1. Introduction
Brazil stands out worldwide in terms of agricultural production. The sector is the driving force of the economy and employed 19 million people in 2018 [
1]. Furthermore, in 2020, according to the Center for Advanced Studies in Applied Economics (CEPEA), the sector was responsible for 26% of the Gross Domestic Product (GDP) and 52% of everything that was exported. However, this feat is linked to the intense use of agricultural pesticides. Data from the Ministry of Health [
2] show that between 2007 and 2014 the sale of agricultural pesticides grew by 149% and the planted area increased by 22.31%. There is no doubt that the use of agricultural pesticides was one of the factors that led to an increase in field productivity, but their intensive use also leads to harmful effects, such as exposure of workers and consumers, negative impacts on water quality, soil and human health, which can cause chronic and acute poisoning [
3].
Due to its non-selective and efficient action in eliminating weeds, glyphosate is the most used agricultural pesticide in Brazil, representing 62% of the total agricultural pesticides used [
4].
The World Health Organization (WHO) cited in 2015, that there is evidence that glyphosate is potentially carcinogenic to humans [
3]. Samsel and Sneff [
5] and Bruce et al [
6] are, among others, important studies that correlate the use of glyphosate-based pesticides with an increase in cancer cases. Glyphosate [N-(phosphonomethyl) glycine] is a broad-spectrum, non-selective, systemic, and post-emergent agricultural pesticide, used in various food and non-food crops. It belongs to the group of phosphorus amino acids or substituted glycines. It has remained for years as the most consumed pesticide worldwide [
7]. It appears as a white, crystalline salt, very soluble in water (12 g L
-1 at 25 °C) and slightly soluble in organic solvents. Its functional groups (phosphate and carboxylic) are acidic in nature [
8]. Microorganisms present in the soil are mainly responsible for the degradation of glyphosate. Approximately 50% of the original molecule can be metabolized in 28 days, reaching 90% in 90 days. For this reason, metabolites or degradation products of pesticides have been identified and extensively studied. The first and main metabolite of glyphosate degradation in soil is aminomethylphosphonic acid (AMPA), which is formed by microbial action. AMPA has slightly greater toxicity than glyphosate [
9].
In glyphosate-based pesticide formulations, the toxic effects are increased by the presence of other ingredients. Mesnage et al. [
10] showed that some formulations can be up to a thousand times more toxic than the isolated principle, especially those that use the surfactant polyoxyethylene amine.
The residual limits of glyphosate are variable; for drinking water, Brazilian legislation states that the maximum permitted value (MPV) is 500 µg L
-1 and in Europe, this value is 0.1 µg L
-1, however, some other countries that have these limits in their potability criteria, it is concluded that Brazilian values are not that far from the others. For this criterion, the countries/continents that differ are Europe and Japan, the first being very restrictive and the second having a very high permitted value compared to the others [
11].
Carneiro
et al [
3] presented results on the absorption of glyphosate by chitosan membranes. Espinoza-Montero reviewed the literature on treatments for glyphosate-contaminated water. Lita
et al [
4] evaluated glyphosate removal by Biochar Based Coffee Husk Loaded Fe
3O
4. None of the studies cited used nanocellulose for this purpose. Nanocellulose is a promising new class of adsorbents and Morales Quintana
et al [
5] synthesized and characterized a new inexpensive resin from melamine and glyoxal for glyphosate removal. It is classified as a sustainable material, as it is of biological/natural origin, capable of regeneration, whose source is renewable, and the raw material is most often destined for disposal. Many of the new lines of research for this material are aimed at surface modifications, aiming to increase its adsorption efficiency [
12]. Nanocellulose is a lightweight material, with strong mechanical resistance, low production costs, and safe handling compared to synthetic nanoparticles. Many classes of substances can be adsorbed by nanocellulose, including organic products such as pesticides, dyes, and various effluents [
13].
Nanocellulose is a material still under development, but promising from an environmental and added value point of view. Kim et al [
14] evaluated the cytotoxicity of nanocellulose and the results indicated that these materials are promising for an environmentally friendly and relatively safe material. Therefore, this work proposed to develop a material with potential for environmental removal, to assist in the treatment of aquatic systems contaminated by glyphosate. The specific purpose of this work was to use cellulose from eucalyptus pulp, which presents characteristics for obtaining nanocellulose and its evaluation of glyphosate removal in aquatic environments.
2. Materials and Methods
All reagents used are of analytical grade and the glyphosate standard, as well as sodium molybdate and ninhydrin are from the Sigma-Aldrich brand. All solutions used were prepared using ultrapure water (18.18 MΩ cm-1).
2.1. Obtaining and Characterizing Nanocellulose and Organ Modification
Nanocellulose was obtained from a cellulose sample obtained by the kraft pulping method from eucalyptus wood, sieved, cooked, and bleached. The pulp passed through a defibrillator mill (Masuko Sangyo - Super Masscolloider) for 15 to 20 complete cycles. This procedure reduced the dimensions of cellulose to the nanometric scale.
Organomodified nanocellulose was obtained by adding tetraethylorthosilicate solution (TEOS 98% w/v) to the nanocellulose suspension (4 hours of stirring). After that, the solution was acidified with nitric acid (HNO3 0.1 mol L-1) (30 minutes of stirring). Finally, the 3-aminopropyltriethoxysilane (3-ATPS) solution was pipetted into the nanocellulose suspension (4 hours of stirring). At each step with the addition of reagents, the solution remained under moderate agitation at room temperature of 25 °C, 8 hours, and 30 minutes of reaction. The One Pot methodology was adopted to make this modification, adding amino groups to the original nanocellulose structure through sequenced reactions carried out by adding different reagents, using just one “pot” (glassware where the nanocellulose was from the beginning). This methodology was based on Goveia
et al [
15] and modified to the nanometric scale.
To characterize the materials, the films were analyzed by scanning and double-beam electron microscopy (JEOL high-resolution field emission electron microscope (SEM) model JSM-7500F), scanning microscopy with an energy dispersive detector (scanning microscope JEOL model JSM-6010) and Fourier Transform Infrared (FTIR) spectroscopy (Jasco FTIR Spectrophotometer – 41).
2.2. Kinetic Studies of Glyphosate Removal by Nanocellulose
Nanocellulose and modified nanocellulose were then evaluated for their adsorption capabilities by applying them to glyphosate solution. Different presentation forms of nanocellulose were tested in 0.1 L (100 mL) of 5 mg L-1 aqueous glyphosate solution, pH= 7.0, under light agitation (around 50 rpm) on a shaking table, at room temperature ( 25 °C) for a period of 24 hours. To standardize the amount of nanocellulose in each form of presentation tested at 50 mg, the density of the 3% solution was previously calculated, obtaining the value of 1.52 g mL-1, therefore for every 1.1 mL of the solution 3% nanocellulose we have 0.05g (50mg) of nanocellulose.
The presentation forms used with unmodified nanocellulose were: a) directly dissolved in the solution – 1.1 mL of 3% nanocellulose solution was pipetted directly into the glyphosate solution and then the final aliquot was centrifuged/ b) on a membrane to SERVAPOR MWCO dialysis, pore size 0.25 nm: 1.1 mL of 3% nanocellulose solution was pipetted into the membrane, which was closed with clips/ c) as a previously dried film, obtained by evaporation of water after placing approximately 50mg of nanocellulose and spread them in 25 mm diameter Petri dishes, placing them in an oven at 35 °C for approximately 5 hours, until completely dry / d ) in a glass column with 0.6 cm in diameter, the nanocellulose was packed to a height of 3.9 cm, where a 5 mg.L-1 glyphosate solution was passed through the column, with perfusion controlled at 0. 05 mL per minute, using a peristaltic pump for this. All these preliminary tests, with different ways of using nanocellulose to remove glyphosate in solution, were carried out in duplicate to check whether the results were reproducible. The concentrations of glyphosate were read in the solutions: initially and after 24 hours of reaction with nanocellulose, thus obtaining the values of glyphosate remaining in solution, of the unadsorbed glyphosate.
With modified nanocellulose, they were made by grafting onto column and film, in duplicate. Analyzing initial concentration and after 24 hours of complexation. The kinetic analysis was carried out with film, using a batch procedure with the removal of 8 mL aliquots at each pre-defined interaction time (0, 30, 60, 180, 360, 720, and 1440 minutes). The entire test took place under slow agitation (50 RPM on a shaking table) and a temperature of 25 °C.
The aliquots were quantified through the reaction of free glyphosate with ninhydrin and sodium molybdate at a temperature of 100 °C, in triplicate [
16]. The mean and standard deviation are calculated.
The values of glyphosate adsorbed per gram of nanocellulose and modified nanocellulose were calculated for each time at which the aliquots were removed (qt). The value of glyphosate adsorbed per gram of nanocelluloses at the equilibrium point (qe) for the two nanocellulose materials was also calculated. The 8 mL aliquots taken were considered, adjusting the volume of the solution (VSol) (Equations 1 and 2).
Equation 1 Calculation of the amounts of Glyphosate (mg) adsorbed per amount of nanocelluloses (g), made for each time that aliquots were removed.
Where:
[ ] initial: initial concentration of glyphosate in solution;
Vsol.: Volume of solution. Initially 0.1 L, but it changed with each aliquot removed;
[ ] tx: concentration of glyphosate in solution at each time x;
Adsorbent mass: masses in grams of nanocelluloses. For this work 0.05 g.
Equation 2 Calculation of the total glyphosate (mg) absorbed at the equilibrium point of the reaction.
On what:
[ ] te: concentration of glyphosate in solution at the time at which equilibrium occurred;
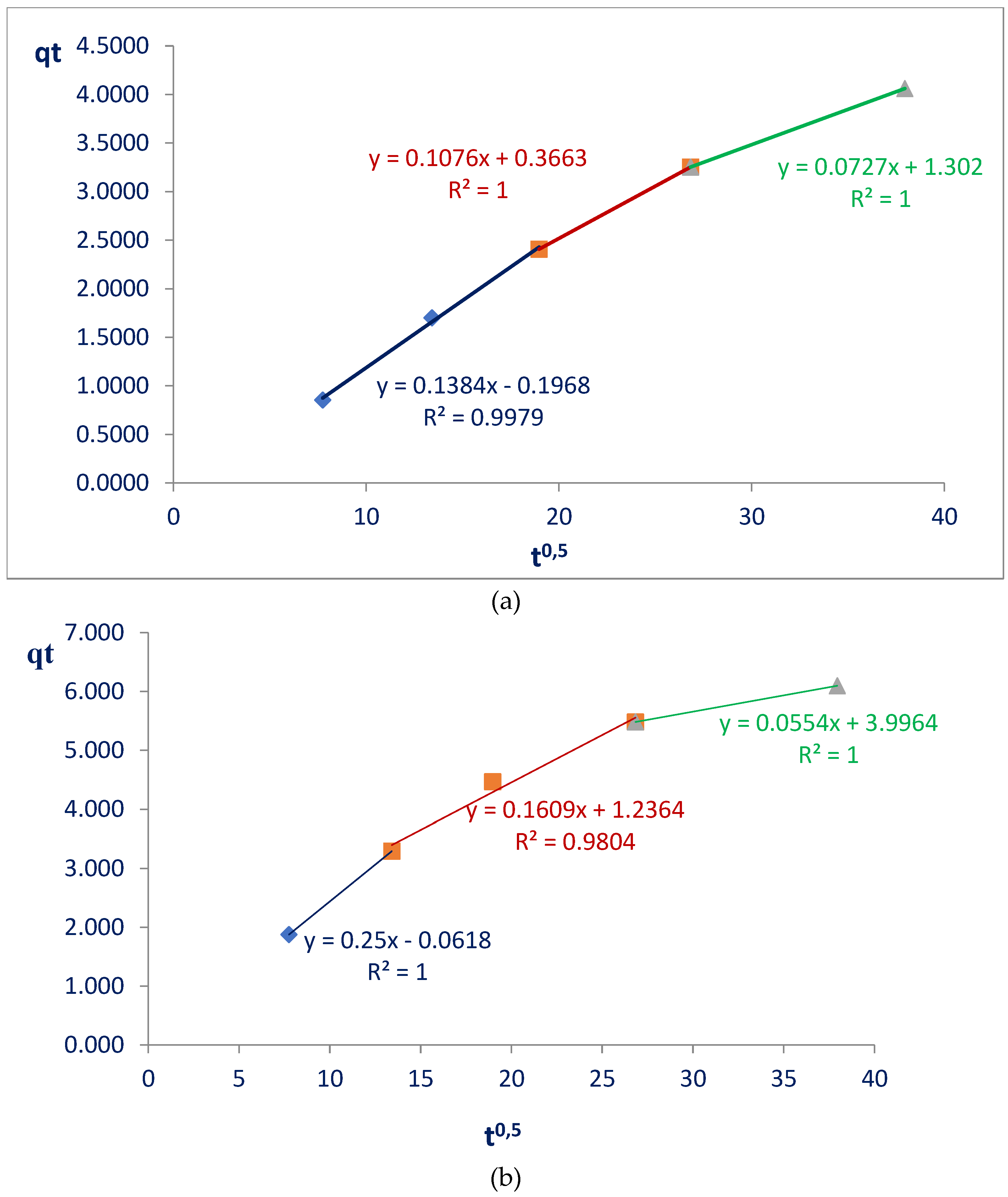
The pseudo-first and pseudo-second order models were plotted, as described in the literature: ln(qe-qt) versus time and (t/qt) versus time, respectively, and provided information about the types of bonds between nanocelluloses and glyphosate. The Weber and Morris model was obtained by the graph qt versus t0.5 and from this, it was possible to understand where the interactions occurred with greater prevalence: in the pores or on the surface of the nanocelluloses.
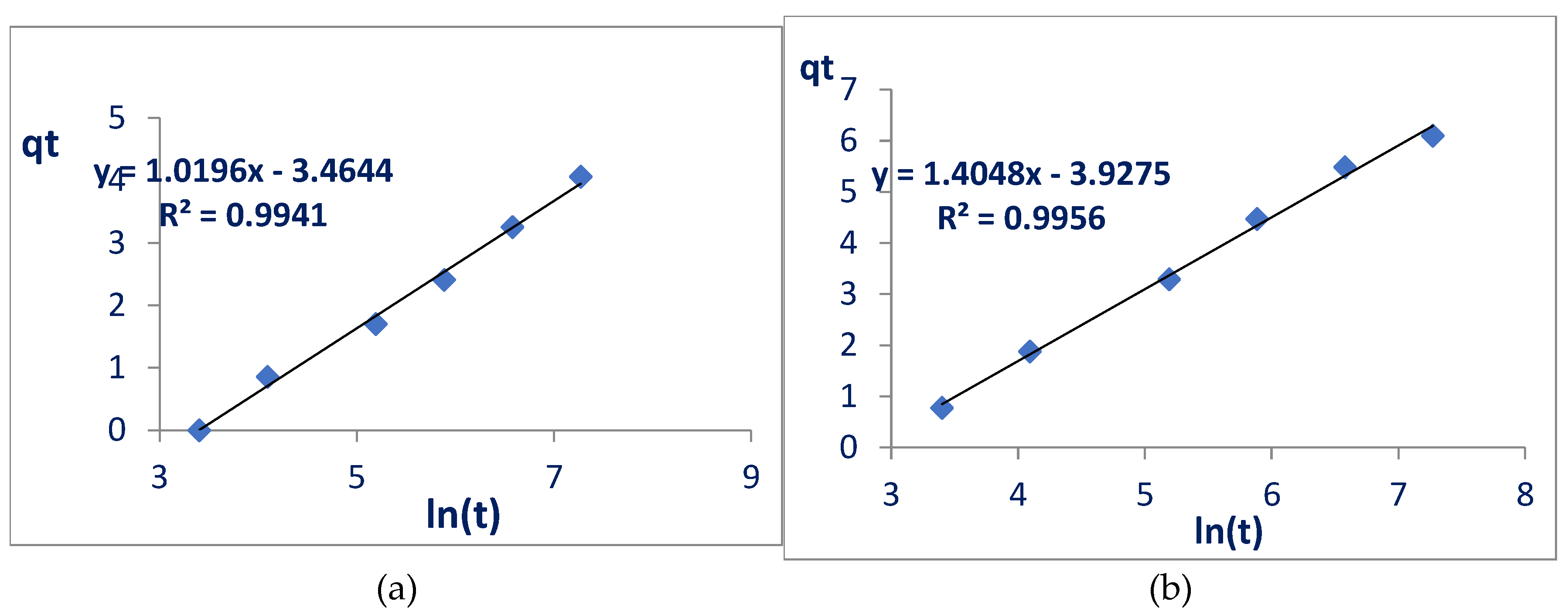
The last model calculated was that of Elovich, obtained by the graph formed with the values of qt versus ln(t), with this model it was possible to calculate the adsorption rates of glyphosate on nanocelluloses (the speed at which interactions occur) and the constants of desorption (value of glyphosate that can be desorbed, in mg of glyphosate per g of adsorbent).
The quantification of glyphosate consisted of the reaction with ninhydrin and subsequent determination in a spectrophotometer (Hach brand, model DR3900) at 570 nm. The calibration curve varied from 0.25 to 10.0 mg L-1. Ninhydrin (reagent responsible for the developed color) and sodium molybdate (catalyst) were added to the glyphosate standards and heated, standardizing the reaction time to 30 minutes. The purplish color was proportional to the concentration of Glyphosate present in solution (Bhaskara and Nagaraja 2006).
To verify the sensitivity of the method, its precision and repeatability, the values of the limits of detection (LD) and quantification (LQ) and the relative standard deviation (%DPR) were calculated. Experiments with the blank were carried out for 48h to evaluate the stability of glyphosate in solution.
3. Results and Discussion
The characterization of nanocellulose is an important factor in understanding the interaction mechanisms that can occur with contaminants, specifically when referring to the proposal of a methodology aimed at environmental remediation.
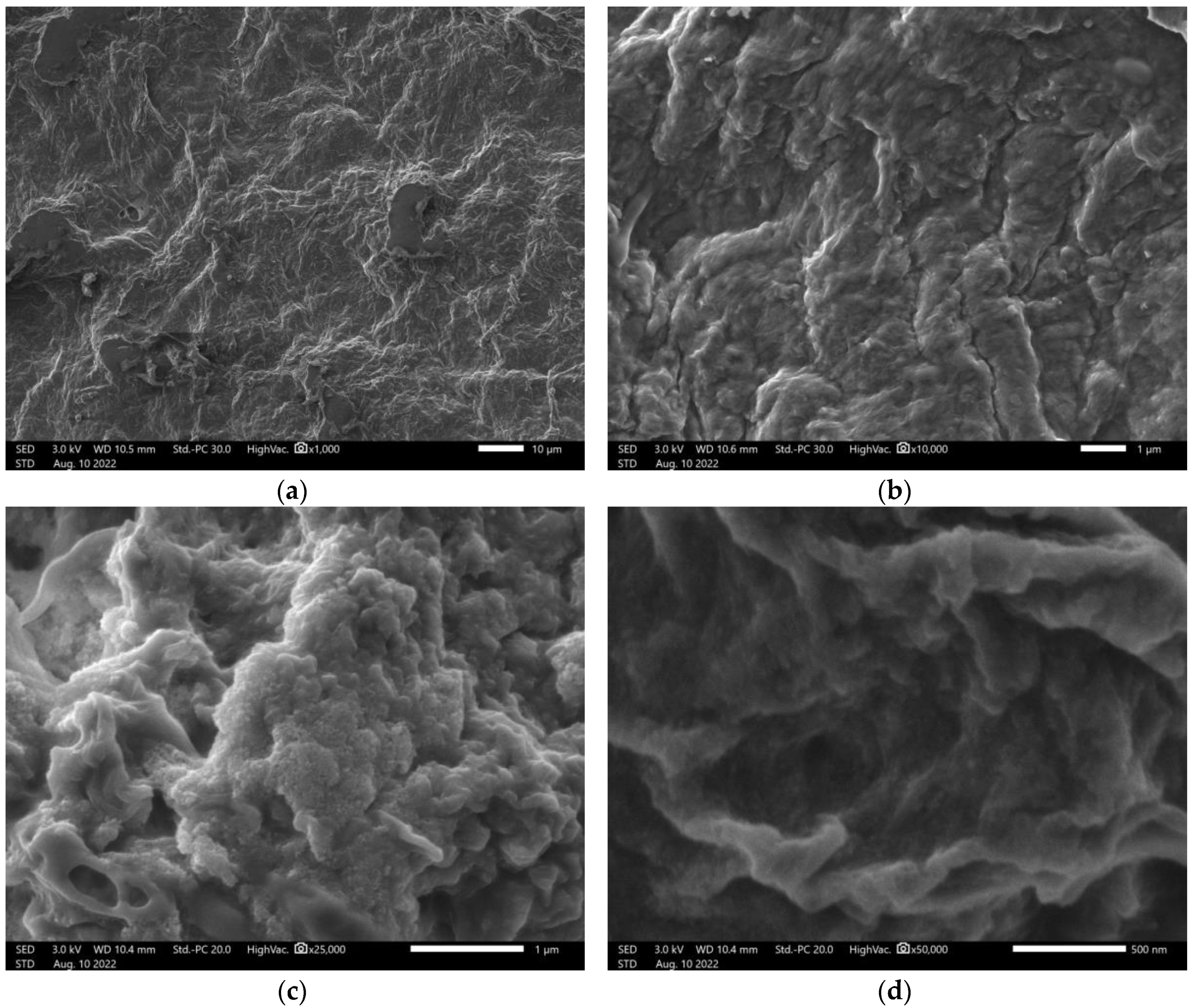
From the scanning electron microscopy images obtained for nanocellulose, the presence of fibrils was observed, common in the mechanical obtaining process (
Figure 1a). After organomodification, the structure changes, with microfibrils no longer visible, but the incorporation of the organosilicate material (
Figure 1b).
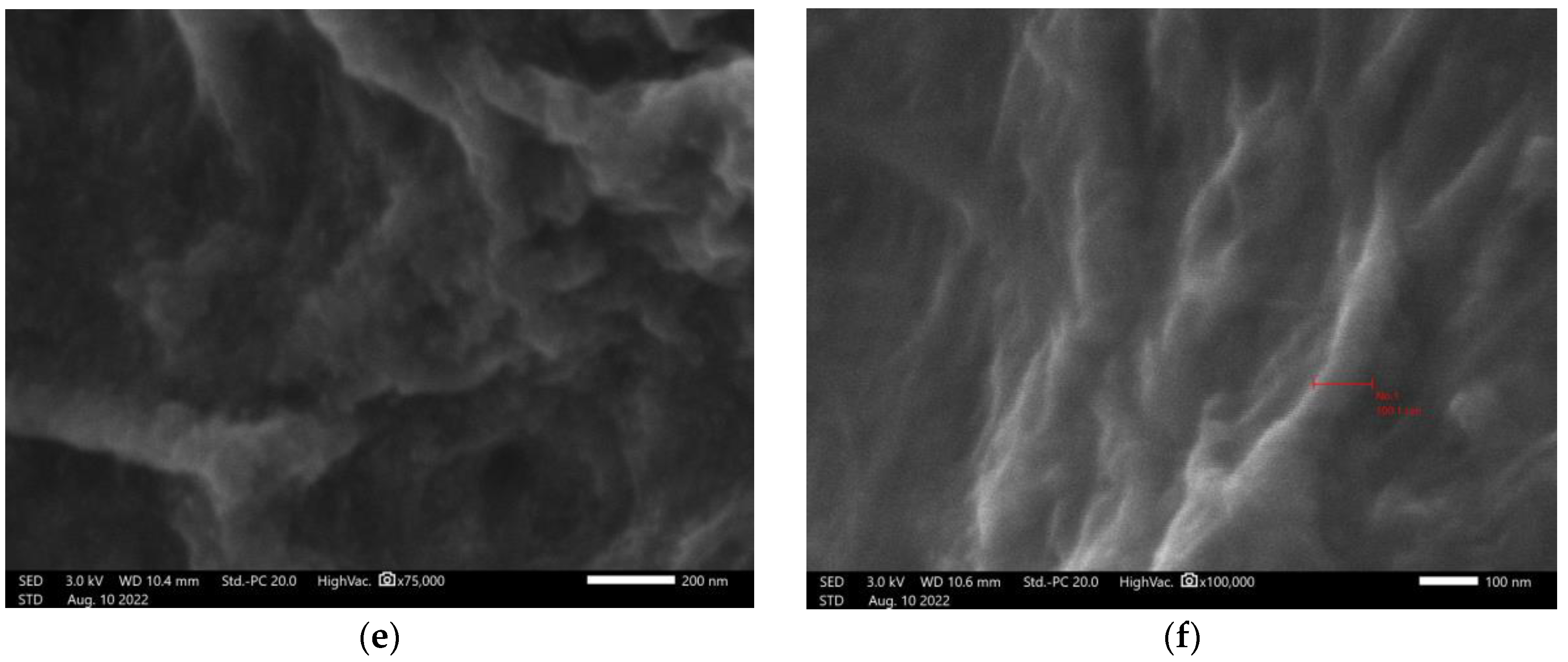
Organomodified nanocellullose was evaluated at 1,000- to 100,000-fold magnification (
Figure 2). The image magnified 100,000 times indicates that one of the measurements of the fibrils has a diameter smaller than 100 nm, indicating that after organomodification the nanocellulose presents nanoscale parameters. A special arrangement of greater roughness and apparent relief is observed.
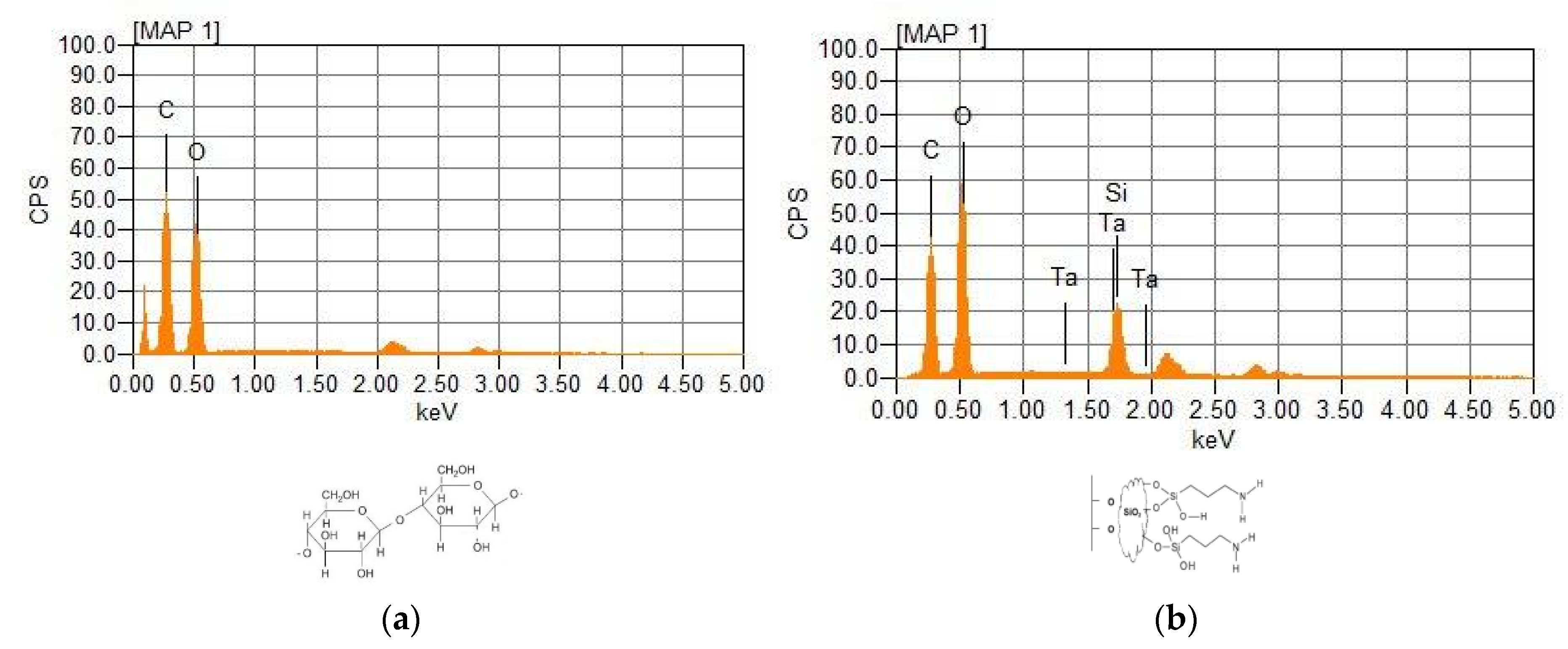
By Energy Dispersive Spectroscopy (EDS) it can be observed that there was the formation of a material where the nanocellulose was coated by SiO2 groups present in the tetraorthosilicate (
Figure 3). Nitric acid promoted hydrolysis with the formation of silanol groups (Si-OH) and the Si-OH bond being more reactive than C-OH, so silicon became a strong nucleophile where substitution occurred. The nanocellulose then reacted with 3-aminopropyltriethoxysilane, incorporating the amino group into the original nanocellulose structure [
15].
The main elements identified in nanocellulose were carbon (approximately 45%) and oxygen (approximately 40%). In modified nanocellulose, it was also possible to observe the presence of silicon and a value of 53% for the percentage of oxygen, which is higher than that of nanocellulose (around 40%). This is due to the organomodification of nanocellulose, which includes 3-aminopropyltriethoxysilane. to the original molecule, increasing the oxygen content, in addition to silicon and nitrogen becoming part of the new molecule. The presence of nitrogen is difficult to observe by EDS, due to its low atomic mass, but it was confirmed by spectroscopy in the Fourier transform infrared (FTIR) region (
Figure 4).
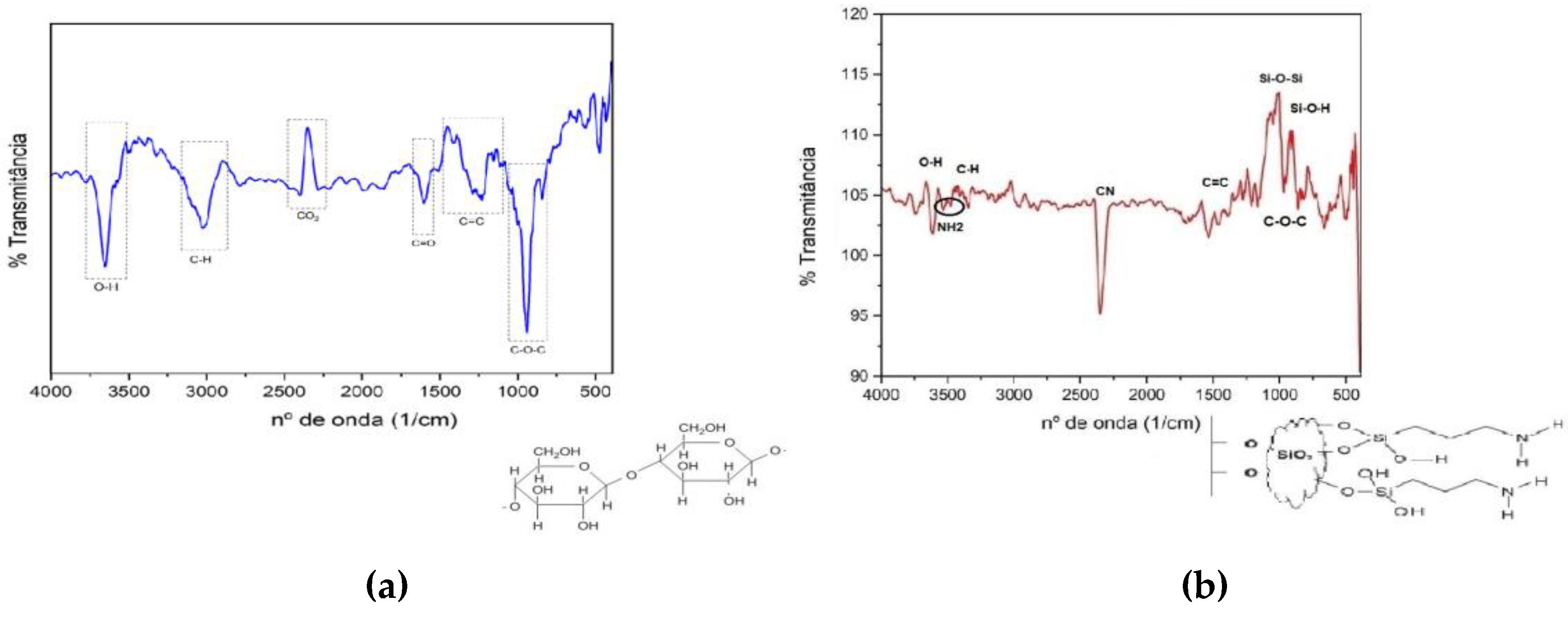
From the spectra in the infrared region (
Figure 4) it is possible to observe the difference in the functional groups present in nanocellulose (
Figure 4a) and in modified nanocellulose (
Figure 4b), highlighting the presence of amino groups. In the infrared spectrum for nanocellulose, in addition to the expected groups, it was possible to verify some groups that can be attributed to the residual presence of lignin from eucalyptus pulp, the raw material for nanocellulose. They are C=C between 1440 and 1100 cm
-1 and C=O at 1600 cm
-1. Furthermore, a very characteristic band at 2340-2400 cm
-1 indicates O=C=O, and it is most likely that atmospheric carbon dioxide was adsorbed to the nanocellulose film. The other groups identified prove the expected bonds in the nanocellulose structure: O-H between 3220, and 3500 cm
-1, C-H at 2900 cm
-1, and C-O-C close to 1000 cm
-1 [
17,
18,
19].
In the spectrum for the modified nanocellulose, in addition to the original nanocellulose bands, proof of the success of the reaction can be seen with the band at 964 cm
-1 referring to the Si-O-H bond and between 1020 – 1090 cm
-1 is attributed to the Si-O-Si bond which is responsible for the coupling of 3-aminopropyltriethoxysilane to the O- groups of nanocellulose. Double stretching between 3300 and 3500 cm
-1 is also observed, proving the incorporation of the NH
2 group into nanocellulose. The accentuated stretching downwards close to 2250 cm
-1 is characteristic of the triple bond between Carbon and Nitrogen, one justification being the possible dehydration of the modified nanocellulose film [
20].
The UV-VIS spectrophotometric determination method for glyphosate is an alternative that is easy to handle, quick, low cost, sensitive, and reproducible. The calibration curve presented a linear coefficient of determination of 0.9908 and the following straight line equation: C = -2.003 + 15.505A, where C is the glyphosate concentration, -2.003 is the linear coefficient, 15.505 is the angular coefficient and A is the absorbance read at 570 nm. The Limit of Detection (LD) and the Limit of Quantification (LQ) were: 0.004 and 0.013 mg L
-1 respectively. The value of the Relative Standard Deviation (DPR) was: 3.41%, a result acceptable by ANVISA, which is a DPR of up to 5% [
21]. Bhaskara and Nagaraja [
22] presented the validation of the method obtaining: Limit of Detection (LD): 0.04 mg L
-1, Limit of Quantification (LQ): 0.11 mg L
-1 and Relative Standard Deviation for the average of seven determinations of a standard (DPR): 1.74%.
For kinetic studies, adsorption analyses were carried out on dialysis membranes by immersion in a glyphosate solution for 24 hours, with no recovery of glyphosate. Then a new test, for the same period, with the nanocellulose in direct contact with glyphosate in aqueous solution, the results indicated that 9% of glyphosate was removed. We continued with new ways to verify the ability of nanocellulose to adsorb glyphosate: in a packed column, perfusing glyphosate solution (with the aid of a peristaltic pump), and in the form of a dry film, the percentages of glyphosate removal were maintained. were practically the same as the test with nanocellulose directly dispersed in solution: 8.9 and 9%, respectively.
Nanocellulose showed greater glyphosate removal capacity when dispersed in solution and in the form of a dry film, with practically equal values. From the results obtained, it can be seen that the dialysis membrane interfered with the interaction between nanocellulose and glyphosate, making it impossible to remove glyphosate from the solution. It is believed that the membrane (SERVAPOR MWCO, pore size 0.25 nm) was not capable of promoting the diffusion of glyphosate so that it could interact with the nanocellulose contained in the membrane, due to the size of its pores.
The modified nanocellulose proved to be better for removing glyphosate when compared to the unmodified one, showing 26.9% removal when placed in a glass column and 27.9% when used in the form of a dry film.
The two tested forms of modified nanocellulose had practically the same performance (in column or dry film) regarding glyphosate adsorption, however the dry film test was slightly better. For environmental applications, it is interesting to use it in films, which is why adsorption studies were carried out on films.
The adsorption values obtained in this work were efficient when compared to other results in the literature (
Table 1).
The results of the kinetic studies (
Table 2) indicated correlation coefficient values (R²) greater than 0.9, and this indicates that the two linear models fit the data obtained.
These results indicate that physical and chemical interactions occur with the two materials, with pseudo-second-order chemical interactions prevailing, indicated by the highest correlation coefficient (
Table 3).
The pseudo-first-order model considers that interactions between species occur through the occupation of unoccupied sites on the adsorbent, that is, the interactions are weaker because they are physical-chemical type bonds (physiosorption interactions). In the pseudo-second-order model, adsorption is proportional to the square of the number of active sites on the surface and the bonds are chemical, that is, more effective involving the exchange or donation of electrons between the adsorbate and adsorbent. In this type of adsorption, exchanges occur at specific binding sites and initially form a single layer. However, the formation of other layers may occur through physisorption. The results obtained indicate that this is what happens with both nanocelluloses.
The chemical and structural modification promoted in the organomodification of nanocellulose enhanced the chemisorption processes, as it increased the pseudo-second-order R2, from 0.99 to 1. As for the physisorption interactions, it is assumed that they also occurred with more intensity, as the adjustment of the straight line (R2 of the pseudo-second-order model) increased from 0.96 to 0.99.
The observations obtained by the models are consistent with what is observed in the EDS images (
Figure 3): a much greater complexity in the structural arrangement of the modified nanocellulose when compared to the unmodified nanocellulose. It is believed that this complexity increases the active sites for physicochemical interactions promoted by “empty” spaces and induced and/or temporary charges. Furthermore, the modified nanocellulose now has another functional group, the amino, capable of chemical interaction with glyphosate.
The Weber and Morris model (
Figure 5) to verify how intraparticle diffusion occurs and the Elovich model to understand the initial adsorption rate on the surface of nanocellulose and modified nanocellulose and also how much glyphosate is subject to desorption indicated more details about the adsorption process.
In the Weber and Morris models, when observing the straight lines formed by the first points (initial stages of adsorption, referring to the blue lines), a linear coefficient equal to zero would indicate that intrapore diffusion would be controlling the adsorption process. The results obtained are different from zero: 0.2 and 0.06 for nanocellulose and modified nanocellulose respectively, therefore intrafilm diffusions are occurring (
Figure 5). This was already expected since nanocelluloses have a film format made up of nanofibrils in the shape of threads.
By applying this model it is also possible to determine the rate-limiting steps of the entire adsorption process [
23]. The results for the two nanocelluloses indicate three stages, with different interaction speeds, defined by the angular coefficients which are, in this model, the intraparticle diffusion coefficients (Kd) of each straight line. Following the straight lines (1st: blue, 2nd: red, and 3rd: green) it is possible to verify, in both materials, a decrease in the speed of interactions between nanocelluloses and glyphosate (
Figure 5).
Nanocellulose: Kd1 = 0.14 > Kd2 = 0.11 > Kd3 = 0.07.
Modified Nanocellulose: Kd1 = 0.25 > Kd2 = 0.16 > Kd3 = 0.05.
Diffusion speeds decrease in both cases studied. The drop in velocities from the first to the second stage is also more pronounced for the modified nanocellulose, which may indicate large adsorption on the external surface.
For each of the stages with very different speeds, we have (
Figure 5):
1. BLUE STRAIGHTS: diffusion of glyphosate on the external surface of nanocelluloses;
2. RED STRAIGHTS: glyphosate diffuses through the voids and towards the internal surface;
3. GREEN STRAIGHTS: the last stage of adsorption. It approaches equilibrium, where adsorption and desorption occur simultaneously and in a balanced way [
23].
Another information that the model provides us is that the greater the linear coefficient of each straight segment, the greater the thickness of the film. The values increase in each straight segment, that is, the thickness of the film would become greater over time and this is expected since diffusions are occurring and glyphosate is accumulating on the surface of the film. This increase in film thickness is also more significant in modified nanocellulose, varying from 0.06 to 3.99. With nanocellulose this variation went from 0.2 to 1.3. These values show that glyphosate diffuses more intensely on the surface of the modified nanocellulose and causes a large thickening of the film.
Equation 3. Representation of the straight line equation obtained by the Weber and Morris model.
Where:
qt: amount of adsorbed glyphosate (mg) per amount of nanocellulose (g) at each time;
Kd: intraparticle diffusion coefficient;
C: constant that relates to resistance to diffusion (its value gives an idea of the thickness of the boundary layer of the film formed).
From the straight line equation in the graphs of the Elovich model (
Figure 6) it is possible to calculate the initial adsorption rate constants (α) and the desorption constant (β). α refers to the adsorption rate and is expressed in mg.g
-1.min
-1, whereas β is the desorption constant and indicates the amount of glyphosate that is likely to undergo desorption (mg.g
-1). The values of α and β (
Table 3) calculated from the Elovich linearization line, given by equation 4, indicated values consistent with the literature.
Where:
qt: amount of adsorbed glyphosate (mg) per amount of nanocellulose (g) at each time;
α: initial adsorption rate (mg g-1min-1);
β: desorption constant (mg g-1);
t: time.
The calculated values for α and β once again indicate a better performance of the modified nanocellulose when compared to the unmodified nanocellulose, as the adsorption rate triples (from 0.03 mg g-1 min-1 to 0.09 mg g-1 min-1) and the desorption constant decreases (from 0.98 mg g-1 to 0.71 mg g-1). When compared with works available in the literature, it is observed that the initial adsorption rates for the two nanocelluloses are much better, adsorption occurs in the range of 10x faster, and the desorption constants are higher than for nickel and aluminum nitrate, but much smaller than alkaline fiber. Considering the two materials in this work, Elovich's models demonstrate that the adsorption of modified nanocellulose is faster and more stable.