1. Introduction
African swine fever (ASF), caused by African swine fever virus (ASFV), is a highly contagious and hemorrhagic disease of swine with high mortality up to 100% [
1]. Since its initial outbreak in Kenya in the 1920s, ASF had predominantly manifested as an epidemic in Europe, South America and the Caribbean [
2]. However, the disease was introduced into China in 2018 and resulted in huge economic losses due to the lack of effective vaccines and antiviral drugs [
3,
4]. ASFV belongs to the family
Asfarviridae and is a large enveloped virus with a genome of double-stranded DNA, encoding more than 150 proteins [
5]. However, the functional role of the structural protein B169L (pB169L) remains elusive. The open reading frame (ORF) of
B169L comprises 495 base pairs (bp) and is translated into pB169L with 169 amino acids (aa). It is noteworthy that the transcription of
B169L gives rise to two mRNAs: a full-length of
B169L mRNA and an alternatively spliced
B169L mRNA (referred to as
B169L mRNA2) encoding a truncated pB169L (tpB169L) (aa 92–169) [
6]. However, the TSS and the promoter responsible for the generation of
B169L mRNA2 remain unknown. Notably, the
B169L gene is arranged adjacent with its downstream gene
B438L in the ASFV genome. Therefore, we postulated that the promoter sequence of the downstream gene
B438L is located within the
B169L gene.
A previous study has revealed that the ASFV B438L protein (pB438L) is indispensable for the construction of the icosahedral capsid of ASFV, and the gene-deletion of
B438L leads to a significant alteration in virion assembly, resulting in the formation of tubular viral particles instead of the characteristic icosahedral capsid, thereby disrupting the icosahedral symmetry [
7,
8]. The morphological perturbation also affects the infectivity and pathogenicity of viruses. It has been shown that the transcriptional machinery of ASFV exhibits complexity, which is involved in the complex interplay between enzymes and cofactors encoded by ASFV [
9]. The ORFs of ASFV
B438L and
B169L are densely distributed in an organized manner, it is possible that the upstream gene encompasses promoters to govern the expression of the downstream gene of ASFV, and an individual gene may harbor multiple promoters for the initiation of downstream viral gene transcription. Therefore, it is essential to decipher the transcriptional regulation patterns of the downstream
B438L gene, which will facilitate the design of the
B169L-deleted ASFV.
In this study, we have elucidated an intricate interrelationship between the ASFV B438L gene and its adjacent upstream and downstream genes. Notably, the B438L promoter region is located in the upstream of its ORF and encompasses two distinct promoters, and the B438L distal promoter also initiates the transcription of alternatively spliced B169L (B169L-2). Moreover, we have discerned that the transcriptional activity of the B438L gene depends on the viral transcriptional machinery, in conjunction with relevant cofactors, and the transcription efficiency of the gene was also affected by the ATG located upstream of B169L-2. The findings enhance our understanding of transcriptional mechanism underlying ASFV, thereby providing novel insights into the development of antiviral strategies.
2. Materials and Methods
2.1. Cells and Viruses
Primary porcine alveolar macrophages (PAMs) or HEK293T cells were cultured in RPMI 1640 or Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin and 100
μg/mL streptomycin at 37°C with 5% CO
2. The ASFV HLJ/2018 strain was isolated from field pig samples in China and propagated in the PAMs as described previously [
10].
2.2. 5′ and 3′ Rapid Amplification of cDNA Ends (RACE) Assay
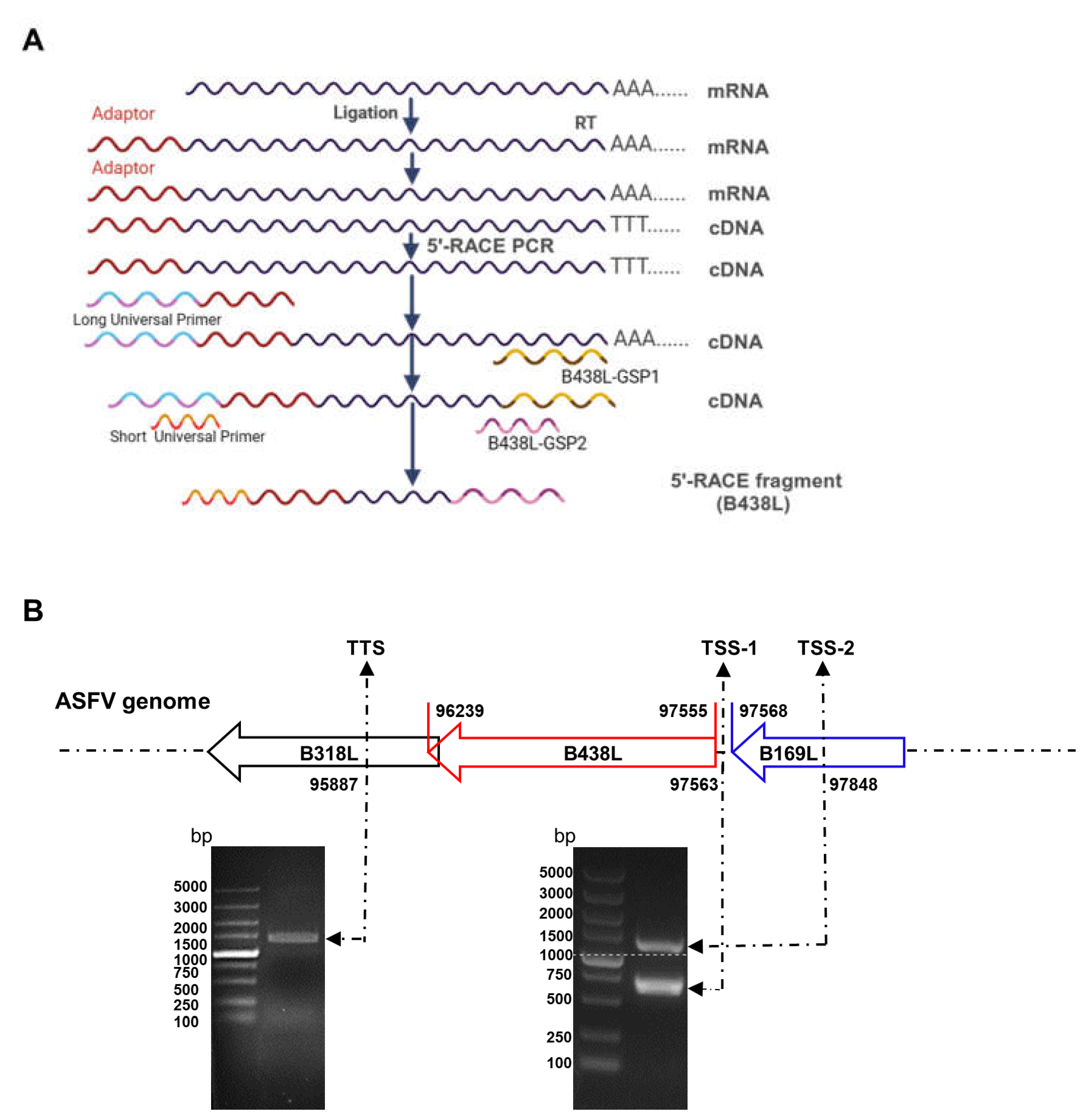
The total RNA was extracted from the ASFV-infected PAMs at a multiplicity of infection (MOI) of 2 using an RNA purification kit (catalog no. BSC52M1; BioFlux) according to manufacturer’s instructions. The 5′RACE was performed using the 5′-Full RACE kit (catalog no. 638858; TaKaRa) and the B438L-specific primers (B438L-GSP1 and -GSP2 primers). The schematic diagram of the B438L-GSP 1 and -GSP 2 for 5′RACE was shown in
Figure 1. The gene products of 5′RACE polymerase chain reaction (PCR) were cloned into the pMD19-T vector (catalog no. 639648; TaKaRa) and sequenced using the M13 primers. All the primers in this study are shown in
Table 1.
2.3. Construction of Plasmids
The genomic DNA of ASFV was extracted using a MagaBio plus virus DNA purification kit (catalog no. 9109; BioFlux) according to the manufacturer’s instructions. Six primer pairs were designed for the amplification of the
B438L-1 to
-6 genes using the genomic DNA as a template. To generate the gene constructs of
B169L-2 and
B169L-mATG-2 (the mutation of the second ATG in
B169L), a series of reverse primers and intermediate primers were generated and subjected to PCR using the
B438L-1 gene as a template. The mutation and deletion of the GC and TATA boxes in
B438L-7 and
-5 were obtained by sequence overlapping extension (SOE) PCR, and the primers used in this study are shown in
Table 1. All the PCR products were purified by using a gel extraction kit (catalog no. 740609.10; TaKaRa) and subsequently digested with the endonucleases
KpnI and
XhoI, followed by a second gel purification for subsequent assays. The sequences of the
B438L promoters were cloned into the pGL3-Basic luciferase reporter plasmid (catalog no. E1751; Promega). The recombinant plasmids were subjected to DNA sequencing.
2.4. Luciferase Reporter Assay
HEK293T cells cultured in 24-well plates were transfected with 0.5 μg of a series of reporter plasmids harboring different B438L promoters, a negative control (pGL3-Basic), and a positive control (pGL3-Control) together with 0.05 μg of an internal control plasmid pRL-TK expressing the Renilla luciferase.
2.5. Western Blotting Analysis
The HEK293T transfected with the plasmids were lysed with RIPA buffer, and subjected to SDS-PAGE analysis, and then the protein bands were electro-transferred onto nylon membranes. Subsequently, the membranes were blocked in TBS containing 5% skimmed milk for 2 hours at room temperature, and incubated with a mouse anti-Flag monoclonal antibody (MAb) or mouse anti-β-actin polyclonal antibodies (PAb) (catalog no. ZMS1156; Sigma-Aldrich) for 2 hours. The membranes were rinsed three times using TBS containing 0.05% Tween 20 (TBST) and incubated with IRDye 800CW goat anti-mouse IgG secondary antibody (catalog no. 926-32210; LI-COR) for 1 hour at room temperature. Next, the membranes were scanned using an Odyssey infrared imaging system (LiCor BioSciences).
2.6. RNA Extraction and RT-qPCR
Total RNA was extracted from the PAMs treated with specific inhibitors or virus infection by a Simply P total RNA extraction kit (catalog no. BSC52M1; BioFlux). The isolated RNA was reverse transcribed to cDNA using a FastKing gDNA Dispelling RT SuperMix (catalog no. KR118-02; Tiangen) according to the manufacturer’s protocols. The cDNAs were used to analyze the mRNA transcription of
B169L-2 by RT-qPCR using a QuantStudio system (Applied Biosystems) as described previously [
11]. The relative mRNA levels of the target genes were normalized to the internal reference GAPDH, and all primers are listed in
Table 1. The relative fold changes in gene expression were determined by the threshold cycle (2
–ΔΔCT) method [
12].
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0 (San Diego, CA). Statistical differences between groups were assessed by Student’s t test. All the experiments were performed independently in triplicates. Error bars represent standard deviations (SD) or standard errors of the mean (SEM) in each group, as indicated in the figure legends (ns, not significant, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001). A P-value of < 0.05 was considered significant.
3. Results
3.1. Two Distinct Initiation Sites of B438L Transcription
To analyze the transcription start site (TSS) located upstream of the
B438L translation initiation codon (ATG), 5′RACE assay was conducted using the total RNA of the PAMs infected with the ASFV HLJ/2018 strain at 24 hours postinfection (hpi). The first round of amplification was run by using the B438L-GSP1 primer pairs. The product of the first round of PCR (5
μL) was used as a template to perform the second round of amplification using the B438L-GSP2 primer pairs (
Figure 1A). As shown in
Figure 1B, PCR products were generated. No PCR products were obtained from the parallel reactions without reverse transcriptase or DNA template (data not shown), indicating that the PCR products were derived from the ASFV transcript. Approximately 20–25 bacterial clones from each 5′RACE product were sequenced and confirmed to harbor an identical 5′-ends, indicating that they were derived from the same
B438L mRNA transcript. The two positive bands with 700 and 1100 bp, respectively, were mainly amplified by PCR (
Figure 1B). Upon sequence alignment, we identified two distinct TSSs located at nt 97563 (TSS-1) and 97848 (TSS-2) of the genome of the ASFV HLJ/2018 strain (
Figure 1B). In addition, 3′RACE assay was performed to ascertain the transcription termination site of
B438L, which was determined to be located at nt 95887 (
Figure 1B). Collectively, these findings suggest the presence of two TSSs for the
B438L gene.
3.2. Identification of the B438L Promoter Region Located in the B169L Gene
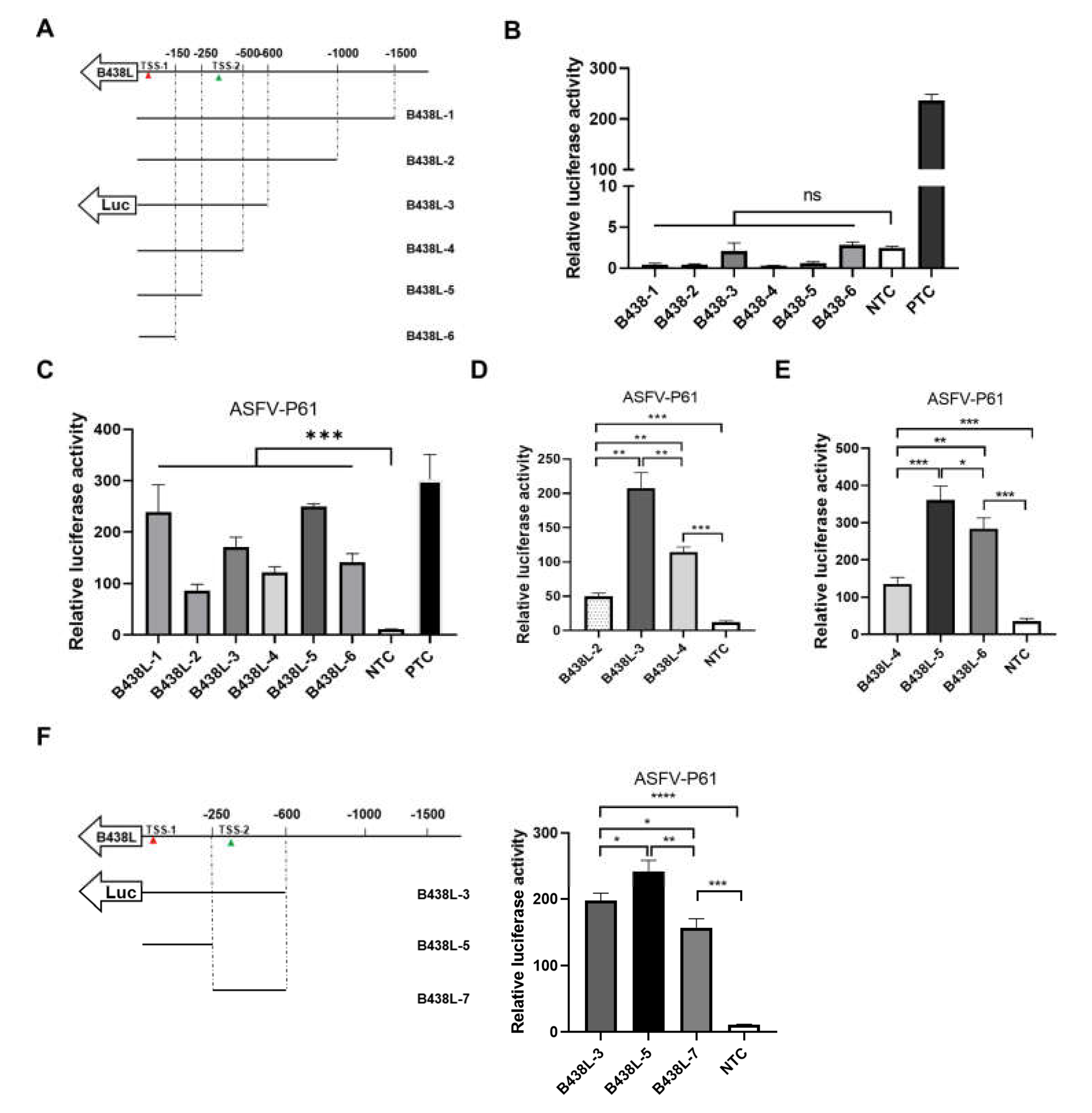
To delineate the promoter regions of ASFV
B438L, a series of primers were designed to amplify seven DNA fragments of the
B438L gene upstream, using the ASFV genomic DNA as the template (
Table 1). Each promoter region was delineated with respect to an individual TSS position, as illustrated in
Figure 2A. Notably, these selected promoter regions were designed to lack the ATG codon in the 3′ flanking sequence downstream of the TSS, thereby preventing any potential interference with the translation of the luciferase gene. To evaluate the potential promoter activity of these regions, the DNA fragments were cloned into pGL3-Basic to give rise to the luciferase reporter plasmids.
Subsequently, HEK293T cells were transfected with each the reporter plasmids of different promoter regions and the internal control plasmid pRL-TK for 24 hours. Afterward, the cells were lysed with the passive buffer, and the cell lysates were subjected to the examination of the luciferase activities using the dual-luciferase reporter assay system according to the manufacturer’s instructions. As shown in
Figure 2B, all the reporter plasmids did not display any promoter activities. In light of this observation, we presumed that the transcription of the
B438L gene is likely to depend on ASFV-specific RNA polymerases and other essential transcription factors. To verify the hypothesis, HEK293T cells were transfected with the
B438L gene reporter plasmids for 12 hours. Subsequently, the cells were infected with an HEK293T cells-adapted ASFV, ASFV-P61 strain [
13], at an MOI of 0.1 for 24 hours. Next, the luciferase activities were examined as described above. The results showed that all the reporter plasmids displayed the promoter activities, while the
B438L-1,
B438L-3, and
B438L-5 exhibited the higher activities in comparison with
B438L-2,
B438L-4, and
B438L-6 (
Figure 2C). More specifically, the promoter activities of
B438L-2 and
B438L-4 were shown to be lower than those of
B438L-3 and
B438L-5, respectively (
Figure 2D and E). To further determine whether a promoter exists between
B438L-3 and
B438L-5, we constructed a luciferase reporter plasmid in the genome region, the data showed that
B438L-7 also exhibited higher activity compared with the negative control (
Figure 2F). Taken together, these findings suggest that
B438L-5 and
B438L-7 are responsible for initiating
B438L transcription at TSS-1 and TSS-2, respectively.
3.3. Identification of the TATA and GC Boxes in the B438L Promoter
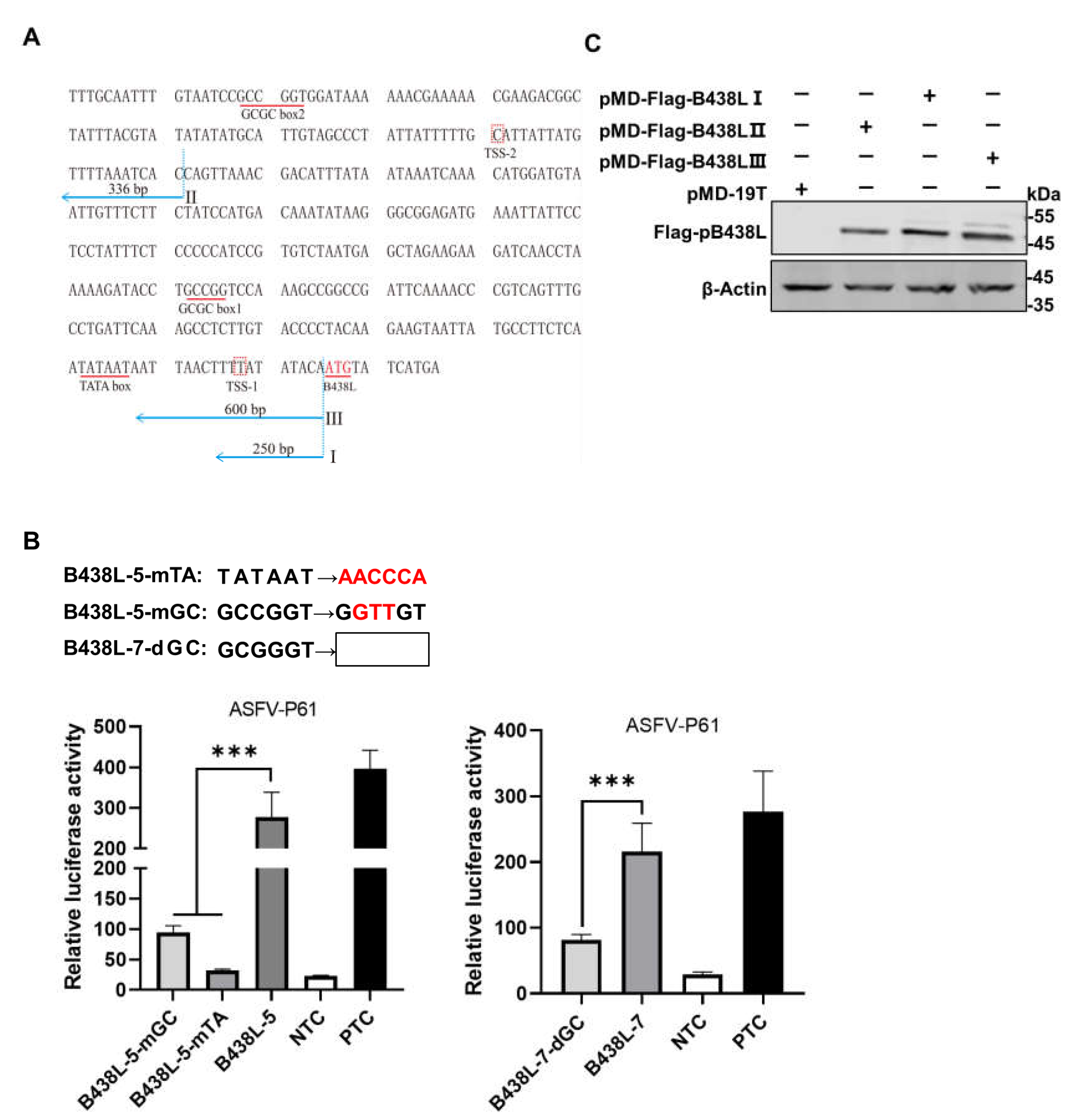
We have identified two upstream fragments of the
B438L TSS as promoters. Utilizing a comprehensive analysis of the locations of TATA or GC box within the promoter regions, along with computational analysis [
14,
15,
16,
17], we have predicted that the putative TATA and GC boxes positioned between 10 and 100 nt, respectively, upstream of TSS (
Figure 3A). The TSSs of ASFV-
B438L were determined at nt 97563 and 97848 in the ASFV HLJ/2018 strain
We predicted its GC and TATA boxes. The primers for amplifying the
B438L promoter regions are shown in
Table 1. The B438L-GSP1 outer and -GSP2 primers were designed based on the genome of ASFV HLJ/2018 strain, while the 5′RACE long and short Universal Primers were provided by the 5′RACE kit. The TATA box or GC box was predicted as described above.
To identify the GC box or TATA box in the regions of
B438L-7 and
B438L-5, we introduced mutations or deletions (
Figure 3B, upper panel) in these cis-acting elements using the mutant primers (
Table 1). As shown in
Figure 3B, the mutation (
B438L-5-mGC) or deletion (
B438L-7-dGC) of the GC box and the mutation of TATA box (
B438L-5-mTA) resulted in the reduction of the promoter activity. The data indicate that the
B438L promoters contain functional TATA and GC boxes, which are indispensable for the promoter activity.
To further validate the identified promoter regions, we generated a series of recombinant plasmids expressing
B438L using the clonal vector pMD-19T, each of which carries one of these two
B438L promoter regions or two promoter regions. Subsequently, HEK293T cells were transfected with the expressing plasmids of
B438L ORF with various regions of promoters, pMD-Flag-B438L-I (the region of
B438L-5), -II (the region of
B438L-7), and -III (the region of
B438L-3) or the pMD-19T plasmid, respectively. At 36 hours post transfection (hpt), the cell lysates were analyzed by western blotting to verify the expression of pB438L. Notably, pB438L was efficiently expressed in cells transfected with both plasmids (
Figure 3C), indicating that both the distal and proximal promoters of
B438L can initiate the expression of pB438L.
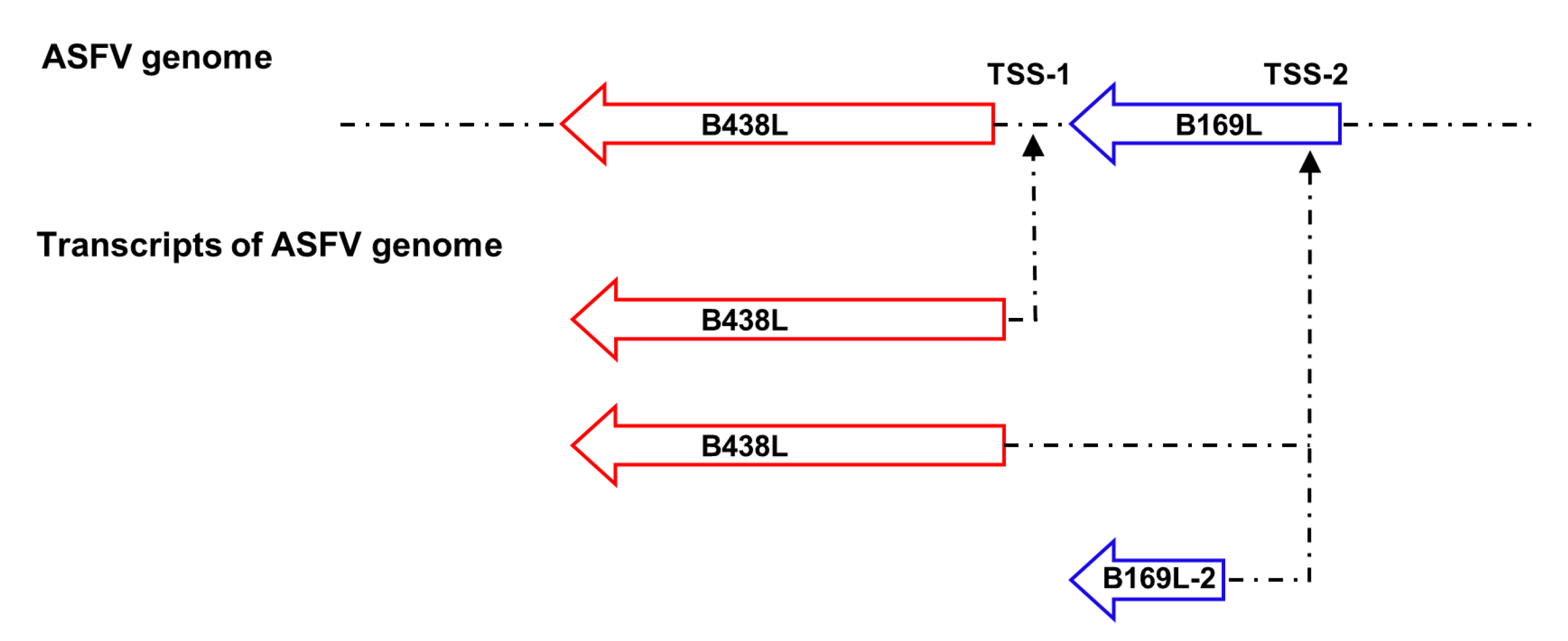
3.4. Identification of the B438L Distal Promoter that Initiates Transcription of B169L-2
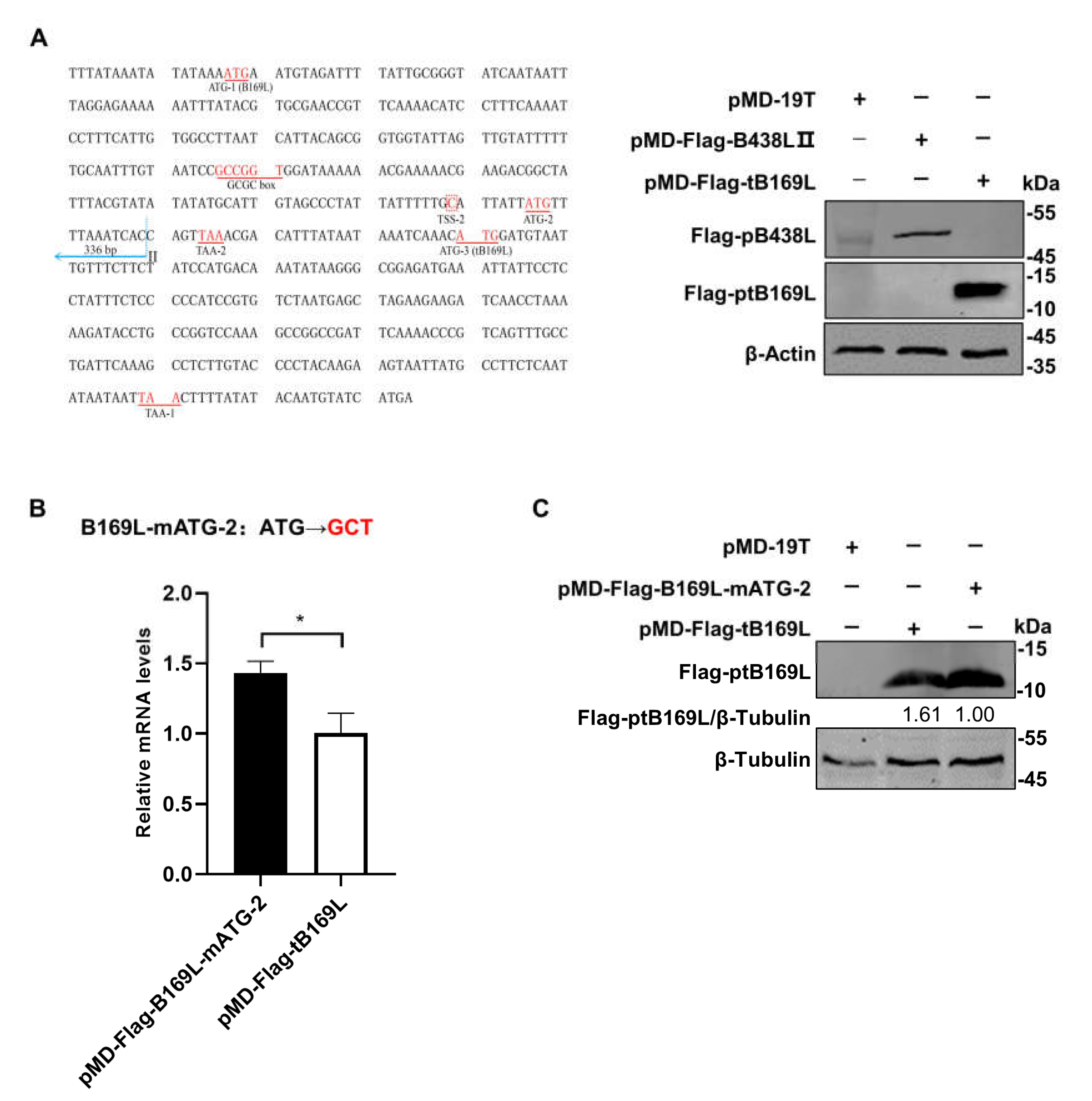
Since the
B438L distal promoter is located in the
B169L gene, we investigate whether the promoter affects the
B169L expression. To this end, we reviewed the relevant data and found that there is
B169L-mRNA2 encoding a truncated pB169L (tpB169L) (amino acids 92–169). The sequence of
B169L-2 is located in the downstream of the
B438L distal promoter. Thus, we verified whether the
B438L distal promoter is capable of initiating the transcription of
B169L-2. Firstly, we constructed the recombinant plasmid pMD-Flag-tB169L, which carries the gene sequence of
B169L-2 as well as the distal promoter sequence of
B438L. Secondly, HEK293T cells were transfected with pMD-Flag-tB169L or pMD-Flag-B438L-II and infected with ASFV-P61. At 24 hpi, the cell lysates were analyzed by western blotting to verify the expression of tpB169. Notably, tpB169L was expressed in the pMD-Flag-tB169L-transfected HEK293T cells (
Figure 4A), indicating that the distal promoter of
B438L can initiate the expression of tpB169L.
We further analyzed the sequences of
B169L-2 and revealed that the gene contains two initiation codon (ATG-2 and ATG-3) located in the region between TSS-2 and the termination codon TAA-1 (
Figure 4A). Further study demonstrated that there is a termination codon (TAA-2) located in the 15 nt downstream of ATG-2, and ATG-3 is required for the translation of
B169L-2. We speculated that the transcription efficiency of
B169L-2 was impaired due to the presence of a mini transcript (located in the region of ATG-2 and TAA-2). Thus, we mutated the ATG-2 of
B169L and constructed the Flag-tagged mutant plasmid pMD-B169L-mATG-2. Next, HEK293T cells were transfected with pMD-tB169L, pMD-B169L-mATG-2 and infected with ASFV-P61. At 24 hpi, cell lysates were collected to examine the protein expression of pMD-Flag-tB169L and pMD-tB169L-mATG2 by western blotting, and total RNA was extracted for reverse transcription-quantitative PCR (RT-qPCR) to determine the mRNA transcription. The results showed that the transcription and translation efficiency of pMD-tB169L-mATG2 were significantly higher than that of pMD-Flag-tB169L (
Figure 4B and C), indicating that the
B438L distal promoter is capable of initiating the transcription of
B438L as well as
B169L-2, and that the mutation of the ATG-2 located between the TSS-2 of
B438L and the ATG of
B169L-2 (ATG-3) can efficiently enhance the expression of
B169L-2.
4. Discussion
Viruses have evolved multiple strategies to regulate the viral gene expression to complete the virus life cycle. Multiple promoters enable viruses to selectively activate or repress genes depending on different cell types, microenvironment, or the stages of the virus life cycle, thereby enhancing viral adaptation in host cells. For example, the HIV-1
Nef gene contains multiple promoters, each of which plays a different regulatory role in different cell types and conditions [
18,
19].
In this study, we revealed that two promoter regions responsible for the ASFV B438L transcription located upstream of the B438L ORF. In addition, we demonstrate the B169L gene (the upstream gene of B438L) contains a distal promoter for B438L. Interestingly, this distal promoter not only initiates the transcription of the B438L gene but also governs the transcription of B169L-2, suggesting the precise regulatory association of the adjacent viral genes.
Furthermore, we have also observed an initiation codon (ATG-2) located upstream of
B169L-2 (
Figure 4A). Unexpectedly, substitution of the ATG with GCT resulted in an enhanced transcription and translation efficiency for
B169L-2 (
Figure 4B). The presence of the ATG-2 is likely to be involved in modulating the expression of the downstream
B169L-2, while the mutation of ATG-2 is speculated to abolish the binding between transcription factors and the promoter, thereby releasing the transcription factors for favoring the downstream gene transcription. Besides, in the absence of viral infection, all of the luciferase reporter plasmids were devoid of promoter activity. In contrast, in the presence of viral infection, all of the luciferase reporter plasmids exhibited the promoter activities, suggesting that regulation of these two promoters requires the participation of the ASFV-encoded polymerases and their associated factors. Here, we identified for the first time the transcriptional regulatory element for
B169L-2, indicating that ASFV employs multiple strategies to regulate gene expression. Future studies are required to investigate the functional role of
B169L-2 during ASFV replication. Furthermore, the findings will facilitate the design of the
B169L gene-deleted ASFV. The phenomenon of multiple promoters regulating the transcription of viral genes is an interesting topic in virology.
Additionally, multiple promoters enhance viral adaptation to host diversity, exhibiting the distinct biological characteristics. It has been shown that the
X gene of hepatitis B virus (HBV) contains multiple promoters, therefore, regulating gene expression under different conditions [
20,
21]. Viruses also utilize multiple promoters to regulate gene expression patterns for the immune evasion of host cells. The herpes simplex virus 1 (HSV-1)
UL34 gene (ICP0) contains multiple promoters, which functions at multiple stages of the virus life cycle [
22,
23].
Moreover, the presence of multiple promoters contributes to the genetic diversity of viral genes, thereby facilitating immune-evasion of host cells. The
IE1 gene of human cytomegalovirus (CMV) has multiple promoters, with these promoters playing a pivotal role in viral infection, enhancing our understanding of CMV gene regulation [
24,
25]. Finally, different promoters are activated during various stages of the viral life cycle, favoring virus replication and transmission. The
LMP1 gene of Epstein-Barr virus (EBV) contains multiple promoters, resulting in the expression of various LMP1 proteins, which are crucial for comprehending the complex regulatory network of EBV [
26,
27]. Taken together, we demonstrated that the distal promoter of
B438L gene initiates the transcription of both the
B438L mRNA and
B169L mRNA2.
5. Conclusions
In conclusion, we demonstrated for the first time that the ORF of the ASFV
B438L gene was governed by two different promoters, and the distal promoter could also initiate the transcription of both
B438L and
B169L-2 genes (
Figure 5). A comprehensive understanding of the multiple promoters of viral genes can facilitate the development of more effective antiviral strategies.
Author Contributions
Conceptualization, H.C., H.D., and Y.W.; methodology, H.C., H.D., and Y.W.; software, H.C., H.D., and D.L.; validation, H.C., H.D., and Y.W.; formal analysis, H.C., M.L., and L.L.; investigation, H.C.; resources, H.Q.; data curation, S.L.; writing—original draft preparation, H.C.; writing—review and editing, H.Q., and S.L; visualization, D.P., J.D., and J.J.; supervision, S.L.; project administration, H.Q.; funding acquisition, H.Q., and S.L. All authors have read and agreed to the published version of the manuscript.”.
Funding
This work was supported by the Natural Science Foundation of China (grants 32072866 and U20A2060), and the Heilongjiang Provincial Natural Science Foundation of China (grant YQ2022C043).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results are available in this article.
Conflicts of Interest
The authors declare no conflict of interest in this work.
References
- Iyer, L.M.; Aravind, L.; Koonin, E.V. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 2001, 75, 11720–11734. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Andrés, G.; Salas, M.L. African swine fever virus proteinase is essential for core maturation and infectivity. J. Virol. 2003, 77, 5571–5577. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.L.; Bustos, M.J.; de Leon, P. Methods for growing and titrating African swine fever virus: field and laboratory samples. Curr. Protoc. Cell Biol. 2011, 26, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef]
- Cackett, G.; Matelska, D.; Sýkora, M.; Portugal, R.; Malecki, M.; Bähler, J.; Dixon, L.; Werner, F. The African swine fever virus transcriptome. J. Virol. 2020, 94, e00119–20. [Google Scholar] [CrossRef] [PubMed]
- Epifano, C.; Krijnse-Locker, J.; Salas, M.L.; Salas, J.; Rodríguez, J.M. Generation of filamentous instead of icosahedral particles by repression of African swine fever virus structural protein pB438L. J. Virol. 2006, 80, 11456–11466. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhang, L.H.; Lin, Y.; Cai, Y.F.; Zou, Y.W.; Hao, Z.Y.; Luo, Z.H.; Wang, N.D.; Deng, Z.B.; Yang, Y.; et al. Development and preliminary testing of a probe-based duplex real-time PCR assay for the detection of African swine fever virus. Mol. Cell. 2021, Probes 59, 101764. [Google Scholar] [CrossRef]
- Cackett, G.; Sýkora, M.; Werner, F. Transcriptome view of a killer: African swine fever virus. Biochem. Soc. Trans. 2020, 48, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Zhou, P.; Li, L.F.; Zhang, K.; Wang, B.; Tang, L.; Li, M.; Wang, T.; Sun, Y.; Li, S.; Qiu, H.J. Deletion of the H240R gene of African swine fever virus decreases infectious progeny virus production due to aberrant virion morphogenesis and enhances inflammatory cytokine expression in porcine macrophages. J. Virol. 2022, 96, e0166721. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, Y.; Huang, S.; Qiu, H.J. Multifaceted immune responses to African swine fever virus: implications for vaccine development. Vet. Microbiol. 2020, 249, 108832. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, W.C.; Hsu, J.B.; Chang, T.H.; Shien, D.M. GPMiner: an integrated system for mining combinatorial cis-regulatory elements in mammalian gene group. BMC. Genomics. 2012, 13 Suppl 1, S3. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bhanja, C.J.; Steele, R.; Ray, R.; Ray, R.B. Hepatitis C virus upregulates Beclin1 for induction of autophagy and activates mTOR signaling. J. Virol. 2012, 86, 8705–8712. [Google Scholar] [CrossRef]
- Smale, S.T.; Kadonaga, J.T. The RNA polymerase II core promoter. Annu. Rev. Biochem. 2003, 72, 449–479. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, W.W.; Sandelin, A. Applied bioinformatics for the identification of regulatory elements. Nat. Rev. Genet. 2004, 5, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.R.; Balachandran, R.; Gupta, P.; Wolinsky, S.M. Analysis of alternatively spliced human immunodeficiency virus type-1 mRNA species, one of which encodes a novel tat-env fusion protein. Virology 1991, 185, 258–270. [Google Scholar] [CrossRef]
- Telwatte, S.; Lee, S.; Somsouk, M.; Hatano, H.; Baker, C.; Kaiser, P.; Kim, P.; Chen, T.H.; Milush, J.; Hunt, P.W.; et al. Gut and blood differ in constitutive blocks to HIV transcription, suggesting tissue-specific differences in the mechanisms that govern HIV latency. PLoS Pathog. 2018, 14, e1007357. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, A.; Sugata, F.; Chen, H.S.; Miller, R.H.; Purcell, R.H. Evidence for a bidirectional promoter complex within the X gene of woodchuck hepatitis virus. Virus Res. 1998, 56, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Slagle, B.L.; Bouchard, M.J. Hepatitis B virus X and regulation of viral gene expression. Cold Spring Harb. Perspect. Med. 2016, 6, a021402. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tanaka, M.; Yokoymama, A.; Matsuda, G.; Kato, K.; Kagawa, H.; Hirai, K.; Roizman, B. Herpes simplex virus 1 alpha regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, N.; Hazell, N.; Jones, C. Independent cis-regulatory modules within the herpes simplex virus 1 infected cell protein 0 (ICP0) promoter are transactivated by Krüppel-like factor 15 and glucocorticoid receptor. Viruses 2022, 14, 1284. [Google Scholar] [CrossRef] [PubMed]
- Jeang, K.T.; Rawlins, D.R.; Rosenfeld, P.J.; Shero, J.H.; Kelly, T.J.; Hayward, G.S. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J. Virol. 1987, 61, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Stinski, M.F.; Isomura, H. Role of the cytomegalovirus major immediate early enhancer in acute infection and reactivation from latency. Med. Microbiol. 2008, 197, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Khanna, R. Role of LMP1 in immune control of EBV infection. Semin. Cancer Biol. 2001, 11, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Morgan, D.R.; Dominguez, R.L.; Thorne, L.B.; Elmore, S.H.; Mino-Kenudson, M.; Lauwers, G.Y.; Booker, J.K.; Gulley, M.L. High levels of Epstein-Barr virus DNA in latently infected gastric adenocarcinoma. Lab. Invest. 2009, 89, 80–90. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).