de Segurança de Produtos Químicos (FISPQ). Fox Xpro. 2022. Available online: https://www.bayerfispq.com.br/Downloads/DownloadFile?idForm=4608 (accessed on 15 April 2024).
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, D.J.; Robertson, J.L.; Mullin, C.; Frazier, J.; Ashcraft, S.A.; Rajotte, E.G.; Joshi, N.K.; Vaughn, M. Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS One 2013, 8, e72587. [Google Scholar] [CrossRef]
- Bokšová, A.; Kazda, J.; Stejskalová, M.; Šubrt, T.; Uttl, L.; Mráz, P.; Bartoška, J. Findings of herbicide and fungicide residues in bee bread. Plant, Soil Environment 2021, 67, 343–352. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidol. 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Bus, J.S. “The dose makes the poison”: key implications for mode of action (mechanistic), research in a 21st century toxicology paradigm. Curr. Opin. Toxicol. 2017, 3, 87e91. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of bee-pollen? J. ApiProd ApiMed Sci. 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Pesticide chemical research in toxicology: lessons from nature. Chem. Res. Toxicol. 2016, 30, 94–104. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Rana, S.; Sarkar, S.; Smith, B.; Basu, P. Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidol. 2015, 46, 107–129. [Google Scholar] [CrossRef]
- Christen, V.; Krebs, J.; Fent, K. Fungicides chlorothanolin, azoxystrobin and folpet induce transcriptional alterations in genes encoding enzymes involved in oxidative phosphorylation and metabolism in honey bees (Apis mellifera) at sublethal concentrations. J. Hazard. Mater. 2019, 5, 215–226. [Google Scholar] [CrossRef]
- Claudianos, C.; Ranson, H.; Johnson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Corona, M.; Robinson, G.E. Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol. Biol. 2006, 15, 687–701. [Google Scholar] [CrossRef]
- David, A.; Botías, C.; Abdul-Sada, A.; Nicholls, E.; Rotheray, E.L.; Hill, E.M.; Goulson, D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016, 88, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed]
- D’Hose, D.; Isenborghs, P.; Brusa, D.; Jordan, B.F.; Gallez, B. The short-term exposure to sdhi fungicides boscalid and bixafen induces a mitochondrial dysfunction in selective human cell lines. Molecules 2021, 26, 5842. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Fang, Q.; Yan, Z.; Zhang, C.; Shi, Y.; Zhang, Z.; Gao, Q.; Xiao, J.; Liao, M.; Qi, C.; Cao, H. Insights into the fungicide prothioconazole and its metabolite in wheat: residue behavior and risk assessment. Agron. 2023, (12), 2906. [Google Scholar] [CrossRef]
- Fisher, A.; Coleman, C.; Hoffmann, C.; Fritz, B.; Rangel, J. The synergistic effects of almond protection fungicides on honey bee (Hymenoptera: Apidae) forager survival. J. Econ. Entomol. 2017, 110, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Degrandi-Hoffman, G.; Liao, L.H.; Tadei, R.; Harrison, J.F. The challenge of balancing fungicide use and pollinator health. Adv. Insect Physiol. 2023, 117–190. [Google Scholar]
- Frumkin, I.; Schirman, D.; Rotman, A.; Li, F.; Zahavi, L.; Mordret, E.; Asraf, O.; Wu, S.; Levy, S.F.; Pilpel, Y. Gene architectures that minimize cost of gene expression. Mol. Cell 2017, 65, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Giannini, T.C.; Cordeiro, G.D.; Freitas, B.M.; Saraiva, A.M.; Imperatriz-Fonseca, V.L. The dependence of crops for pollinators and the economic value of pollination in Brazil. J. Econ. Entom. 2015, 108, 849–857. [Google Scholar] [CrossRef]
- Gomes, C.R.A.; Freitas, T.A.L.; Kato, A.Y.; Silva, M.A.G.; Ferraz, Y.M.M.; Alves, T.R.R.; Serafim, J.A.; De Jong, D.; Prado, E.P.; Vicente, E.F.; et al. Contact contamination of honey bees in controlled fungicide spraying using fine and coarse droplets. In: 48th Apimondia, 2023, Santiago. Abstract Book - 48th Apimondia 2023, 233.
- Haas, J.; Zaworra, M.; Glaubitz, J.; Hertlein, G.; Kohler, M.; Lagojda, A.; Lueke, B.; Maus, C.; Almanza, M.T.; Davies, T.G.E.; et al. A toxicogenomics approach reveals characteristics supporting the honey bee (Apis mellifera L.) safety profile of the butenolide insecticide flupyradifurone. Ecotoxicol. Environ. Saf. 2021, 217, 112247. 217.
- Ibama. Vendas de ingredientes ativos por unidade da federação em 2022. In: Relatórios de comercialização de agrotóxicos. 2023. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos (accessed on 11 April 2024).
- Iwasaki, J.M.; Hogendoorn, K. Non-insecticide pesticide impacts on bees: A review of methods and reported outcomes. Agr. Ecosyst. Environ. 2021, 314, 107423. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Cha, J.; Lee, S.H.; Kim, Y.H. Validation of quantitative real-time PCR reference genes and spatial expression profiles of detoxication-related genes under pesticide induction in honey bee, Apis mellifera. PLoS One 2022, 17, e0277455. [Google Scholar] [CrossRef]
- LIAO, L.-H.; WU, W.-Y.; DAD, A.; BERENBAUM, M.R. Fungicide suppression of flight performance in the honeybee (Apis mellifera) and its amelioration by quercetin. Proc. R. Soc. Lond. B. Biol. Sci. 2019, 286, 20192041. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.P.; Mackert, A.; Cristino, A.S.; Simões, Z.L.P. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidol. 2008, 39, 372–385. [Google Scholar] [CrossRef]
- MATTOS, I.M.; SOUZA, J.; SOARES, A.E. E. Analysis of the effects of colony population size and feeding supplementation on bee pollen production, Journal of Apicultural Research, v.54, p.411-419. 2015.
- National Honey Bee Health Stakeholder. Conference Steering Committee. Report on the National Stakeholders Conference on Honey Bee Health. US Department of Agriculture, Washington, DC. 2012.
- Nicodemo, D.; Mingatto, F.E.; De Jong, D.; Bizerra, F.V.B.; Tavares, M.A.; Bellini, W.; Vicente, E.F.; Carvalho, A. Mitochondrial respiratory inhibition promoted by pyraclostrobin in fungi is also observed in honey bees. Environ. Toxicol. Chem. 2020, 39, 1267–1272. [Google Scholar] [CrossRef]
- OECD. Test No. 214: Honeybees, Acute Contact Toxicity Test. OECD Guidelines for the Testing of Chemicals 1998, 2, 2–4. [Google Scholar]
- Olgun, T.; Dayioğlu, M.; Özsoy-Taġkiran, N. Pesticide and pathogen induced oxidative stress in honey bees (Apis mellifera L.). Mellifera 2020, 20, 32–52. [Google Scholar]
- Oliver, R.; Hewitt, H.G. Fungicides in crop protection. 2.ed. CABI: Boston, United States, 2014, 189.
- Omar, E.M.; Darwish, H.Y.A.; Othman, A.A.; El-Seedi, H.R.; Naggar, Y.A. Crushing corn pollen grains increased diet digestibility and hemolymph protein content while decreasing honey bee consumption. Apidol. 2022, 53, 52–2022. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper - Excel - based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; Vanengelsdorp, D. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS One.
- Picard, M.; Taivassalo, T.; Gouspillou, G.; Hepple, R.T. Mitochondria: isolation, structure and function. Physiol. J. 2011, 598, 4413–4421. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.; Pioz, M.; Vidau, C.; Requier, F.; Jury, M.; Crauser, D.; Brunet, J.L.; Le Conte, Y.; Alaux, C. Exposure to pollen-bound pesticide mixtures induces longer-lived but less efficient honey bees. Sci. Total Environ. 2019, 650, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.M.; Grozinger, C.M. Honey bee nutritional ecology: from physiology to landscapes. Adv. Insect Physiol. 2023, 64, 289–345. [Google Scholar]
- Raimets, R.; Bontšutšnaja, A.; Bartkevics, V.; Pugajeva, I.; Kaart, T.; Puusepp, L.; Pihlik, P.; Keres, I.; Viinalass, H.; Mänd, M.; et al. Pesticide residues in beehive matrices are dependent on collection time and matrix type but independent of proportion of foraged oilseed rape and agricultural land in foraging territory. Chemosphere 2020, 238, 124555. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, E.D.L.; Costa, J.J.D.N.; Passos, M.J.; Passos, J.R.D.S.; Hurk, R.V.D.; Silva, J.R.V. Real time PCR and importance of housekeepings genes for normalization and quantification of mRNA expression in different tissues. Braz. Arch. Biol. Technol. 2013, 56, 143–154. [Google Scholar] [CrossRef]
- Rehman, S.; Waliullah, M.I.S. Chlorpyrifos-induced neuro-oxidative damage in bee. J. Toxicol. Environ. Health Sci. 2012, 4, 30–36. [Google Scholar] [CrossRef]
- Rondeau, S.; Raine, N.E. Fungicides and bees: a review of exposure and risk. Environ. Int. 2022, 165, 107311. [Google Scholar] [CrossRef]
- Roszko, M.L.; Kamińska, M.; Szymczyk, K.; Jędrzejczak, R. Levels of selected persistent organic pollutants (PCB, PBDE) and pesticides in honey bee pollen sampled in Poland. PLoS One 2016, 11, e0167487. [Google Scholar] [CrossRef] [PubMed]
- Salmela, H.; Harwood, G.P.; Münch, D.; Elsik, C.G.; Herrero-Galán, E.; Vartiainen, M.K.; Amdam, G.V. Nuclear translocation of vitellogenin in the honey bee (Apis mellifera). Apidol. 2022, 53, 13. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS 9.2 online documentation. SAS Institute Inc., Cary, NC. 2013.
- SCHMEHL, D.R.; TEAL, P.E.A.; FRAZIER, J.L.; GROZINGER, C.M. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 2014, 71, 177–190. [Google Scholar] [CrossRef]
- Schuhmann, A.; Schmid, A.P.; Manzer, S.; Schulte, J.; Scheiner, R. Interaction of insecticides and fungicides in bees. Front. Insect Sci. 2022, 1, 808335. [Google Scholar] [CrossRef]
- SGOLASTRA, F.; MEDRZYCKI, P.; BORTOLOTTI, L.; MAINI, S.; PORRINI, C.; SIMON-DELSO, N.; BOSCH, J. Bees and pesticide regulation: lessons from the neonicotinoid experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Siede, R.; Meixner, M.D.; Buchler, R. Comparison of transcriptional changes of immune genes to experimental challenge in the honey bee (Apis mellifera). J. Apic. Res. 2012. [Google Scholar]
- Silva-Neto, C.M.; Ribeiro, A.C.C.; Gomes, F.L.; Neves, J.G.; Melo, A.P.C.; Calil, F.N.; Nascimento, A.R.; Franceschinelli, E.V. Interaction between biological and chemistry fungicides and tomato pollinators. Rev. Colomb. Cienc. Hortic. 2018, 12, 425–435. [Google Scholar] [CrossRef]
- Simon-Delso, N.; San Martin, G.; Bruneau, E.; Hautier, L. Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci. Rep. 2018, 8, 7241. [Google Scholar] [CrossRef]
- SIMONE, M.; EVANS, J.D.; SPIVAK, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef]
- Somerville, D. Fat bees skinny bees: a manual on honey bee nutrition for beekeepers. GOULBURN: Australian Government Rural Industries Research and Development Corporation. 2005. 142p.
- Tadei, R.; Menezes-Oliveira, V.; Silva-Zacarin, E.C. M. Silent effect of the fungicide pyraclostrobin on the larval exposure of the non-target organism Africanized Apis mellifera and its interaction with the pathogen Nosema ceranae in adulthood. Environ. Pollut. 2020, 267, 115622. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Weirich, G.F.; Collins, A.M.; Williams, V.P. Antioxidant enzymes in the honey bee, Apis mellifera. Apidol. 2022, 33, 3–14. [Google Scholar] [CrossRef]
- Wood, S.C.; Chalifour, J.C.; Kozii, I.V.; Medici De Mattos, I.; Klein, C.D.; Zabrodski, M.W.; Moshynskyy, I.; Guarna, M.M.; Wolf Veiga, P.; Epp, T.; et al. In vitro effects of pesticides on european foulbrood in honeybee larvae. Insects 2020, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Meng, F.; Jia, H.; Guo, X.; Xu, B. The identification and oxidative stress response of a zeta class glutathione S-transferase (GSTZ1), gene from Apis cerana cerana. J. Insect Physiol. 2012, 58, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: an overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
| Genes | Sequence (5’-3’) | References |
|---|---|---|
| Ribosomal Protein L32 (RPL32) (former rp49) | F: CGTCATATGTTGCCAACTGGT R: TTGAGCACGTTCAACAATGG |
Lourenço et al. (2008) |
| Superoxide Dismutase 1 (SOD-1) |
F: GGTGGTGGTCATTTGAATCATTC R: AAGAAGTGCAGCGTCTGGTTTAC |
Kim et al. (2022) |
| Catalase (CAT) |
F: TGGAGCAAGTCCTGATAAAATGC R: TGGGCCAAGACGATGTCTATG | Kim et al. (2022) |
| Glutathione Peroxidase 1 (GPX-1) |
F: CGACAACTATAAGGAAGCGAAA R: AGATAGAAAAACGTCTTCGCCT |
Corona and Robinson (2006) |
| Glutathione S-transferase 1 (GST-1) | F: TGCCGATCGATTTTTATCAACTT R: AGCCGTCAACGCAACTGC |
Corona and Robinson (2006) |
| Cytochrome P450 306A1 (CYP306A1) |
F: CGTCGATGGGAAGGATAAAA R: TCGGTGAAATATCCCGATTC |
Corona and Robinson (2006) |
| Vitellogenin | F: TCGACAACTGCGATCAAAGGA R: TGGTCACCGACGATTGGATG |
Simone et al. (2009) |
| Defensin-1 | F: TGCGCTGCTAACTGTCTCAG R: AATGGCACTTAACCGAAACG |
Siede et al. (2012) |
| Abaecin | F: AGATCTGCACACTCGAGGTCTG R: TCGGATTGAATGGTCCCTGA |
Siede et al. (2012) |
| Hymenoptaecin | F: CTCTTCTGTGCCGTTGCATA R: ′GCGTCTCCTGTCATTCCATT |
Siede et al. (2012) |
| Apidaecin | F: CTTTGTAGTCGCGGTATTTGG R: AGGCGCGTAGGTCGAGTAG |
Siede et al. (2012) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





