Submitted:
02 May 2024
Posted:
03 May 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Nomenclature and Configurations
Physiology of Extracorporeal Membrane Oxygenation
Patient Selection
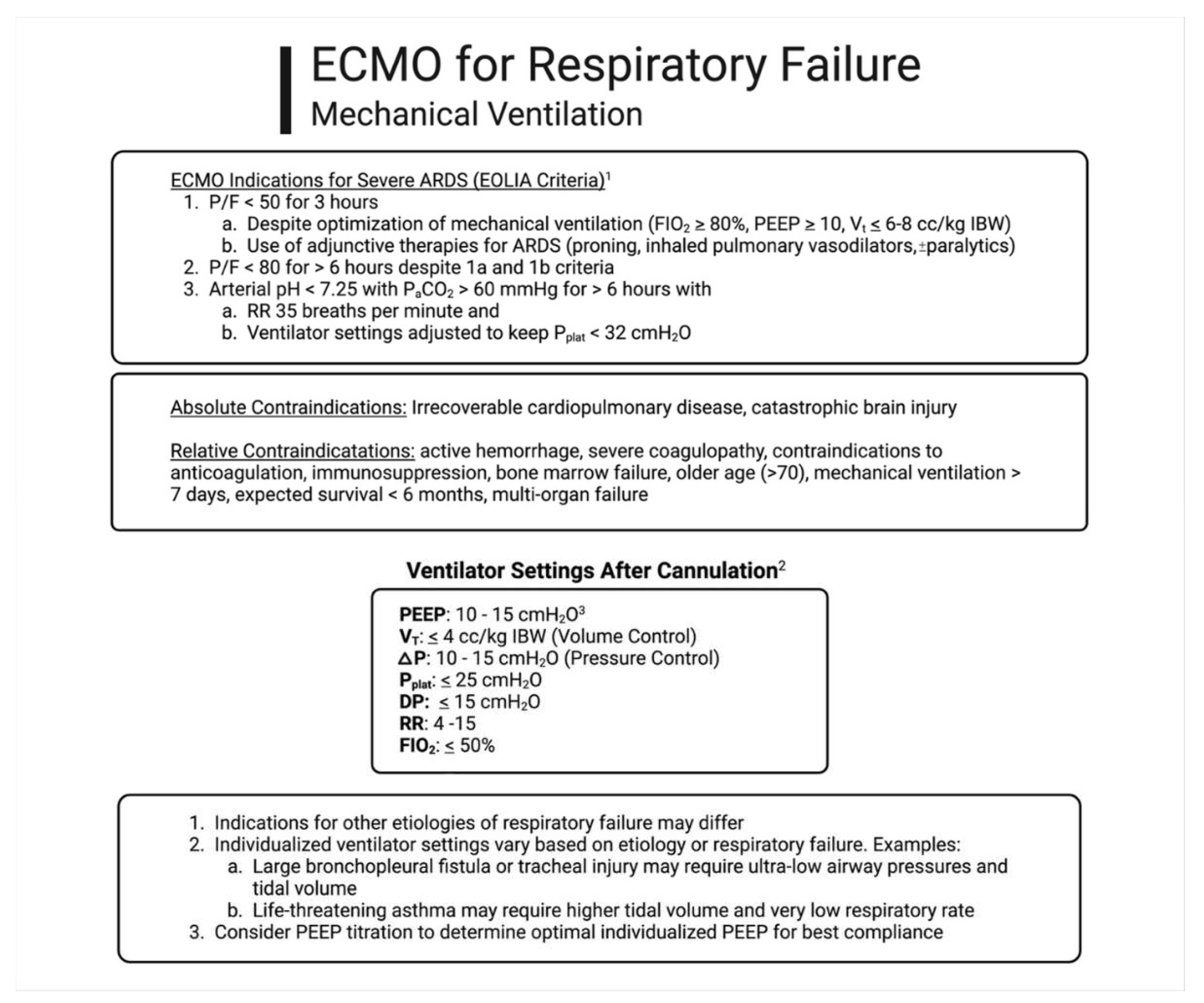
Ventilator Management
Management and Troubleshooting of the Extracorporeal Circuit
Physiologic Goals and Monitoring
Circuit Pharmacology
Hypoxemia and Tissue Hypoxia
Complications
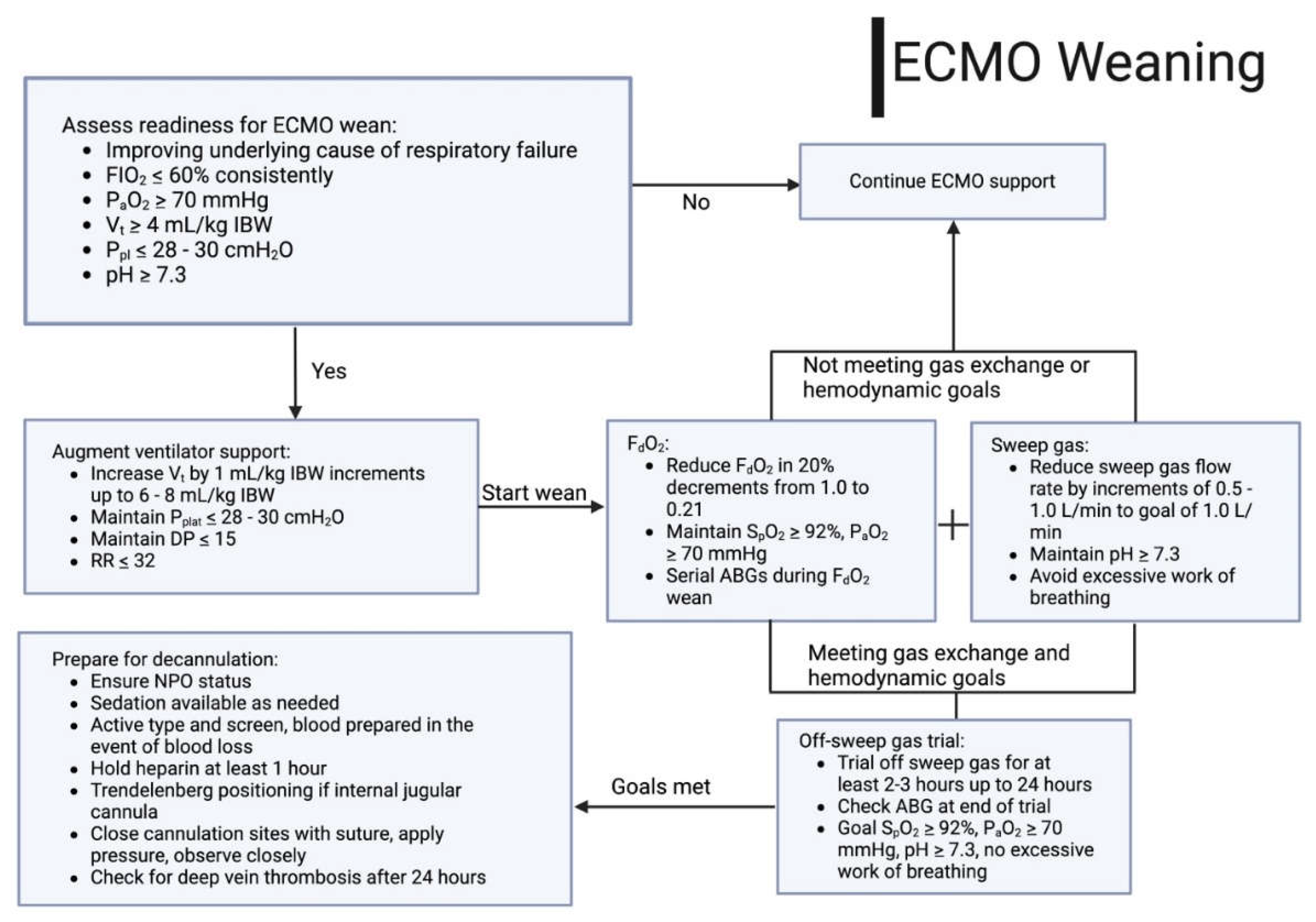
Weaning of Extracorporeal Support
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan E, Gattinoni L, Combes A, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory failure: A clinical review from an international group of experts. Intensive Care Med 2016;42(5):712–724.
- Ventetuolo CE, Muratore CS. Extracorporeal life support in critically III adults. Am J Respir Crit Care Med 2014;190(5):497–508.
- Cai, T.; Swaney, E.; Helm, S.V.D.; Brown, G.; MacLaren, G.; Horton, S.; Monagle, P.; Ignjatovic, V. The Evolution of Extracorporeal Membrane Oxygenation Circuitry and Impact on Clinical Outcomes in Children: A Systematic Review. ASAIO J. 2023, 69, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.D.; O'Brien, T.G.; Murray, J.J.; Dontigny, L.; Bramson, M.L.; Osborn, J.J.; Gerbode, F. Prolonged Extracorporeal Oxygenation for Acute Post-Traumatic Respiratory Failure (Shock-Lung Syndrome). New Engl. J. Med. 1972, 286, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Boonstra, P.S.; MacLaren, G.; Paden, M.; Brodie, D.; Anders, M.; Hoskote, A.; Ramanathan, K.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal Life Support Organization Registry International Report 2022: 100,000 Survivors. ASAIO J. 2024, 70, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Broman, L.M.; Taccone, F.S.; Lorusso, R.; Malfertheiner, M.V.; Pappalardo, F.; Di Nardo, M.; Belliato, M.; Bembea, M.M.; Barbaro, R.P.; Diaz, R.; et al. The ELSO Maastricht Treaty for ECLS Nomenclature: abbreviations for cannulation configuration in extracorporeal life support - a position paper of the Extracorporeal Life Support Organization. Crit. Care 2019, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dessap, A.M.; Boissier, F.; Charron, C.; Bégot, E.; Repessé, X.; Legras, A.; Brun-Buisson, C.; Vignon, P.; Vieillard-Baron, A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensiv. Care Med. 2016, 42, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Chad, T.; Yusuff, H.; Zochios, V.; Pettenuzzo, T.; Fan, E.; Schmidt, M.; (Prorvnet), F.T.P.T.R.V.N. Right Ventricular Injury Increases Mortality in Patients With Acute Respiratory Distress Syndrome on Veno-Venous Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. ASAIO J. 2023, 69, e14–e22. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Park, S.; Zundel, M.T.; Dong, H.; Szabo, A.; Cain, M.T.; Durham, L.A. Extracorporeal membrane oxygenation for COVID-19: An evolving experience through multiple waves. Artif. Organs 2022, 46, 2257–2265. [Google Scholar] [CrossRef]
- Tatooles, A.J.; Mustafa, A.K.; Joshi, D.J.; Pappas, P.S. Extracorporeal membrane oxygenation with right ventricular support in COVID-19 patients with severe acute respiratory distress syndrome. JTCVS Open 2021, 8, 90–96. [Google Scholar] [CrossRef]
- Kuroda, T.; Miyagi, C.; Fukamachi, K.; Karimov, J.H. Mechanical circulatory support devices and treatment strategies for right heart failure. Front. Cardiovasc. Med. 2022, 9, 951234. [Google Scholar] [CrossRef] [PubMed]
- Grotberg, J.C.; Greenberg, J.; Sullivan, M.; A Pawale, A.; Kotkar, K.D.; Masood, M.F. Physiologic benefits of veno-pulmonary extracorporeal membrane oxygenation for COVID-19 ARDS: A single center experience. Int. J. Artif. Organs 2024, 47, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.H. Physiology of Gas Exchange During ECMO for Respiratory Failure. J. Intensiv. Care Med. 2017, 32, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Vincent J, Backer D De. Oxygen transport— the oxygen delivery controversy. Intensive Care Med 2004;30(11):1990–1996.
- D. B, M. D. B, M. B, I. H, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360(9328):219–223.
- Callahan, L.A.; Supinski, G.S. Sepsis Induces Diaphragm Electron Transport Chain Dysfunction and Protein Depletion. Am. J. Respir. Crit. Care Med. 2005, 172, 861–868. [Google Scholar] [CrossRef]
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Bréchot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.-L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensiv. Care Med. 2013, 39, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Mateo-Sidron, J.A.R.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Abrams D, Brodie D. Extracorporeal Membrane Oxygenation for Adult Respiratory Failure: 2017 Update. Chest 2017;152(3):639–649.
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoue, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. New Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Munshi L, Sorbo L Del, Adhikari NKJ, et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2017;14(4):280–288.
- Goligher, E.C.; Tomlinson, G.; Hajage, D.; Wijeysundera, D.N.; Fan, E.; Juni, P.; Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA 2018, 320, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Burrell A, Kim J, Alliegro P, et al. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev 2023;2023(9).
- Yeo, H.J.; Kim, D.; Jeon, D.; Kim, Y.S.; Rycus, P.; Cho, W.H. Extracorporeal membrane oxygenation for life-threatening asthma refractory to mechanical ventilation: analysis of the Extracorporeal Life Support Organization registry. Crit. Care 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schmidt M, Hajage D, Landoll M, et al. Comparative outcomes of extracorporeal membrane oxygenation for COVID-19 delivered in experienced European centres during successive SARS-CoV-2 variant outbreaks ( ECMO-SURGES ): an international, multicentre, retrospective cohort study. 2023;2600(22):1–13.
- Schmidt, M.; Langouet, E.; Hajage, D.; James, S.A.; Chommeloux, J.; Brechot, N.; Barhoum, P.; Lefevre, L.; Troger, A.; de Chambrun, M.P.; et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit. Care 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Docherty AB, Mulholland RH, Lone NI, et al. Changes in in-hospital mortality in the first wave of COVID-19: a multicentre prospective observational cohort study using the WHO Clinical Characterisation Protocol UK. Lancet Respir Med 2021;9(7):773–785.
- Grotberg, J.C.; Kraft, B.D.; Sullivan, M.; Pawale, A.A.; Kotkar, K.D.; Masood, M.F. Advanced Respiratory Support Days as a Novel Marker of Mortality in COVID-19 Acute Respiratory Distress Syndrome Requiring Extracorporeal Membrane Oxygenation. ASAIO J. 2024. [Google Scholar] [CrossRef]
- Peetermans, M.; Guler, I.; Meersseman, P.; Wilmer, A.; Wauters, J.; Meyns, B.; Vlaar, A.P.J.; Combes, A.; Hermans, G. Impact of BMI on outcomes in respiratory ECMO: an ELSO registry study. Intensiv. Care Med. 2023, 49, 37–49. [Google Scholar] [CrossRef]
- Rudym, D.; Pham, T.; Rackley, C.R.; Grasselli, G.; Anderson, M.; Baldwin, M.R.; Beitler, J.; Agerstrand, C.; Serra, A.; Winston, L.A.; et al. Mortality in Patients with Obesity and Acute Respiratory Distress Syndrome Receiving Extracorporeal Membrane Oxygenation: The Multicenter ECMObesity Study. Am. J. Respir. Crit. Care Med. 2023, 208, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Zogheib, E.; Rozé, H.; Repesse, X.; Lebreton, G.; Luyt, C.-E.; Trouillet, J.-L.; Bréchot, N.; Nieszkowska, A.; Dupont, H.; et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensiv. Care Med. 2013, 39, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Hilder, M.; Herbstreit, F.; Adamzik, M.; Beiderlinden, M.; Bürschen, M.; Peters, J.; Frey, U.H. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the PREdiction of Survival on ECMO Therapy-Score (PRESET-Score). Crit. Care 2017, 21, 301–301. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting Survival after Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Moyon Q, Chambrun MP De, Lebreton G, Chaieb H, Combes A, Schmidt M. Validation of survival prediction models for ECMO in Sars - CoV - 2 - related acute respiratory distress syndrome. Crit Care 2022;26(187):1–4.
- Joshi, H.; Flanagan, M.; Subramanian, R.; Drouin, M. Respiratory ECMO Survival Prediction (RESP) Score for COVID-19 Patients Treated with ECMO. ASAIO J. 2022, 68, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Schmidt M, Stewart C, Bailey M, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: A retrospective international multicenter study. Crit Care Med 2015;43(3):654–664.
- Belliato, M.; Epis, F.; Cremascoli, L.; Ferrari, F.; Quattrone, M.G.; Fisser, C.; Malfertheiner, M.V.; Taccone, F.S.; Di Nardo, M.; Broman, L.M.; et al. Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study. Membranes 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.-C.; Lin, S.-W.; Chuang, L.-P.; Li, H.-H.; Liu, P.-H.; Tsai, F.-C.; Chang, C.-H.; Hung, C.-Y.; Lee, C.-S.; Leu, S.-W.; et al. Mechanical power during extracorporeal membrane oxygenation and hospital mortality in patients with acute respiratory distress syndrome. Crit. Care 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Marhong, J.D.; Munshi, L.; Detsky, M.; Telesnicki, T.; Fan, E. Mechanical ventilation during extracorporeal life support (ECLS): a systematic review. Intensiv. Care Med. 2015, 41, 994–1003. [Google Scholar] [CrossRef]
- Assouline, B.; Combes, A.; Schmidt, M. Setting and Monitoring of Mechanical Ventilation During Venovenous ECMO. Crit. Care 2023, 27, 1–8. [Google Scholar] [CrossRef]
- Magunia, H.; Haeberle, H.A.; Henn, P.; Mehrländer, M.; Vlatten, P.O.; Mirakaj, V.; Rosenberger, P.; Koeppen, M. Early Driving Pressure Changes Predict Outcomes during Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Crit. Care Res. Pr. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Serpa Neto A, Schmidt M, Azevedo LCP, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis: Mechanical ventilation during ECMO. Intensive Care Med 2016;42(11):1672–1684.
- Guérin, C. Individualization of Positive End-Expiratory Pressure Setting in Patients with Acute Respiratory Distress Syndrome under Extracorporeal Membrane Oxygenation. Inputs from Electrical Impedance Tomography. Am. J. Respir. Crit. Care Med. 2017, 196, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Soulé, C.; Crognier, L.; Puel, F.; Ruiz, S.; Seguin, T.; Fourcade, O.; Georges, B.; Conil, J.-M.; Minville, V.; Vardon-Bounes, F. Assessment of Electrical Impedance Tomography to Set Optimal Positive End-Expiratory Pressure for Venoarterial Extracorporeal Membrane Oxygenation-Treated Patients*. Crit. Care Med. 2021, 49, 923–933. [Google Scholar] [CrossRef]
- Puel, F.; Crognier, L.; Soulé, C.; Vardon-Bounes, F.; Ruiz, S.; Seguin, T.; Fourcade, O.; Minville, V.; Conil, J.-M.; Georges, B. Assessment of electrical impedance tomography to set optimal positive end-expiratory pressure for veno-venous ECMO-treated severe ARDS patients. J. Crit. Care 2020, 60, 38–44. [Google Scholar] [CrossRef]
- Meservey, A.; Krishnan, G.; Green, C.L.; Morrison, S.; Rackley, C.R.; Kraft, B.D. U-Shaped Association Between Carboxyhemoglobin and Mortality in Patients With Acute Respiratory Distress Syndrome on Venovenous Extracorporeal Membrane Oxygenation. Crit. Care Explor. 2023, 5, e0957. [Google Scholar] [CrossRef] [PubMed]
- Cavayas, Y.A.; Munshi, L.; del Sorbo, L.; Fan, E. The Early Change in PaCO2after Extracorporeal Membrane Oxygenation Initiation Is Associated with Neurological Complications. Am. J. Respir. Crit. Care Med. 2020, 201, 1525–1535. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Yang, X.; Zou, X.; Shu, H.; Liu, Z.; Shang, Y. Narrative Review of Neurologic Complications in Adults on ECMO: Prevalence, Risks, Outcomes, and Prevention Strategies. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- Martucci, G.; Schmidt, M.; Agerstrand, C.; Tabatabai, A.; Tuzzolino, F.; Giani, M.; Ramanan, R.; Grasselli, G.; Schellongowski, P.; Riera, J.; et al. Transfusion practice in patients receiving VV ECMO (PROTECMO): a prospective, multicentre, observational study. Lancet Respir. Med. 2023, 11, 245–255. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wu, X. Liberal or restrictive transfusion for VV ECMO. Lancet Respir. Med. 2023, 11, e20. [Google Scholar] [CrossRef]
- Peters, T.; Wille, K.; Kulkarni, T.; Diaz-Guzman, E.; Sharma, N. Safety of Flexible Bronchoscopy in Critically ill Adult Patients Supported With Extracorporeal Membrane Oxygenation. Chest 2015, 148, 290A–290. [Google Scholar] [CrossRef]
- Cheng, V.; Abdul-Aziz, M.-H.; Roberts, J.A.; Shekar, K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10 (Suppl S5), S629–S641. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 2012, 27, 741–e9. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; A Roberts, J.; I Mcdonald, C.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T.; et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, R194–R194. [Google Scholar] [CrossRef]
- Lemaitre, F.; Hasni, N.; Leprince, P.; Corvol, E.; Belhabib, G.; Fillâtre, P.; Luyt, C.-E.; Leven, C.; Farinotti, R.; Fernandez, C.; et al. Propofol, midazolam, vancomycin and cyclosporine therapeutic drug monitoring in extracorporeal membrane oxygenation circuits primed with whole human blood. Crit. Care 2015, 19, 1–6. [Google Scholar] [CrossRef]
- Landolf, K.M.; Rivosecchi, R.M.; Goméz, H.; Sciortino, C.M.; Murray, H.N.; Padmanabhan, R.R.; Sanchez, P.G.; Harano, T.; Sappington, P.L. Comparison of Hydromorphone versus Fentanyl-based Sedation in Extracorporeal Membrane Oxygenation: A Propensity-Matched Analysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, D.L.; Wengenmayer, T.; Schmidt, M. Beta-blockers in refractory hypoxemia on venovenous extracorporeal membrane oxygenation: a double-edged sword. Crit. Care 2023, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Abrams D, Brodie D, Brechot N, Fan E, Pellegrino V, Brodie D. Extracorporeal Life Support Organization ( ELSO ) for Identification and management of recirculation in venovenous ECMO. Extracorpor Life Support Organ 2015;(May 2015):1–7.
- Abrams, D.; Bacchetta, M.; Brodie, D. Recirculation in Venovenous Extracorporeal Membrane Oxygenation. ASAIO J. 2015, 61, 115–121. [Google Scholar] [CrossRef]
- Lubnow, M.; Philipp, A.; Foltan, M.; Enger, T.B.; Lunz, D.; Bein, T.; Haneya, A.; Schmid, C.; Riegger, G.; Müller, T.; et al. Technical Complications during Veno-Venous Extracorporeal Membrane Oxygenation and Their Relevance Predicting a System-Exchange – Retrospective Analysis of 265 Cases. PLOS ONE 2014, 9, e112316. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.D.; Giosa, L.; Camarda, V.; Camporota, L. Physiological adaptations during weaning from veno-venous extracorporeal membrane oxygenation. Intensiv. Care Med. Exp. 2023, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, A.; Protos, A.N.; Saikus, C.E.; Jeyakumar, A.K.C. Fundamentals of weaning veno-arterial and veno-venous extracorporeal membrane oxygenation. Indian J. Thorac. Cardiovasc. Surg. 2023, 39, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wieruszewski, P.M.; Ortoleva, J.P.; Cormican, D.S.; Seelhammer, T.G. Extracorporeal Membrane Oxygenation in Acute Respiratory Failure. Pulm. Ther. 2023, 9, 109–126. [Google Scholar] [CrossRef]
- Gattinoni L, Vassalli F, Romitti F, et al. Extracorporeal gas exchange: When to start and how to end? Crit Care 2019;23(1):1–7.
- Pratt, E.H.; Mausert, S.M.; Wilson, M.D.R.-A.; Emerson, L.J.M.; Navuluri, N.; Pulsipher, A.M.; Brucker, A.; Green, C.L.; Bonadonna, D.K.M.; Bryner, B.S.; et al. A Daily, Respiratory Therapist Assessment of Readiness to Liberate From Venovenous Extracorporeal Membrane Oxygenation in Patients With Acute Respiratory Distress Syndrome. Crit. Care Explor. 2021, 3, e0584. [Google Scholar] [CrossRef]


| Acute Respiratory Distress Syndrome (ARDS) |
|---|
| Bacterial |
| Viral |
| Fungal |
| Aspiration |
| Transfusion-related Acute Lung Injury (TRALI) |
| Life-threatening Asthma |
| Acute Eosinophilic Pneumonia |
| Acute Interstitial Pneumonia |
| Diffuse Alveolar Hemorrhage (DAH) |
| Acute Chest Syndrome |
| Thoracic Trauma |
| Parenchymal Lung Injury |
| Tracheal Injury |
| Bronchopleural Fistula |
| Peri-Lung Transplant |
| Bridge to Transplant |
| Primary Graft Dysfunction |
| RESP Score Parameter | Score |
|---|---|
| Age, yr | |
| 18-49 | 0 |
| 50-59 | -2 |
| ≥ 60 | -3 |
| Immunocompromised status | -2 |
| Mechanical ventilation prior to the initiation of ECMO | |
| < 48 h | 3 |
| 48 h to 7 d | 1 |
| > 7 d | 0 |
| Acute respiratory diagnosis group | |
| Viral pneumonia | 3 |
| Bacterial pneumonia | 3 |
| Asthma | 11 |
| Trauma and burn | 3 |
| Aspiration pneumonitis | 5 |
| Other acute respiratory diagnosis | 1 |
| Non-respiratory and chronic respiratory diagnoses | 0 |
| Central nervous system dysfunction | -7 |
| Acute associated non-pulmonary infection | -3 |
| Neuromuscular blockade agents before ECMO | 1 |
| Nitric oxide use before ECMO | -1 |
| Bicarbonate infusion before ECMO | -2 |
| Cardiac arrest before ECMO | -2 |
| PaCO2, mmHg | |
| <75 | 0 |
| ≥ 75 | -1 |
| Peak inspiratory pressure, cmH2O | |
| < 42 | 0 |
| ≥ 42 | -1 |
| Total Score | -22 to 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




