1. Introduction
Diabetes Mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia and complications caused by impairments in any or all of the production, release, and effects of insulin, a peptide hormone secreted by the β cells of the islets of Langerhans of the pancreas [
1]. It has been reported that 90% of diabetes cases in the world are Type 2 DM cases [
2]. According to the Diabetes Atlas published periodically by the International Diabetes Federation, the number of people diagnosed with diabetes globally by the end of 2021 was recorded as 537 million. . Even more remarkably, this number is estimated to reach 783 million by 2045 [
3]. Chronic complications of type 2 DM are categorized under two main headings as micro-macro vascular complications: nephropathy, retinopathy, and neuropathy are defined as microvascular complications, while coronary artery disease (CAD), peripheral arterial disease (PAD) and cerebrovascular diseases (CVD) are defined as macrovascular complications [
4,
5]. Chronic hyperglycemia is a critical factor in the formation of these complications. Duration of diabetes and comorbidities are other factors affecting the development of complications. Diabetic retinopathy (DR) is the most common microvascular complication and is a precursor of other microvascular complications. One out of every three patients with type 2 DM develops DR. It is also the most common cause of preventable new cases of blindness in people aged 20-74 years [
6].
Fundus examination is critical to detect the first signs of DR. Initial signs include damage to the endothelium of the retinal vasculature, microaneurysms, hemorrhage, and exudate development. With DR progression, capillary occlusion, neovascularization, and subsequent retinal detachment may develop. Retinal lesions are detected with an ophthalmoscope. Retinal lesions are divided into two types according to their intensity: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Fundus fluorescein angiography (FFA) and optical coherence tomography (OCT) are used for diagnosis and follow-up. These are costly, invasive, and specialized methods. At the same time, each of the methods used to monitor the development of other micro-macro complications in patients with Type 2 DM increases the financial burden. Therefore, it is important to have additional biomarkers for the diagnosis and follow-up of both DR and other complications in Type 2 DM patients with DR in many peripheral regions.
Hyperglycemia-induced glycosylated proteins damage cells, causing dysfunction and initiating the inflammatory process by inducing the release of Tumor Necrosis Factor-alpha (TNF-α), free radicals, Interleukin-6 (IL-6), C-reactive protein (CRP). This is recognized as one of the main mechanisms of vascular damage, which is the cause of chronic complications of diabetes [
2].
During the inflammatory response, platelets mediate the activation of circulating leukocytes and their adhesion to the endothelial surface, leading to both changes in the number of circulating leukocytes and endothelial damage [
7].
Determination of SII cut-off values in patients with DR may be a warning for the prevention of other complications and mortality.
Systemic immune inflammation (SII) derived from laboratory parameters has recently come to the forefront in determining the prognosis of many diseases. SII, which can be calculated simply by using platelet, lymphocyte, and neutrophil values from complete blood count (CBC) values, was first developed in 2014 to predict survival rates of individuals with hepatocellular carcinoma [
8,
9].
There is increasing evidence that there may be an association between SII and metabolic derangements and their components [
10]. Moreover, considering that macro- and microvascular complications of Type 2 DM have chronic inflammation and metabolic derangements as common risk factors, it is a valid assumption that people with high SII levels have a higher risk of these complications.
Numerous studies have examined the relationship between type 2 DM, its complications, and SII. However, no study has addressed the association of SII with duration in predicting macrovascular and other microvascular complications and mortality rates in type 2 diabetic retinopathy patients.
This study was conducted to fill the existing literature gap and examine the relationship between SII levels, complications, and mortality rates in type 2 diabetic retinopathy patients during the first year of follow-up and the subsequent three years after the index was calculated.
2. Materials and Methods
This study was designed as a single-center and retrospective study. Between January 2019 and December 2019, 523 patients over 18 years old who were admitted to the endocrinology outpatient clinic of our hospital with a DR diagnosis of Type 2 DM were included in the study. Patients diagnosed with type 1 DM, patients with acute infectious disease, sepsis, chronic inflammatory and rheumatologic diseases, active malignancies, other endocrinologic disorders, patients under 18 years of age, pregnant women, and breastfeeding women were excluded. For this study, TÜTF-GOBAEK 2023/341 approval was received from the ethics committee of our faculty of medicine, dated 29/09/2023. DR progression was defined as increased macular edema, decreased visual acuity, and conversion of NPDR to PDR.
Demographic information, laboratory data at the time of admission, medical history, and examination findings of the patients included in the study were obtained from the patient file records of the hospital. Demographic information of the patients was determined as age, gender, alcohol, and smoking. The diagnosis of DR was confirmed according to Euretina guidelines, and Type 2 DM was confirmed according to the American Diabetes Association 2019 criteria [
11,
12]. The medical history of the patients included duration of DM, diagnosis and duration of hypertension, diagnosis of heart failure, diagnosis of cerebrovascular disease, insulin use, anti-triglycemic and anti-hyperlipidemic drug use. Laboratory measurements in the study were performed by an automated analyzer (Roche Diagnostic Modular Systems, Tokyo, Japan). The SII value was obtained using (platelet count at admission x neutrophil count/lymphocyte count ratio) [
13,
14]. Macro-micro complications and clinical findings of the patients during follow-up were obtained through outpatient clinic records, phone calls, and an e-pulse system [
15].
Descriptive statistics regarding demographic and clinical characteristics of patients were shown as shown as (mean, standard deviation, number, %). The suitability of quantitative data for normal distribution was examined with the Shapiro-Wilk test. The Mann-Whitney U test was used to compare SII values between groups. The Mc Nemar Chi-square test was used in comparison of complication rates between first and the following three years. The Related-Samples Cochran's Q test was used in comparison of complication rates between baseline, first and the following three years. Using the Receiver Operating Characteristic (ROC) analysis method, area under the curve (AUC), cut-off points, sensitivity and specificity values were calculated for SII values. P<0.05 value was accepted as statistical significance. SPSS 20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) package program was used to analyze the data.
3. Results
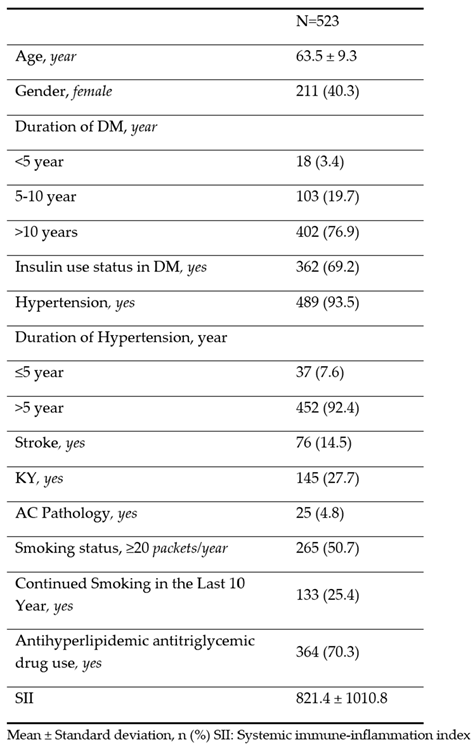
The demographic and clinical characteristics of the patients are shown in
Table 1. The average age of the patients was 63.5 ± 9.3 years and 40.3% were women. The average SII values were found to be 821.4 ± 1010.8.
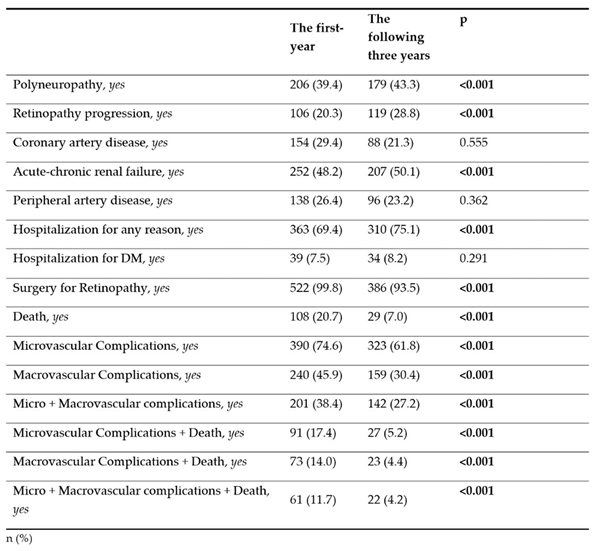
The rate of polyneuropathy was 39.4% in the first year and 43.3% in the following three years. Retinopathy progression was detected as 20.3% in the first year and 28.8% in the following three years. While the rate of microvascular complications was 74.6% in the first year, this rate decreased to 61.8% in the the following three years (p<0.001). While the rate of macrovascu lar complications was 45.9% in the first year, this rate decreased to 30.4% in the the following three years (p<0.001). Similarly, a significant decrease was observed in the rates of micro + macrovascular complications, microvascular complications + death, macrovascular complications + death, micro + macrovascular complications + death combinations in the the following three years (p < 0.001) (
Table 2).
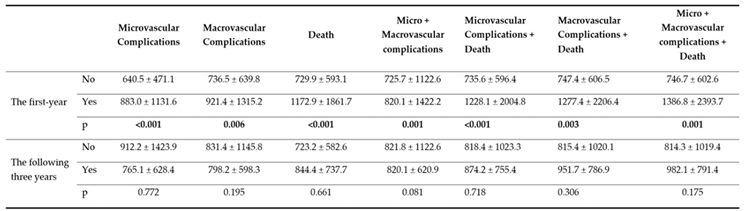
Comparison of patients' SII values in the first and the following three years according to the presence of micro-macro complications, hospitalization for any reason, hospitalization for DM parameters is shown in Table 3. Accordingly, the SII values of those with Acute-chronic renal failure, Peripheral Artery Disease and Hospitalization were found to be significantly higher both in the first year and in the the following three years (p<0.05 for all). No significant difference was found in SII values in terms of Polyneuropathy, CAD, Retinopathy Progression, Hospitalization Due to DM (p>0.05 for all).
Table 4.
Comparison of SII values according to microvascular, macrovascular complications and death.
Table 4.
Comparison of SII values according to microvascular, macrovascular complications and death.
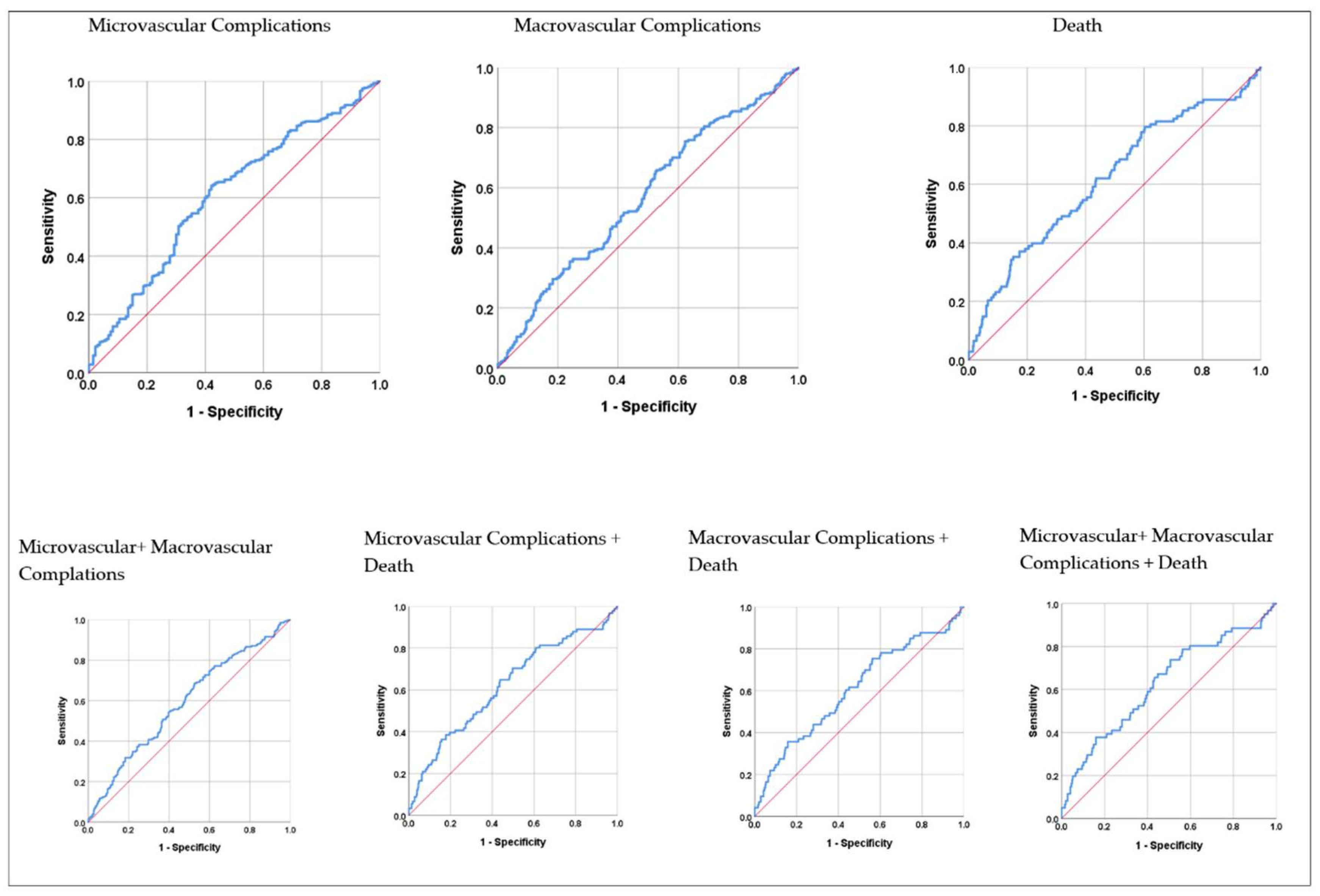
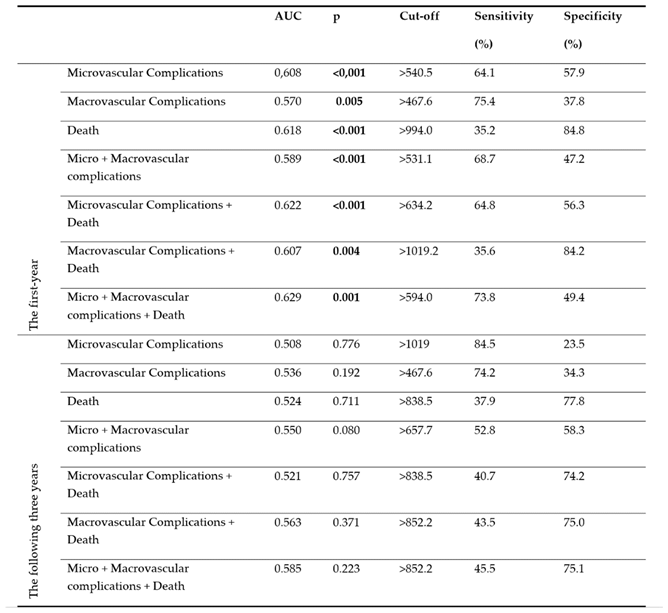
Results of ROC analysis of SII values regarding micro-macrovascular complications and death are shown in
Table 5. Accordingly, the area under the curve (AUC) values in the first year were 0.608 (p<0.001) for microvascular complications, 0.570 (p=0.005) for macrovascular complications, 0.618 (p<0.001) for death, 0.589 (p<0.001), for micro + macrovascular complications and 0.629 (p=0.001) for micro + macrovascular complications + death. The area under the curve values in the the following three years were not significant (p>0.05 for all).
Results of ROC analysis of SII values regarding micro-macrovascular complications and death in the first year are shown graphically in
Figure 1. It was found that SII values reached the highest diagnostic accuracy rate in the first year in the Micro + Macrovascular complications + Death combination, and at the cut-off point calculated as >594.0, the sensitivity value was 73.8%, and the specificity value was 49.4%.
4. Discussion
This study investigated the relationship between the predictive value of SII on other microvascular complications of Type 2 DM (nephropathy, neuropathy), macrovascular complications (coronary artery disease, peripheral artery disease, cerebrovascular disease), and mortality rate in patients diagnosed with DR, which is one of the most common microvascular complications of Type 2 DM, with early and late period (the first year and the following three year). The findings of our study showed that high SII values were associated with increased mortality and development of other microvascular and macrovascular complications and were statistically significant in predicting the likelihood of mortality and development of complications, especially in the first year following the calculation of the SII value.
Type 2 DM is a chronic disease characterized by persistent hyperglycemia. Due to hyperglycemia, glucose reacts non-enzymatically with lipids and proteins, releasing advanced glycation end products (AGEs) and causing oxidative stress. The resulting oxidative stress increases the levels of reactive oxygen species (ROS), leading to impaired blood flow in small blood vessels and impaired tissue nutrition, leading to microvascular complications. In large blood vessels, it facilitates the development of atherosclerotic plaques, which narrow the arteries and restrict blood flow, leading to macrovascular complications [
16,
17]. Sustained hyperglycemia triggers the immune response, leading to the synthesis of pro-inflammatory cytokines C-reactive protein, tumor necrosis factor (TNF)-α and inter-leukin (IL)-6 and chemokines. Chronic inflammation disrupts the structure of small vessels and contributes to the development of microvascular complications. In large vessels, they enter atherosclerotic plaques and destabilize them. They cause plaque ruptures [
18]. Endothelial dysfunction develops. Another condition that contributes to endothelial dysfunction is adipokines (adiponectin, leptin, and resistin). Adiponectin is anti-inflammatory, whereas leptin and resistin are inflammatory. Adiponectins increase nitric oxide (NO) levels. However, in type 2 DM, adiponectin is decreased, which leads to a decrease in NO level and an increase in endothelin-1 level, which disrupts the balance in small vessels in favor of vasoconstriction and leads to the development of microvascular complications. In large vessels, endothelial dysfunction plays an important role in the emergence of macrovascular complications by facilitating vasoconstriction, inflammation, and oxidative stress [
19].
DR is a microvascular complication of diabetes in the eye with an increasing prevalence. Once it occurs, it is irreversible. It can progress to vision loss. Current treatments are aimed at halting its progression. Chronic minimal inflammation plays a role in its pathogenesis. An animal model study in diabetic mice showed that the progression of DR was slowed when inflammatory pathways were blocked [
20]. Another study examined the levels of inflammatory proteins in patients with DR using proteomic techniques and found an increase in these levels [
21]. These studies demonstrate the role of chronic minimal inflammation in the development and progression of DR and suggest that inhibition of inflammation may prevent the progression of the disease.
Many inflammatory cells are involved in developing and maintaining chronic minimal inflammation. The most prominent feature of this inflammatory process is the increased permeability of the vascular wall secondary to inflammation, allowing inflammatory cells to pass from the vessels to the tissues. Neutrophils are the first cells to arrive when inflammation occurs. Under chronic inflammation, neutrophils are constantly activated, leading to the formation and accumulation of neutrophil extracellular traps (NETs). This leads to vascular occlusion, tissue damage, and exacerbation of inflammation, increasing damage at the site of inflammation. In a study comparing the neutrophil levels of patients with type 2 DM and healthy individuals, it was found that the neutrophil levels of the patient group were significantly higher [
22]. In another study, it was concluded that NET levels were elevated in Type 2 DM patients and that NETs play a role in the pathogenesis of both DM and microvascular complications, especially diabetic nephropathy and cardiac disease associated with DM [
23]. One of the other cells involved in chronic minimal inflammation is monocyte-macrophages, which maintain and exacerbate the inflammatory process by secreting pro-inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-8, MCP-1, IL-1) [
24]. Another cell is platelets, which both form thrombosis with their own activation and increase their activation by binding to leukocyte subcellular groups [
25]. An increase in neutrophil, monocyte-macrophage, and platelet cell numbers indicates inflammation. These results are compatible with our study because neutrophil, monocyte, and platelet values were found to be high in patients with DR in our study (SII: 821.4 ± 1010.8;
Table 1).
SII, one of the most frequently used inflammatory markers in recent years, is less affected by physiopathologic changes. This makes SII more reliable and more sensitive in showing existing inflammation [
26]. Other advantages are that it is cost-effective and can be calculated with hemogram results that can be performed even in peripheral areas. In a study of 500 Type 2 DM patients with and without DR, SII was found to be an inflammatory marker that can be used for early diagnosis of DR [
27]. In our study, our patients had a diagnosis of DR, but we found that SII had no predictive value in DR progression in the first and the following three years of follow-up (Retinopathy progression P > 0.05; Table 3). This may be because the SII values of all patients already diagnosed with DR were higher than the SII of only patients with Type 2 DM due to inflammation. In a study in which 100 uncomplicated Type 2 DM patients were included, and many biomarkers were examined, the mean SII value was found to be 470.91 [
28]. In our study, all of our patients were Type 2 DM patients with DR diagnosis, and the mean value for SII was found to be 821.4. Because of the existing microvascular complications, the mean SII value in our study was almost twice as high compared to uncomplicated Type 2 DM in the other study. For this reason, chronic minimal inflammation may have continued in the following years but may not have reached very high levels with progression. Another reason may be that 108 patients died within one year after the calculated SII values, and 29 patients died within the following three years (
Table 2). Because we do not know how many of these deceased patients had DR progression. In a study of 300 Type 2 DM patients on whether SII has a predictive value for diabetic nephropathy and cardiovascular diseases in patients with Type 2 DM, predictive SII values were found for cardiovascular diseases and diabetic nephropathy [
28]. In a study investigating the relationship between SII and diabetic neuropathy in 1460 hospitalized type 2 DM patients in China, it was found that SII levels were significantly higher in patients with diabetic neuropathy [
29]. In another study in which 584 patients with diabetic nephropathy (DN) due to Type 2 DM, Type 2 DM patients without DN, and a control group were investigated for the relationship between SII and DN, it was found that high SII levels were associated with DN [
30]. The results of our study are consistent with the literature. SII was found to be statistically significant in predicting type 2 diabetic retinopathy patients to be diagnosed with acute or chronic renal failure due to diabetic nephropathy in the first year and in the following three years, development of peripheral arterial disease, and hospitalization of patients for any reason (Table 3). It was not statistically significant in predicting coronary artery disease, hospitalization due to diabetes, and development of polyneuropathy both in the first year and in the following three years (Table 3). This may be due to the fact that the basal inflammation was high in those who died, chronic minimal inflammation was ongoing due to DR, and therefore, the patient's immune systems were active, or other microvascular complications may have started due to the development of DR but were not detected because they were not yet diagnosed. In addition, it has been observed that type 2 diabetic retinopathy patients are more likely to be hospitalized due to complications. Because patients who develop micro and macrovascular complications are mostly patients with the poor glycemic index, who do not pay attention to their diet and do not follow the controls. It is natural that they are frequently hospitalized due to complications that develop as a result. Another reason may be the need for a larger sample group, which is also one of the limitations of our study.
The focus of our study was to examine the relationship between the SII values of patients with type 2 diabetic retinopathy and the development of micro-macro vascular complications and death in the first year and the following three years. When the SII values of the patients were analyzed by ROC analysis for microvascular complications, macrovascular complications, death, micro+macrovascular complications, microvascular complications+death, macrovascular complications+death, micro+macrovascular complications+death, and micro+macrovascular complications+death (composite endpoint) for the first year and the the following three years, a statistically significant optimal SII value was confirmed for all parameters in the first year (P<0.05;
Table 5). For the the following three years, the SII values determined were not statistically significant (P>0.05;
Table 5). This may be due to the relationship between the existing inflammation and duration. In general, our SII values were found to be high because Type 2 DM, DR, and its complications are based on chronic minimal inflammation, and SII is an inflammatory marker. This is consistent with the literature. ROC curve analysis confirmed that the optimal SII value for the composite endpoint, >594.0, predicted the composite endpoint with a higher sensitivity of 73.8%, specificity of 49.4%, and AUC= 0.629 compared to other parameters (P=0.001;
Table 5;
Figure 1).
Our study had several limitations, the most important of which was that it was retrospective and single-center. Another one is the small number of participants.
5. Conclusions
Our research is significant in determining the predictive cut-off value of the Systemic Immune Inflammation (SII) Index for microvascular and macrovascular complications, mortality, and composite endpoints in the first year among patients with type 2 diabetic retinopathy. Additionally, both in the first year and in the following three years, it is valuable for identifying SII values that can predict the development of acute or chronic renal failure due to diabetic nephropathy, peripheral arterial disease, and hospital admissions for any reason among these patients. This capability enables early diagnosis of potential complications in patients with type 2 diabetic retinopathy, leading to timely treatment. Thus, patients at risk can be identified through simple hemogram parameters used to calculate the SII, potentially preventing or halting the progression of diabetic complications. This not only helps prevent a decline in patients' quality of life but also results in significant health care cost savings. Moreover, predicting the risk of death within the first year could also enhance patient survival rates.
Author Contributions
Conceptualization, N.T.T. and M.C.; methodology, N.T.T.; software, N.T.T.; validation, N.T.T. and M.C.; formal analysis, N.T.T.; investigation, N.T.T.; resources, N.T.T.; data curation, N.T.T.; writing—original draft preparation, N.T.T. and M.C.; writing—review and editing, N.T.T. and M.C.; visualization, N.T.T.; supervision, N.T.T and M.C..; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Institutional Review Board Statement
Ethics committee approval for this study was obtained from the Ethics Committee of the Faculty of Medicine of our University with the decision number and protocol code TÜTF-GOBAEK 2023/341 dated 29/09/2023, and all procedures in this study were performed under the Declaration of Helsinki and its subsequent amendments.
Informed Consent Statement
This study was conducted retrospectively as a file review.
Data Availability Statement
Our study data contain personal information of patients, and therefore, are not available for sharing due to the 'Personal Data Protection Law' and ethical reasons.
Acknowledgments
We would like to thank Prof. Dr. Necdet Süt for his assistance in conducting this study.
Conflicts of Interest
The authors declare no conflicts of interest
References
- U. D. K. Group, Diabetes Mellitus Tanı, Sınıflama ve İzlem İlkeleri. Diyabet Tanı ve Tedavi Rehberi 2019, 9nd ed.; Balcı, M. K., Ed.; Turkish Diabetes Foundation: İstanbul, Türkiye, 2020; pp. 16–17. [Google Scholar]
- Bhattacharyya, S.; Jain, N.; Verma, H.; Sharma, K. A Cross-sectional Study to Assess Neutrophil Lymphocyte Ratio as a Predictor of Microvascular Complications in Type 2 Diabetes Mellitus Patients. JCDR 2021, 15, OC59–OC62. [Google Scholar] [CrossRef]
- Kocaeli, A.A.; Gül, Ö.Ö. Diabetes Mellitusun Epidemiyolojisi. Diabetes Mellitusun Tanı, Tedavi ve İzlemi, 1nd ed.; İmamoğlu, Ş., Ersoy, C.Ö., Eds.; Bursa Uludağ University: Bursa, Türkiye, 2022; pp. 43–57. [Google Scholar]
- De Ferranti, S.D.; De Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 2014, 130, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J. ; Fauci,A.S.; Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; Harrison's principles of internal medicine, 20rd ed.; McGraw-hill education: Online, United States of America, 2018; pp. 2850–2889. [Google Scholar]
- Lonardo, A. Liver Fibrosis: more than meets the eye. Ann. Hepatol. 2024, 101479. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; He, J.; Zhang, R.; Yuan, S.; Dou, K. The Combined Effect of Systemic Immune-Inflammation Index and Type 2 Diabetes Mellitus on the Prognosis of Patients Undergoing Percutaneous Coronary Intervention: A Large-Scale Cohort Study. J. Inflamm. Res. 2023, ume 16, 6415–6429. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Li, H.; Wang, L.; Geng, J.; Yang, Q.; Su, B.; Liao, R. Systemic Immune-Inflammation Index Is Associated With Increased Urinary Albumin Excretion: A Population-Based Study. Front. Immunol. 2022, 13, 863640. [Google Scholar] [CrossRef]
- Wang, P.; Guo, X.; Zhou, Y.; Li, Z.; Yu, S.; Sun, Y.; Hua, Y. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front. Endocrinol. 2022, 13, 944991. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162. [Google Scholar] [CrossRef]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2019, 43, 487–493. [Google Scholar] [CrossRef]
- Wang, R.-H.; Wen, W.-X.; Jiang, Z.-P.; Du, Z.-P.; Ma, Z.-H.; Lu, A.-L.; Li, H.-P.; Yuan, F.; Wu, S.-B.; Guo, J.-W.; et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front. Immunol. 2023, 14, 1115031. [Google Scholar] [CrossRef]
- Ozkan, U.; Gurdogan, M. TyG index as a predictor of spontaneous coronary artery dissection in young women. Postgrad. Med. 2023, 135, 669–675. [Google Scholar] [CrossRef] [PubMed]
- e-nabız personal health system.Turkish ministry of health. https://www.enabiz.gov.tr/ (accessed 21.04.2024).
- Herrera, M.C.A.; Subhan, F.B.; Chan, C.B. Dietary Patterns and Cardiovascular Disease Risk in People with Type 2 Diabetes. Curr. Obes. Rep. 2017, 6, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Aubin, A.; Loomba, R. The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr. Diabetes Rep. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Shaw, L.C.; Grant, M.B. Inflammation in the pathogenesis of microvascular complications in diabetes. Front. Endocrinol. 2012, 3, 170. [Google Scholar] [CrossRef]
- Zitouni, K.; Steyn, M.; Earle, K.A. Residual renal and cardiovascular disease risk in conventionally-treated patients with type 2 diabetes: the potential of non-traditional biomarkers. Minerva Medica 2018, 109, 103–115. [Google Scholar] [CrossRef]
- Portillo, J.-A.C.; Yu, J.-S.; Vos, S.; Bapputty, R.; Corcino, Y.L.; Hubal, A.; Daw, J.; Arora, S.; Sun, W.; Lu, Z.-R.; et al. Disruption of retinal inflammation and the development of diabetic retinopathy in mice by a CD40-derived peptide or mutation of CD40 in Müller cells. Diabetologia 2022, 65, 2157–2171. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef]
- Giovenzana, A.; Carnovale, D.; Phillips, B.; Petrelli, A.; Giannoukakis, N. Neutrophils and their role in the aetiopathogenesis of type 1 and type 2 diabetes. Diabetes/Metabolism Res. Rev. 2021, 38, e3483. [Google Scholar] [CrossRef] [PubMed]
- Njeim, R.; Azar, W.S.; Fares, A.H.; Azar, S.T.; Kassouf, H.K.; Eid, A.A. NETosis contributes to the pathogenes of diabetes and its complications. J. Mol. Endocrinol. 2020, 65, R65–R76. [Google Scholar] [CrossRef]
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef]
- Klisic, A.; Scepanovic, A.; Kotur-Stevuljevic, J.; Ninic, A. Novel leukocyte and thrombocyte indexes in patients with prediabetes and type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, E.A.; Gąsior, J.S.; Tuzimek, A.; Paleczny, J.; Junka, A.; Dąbrowski, M.; Jankowski, P. Investigation of the Associations of Novel Inflammatory Biomarkers—Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)—With the Severity of Coronary Artery Disease and Acute Coronary Syndrome Occurrence. Int. J. Mol. Sci. 2022, 23, 9553. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, X.; Jia, B.; Chen, S. Exploring the Correlation Between the Systemic Immune Inflammation Index (SII), Systemic Inflammatory Response Index (SIRI), and Type 2 Diabetic Retinopathy. Diabetes Metab Syndr Obes, 2023; 3827–3836. [Google Scholar] [CrossRef]
- Suvarna, R.; Biswas, M.; Shenoy, R.P.; Prabhu, M.M. Association of clinical variables as a predictor marker in type 2 diabetes mellitus and diabetic complications. J. Biomed. 2023, 43, 335–340. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhang, Y.; Dan, X.; Wu, X.; Yang, Y.; Chen, X.; Li, S.; Xu, Y.; Wan, Q.; et al. Increased Systemic Immune-Inflammation Index Was Associated with Type 2 Diabetic Peripheral Neuropathy: A Cross-Sectional Study in the Chinese Population. J. Inflamm. Res. 2023, ume 16, 6039–6053. [Google Scholar] [CrossRef]
- Duman, T.T.; Ozkul, F.N.; Balci, B. Could Systemic Inflammatory Index Predict Diabetic Kidney Injury in Type 2 Diabetes Mellitus? Diagnostics 2023, vol. 13, p. 2063.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).