Submitted:
02 May 2024
Posted:
06 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Equipments
2.2. Extraction of Prenylated Flavonoids from Humulus lupulus var. Polaris
2.3. Determination of the Total Content of Polyphenols
2.4. Determination of the Total Antioxidant Capacity by the ABTS Method
2.5. Determination of Antioxidant Activity by the DPPH Method
2.6. Quantitative Determination of Prenylated Flavonoids by HPLC-DAD
3. RESULTS AND DISCUSSION
3.1. Analysis of ASE Extract from Hops of the Polaris Variety
3.2. Functional Unfiltered Beers
3.3. Functional Filtered Beers
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, F., Watanabe., Y., Nozawa, H., Daikonnya, A., Kondo, K., Kitanaka. S. (2005). Prenylflavonoids and Phloroglucinol Derivatives from Hops (Humulus lupulus). Journal of Natural Products, 68, 1, 43–49. [CrossRef]
- Żołnierczyk, A. K., Mączka, W. K., Grabarczyk, M. ł., Wińska, K., Woźniak, E., Anioł, M. ł. (2015). Isoxanthohumol — Biologically active hop flavonoid. Fitoterapia, 103, 71–82. [CrossRef]
- Karabín, M., Hudcová, T., Jelínek, L., Dostálek, P. (2012). Význam chmelových prenylflavonoidů pro lidské zdraví. Chemické listy, 106(12), 1095-1103. http://www.chemicke-listy.cz/docs/full/2012_12_1095-1103.pdf.
- Santarelli, V., Neri, L., Carbone, K., Macchioni, V., Faieta, M., & Pittia, P. (2023). Conventional and innovative extraction technologies to produce food-grade hop extracts: Influence on bitter acids content and volatile organic compounds profile. Journal of Food Science, 88(4), 1308-1324. [CrossRef]
- Rošul, M. Đ., Mandić, A. I., Mišan, A. Č., Đerić, N. R., & Pejin, J. D. (2019). Review of trends in formulation of functional beer. Food and Feed research, 46(1), 23-35. [CrossRef]
- Nardini, M. (2023). An Overview of Bioactive Phenolic Molecules and Antioxidant Properties of Beer: Emerging Trends. Molecules, 28(7), 3221. [CrossRef]
- de Andrade Silva, G. V., Arend, G. D., Zielinski, A. A. F., Di Luccio, M., Ambrosi, A. (2023). Xanthohumol properties and strategies for extraction from hops and brewery residues: A review. Food Chemistry, 404, 134629. [CrossRef]
- Weiskirchen, R., Mahli, A., Weiskirchen, S., & Hellerbrand, C. (2015). The hop constituent xanthohumol exhibits hepatoprotective effects and inhibits the activation of hepatic stellate cells at different levels. Frontiers in Physiology, 6, 140. [CrossRef]
- Karabín, M., Jelínek, L., Kinčl, T., Hudcová, T., Kotlíková, B., & Dostálek, P. (2013). New approach to the production of xanthohumol-enriched beers. Journal of the Institute of Brewing, 119(3), 98-102. [CrossRef]
- Magalhães, P. J., Guido, L. F., Cruz, J. M., Barros, A. A. (2007). Analysis of xanthohumol and isoxanthohumol in different hop products by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Journal of Chromatography A, 1150(1–2), 295–301. [CrossRef]
- Wunderlich, S., Zürcher, A., & Back, W. (2005). Enrichment of xanthohumol in the brewing process. Molecular Nutrition & Food Research, 49(9), 874-881. [CrossRef]
- Protsenko, L., Rudyk, R., Hryniuk, T., Vlasenko, A., Prostenko, A., Litvynchuk, S.,. Ovadenko, O. (2018). Beer enrichment with biologically active hop compounds. Ukrainian Food Journal, 7(1). [CrossRef]
- Kyselová, L., & Brányik, T. (2015). Quality improvement and fermentation control in beer. In Advances in fermented foods and beverages (pp. 477-500). Woodhead Publishing. [CrossRef]
- Habschied, K., Živković, A., Krstanović, V., Mastanjević, K. (2020). Functional Beer- A Review on Possibilities. Beverages, 6(3). [CrossRef]
- Yang, S., Chen, R., Cao, X., Wang, G., & Zhou, Y. J. (2024). De novo biosynthesis of the hops bioactive flavonoid xanthohumol in yeast. Nature Communications, 15(1), 253. [CrossRef]
- Rosaria Ciriminna, R., Albanese, L., Di Stefano, V., Delisi, R., Avellone, G., Meneguzzo, F., Pagliaro, M. (2018). Beer produced via hydrodynamic cavitation retains higher amounts of xanthohumol and other hops prenylflavonoids. In LWT, 91, 160-167. [CrossRef]
- Silva, K. F. C. E., Strieder, M. M., Pinto, M. B. C., Rostagno, M. A., & Hubinger, M. D. (2023). Processing strategies for extraction and concentration of bitter acids and polyphenols from Brewing By-Products: A Comprehensive Review. Processes, 11(3), 921. [CrossRef]
- Wasmuht, I. (2024). Relevant process steps influencing wort and beer composition and its quality: A review. Brewing Science Yearbook 2023, 113.
- Stevens, J. F., Taylor, A. W., Deinzer, M. L. (1999). Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography–tandem mass spectrometry. Journal of Chromatography A, 832(1–2), 97–107. [CrossRef]
- Deng, Y., Lim, J., Nguyen, T. T. H., Mok, I. K., Piao, M., & Kim, D. (2020). Composition and biochemical properties of ale beer enriched with lignans from Schisandra chinensis Baillon (omija) fruits. Food Science and Biotechnology, 29, 609-617. [CrossRef]
- Nardini, M., & Foddai, M. S. (2020). Phenolics profile and antioxidant activity of special beers. Molecules, 25(11), 2466. [CrossRef]
- Pluháčková, H., Gregor, T., Boško, R., Běláková, S., Svoboda, Z., & Benešová, K. (2020). Fortification of beer with extracts of the selected Czech medicinal herbs and plants. Kvasny prumysl, 66(4), 314-319. [CrossRef]
- Cho, J. H., Kim, I. D., Dhungana, S. K., Do, H. M., & Shin, D. H. (2018). Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food science and biotechnology, 27, 1067-1073. [CrossRef]
- Gil-Ramírez, A., Mendiola, J., Arranz, E., Ruíz-Rodriguéz, A., Reglero, G., Ibánez, E., Marín, F. (2012). Highly isoxanthohumol enriched hop extract obtained by pressurized hot water extraction (PHWE). Chemical and functional characterization. Innovative Food Science & Emerging Technologies, 16, 54-60. [CrossRef]
- Adebiyi, O. E., Olayemi, F. O., Ning-Hua, T., & Guang-Zhi, Z. (2017). In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef University Journal of Basic and Applied Sciences, 6(1), 10-14. [CrossRef]
- Rajbhar, K., Dawda, H., Mukundan, U. (2015). Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun, 5(1), 1-6.
- Stevens, J. F., Page, J. E. (2004). Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry, 65(10), 1317–1330. [CrossRef]
- Roth, C. (2023). American Amber. On Tap, 2023(3), 46-47.
- Villacreces, S., Blanco, C. A., & Caballero, I. (2022). Developments and characteristics of craft beer production processes. Food bioscience, 45, 101495. [CrossRef]
- Heath, E., & Milam, J. (2023). Perceptions of China and Russian chinoiserie under Empress Elisabeth Petrovna. In Russian Orientalism in a global context (pp. 50-74). Manchester University Press.
- https://www.uvartesipivo.sk/Sladovy-vytazok-PALE-ALE-1-7-kg-d691.htm.
- Paszkot, J., Kawa-Rygielska, J., & Anioł, M. (2021). Properties of Dry Hopped Dark Beers with High Xanthohumol Content. Antioxidants, 10(5), 763. [CrossRef]
- https://www.kupsito.sk/i/13912478418/supravy-na-varenie-piva/tmavy-pivny-sladovy-extrakt-1-2kg-brewa.
- Tretyak, L., Rebezov, M., Kenijz, N., Khayrullin, M., Gribkova, V., & Goncharov, A. (2020). Controlled Glycolysis as the Basis of Beer Technology with Specified Consumer Properties. Systematic Reviews in Pharmacy, 11(5). [CrossRef]
- Paoeltta, C., Balog, C., Higgs, A., Liskin, D., Kingsbury, K., Brehm, A., & Quinlan, R. A. (2023). Science of Brewing: An Introduction to the Impact of Local Regions on a Favorite Fermented Beverage. In Chemistry of Alcoholic Beverages. 10, 187-215. DOI: 10.1021/bk-2023-1455.ch010. [CrossRef]
- Alves, E. M., de Souza, J. F., de Oliva Neto, P., (2021). Advances in yeast autolysis technology - a faster and safer new bioprocess. Braz. J. Food Technol. 24. [CrossRef]
- Wang, J., Li, M., Zheng, F., Niu, C., Liu, C., Li, Q., & Sun, J. (2018). Cell wall polysaccharides: Before and after autolysis of brewer’s yeast. World Journal of Microbiology and Biotechnology, 34, 1-8. [CrossRef]
- Jaeger, A., Arendt, E. K., Zannini, E., & Sahin, A. W. (2020). Brewer’s spent yeast (BSY), an underutilized brewing by-product. Fermentation, 6(4), 123. [CrossRef]
- Yang, F., Chen, C., Ni, D., Yang, Y., Tian, J., Li, Y., & Wang, L. (2023). Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods, 12(17), 3315. [CrossRef]
- Silva, S., Oliveira, A. I., Cruz, A., Oliveira, R. F., Almeida, R., & Pinho, C. (2022). Physicochemical Properties and Antioxidant Activity of Portuguese Craft Beers and Raw Materials. Molecules, 27(22), 8007. [CrossRef]
- Pilcher, J. M. (2024). Imperial Hops: Beer in the Age of Empire. Global Food History, 10(1), 52-67. [CrossRef]
- Wang, X., Lu, J., Cai, G., & Wu, D. (2023). Advances in Maillard reaction products on color, flavor and biological activity of specialty malt. Systems Microbiology and Biomanufacturing, 1-11. [CrossRef]
- Nakayama, T., Takahashi, S., & Waki, T. (2019). Formation of flavonoid metabolons: functional significance of protein-protein interactions and impact on flavonoid chemodiversity. Frontiers in Plant Science, 10, 460916. [CrossRef]
- Mukai, R. (2018). Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Bioscience. Biotechnology and Biochemistry, 82(2), 207-215. [CrossRef]
- Wunderlich, S., Wurzbacher, M., & Back, W. (2013). Roasting of malt and xanthohumol enrichment in beer. European Food Research and Technology, 237, 137-148. [CrossRef]
- Adamczyk, B., Simon, J., Kitunen, V., Adamczyk, S., & Smolander, A. (2017). Tannins and their complex interaction with different organic nitrogen compounds and enzymes: old paradigms versus recent advances. ChemistryOpen, 6(5), 610-614. [CrossRef]
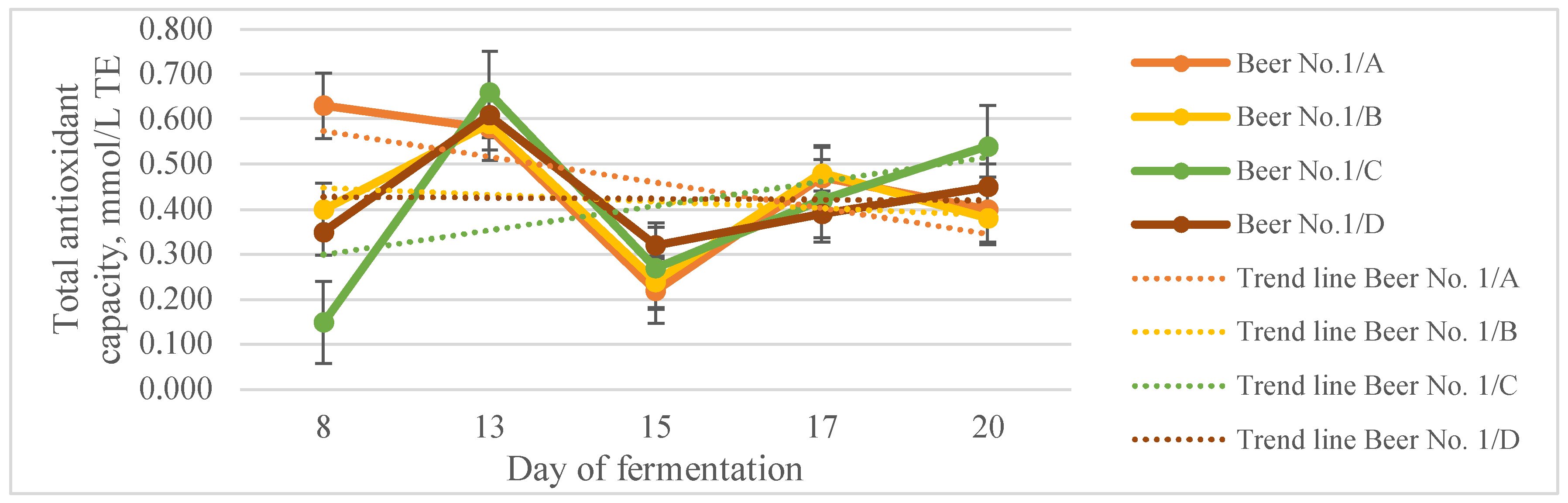
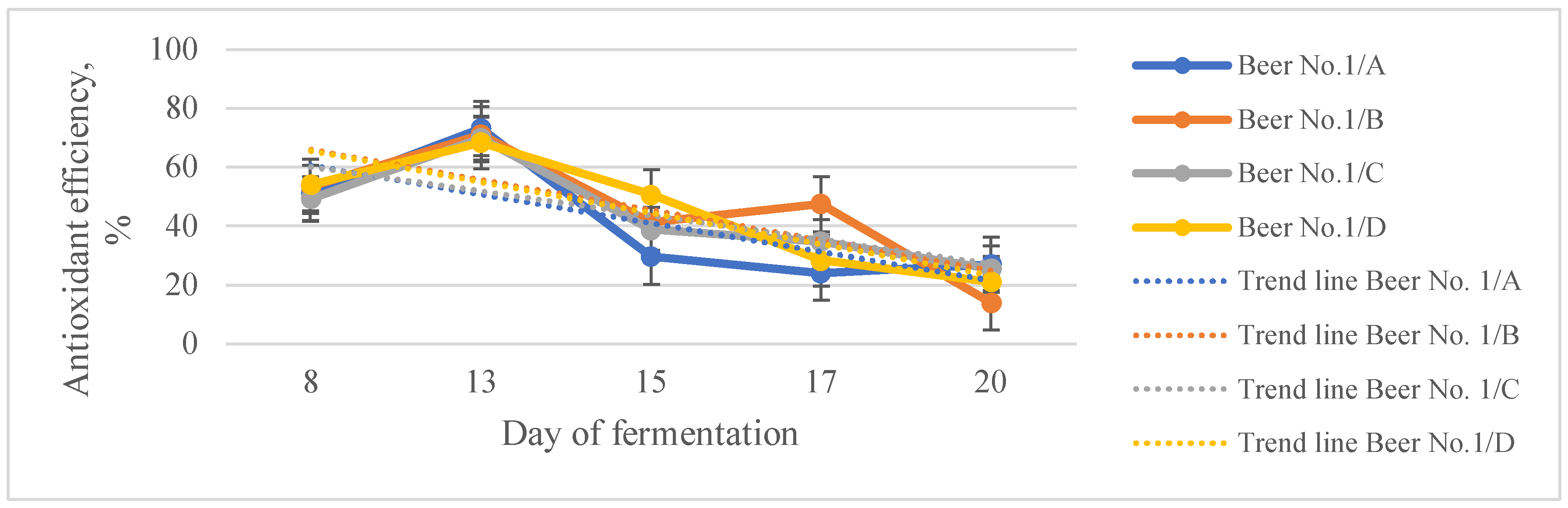
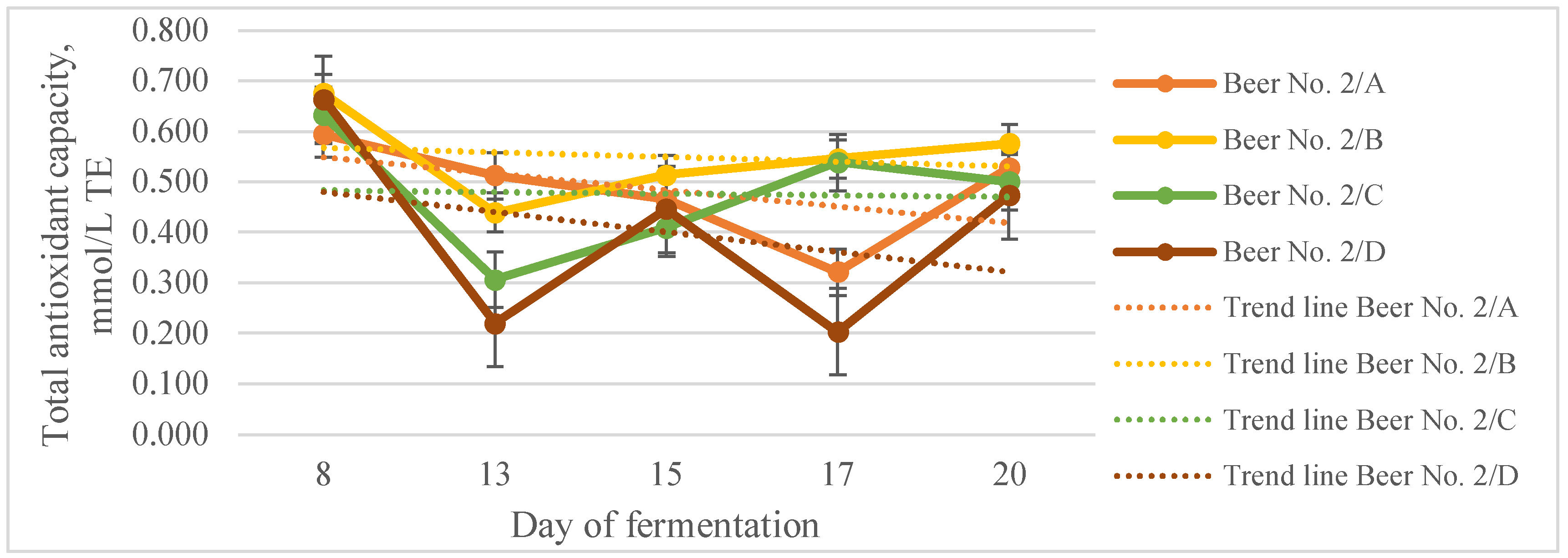
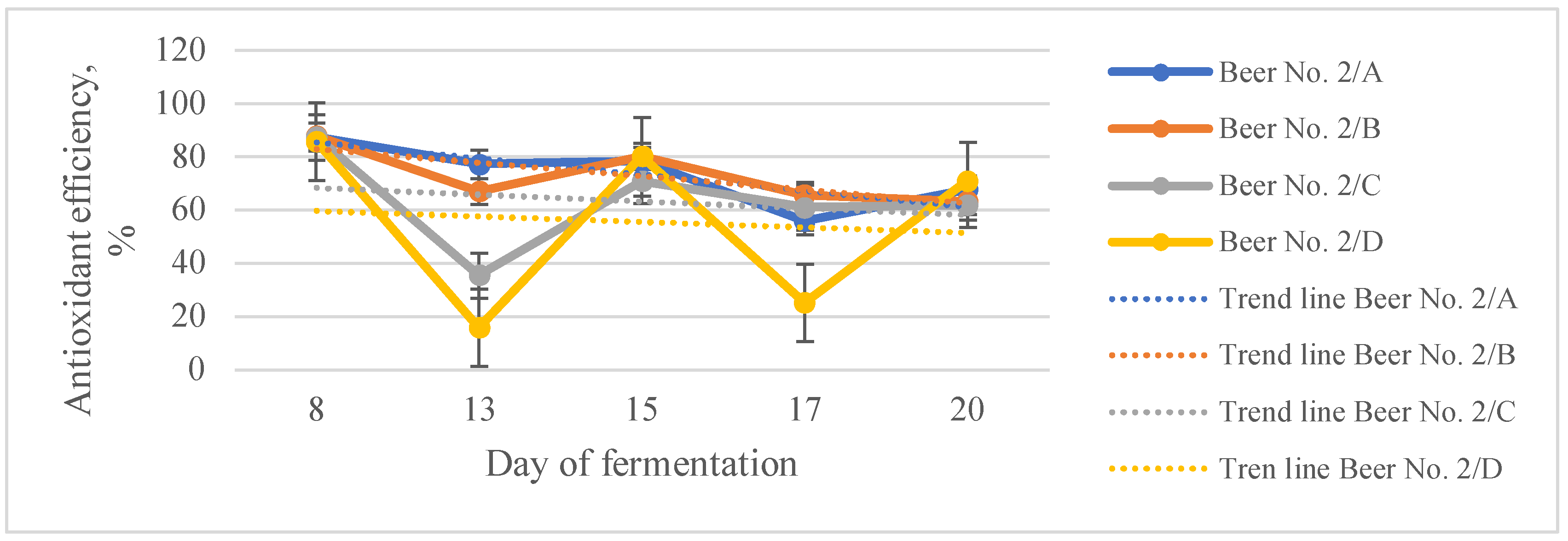
| Total polyphenols content, g/L GAE | Total antioxidant capacity, mmol/L TE | Antioxidant efficiency, % | |
|---|---|---|---|
| ẋ (n=6) | 1.180 | 15.960 | 85.63 |
| σ | 0.025 | 0.289 | 0.45 |
| Xanthohumol, mg/mL | Izoxanthohumol, mg/mL | |
|---|---|---|
| (n=6) | 5.765 | 1.096 |
| σ | 0.397 | 0.177 |
| Variant of beer | Total polyphenols content, g/L GAE | |||||
| Day of fermentation | ||||||
| 8 | 13 | 15 | 17 | 20 | ||
| Beer No. 1/A | ẋ (n=6) | 2.220 | 2.280 | 3.600 | 2.290 | 2.500 |
| σ | 0.043 | 0.564 | 0.320 | 0.078 | 0.007 | |
| Beer No. 1/B | ẋ (n=6) | 2.190 | 0.260 | 2.700 | 3.180 | 2.340 |
| σ | 0.076 | 0.023 | 0.433 | 0.065 | 0.432 | |
| Beer No. 1/C | ẋ (n=6) | 2.350 | 2.400 | 2.400 | 2.330 | 2.440 |
| σ | 0.022 | 0.065 | 0.005 | 0.008 | 0.021 | |
| Beer No. 1/D | ẋ (n=6) | 2.470 | 2.460 | 1.640 | 2.410 | 3.010 |
| σ | 0.041 | 0.022 | 0.008 | 0.043 | 0.032 | |
| Variant of beer | Total polyphenols content, g/L GAE | |||||
| Day of fermentation | ||||||
| 8 | 13 | 15 | 17 | 20 | ||
| Beer No. 2/A | ẋ (n=6) | 1.420 | 1.340 | 1.480 | 1.400 | 1.440 |
| σ | 0.010 | 0.070 | 0.770 | 0.040 | 0.210 | |
| Beer No. 2/B | ẋ (n=6) | 1.630 | 1.420 | 1.720 | 1.380 | 1.510 |
| σ | 0.028 | 0.010 | 0.120 | 0.090 | 0.060 | |
| Beer No. 2/C | ẋ (n=6) | 1.500 | 1.360 | 1.490 | 1.370 | 1.500 |
| σ | 0.010 | 0.070 | 0.077 | 0.040 | 0.021 | |
| Beer No. 2/D | ẋ (n=6) | 1.350 | 1.230 | 1.730 | 1.270 | 1.250 |
| σ | 0.076 | 0.023 | 0.043 | 0.065 | 0.043 | |
| Variant of beer | Xanthohumol, mg/L | Izoxanthohumol, mg/L | |||
| Day of fermentation | |||||
| 8 | 13 | 8 | 13 | ||
| Beer No. 2/A | ẋ (n=6) | 1.040 | 0.000 | 1.245 | 0.902 |
| σ | 0.005 | 0.000 | 0.005 | 0.004 | |
| Beer No. 2/B | ẋ (n=6) | 2.050 | 1.640 | 2.819 | 0.486 |
| σ | 0.008 | 0.038 | 0.006 | 0.002 | |
| Beer No. 2/C | ẋ (n=6) | 1.920 | 1.860 | 1.598 | 0.752 |
| σ | 0.052 | 0.028 | 0.001 | 0.006 | |
| Beer No. 2/D | ẋ (n=6) | 1.880 | 0.000 | 1.082 | 0.513 |
| σ | 0.005 | 0.000 | 0.012 | 0.076 | |
| Variant of beer | Xanthohumol, mg/L | Izoxanthohumol, mg/L | |
| Beer No. 1/FA | ẋ (n=6) | 8.315 | 0.654 |
| σ | 0.006 | 0.006 | |
| Beer No. 1/FB | ẋ (n=6) | 15.210 | 0.922 |
| σ | 0.053 | 0.007 | |
| Beer No. 2/FA | ẋ (n=6) | 15.300 | 0.982 |
| σ | 0.005 | 0.009 | |
| Beer No. 2/FB | ẋ (n=6) | 32.375 | 1.877 |
| σ | 0.032 | 0.043 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





